Summary
Converting resident glia into functional and subtype-specific neurons in vivo by delivering reprogramming genes directly to the brain provides a step forward toward the possibility of treating brain injuries or diseases. To date, it has been possible to obtain GABAergic and glutamatergic neurons via in vivo conversion, but the precise phenotype of these cells has not yet been analyzed in detail. Here, we show that neurons reprogrammed using Ascl1, Lmx1a, and Nurr1 functionally mature and integrate into existing brain circuitry and that the majority of the reprogrammed neurons have properties of fast-spiking, parvalbumin-containing interneurons. When testing different combinations of genes for neural conversion with a focus on pro-neural genes and dopamine fate determinants, we found that functional neurons can be generated using different gene combinations and in different brain regions and that most of the reprogrammed neurons become interneurons, independently of the combination of reprogramming factors used.
Keywords: in vivo reprogramming, interneurons, striatum, NG2-glia, functional neurons, environmental effect, midbrain
Highlights
-
•
Reprogramed striatal NG2 glia yield neurons similar to fast-spiking interneurons
-
•
Reprogrammed neurons reach functional maturation after 12 weeks
-
•
Dopamine (DA) denervation leads to TH expression but no reprogramming into DA neurons
-
•
Variation in pro-neural genes or fate specifiers does not affect neuronal phenotype
In this study, Parmar, Ottosson, and colleagues show how endogenous NG2 glia can be reprogrammed into GABAergic interneurons of different subtypes, the majority of them with properties of fast-spiking parvalbumin-containing interneurons. This neuronal subtype has been implicated in several neurological diseases, and the findings can open up new therapeutic options.
Introduction
Direct cellular reprogramming provides a route to generate neurons from somatic cells in vitro (Vierbuchen et al., 2010) that opens up new possibilities to obtain patient- and disease-specific neurons, and several groups have reported successful reprogramming into functional neurons of distinct subtypes in vitro (reviewed in Masserdotti et al., 2016). More recently, it has been shown that non-neural cells can be reprogrammed into functional neurons in situ (reviewed in Grealish et al., 2016). Many of the neurons obtained acquire a GABAergic or glutamatergic identity (Grande et al., 2013, Torper et al., 2015), but the exact subtype identity and how fate specification is controlled during in vivo conversion remains an important question.
In this study, we performed a time-course analysis of NG2 glia reprogrammed into neurons using Ascl1, Lmx1a, and Nurr1 (ALN). We show that in vivo reprogrammed neurons functionally mature over time and that their ability to fire action potentials (AP) precedes circuitry integration. We also reprogrammed neurons in the dopamine (DA)-depleted striatum and in the midbrain, and tested different combinations of pro-neural genes and DA fate determinants. In all these conditions, we found only minor differences in the phenotype of the reprogrammed cells. A detailed analysis using electrophysiology, immunohistochemistry, and transcriptional profiling showed that most of the reprogrammed neurons acquire properties of fast-spiking (FS), parvalbumin (PV)+ interneurons (IntNs), a neuronal subtype that plays a highly interesting role in striatal function and with potentially important therapeutic roles.
Results
Gradual Maturation into Functional Neurons
We injected NG2-Cre mice with CRE-dependent ALN conversion vectors and a GFP reporter that specifically labels reprogrammed neurons (Torper et al., 2015). To estimate the conversion efficiency, we also injected animals (n = 3) with a Cre-dependent GFP under the ubiquitous chicken beta-actin (cba) promoter rendering all targeted cells GFP+. We found that the vectors efficiently targeted NG2 glia (Figure S1A), and estimated that 66.81% ± 38.38% of targeted cells converted into neurons (Figure S1B).
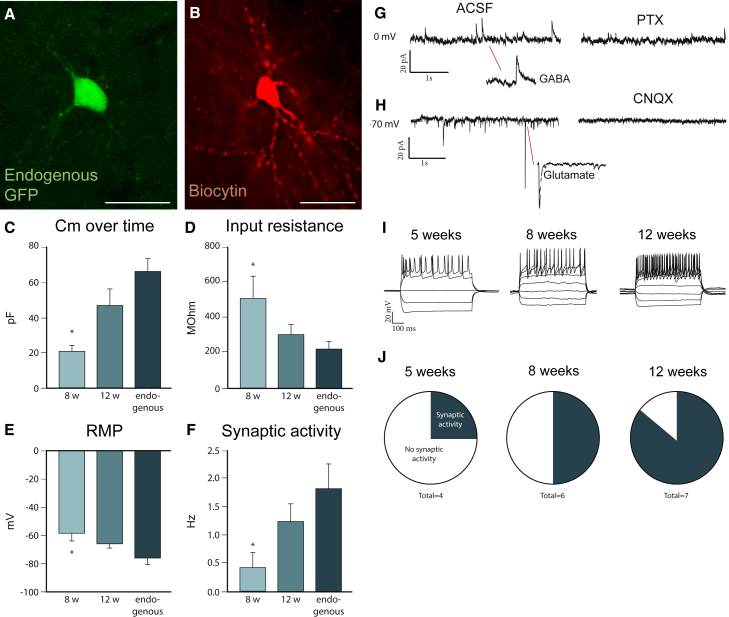
Reprogrammed neurons were detected by their endogenous GFP expression (Figure 1A). Biocytin neuronal filling of such GFP + neurons revealed mature neuronal morphologies and extensive dendritic trees of the reprogrammed neurons (Figure 1B). Electrophysiological recordings performed on the GFP-expressing neurons 5, 8, or 12 weeks post-injection (w.p.i.). showed that membrane-intrinsic properties gradually matured: Membrane capacitance (Cm) increased (Figure 1C), while the input resistance and the resting-membrane potential (RMP) decreased (Figures 1D and 1E), indicating that the cells acquired more ion channels, increased in size, and gained more elaborate morphology with time. During the same time period, the frequency of spontaneous postsynaptic activity increased, suggesting added postsynaptic connections (Figure 1F). For all these parameters, cells were not significantly different from endogenous cells by 12 w.p.i. (Figures 1C–1F).
Figure 1.
Reprogrammed Neurons Functionally Mature over Time
(A) Endogenous GFP expression can be detected in a reprogrammed neuron (unstained tissue) and was used to identify cells for recordings.
(B) Post-recorded biocytin-filled neuron shows mature neuronal morphology including dendritic spines.
(C–F) The reprogrammed neurons show maturation in membrane-intrinsic properties from 8 to 12 w.p.i., as shown for (C) membrane capacitance (Cm), (D) input resistance, (E) resting-membrane potential (RMP), and (F) frequency in spontaneous postsynaptic activity (for Cm, input resistance, and RMP, n = 6 [8 w.p.i.], n = 7 [12 w.p.i.], and n = 7 [endogenous]; for spontaneous postsynaptic activity, n = 4 [5 w.p.i.], n = 6 [8 w.p.i.], n = 7 [12 w.p.i.], and n = 5 [endogenous]).
(G and H) Traces showing (G) inhibitory (GABAergic) activity that was blocked with picrotoxin, a GABAA receptor antagonist and (H) excitatory activity that was blocked with CNQX, an AMPA receptor antagonist.
(I) Reprogrammed neurons show repetitive firing already at 5 w.p.i. and continue to show that at 8 and 12 w.p.i.
(J) The number of neurons with postsynaptic activity increases over time.
All data are presented as means ± SEM; ∗p < 0.05 in one-way ANOVA. Scale bars: (A and B), 25 μm. See also Figure S1.
Spontaneous inhibitory currents could be blocked with picrotoxin, and spontaneous excitatory currents could be blocked with CNQX (Figures 1G and 1H), indicating that reprogrammed neurons received synaptic input from both inhibitory and excitatory terminals, probably from nearby striatal neurons (GABAergic) and from more distal glutamatergic terminals (corticostriatal and thalamostriatal). While all cells but one (n = 17) showed repetitive firing from 5 w.p.i. (Figures 1I and S1C–S1F), few neurons at this time point showed postsynaptic activity. The proportion of neurons that displayed postsynaptic activity increased from 5 to 12 w.p.i. (Figures 1J and S1C–S1S).
ALN Reprogrammed Neurons in the Striatum Display Functional, Histological, and Molecular Properties Similar to Interneurons
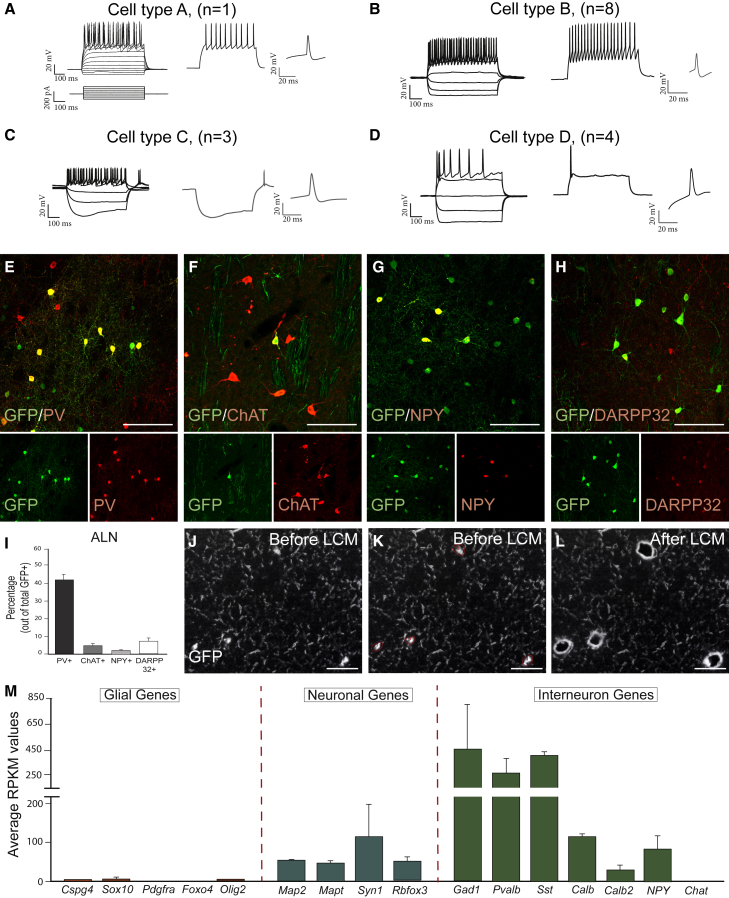
A more detailed analysis revealed several distinct firing patterns. Only one of the patched cells showed firing properties characteristic of striatal medium-spiny projection neurons (MSNs, cell type A in Figure 2A), while most cells showed a firing pattern similar to striatal IntNs (Figures 2B–2D) (Kawaguchi, 1993). Most cells displayed hyperpolarized resting membrane potential and similar firing frequency and input resistance as fast-spiking IntN (FSI) (Povysheva et al., 2013), with relatively short AP even though the AP duration and spike after-hyperpolarization were not yet in the range of their endogenous counterpart (Kawaguchi, 1993) (cell type B in Figure 2B and Table S1). Some cells showed other firing patterns reminiscent of long-lasting (LA) after-hyperpolarization or persistent and low-threshold spiking (PLTS) cells (cell type C and D, Figures 2C and 2D and Table S1). Immunohistochemical analysis at 12w.p.i. revealed the presence of markers common to IntNs such as PV (marker of FS cells), ChAT (marker of cholinergic, LA cells), NPY (marker of PLTS cells), or the striatal projection neuron marker DARPP32 (Figures 2E–2H). Quantifications showed that the majority (41.27% ± 2.99%) co-expressed PV, whereas less than 10% of the GFP+ cells were co-labeled with any of the other markers (Figure 2I). Thus, many of the IntN-specific markers that are not present at 6 w.p.i. (Torper et al., 2015) appear after additional maturation time in vivo.
Figure 2.
Phenotypic Identities of Reprogrammed Neurons
(A–D) The firing patterns were of four distinct types: (A) similar to endogenous medium-spiny neuron (1/16; cell type A); (B) similar to FSI (8/16, cell type B); (C) similar to low-threshold spiking cells with prominent sag (3/16, cell type C); (D) firing with large after-hyperpolarization (4/16, cell type D).
(E–H) Confocal images showing co-localization of GFP and the interneuron markers PV (E), ChAT (F), NPY (G), and projection neuron marker DARPP32 (H).
(I) Percentage of neurons expressing the markers from (E–H) shows that the majority of ALN-converted neurons are PV+ (n = 9 brains).
(J–L) LCM was performed on nc-GFP-expressing cells: (J) before LCM; (K) before LCM; (L) after LCM.
(M) RNA-seq results, presented as average RPKM (reads per kilobase per million) values, show the downregulation of glial genes and upregulation of pan-neuronal genes and IntN-linked genes (n = 12–65 cells from n = 2–3 brains).
All data are presented as means ± SEM. Scale bars: (E–H), (J–L), 50 μm. See also Figure S2.
To generate a transcriptional profile, we isolated ncGFP-expressing reprogrammed cells using laser capture microdissection (LCM) (Figures 2J–2L). Collected cells were pooled (n = 12–65 cells/sample) and prepared for polyA-based RNA sequencing (LCM-seq) (Nichterwitz et al., 2016). The sequencing data confirmed high expression levels of the reprogramming genes ALN (Figure S2E), downregulation of glial genes, and upregulation of pan-neuronal genes (Figure 2M). We also detected expression of IntN-linked genes (Figure 2M), confirming the histological and electrophysiological analysis.
DA Denervation or Reprogramming Region Does Not Affect Reprogramming Efficiency, Maturation, or Phenotype
We next tested if DA denervation that radically changes the striatal compartment and induces glia activation (Walsh et al., 2011) could affect reprogramming in vivo. NG2-Cre mice received a unilateral 6-OHDA toxin injection into the medial forebrain bundle (mfb lesion, n = 9), which produced a substantial loss of DA neurons in the SNc (Figures S3A and S3B) and subsequent loss of their projections to the dorsolateral striatum. Littermate control animals were left intact (n = 10).
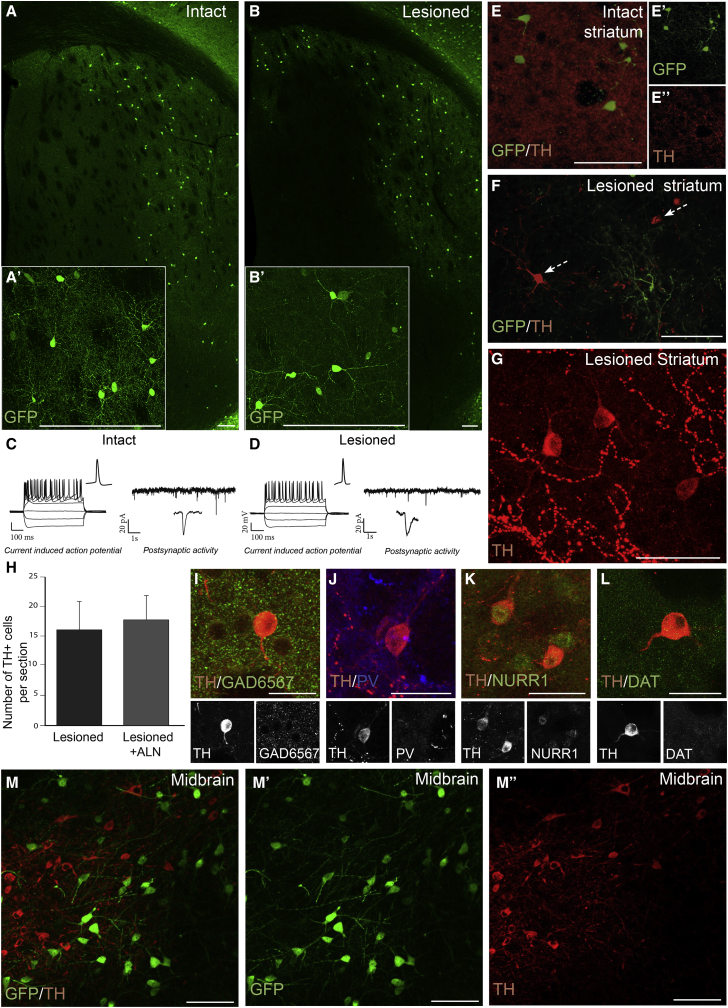
Three weeks after lesions, animals were injected with ALN into the striatum and analyzed 12 w.p.i. GFP+ neurons were abundant in the lateral striatum and found in equal numbers in both intact and lesioned animals (Figures 3A, 3B, and S3C). TH+ cell bodies were found in the striatum of lesioned animals (Figure 3F) but not in intact controls (Figure 3E), which could be indicative of reprogramming into DA neurons under these conditions, as has been suggested in a recent study using similar DA conversion factors to reprogram resident mouse astrocytes (Rivetti di Val Cervo et al., 2017). However, none of the TH+ neurons were co-labeled with the GFP reporter (Figure 3F), suggesting that these were not in vivo reprogrammed neurons. Indeed, ectopic TH+ cell bodies were present in similar numbers in the striatum in control animals that were lesioned but not reprogrammed 15 weeks after lesion (Figures 3G and 3H). Like in the study by Rivetti di Val Cervo et al. (2017), most TH+ neurons in the control lesioned animals were negative for GABAergic IntN markers (Figures 3I and 3J) and positive for other DA markers, such as Nurr1 (Figure 3K), but only weakly expressing DAT (Figure 3L). Such ectopic striatal TH+ cell bodies have been found after lesion as reported in a number of studies (reviewed in Tepper and Koós, 2010), and we also confirmed their presence after 15 weeks in lesioned wild-type mice from a separate experiment (Figure S3K).
Figure 3.
In Vivo Reprogramming of Resident NG2 Glia in a DA-Denervated Striatum and in the Midbrain
(A and B) Confocal images (stitched into a tile) of striatal sections showing GFP+ reprogrammed neurons that occur with similar efficiency (A and B) and morphology (A′ and B′) in the intact (A) and 6-OHDA lesioned (B) brain.
(C and D) Neurons in both conditions, (C) intact and (D) lesioned, showed repetitive current-induced action potentials (AP; traces on the left) and spontaneous postsynaptic events (traces on the right), in the absence of any drugs or stimulation.
(E) Reprogrammed neurons in the intact brain express GFP (E′), but not TH (E″).
(F) TH+ neurons were observed in the striatum after 6-OHDA mfb lesion, but these cells do not co-express GFP (arrows).
(G) Confocal image of abundant TH+ cells appearing in control animals (lesioned but no reprogramming factors).
(H) Quantification of the average number of TH+ cells found per section in control lesioned versus lesioned + reprograming factors groups (n = 6/group).
(I–L) Analysis of TH+ cells show that they do not express the striatal markers GAD65/67 (I) or PV (JK), but do express Nurr1 (K), and low levels of DAT (L).
(M–M″) In vivo reprogramming of resident NG2 glia in the midbrain results in endogenous TH+ cells (red) intermingled with GFP+ (green) reprogrammed neurons, with no overlap.
Data are presented as means ± SEM. Scale bars: (A–F) 100 μm; (A′ and B′) 100 μm; (G) 50 μm; (I–L) 25 μm; (M–M″) 50 μm. See also Figure S3.
The reprogrammed neurons in both the intact and lesioned brains were analyzed using whole-cell patch clamp recordings. All neurons (n = 12) showed similar physiological properties with the ability to induce repetitive APs (Figures 3C and 3D) and also contained voltage-gated sodium and potassium currents (Figure S3D). Furthermore, the majority of cells (n = 9) displayed spontaneous postsynaptic activity (Figure S3E). The cells in the lesioned mice showed similar frequency in spontaneous activity as in intact mice (1.44 ± 0.36 Hz for intact, 1.52 ± 0.14 Hz for lesioned) (Figures 3C and 3D). None of the reprogrammed neurons recorded from displayed any DA-specific functional properties (Figure S3F).
We next tested if injecting the factors into the midbrain (homotopic environment for DA neurons) would influence the subtype toward DA identity. Analysis at 12 w.p.i. revealed the presence of GFP+ cells intermingled with endogenous nigral TH-expressing DA neurons (Figure 3M). None of the reprogrammed neurons co-expressed GFP and TH (Figures 3M–M″), even though a significant number of cells co-expressed GFP and the reprogramming factors ALN (Figures S3G–S3J).
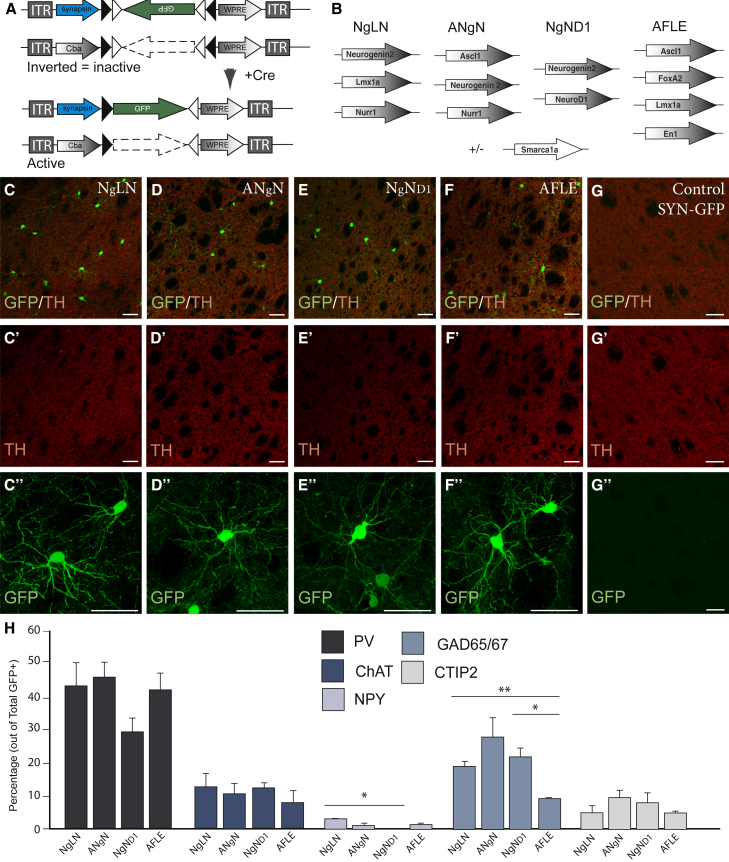
Overexpression of Different Gene Combinations in Striatal NG2 Glia
The appearance of IntNs after reprogramming using factors that give rise to DA neurons in vitro is intriguing and raises the question of how cell-fate conversion is controlled during in vivo conversion. We therefore next tested if different combinations of pro-neural (Ascl1, Ngn2, NeuroD1) and DA fate-specifying genes (Lmx1a, Nurr1, FoxA2, En1), could affect the phenotype of the converted neurons. Four different combinations of conversion factors, NgLN (Neurogenin2, Lmx1a, and Nurr1), ANgN (Ascl1, Neurogenin2, and Nurr1), NgND1 (Neurogenin2 and NeuroD1), and AFLE (Ascl1, FoxA2, Lmx1a, and En1), were injected either alone or together with the midbrain-specific chromatin remodeler Smarca1 (Metzakopian et al., 2015) into the striatum of intact NG2-CRE mice (Figures 4A and 4B). GFP+ cells with complex neuronal morphology (Figures 4C″–4F″) could be observed in all groups. However, no co-expression of TH and GFP reporter could be detected (Figures 1C–1F and Figures 1C′–1F′). No GFP+ neurons were detected in control animals injected with the reporter vector alone (Figures 1G–1G″).
Figure 4.
Different Gene Combinations Expressed in Striatal NG2 Glia Lead to Minor Differences in Neuronal Phenotype
(A) Schematics of AAV5 constructs used for in vivo reprogramming.
(B) Genes were grouped into four different combinations: NgLN, ANgN, NgND1, and AFLE. These groups of factors were used alone or in combination with Smarca1a.
(C–F) Confocal images showing the presence of GFP+ neurons 12 w.p.i. in the brains of NG2-Cre mice, when different combinations of genes were used for in vivo reprogramming: (C) NgLN, (D) ANgN, (E) NgND1, and (F) AFLE. (C″–F″) The reprogrammed neurons show a mature morphology but did not express TH.
(G–G″) In control animals where only SYN-GFP was injected in the brain of NG2-Cre mice, no GFP+ neurons were detected.
(H) Quantification of neurons reprogrammed with different factor combinations that express the markers PV, ChAT, NPY, GAD65/67, and CTIP2 shows that the majority of neurons obtained are PV-positive in all conditions (n = 3 brains/combination).
All data are presented as means ± SEM, and an unpaired t test was performed to evaluate differences between percentages of double-positive cells found among the four conditions (NgLN, ANgN, NgND1, AFLE); ∗p < 0.005 (p = 0.0018 for differences in NPY expression, and p = 0.0019 for differences in GAD65/67 expression); ∗∗p < 0.05 (p = 0.0081 for differences in GAD65/67 expression). Scale bars: all 25 μm. See also Figure S4.
A more detailed phenotypic analysis of the reprogrammed neurons using the different factor combinations revealed that 9.03% to 27.01% of the reprogrammed cells expressed GAD65/67, while no VGlut1+ neurons were identified in any condition (data not shown). Similar to ALN, the largest proportion expressed the interneuron marker PV. ChAT+ and NPY+ neurons were found in lower percentages and CTIP2 was found in less than 10% of the reprogrammed neurons (Figures 4H and S4A–S4E).
Discussion
In vivo reprogramming has emerged as a future possibility for brain repair. However, the phenotype of the reprogrammed cells obtained in vitro often differs from that obtained in vivo (Su et al., 2014). For example, several factors that convert astrocytes into neurons in vitro (Berninger et al., 2007, Heinrich et al., 2010) fail to do so in vivo (Grande et al., 2013). Our group has previously reported successful reprogramming of resident NG2 glial cells into neurons in vivo, using ALN. Despite the fact that these genes give rise to TH-expressing DA neurons when fibroblasts and astrocytes are reprogrammed in vitro (Addis et al., 2011, Caiazzo et al., 2011), no TH-expressing neurons were generated via in vivo reprogramming (Torper et al., 2015). Here, we reprogrammed NG2 glia in the 6-OHDA lesion mouse model, a condition that was also used in a study published during the revision of this manuscript, to reprogram mouse astrocytes using a slightly different combination of genes and miRNAs (Rivetti di Val Cervo et al., 2017). In both these studies, reprogramming was shown to be achievable in the DA-denervated striatum, which supports the use of in vivo reprogramming for brain repair. However, the interpretation of the finding that TH+ cells appear after reprogramming in the lesioned striatum (this study and the Rivetti di Val Cervo et al., 2017) is complicated as non-dopaminergic TH+ neurons appear spontaneously in response to the DA-denervating lesion (Tepper and Koós, 2010). We include a GFP reporter for identification of reprogrammed neurons and found that GFP did not co-label with TH. We conclude therefore that the neurons we observe are not TH+ DA neurons generated via reprogramming but rather striatal neurons that express TH in response to the lesion. In the Rivetti di Val Cervo et al (2017) study, the origin of the TH+ cells is unclear as no reporter was used, and no significant effect on DA-dependent behavior was observed. Thus, more work is needed in order to obtain functional DA neurons via in vivo reprogramming.
The functional properties of the reprogrammed neurons mature over time, and by 12 weeks the reprogrammed cells have phenotypic and functional properties of IntNs. The striatum is mainly composed of MSNs (95%) and, to a lesser extent, IntNs of different subtypes. Given the endogenous subtype distribution of striatal neurons, the selective conversion into neurons with properties of FSI expressing PV, which normally accounts for less than 1% in the striatum, is noteworthy. This raises the question of how cell fate is influenced during in vivo conversion. We tested three different pro-neural genes (Ascl1, Ngn2, and NeuroD1), which have all been used previously for neural conversion (Grande et al., 2013, Guo et al., 2014, Liu et al., 2015), and found that independently of which neurogenic genes or fate determinants are used, the reprogrammed cells still were of an interneuron identity, and the different combinations had only minor impact on the subtype identity.
The presence of GABAergic neurons when Ngn2 was used for conversion may seem counterintuitive. However, during in vivo reprogramming, Ngn2 has previously been shown to drive both GABAergic and glutamatergic phenotypes (Grande et al., 2013, Gascon et al., 2016). This may be explained by differences in starting cell types that can have different transcriptional accessibility of target genes (Wapinski et al., 2013), or differences in the level of expression as high levels of Ngn2 drive glutamatergic differentiation in the developing forebrain but support GABAergic neuron formation when expressed at low levels (Parras et al., 2002).
GABAergic IntNs have previously been generated from resident glia, both in the latent state or after trauma such as stroke (Grealish et al., 2016). Our study shows the direct reprogramming of resident NG2 glia into neurons similar to FS, PV-containing IntNs, a subtype that plays a highly interesting role in striatal function. An initial deprivation of DA input in the striatum does create an imbalance in local circuits that involve IntNs (Martinez-Cerdeno et al., 2010), and GABAergic stimulation in the striatum could support and enhance the effects from intrastriatal DA transplants (Winkler et al., 1999). In line with this, intrastriatal transplantation of FSI precursors has even shown motor improvement in a rat Parkinson's disease (PD) model (Mallet et al., 2006). Moreover, striatal FSI dysfunction may underlie some forms of dystonia in PD (Gittis et al., 2011). This type of IntN has implications also in several diseases affecting the human striatum such as Tourette's syndrome, Huntington's disease, and paroxysmal dystonia (Reiner et al., 2013, Kalanithi et al., 2005, Gernert et al., 2000), which makes these neurons interesting from a therapeutic point of view (Spatazza et al., 2017).
Experimental Procedures
Cloning and Viral Vector Production
Cre-inducible AAV5 vectors were created using a similar approach as in Torper et al. (2015).
Animals and Surgery
All experimental procedures were carried out under the European Union Directive (2010/63/EU) and approved by the ethical committee for the use of laboratory animals at Lund University and the Swedish Department of Agriculture (Jordbruksverket). Surgeries were performed under general anesthesia using 2% isoflurane mixed with air at a 2:1 ratio. For in vivo conversion experiments, 1 μL of vector mix (AAV5) was injected into the striatum of each animal at a rate of 0.4 μL/min with a diffusion time of 2 min.
Electrophysiology
Patch-clamp electrophysiology was performed on striatal brain slices from ALN-injected mice using same methods as in Torper et al. (2015).
RNA Sequencing of Nuclear GFP+ Cells Isolated by Laser Capture Microdissection
Mouse brains were dissected (n = 2–3 animals/group) after decapitation and snap frozen in 2-methylbutane (Sigma-Aldrich) on dry ice and stored at −80°C until further processed. Brains were sectioned in a cryostat at −20°C, and striatal sections with target GFP-positive cells placed onto PEN membrane glass slides (Zeiss) (three replicate slides per animal), which were kept at −20°C during the sectioning and subsequently stored at −80°C. Transcriptomic data were generated using LCM-seq (Nichterwitz et al., 2016).
For more detailed information, see Supplemental Information.
Author Contributions
M. Parmar, D.R.O., M. Pereira, J.A.B., and E.H. planned and structured the experiments. M. Pereira, S.S., M.B., J.A.B., and D.R.O. performed experiments. M. Pereira, D.R.O., and M. Parmar analyzed data and wrote the manuscript. All authors had input and gave final approval of the manuscript.
Acknowledgments
We thank Bengt Mattsson, Michael Sparrenius, Ulla Jarl, Elsy Ling, and Jenny Johansson for technical assistance, Angela Cencci Nilsson and Laura Andreoli for providing sections from lesioned wild-type mice, and Isak Wernehov for help with histology and analysis. The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Program: FP/2007–2013 NeuroStemcellRepair (no. 602278) and ERC Grant Agreement no. 30971, the New York Stem Cell Foundation, the Swedish Research Council (grants to M.P. and E.H.), the Swedish Parkinson Foundation (M.P. and E.H.), and the Strategic Research Area at Lund University Multipark. D.R.O. was funded by the Parkinson Research Foundation, Svenska sällskapet för medicinsk forskning, Fysiografen, and Åhléns-stiftelsen in Sweden. M.B. and S.S. are funded via BrainMatTrain, European Union Horizon 2020 Program (H2020-MSCA-ITN-2015) under the Marie Skłodowska-Curie Initial Training Network and Grant Agreement No. 676408. M.P. is a New York Stem Cell Foundation - Robertson Investigator.
Published: August 24, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.07.023.
Contributor Information
Malin Parmar, Email: malin.parmar@med.lu.se.
Daniella Rylander Ottosson, Email: daniella.ottosson@med.lu.se.
Accession Numbers
The accession numbers for the gene sequences (the vectors avaliable via Addgene) are as follows: Ascl1, RefSeq: NM_008553.4; Lmx1a, RefSeq: NM_033652.5; Nurr1, RefSeq: NM_013613.2; Ngn2, RefSeq: NM_009718.2; NeuroD1, RefSeq: NM_010894.2; FoxA2, RefSeq: NM_001291065.1; En1, RefSeq: NM_010133.2; Smarca1, RefSeq: NM_053123.4.
Supplemental Information
References
- Addis R.C., Hsu F.C., Wright R.L., Dichter M.A., Coulter D.A., Gearhart J.D. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS One. 2011;6:e28719. doi: 10.1371/journal.pone.0028719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B., Costa M.R., Koch U., Schroeder T., Sutor B., Grothe B., Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M., Dell'Anno M.T., Dvoretskova E., Lazarevic D., Taverna S., Leo D., Sotnikova T.D., Menegon A., Roncaglia P., Colciago G. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Gascon S., Murenu E., Masserdotti G., Ortega F., Russo G.L., Petrik D., Deshpande A., Heinrich C., Karow M., Robertson S.P. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell. 2016;18:396–409. doi: 10.1016/j.stem.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Gernert M., Hamann M., Bennay M., Loscher W., Richter A. Deficit of striatal parvalbumin-reactive GABAergic interneurons and decreased basal ganglia output in a genetic rodent model of idiopathic paroxysmal dystonia. J. Neurosci. 2000;20:7052–7058. doi: 10.1523/JNEUROSCI.20-18-07052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis A.H., Hang G.B., LaDow E.S., Shoenfeld L.R., Atallah B.V., Finkbeiner S., Kreitzer A.C. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71:858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande A., Sumiyoshi K., Lopez-Juarez A., Howard J., Sakthivel B., Aronow B., Campbell K., Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealish S., Drouin-Ouellet J., Parmar M. Brain repair and reprogramming: the route to clinical translation. J. Intern. Med. 2016;280:265–275. doi: 10.1111/joim.12475. [DOI] [PubMed] [Google Scholar]
- Guo Z., Zhang L., Wu Z., Chen Y., Wang F., Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C., Blum R., Gascon S., Masserdotti G., Tripathi P., Sanchez R., Tiedt S., Schroeder T., Gotz M., Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanithi P.S., Zheng W., Kataoka Y., DiFiglia M., Grantz H., Saper C.B., Schwartz M.L., Leckman J.F., Vaccarino F.M. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl. Acad. Sci. USA. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Miao Q., Yuan J., Han S., Zhang P., Li S., Rao Z., Zhao W., Ye Q., Geng J. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J. Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N., Ballion B., Le Moine C., Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J. Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V., Noctor S.C., Espinosa A., Ariza J., Parker P., Orasji S., Daadi M.M., Bankiewicz K., Alvarez-Buylla A., Kriegstein A.R. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserdotti G., Gascon S., Gotz M. Direct neuronal reprogramming: learning from and for development. Development. 2016;143:2494–2510. doi: 10.1242/dev.092163. [DOI] [PubMed] [Google Scholar]
- Metzakopian E., Bouhali K., Alvarez-Saavedra M., Whitsett J.A., Picketts D.J., Ang S.L. Genome-wide characterisation of Foxa1 binding sites reveals several mechanisms for regulating neuronal differentiation in midbrain dopamine cells. Development. 2015;142:1315–1324. doi: 10.1242/dev.115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichterwitz S., Chen G., Aguila Benitez J., Yilmaz M., Storvall H., Cao M., Sandberg R., Deng Q., Hedlund E. Laser capture microscopy coupled with Smart-seq2 for precise spatial transcriptomic profiling. Nat. Commun. 2016;7:12139. doi: 10.1038/ncomms12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras C.M., Schuurmans C., Scardigli R., Kim J., Anderson D.J., Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva N.V., Zaitsev A.V., Gonzalez-Burgos G., Lewis D.A. Electrophysiological heterogeneity of fast-spiking interneurons: chandelier versus basket cells. PLoS One. 2013;8:e70553. doi: 10.1371/journal.pone.0070553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A., Shelby E., Wang H., Demarch Z., Deng Y., Guley N.H., Hogg V., Roxburgh R., Tippett L.J., Waldvogel H.J., Faull R.L. Striatal parvalbuminergic neurons are lost in Huntington's disease: implications for dystonia. Mov. Disord. 2013;28:1691–1699. doi: 10.1002/mds.25624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivetti di Val Cervo P., Romanov R.A., Spigolon G., Masini D., Martin-Montanez E., Toledo E.M., La Manno G., Feyder M., Pifl C., Ng Y.H. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson's disease model. Nat. Biotechnol. 2017;35:444–452. doi: 10.1038/nbt.3835. [DOI] [PubMed] [Google Scholar]
- Spatazza J., Mancia Leon W.R., Alvarez-Buylla A. Transplantation of GABAergic interneurons for cell-based therapy. Prog. Brain Res. 2017;231:57–85. doi: 10.1016/bs.pbr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Niu W., Liu M.L., Zou Y., Zhang C.L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper J.M., Koós T. GABAergic Interneurons of the Striatum. In: Steiner H., Tseng K.Y., editors. Handbook of Behavioral Neuroscience. Elsevier; 2010. pp. 157–178. [Google Scholar]
- Torper O., Ottosson D.R., Pereira M., Lau S., Cardoso T., Grealish S., Parmar M. In vivo reprogramming of striatal ng2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Sudhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S., Finn D.P., Dowd E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson's disease in the rat. Neuroscience. 2011;175:251–261. doi: 10.1016/j.neuroscience.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Wapinski O.L., Vierbuchen T., Qu K., Lee Q.Y., Chanda S., Fuentes D.R., Giresi P.G., Ng Y.H., Marro S., Neff N.F. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell. 2013;155:621–635. doi: 10.1016/j.cell.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C., Bentlage C., Nikkhah G., Samii M., Bjorklund A. Intranigral transplants of GABA-rich striatal tissue induce behavioral recovery in the rat Parkinson model and promote the effects obtained by intrastriatal dopaminergic transplants. Exp. Neurol. 1999;155:165–186. doi: 10.1006/exnr.1998.6916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.