Abstract
Donor lung shortage has been the main reason to the increasing number of patients waiting for lung transplant. Ex vivo lung perfusion (EVLP) is widely expanding technology to assess and prepare the lungs who are considered marginal for transplantation. the outcomes are encouraging and comparable to the lungs transplanted according to the standard criteria. in this article, we will discuss the history of development, the techniques and protocols of ex vivo, and the logics and rationales for ex vivo use.
Keywords: Ex vivo lung perfusion (EVLP), ex vivo, lung transplant, lung reconditioning
Introduction
Lung transplantation is the ultimate solution for patients with end stage respiratory failure. Lung organ procurement rates from deceased donors are considerably lower than other solid organ procurement rates. Lungs are harvested from only 15–20% compared with 30% of deceased donors for hearts (1-3). This low percentage of transplanted lung is likely due to the potential complications of the lung that might occur before and after donor brain death such as thoracic trauma, aspiration, ventilator associated barotrauma injury, ventilator associated pneumonia, and neurogenic pulmonary edema (4-7). However, 40% of rejected donor lungs may have been suitable for transplantation (6,8). The number of patients awaiting transplant is expanding, and waitlist mortality is increasing concern due to the donor lung shortage (3-5). Multiple ways are used to expand the donor pool as extended criteria donors, donation after cardiac death (DCD) (9,10), and aggressive use of ECMO post-transplantation for marginal lungs (6,11-13), lobar lung transplantations were used for patients with small thoracic volume as well (14). more marginal lungs are used after assessment by ex vivo lung perfusion (EVLP), these extra marginal lungs used will expand the donor pool. using EVLP may ameliorate lung injury in some cases and allow transplantation from donors previously deemed unsuitable (15,16).
Ex vivo and lung transplant development history
The first normothermic ex vivo organ perfusion was described by Carrel and Lindbergh (17) in 1935. When they explanted thyroid glands of cats and rabbits and perfused them up to a week.
The first human lung transplantation was performed in 1963, and the recipient survived 18 days (18). Over the subsequent two decades, around 40 lung transplantations were performed, with a very low survival rate, as the majority of recipients died preoperatively because rejection, infections and of bronchial anastomotic complications. The first successful heart/lung transplantation was performed for idiopathic pulmonary arterial hypertension in 1981 (19). This was followed by successful single lung transplantation for idiopathic pulmonary fibrosis in 1983 (20), and double lung transplantation for emphysema in 1986 (21).
Normothermic EVLP was studied clinically in the 1980s by Hardesty, but it was abandoned due to lower outcomes (22).
Steen et al. in Sweden (9,23-25) developed a new method for ex vivo lung assessment in the mid 1990s allowing for objective evaluation for some hours to the lungs of non-heart-beating, this technique led to the first in human lung transplantation from a non-heart-beating donor in 2000 after successful evaluation by ex vivo (9). The same team performed in 2005 the first transplant of initially rejected lung after ex vivo lung “reconditioning” (26), this concept was proven in a study published in 2006 (27), by using EVLP on six lungs which were initially rejected then implanted with good outcomes. Same results were obtained by another team in the USA using a similar methodology (28). In 2009 Cypel et al. (16) proposed extended EVLP reassessment of lung function for transplant using a new protocol (Toronto protocol).
The rational and the indications of ex vivo use
EVLP will give a window of time to evaluate and recondition lungs of inferior quality outside the donor body before transplantation (27). During EVLP evaluation, the Lungs remain viable without additional injury during EVLP, as it is done at body temperature (37 °C), this makes the lungs metabolically active and viable for hours, putting the lungs in cold static will decrease cellular metabolism and will lead to decrease in oxygen and nutrients requirements, this will keep lungs in physiologic conditions prior to transplantation. Early favorable outcomes have been reported in recipients who underwent transplantation after EVLP to those with conventionally selected and transplanted lungs (27,29-31).
The EVLP is used in high-risk donor lungs which meet any one of the following five criteria: (I) best ratio of the partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FIO2) of less than 300 mmHg; (II) pulmonary edema, detected on the last chest radiograph; (III) poor lung deflation or inflation during intraoperative lung harvest; (IV) blood transfusions exceeding 10 units; (V) donor after cardiac death (DCD) (4,9,32).
EVLP should not be used if donor lungs have established pneumonia, severe mechanical lung injury including multi lobes trauma, and/ or gross gastric aspiration (9,32).
EVLP circuit and operating models
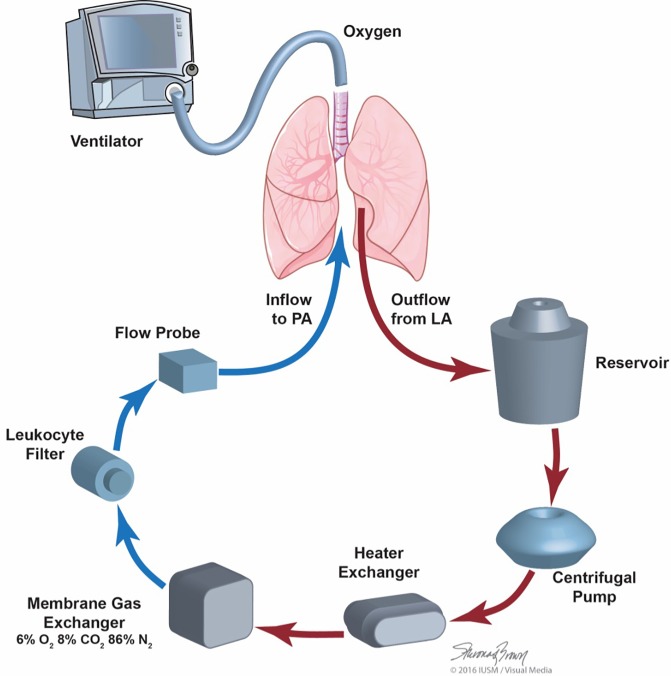
The EVLP system consists of multiple components available from the perfusion and respiratory departments. The basic components of an EVLP circuit include a ventilator, endotracheal tube, perfusion solution, reservoir, oxygenator, air filter, O2 sensor, and pump. In addition, a tank of de-oxygenating gas, tubing pack, cannulas to be connected to the pulmonary artery and left atrial cuff (Figures 1,2) (33).
Figure 1.
EVLP circuit with permission of Makdisi and Wozniak (33). O2, oxygen; CO2, carbon dioxide; N2, nitrogen; EVLP, ex vivo lung perfusion.
Figure 2.
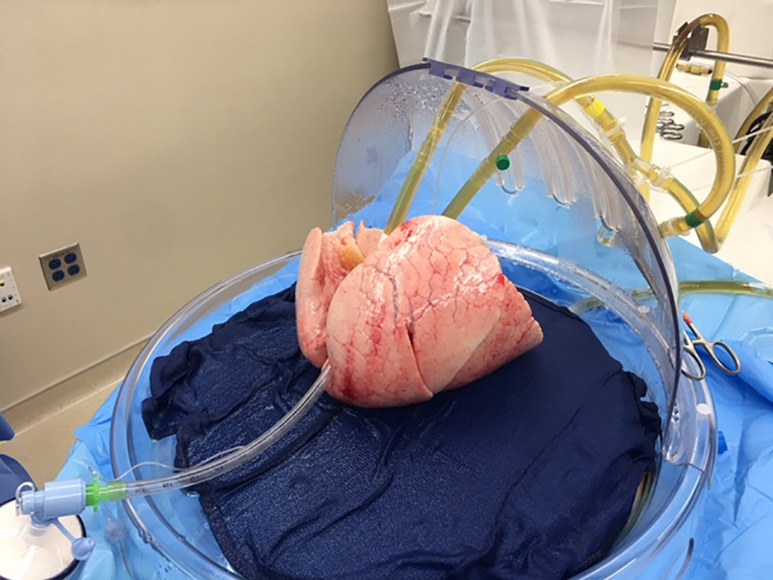
Lungs assessed by EVLP (Toronto protocol). EVLP, ex vivo lung perfusion.
There are 4 commercialized devices for clinical EVLP use: Organ Care System™ Lung (OCS); XPS™ (XVIVO Perfusion AB); Lung Assist® (Organ Assist); and Vivoline® LS1. OCS™ Lung is the only pulsatile and transportable device, the assessment starts at the donor hospital, OCS™ Lung and XPS™ are available in both US and CE (Conformité Européene) market, the Lung Assist® and Vivoline® LS1 are only available in the CE market. There are diverse differences among these devices in design and in clinical use.
There are three different EVLP protocols used to prepare and assess the lungs: the Toronto protocol which is the most commonly used protocol; the Lund protocol which is the original protocol of ex vivo, and the OCS™ protocol. The first 2 protocols are similar in a way that after the cold pulmonary flush and the lungs harvest, the lungs are kept in static cold storage (ice) during the transportation time to the recipient hospital when the lungs are connected to the ex vivo device and being assessed, this transport time while on ice increases the overall cold ischemia time.
On the other hand, the OCS can provide lung assessment starting immediately after the cold pulmonary flush and the lung harvest; sub sequentially it reduces the cold ischemic time during transportation (34), Table 1 summarize the most important variables among these three protocols.
Table 1. Comparison between the three different protocols of EVLP.
| Parameters | Lund | Toronto | OCS |
|---|---|---|---|
| Perfusion | |||
| Target flow | 40% of CO | 40% of CO | 2–2.5 L/min |
| Pressure | |||
| PA | Flow dictated | ≤20 mmHg | ≤20 mmHg |
| LA | 3–5 mmHg (closed) | 0 mmHg (open) | 0 mmHg (open) |
| Perfusate | Steen TM solution | Steen TM solution and RBCP Hct 14% | OCS TM solution and PRBC Hct 15–25% |
| Pump | Roller | Centrifugal | Piston (pulsatile) |
| Ventilation | |||
| Start temp (°C) | 32 | 32 | 34 |
| Tidal volume | 7 mL/kg | 5–7 mL/kg | 7 mL/kg |
| RR | 7 | 20 | 10 |
| PEEP | 5 cmH2O | 5 cmH2O | 5–7 cmH2O |
| FiO2 (%) | 21 | 50 | 12 |
| Sweep gas flow | Titrate to PA pCO2 34–38 mmHg | Titrate to PA pCo2 34–38 mmHg | |
| Temperature (°C) | |||
| Start of ventilation | 32 | 32 | 32 |
| Start of perfusion | 15 | 25 | 32 |
| Start of evaluation | 37 | 37 | 37 |
EVLP, ex vivo lung perfusion; OCS, Organ Care System; CO, cardiac output; PA, pulmonary artery; LA, left atrial; RBCP, red blood cell products; Hct, hematocrit; RR, respiratory rate; PEEP, positive end expiratory pressure; FiO2, fraction of inspired oxygen.
Assessment during EVLP
The graft can be examined, palpated, and evaluated clinically, bronchoscopically and radiographically allowing the surgeon to rule out tumors, areas of contusion, edema, infection, emboli, or other interstitial parenchymal abnormalities. Lung function including gas exchange, hemodynamics, and ventilatory mechanics is clinically evaluated for some hours (4,6). During these hours microbiologic, molecular, and morphological analysis are performed on any sampling obtained by bronchoalveolar lavage or any lung tissue specimens. These may help to guide the selection process of suitable organs in the future (35,36). Most important parameters monitored during assessment are listed in Table 2.
Table 2. Multiple parameters need to be monitored during assessments period.
| Gross anatomy: direct evaluation by the surgeon (weight, inspects of atelectasis, consolidation, dynamic compliance etc.), airway pressure |
| Radiologic CXR: assessment of lung improvement (reduction of lung edema) |
| Bronchoscopy: checking for clear secretions, edema, contusion |
| ABGs: the numbers, the tendency & curves, along with pO2/FiO2 less than 350 mmHg |
| Other factors: monitored are any decline of pulmonary vascular resistant, significant changes of peek inspiratory pressure |
pO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; CXR, chest X-ray; ABG, Arterial blood gas.
EVLP evidence-based
The role of EVLP for assessment and reconditioning of questionable donor lungs was investigated in multiple studies and currently ongoing trials. The results from the first prospective clinical trial the “HELP” trial was published in 2011, in this trial a group of 23 high-risk donors underwent EVLP and among these; 20 sets of lungs (87%) were considered suitable for transplantation. These were compared to a group consisting of 116 patients underwent transplantation according to the standard criteria. The study group didn’t observe any significant differences in terms of primary graft dysfunction (PGD), days on mechanical ventilation after transplant, ICU stay, hospital stay, and 30-day mortality (32).
Aigner et al. (30) reported 9 double-lung transplants with EVLP assessment, compared to 119 standard preservation transplants with similar short term results between the 2 groups including days on mechanical ventilation, ICU stay, hospital stay and 30-day mortality.
Same results were obtained by Zych et al. (37) at 3 and 6 months comparing a group of 6 implanted lungs after EVLP assessment with second group of 86 patients transplanted according to the standard criteria.
At the ISHLT meeting in 2013, the Toronto, Paris and Vienna groups presented their EVLP experience combined (125 transplantation after EVLP assessment). The overall transplantation rate of 82.5% of. Comparable to earlier reports, the occurrence of PGD at 72 hours was at 5%, the 12-month mortality at 12% (38).
The NOVEL Lung Trial, which is a FDA-mandated multicenter, non-randomized comparing 2 groups of reconditioned EVLP lungs versus standard-criteria lungs, the study included 76 EVLPs resulting in 42 lung transplants compared to 42 controls, early results and 1-year survival were comparable (39,40).
Fisher et al. (41) reported the outcomes of DEVELOP-UK which is a nonrandomized Reconditioned extended-criteria lungs versus standard-criteria lungs, among 53 donors with EVLP, only 18 (34%) were subsequently transplanted. The study concluded the estimated survival over one year was lower than in the standard group, but this was not statistically significant. Patients receiving these additional transplants experienced a higher rate of early graft injury and need for unplanned ECMO support, at increased cost.
Most recently, Yeung et al. (42) presented retrospective study comparing the outcomes between 2 groups the first combined of transplanted patients who received lungs preserved for more than 12 hours including EVLP time (97 patients) with the second group with total preserved lung less than 12 hours (809 patients). The average preservation time for group one was 14.6 hours for 97, and 6.7 hours for group 2. Early post-transplant outcomes were similar between the two groups despite high-risk lungs. No differences were seen in PGD or length of hospital and ICU stay between the two groups. These results are extremely encouraging, as lifesaving transplants can now be performed across larger geographic areas without the risk of poorer outcomes.
Currently, the Normothermic EVLP as an assessment of extended/marginal donor Lungs trial is being conducted in the USA to approve the clinical use of EVLP assessing high-risk donors pre-transplantation, and the outcomes are being compared to a group of patients who received lungs according to the standard criteria. Inclusion and exclusion criteria for EVLP are based on those previously used in HELP trial. The study still ongoing, less than handful of patients still needed before closing the study.
Finally, several groups have used lungs from DCDs after ex vivo assessment with outcomes comparable to using without ex vivo and some of these proclaimed using ex vivo will improve DCD lung selection, and increased the uses of DCD lungs (9,16,43-45). The conversion rate from EVLP to transplantation varies between 46 and 87% (15,30-32,37-43).
Conclusions
The usage of ELVP resulted in increased of lung transplants using grafts from marginal donor lungs pool, the performance of these suboptimal lungs evaluated by EVLP is considered equal to those lungs transplanted according to the standard criteria. The Expectations are high that EVLP will overcome some fundamental limitations of current lung transplantation protocols, and push to the cutting edge.
There are some unanswered questions: What is the optimal time needed to keep the lungs on EVLP device? When is the optimal time to start EVLP assessment? Is it directly after the harvest in donor hospital? or after transportation to the recipient hospital? In case of double lung transplant what we should do with the second lung? should we keep it on EVLP device? or put it on cold environment (ice)? All these questions warrant further research studies to find the optimal way in managing this booming technology.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Valapour M, Skeans MA, Smith JM, et al. OPTN/SRTR 2015 Annual Data Report: Lung. Am J Transplant 2017;17 Suppl 1:357-424. 10.1111/ajt.14129 [DOI] [PubMed] [Google Scholar]
- 2.Colvin M, Smith JM, Skeans MA, et al. OPTN/SRTR 2015 Annual Data Report: Heart. Am J Transplant 2017;17:286-356. 10.1111/ajt.14128 [DOI] [PubMed] [Google Scholar]
- 3.Popov AF, Sabashnikov A, Patil NP, et al. Ex vivo lung perfusion - state of the art in lung donor pool expansion. Med Sci Monit Basic Res 2015;21:9-14. 10.12659/MSMBR.893674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machuca TN, Cypel M. Ex vivo lung perfusion. J Thorac Dis 2014;6:1054-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Punch JD, Hayes DH, LaPorte FB, et al. Organ donation and utilization in the United States, 1996-2005. Am J Transplant 2007;7:1327-38. 10.1111/j.1600-6143.2007.01779.x [DOI] [PubMed] [Google Scholar]
- 6.Van Raemdonck D, Neyrinck A, Verleden GM, et al. Lung donor selection and management. Proc Am Thorac Soc 2009;6:28-38. 10.1513/pats.200808-098GO [DOI] [PubMed] [Google Scholar]
- 7.Yeung JC, Cypel M, Waddell TK, et al. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and Ex Vivo donor lung perfusion. Thorac Surg Clin 2009;19:261-74. 10.1016/j.thorsurg.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 8.Ware LB, Wang Y, Fang X, et al. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet 2002;360:619-20. 10.1016/S0140-6736(02)09774-X [DOI] [PubMed] [Google Scholar]
- 9.Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. 10.1016/S0140-6736(00)04195-7 [DOI] [PubMed] [Google Scholar]
- 10.Cypel M, Levvey B, Van Raemdonck D, et al. International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry Report. J Heart Lung Transplant 2015;34:1278-82. 10.1016/j.healun.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 11.Cypel M, Keshavjee S. Strategies for safe donor expansion: donor management, donations after cardiac death, ex-vivo lung perfusion. Curr Opin Organ Transplant 2013;18:513-7. 10.1097/MOT.0b013e328365191b [DOI] [PubMed] [Google Scholar]
- 12.Van Raemdonck DE, Rega FR, Neyrinck AP, et al. Non-heart-beating donors. Semin Thorac Cardiovasc Surg 2004;16:309-21. 10.1053/j.semtcvs.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Pierre AF, Sekine Y, Hutcheon MA, et al. Marginal donor lungs: a reassessment. J Thorac Cardiovasc Surg 2002;123:421-7; discussion 427-8. 10.1067/mtc.2002.120345 [DOI] [PubMed] [Google Scholar]
- 14.Mitilian D, Sage E, Puyo P, et al. Techniques and results of lobar lung transplantations. Eur J Cardiothorac Surg 2014;45:365-9; discussion 369-70. 10.1093/ejcts/ezt353 [DOI] [PubMed] [Google Scholar]
- 15.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning Ex Vivo. Ann Thorac Surg 2009;87:255-60. 10.1016/j.athoracsur.2008.09.049 [DOI] [PubMed] [Google Scholar]
- 16.Cypel M, Rubacha M, Yeung J, et al. Normothermic Ex Vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant 2009;9:2262-9. 10.1111/j.1600-6143.2009.02775.x [DOI] [PubMed] [Google Scholar]
- 17.Carrel A, Lindbergh C. The culture of whole organs. Science 1935;81:621-3. 10.1126/science.81.2112.621 [DOI] [PubMed] [Google Scholar]
- 18.Hardy JD, Webb WR, Dalton ML, Jr, et al. Lung homotransplantations in man. JAMA 1963;186:1065-74. 10.1001/jama.1963.63710120001010 [DOI] [PubMed] [Google Scholar]
- 19.Reitz BA, Wallwork JL, Hunt SA, et al. Heart-lung transplantation: successful therapy for patients with pulmonary vascular disease. N Engl J Med 1982;306:557-64. 10.1056/NEJM198203113061001 [DOI] [PubMed] [Google Scholar]
- 20.Toronto Lung Transplant Group Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. 10.1056/NEJM198605013141802 [DOI] [PubMed] [Google Scholar]
- 21.Cooper JD, Patterson GA, Grossman R, et al. Double-lung transplant for advanced chronic obstructive lung disease. Am Rev Respir Dis 1989;139:303-7. 10.1164/ajrccm/139.2.303 [DOI] [PubMed] [Google Scholar]
- 22.Hardesty RL, Griffith BP. Autoperfusion of the heart and lungs for preservation during distant procurement. J Thorac Cardiovasc Surg 1987;93:11-8. [PubMed] [Google Scholar]
- 23.Steen S, Ingemansson R, Budrikis A, et al. Successful transplantation of lungs topically cooled in the non-heartbeating donor for 6 hours. Ann Thorac Surg 1997;63:345-51. 10.1016/S0003-4975(96)01101-0 [DOI] [PubMed] [Google Scholar]
- 24.Wierup P, Bolys R, Steen S. Gas exchange function one month after transplantation of lungs topically cooled for 2 hours in the nonheart- beating cadaver after failed resuscitation. J Heart Lung Transplant 1999;18:133-8. 10.1016/S1053-2498(98)00011-4 [DOI] [PubMed] [Google Scholar]
- 25.Wierup P, Andersen C, Janciauskas D, et al. Bronchial healing, lung parenchymal histology, and blood gases one month after transplantation of lungs topically cooled for 2 hours in the non-heart-beating cadaver. J Heart Lung Transplant 2000;19:270-6. 10.1016/S1053-2498(99)00132-1 [DOI] [PubMed] [Google Scholar]
- 26.Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg 2007;83:2191-4. 10.1016/j.athoracsur.2007.01.033 [DOI] [PubMed] [Google Scholar]
- 27.Wierup P, Haraldsson A, Nilsson F, et al. Ex Vivo evaluation of nonacceptable donor lungs. Ann Thorac Surg 2006;81:460-6. 10.1016/j.athoracsur.2005.08.015 [DOI] [PubMed] [Google Scholar]
- 28.Egan TM, Haithcock JA, Nicotra WA, et al. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006;81:1205-13. 10.1016/j.athoracsur.2005.09.034 [DOI] [PubMed] [Google Scholar]
- 29.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 Ex Vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. 10.1016/j.jtcvs.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 30.Aigner C, Slama A, Hötzenecker K, et al. Clinical Ex Vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. 10.1111/j.1600-6143.2012.04027.x [DOI] [PubMed] [Google Scholar]
- 31.Inci I, Schuurmans MM, Boehler A, et al. Zurich University Hospital lung transplantation programme: update 2012. Swiss Med Wkly 2013;143:w13836. [DOI] [PubMed] [Google Scholar]
- 32.Cypel M, Yeung JC, Liu M, et al. Normothermic Ex Vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. 10.1056/NEJMoa1014597 [DOI] [PubMed] [Google Scholar]
- 33.Makdisi G, Wozniak T. How to Establish a Successful Ex-Vivo Lung Perfusion Program. Ann Transl Med 2017;5:S12. 10.21037/atm.2017.03.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet 2012;380:1851-58. 10.1016/S0140-6736(12)61344-0 [DOI] [PubMed] [Google Scholar]
- 35.Fisher AJ, Dark JH, Corris PA. Improving donor lung evaluation: a new approach to increase organ supply for lung transplantation. Thorax 1998;53:818-20. 10.1136/thx.53.10.818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneda H, Waddell TK, de Perrot M, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant 2006;6:544-51. 10.1111/j.1600-6143.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 37.Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after Ex Vivo lung evaluation and reconditioning. J Heart Lung Transplant 2012;31:274-81. 10.1016/j.healun.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 38.Cypel M, Aigner C, Sage E, et al. Three center experience with clinical normothermic Ex Vivo lung perfusion. J Heart Lung Transplant 2013;32:S16 10.1016/j.healun.2013.01.021 [DOI] [Google Scholar]
- 39.Sanchez PG, Davis RD, D’Ovidio F, et al. Normothermic Ex Vivo. lung perfusion as an assessment of marginal donor lungs – the NOVEL lung trial. J Heart Lung Transplant 2013;32:S16-17. 10.1016/j.healun.2013.01.022 [DOI] [Google Scholar]
- 40.Sanchez PG, Davis RD, D’Ovidio F, et al. The NOVEL Lung Trial One-Year Outcomes. J Heart Lung Transplant 2014;33:S71-72. 10.1016/j.healun.2014.01.226 [DOI] [Google Scholar]
- 41.Fisher A, Andreasson A, Chrysos A, et al. An observational study of Donor Ex Vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess 2016;20:1-276. 10.3310/hta20850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung JC, Krueger T, Yasufuku K, et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med 2017;5:119-24. 10.1016/S2213-2600(16)30323-X [DOI] [PubMed] [Google Scholar]
- 43.Pêgo-Fernandes PM, de Medeiros IL, Mariani AW, et al. Ex Vivo lung perfusion: early report of Brazilian experience. Transplant Proc 2010;42:440-3. 10.1016/j.transproceed.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 44.Charles EJ, Huerter ME, Wagner CE, et al. Donation After Circulatory Death Lungs Transplantable Up to Six Hours After Ex Vivo Lung Perfusion. Ann Thorac Surg 2016;102:1845-53. 10.1016/j.athoracsur.2016.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machuca TN, Mercier O, Collaud S, et al. Lung Transplantation With Donation After Circulatory Determination of Death Donors and the Impact of Ex Vivo Lung Perfusion. Am J Transplant 2015;15:993-1002. 10.1111/ajt.13124 [DOI] [PubMed] [Google Scholar]