Abstract
The incidence of diabetes has more than doubled in the United States in the last 30 years and the global disease rate is projected to double by 2030. Cognitive impairment has been associated with diabetes, worsening quality of life in patients. The structural and functional interaction of neurons with the surrounding vasculature is critical for proper function of the central nervous system including domains involved in learning and memory. Thus, in this review we explore cognitive impairment in patients and experimental models, focusing on links to vascular dysfunction and structural changes. Lastly, we propose a role for the innate immunity--mediated inflammation in neurovascular changes in diabetes.
Keywords: cerebral vasculature, diabetes, vascular, cognitive impairment
Introduction
The incidence of diabetes has more than doubled in the United States in the last 30 years (1). Moreover, the global disease incidence is expected to double by 2030 (2). The increase in diabetes is not only in type 2 diabetes (T2D); there is an equally alarming increase in the number of younger patients diagnosed with type 1 diabetes (T1D) (3). The consequences of this disease burden indicate a massive worldwide health problem that poses many issues, including social and economic factors. Elevated blood glucose levels, both as a result of poor glycemic control or undiagnosed pathology, leads to many serious and debilitating heath complications, such as chronic kidney disease, retinopathy, and peripheral neuropathy (4). The presence of central neuropathy and the predisposition of diabetic individuals to develop cognitive decline has been observed, with a prevalence as high as 40% in poorly controlled and long-standing diabetes (5). However, cognitive impairment remains to be one of the less understood and less studied complication of diabetes. This is in part due the wide spectrum of deficits observed and the varying impact of disease duration and severity on the symptoms. Most patients, even children, experience impaired performance on multiple domains affecting their mental speed and ultimately quality of life (6). Older diabetic individuals have an increased risk of the development of cognitive impairment which can progress to dementia.
There are various pathophysiological mechanisms that contribute to this cognitive decline, one of which is diabetic vascular disease. The importance of vascular disease in diabetic cognitive impairment is becoming an increasingly recognized process, as can be seen in the recently issued statement by the American Heart Association entitled “Vascular contributions to cognitive impairment and dementia” (7). VCI encompasses all cognitive disorders that stem from a vascular origin and is defined as a syndrome with evidence of clinical stroke or subclinical vascular brain injury and cognitive impairment affecting at least 1 cognitive domain (7). Understanding how certain cerebrovascular pathologies occur and contribute to the development of VCI is an ever-evolving area of focus. The major cerebrovascular risk factors that contribute VCI are hypertension, aging, and diabetes (8), with an undeniable comorbid effect occurring between these causes. Diabetes is the most rapidly increasing risk factor in the patient population and leads to profound vascular disease throughout the body. As such, assessing the contribution of this disease to the development of VCI is highly critical.
The overall goal of this review is to describe the potential vascular contributions to cognitive impairment in diabetes, examining data from both clinical and preclinical animal studies. There are many potential mechanisms that contribute to the vascular complications present in diabetes, and in this review we present a novel role of the innate immune system, demonstrating a link between TLR2 and NLRP3-mediated inflammation and neurovascular changes in diabetes. However, this review primarily attempts to provide an overview linking vascular disease to diabetic cognitive impairment, rather than provide an in-depth review of the vascular pathophysiology itself.
A. DIABETES AND COGNITIVE DYSFUNCTION: EVIDENCE FROM CLINICAL STUDIES
1. T1D
a. Cognitive Function
The overall cognitive deficits seen in patients with T1D are commonly found in the domains of intelligence, attention, psychomotor efficiency, information processing speed, cognitive flexibility and visual perception (9). The effect of T1D on cognitive function in general has been shown to be relatively mild, somewhere in the range of 0.3–0.7 standard deviations per cognitive domain less than observed in non-diabetic groups. However, even small impairments could lead to issues such as poor compliance with standard insulin treatment or a reduction in the efficiency of activities of daily living. These issues greatly contribute to the burden of cognitive dysfunction in the T1D patient population, and make research in this area a crucial necessity in the broad scope of diabetic complications.
While the peak age of onset of T1D is 14 years old, the disease can occur as early as a few years of age, which holds potential consequences for the effect of hyperglycemia on the developing brain. Studies have shown that those patients who develop diabetes at an early age score worse on cognitive tests relative to their peers, affecting the development of intelligence, attention, executive function, and psychomotor speed (10–13). The worsening of cognitive function in these children is possibly due to the effects of hyperglycemia on both the structural and functional development of the brain, which can leave the patients more susceptible to future insults and further impairment (14). Children who develop diabetes earlier than 7 years of age are also at a higher risk of cognitive impairment than individuals who develop diabetes in adulthood, indicating that children’s brains are potentially more susceptible to the effects of diabetes such as hyper- or hypoglycemia (15). The negative effects of diabetes on cognitive abilities are often apparent as early as 2 years post-diagnosis (16), and progress slowly thereafter (17), demonstrating a difference from the more rapid decline observed in dementia and Alzheimer’s.
b. Hyperglycemia, Microvascular Disease, and Cognitive Function
One of the most consistent links between diabetes and cognitive impairment is the effect of hyperglycemia on microvascular function, which is also important in other diabetic complications such as retinopathy, neuropathy, and nephropathy (18). Diabetic patients with these microvascular complications were shown to have an increase in the rate of impairment in psychomotor efficiency when compared to diabetics without microvascular disease (19). Examination of the retinal vessels provides an excellent surrogate marker for cerebral microvascular disease, as both retinal and cerebral vessels share similar embryologic and anatomic characteristics (20). The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) demonstrated a significant association between mild cognitive dysfunction, characterized by impaired motor speed and psychomotor efficiency, and chronic hyperglycemia in relatively healthy young and middle aged adults with T1D (21). In this study, the presence of retinopathy exhibited the strongest association with cognitive dysfunction. However, the presence of retinopathy and other microvascular diseases are not readily apparent until hyperglycemia has been present for a prolonged period, while the cognitive decrements usually occur early on in the process. This suggests that even subclinical microvascular disease can have an effect on cognitive ability, or that perhaps there are multifactorial contributions of the various complications associated with diabetes.
The presence of microvascular disease in diabetic patients is associated with reduced cerebral autoregulation, which is the maintenance of cerebral blood flow (CBF) across a wide range of arterial perfusion pressures (22). As appropriate CBF is necessary to maintain healthy structure and function of the brain, any disturbance in flow can potentially lead to damage and subsequent cognitive impairment. Impaired cerebral autoregulation was shown using administration of vasodilatory acetazolamide, where the presence of microvascular disease and longstanding diabetes were strongly associated with reductions in vasodilatation (23,24). Other studies in T1D have reported increases in total CBF compared to non-diabetic patients when controlled for relative brain atrophy (25). It was suggested that this process in the brain is similar to the hyperperfusion that occurs in the kidney in the early stages of diabetes, thus leading to additional pulsatile flow damaging the microvasculature.
Advances in imaging techniques have allowed for a more effective means of studying how microvascular disease leads to reductions in CBF and subsequent cognitive impairment. Microvascular disease leads to a reduction in nutrient delivery to neuronal tissue and removal of metabolic waste (26), and such reductions in CBF have been demonstrated in T1D compared to non-diabetics (27). Single-photon emission computed tomography (SPECT) was performed in both children and adults with T1D to measure CBF, revealing areas of both increased and decreased perfusion relative to non-diabetics. The cerebral areas most affected were the cerebellum, frontotemporal brain and frontal brain, and were correlated with poor glycemic control indicated by elevated HA1C% and the presence of microvascular complications (27–29). Additionally, the duration of hyperglycemia was also correlated with reductions in blood flow (30). MRI performed using voxel-based morphometry demonstrated that T1D patients exhibited a decrease in grey matter density in the posterior, temporal, and cerebellar regions of the brain (31). Importantly, the degree of microvascular disease indicated by the degree of retinopathy was correlated with brain matter loss in the medial and superior frontal gyri bilaterally, the right middle temporal and parahippocampal gyri, and the left insula, indicating a possible effect on areas important for cognition. This association between retinopathy and gray matter atrophy has also been demonstrated to occur in the cerebellum, the frontal gyri, and the occipital lobe (32). In the study by Musen et al.(31), several cognitive deficits in the diabetic population were observed in addition to an association between gray matter atrophy and retinopathy, though a full examination of cognitive impairment was not performed.
White matter hyperintensities (WMH) and microbleeds are consequences of vascular pathology in the brain, and can be assessed on MRI as indirect indices of microvascular disease. However, the association between the presence of these pathologies on MRI, T1D, and subsequent cognitive dysfunction is still inconclusive. Weinger et al. (33) found only mild WMH were present in a sample of young adult T1D patients, and the lesion burden was comparable to that observed in nondiabetics. Neither glycemic control, duration of disease, or retinopathy was associated with the WMH in the diabetic patient population, nor was cognitive functional assessments related. In contrast, a recent study by Nunley et al. (34) observed a significantly greater severity of WMH and slower information processing in middle-aged patients with T1D compared with similarly aged non-diabetic adults, independent of hyperglycemia, hypertension, and other risk factors. Whether these risk factors played a role in the development of the WMH was uncertain, but it was proposed that these diabetic complications may play a greater role in the development of WMH at younger ages than were assessed in this study. Given the disparity between studies examining the association of WMH, diabetes, and cognitive function, future longitudinal studies begun around the onset of the disease are warranted.
2. T2D
a. Cognitive Function
Given the increasing prevalence of T2D, several prospective longitudinal studies have been conducted to address what the effects of the disease may be on cognition. The Rotterdam study of over 6000 patients with T2D showed a 2-fold increase in the risk for dementia (35). The Honolulu-Asia Aging Study also showed an increase risk of developing dementia, with the highest risk being that for vascular dementia, a finding that was observed by others as well (36,37). The most common deficits have been shown to occur in the areas of memory, attention, executive function, and information processing speed (38–40), and through meta-analysis studies the effect sizes of these decrements have been shown to be anywhere from 0.3–0.4 (38) up to 1.9(39). These pathologies occur early in the disease course of T2D (41), and even in pre-diabetic stages where hyperinsulinemia and impaired glucose tolerance are present (30,42). Given the advanced age of the participants in these studies and the well-known relationship between cognitive decline and aging, it is necessary to control for this effect when examining the association between diabetes and cognitive impairment. An increased risk of cognitive impairment is present in T2D than can be attributed to aging alone (43), but whether the rate of cognitive decline is increased is unclear. Patients with T2D have been reported to exhibit an increased rate of cognitive decline over an average duration of 4 years (44), however other studies including the Utrecht Diabetic Encephalopathy Study failed to identify such an effect (45–47). These data taken together indicate a need for further longitudinal studies for durations of longer than 4–5 years to more thoroughly characterize this relationship.
Cross-sectional studies examining cognitive function in T2D demonstrate similar cognitive impairment to that found in the longitudinal studies, particular in the areas of memory, information processing speed, attention, and executive function (48–51). The effect sizes of these impairments are also similar, ranging from 0.2 to 0.8 (39). Again, these effects were shown to occur even in the pre-diabetic and early stages of the disease (41,52). It has been suggested that disease duration correlates with the degree of cognitive impairment, which is supported by studies showing that individuals older than 60 years of age display more prominent cognitive deficits (17,51). However, studies examining the overall rate of cognitive decline indicate that there is no difference between diabetic patients and age-matched controls (45,53). In a review of diabetes and cognitive dysfunction, McCrimmon et al. (54) hypothesized that the absence of a difference in the rate of cognitive decline in the presence of significant cognitive impairment suggests that diabetic patients may experience a demarcated onset of cognitive dysfunction that then progresses similarly. This could occur during a critical period of cognitive development or could occur as a result of the pathological consequences of diabetes such as microvascular disease.
b. Hyperglycemia, Vascular Disease, and Cognitive Function
The evident vascular damage, inflammation, and oxidative stress observed due to hyperglycemia in T1D is similarly present in T2D, however the impact of other frequent comorbid conditions such as hypertension, obesity, and hyperlipidemia make identifying the individual contribution of hyperglycemia to cognitive dysfunction more difficult. Studies have shown that patients with a HA1C of greater than 7% have a 4-fold increase in the development of mild cognitive impairment (55), with higher HA1C being associated with decreased function in the domains of memory, psychomotor skills, and executive function (49,56). The predominant vascular issue in T2D is macrovascular disease and atherosclerosis (57), largely due to hyperglycemia-mediated endothelial dysfunction (58). Endothelial function in T2D is often compromised, leading to vascular dysfunction characterized by reductions in flow-mediated vasodilation (59). Additionally, hyperglycemia leads to increases in diacylglycerol and protein kinase C (PKC), which decreases the levels of endothelial nitric oxide synthase (eNOS) (60) and thus lowers the bioavailability of nitric oxide (NO) leading to impaired vasorelaxation. PKC also leads to the proliferation and migration of vascular smooth muscle cells (VSMCs), an important step in the development of atherosclerosis (61). Along with these effects, advanced glycation end products induce an increase in inflammatory cytokines like tumor-necrosis factor-α and interlekin-1, which causes a decrease in endothelium dependent vasodilation and an increase in VSMC migration and proliferation (62), further contributing to the development of the pro-atherogenic state. This endothelial dysfunction in T2D can potentially explain the reductions in CBF observed in patients, as was shown in a clinical study by Nazir et al. (63) where assessment of decreased CBF revealed that basal NO activity is attenuated in the cerebral circulation of established T2D patients.
The cerebrovasculature is affected similarly to both the coronary and peripheral arterial circulation, with increases in carotid atheromas particularly noted in diabetic patients (64). Atherosclerotic disease has been associated with cognitive dysfunction in T2D (65), and importantly the relative risk of stroke in diabetes is up to 4 times greater than non-diabetic age matched-controls as demonstrated in the Atherosclerosis Risk in Communities (ARIC) Study (66). The increase in relative risk for stroke was shown to correlate with the degree of hyperglycemia, with an increase of 1.15 for every 1% increase in HA1C (67). Additionally, the presence of diabetes at midlife in this study was associated with a 19% greater decline in cognitive function in the areas of processing speed and executive function over a 20 year duration (68). Based on this association, several studies have investigated whether glycemic control could help to reduce cerebrovascular disease and events in diabetes, and thus potentially improve the occurrence of cognitive dysfunction. One of the most recent of these studies was the ACCORD Memory in Diabetes (ACCORD-MIND) Study (69), which treated T2D patients with either standard glycemic control therapy (target HA1C 7–7.9%) or intensive therapy (target HA1C <6%). Over a follow-up period of 40 months, no differences were observed in cognitive function between either treatment groups; however the intensive treatment group did have an improvement in reducing the loss of total brain volume. This suggests that continued follow-up is warranted, as decreases in brain volume could precede the presence of clinical symptoms of cognitive impairment. Additional studies such as the VA Diabetes Trial (VADT), the United Kingdom Prospective Diabetes Study (UKPDS), and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial, all similarly examined whether intensive glycemic control would improve stroke and cardiovascular outcomes in diabetes (70–72). The findings of these studies did not indicate that intensive glycemic control had a significant reduction in the rate of stroke among diabetic patients, however other cardiovascular outcomes such as myocardial infarction and microvascular complications were individually improved. The cognitive domains that were associated with impairment in these and other clinical studies examining cognition in diabetes are summarized in Table 1.
Table 1.
Major Clinical Studies Examining Individual Cognitive Domains in Diabetes *
| Diabetes | Clinical Study | Cognitive Impairment | Reference |
|---|---|---|---|
| Type 1 | Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) |
|
(179) |
| Type 2 | Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) |
|
(180) |
| Anglo-Danish- Dutch Study of Intensive Treatment in People with Screen Detected Diabetes in Primary Care (ADDITION) |
|
(41) | |
| Utrecht Diabetic Encephalopathy Study |
|
(47) | |
| Atherosclerosis Risk in Communities Study (ARIC) |
|
(181) | |
| The Age, Gene/Environment Susceptibility–Reykjavik Study |
|
(182) | |
| Edinburgh Type 2 Diabetes Study |
|
(183) |
Major clinical studies that assessed only global cognitive function or presence of dementia are not included in this table.
While macrovascular disease and atherosclerosis are most commonly linked to cognitive impairment in T2D, the microvasculature also contributes to the overall cognitive profile. Cerebral microvascular disease may play a role in the acceleration of cognitive decline in diabetes (48,73). The Edinburgh Type 2 Diabetes Study examined whether microvascular disease, as indicated by assessment of diabetic retinopathy, would be associated with cognitive decline in T2D (74). They observed that the increasing severity of diabetic retinopathy was associated with decreases in the cognitive domains of verbal fluency, information processing speed and mental flexibility. Interestingly, these findings were only present in the male patients, implying that there could be sex-dependent effects such as a vasculoprotective role of estrogen that merits further consideration. The overall results suggest that diabetic retinopathy in T2D patients is a useful indicator of microvascular disease contributing to cognitive impairment, and are in agreement with findings from the ARIC study that found at 14 years follow up that diabetic patients with retinopathy had a greater decline in verbal fluency and information processing speed than those without retinopathy (75).
The contribution of both macro- and microvascular disease in patients with T2D is linked to decreases in CBF, which could contribute to the overall pattern of cognitive impairment in these individuals. Increased HA1C is associated with lower CBF (76), and can directly lead to reductions in CBF due to vasogenic edema, which impairs collateral flow and can lead to reductions in cerebral autoregulation (77). The decreases in CBF have been demonstrated using both SPECT (78,79) and arterial spin labeling (ASL) MRI (76). Decreases in CBF are correlated with reductions in cognitive function, however when concomitant decreases in brain volume were corrected for, the association between diabetes and decreased CBF was not significant (80). In one recent longitudinal study, CBF and cerebrovascular reactivity were both associated with impaired cognition and total brain volume, but they did not predict cognitive decline over a period of 4 years (81). Conversely, a recent study by Chung et al. showed that patients with T2D exhibited a reduction in cerebrovascular reactivity and cognitive function in a 2 year longitudinal study (82). Utilizing PET imaging, it has been observed that T2D is associated with reductions in CBF to the insular cortex (83), and that overall glucose intolerance leads to reductions in perfusion to the orbitofrontal cortex, inferior parietal lobe, and the superior temporal gyrus (84).
Cerebral imaging can also identify vascular infarcts, which occur in both small vessels such as cerebral arterioles and capillaries as well as in large vessels. Lacunar infarcts, which are subcortical fluid filled cavities up to 15 mm., occur due to occlusion of a single perforating arteriole and are observed by MRI in diabetic patients at a frequency almost two times more than observed in non-diabetic patients (73). Additionally, these small vessel subcortical infarcts occur more commonly when compared to the large vessel cortical infarcts among the T2D patients population (85). It is believed that there are multiple factors contributing to the increase in infarcts such as the duration of diabetes in the patient population, insulin resistance, or the presence of microvascular disease (86,87). In T2D patients, an association between the presence of infarcts and cognitive decline was shown to exist, particularly in the area of information processing speed (48).
Taken together, these findings suggest that T2D leads to pronounced macro- and microvascular disease that likely contributes to the overall cognitive decline in these patients. While there is a preponderance of evidence examining this association, there are a multitude of factors that require further investigation. In particular, the impact of glycemic control at the onset of disease or even in the pre-diabetic state is a pressing area of research. It is possible that treatment during this critical period could prevent not only the influence of hyperglycemia on vascular disease, but also other pathological influences such as hyperinsulinemia. The exploration of individual pathological contributions to diabetic vascular disease and how they lead to the development of cognitive impairment necessitates a wide array of experimental animal studies, which are reviewed in the following sections.
B. DIABETES AND COGNITIVE DYSFUNCTION: EVIDENCE FROM PRECLINICAL STUDIES
In this section, we first review cognitive deficits in animal models of T1D and T2D and how these pathologies relate to deficits observed in experimental models of VCI. Diabetes-mediated microvascular dysfunction is thought to initiate a cascade of event leading to blood brain barrier (BBB) disruption, neuroinflammation, and white matter damage. While the mechanisms are multifactorial, cerebrovascular dysfunction and reduced CBF is believed to precede the negative changes in cognitive function observed in both patients and experimental models (88,89). Thus, we next summarize changes in cerebrovascular function and structure that may contribute to the pathogenesis and progression of cognitive dysfunction in diabetes. Finally, evidence linking vascular dysfunction and cognitive impairment to inflammation is reviewed in an effort to delineate potential underlying mechanisms.
1. Cognitive Deficits in Experimental Models of Diabetes
a. T1D
The hippocampus, a major domain in learning and memory, has been the focus of numerous studies relating cognitive impairment and diabetes. The hippocampus has lesser number of capillaries, compared to the cortex and striatum (90), yet remains more susceptible to vascular dysfunction. Cognitive decline in T1D has been associated with reduction of neurogenesis, hippocampal neuronal loss and elevated astrocytic reactivity in multiple animal models (91–93). While inflammation levels in the brain are elevated in diabetes, glial activation in the hippocampus has seen to be correlated to cognitive impairment (94). Using Morris water maze (MWM), Y-maze and the novel object-placement recognition task, T1D models showed deficiencies in hippocampal-based memory (95–97). Recently, a reduction of VEGF signaling in the hippocampus of diabetic mice has been reported to contribute to cognitive impairment, which was recovered with chronic low dose infusion of VEGF in these mice (98). STZ-induced diabetic rats have deficits in NMDA-dependent long-term potentiation (LTP) as early as 6 weeks after induction of diabetes, while long-term depression was enhanced in the CA1 region of the hippocampus (99–101). For a more in depth view of hippocampal changes in diabetes, readers are referred to a recent review by Stranahan which explores changes and underlying mechanisms for hippocampal dysfunction in obesity and diabetic animal models (102).
While hippocampus is a major domain in learning and memory, both the hippocampus and cortex pathologies contribute to learning and memory deficits seen in diabetes. Working memory has been studied using novel object recognition in diabetic animals, mainly targeting cortical function. A reduced discrimination index has been observed through the novel object recognition task in STZ-induced diabetic animals (103). Poor learning performance has been observed in STZ-induced diabetes through the passive avoidance test, which suggests a reduction of memory retention in these animals (103). STZ-induced diabetic mice showed less freezing behavior than non-diabetic counterparts using the fear conditioning paradigm, suggesting amygdala-based cognitive dysfunction (104).
b. T2D
Hippocampal-driven cognitive deficits have been reported in multiple T2D experimental models such as the spontaneously diabetic, lean Goto-Kakizaki (GK) rats, Zucker rats and db/db mice (93,105,106). T2D animal models have reported functional and structural changes to the CA1 and dentate gyrus (DG) regions of the hippocampus. Beaquis et al. reported a reduction in microvessels in the hippocampus, in particular the DG region in GK diabetic rats (107). Reduced LTP in the CA1 and DG hippocampal regions, reduced synaptic plasticity and behavioral deficits have also been reported in the db/db mice (93,105). Deposition of amyloid beta has been seen in the hippocampus of HFD/STZ, which may contribute to cognitive deficits (108), yet neurovascular dysfunction has not been reported. Although hippocampal changes in diabetes have been studied across many different models with major emphasis on neuronal changes, the interaction between microvessels, neurons and glial cells in cognitive decline needs to be further explored.
Neurodegeneration in T2D has not been seen exclusively in the hippocampus, but in cortical regions as well. Recently a reduction of neurons in the cerebral cortex (approximately 11%) in GK rats was reported, while astrocyte numbers remained unchanged from non-diabetic controls (109). Our lab has shown cognitive dysfunction in GK rats, which is associated with cerebrovascular dysfunction and pathological neovascularization (110). We recently reported similar changes in the HFD/STZ-induced model of diabetes, with pathological neovascularization occurring in the cortex and striatum, and cognitive impairment seen through the novel object recognition task after 8 weeks of diabetes (111,112). While we saw cognitive changes as early as 8 weeks in the HFD/STZ model, others have reported seeing cognitive impairment begin at 15 weeks of age using MWM, Y-maze and the passive avoidance task, although earlier time points were not examined (113). Activation of microglia in male GK rats is elevated and is thought to contribute to cognitive dysfunction and neurodegeneration (109). Episodic memory was impaired in 28-week old db/db mice compared to non-diabetic control, but not at 4 or 14 weeks of age. In the same study, pre-diabetic animals fed a HFD showed no impairment in episodic memory, suggesting that changes to episodic memory is evident later in diabetes rather than in pre- or early diabetes (106).
c. Models of Cognitive Impairment
Cognitive impairment has been examined through a variety of animal models from rodents to primates. As briefly mentioned above, cerebral hypoperfusion precedes changes in cognitive function and hence most models of VCI have been based on a cerebral hypoperfusion approach, which can be induced chronically, transiently or focally. For a more in depth description of vascular cognitive impairment experimental models, refer to Jiwa et al. (114). A common animal model to mimic chronic cerebral hypoperfusion seen in VCI is the occlusion of the common carotid arteries. In rats, bilateral carotid artery occlusion (BCCAO) or placement of ameroid constrictors bilaterally is used. Ameroid constrictors contain casein, which gradually absorb water and slowly occlude the vessel (115). Bilateral common carotid artery stenosis (BCAS) induced through microcoils placed on both CCAs to globally restrict blood flow or unilateral common carotid occlusion (UCCAO) is used in mice (116,117). A new model combining the microcoil of BCAS on the left CCA and an ameroid constrictor on the right CCA, termed asymmetrical common carotid artery surgery, has been implemented to mimic the white matter hyper-intensities and cognitive deficits (118). Transient cerebral hypoperfusion has been used in rats, mice and gerbils through 4 or 2-vessel occlusion. Although these models are widely used, they decrease cerebral blood flow rapidly; creating a hypoxic-ischemic condition that may become too severe to be considered chronic cerebral hypoperfusion (119). Rather than these models, ameroid constrictor devices can be placed on both common carotid arteries, leading to acute decrease in CBF for a model termed 2-vessel gradual occlusion, although eventually these vessel will become completely occluded (115).
Experimental models of VCI are not only induced through surgical methods, but also through diet. Induction of hyperhomocysteinemia by a diet with low folate, vitamin B6 and B12 and supplemented with methionine models VCI through the presence of microhemorrhages, elevated neuroinflammation and impaired spatial memory (120,121). These microhemorrhages were more prominent in the entorhinal and parietal cortex compared to the hippocampus and frontal cortex. Microglia reactivity was assessed in the frontal cortex and hippocampus, and M1 microglia (produced proinflammatory cytokines) increased while M2 microglia (produce anti-inflammatory cytokines) expressed no difference in hyperhomocysteinemic mice (122).
Metabolic and vascular risk factors in diabetes have been associated with increased cerebrovascular disease, cognitive impairment and dementia. Pathologies present in VCI models express similarities to cognitive dysfunction in diabetes. While learning and memory is the most prominent form of cognitive impairment, VCI models show more diverse impairment span from deficits in spatial and working memory, as well as executive function and attention (123,124). Similar to VCI, diabetes has reduced CBF and increased oxidative stress leading to loss of vascular integrity (125). Macro and microvascular alterations, such as changes in tortuosity and the thickening of the basement membrane, contribute to decreased CBF in both VCI and diabetes. While arteriole and capillary numbers are decreased in VCI, some diabetic models have shown a pathological neovascularization in the cortex and striatum. These vascular changes in both VCI and diabetes have been shown to precede neurovascular uncoupling and neuronal dysfunction, ultimately resulting in cognitive impairment (126). White matter changes such as gliosis, axonal damage and white matter rarefaction, have been well documented in VCI experimental models (123,124). Diabetes increases the risk of small vessel disease and can lead to lacunar strokes in the white matter, as mentioned above, suggesting similar pathologies between VCI and diabetes. Although many parallels occur between VCI and diabetes, few studies have reported structural and functional changes in combination (Table 2). In db/db mice exposed to BCAS, increased subcortical white matter rarefaction and reduction of oligodendrocyte progenitor cells was observed, although cognitive deficits were not explored (127). A HFD model of diabetes along with UCCAO showed reduced CBF, impaired spatial memory, reduced cue fear memory, increased neurodegeneration and gliosis (128). Lastly, BCCAO was induced in T2D Otsuka Long-Evans Tokushima Fatty (OLETF) rats, expressing exacerbated spatial memory deficits compared to diabetic or BCCAO alone, with elevated hippocampal neuronal death (129). Although diabetes has been associated as a risk factor of VCI, most studies of VCI are explored in mostly male and otherwise healthy animals. Therefore, further exploration of diabetic contributions to VCI is warranted.
Table 2.
Cognitive deficits in diabetic animal models in the presence or absence of VCI.
| Diabetes | Diabetic Model | VCI Model | Cognitive Impairment | Ref. |
|---|---|---|---|---|
| Type 1 | STZ | --- |
|
(91,92,95–97,99–101,103, 104) |
| NOD | --- |
|
(91,92) | |
| Type 2 | GK rats | --- |
|
(110,184) |
| Zucker rats | --- |
|
(105) | |
| db/db mice | --- |
|
(93,105, 106) | |
| BCAS |
|
(127) | ||
| HFD+STZ | --- |
|
(108,111–113) | |
| OLETF rats | BCCAO |
|
(129) |
2. Diabetes-Induced Cerebrovascular Dysfunction and Neurovascular Remodeling: Link to Cerebral Hypoperfusion
Regulation of structure and function of the cerebrovasculature is essential to cerebral perfusion and neuronal function. Maintenance of CBF allows delivery of nutrients and removal of metabolites from the brain, which is highly metabolically active. Therefore, diseases such as diabetes, contributing to both micro- and macrovascular complications, can have a profound effect on proper neurologic function. Here, we will provide an overview of diabetes-induced changes to cerebrovascular function as well as structure.
a. Cerebrovascular Dysfunction and CBF
Causes of vascular dysfunction are multifactorial, with increased oxidative stress (130–132), disturbances in NO synthesis and production (133), ion channel impairment in VSMCs (134,135), as well as stimulation of Rho-kinase activity contributing to this dysfunction (132). Cerebrovascular dysfunction in diabetes has been shown in experimental models to have enhanced constriction, reduced dilatory function and impaired myogenic tone, which contribute to a global reduction in CBF (136).
Changes in the vascular constriction response in diabetes have been shown using vasoconstrictors such as endothelin-1 (ET-1), 5-hydroxytryptamine (5-HT), norepinephrine (NE) and changes in ion channels. We have shown heightened ET-1 sensitivity in middle cerebral arteries (MCAs) from T2D rats, which is eliminated by chronic ETA receptor blockade (137). Initial reports suggested 5-HT response was not affected within 10 weeks of diabetes (138), yet it was later reported that short-term diabetes (4 weeks) expressed elevated sensitivity to 5-HT, while long-term diabetes (40 weeks) showed reduced sensitivity (139). In STZ-treated rats vasoconstriction of MCAs developed from reduced sensitivity to ATP-sensitive K+ channel openers in an endothelium-dependent manner (140). STZ/high fructose diet-induced diabetic rats showed reduced conductance of Ca2+-activated K+ (BKCa) channels, contributing to elevated vascular tone and blood pressure (141). NE and potassium ions constricting responses in basilar arteries (BAs) expressed no difference from non-diabetic controls (142) and responses to ET-1, angiotensin II, arginine vasopressin, and thromboxane in BAs isolated from STZ-induced diabetic rats was unchanged at 12–16 weeks of diabetes and unaffected by L-NMMA. These results suggest that these constricting responses are independent of nitric oxide synthesis or release (138,143).
Attenuated endothelium-dependent relaxation is another mechanism of cerebrovascular dysfunction in diabetes (132,144). Cerebral arteries express decreased dilator responses to acetylcholine, adenosine 5’-diphosphate, and beta-adrenergic receptor activation (131,133). Impairment of NOS-dependent reactivity has been rescued through treatment of enalapril (145), apocynin (131), tempol (146), chronic ETA receptor blockade (147), and poly (ADP-ribose) polymerase (PARP) inhibition (146). The duration of diabetes has been seen to impact vessel function and reactivity to vasoactive agents. Dilatory responses to acetylcholine are weakened after 5–6 weeks of diabetes in cerebral arterioles (148). Acetylcholine-induced dilation in BAs from rats is impaired after 8–12 weeks of diabetes, which was reversed by insulin treatment (149). These functional changes highlight the multifactorial nature of cerebrovascular dysfunction in diabetes.
Myogenic tone, the basal tension of the cerebral VSMCs, in diabetic animals has been shown to impair function compared to non-diabetic controls. In T2D rats, we observed reduced myogenic tone in MCAs 12–14 weeks after induction of diabetes compared to MCAs in short-term diabetic rats (4–6 weeks). Furthermore, treatment with metformin starting at the onset of diabetes restored myogenic tone in 18 week-old rats (150). Posterior cerebral arteries isolated from T2D rats were shown to have altered myogenic reactivity as well as increased tone (151).
The combination of impaired dilatory responses, increased cerebrovascular constriction and dysfunctional myogenic tone observed in diabetes contributes to the decreased CBF (152). Furthermore, we showed reduced response to whisker stimulation compared to controls, suggesting impaired neurovascular coupling. Reduction of the CBF and subsequent neurovascular coupling dysfunction can lead to neuronal injury and death, contributing to cognitive deficits seen in diabetes.
b. Cerebrovascular Restructuring
Emerging evidence has shown cerebral microvasculature is affected by diabetes in many regions in the body, including the brain (6,153). A thickening of the basement membrane of cerebral microvasculature has been reported in various studies, reporting collagen deposition and amorphous nodules termed “cotton tufts” (154–156). This thickening of the basement membrane impacts adjacent VSMCs, pericytes and astrocytic end feet, compromising the integrity and function (154). Changes to these adjacent cells include diffuse swelling of the astrocytic end feet (154,157), swelling of the mitochondria and endoplasmic reticulum of the VSMCs, and degeneration of the endothelium (158).
Not only do these ultra-structural alterations arise, but significant vascular remodeling occurs as early as 4 weeks after induction of diabetes with STZ injection (154). In GK rats, a mild and lean T2D model these changes occur as early as 5–6 weeks after onset of diabetes. There is extensive vascular remodeling illustrated by increased tortuosity, collateral numbers and collateral size in this model (159,160). In animals with a longer duration of diabetes (~10–12 weeks), extracellular matrix deposition and the wall thickness of MCAs increased in an ET-1-dependent manner (161), which was prevented with either ET-receptor antagonism or glycemic control with metformin (150,162).
Cerebrovasculature provides unique structures through tight junctions on endothelial cells, astrocytic end feet and the basal lamina (163), providing necessary communication between the endothelial cells, glial cells and neurons. This communication characterizes the integrity of the BBB, which is susceptible to injury due to remodeling of the microvasculature in diabetes. Increased BBB permeability occurs as early as 2 weeks after the induction of T1D (164). While some reports did not find evidence for increased FITC-labeled albumin leakage using the STZ model (164), others observed increased BBB permeability and altered tight junctions 2 weeks after STZ-induced diabetes(165). Reduction of tight junction proteins, occludin and zona occludens proteins along with an increase in matrix metalloproteinase (MMP) levels has been reported as the underlying mechanism in BBB dysfunction, although another group reported no decrease in zona occludens proteins (166). BBB leakage has been prevented when treated with insulin a week after induction of diabetes.
Diabetes also promotes pathological neovascularization in the brain. In multiple models of diabetes including GK rats, db/db mice and HFD/STZ model, there is immature new vessel formation and remodeling of the existing vasculature (110–112,160,161,167). All these changes may contribute to the impaired regulation of CBF and hence lead to cerebral hypoperfusion in diabetes. Understanding how these structural changes occur may provide insight on the contribution of vascular dysfunction on cognitive impairment in diabetes.
3. Potential Mechanisms: Inflammation Linking Cerebrovascular and Cognitive Dysfunction
Diabetes is associated with activation of the immune system and inflammation in a wide spectrum of events involved in the pathogenesis and the progression of the disease in both T1D and T2D. Similarly, cognitive impairment is also associated with inflammation. While the role(s) of the adaptive immune system in these diseases are relatively more studied, the role(s) of innate immunity are far less understood. The innate immune system is activated through pattern recognition receptors such as membrane-bound toll-like receptors (TLRs) and cytosolic NOD-like receptors (NLRs). Interestingly, TLR2 has been implicated in both cognitive function and diabetes-mediated vascular dysfunction.
Several studies have examined the impact of TLR2 signaling on cognitive function specifically. The developing brain can be extremely susceptible to inflammation, and postnatal activation of TLR2 has been shown to induce neuroinflammation that impairs cognitive spatial and fear learning, as well leading to the impairment of motor skills in adulthood (168). Additionally, they observed a reduction in exploratory behavior, and it was hypothesized that TLR2 could play a critical role in the plasticity of cognitive behavior during developmentally crucial periods.
TLR2 is implicated in classical microglial cell activation, and this neuroinflammation is associated with decreased cognitive and memory function. Blockade of downstream pro-inflammatory cytokines such as TNF-α restored this impairment (169). Additionally, MyD88 (an essential signaling pathway for all TLRs except TLR3) knockout was shown to enhance spatial learning in a MWM test and ameliorate spatial cognitive effects in a mouse model of Alzheimer’s (170). However, the overall picture in regards to the role of inflammation is not quite so simplistic as to say that removal of TLR2 is completely beneficial. TLR2 knockout mice also have been shown to exhibit a cognitive phenotype that is seen in schizophrenia; the mice exhibit psychotic symptoms such as hyperlocomotion, anxiolytic behavior, pre-pulse inhibition effects, social withdrawal, and cognitive impairments in the domains of attention, conditioning memory, learning, and spatial ability (171). These results are contradictory to the decreases in exploratory behavior and motor skills in the previously mentioned studies (168). Findings from our lab on TLR2 knockout mice on a C57Bl/6 background suggest that they do indeed show increases in exploratory behavior during open field testing, as well as hyperlocomotion during overall cognitive assessments (Unpublished data).
The evidence for the involvement of TLR2 in diabetic vascular dysfunction originally came from studies involving renal vasculature. The expression of TLR2 in endothelial cells is involved in the HFD induction of pro-inflammatory cytokines, and most notably activation of TLR2 impairs insulin-mediated vascular relaxation (172). This suggests that TLR2 plays a role in endothelium dependent vascular function. However, whether TLR2 activation in the cerebrovasculature is an early event linking vascular dysfunction and inflammation to cognitive impairment is unknown. Studies from our group suggest that genetic deletion of TLR2 confers a protective effect on cerebral perfusion; STZ-induced T1D mice exhibit a reduction in CBF that was significantly attenuated in those mice that had genetic deletion of TLR2. Additionally, we have observed that endothelium-dependent relaxation in cerebral vessels is also impaired in STZ-induced T1D mice and that in mice lacking TLR2 this vascular dysfunction is ameliorated (Unpublished data). The involvement of TLR2 on the cerebral microvasculature in diabetes and subsequent cognitive impairment remains to be clarified, but given the links between TLR2 mediated neuroinflammation and cognitive function it is a promising new area of study.
Another common link among diabetes-mediated vascular dysfunction, cognition and innate system activation is the inflammasome. Stimulation of the innate immune system through cytosolic NLRs enables the formation and activation of inflammasome complexes. NLR containing a pyrin domain 3 (NLRP3) inflammasome has been the best studied and characterized. Activation of these complexes leads to cleavage of the inflammatory cytokines pro-IL-1β and pro-IL-18 into their active forms by caspase-1, and within the brain has been reported in neurons, astrocytes, microglia and endothelial cells. As a sensor for abnormal changes in the intracellular environment, a wide array of signals contributes to activation of the NLRP3 inflammasome including oxidative stress, cell edema, ATP, potassium efflux, acidosis and amyloid beta (173). In long-term diabetic patients, the presence of gain-of-function polymorphism NLRP3 re35829419 has been associated with increased risk for development of macrovascular complications, especially myocardial infarction (174). NLRP3 plays a role in tight-junction protein expression and endothelial cell permeability, suggesting a target for neurovascular dysfunction (175). NLRP3 has also been implicated in diabetic retinopathy (176), a complication that involves microvascular disease and neuronal death.
Although NLRP3 has yet to be explored in the cerebrovasculature in the diabetic brain, the inflammasome has been studied with respect to numerous neurodegenerative disorders including stroke (175), Alzheimer’s disease (173) and traumatic brain injury (177). In nlrp3−/− mice, neurodegeneration was ameliorated and improved cognitive outcome compared to control animals (175). Cytokine elevation through NLRP3 activation has been implicated in upregulation of BACE1 and amyloid beta deposition, and NLRP3 knockout mice were protected in an Alzheimer’s model (178). These results suggest a role for the NLRP3 inflammasome in cognition through neuronal and vascular effects. Further exploration of the innate immune system, specifically TLR2 and NLRP3 inflammasome, may provide novel therapeutics to cognitive impairment and microvascular dysfunction induced by diabetes.
Conclusions
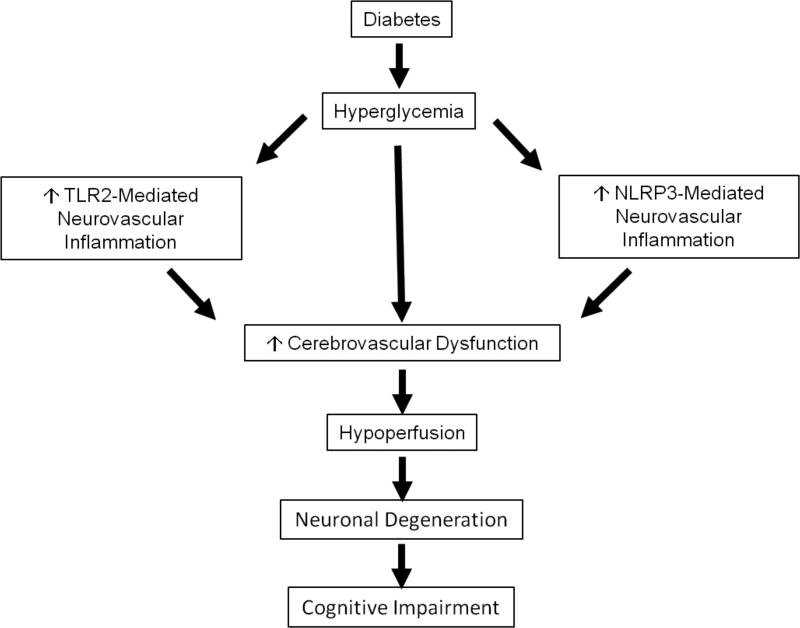
There is no doubt that structural and functional interaction of neurons with the surrounding vasculature is critical for proper function of the central nervous system, including domains involved in learning and memory. Early activation of the innate immune response as a compensatory response may result in pathological vascular remodeling and dysfunction that contribute to the development and progression of cognitive decline by decreasing CBF, creating a hypoxic milieu that further promotes inflammation and triggers a vicious cycle (Figure 1). Better understanding of the role and mechanisms by which cerebrovascular dysfunction leads to cognitive impairment in diabetes and whether there is a critical window to correct vascular dysfunction before and after onset of cognitive deficits are key questions to be answered.
Figure 1.
Schematic of the role the innate immune system, specifically TLR2 and NLRP3 inflammasome, in neurovascular dysfunction leading to cognitive impairment in diabetes.
Summary Statement.
Diabetes is an increasingly prevalent issue in the United States, and the vascular issues that arise from this disease can contribute to cognitive impairment. This review highlights evidence from both clinical and basic science research regarding diabetic vascular cognitive impairment.
Acknowledgments
Adviye Ergul is a Research Career Scientist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. This work was supported in part by VA Merit Award (BX000347), VA Research Career Scientists Award, and NIH award (NS070239, R01NS083559) to Adviye Ergul; American Heart Association Predoctoral Fellowship (15PRE25760034) to Trevor Hardigan. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
DISCLOSURE
Authors declare no conflict of interest.
References
- 1.Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion C. Crude and Age-Adjusted Incidence of Diagnosed Diabetes per 1,000 Population Aged 18–79 Years, United States, 1980–2013. 2015 [Google Scholar]
- 2.Wild S, et al. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Ehehalt S, Popovic P, Muntoni S, Muntoni S, Willasch A, Hub R, et al. Incidence of diabetes mellitus among children of Italian migrants substantiates the role of genetic factors in the pathogenesis of type 1 diabetes. Eur J Pediatr. 2009;168(5):613–7. doi: 10.1007/s00431-008-0808-9. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association 2014. National Diabetes Fact Sheet. 2014 [Google Scholar]
- 5.Dejgaard A, Gade A, Larsson H, Balle V, Parving A, Parving HH. Evidence for diabetic encephalopathy. Diabet Med. 1991;8(2):162–7. doi: 10.1111/j.1464-5491.1991.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 6.Stiles MC, Seaquist ER. Cerebral structural and functional changes in type 1 diabetes. Minerva Medica. 2010;101:105–14. [PubMed] [Google Scholar]
- 7.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5(12):649–58. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 9.Brands A, Biessels G. The effects of type 1 diabetes on cognitive performance A meta-analysis. Diabetes …. 2005;28(3):726–35. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 10.Desrocher M, Rovet J. Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol. 2004;10(1):36–52. doi: 10.1076/chin.10.1.36.26241. [DOI] [PubMed] [Google Scholar]
- 11.Northam Ea, Rankins D, Cameron FJ. Therapy insight: the impact of type 1 diabetes on brain development and function. Nat Clin Pract Neurol. 2006;2(2):78–86. doi: 10.1038/ncpneuro0097. [DOI] [PubMed] [Google Scholar]
- 12.Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: A 7-year prospective study. J Pediatr. 1999;134(4):503–6. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- 13.Schoenle EJ, Schoenle D, Molinari L, Largo RH. Impaired intellectual development in children with Type I diabetes: association with HbA 1 c, age at diagnosis and sex. Diabetologia. 2002;45(1):108–14. doi: 10.1007/s125-002-8250-6. [DOI] [PubMed] [Google Scholar]
- 14.Ryan CM. Why is cognitive dysfunction associated with the development of diabetes early in life? The diathesis hypothesis. Pediatr Diabetes. 2006;7(5):289–97. doi: 10.1111/j.1399-5448.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 15.Ryan CM. Diabetes, aging, and cognitive decline. Neurobiol Aging. 2005;26(SUPPL):21–5. doi: 10.1016/j.neurobiolaging.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Ryan CM. Does severe hypoglycaemia disrupt academic achievement in children with early onset diabetes? Dev Med Child Neurol. 2012;54(5):393–4. doi: 10.1111/j.1469-8749.2012.04256.x. [DOI] [PubMed] [Google Scholar]
- 17.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7(2):184–90. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJK, Wardlaw J, et al. Cognitive ability and brain structure in type 1 diabetes: Relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52(1):149–56. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CM, Geckle MO, Orchard TJ. Cognitive efficiency declines over time in adults with Type 1 diabetes: Effects of micro- and macrovascular complications. Diabetologia. 2003 Jul;46(7):940–8. doi: 10.1007/s00125-003-1128-2. [DOI] [PubMed] [Google Scholar]
- 20.Klein BEK, Heiss G, Hubbard LD, Duncan BB. Retinal Microvascular Abnormalities and Renal Dysfunction: The Atherosclerosis Risk in Communities Study. J Am Soc Nephrol. 2004;15(9):2469–76. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: An 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2011;54(2):245–55. doi: 10.1007/s00125-010-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastrup J, Rørsgaard S, Parving HH, Lassen NA. Impaired autoregulation of cerebral blood flow in long-term type I (insulin-dependent) diabetic patients with nephropathy and retinopathy. Clin Physiol. 1986;6(6):549–59. doi: 10.1111/j.1475-097x.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 23.Fiilesdi B, Limburg M, Bereczki D, Michels RPJ, Neuwirth G, Legemate D. Impairment of Cerebrovascular Reactivity in Long-Term T^pe 1 Diabetes. Diabetes. 1997 Nov;46:1840–5. doi: 10.2337/diab.46.11.1840. [DOI] [PubMed] [Google Scholar]
- 24.Keymeulen B, Jacobs A, de Metz K, de Sadeleer C, Bossuyt A, Somers G. Regional cerebral hypoperfusion in long-term type 1 (insulin-dependent) diabetic patients: relation to hypoglycaemic events. Nucl Med Commun. 1995;16(1):10–6. doi: 10.1097/00006231-199501000-00005. [DOI] [PubMed] [Google Scholar]
- 25.van Elderen SG, Brandts A, van der Grond J, Westenberg JJ, Kroft LJ, van Buchem MA, et al. Cerebral perfusion and aortic stiffness are independent predictors of white matter brain atrophy in type 1 diabetic patients assessed with magnetic resonance imaging. Diabetes Care. 2011;34(2):459–63. doi: 10.2337/dc10-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wessels AM, Scheltens P, Barkhof F, Heine RJ. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur J Pharmacol. 2008;585(1):88–96. doi: 10.1016/j.ejphar.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 27.Salem MaK, Matta LF, Tantawy AaG, Hussein M, Gad GI. Single photon emission tomography (SPECT) study of regional cerebral blood flow in normoalbuminuric children and adolescents with type 1 diabetes. Pediatr Diabetes. 2002;3(3):155–62. doi: 10.1034/j.1399-5448.2002.30306.x. [DOI] [PubMed] [Google Scholar]
- 28.Jimenéz-Bonilla JF, Quirce R, Hernández A, Vallina NK, Guede C, Banzo I, et al. Assessment of cerebral perfusion and cerebrovascular reserve in insulin-dependent diabetic patients without central neurological symptoms by means of 99mTc-HMPAO SPET with acetazolamide. Eur J Nucl Med. 2001;28(11):1647–55. doi: 10.1007/s002590100595. [DOI] [PubMed] [Google Scholar]
- 29.Quirce R, Carril JM, Jimenez-Bonilla JF, Amado JA, Gutierrez-Mendiguchia C, Banzo I, et al. Semi-quantitative assessment of cerebral blood flow with 99mTc-HMPAO SPET in type I diabetic patients with no clinical history of cerebrovascular disease. Eur J Nucl Med. 1997;24(12):1507–13. doi: 10.1007/s002590050181. [DOI] [PubMed] [Google Scholar]
- 30.Schwab KO, Doerfer J, Hecker W, Grulich-Henn J, Wiemann D, Kordonouri O, et al. Spectrum and Prevalence of Atherogenic Risk Factors in 27,358 Children, Adolescents, and Young Adults With Type 1 Diabetes. Diabetes Care. 2006;29(2):218–25. doi: 10.2337/diacare.29.02.06.dc05-0724. [DOI] [PubMed] [Google Scholar]
- 31.Musen G. Effects of Type 1 Diabetes on Gray Matter Density as Measured by Voxel-Based Morphometry. Diabetes. 2006;55(2):326–33. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- 32.Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, et al. Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia. 2006;49(10):2474–80. doi: 10.1007/s00125-006-0283-7. [DOI] [PubMed] [Google Scholar]
- 33.Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia. 2008;51(3):417–25. doi: 10.1007/s00125-007-0904-9. [DOI] [PubMed] [Google Scholar]
- 34.Costacou T, Rosano C, Aizenstein H, Mettenburg JM, Nunley K, Ferrell RE, et al. The haptoglobin 1 allele correlates with white matter hyperintensities in middle-aged adults with type 1 diabetes. Diabetes. 2015;64(2):654–9. doi: 10.2337/db14-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ott A, Stolk RP, van Harskamp F, Pols Hofman A, Breteler M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;59(9):1937–42. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 36.Xu WL, Qiu CX, Wahlin a, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181–6. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 37.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51(4):1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 38.Palta P, Schneider ALC, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20(3):278–91. doi: 10.1017/S1355617713001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Berg E, Kloppenborg RP, Kessels RPC, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim Biophys Acta - Mol Basis Dis. 2009;1792(5):470–81. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Kanaya AM. Change in Cognitive Function by Glucose Tolerance Status in Older Adults<subtitle>A 4-Year Prospective Study of the Rancho Bernardo Study Cohort</subtitle>. Arch Intern Med. 2004;164(12):1327. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- 41.Ruis C, van den Donk M, Biessels GJ, Kapelle LJ, Gorter K, Rutten GEH. Cognition in the Early Stage of Type 2. Diabetes Care. 2009;32(7):3–7. doi: 10.2337/dc08-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Messier C, Tsiakas M, Gagnon M, Desrochers A, Awad N. Effect of age and glucoregulation on cognitive performance. Neurobiol Aging. 2003;24(7):985–1003. doi: 10.1016/s0197-4580(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 43.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–9. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 44.Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: Results of the epidemiology of vascular aging study. Diabetes Care. 2001;24(2):366–70. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- 45.Van Den Berg E, Reijmer YD, De Bresser J, Kessels RPC, Kappelle LJ, Biessels GJ. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(1):58–65. doi: 10.1007/s00125-009-1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer AL, de Frias CM, Yeung SE, Dixon RA. Short-term longitudinal trends in cognitive performance in older adults with type 2 diabetes. J Clin Exp Neuropsychol. 2009;31(7):809–22. doi: 10.1080/13803390802537636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reijmer YD, van den Berg E, de Bresser J, Kessels RPC, Kappelle LJ, Algra A, et al. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;(30):96–102. doi: 10.1002/dmrr.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manschot SM. Brain Magnetic Resonance Imaging Correlates of Impaired Cognition in Patients With Type 2 Diabetes. Diabetes. 2006;55(4):1106–13. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 49.Reaven GM, Thompson LW, Nahum D, Haskins E. Relationship between hyperglycaemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;13(1):16–21. doi: 10.2337/diacare.13.1.16. [DOI] [PubMed] [Google Scholar]
- 50.Ebady SA, Arami MA, Shafigh MH. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res Clin Pract. 2008;82(3):305–9. doi: 10.1016/j.diabres.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Ryan CM, Geckle MO. Circumscribed cognitive dysfunctiion in Middle-Aged Adults With Type 2 Diabetes. Diabetes Care. 2000;23(10):1486–93. doi: 10.2337/diacare.23.10.1486. [DOI] [PubMed] [Google Scholar]
- 52.Crichton G, Elias M, Buckley J, Murphy K, Frisardi V. Metabolic syndrome, cognitive performance, and dementia. J Alzheimers Dis. 2012;30(Suppl 2):S77–87. doi: 10.3233/JAD-2011-111022. [DOI] [PubMed] [Google Scholar]
- 53.Haan M. Therapy Insight: type 2 diabetes mellitus and the risk of late-onset Alzheimer’s disease. Nat Clin Pr Neurol. 2006;2(3):159–66. doi: 10.1038/ncpneuro0124. [DOI] [PubMed] [Google Scholar]
- 54.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–9. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 55.Yaffe K, Blackwell T, Whitmer Ra, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10(4):293–5. [PubMed] [Google Scholar]
- 56.Munshi M, Grande L, Hayes M, Ayres D. Cognitive dysfunction is associated with poor diabetes control in older adults. Diabetes …. 2006 doi: 10.2337/dc06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287(19):2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 58.Vehkavaara S, Seppala-Lindroos A, Westerbacka J, Groop PH, Yki-Jarvinen H. In vivo endothelial dysfunction characterizes patients with impaired fasting glucose. Diabetes Care. 1999;22(12):2055–60. doi: 10.2337/diacare.22.12.2055. [DOI] [PubMed] [Google Scholar]
- 59.Ohsugi K, Sugawara H, Ebina K, Shiga K, Kikuchi N, Mori M, et al. Comparison of brachial artery flow-mediated dilation in youth with type 1 and type 2 diabetes mellitus. J Diabetes Investig. 2014;5(5):615–20. doi: 10.1111/jdi.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991;87(5):1643–8. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams B. Factors regulating the expression of vascular permeability/vascular endothelial growth factor by human vascular tissues. Diabetologia. 1997;40(Suppl 2):S118–20. doi: 10.1007/s001250051423. [DOI] [PubMed] [Google Scholar]
- 62.Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87(2):432–8. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nazir FS, Alem M, Small M, Connell JMC, Lees KR, Walters MR, et al. Blunted response to systemic nitric oxide synthase inhibition in the cerebral circulation of patients with Type 2 diabetes. Diabet Med. 2006;23(4):398–402. doi: 10.1111/j.1464-5491.2006.01815.x. [DOI] [PubMed] [Google Scholar]
- 64.Ezoddini AF, Afkhami AM, Z M, MH S. Evaluating calcified carotid artery atheromas in panoramic radiographs of patients with type 2 diabetes mellitus. Oral Radiol. 2007;23(1):6–9. [Google Scholar]
- 65.Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Foster JK, et al. Predictors of cognitive impairment and dementia in older people with diabetes. Diabetologia. 2008;51(2):241–8. doi: 10.1007/s00125-007-0894-7. [DOI] [PubMed] [Google Scholar]
- 66.Folsom AR, Rasmussen ML, Chambless LE, Howard G, Cooper LS, Schmidt MI, et al. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care. 1999;22(7):1077–83. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- 67.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes. 2010;2(2):118–24. doi: 10.1111/j.1753-0407.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rawlings AM, Sharrett AR, Schneider ALC, Coresh J, Albert M, Couper D, et al. Diabetes in midlife and cognitive change over 20 years: A cohort study. Ann Intern Med. 2014;161(11):785–93. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, Cukierman-Yaffee T, et al. Poor Cognitive Function and Risk of Severe Hypoglycemia in Type 2 Diabetes. Diabetes Care. 2012;35:1–7. doi: 10.2337/dc11-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven P, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 71.Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes — NEJM. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 72.Holman RR, Paul SK, Bethel MA, Matthews DR. Long-Term Follow-up after Tight Control of Blood Pressure in Type 2 Diabetes. N Engl J Med. 2008;359:1–12. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 73.Van Harten B, Leeuw F De. Brain imaging in patients with diabetes a systematic review. Diabetes Care. 2006;29(11):2539–48. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 74.Ding J, Strachan MWJ, Reynolds RM, Frier BM, Deary IJ, Fowkes FGR, et al. Diabetic Retinopathy and Cognitive Decline in Older The Edinburgh Type 2 Diabetes Study. 2010 Nov;59:2883–9. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lesage SR, Mosley TH, Wong TY, Szklo M, Knopman D, Catellier DJ, et al. Retinal microvascular abnormalities and cognitive decline: The ARIC 14-year follow-up study. Neurology. 2009;73(11):862–8. doi: 10.1212/WNL.0b013e3181b78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Last D, Alsop D, Abduljalil A, Marquis R, de Bazelaire C, Hu K, et al. Global and Regional Effects of Type 2 Diabetes on Brain Tissue Volumes and Cerebral Vasoreactivty. Diabetes Care. 2007;30(5):1193–9. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010;67(5):570–8. doi: 10.1002/ana.21983. [DOI] [PubMed] [Google Scholar]
- 78.Nagamachi S, Nishikawa T, Ono S, Ageta M, Matsuo T, Jinnouchi S, et al. Regional cerebral blood flow in diabetic patients: evaluation by N-isopropyl-123I-IMP with SPECT. Nucl Med Commun. 1994;15(6):455–60. doi: 10.1097/00006231-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Sabri O, Hellwig D, Schreckenberger M, Schneider R, Kaiser HJ, Wagenknecht G, et al. Influence of diabetes mellitus on regional cerebral glucose metabolism and regional cerebral blood flow. Nucl Med Commun. 2000;21(1):19–29. doi: 10.1097/00006231-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Tiehuis AM, Vincken KL, van den Berg E, Hendrikse J, Manschot SM, Mali WPTM, et al. Cerebral perfusion in relation to cognitive function and type 2 diabetes. Diabetologia. 2008;51(7):1321–6. doi: 10.1007/s00125-008-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brundel M, Van Den Berg E, Reijmer YD, De Bresser J, Kappelle LJ, Biessels GJ. J Diabetes Complications. 3. Vol. 26. Elsevier Inc.; 2012. Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes; pp. 205–9. [DOI] [PubMed] [Google Scholar]
- 82.Chung C, Pimentel D, Jor’dan AJ, Hao Y, Milberg W, Novak V. Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology. 2015:1–9. doi: 10.1212/WNL.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beason-Held LL, Thambisetty M, Deib G, Sojkova J, Landman BA, Zonderman AB, et al. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke. 2012;43(6):1542–7. doi: 10.1161/STROKEAHA.111.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thambisetty M, Beason-Held LL, An Y, Kraut M, Metter J, Egan J, et al. Neurobiol Aging. 10. Vol. 34. Elsevier Ltd; 2013. Impaired glucose tolerance in midlife and longitudinal changes in brain function during aging; pp. 2271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shah IM, Ghosh SK, Collier A. Stroke presentation in Type 2 diabetes and the metabolic syndrome. Diabetes Res Clin Pract. 2008;79(1):2–5. doi: 10.1016/j.diabres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Saczynski JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Jonsson PV, Garcia ME, et al. Cerebral infarcts and cognitive performance importance of location and number of infarcts. Stroke. 2009;40(3):677–82. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsumoto K, Miyake S, Yano M, Ueki Y, Miyazaki a, Hirao K, et al. Insulin resistance and classic risk factors in type 2 diabetic patients with different subtypes of ischemic stroke. Diabetes Care. 1999;22(7):1191–5. doi: 10.2337/diacare.22.7.1191. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke [Internet] 2011;42:3323–8. doi: 10.1161/STROKEAHA.110.608257. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3584169&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab [Internet] 2012;32(7):1139–51. doi: 10.1038/jcbfm.2011.197. Available from: http://dx.doi.org/10.1038/jcbfm.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Czeh B, Abumaria N, Rygula R, Fuchs E. Quantitative changes in hippocampal microvasculature of chronically stressed rats: No effect of fluoxetine treatment. Hippocampus. 2010;20:174–85. doi: 10.1002/hipo.20599. [DOI] [PubMed] [Google Scholar]
- 91.Revsin Y, Saravia F, Roig P, Lima A, de Kloet ER, Homo-Delarche F, et al. Neuronal and astroglial alterations in the hippocampus of a mouse model for type 1 diabetes. [cited 2016 Jan 19];Brain Res [Internet] 2005 Mar 15;1038(1):22–31. doi: 10.1016/j.brainres.2004.12.032. Available from: http://www.sciencedirect.com/science/article/pii/S0006899304019584. [DOI] [PubMed] [Google Scholar]
- 92.Saravia FE, Revsin Y, Gonzalez Deniselle MC, Gonzalez SL, Roig P, Lima A, et al. Increased astrocyte reactivity in the hippocampus of murine models of type 1 diabetes: the nonobese diabetic (NOD) and streptozotocin-treated mice. [cited 2015 Dec 6];Brain Res [Internet] 2002 Dec 13;957(2):345–53. doi: 10.1016/s0006-8993(02)03675-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12445977. [DOI] [PubMed] [Google Scholar]
- 93.Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11(3):309–17. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagayach A, Patro N, Patro I. Astrocytic and microglial response in experimentally induced diabetic rat brain. Metabolic Brain Disease. 2014 doi: 10.1007/s11011-014-9562-z. [DOI] [PubMed] [Google Scholar]
- 95.De Senna PN, Ilha J, Baptista PPA, Do Nascimento PS, Leite MC, Paim MF, et al. Effects of physical exercise on spatial memory and astroglial alterations in the hippocampus of diabetic rats. Metab Brain Dis. 2011;26:269–79. doi: 10.1007/s11011-011-9262-x. [DOI] [PubMed] [Google Scholar]
- 96.Stranahan AM, Lee K, Pistell PJ, Nelson CM, Readal N, Miller MG, et al. Accelerated cognitive aging in diabetic rats is prevented by lowering corticosterone levels. Neurobiol Learn Mem [Internet] 2008;90(2):479–83. doi: 10.1016/j.nlm.2008.05.005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18579418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamal A, Biessels GJ, Duis SE, Gispen WH. Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing. Diabetologia [Internet] 2000;43(4):500–6. doi: 10.1007/s001250051335. Available from: http://download.springer.com/static/pdf/502/art%3A10.1007%2Fs001250051335.pdf?auth66=1385727082_816e8e8e7f3eeb35dc0226aadd24dbe7&ext=.pdf. [DOI] [PubMed] [Google Scholar]
- 98.Taylor SL, Trudeau D, Arnold B, Wang J, Gerrow K, Summerfeldt K, et al. VEGF can protect against blood brain barrier dysfunction, dendritic spine loss and spatial memory impairment in an experimental model of diabetes. Neurobiol Dis. 2015;78:1–11. doi: 10.1016/j.nbd.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 99.Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, et al. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45(9):1259–66. doi: 10.2337/diab.45.9.1259. [DOI] [PubMed] [Google Scholar]
- 100.Kamal A, Biessels GJ, Urban IJA, Gispen WH. Hippocampal synaptic plasticity in streptozotocin-diabetic rats: Impairment of long-term potentiation and facilitation of long-term depression. Neuroscience. 1999;90(3):737–45. doi: 10.1016/s0306-4522(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 101.Artola A, Kamal A, Ramakers GMJ, Biessels GJ, Gispen WH. Diabetes mellitus concomitantly facilitates the induction of long-term depression and inhibits that of long-term potentiation in hippocampus. Eur J Neurosci. 2005;22(1):169–78. doi: 10.1111/j.1460-9568.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 102.Stranahan AM. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience. 2015 Nov;309:125–39. doi: 10.1016/j.neuroscience.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jabbarpour Z, Shahidi S, Saidijam M, Sarihi A, Hassanzadeh T, Esmaeili R. Effect of tempol on the passive avoidance and novel object recognition task in diabetic rats. [cited 2016 Jan 19];Brain Res Bull [Internet] 2014 Feb;101:51–6. doi: 10.1016/j.brainresbull.2013.12.013. Available from: http://www.sciencedirect.com/science/article/pii/S0361923014000021. [DOI] [PubMed] [Google Scholar]
- 104.Kawamoto EM, Cutler RG, Rothman SM, Mattson MP, Camandola S. TLR4-dependent metabolic changes are associated with cognitive impairment in an animal model of type 1 diabetes. [cited 2016 Jan 19];Biochem Biophys Res Commun [Internet] 2014 Jan 10;443(2):731–7. doi: 10.1016/j.bbrc.2013.12.039. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3916215&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li X-L, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience [Internet] 2002;113(3):607–15. doi: 10.1016/s0306-4522(02)00162-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12150780. [DOI] [PubMed] [Google Scholar]
- 106.Ramos-Rodriguez JJ, Ortiz O, Jimenez-Palomares M, Kay KR, Berrocoso E, Murillo-Carretero MI, et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology. 2013 Nov;38(11):2462–75. doi: 10.1016/j.psyneuen.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Beauquis J, Homo-Delarche F, Giroix M-H, Ehses J, Coulaud J, Roig P, et al. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010;222(1):125–34. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 108.Cai Z, Zhao Y, Yao S, Bin Zhao B. Increases in β-amyloid protein in the hippocampus caused by diabetic metabolic disorder are blocked by minocycline through inhibition of NF-κB pathway activation. [cited 2015 Dec 18];Pharmacol Rep [Internet] 2011 Jan;63(2):381–91. doi: 10.1016/s1734-1140(11)70504-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21602593. [DOI] [PubMed] [Google Scholar]
- 109.Hussain S, Mansouri S, Sjöholm Å, Patrone C, Darsalia V. Evidence for cortical neuronal loss in male type 2 diabetic Goto-Kakizaki rats. [cited 2016 Feb 1];J Alzheimers Dis [Internet] 2014 Jan;41(2):551–60. doi: 10.3233/JAD-131958. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24643136. [DOI] [PubMed] [Google Scholar]
- 110.Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral Neovascularization and Remodeling Patterns in Two Different Models of Type 2 Diabetes. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qu Z, Li W, Prakash R, Fagan SC, Ergul A. Stroke [Internet] Suppl 1. Vol. 45. Lippincott Williams & Wilkins; 2014. Feb 1, [cited 2015 Oct 22]. Abstract 169: Diet-induced Diabetes Impairs Neovascularization and Functional Recovery After Ischemic Stroke; p. A169. Available from: http://stroke.ahajournals.org/content/45/Suppl_1/A169.abstract. [Google Scholar]
- 112.Valenzuela JP, Qu Z, Prakash R, Li W, Ward R, Fagan SC, et al. Abstract W P412: Metabolic Syndrome Alters Cerebrovascular Architecture: Relevance to Cognitive Deficits and Stroke Outcomes. [cited 2015 Oct 22];Stroke [Internet] 2015 Feb 1;46(Suppl_1):AWP412. –. Available from: http://stroke.ahajournals.org/content/46/Suppl_1/AWP412.abstract. [Google Scholar]
- 113.Datusalia AK, Sharma SS. Amelioration of diabetes-induced cognitive deficits by GSK-3β inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Mol Neurobiol. 2014 Oct;50(2):390–405. doi: 10.1007/s12035-014-8632-x. [DOI] [PubMed] [Google Scholar]
- 114.Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. Journal of Neurochemistry. 2010;115:814–28. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- 115.Kitamura A, Fujita Y, Oishi N, Kalaria RN, Washida K, Maki T, et al. Selective white matter abnormalities in a novel rat model of vascular dementia. Neurobiol Aging. 2012;33 doi: 10.1016/j.neurobiolaging.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 116.Nishio K, Ihara M, Yamasaki N, Kalaria RN, Maki T, Fujita Y, et al. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke. 2010;41(6):1278–84. doi: 10.1161/STROKEAHA.110.581686. [DOI] [PubMed] [Google Scholar]
- 117.Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, et al. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210(2):585–91. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 118.Hattori Y, Enmi J, Kitamura A, Yamamoto Y, Saito S, Takahashi Y, et al. A novel mouse model of subcortical infarcts with dementia. [cited 2015 Jul 25];J Neurosci [Internet] 2015 Mar 4;35(9):3915–28. doi: 10.1523/JNEUROSCI.3970-14.2015. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25740520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marosi M, Rákos G, Robotka H, Németh H, Sas K, Kis Z, et al. Hippocampal (CA1) activities in Wistar rats from different vendors. Fundamental differences in acute ischemia. [cited 2015 Jul 26];J Neurosci Methods [Internet] 2006 Sep 30;156(1–2):231–5. doi: 10.1016/j.jneumeth.2006.03.010. Available from: http://www.sciencedirect.com/science/article/pii/S016502700600149X. [DOI] [PubMed] [Google Scholar]
- 120.Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cereb Blood Flow Metab [Internet] 2012 Oct;33:708–15. doi: 10.1038/jcbfm.2013.1. 2013; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3652696&tool=pmcentrez&rendertype=abstract\n http://www.ncbi.nlm.nih.gov/pubmed/23361394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A [Internet] 2008;105(34):12474–9. doi: 10.1073/pnas.0805350105. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18711131\n http://www.pnas.org/content/105/34/12474.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sudduth TL, Powell DK, Smith CD, Greenstein A, Wilcock DM. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J Cereb Blood Flow Metab. 2012 Oct;33:708–15. doi: 10.1038/jcbfm.2013.1. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35(11):2598–603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 124.Coltman R, Spain A, Tsenkina Y, Fowler JH, Smith J, Scullion G, et al. Selective white matter pathology induces a specific impairment in spatial working memory. Neurobiol Aging. 2011;32(12) doi: 10.1016/j.neurobiolaging.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 125.Mogi M, Horiuchi M. Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ J. 2011;75:1042–8. doi: 10.1253/circj.cj-11-0121. [DOI] [PubMed] [Google Scholar]
- 126.Liu H, Zhang J. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci [Internet] 2012;122(9):494–9. doi: 10.3109/00207454.2012.686543. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22519891. [DOI] [PubMed] [Google Scholar]
- 127.Yatomi Y, Tanaka R, Shimada Y, Yamashiro K, Liu M, Mitome-Mishima Y, et al. Type 2 diabetes reduces the proliferation and survival of oligodendrocyte progenitor cells in ishchemic white matter lesions. Neuroscience. 2015;289:214–23. doi: 10.1016/j.neuroscience.2014.12.054. [DOI] [PubMed] [Google Scholar]
- 128.Zuloaga KL, Johnson LA, Roese NE, Marzulla T, Zhang W, Nie X, et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab [Internet] 2015 doi: 10.1177/0271678X15616400. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26661233. [DOI] [PMC free article] [PubMed]
- 129.Kwon KJ, Lee EJ, Kim MK, Kim SY, Kim JN, Kim JO, et al. Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: Implication of cilostazol for diabetes mellitus-induced dementia. [cited 2014 Oct 31];Neurobiol Dis [Internet] 2014 Oct 2;73C:12–23. doi: 10.1016/j.nbd.2014.08.034. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25281785. [DOI] [PubMed] [Google Scholar]
- 130.Erdös B, Snipes Ja, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol [Internet] 2006;290(3):H1264–70. doi: 10.1152/ajpheart.00804.2005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16284235. [DOI] [PubMed] [Google Scholar]
- 131.Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H Oxidase Alleviates Impaired NOS-Dependent Responses of Pial Arterioles in Type 1 Diabetes Mellitus. Microcirculation. 2006;13(7):567–75. doi: 10.1080/10739680600885194. [DOI] [PubMed] [Google Scholar]
- 132.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36(2):342–7. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 133.Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res [Internet] 2007;1184:365–71. doi: 10.1016/j.brainres.2007.10.004. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2174607&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Zhang H-T, Su X-L, Deng X-L, Yuan B-X, Zhang W, et al. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res. 2010 May;7(2):75–84. doi: 10.2174/156720210791184925. [DOI] [PubMed] [Google Scholar]
- 135.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81(6):996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 136.Ergul A, Kelly-Cobbs A, Abdalla MC, Fagan S. Cerebrovascular Complications of Diabetes: Focus on Stroke. Endocrine, Metabolic & Immune Disorders - Drug Targets. 2012;12:148–58. doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol [Internet] 2008;294(4):R1213–9. doi: 10.1152/ajpregu.00885.2007. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3791629&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mayhan WG. Effect of diabetes mellitus on responses of the basilar artery in rats to products released by platelets. Stroke. 1992 Oct 2;(10):1499–503. doi: 10.1161/01.str.23.10.1499. 1992. [DOI] [PubMed] [Google Scholar]
- 139.Van Buren T, Vleeming W, Krutzen MM, Van de Kuil T, Gispen WH, De Wildt DJ. Vascular responses of isolated mesenteric resistance and basilar arteries from short- and long-term diabetic rats. Naunyn Schmiedebergs Arch Pharmacol [Internet] 1998;358(6):663–70. doi: 10.1007/pl00005309. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9879726. [DOI] [PubMed] [Google Scholar]
- 140.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res [Internet] 1997;81(6):996–1004. doi: 10.1161/01.res.81.6.996. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9400380. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Zhang H-T, Su X-L, Deng X-L, Yuan B-X, Zhang W, et al. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. [cited 2015 Dec 18];Curr Neurovasc Res [Internet] 2010 May;7(2):75–84. doi: 10.2174/156720210791184925. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20334613. [DOI] [PubMed] [Google Scholar]
- 142.Abiru T, Kamata K, Miyata N, Kasuya Y. Differences in vascular responses to vasoactive agents of basilar artery and aorta from rabbits with alloxan-induced diabetes. [cited 2015 Dec 18];Can J Physiol Pharmacol [Internet] 1990 Jul;68(7):882–8. doi: 10.1139/y90-134. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1696518. [DOI] [PubMed] [Google Scholar]
- 143.Mayhan WG. Constrictor responses of the rat basilar artery during diabetes mellitus. Brain Res [Internet] 1998;783(2):326–31. doi: 10.1016/s0006-8993(97)01387-5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9507178. [DOI] [PubMed] [Google Scholar]
- 144.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, et al. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55(4):1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 145.Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthasedependent dilatation of cerebral arterioles in diabetic rats. Stroke [Internet] 2003;34(11):2698–703. doi: 10.1161/01.STR.0000092121.62649.DC. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14563973. [DOI] [PubMed] [Google Scholar]
- 146.Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res. 2007;1184:365–71. doi: 10.1016/j.brainres.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arrick DM, Mayhan WG. Inhibition of endothelin-1 receptors improves impaired nitric oxide synthase-dependent dilation of cerebral arterioles in type-1 diabetic rats. Microcirc (New York, NY 1994) [Internet] 2010;17(6):439–46. doi: 10.1111/j.1549-8719.2010.00042.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kitayama J, Faraci FM, Gunnett Ca, Heistad DD. Impairment of dilator responses of cerebral arterioles during diabetes mellitus: role of inducible NO synthase. Stroke [Internet] 2006;37(8):2129–33. doi: 10.1161/01.STR.0000231654.79017.df. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16809563. [DOI] [PubMed] [Google Scholar]
- 149.Mayhan WG, Trauernicht aK, Irvine SD. Insulin reverses impaired acetylcholine-induced dilatation of the rat basilar artery during diabetes mellitus. Brain Res [Internet] 2001;893(1–2):195–201. doi: 10.1016/s0006-8993(00)03314-x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11223007. [DOI] [PubMed] [Google Scholar]
- 150.Kelly-Cobbs a, Elgebaly MM, Li W, Ergul a. Pressure-independent cerebrovascular remodelling and changes in myogenic reactivity in diabetic Goto-Kakizaki rat in response to glycaemic control. Acta Physiol. 2011;203:245–51. doi: 10.1111/j.1748-1716.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jarajapu YPR, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. [cited 2015 Dec 18];Eur J Pharmacol [Internet] 2008 Jan 28;579(1–3):298–307. doi: 10.1016/j.ejphar.2007.10.028. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3008571&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kelly-Cobbs AI, Prakash R, Coucha M, Knight Ra, Li W, Ogbi SN, et al. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther [Internet] 2012;342(2):407–15. doi: 10.1124/jpet.111.191296. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3400801&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frier BM. Cognitive functioning in type 1 diabetes: The Diabetes Control and Complications Trial (DCCT) revisited. Vol. 54. Diabetologia. 2011:233–6. doi: 10.1007/s00125-010-1983-6. [DOI] [PubMed] [Google Scholar]
- 154.McCuskey PA, McCuskey RS. In vivo and electron microscopic study of the development of cerebral diabetic microangiography. Microcirc Endothelium Lymphatics. 1984 Apr;1(2):221–44. [PubMed] [Google Scholar]
- 155.Mukai N, Hori S, Pomeroy M. Cerebral lesions in rats with streptozotocin-induced diabetes. Acta Neuropathol. 1980;51(1):79–84. doi: 10.1007/BF00688853. [DOI] [PubMed] [Google Scholar]
- 156.Tomassoni D, Bellagamba G, Postacchini D, Venarucci D, Amenta F. Cerebrovascular and brain microanatomy in spontaneously hypertensive rats with streptozotocin-induced diabetes. Clin Exp Hypertens. 2004;26:305–21. doi: 10.1081/ceh-120034136. [DOI] [PubMed] [Google Scholar]
- 157.Mayhan WG. Cerebral circulation during diabetes mellitus. Pharmacol Ther. 1993 Jan 57;(2–3):377–91. doi: 10.1016/0163-7258(93)90062-i. [DOI] [PubMed] [Google Scholar]
- 158.Moore Sa, Bohlen HG, Miller BG, Evan aP. Cellular and vessel wall morphology of cerebral cortical arterioles after short-term diabetes in adult rats. Blood Vessels. 1985;22(6):265–77. doi: 10.1159/000158613. [DOI] [PubMed] [Google Scholar]
- 159.Ergul A, Elgebaly MM, Middlemore M-L, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. [cited 2015 Dec 18];BMC Neurol [Internet] 2007 Jan;7:33. doi: 10.1186/1471-2377-7-33. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2098774&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes [Internet] 2010;59(1):228–35. doi: 10.2337/db09-0902. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2797926&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, et al. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes [Internet] 2005;54(9):2638–44. doi: 10.2337/diabetes.54.9.2638. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16123352. [DOI] [PubMed] [Google Scholar]
- 162.Kelly-Cobbs AI, Harris AK, Elgebaly MM, Li W, Sachidanandam K, Portik-Dobos V, et al. Endothelial endothelin B receptor-mediated prevention of cerebrovascular remodeling is attenuated in diabetes because of up-regulation of smooth muscle endothelin receptors. J Pharmacol Exp Ther. 2011 Apr;337(1):9–15. doi: 10.1124/jpet.110.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.del Zoppo GJ. Stroke and neurovascular protection. [cited 2015 Dec 18];N Engl J Med [Internet] 2006 Feb 9;354(6):553–5. doi: 10.1056/NEJMp058312. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16467542. [DOI] [PubMed] [Google Scholar]
- 164.Stauber WT, Ong SH, McCuskey RS. Selective extravascular escape of albumin into the cerebral cortex of the diabetic rat. Diabetes [Internet] 1981;30(6):500–3. doi: 10.2337/diab.30.6.500. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7014313. [DOI] [PubMed] [Google Scholar]
- 165.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: Contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50(1):202–11. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 166.Chehade JM, Haas MJ, Mooradian AD. Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. [cited 2015 Dec 18];Neurochem Res [Internet] 2002 Mar;27(3):249–52. doi: 10.1023/a:1014892706696. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11958524. [DOI] [PubMed] [Google Scholar]
- 167.Ergul A, Abdelsaid M, Fouda AY, Fagan SC. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab [Internet] 2014;34:553–63. doi: 10.1038/jcbfm.2014.18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24496174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Madar R, Rotter A, Ben-Asher HW, Mughal MR, Arumugam TV, Wood WH, et al. Brain Behav Immun. Vol. 1. Elsevier Inc.; 2015. Postnatal TLR2 activation impairs learning and memory in adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, et al. J Neuroinflammation. 1. Vol. 9. BioMed Central Ltd; 2012. TNF- a protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation; p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lim JE, et al. The effects of MyD88 deficiency on exploratory activity, anxiety, motor coordination, and spatial learning in C57BL/6 and APPswe/PS1dE9 mice. Behav Brain Res. 2012;227(1):36–42. doi: 10.1016/j.bbr.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Park SJ, Lee JY, Kim SJ, Choi S-Y, Yune TY, Ryu JH. Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci Rep. 2015;5:8502. doi: 10.1038/srep08502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jang H-JJ, Kim H-SS, Hwang DH, Quon MJ, Kim J. Toll-like receptor 2 mediates high-fat dietinduced impairment of vasodilator actions of insulin. Am J Physiol - Endocrinol Metab. 2013 May;304(10):E1077–88. doi: 10.1152/ajpendo.00578.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT. Inflammasomes in neuroinflammation and changes in brain function: a focused review. [cited 2014 Oct 24];Front Neurosci [Internet] 2014 Jan;8:315. doi: 10.3389/fnins.2014.00315. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4188030&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Klen J, Goričar K, Janež A, Dolžan V. NLRP3 Inflammasome Polymorphism and Macrovascular Complications in Type 2 Diabetes Patients. [cited 2015 Dec 18];J Diabetes Res [Internet] 2015 Jan 14;2015(6):616747. doi: 10.1155/2015/616747. Available from: https://www.researchgate.net/publication/281056438_NLRP3_Inflammasome_Polymorphism_and_Macrovascular_Complications_in_Type_2_Diabetes_Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang F, Wang Z, Wei X, Han H, Meng X, Zhang Y, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab [Internet] 2014;34:660–7. doi: 10.1038/jcbfm.2013.242. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24424382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Devi TS, Lee I, Hüttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. [cited 2015 Jul 14];Exp Diabetes Res [Internet] 2012 Jan;2012:438238. doi: 10.1155/2012/438238. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3313582&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu H-D, Li W, Chen Z-R, Hu Y-C, Zhang D-D, Shen W, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res [Internet] 2013;38:2072–83. doi: 10.1007/s11064-013-1115-z. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23892989. [DOI] [PubMed] [Google Scholar]
- 178.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. [cited 2015 Dec 7];Nature [Internet] 2013 Jan 31;493(7434):674–8. doi: 10.1038/nature11729. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3812809&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Long-Term Effect of Diabetes and Its Treatment on Cognitive Function. N Engl J Med. 2007;356(18):1842–52. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. Effects of randomization to intensive glucose lowering on brain structure and function in type 2 diabetes. ACCORD Memory in Diabetes Study. 2012;10(11):969–77. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Knopman DS, Mosley TH, Catellier DJ, Coker LH. Alzheimer’s Dement. 3. Vol. 5. Elsevier Ltd; 2009. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition : The ARIC MRI Study; pp. 207–14. [DOI] [PubMed] [Google Scholar]
- 182.Qiu C, Sigurdsson S, Zhang Q, Jonsdottir MK, Kjartansson O, Eiriksdottir G, et al. Diabetes, markers of brain pathology and cognitive function: The Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol. 2014;75(1):138–46. doi: 10.1002/ana.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Feinkohl I, Aung PP, Keller M, Robertson CM, Morling JR, McLachlan S, et al. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: The edinburgh type 2 diabetes study. Diabetes Care. 2014;37(2):507–15. doi: 10.2337/dc13-1384. [DOI] [PubMed] [Google Scholar]
- 184.Beauquis J, Homo-Delarche F, Giroix M-H, Ehses J, Coulaud J, Roig P, et al. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol [Internet] 2010;222(1):125–34. doi: 10.1016/j.expneurol.2009.12.022. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20045412. [DOI] [PubMed] [Google Scholar]