Abstract
The goal of this study was to investigate the neural dynamics of error processing and post-error adjustments in cognitive control and attention to a cognitive task in schizophrenia. We adopted a time-frequency approach in order to examine activity in the theta and alpha frequency bands as indices of cognitive control and attentional engagement. The results showed that error processing was characterized by increases in theta-band activity, accompanied by decreases in alpha-band activity, in both healthy control participants and participants with schizophrenia. However, both the theta and alpha effects were significantly reduced in participants with schizophrenia. Post-error increases in theta activity were associated with improved accuracy on subsequent trials in control participants but not in participants with schizophrenia. In addition, increases in alpha-band activity were found in the prestimulus period before partial attention lapses, but only for control participants and participants with schizophrenia with relatively low positive symptom severity. These results provide evidence for a deficit in cognitive control mechanisms mediated by midfrontal theta activity in schizophrenia, and suggest a particularly pronounced deficit in patients' ability to engage adaptive control mechanisms following errors. Our results also indicate that partial attention lapses can be indexed in both control participants and participants with schizophrenia by increases in alpha activity, but that in schizophrenia this varies as a function of positive symptom severity. We suggest that disrupted theta-band function represents a key deficit of schizophrenia, whereas disruptions in the alpha band may be the byproduct of atypically regulated attention.
Descriptors: Cognitive control, Error processing, Schizophrenia, EEG, Alpha rhythm, Theta rhythm
Schizophrenia is a severe mental disorder characterized not only by psychotic symptoms such as hallucinations and delusions, but by a variety of cognitive impairments that are closely related to functional outcome (Carter, 2006; Green, 1996; Green, Kern, Braff, & Mintz, 2000; Green, Kern, & Heaton, 2004; Green & Nuechterlein, 1999). One of the most significant areas of impairment is in cognitive control, including inhibition, monitoring, and the ability to direct and maintain attentional focus (Lesh, Niendam, Minzenberg, & Carter, 2011). Previous work has shown behavioral, ERP, and time-frequency correlates of these deficits during task performance (e.g., Minzenberg et al., 2010; Minzenberg, Gomes, Yoon, Swaab, & Carter, 2013). In the current study, EEG and behavioral measures were used in order to examine the neural dynamics of error processing and post-error adjustments in cognitive control and attention to a cognitive task in schizophrenia, in real time. Our focus was on prestimulus and response-related oscillatory activity in the theta and alpha frequency bands, as activity in these bands has been related to modulations of cognitive control and attention processes, respectively. Below, we briefly introduce previous work suggesting that oscillations in the theta and alpha frequency bands index cognitive control and attention processes. We then detail the current study and our predicted results.
Theta Oscillations and Cognitive Control
A variety of situations and tasks that engage cognitive control mechanisms elicit a similar midfrontal theta-band (~4–7 Hz) signature in the EEG signal (Cavanagh & Frank, 2014; Cavanagh, Zambrano-Vazquez, & Allen, 2011). For example, ERP studies have shown that the error-related negativity (ERN) and conflict-related negativity (CRN) occur with similar timing (closely tied to error or high-conflict responses), have similar scalp distributions (maximal at midfrontal electrode sites), and have similar task sensitivities (elicited by increased cognitive control demands; Cavanagh & Frank, 2014; Cavanagh et al., 2011; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000). Source localization and neuroimaging studies suggest that the neural generator of the ERN is in the anterior cingulate cortex (ACC; Carter, Van Veen, Botvinick, Cohen, & Stenger, 2001; Dehaene, Posner, & Tucker, 1994; van Veen & Carter, 2002), and this association with the ACC and with error processing has led researchers to posit that the ERN reflects the detection of response conflict (Yeung, Botvinick, & Cohen, 2004). When a time-frequency approach is used to analyze oscillatory activity in the EEG signal under the conditions that produce an ERN or CRN, an increase in midfrontal theta oscillations can be observed in both cases (e.g., Cavanagh et al., 2011). Likewise, midfrontal theta-band activity has been observed under a variety of other conditions requiring increased cognitive control, such as when comparing high conflict to low conflict stimuli (e.g., Cavanagh et al., 2011). Increased theta-band activity has also been associated with increased working memory maintenance demands and task difficulty (Gevins, Smith, McEvoy, & Yu, 1997; Jensen & Tesche, 2002; Meltzer et al., 2008).
On the basis of studies such as these, midfrontal theta activity has been put forward as a candidate neural signal of cognitive control (Cavanagh & Frank, 2014). Moreover, it has been proposed that a relatively low-frequency, high-amplitude signal generated by a well-connected cortical source (i.e., midfrontal theta) would be computationally capable of communicating the need for increased cognitive control across distant regions of the cortex (Cavanagh & Frank, 2014). This would make it possible for midfrontal theta to flexibly engage a variety of cortical networks as needed based on task demands.
As noted above, individuals with schizophrenia show a range of deficits in cognitive control mechanisms, including impaired action monitoring and adaptive control. For example, individuals with schizophrenia show a reduced ability to slow down their responses following errors, and exhibit reduced ERP correlates of error processing, such as the ERN (Bates, Kiehl, Laurens, & Liddle, 2002; Kopp & Rist, 1999; Mathalon et al., 2002; Minzenberg et al., 2013). Recent work has also shown a reduced theta-band response to errors or increased working memory load in individuals with schizophrenia (Reinhart, Zhu, Park, & Woodman, 2015; Schmiedt, Brand, Hildebrandt, & Basar-Eroglu, 2005). In one study, participants with schizophrenia and healthy control participants completed a version of a go/no-go task (Reinhart et al., 2015). The results showed a post-error slowing effect in control participants that was not present in participants with schizophrenia, and the schizophrenia group also showed reduced oscillatory activity in the theta band. Interestingly, in this study, transcranial direct current stimulation (tDCS) applied prior to task completion enhanced both the behavioral (post-error slowing) and neural (midfrontal theta activity) correlates of adaptive control in both groups, such that the post-tDCS pattern in the schizophrenia group was indistinguishable from the no-tDCS pattern in the control group (Reinhart et al., 2015). These results are consistent with the hypothesis described above, suggesting that post-error adjustments may be signaled via midfrontal theta-band activity. Further, these results suggest that the adaptive control deficits that have long been observed in schizophrenia can at least partially be attributed to dysfunctional theta-band activity (Reinhart et al., 2015).
Alpha Oscillations and Attention
Changes in EEG alpha activity (~8–12 Hz) have long been associated with changes in attentional focus (Adrian & Matthews, 1934; Lemere, 1936). Two lines of research suggest that alpha is sensitive to attention during the performance of complex cognitive tasks. One line of research has correlated reductions in alpha power with attention directed toward processing stimuli from the external environment in visual attention tasks (Bengson, Mangun, & Mazaheri, 2012; Jensen & Mazaheri, 2010; Mazaheri et al., 2010; Mazaheri & Jensen, 2010; Mazaheri, Nieuwenhuis, van Dijk, & Jensen, 2009). The second has linked increases in alpha power to the suppression of external, potentially distracting stimuli during the retention interval of working memory tasks (Jensen, Gelfand, Kounios, & Lisman, 2002; Roux & Uhlhaas, 2014). Increases in alpha power and changes in alpha phase have also been linked to poor performance on visual target detection tasks (Mathewson, Gratton, Fabiani, Beck, & Ro, 2009; Mathewson et al., 2011). Together, these findings indicate that increases in alpha reflect the direction of attention inward, away from external stimuli.
Changes in alpha power during a language comprehension task have recently been used as an index of attentional engagement to the task, and shown to be predictive of comprehension performance (Boudewyn et al., 2015). In that study, increases in alpha activity when critical information was presented in a story comprehension paradigm were associated with decreases in the ability to access that information later on in the stories. Other studies have found that increases in alpha activity preceded errors on sustained visual attention tasks, which are often taken as behavioral indicators of lapses in attention (O'Connell, Dockree, Bellgrove et al., 2009; O'Connell, Dockree, Robertson et al., 2009). This pattern supports the use of alpha activity as a marker of lapses in attention from a cognitive task in which good task performance requires attentional engagement to external stimuli (as in the current study). Thus, when a task requires attention to an external stimulus, decreases in alpha activity may reflect attentional engagement or focusing on the task, whereas increases in alpha activity may reflect attention disengagement or lapsing.
In schizophrenia, abnormalities in the alpha frequency band have been documented at rest (Iacono, 1982; Miyauchi et al., 1990; Sponheim, Clementz, Iacono, & Beiser, 1994, 2000) and during visual attention tasks (Martínez et al., 2015). In studies of resting-state EEG, reductions in alpha activity have been observed in participants with schizophrenia compared to control participants (Iacono, 1982; Miyauchi et al., 1990; Sponheim et al., 1994, 2000). Fewer studies have focused on alpha-band activity during a cognitive task in schizophrenia. In one recent study, participants were shown sinusoidal gratings of either high spatial frequency or low spatial frequency, and asked to attend selectively to one grating type or the other (Martínez et al., 2015). While the primary focus of this study was on selectively reduced performance and theta-band activity in the schizophrenia group for low spatial frequency gratings, participants with schizophrenia also showed reduced alpha-band modulation following presentation of either type of grating compared to control participants (Martínez et al., 2015). As noted above, presentation of a visual stimulus typically leads to a reduction in alpha-band activity, often taken to indicate the direction of attention outward, toward the external stimulus. In Martínez et al. (2015), participants with schizophrenia showed reduced alpha-band modulation compared to control participants, and, in addition, diminished reductions in alpha activity in response to a visual stimulus were associated with relatively low scores on attention-vigilance and visual learning neurocognitive tasks (Martínez et al., 2015). This is suggestive of a possible impairment in the ability to focus visual attention in a task-appropriate manner in schizophrenia.
The Current Study
The goal of the current study was to examine the neural dynamics of error processing and post-error adjustments in cognitive control and attention to a cognitive task in schizophrenia. We focused on prestimulus and response-related oscillatory activity in the theta and alpha frequency bands during completion of the Stroop task. ERP results using this task have been reported in Minzenberg et al. (2013), who found attenuated ERP correlates of error and conflict processing in participants with schizophrenia (reduced ERN and N450 effects) compared to control participants. Here, we adopted a time-frequency approach in order to investigate activity in the theta and alpha frequency bands as indices of cognitive control and attentional engagement.
In this task, color words (red, green, blue) were presented to participants in either congruent (matching) or incongruent (mismatching) font colors. Participants were asked to respond via button press to the font color in which the word was printed. Congruent trials are considered to be instances of low response conflict, while incongruent trials are considered to be instances of high response conflict.
If midfrontal theta activity provides an index of the engagement of cognitive control, we expected to observe the following pattern of results in healthy adults. First, we predicted increased theta activity following an error response compared to following a correct response. To the extent that the commission of an error leads to post-error adjustments in cognitive control, we predicted that control participants would show an increase in theta-band activity in the prestimulus period of trials following error responses compared to trials following correct responses. Similarly, we expected to observe an increase in theta-band activity in the prestimulus period of trials following incongruent trials compared to trials following congruent trials, if processing incongruent trials engages similar adaptive control mechanisms.
If alpha activity can provide an index of external task engagement, we predicted that, in control participants, alpha-band activity would decrease following the commission of an error, to the extent that making an error serves to refocus attention to the task. As with our theta-band predictions, if commission of an error also leads to post-error adjustments in task engagement, then we predicted a decrease in alpha-band activity in the prestimulus period of trials following error responses compared to trials following correct responses. Similarly, decreases in alpha activity in the prestimulus period of trials following incongruent trials compared to trials following congruent trials were expected if encountering an incongruent trial jogs participants' attention enough to increase task engagement on the subsequent trial.
In participants with schizophrenia, we predicted that the error-related increases in theta-band activity we expected to observe in control participants would be attenuated or absent, due to deficits in the ability to monitor for conflict and implement cognitive control. However, if error-related processing exerts a similar influence on attention and task engagement in schizophrenia, we predicted that participants with schizophrenia would show a similar pattern of results in the alpha band as in control participants.
In addition to examining error processing and post-error adjustments, we sought to investigate attention lapses and their relation to alpha activity in schizophrenia. If alpha activity can provide an index of external task engagement, then behavioral correlates of lapses in attention to the external task may be accompanied by changes in alpha activity. In the current task, congruent trials provided an opportunity to examine neural processing associated with behavioral markers of lapses in attention. This is because congruent trials on the Stroop task are not as demanding of cognitive processes as are incongruent trials, and performance should be near ceiling on these trials when participants are attending to the task. Here, we considered congruent hit trials on which participants responded particularly slowly to be indicative of partial lapses in attention to the task. We were primarily interested in comparing alpha activity preceding congruent trials on which participants responded particularly slowly (partial lapse trials) to congruent trials on which participants responded particularly quickly (on-task trials). Slow congruent hits and fast congruent hits were defined for each participant using that participant's congruent hit reaction times (see Method for additional details).
We hypothesized that, in control participants, partial lapses may largely be attributed to attentional drift away from the external task (e.g., mind wandering). If this is the case, we predicted increased alpha-band activity in the prestimulus period prior to a slow congruent hit compared to a fast congruent hit. If attention lapses in schizophrenia are driven by the same loss of attentional focus on the external task, then participants with schizophrenia should show a similar pattern in the alpha band as seen in control participants. However, it is possible that a greater portion of attention lapses in schizophrenia may be attributed to shifting attention to a task-irrelevant external distractor rather than a task-irrelevant internal distractor, compared to control participants. In this case, we predicted that lapses in the schizophrenia group would not have the same neural signature as in the control group.
Method
Participants
These data were culled from a larger, multimodal longitudinal study. Included in the current sample are 75 participants with first-episode schizophrenia or a schizophrenia-spectrum disorder, and 57 healthy control participants who completed the EEG task described below at the first time point in the study. Results of analyses of ERPs on a largely overlapping sample have previously been published (Minzenberg et al., 2013). A subset of participants in that analysis was excluded from the present one because of failure to meet the exclusion criteria listed below (10; 2 control participants and 8 participants with schizophrenia), or because of data loss (4; 2 control participants and 2 participants with schizophrenia). In addition, the current sample includes 19 new participants (7 control participants and 12 participants with schizophrenia) that did not take part in the Minzenberg et al., 2013, study. Participants with schizophrenia were recruited through the Early Diagnosis and Preventive Treatment of Psychosis clinic at the University of California, Davis Medical Center. Control participants were recruited from the community through advertisements. All participants provided informed consent, with approval of the Institutional Review Board at the University of California, Davis. All participants were compensated at a rate of $15 per hour.
Participants with first-episode schizophrenia were within 1 year of the onset of their psychotic symptoms and ranged in age from 16–30 years (mean: 20.2); 18.7% were female. Control participants ranged in age from 16–27 years (mean: 19.9); 43.9% were female. All participants were recruited with the following exclusion criteria: (a) IQ below 70, as measured by the Wechsler Abbreviated Scale of Intelligence; (b) history of neurological illness, including head injury; (c) substance-related disorder in the previous 6 months; (d) uncontrolled medical illness; (e) history of electrocon-vulsive therapy. Control participants were evaluated using the Structured Clinical Interview for DSM-IV-TR (SCID) to exclude those with a history of an Axis I disorder or first-degree relatives with a psychotic disorder. All participants tested negative on a urine drug screen test immediately prior to completion of the study. Participants were matched on age and parental education level. See Table 1 for additional demographic information.
Table 1. Demographic and Symptom Severity Information for All Participants.
| Demographics | Control participants (N = 57) | Patient participants (N = 75) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean | Range | SD | Mean | Range | SD | |
| Age | 19.9 | 16–27 | 2.4 | 20.2 | 16–30 | 2.8 |
| Participant education (years) | 13.7 | 9–17 | 2.1 | 12.6** | 9–19 | 2 |
| Parental education (years) | 14.3 | 6.5–18 | 2.4 | 14.4 | 4.5–21.5 | 3 |
| IQ (WASI) | 116 | 85–142 | 10.5 | 101*** | 75–131 | 13.4 |
| Symptom ratings | ||||||
| BPRS total | – | – | – | 41.6 | 24–70 | 9.6 |
| SAPS total | – | – | – | 5.8 | 0–12 | 3.1 |
| SANS total | – | – | – | 9.5 | 0–19 | 4.1 |
p<.01.
p<.001 (t tests between control and patient groups).
Clinical and Functional Characteristics
Participants with schizophrenia were within 1 year of their first psychotic episode. Diagnoses were established using the SCID, or, for participants under 18 years of age, the Kiddie-SADS-Present and Lifetime Version. Clinicians conducted all diagnostic evaluations, and diagnoses were confirmed by consensus conference. All clinicians had demonstrated reliability, defined by > 0.8 intraclass correlation coefficients for continuous measures, and kappa > 0.7 for categorical measures; all participated in monthly reliability checks to prevent drift from these standards. Clinical symptom scores were measured using the Brief Psychiatric Rating Scale (BPRS), and the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS).
Stimuli
EEG was recorded while participants completed the Stroop task as described in Minzenberg et al., 2013. In this task, words (red, green, blue) were presented to participants in one of three possible font colors (red, green, blue). Trials in which the word and color matched were considered congruent trials, and trials in which the word and color mismatched were considered incongruent trials.
Procedure
Stimuli were presented using E-Prime (www.pstnet.com/eprime). Words appeared in the center of a computer screen. The intertrial interval (ITI) varied randomly from 1,000–2,000 ms. Words were presented for 500 ms, unless a response was made within that time; in this case, the stimulus disappeared and the 1,000–2,000 ms ITI began. For responses made between 500 and 1,000 ms, the stimulus disappeared at 500 ms, and the remainder of the 1,000-ms response period (from stimulus) elapsed before the 1,000–2,000 ms ITI began. For responses made over 1,000 ms, the 1,000–2,000 ms ITI began at the response.
The task consisted of eight blocks of 120 trials each (70% congruent and 30% incongruent). Participants were instructed to respond to the font color and to prioritize both speed and accuracy. Responses (red, green, blue) were mapped buttons on a keypad in a fixed order for each participant that was randomized across participants. Congruent and incongruent trials were equally distributed across the three possible font colors. Two practice sessions were completed prior to the experimental session, consisting of 20 trials (80% congruent and 20% incongruent). Practice sessions were repeated until participants reached at least 80% accuracy.
EEG Recording and Processing
EEG was acquired with a Neuroscan 128-electrode Quik-Cap and Neuroscan Synamps2 system. The EEG signal was digitized with a 1000 Hz sampling rate, with a low-pass filter of 100 Hz. Impedances were below 5 kΩ. All channels were referenced to the electrode immediately behind channel 63 (Cz). EEG data were visualized and processed using EEGLAB. Malfunctioning electrodes were excluded based upon a combination of visual inspection and automated procedures. Data were rereferenced offline using the average reference, downsampled to 250 Hz, and high-pass filtered using a 0.05 Hz half-amplitude cutoff.
Data processing was performed using MATLAB (MathWorks) with the EEGLAB toolbox (Delorme & Makeig, 2004). Independent component analysis (ICA) was used to correct for eyeblink artifacts. Single-trial waveforms were screened for amplifier blocking, horizontal eye movements, and any remaining blinks or movement-related artifacts over epochs of 6,000 ms, starting 3,000 ms before the onset of stimulus or response.
Time-Frequency Analysis
EEG spectral power was calculated using the EEGLAB toolbox, by convolving single-trial epochs with six-cycle complex Morlet wavelets. Power for 52 log-spaced frequencies from 4–30 Hz was averaged across trials within a condition and log-transformed. Power estimates were binned into theta (5–7 Hz) and alpha (8–12 Hz) frequency bands. We focused on three midline electrodes: Fz, Cz, and Pz. For analysis, power estimates in the theta and alpha frequency bands were averaged across seven-electrode clusters surrounding each electrode of interest (e.g., the Fz cluster consisted of Fz and the six immediately surrounding electrodes).
Statistical Analyses
Repeated measures analyses of variance (ANOVAs) with the within-participant factors trial type (two levels) and electrode (three levels) and the between-participants factor group (control participants, patient participants) were conducted. For EEG analyses, average power estimates for each electrode cluster across the time window of interest (specified for each analysis below) were used as the dependent measure. Significant interactions with trial type were followed up with simple effects comparisons. The Greenhouse-Geisser correction was applied to all analyses with more than one degree of freedom in the numerator. For all analyses, outliers were defined as values exceeding 2.5 standard deviations of each group's average, and excluded from further analysis. Thus, sample sizes varied slightly for each set of analyses. In all cases, the groups remained demographically matched (age and parental education) as described above. In addition, for the error versus hit analysis, participants with fewer than 15 total error trials were excluded, leaving 49 control participants and 58 participants with schizophrenia included in this analysis. For the previous error versus previous correct analysis, 53 control participants and 63 participants with schizophrenia were included. For the incongruent-incongruent versus congruent-incongruent analysis, 54 control participants and 61 participants with schizophrenia were included. Finally, for the slow congruent hit versus fast congruent hit analysis, 55 control participants and 71 participants with schizophrenia were included. For the slow congruent hits versus fast congruent hits analysis, slow congruent hits were defined as those congruent hit trials with reaction times in the slowest quintile of each participant's congruent hit trials; fast congruent hits were defined as those congruent hit trials with reaction times in the fastest quintile of each participant's congruent hit trials Thus, fast and slow trials were identified on an individual participant basis.
For analyses investigating response-locked data, we used a time window of −50 to 300 ms relative to the response to measure theta-band activity, consistent with prior work (e.g., Cavanagh, Cohen, & Allen, 2009; Reinhart et al., 2015; van Driel, Ridderinkhof, & Cohen, 2012). To measure response-locked alpha-band activity, we used a time window of 300 to 500 ms following the response, consistent with previous studies (e.g., Martínez et al., 2015; van Driel et al., 2012). For analyses investigating activity in the prestimulus period (stimulus-locked data), we used a measurement window of −1,500 to 0 ms relative to the stimulus to examine both theta and alpha activity. Results are summarized below; see online supporting information fzor tables reporting statistics in full.
Results
Behavioral Data
Behavioral data are summarized in Table 2.
Table 2. Post-Error and Postconflict Adjustments in Behavior.
| Control participants | Participants with schizophrenia | ||
|---|---|---|---|
| Post-error changes in accuracy | |||
| Previous error | 88.79 (8.06) | 74.19 (15.82) | |
| Previous hit | 92.71 (6.22) | 85.05 (8.82) | |
| Previous error minus previous hit | −3.91**** | 7**** | |
| Group difference | **** | ||
| Post-error changes in speed | |||
| Previous error | 695.96 (88.37) | 728.79 (127.28) | |
| Previous hit | 641.79 (74.68) | 678.93 (109.94) | |
| Previous error minus previous hit | 54.17**** | 49.86**** | |
| Group difference | n.s. | ||
| Postconflict changes in accuracy | |||
| Incongruent-incongruent | 92.01 (6.07) | 83.84 (11.92) | |
| Congruent-incongruent | 91.84 (6.06) | 83.74 (10.69) | |
| inc:inc minus con:inc | 0.17 | 0.1 | |
| Group difference | n.s. | ||
| Postconflict changes in speed | |||
| Incongruent-incongruent | 708.22(104.88) | 743.67 (126.76) | |
| Congruent-incongruent | 719.07 (98.95) | 753 (127.73) | |
| inc:inc minus con:inc | −10.85**** | −9.33**** | |
| Group difference | n.s. |
Note. Standard deviations are displayed in parentheses.
p<.0001.
Errors versus hits
Participants with schizophrenia had a significantly higher error rate than control participants, committing errors on 15.96% compared to 7.73% of trials, F(1,105)=20.28; p<.0001.
Post-error changes in accuracy
Comparing accuracy on previous error trials and previous hit trials yielded a significant main effect of trial type, F(1,114)=80.66; p<.0001, such that accuracy was reduced on previous error trials compared to previous hit trials. This effect interacted with group, F(1,114)= 17.79; p<.0001. Follow-up analyses showed that the effect of trial type was significant in both groups (controls: F(1,52)=26.94; p<.0001; patients: F(1,62)=62.97; p<. 0001), although more robust in participants with schizophrenia. In other words, while individuals in both groups tended to commit strings of errors, participants with schizophrenia were significantly more likely to do so as compared to control participants.
Post-error changes in speed
Comparing reaction time on previous error trials and previous hit trials yielded a significant main effect of trial type, F(1,114)=109.36; p<.0001, such that reaction times were increased on previous error trials compared to previous hit trials. This effect did not interact with group.
Post-conflict changes in accuracy
Comparing accuracy on incongruent-incongruent (inc:inc) and congruent-incongruent (con:inc) trials yielded no significant effects.
Post-conflict changes in speed
Comparing reaction time on inc:inc and con:inc trials yielded a significant main effect of trial type, F(1,113)=9.22; p<.0001, such that reaction times were increased on con:inc trials compared to inc:inc trials. This effect did not interact with group.
EEG Data: Response-Locked Errors Versus Hits
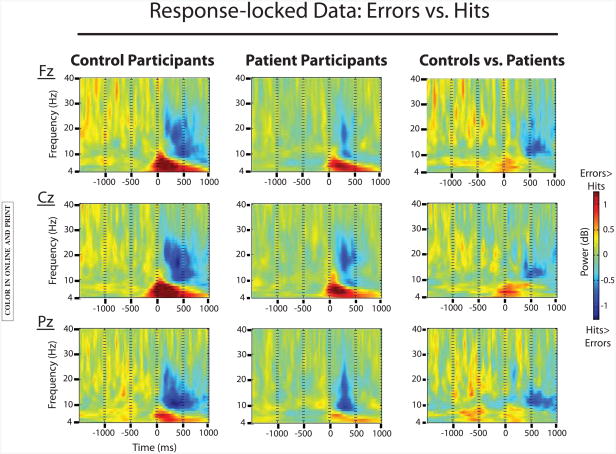
To look at error-related processing, we analyzed response-locked data. See Figure 1 for the plotted results.
Figure 1.
EEG data comparing response-locked error trials to hit trials for both groups. Significant effects of trial type were found in both groups for theta-band activity (error >hit) and alpha-band activity (hit>error). The theta-band effect was further characterized by an interaction with group (controls > patients).
Theta band
Results showed a main effect of trial type, such that response-locked theta power was increased for error trials compared to hit trials, F(1,105)=148.8; p<.0001. This main effect interacted with group, F(1,105)=8.8; p<.01. Follow-up analyses showed that the effect of trial type was significant in both groups (controls: F(1,48)=78.2; p<.0001; patients: F(1,57)=66.5; p<.0001), although weaker in the schizophrenia group compared to the control group. Finally, a significant interaction of trial type and electrode reflected the midfrontal maximum of this effect, F(2,210)=66.6; p<.0001.
Alpha band
Results showed a main effect of trial type, such that response-locked alpha power was decreased for error trials compared to hit trials, F(1,105)=25.4; p<.0001. There was no significant interaction with group. A significant interaction of trial type and electrode reflected the posterior maximum of this effect, F(2,210)=14.2; p<.0001.
EEG Data: Stimulus-Locked Previous Errors Versus Previous Hits
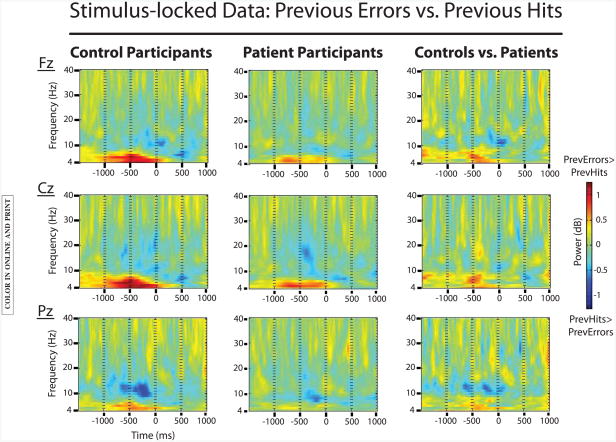
To look at post-error processing adjustments, we analyzed the prestimulus period of correct trials that followed errors compared to correct trials that followed correct responses. See Figure 2 for the plotted results.
Figure 2.
EEG data comparing stimulus-locked previous error trials to previous hit trials for both groups. Significant effects of trial type were found in both groups for theta-band activity (previous error> previous hit) in the prestimulus period. This effect was further characterized by an interaction with group (controls > patients). In contrast, prestimulus effects of trial type in the alpha band were only significant for control participants (previous hit > previous error).
Theta band
Results showed a main effect of trial type, such that stimulus-locked theta power was increased for previous error trials compared to previous hit trials, F(1,114)=29.9; p<.0001. This main effect interacted with group, F(1,114)=5.2; p<.05. Follow-up analyses showed that the effect of trial type was significant in both groups (controls: F(1,52)=26.7; p<.05; patients: F(1,62)=5.8; p<.05), although it was stronger in control participants than participants with schizophrenia. Finally, a significant interaction of trial type and electrode reflected the midfrontal maximum of this effect, F(2,228)=33.5; p<.0001.
In order to follow up on this result, we analyzed the error rate for previous error trials as a function of theta-band activity in the prestimulus period. Namely, for each participant, we divided previous error trials into high theta and low theta based on a median split of theta-band activity in the prestimulus period; 47 control participants and 53 participants with schizophrenia were included in this analysis. Results are plotted in Figure 3.
Figure 3.
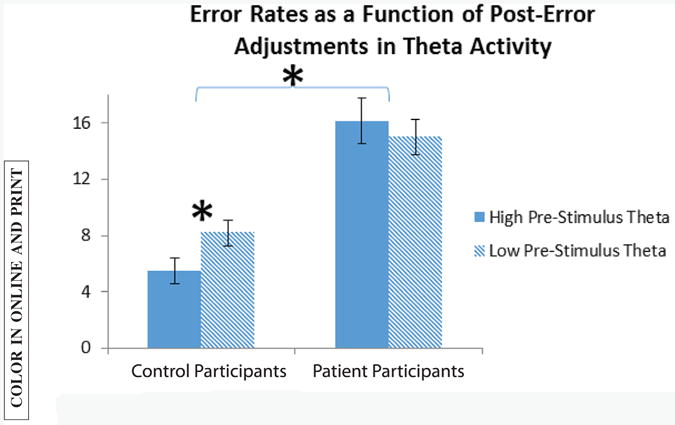
Error rates for previous error trials, as a function of post-error adjustments in theta activity. Each participant's theta activity in the prestimulus period of previous error trials was divided into high and low, using a median split. Error rates based on this split are plotted here for both groups. The effect of post-error adjustments in theta-band activity on accuracy was significant for control participants only.
Results showed no significant main effect of theta, but a significant interaction of theta with group, F(1,98)=6.1; p<.05. Follow-up analyses showed that the effect of theta was significant in control participants only, F(1,46)=9; p<.01, such that increased theta amplitudes in the post-error adjustment period were associated with reduced error rates on the immediately following trials, in control participants but not participants with schizophrenia.
Alpha band
Results showed a main effect of trial type, such that stimulus-locked alpha power was decreased for previous error trials compared to previous hit trials, F(1,114)=7.4; p<.01. There was also a significant three-way interaction of trial type, group, and electrode, F(2,228)=4; p<.05. Follow-up analyses showed that the effect of trial type was significant in control participants, F(1,52)=6; p<.05, but not patient participants (although the effect was marginal in the schizophrenia group at posterior electrode sites (Pz cluster), and was maximal at posterior electrode sites. Thus, decreased alpha amplitudes in the post-error adjustment period led to reduced error rates on the immediately following trials, in control participants but not participants with schizophrenia.
EEG Data: Stimulus-Locked Incongruent-Incongruent Versus Congruent-Incongruent
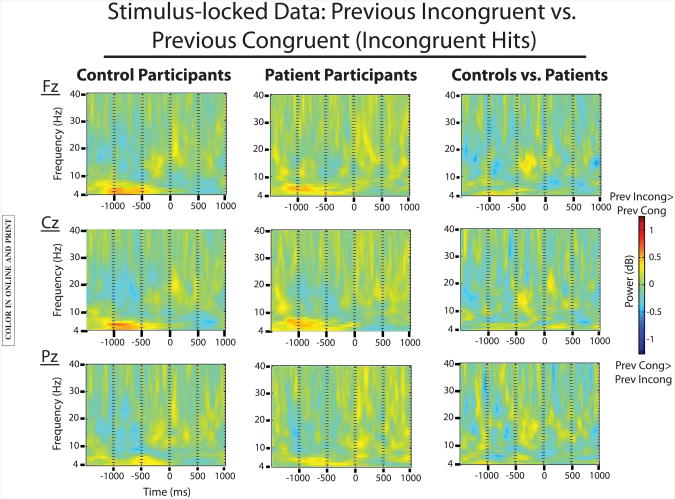
To look at postconflict processing adjustments, we analyzed the prestimulus period of incongruent hits that followed incongruent trials (inc:inc) compared to incongruent hits that followed congruent trials (con:inc) responses. See Figure 4 for the plotted results.
Figure 4.
EEG data comparing stimulus-locked incongruent-incongruent (inc:inc) trials to congruent-incongruent (con:inc) trials for both groups. A significant effect of trial type was present in both groups for theta-band activity (inc:inc > con:inc) in the prestimulus period. This effect did not interact with group. No significant effects were present for this contrast in the alpha band.
Theta band
Results showed a main effect of trial type, such that stimulus-locked theta power was increased for inc:inc trials compared to con:inc trials, F(1,113)=26.9; p<.0001. There were no significant interactions with group. A significant interaction of trial type and electrode reflected the midfrontal maximum of this effect, F(2,226)=11; p<.0001.
Alpha band
No significant effects emerged from this analysis.
EEG Data: Stimulus-Locked Slow Congruent Hits Versus Fast Congruent Hits
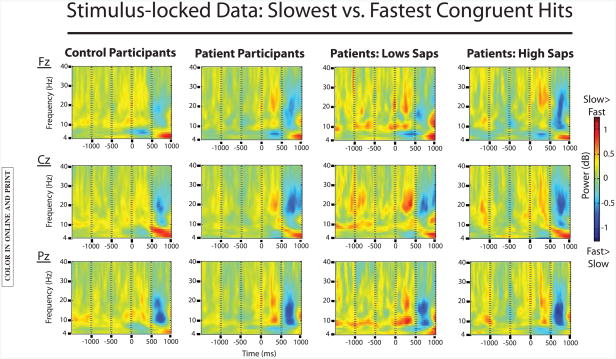
To examine processing associated with partial attention lapses, we analyzed the prestimulus period of slow congruent hits compared to fast congruent hits. See Figure 5 for the plotted results.
Figure 5.
EEG data comparing stimulus-locked slow congruent hit trials to fast congruent hit trials. Data from control participants is plotted on the far left, followed by data from the patient group (second from left). Data from patient participants with low positive symptom severity (low SAPS) and with high positive symptom severity (high SAPS) are plotted in the two rightmost columns. Small but significant effects of trial type were found in both groups for theta-band and alpha-band activity (slow congruent hit >fast congruent hit) in the prestimulus period. In the patient group, presti-mulus alpha activity varied as a function of positive symptom severity: there was a significant effect of trial type on prestimulus alpha activity for patients with low SAPS scores, but not for patients with high SAPS scores.
Theta band
Results showed a main effect of trial type, such that prestimulus theta power was increased for slow congruent trials compared to fast congruent trials, F(1,124)=4.5; p<.05. There were no significant interactions with group. A significant interaction of trial type and electrode reflected the midfrontal maximum of this effect, F(2,248)=5.9; p<.01.
Alpha band
Results showed a main effect of trial type, such that prestimulus alpha power was increased for slow congruent hits compared to fast congruent hits, F(1,124)=16.2; p<.0001. There were no significant interactions of trial type with group or electrode.
In order to further investigate this effect in the schizophrenia group, we conducted an analysis in which we divided participants with schizophrenia into two groups based on a median split of total SAPS scores. SAPS scores, which measure positive symptoms such as hallucinations and delusions, were selected for this analysis because we hypothesized that such symptoms, reflecting internal preoccupation, may influence the nature of lapses in attentional engagement to a cognitive task. The low SAPS group comprised 26 individuals, and the high SAPS group comprised 28 individuals.
Results showed a significant main effect of trial type, F(1,52)=10.4; p<.01, and a marginally significant interaction of trial type with group, F(1,52)=3.8; p=.056. Follow-up analyses showed that the effect of trial type was significant in the low SAPS group only, F(1,25) 5 8.9; p<.01, such that prestimulus alpha power was increased in the prestimulus period for slow congruent hits compared to fast congruent hits (as in the control group); this effect was absent in the high SAPS group.
We also conducted a similar analysis focusing on SANS scores, which measure negative symptoms such as poverty of speech and inattentiveness. The results showed a significant main effect of trial type, F(1,51)=4.2; p<.05, but no significant interaction with group (participants with schizophrenia with low SANS vs. high SANS scores).
Discussion
The goal of this study was to investigate the neural dynamics of error processing and post-error adjustments in cognitive control and visual attentional engagement in schizophrenia. Our focus was on oscillatory activity in the theta and alpha frequency bands in the prestimulus period and following responses during completion of the Stroop task. Consistent with our predictions, control participants demonstrated increased midfrontal theta activity in several contrasts in which cognitive control demands were relatively high compared to when they were relatively low. Specifically, increases in theta-band activity were found following the commission of an error, as well as in the prestimulus period of trials that followed errors and trials that followed incongruent trials. This was accompanied by decreased alpha activity following the commission of an error, and in the prestimulus period of trials that followed errors. In comparison, the pattern of theta and alpha modulations that was associated with error processing and post-error adjustments was significantly reduced in participants with schizophrenia. This attenuated midfrontal theta response following errors is consistent with the impaired ability in schizophrenia to monitor for conflict and implement adaptive cognitive control. The attenuated alpha response suggests that committing an error exerted a weaker influence on attentional engagement to the task in participants with schizophrenia than control participants. We discuss the possible functional significance of our alpha-band results in more detail below.
It is important to note, however, that both our behavioral and EEG results suggest that control participants and participants with schizophrenia were prone to occasional lapses in attention to the task. Specifically, in both groups, accuracy for trials that followed an error was lower than for trials that followed a correct response, although this effect was most marked in participants with schizophrenia. This tendency toward strings of two or more errors is suggestive of temporary lapses during which attention was disengaged from the task. Further, examination of the prestimulus period of partial lapses (defined as congruent trials on which responding was particularly slow) revealed increased activity in the alpha band in both control participants and participants with schizophrenia. This effect was modulated in patient participants by positive symptom severity. Individuals with schizophrenia with relatively low positive symptom severity exhibited a pattern indistinguishable from control participants, whereas partial attention lapses in participants with schizophrenia with relatively high positive symptom severity were not associated with increases in prestimulus alpha activity. In contrast, negative symptom severity was not related to prestimulus alpha activity for partial lapse trials. Together, these data provide insight into the neural dynamics of adaptive control and attentional engagement to a cognitive task in schizophrenia in several ways. Below, we first discuss the findings in the theta band before turning to our results in the alpha band.
Theta Activity and Cognitive Control
As noted in the introduction, midfrontal theta activity has been suggested as a candidate neural mechanism for communicating the need for cognitive control (Cavanagh & Frank, 2014). Our findings of increased theta-band activity under conditions in which cognitive control demands are high is consistent with this hypothesis and with previous empirical work that has observed increased midfrontal theta activity under similar conditions (e.g., Cavanagh et al., 2011). Our results are also in line with previous studies that have found a reduced theta-band response to errors as well as increased working memory load in schizophrenia (Reinhart et al., 2015; Schmiedt et al., 2005). This reduced theta-band response may be the result of disrupted GABA-ergic inhibition. GABA is a major inhibitory neurotransmitter that has been shown to support neural oscillations in multiple frequency bands (Buzsáki, 2005; Lisman & Buzsáki, 2008; Whittington, Traub, & Jefferys, 1995) and to generate theta-band oscillations through cholecystokinin-expressing (CCKb) basket cells (Hartwich, Pollak, & Klausberger, 2009). Multiple abnormalities in GABA-ergic inhibitory interneuron function (including in CCKb basket cells) have been found in schizophrenia (Akbarian et al., 1995; Curley & Lewis, 2012; Gonzalez-Burgos & Lewis, 2008; Hyde et al., 2011; Volk, Austin, Pierri, Sampson, & Lewis, 2001; Woo et al., 2007), which would be expected to lead to reductions in theta-band activity that can be measured using scalp-recorded EEG, as was observed in the current study. Thus, our results add to the substantial literature relating pre-frontally mediated cognitive control impairments to schizophrenia (see Lesh et al., 2011 for a review).
In particular, our theta-band results contribute to a prominent neurocognitive account of context maintenance and updating dysfunction in schizophrenia that draws from control-loop theory, guided activation theory, and empirical work demonstrating a specific context processing deficit in the disorder (Barch, Carter, MacDonald, Braver, & Cohen, 2003; Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004; Cohen, Barch, Carter, & Servan-Schreiber, 1999; Lesh et al., 2011; MacDonald et al., 2005; Servan-Schreiber, Cohen, & Steingard, 1996). During completion of a task, individuals must maintain task-relevant context (such as the goals and rules of the task) over time, and reinstate that context as needed. According to guided activation theory, the dorsolateral-prefrontal cortex (DLPFC) actively maintains such representations of goals and/or rules in order to guide task-appropriate responding (Miller & Cohen, 2001). For example, in the Stroop task, the goal of basing responses on font color (rather than the more automatic tendency to respond to the meaning of the word) must be maintained over the course of the task, and reinstated following an error or attention lapse. Control-loop theory posits that ACC sends signals to the DLPFC in order to communicate the need for increased cognitive control (e.g., after an error), thereby providing the signal to update or reinstate task-relevant contextual representations (Egner & Hirsch, 2005; MacDonald, 2000). As proposed by Cavanagh and Frank (2014), midfrontal theta activity may be the EEG correlate of this ACC-mediated cognitive control signal.
Specific impairments in ACC-mediated detection of error and conflict as well as DLPFC-mediated context representation and maintenance have been reported in schizophrenia, which correspond to significant context processing deficits (Kerns et al., 2005; Kerns, Cohen, Stenger, & Carter, 2004; MacDonald & Carter, 2003; MacDonald et al., 2005). In our data, following the commission of an error, we observed sustained increases in theta activity (and corresponding decreases in alpha activity) in the preparatory period before the start of the following trial. Importantly, this post-error adjustment in theta activity was predictive of behavior on the following trial. Namely, trials with high post-error theta increases were associated with a reduced error rate compared to trials with less pronounced post-error theta increases. However, this effect was only present in control participants: the post-error increase in theta activity during this preparation period was reduced and did not significantly impact behavior on the following trial in participants with schizophrenia. Thus, our results are in line with an account of a control loop in which theta-mediated ACC activity triggers the reinstatement of task-relevant context in the DLPFC in order to guide responding. The differences in post-error adjustments in midfrontal theta activity between the control and patient groups suggest impaired theta-mediated signaling of the need for task-relevant context to be reinstated following an error in schizophrenia, leading to deficits in the ability to appropriately update context representations in order to guide responding on the subsequent trial.
Alpha Activity and Attention to the Task
As noted in the introduction, changes in alpha activity in scalp-recorded EEG have been used to index attentional engagement to external stimuli, based on studies that have associated increases in alpha with the direction of attention inward, away from external stimuli (Bengson et al., 2012; Boudewyn et al., 2015; Jensen et al., 2002; Jensen & Mazaheri, 2010; Mazaheri et al., 2010; Mazaheri & Jensen, 2010; Roux & Uhlhaas, 2014). In the current study, we observed decreased alpha power following the commission of an error and in the prestimulus period of trials that followed errors; this pattern was attenuted in participants with schizophrenia compared to control participants. As noted above, we relate these changes to attentional engagement to the task. Namely, we suggest that increases in alpha reflect disengagement from the task, and that decreases in alpha reflect reengagement to the task. An interesting question, however, concerns what is meant by task engagement, and what aspect of task engagement is reflected by the pattern of alpha responses. One possibility is that alpha serves as an indicator of the implementation of visual attention to the stimulus, while another possibility is that it could represent a cognitive control signal. Although the tendency is to assume the former, it is difficult to disentangle the two possibilities in many tasks, including the one used in the current study. For example, while committing an error may trigger the reinstatement of relatively high-level context such as task-relevant goals and/or rules, a relatively low-level element of this context is the requirement to attend to task-relevant stimuli. Is it possible to separate the regulation of attention from the act of attending to the incoming stimulus?
Although our data cannot adjudicate between these two accounts, the results of recent studies suggest that top-down control of alpha activity may provide one mechanism for regulating visual attention (e.g., Bollimunta, Chen, Schroeder, & Ding, 2008; Samaha, Bauer, Cimaroli, & Postle, 2015). For example, Samaha et al. (2015) observed that expectations about the timing of an upcoming visual stimulus led to changes in alpha phase in order to optimally process that stimulus. Thus, while the alpha signal may reflect shifts in visual attention toward/away from a stimulus, this shift can be driven not only by bottom-up cues (e.g., a stimulus popping into view unexpectedly), but also by top-down cues (e.g., expectations about when a stimulus will appear). In our study, we suggest that committing an error triggered a top-down-mediated refocusing of visual attention to the incoming stimulus. We also suggest that the increased alpha activity that preceded particularly slow congruent responses corresponded to partial lapses in attention to the visual stimulus, due to distraction or mind wandering.
The majority of studies that have focused on oscillations in the alpha band in schizophrenia have documented reductions in alpha-band activity in resting state EEG (Iacono, 1982; Miyauchi et al., 1990; Sponheim et al., 1994, 2000; see Uhlhaas & Singer, 2010, for a review; Wada, Takizawa, Kitazawa, Zheng-Yan, & Yamaguchi, 1994). Reductions in resting-state alpha activity in schizophrenia have been found to be correlated with negative symptoms, such that more severe negative symptom ratings were associated with reduced alpha activity (Merrin & Floyd, 1992, 1996; Sponheim et al., 2000). By contrast, fewer studies have focused on modulations in the alpha band in schizophrenia during a cognitive task. However, reduced modulations of alpha-band activity in response to presentation of a visual stimulus have been observed in individuals with schizophrenia (Martínez et al., 2015). This diminished attenuation of alpha activity following a visual stimulus may suggest reduced attentional engagement to the stimulus in patients, although it may also be consistent with more general alpha-band dysfunction.
Our data support the former possibility and do not indicate a deficit in alpha-band function in schizophrenia per se, as may be the case for frontally mediated theta function in the disorder. Rather, our results suggest that symptoms of schizophrenia—which vary greatly across individuals— can have an impact on attention, particularly on the ability to refocus attention and maintain engagement with a task, and that this can manifest as abnormalities in the alpha band. We favor this interpretation for two reasons. First, committing an error should serve to refocus attention to the task at hand, and we found corresponding reductions in alpha-band activity (often considered to be indicative of engagement to the external stimulus) following errors in both groups. While this error-related alpha response was significantly reduced in participants with schizophrenia compared to control participants, it was still present in both groups. This indicates a similar, albeit weaker, refocusing of attention to the task after an error in the patient group. It may be the case that this diminished attentional refocusing response is the result of deficits in the engagement of cognitive control mechanisms (indexed by theta-band activity). Second, partial lapses in attention to the stimulus (slow congruent hit trials) were associated with increases in alpha-band activity in the prestimulus period in both groups, but this varied as a function of positive symptom severity in the schizophrenia group. Individuals with relatively low positive symptom severity ratings patterned with the control group, showing increases in prestimulus alpha prior to a partial attention lapse. In contrast, individuals with relatively high positive symptom severity ratings did not show this prestimulus alpha signature prior to partial lapses in attention. In other words, there were substantial individual differences among patient participants: whether or not partial attention lapses were associated with prestimulus alpha increases in the patient group was dependent on a given patient's positive symptoms.
Thus, our data suggest that positive symptom severity impacts the locus of an individual's attention during an attention lapse. Specifically, because of the link between increases in alpha activity and the direction of direction inward, away from an external task (or conversely, decreases in alpha activity and the direction of attention to external input), changes in alpha activity can provide a useful marker of attentional engagement to a cognitive task (Boudewyn et al., 2015). However, although momentary lapses in attention to a task may often be characterized by the direction of attention inward, as in mind wandering, it is also possible to become distracted from a task by an external stimulus (or one that is perceived to be external). In this case, task disengagement would not be expected to be associated with increases in alpha activity. Likewise, if individuals with schizophrenia with relatively high positive symptom severity experience heightened attention and/or vigilance to external stimuli, then attentional disengagement from the task in these individuals may not be associated with increases in alpha activity. This was the pattern observed in the current study: attentional disengagement from the task was only linked to increases in alpha activity in control participants and patient participants with relatively low positive symptom severity.
Conclusions
In this study, we investigated the neural dynamics of error processing and post-error adjustments in cognitive control and attentional engagement to a task in healthy adults and individuals with schizophrenia. We found that error processing was characterized by increases in theta-band activity accompanied by decreases in alpha-band activity in both control participants and participants with schizophrenia, but that both the theta and alpha responses were significantly reduced in participants with schizophrenia. Post-error adjustments in processing were characterized by a similar pattern, and, importantly, post-error increases in theta activity were associated with improved accuracy on subsequent trials in healthy adults but not individuals with schizophrenia. Examining the prestimulus period before partial attention lapses showed increases in alpha-band activity, but only for healthy individuals and individuals with schizophrenia with relatively low positive symptom severity.
These results provide evidence for a deficit in cognitive control mechanisms mediated by midfrontal theta activity in schizophrenia, and suggest a particularly pronounced deficit in patients' ability to engage adaptive control mechanisms following errors. Our results also indicate that partial attention lapses can be indexed in both healthy adults and individuals with schizophrenia by increases in alpha activity, although in schizophrenia this varies as a function of positive symptom severity. We suggest that disruption in theta-band function represents a key deficit of schizophrenia, whereas disruptions in the alpha band may be the byproduct of atypically regulated attention. This is an important distinction to make, as it is critical to distinguish between aspects of cognition that are intact and impaired in schizophrenia. These data highlight how electro-physiology can be used in order to investigate dynamic adjustments in cognitive control in schizophrenia, in real time.
Supplementary Material
Table S1: rANOVA results for errors versus hits.
Table S2: rANOVA results for previous errors versus previous correct trials.
Table S3: rANOVA results for incongruent-incongruent versus congruent-incongruent trials.
Table S4: rANOVA results for slow congruent hits versus fast congruent hits.
Table S5: rANOVA results comparing slow congruent hits versus fast congruent hits.
Acknowledgments
This work was supported by the National Institute of Mental Health (Grant No. 5 R01 MH059883) to CSC.
Footnotes
Supporting Information: Additional Supporting Information may be found in the online version of this article.
References
- Adrian ED, Matthews BH. The Berger rhythm: Potential changes from the occipital lobes in man. Brain. 1934;57(4):355–385. doi: 10.1093/brain/57.4. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: Comparison of schizophrenics and controls. Cerebral Cortex. 1995;5(6):550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, MacDonald AW, Braver TS, Cohen JD. Context-processing deficits in schizophrenia: Diagnostic specificity, 4-week course, and relationships to clinical symptoms. Journal of Abnormal Psychology. 2003;112(1):132–143. doi: 10.1037/0021-843X.112.1.132. [DOI] [PubMed] [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clinical Neurophysiology. 2002;113(9):1454–1463. doi: 10.1016/S1388-2457(02)00154-2. [DOI] [PubMed] [Google Scholar]
- Bengson JJ, Mangun GR, Mazaheri A. The neural markers of an imminent failure of response inhibition. NeuroImage. 2012;59(2):1534–1539. doi: 10.1016/j.neuroimage.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Bollimunta A, Chen Y, Schroeder CE, Ding M. Neuronal mechanisms of cortical alpha oscillations in awake-behaving macaques. Journal of Neuroscience. 2008;28(40):9976–9988. doi: 10.1523/JNEURO-SCI.2699-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037//0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Boudewyn MA, Long D, Traxler MJ, Lesh TA, Dave S, Mangun GR, et al. Swaab TY. EEG correlates of fluctuations in attention to discourse context predict sensitivity to referential ambiguity during language comprehension. Journal of Cognitive Neuroscience. 2015;27(12):2309–2323. doi: 10.1162/jocn_a_00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15(7):827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Carter CS. Understanding the glass ceiling for functional outcome in schizophrenia. American Journal of Psychiatry. 2006;163(3):356–358. doi: 10.1176/appi.ajp.163.3.356. [DOI] [PubMed] [Google Scholar]
- Carter CS, Van Veen V, Botvinick M, Cohen J, Stenger VA. Conflict and the evaluative functions of the anterior cingulate: Converging evidence from event-related fMRI and high density ERP. NeuroImage. 2001;13(6):305. doi: 10.1016/S1053-8119(01)91648-9. [DOI] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. Journal of Neuroscience. 2009;29(1):98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology. 2011;49(2):220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: Converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108(1):120–133. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. Journal of Physiology. 2012;590(4):715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5(5):303–305. doi: 10.1111/j.1467-9280.1994.tb00630.x. [DOI] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8(12):1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. doi: 10.1111/j.1467-9280.1993.tb00586.x. [DOI] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7(4):374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocogni-tive deficits in schizophrenia? American Journal of Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia. Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophrenia Research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Should schizophrenia be treated as a neurocognitive disorder? Schizophrenia Bulletin. 1999;25(2):309–319. doi: 10.1016/S0924-977X(99)80104-7. [DOI] [PubMed] [Google Scholar]
- Hartwich K, Pollak T, Klausberger T. Distinct firing patterns of identified basket and dendrite-targeting interneurons in the prefrontal cortex during hippocampal theta and local spindle oscillations. Journal of Neuroscience. 2009;29(30):9563–9574. doi: 10.1523/JNEUROSCI.1397-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Herman MM. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. Journal of Neuroscience. 2011;31(30):11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG. Bilateral electrodermal habituation-dishabituation and resting EEG in remitted schizophrenics. Journal of Nervous and Mental Disease. 1982;170(2):91–101. doi: 10.1097/00005053-198202000-00005. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cerebral Cortex. 2002;12(8):877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience. 2002;15(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict-and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. American Journal of Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Stenger VA, Carter CS. Prefrontal cortex guides context-appropriate responding during language production. Neuron. 2004;43(2):283–291. doi: 10.1016/j.neuron.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Kopp B, Rist F. An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. Journal of Abnormal Psychology. 1999;108(2):337–346. doi: 10.1037/0021-843X.112.1.132. [DOI] [PubMed] [Google Scholar]
- Lemere F. The significance of individual differences in the Berger rhythm. Brain: A Journal of Neurology. 1936;59:366–375. doi: 10.1093/brain/59.3.366. [DOI] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophrenia Bulletin. 2008;34(5):974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Carter CS. Event-related FMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology. 2003;112(4):689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. Cohen JD. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- Martínez A, Gaspar PA, Hillyard SA, Bickel S, Lakatos P, Dias EC, Javitt DC. Neural oscillatory deficits in schizophrenia predict behavioral and neurocognitive impairments. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: An event-related brain potential study. Journal of Abnormal Psychology. 2002;111(1):22–41. doi: 10.1016/j.ijpsycho.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T. To see or not to see: Prestimulus a phase predicts visual awareness. Journal of Neuroscience. 2009;29(9):2725–2732. doi: 10.1523/JNEURO-SCI.3963-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Lleras A, Beck DM, Fabiani M, Ro T, Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Frontiers in Psychology. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, Berry AS, Corbett BA. Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;67(7):617–623. doi: 10.1016/j.biopsych.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Jensen O. Rhythmic pulsing: Linking ongoing brain activity with evoked responses. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis IL, van Dijk H, Jensen O. Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Human Brain Mapping. 2009;30(6):1791–1800. doi: 10.1002/hbm.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, et al. Constable RT. Effects of working memory load on oscillatory power in human intracranial EEG. Cerebral Cortex. 2008;18(8):1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC. Negative symptoms and EEG alpha activity in schizophrenic patients. Schizophrenia Research. 1992;8(1):11–20. doi: 10.1016/0920-9964.(92)90056-B. [DOI] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC. Negative symptoms and EEG alpha in schizophrenia: A replication. Schizophrenia Research. 1996;19(2):151–161. doi: 10.1016/0920-9964(92)90056-B. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharmacology. 2010;35(13):2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, Carter CS. Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Research: Neuroimaging. 2013;11:114–121. doi: 10.1016/j.pscychresns.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T, Tanaka K, Hagimoto H, Miura T, Kishimoto H, Matsushita M. Computerized EEG in schizophrenic patients. Biological Psychiatry. 1990;28(6):488–494. doi: 10.1016/0006-3223(90)90482-H. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Bellgrove MA, Turin A, Ward S, Foxe JJ, Robertson IH. Two types of action error: Elec-trophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no-go tasks. Journal of Cognitive Neuroscience. 2009;21(1):93–104. doi: 10.1162/jocn.2009.21008. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: Electrophysiological signals predict errors up to 20 s before they occur. Journal of Neuroscience. 2009;29(26):8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RM, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proceedings of the National Academy of Sciences. 2015;112(30):9448–9453. doi: 10.1073/pnas.1504196112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: Alpha–gamma versus theta–gamma codes for distinct WM information? Trends in Cognitive Sciences. 2014;18(1):16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Samaha J, Bauer P, Cimaroli S, Postle BR. Top-down control of the phase of alpha-band oscillations as a mechanism for temporal prediction. Proceedings of the National Academy of Sciences. 2015;112(27):8439–8444. doi: 10.1073/pnas.1503686112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Cognitive Brain Research. 2005;25(3):936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context: A test of a theoretical model. Archives of General Psychiatry. 1996;53(12):1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31(1):37–43. doi: 10.1111/j.1469-8986.1994.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Clementz BA, Iacono WG, Beiser M. Clinical and biological concomitants of resting state EEG power abnormalities in schizophrenia. Biological psychiatry. 2000;48(11):1088–1097. doi: 10.1016/S0006-3223(00)00907-0. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van Driel J, Ridderinkhof KR, Cohen MX. Not all errors are alike: Theta and alpha EEG dynamics relate to differences in error-processing dynamics. Journal of Neuroscience. 2012;32(47):16795–16806. doi: 10.1523/JNEUROSCI.0802-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77(4):477–482. doi: 10.1016/S0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vidal F, Hasbroucq T, Grapperon J, Bonnet M. Is the ‘error negativity’specific to errors? Biological Psychology. 2000;51(2):109–128. doi: 10.1016/S0301-0511(99)00032-0. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: Decreased expression in a subset of neurons. American Journal of Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Wada Y, Takizawa Y, Kitazawa S, Zheng-Yan J, Yamaguchi N. Quantitative EEG analysis at rest and during photic stimulation in drug-naive patients with first-episode paranoid schizophrenia. European Archives of Psychiatry and Clinical Neuroscience. 1994;244(5):247–251. doi: 10.1007/BF02190377. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;375:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Woo TUW, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM. Differential alterations of kainate receptor subu-nits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophrenia Research. 2007;96(1):46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111(4):931–959. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: rANOVA results for errors versus hits.
Table S2: rANOVA results for previous errors versus previous correct trials.
Table S3: rANOVA results for incongruent-incongruent versus congruent-incongruent trials.
Table S4: rANOVA results for slow congruent hits versus fast congruent hits.
Table S5: rANOVA results comparing slow congruent hits versus fast congruent hits.