Summary
Regulation of respiratory mucosal immunity by microbial-derived metabolites has been a proposed mechanism that may provide airway protection. Here we examine the effect of oral Lactobacillus johnsonii-supplementation on metabolic and immune response dynamics during respiratory syncytial virus (RSV) infection. L. johnsonii-supplementation reduced airway Th2 cytokines, dendritic cell function, increased T-regulatory cells, and was associated with a reprogrammed circulating metabolic environment, including docosahexanoic acid (DHA) enrichment. RSV-infected bone-marrow derived dendritic cells (BMDC) from L. johnsonii-supplemented mice had altered cytokine secretion, reduced expression of co-stimulatory molecules, and modified CD4+ T cell cytokines. This was replicated upon co-incubation of wild-type BMDCs with either plasma from L. johnsonii-supplemented mice, or DHA. Finally, airway transfer of BMDCs from L. johnsonii-supplemented mice, or with wild-type derived BMDCs pre-treated with plasma from L. johnsonii-supplemented mice, reduced airway pathologic responses to infection in recipient animals. Thus, L. johnsonii-supplementation mediates airway mucosal protection via immunomodulatory metabolites and altered immune function.
Introduction
RSV infection is the leading cause of childhood hospitalization, and increases the risk for developing childhood asthma and recurrent wheezing 1, 2. Factors associated with increased susceptibility to airway inflammation in childhood includes broad-spectrum oral antibiotic use during the first year of life 3-5, known to perturb the gut microbiome 6. In neonates, reduced fecal Bifidobacterium and Lactobacillus burden and increased Escherichia and Clostridium cell counts are associated with childhood allergic sensitization 7, 8 These and other observations support the concept that airway immune status is impacted by the composition and activities of the gastrointestinal microbiome 9, although studies of the protective effect of oral Lactobacillus supplementation on allergic outcomes are inconsistent (9-13). Little is known about the mechanisms associated with the gut microbiome-associated protection from allergic diseases.
Previous studies from our group have shown that oral supplementation of mice with Lactobacillus johnsonii resulted in significantly reduced airway allergic sensitization and RSV-induced pulmonary immunopathology 10. The gut microbiota was significantly altered in these animals, but L. johnsonii was not detected in their airways, with only viable L. johnsonii able to confer protection 10. Other studies have demonstrated that gut microbiome-derived short chain fatty acids, specifically propionate, are associated with airway protection against allergen challenge, and influence hematopoiesis 11. The metabolic potential of the gut microbiome is substantial and extends beyond fatty acid production. Hence, we hypothesized that gastrointestinal L. johnsonii elicits airway protection against RSV infection through programmed changes in the circulating metabolic environment, which concurrently impacts both airway mucosal responses and bone marrow derived immune precursor cell populations.
The findings of our present study extend the evidence for an overall change in immune phenotype that results from gut microbiome manipulation that indicates that L. johnsonii-supplementation leads to broad-scale lipid, carbohydrate and amino acid metabolic re-programming prior to and during pulmonary RSV infection that underpins the associated protective immune responses.
Results
Alteration of immune responses in L. johnsonii-supplemented mice
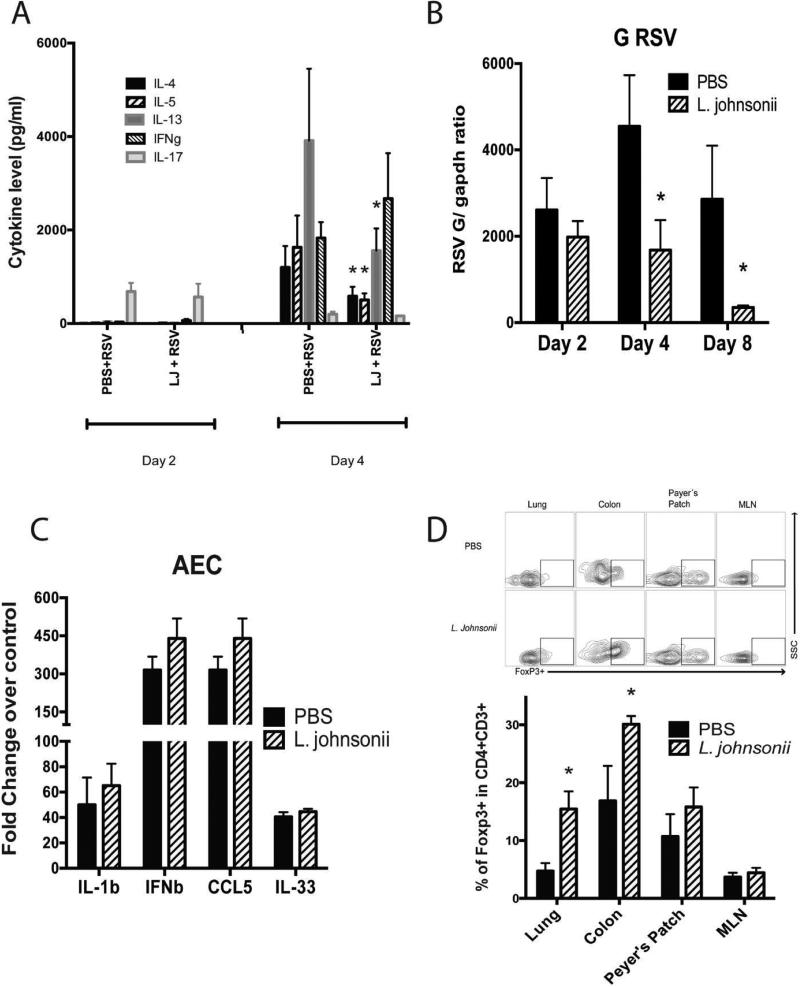
Previously we have demonstrated that oral supplementation with L. johnsonii, isolated from the murine cecum, protects mice against both airway allergen challenge and RSV infection 10. To examine the primary mechanisms associated with the L. johnsonii modified immune responses, we examined immune status prior to and during the early stages of RSV infection. Mice were orally supplemented daily by gavage with L. johnsonii (1×107 CFU/mouse/day) for 7 days and infected with RSV one day after the final supplementation (on day 8). The responses were assessed at day 2, 4, and 8 of infection to identify early changes in the immune responses (Fig. S1A). Lung-draining lymph nodes (LDLN) were isolated from L. johnsonii- and PBS-supplemented animals, from which single cell suspensions were prepared, re-challenged with RSV in vitro and supernatants collected after 48 hrs and examined for cytokine production (Fig.1A). Expression of RSV-induced T cell associated cytokine IL-17 was first evident as early as 2 days in both PBS and L. johnsonii- supplemented animals. At 4 days of infection, IL-4, IL-5, IL-13 and IFN γ, were expressed, however, LDLN cells from L. johnsonii-supplemented mice had significant reductions in the Th2 cytokines, IL-4, IL-5, and IL-13. The production of IFN γ or IL-17 was not significantly altered at either time point. Consistent with previous observations 10, the re-challenged lymph nodes from day 8 of infection also displayed significant reductions in the Th2 cytokines in L. johnsonii treated mice (Fig. S2). Next, viral clearance was examined in supplemented mice at day 2, 4 and 8-post infection with a significant reduction at day 4 and 8 in L. johnsonii-supplemented mice (Fig. 1B). RSV F and N protein mRNA was also altered (Fig. S3A), together demonstrating increased viral clearance. Recently group 2 innate lymphoid cells have been suggested to play a role in RSV immunopathology 12, 13. We did not observed differences in the population of ILC2s in the lung of the mice that were L. johnsonni supplemented (Fig. S3B).
Figure 1. Lactobacillus johnsonii-supplemented mice have altered immune responses.
Eight week old Balb/c/J mice were supplemented with L. johnsonii (1 × 107 cfu/day) or PBS daily for 7 days followed by RSV infection the following day (3 × 105 pfu/mouse) by intratracheal instillation. (A) Lung draining lymph nodes were isolated from the infected mice on day 2 or 4 following RSV infection and single cell suspensions were restimulated with RSV for 48 h and cytokines assessed. (B) Gene expression of RSV G protein was assessed from harvested lungs from infected mice (day 2, 4, and 8 post RSV infection) by RT-PCR and assessed as a ratio of RSV G to GAPDH to standardize the results. (C) Single cell suspensions of enzymatically dispersed lungs were used to grow airway epithelial cells in single cell suspensions and were subsequently infected with RSV for 24 hrs (MOI-1.0) on near confluent cultures. (D) L. johnsonii supplemented mice have increased Treg cells prior to challenge with RSV. Eight week old mice were supplemented with L. johnsonii (1 × 107 cfu/day) or PBS daily for 7 days. Lung, mesenteric lymph nodes (MLN), Peyer's patch, and colons were isolated, dispersed, and assessed for numbers of Treg cells by flow cytometry by identifying those CD4+ cells that also express the Foxp3 protein as the indicative transcription factor for Treg cells. Data represents the Mean ± SE from 4-5 mice in each group (experimental repeats 3-4). *P<0.05.
We have also been interested in whether the lung epithelial cells were impacted by a protective gut microbiome. To this end, we isolated airway epithelial cells, as previously described 14, from animals that had been supplemented with PBS or L. johnsonii prior to RSV infection. Subsequently, the isolated airway epithelial cells (AEC) were infected with RSV in vitro (multiplicity of infection; MOI of 1.0) for 24 hrs. Isolated mRNA analysis demonstrated no alteration of epithelial derived cytokine production, including IL-1β, IFNβ, CCL5 or IL-33 (Fig. 1C). Thus, L. johnsonii supplementation did not result in epithelial cell responsiveness to RSV using this ex vivo analysis.
Several studies have suggested that the host microbiome can alter the development of immune responses through the generation of Treg cells 11, 15. Treg cell numbers were enumerated following seven days of L. johnsonii or PBS supplementation prior to exposure to RSV. A significant increase in Treg cell numbers, assessed by flow cytometry staining of FoxP3, was observed in L. johnsonii- compared to PBS-supplemented mice at the mucosal tissue sites studied, colon and lung (Fig. 1D), but not in mesenteric lymph nodes (MLNs) and Peyer's Patches (PP) (Fig. 1D). Overall there appear to be significant alterations in the L. johnsonii supplemented animals related to mucosal tissue Treg cell numbers prior to infection that could impact the generation of pathogenic Th2 cytokine responses during infection.
Bone marrow-derived DC (BMDC) from L. johnsonii supplemented mice have altered innate cytokines and APC function
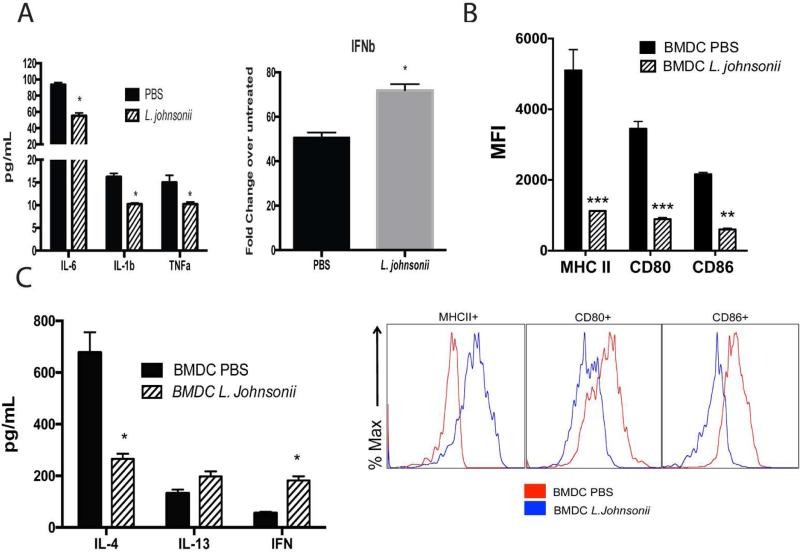
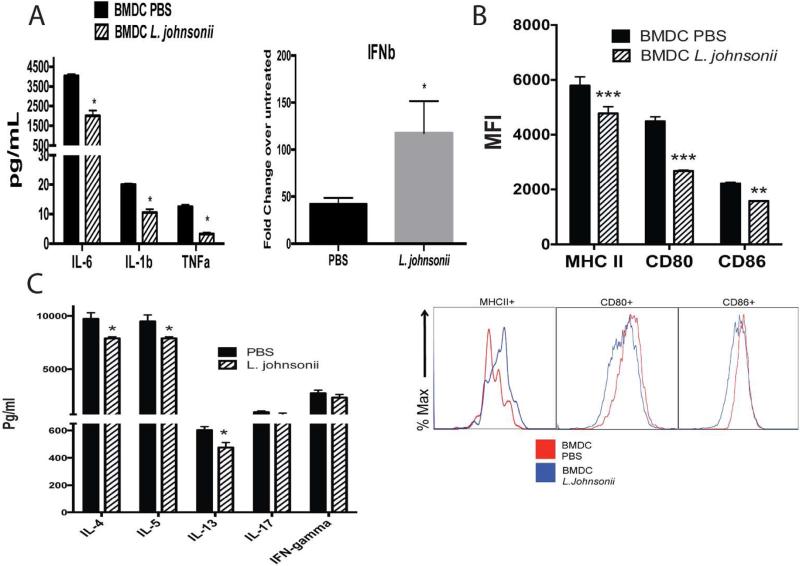
The above studies further support that oral supplementation with L. johnsonii can alter systemic immune cell responses. Since the pulmonary DC numbers were significantly reduced during RSV infection in L. johnsonii-supplemented mice 10 and because DC have a primary effect on the local immune response, we matured BMDC from L. johnsonii- or PBS-supplemented animals. BMDC were activated by RSV infection (MOI of 1.0) for 24 hrs and isolated mRNA from BMDC cultured from L. johnsonii-supplemented mice demonstrated decreased production of innate cytokines, IL-6, IL-1b, TNFα, and an increase in IFNβ mRNA expression compared to BMDC grown from PBS-supplemented animals (Fig. 2A). When maturation markers, MHC class II, CD80, and CD86, were examined, there was a significant decrease in their expression in BMDC infected with RSV from L. johnsonii-supplemented versus PBS supplemented animals (Fig. 2B). In addition, when BMDC from the L. johnsonii supplemented mice were stimulated with TLR ligands, LPS and CpG, they also demonstrated a reduced co-stimulatory molecule expression (Fig. S5). Together these data suggest L. johnsonii supplementation results in an overall decrease in the activation potential that reflects an impact on the bone marrow of supplemented mice. We also analyzed relative ratios of bone marrow progenitor populations in mice that were supplemented prior to and after 2 days of RSV infection. We observed a decrease in only megakaryocyte progenitors in L.Johnsonii-supplemented mice, prior to infection, but not during RSV infection (Fig. S4).
Figure 2. Bone marrow derived DC (BMDC) from L. johnsonii supplemented mice have altered inflammatory and costimulatory activity to promote an modulated T cell activation profile.
BMDC were grown from femurs of animals supplemented daily with L. johnsonii (1 × 107 cfu) or PBS by oral gavage for 7 days. BMDC were infected with RSV (MOI of 1.0) and 24 hrs. later examined for (A) mRNA expression of innate cytokines, (B) upregulation of MHC II and co-stimulatory marker expression by flow cytometry (MFI=mean fluorescence intensity), and (C) for their ability to activate T cells from lung draining lymph nodes of 8 day RSV infected wildtype mice for production of cytokines after 48 h. Data represent mean ± SE from 3 mice with 3-4 repeats. *P<0.05. ***P<0.001.
Next, BMDC from the supplemented mice were used to determine if they differentially altered T cell associated cytokine production. BMDC from supplemented mice were infected with RSV overnight, washed, and combined with isolated CD4+ T cells from LDLN of 8 day RSV infected, unsupplemented mice. When BMDC grown from L. johnsonii supplemented mice were used to re-stimulate the RSV-responsive T cells for 48 hrs a significant reduction in IL-4 and a significant increase in IFNγ was observed, while IL-13 production was not significantly altered (Fig. 2C). These data indicate that significant changes in the innate immune response appear to be manifested in bone marrow progenitor cell populations and alter RSV-induced T cell activation.
Systemic metabolic changes are associated with L. johnsonii oral supplementation
The mechanism(s) of gut microbiome-induced airway protection is presently not clear. Recent studies have begun to outline how the gut microbiome influences available nutrients and metabolites for host utilization, potentially providing an immune altering environment 16, 17. To examine whether supplementation with L. johnsonii for 7 days influences the peripheral metabolite profile, plasma metabolites were examined prior to and following RSV infection. Using 5 mice per group in two independent experiments (for a total n=10 per group) we profiled the plasma metabolites (>400 metabolites) present on day 8 at the end of the supplementation period prior to RSV infection by liquid chromatography/mass spectrometry (LC/MS-MS). Overall, relatively few (n=56) metabolites were significantly altered at baseline prior to RSV infection (Table S1). However, an immunomodulatory ω-3 polyunsaturated fatty acid (PUFA), docosahexaeneoate (DHA) 18, 19, and related metabolite 1-docosahexaenoylglycerophosphocholine (AcedoPC) were significantly increased in the plasma of L. johnsonii-supplemented animals (Table S1). Another notable metabolite, 2-hydroxyisobutyrate, a byproduct of cystathionine incorporation into glutathione 20, was also among the metabolites enriched in plasma of L. johnsonii-supplemented animals (Table S1). Thus, the baseline changes observed in the supplemented animals displayed differences in a discrete spectrum of anti-inflammatory metabolites.
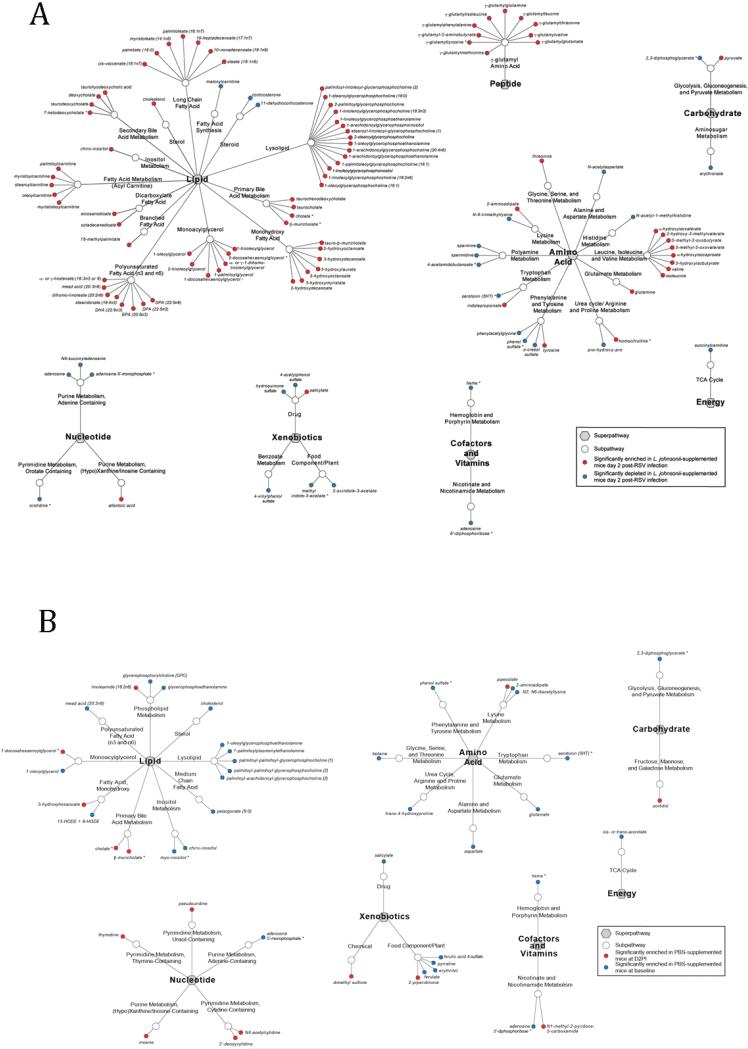
To further understand whether the L. johnsonii vs. control supplemented mice displayed differences in metabolite dynamics during the early phases of RSV infection, plasma from mice at Day 2 post infection with RSV was subjected to LC/MS-MS and compared to the related uninfected plasma profiles from each group at day 0. L. johnsonii supplemented animalsexhibited substantial metabolic reprogramming in response to RSV infection, involving significant increases in a broad range of lipid-, bile-, amino acid and peptide-derived metabolites (Fig. 3A, Table S2). We identified lipids that are known to be able to modify the immune response, especially increases in PUFA/DHA concentrations over baseline. In comparison, PBS supplemented animals exhibited a less substantial and different metabolic response to RSV infection, characterized by decreased concentrations of metabolites compared to baseline (Fig. 3B, Table S3). Thus, at day 2, a time when the innate immune response is most active and early activation events shape the adaptive immune responses, L. johnsonii-supplemented animals exhibit a rapidly altered metabolic environment in response to viral pathogen infection.
Figure 3.
Metabolites significantly enriched (red) or decreased (blue) in the serum of mice supplemented with (A) L. johnsonii or (B) PBS at day 2 post RSV infection compared to baseline levels prior to infection. Data generated by LC/MS-MS and represent significance (Welch's t-test; p<0.05) between the groups (n=5 mice per treatment group).
The ω-3 PUFA, DHA, alters Dendritic Cell function upon RSV infection
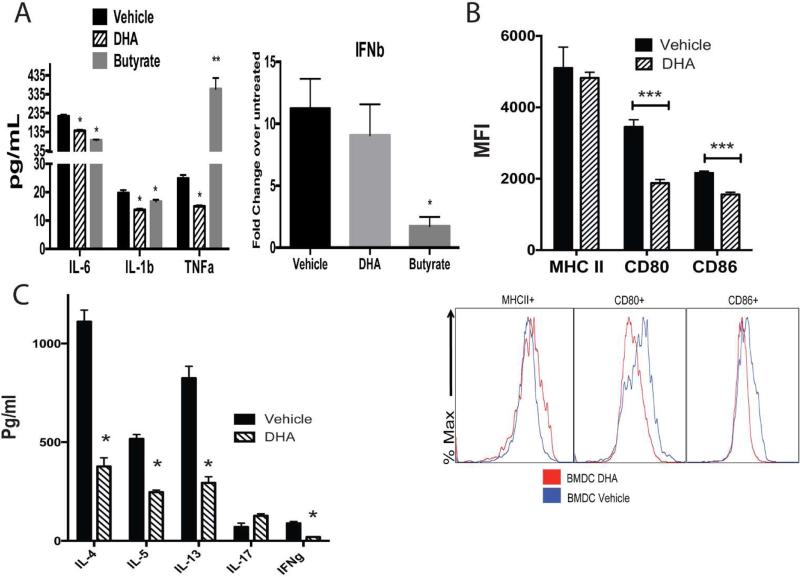
In our metabolic analyses we identified several metabolites altered in the L. johnsonii plasma samples, two of those were metabolites significantly increased; DHA and AcedoDC (related to DHA) discriminated L. johnsonii from PBS-supplemented animals. DHA has been previously described as immune modulatory molecule 21-23. To further investigate whether these metabolites could facilitate the altered DC activation in response to RSV, we co-incubated RSV infected BMDC with DHA. We utilized as a control a short chain fatty acid metabolite, butyrate, which has been shown incapable of inducing protective hematopoietic cell phenotypes during allergic sensitization 11. The addition of DHA significantly reduced RSV-induced inflammatory cytokine production, TNF, IL-1 and IL-6 by BMDCs, while butyrate did not (Fig. 4A). In addition, when we examined IFNβ, an important anti-viral cytokine and TH1 immune mediator, we observed that while DHA did not affect IFNβ, butyrate significantly reduced its expression (Figure 4A). We next co-incubated BMDCs with DHA for 16 h prior to infection with RSV and examined activation marker expression by flow cytometry. The data in Figure 4B show that DHA significantly reduced the ability of BMDCs to up-regulate MHC class II and CD86 in response to RSV as well as baseline expression of MHCII (SF6), which could have a primary effect during RSV infection.
Figure 4. RSV-induced activation of bone marrow-derived DC (BMDC) is inhibited by DHA and alters their ability to activate RSV responsive T cells.
(A) BMDC grown from naïve wildtype mice treated with either DHA (50 uM) or butyrate (1mM) for 16 hour prior to infection with RSV (MOI of 1.0). After 24 hrs supernatants were assessed for innate cytokine levels. (B) DHA (50 uM) treatment of RSV infected BMDC were assessed by flow cytometry for expression of activation/co-stimulatory protein expression. (C) RSV-infected BMDC with or without DHA (50 uM) treatment were combined with CD4 T cells isolated from the lung draining lymph nodes of 8 day RSV infected mice. After 48 h of activation the supernatants were assessed for T cell associated cytokine levels to determine the capacity of the DC for APC function. Data represents Mean ± SE from 3 repeats. *P<0.05.
To examine whether DHA could inhibit BMDC ability to drive T cell activation, CD4+ T cells isolated from LDLNs of unsupplemented, RSV-infected mice at day 8 post infection were incubated for 48 hrs with the DHA-treated, RSV-infected BMDC. Data presented in Fig. 4C demonstrate a significant reduction in the RSV-induced cytokines IL-4, IL-5, IL-13 and IFNγ, compared to the vehicle control. These data indicate that DHA represents at least one of the enriched metabolites after oral supplementation with L. johnsonii that can mediate modified BMDC and in turn alter T cell function to RSV infection. We suggest that DHA, together with other metabolites modified in the L. johnsonii supplemented mice could alter DC and other immune cells upon RSV infection.
Plasma from L. johnsonii supplemented mice alter BMDC activation and function
The above data indicated that the protective effect of L. johnsonii supplementation on the immune response is a systemic effect that influences DC function and is associated with an altered circulating metabolic profile. Hence we reasoned that the observed phenotypic changes in BMDCs are mediated via immunomodulatory metabolites enriched in the circulation of L. johnsonnii-supplemented animals. To test this possibility, BMDCs differentiated from unsupplemented and uninfected mice were incubated with plasma (2% in culture media) from L. johnsonii- or PBS-supplemented animals and infected with RSV in vitro. When the cytokines from the plasma treated BMDCs were examined after RSV infection a significant decrease in IL-6, IL-1β and TNF-α protein, and an increase in type I IFN (IFN-β) was observed in the DC treated with plasma from L. johnsonii-supplemented animals (Fig. 5A). Similar to the BMDCs grown from the supplemented animals pre-RSV infection, RSV infected BMDCs treated with plasma from L. johnsonii-supplemented animals exhibited significantly decreased expression of the co-stimulatory molecules MHC class II, CD80+, and CD86+, compared to BMDC treated with plasma from PBS-supplemented animals (Fig. 5B). No differences were detected at baseline (SF6B). In addition, using other TLR ligands, LPS and CpG, stimulated BMDC from L. johnsonii-supplemented mice also demonstrated a decreased MHC class II and CD80 expression (Fig. S3B). Using BMDCs incubated with plasma from L. johnsonii-supplemented animals for co-stimulation of isolated CD4+ T cells from LDLN cells of 8 day infected unsupplemented mice resulted in a significant decrease in the production of IL-4, IL-5, and IL-13 when compared to BMDC treated with plasma from PBS supplemented mice (Fig. 5C). Thus, similar to DCs grown from bone marrow of L. johnsonii supplemented mice, plasma from these animals altered the wild type BMDC ability to be activated in response to RSV and their ability to co-activate T cells for Th2 cytokine production. Together, these data suggest that plasma from L. johnsonii supplemented animals contain mediators that alter DC function leading to immune modulation in response to RSV infection.
Figure 5. Plasma from L. johnsonii supplemented animals significantly alter the BMDC function compared to plasma from unsupplemented animals.
Plasma was isolated from 2 day RSV infected animals with or without a 7 day L. johnsonii supplementation prior to infection. (A) RSV-infected BMDC from wildtype mice pre-incubated with plasma for 16 h and characterized for co-stimulatory molecule expression by flow cytometry after 24 hrs. Mean fluorescent intensity (MFI) indicates the expression level of each molecule. (B) RSV-infected BMDC incubated with plasma (2%) from animals with or without L. johnsonii supplementation were combined with CD4 T cells isolated from the lung draining lymph nodes of 8 day RSV infected mice. After 48 h of activation the supernatants were assessed for T cell associated cytokine levels to determine the capacity of the DC for APC function. (C) Innate cytokine levels from RSV-infected BMDC incubated with plasma (2%) from animals with or without L. johnsonii supplementation measured after 24 hrs of infection. Data represents Mean ± SE from 3 repeats. *P<0.05, **P<0.01, ***P<0.005.
BMDC from L. johnsonii supplemented animals modulate pulmonary sensitization responses in vivo
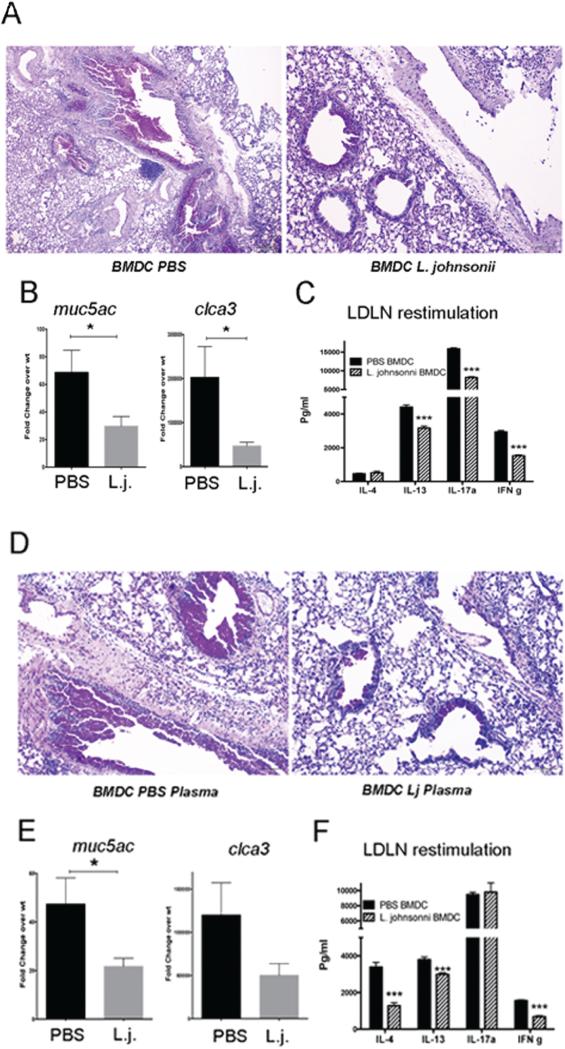
We have previously demonstrated that when we sensitize mice with RSV-infected DCs into the airway followed by a RSV infection challenge 7 days later, an enhanced pathologic response occurs, including increased mucus, enhanced Th2 and Th17 mediated cytokines, and worse inflammation 24-26. We have previously demonstrated that RSV-infected CFSE-labeled DCs migrate to the lymph nodes and since the BMDC do not propagate RSV, no infection of the lungs occurs by the DC transfer 25. Thus, observed changes upon RSV challenge is due to an inappropriate immune response 24, 25 allowing comparison studies to explore differences in DC phenotype. Using the above protocol (outlined in Supplemental Figure 1B), BMDCs from animals supplemented with L. johnsonii were transferred into naïve mice lungs. This resulted in a significant decrease in pathogenic responses, inflammation and mucus (PAS) staining, compared to BMDCs from PBS-treated animals (Fig. 6A). These responses were accompanied by decreased expression of mucus associated genes, mclca3 (gob5) and muc5ac, within the lungs in mice sensitized with BMDC from L. johnsonii- compared with PBS-supplemented animals (Fig. 6B). Furthermore, in a separate study when lung draining lymph nodes (LDLN) were restimulated by RSV ex vivo, there was a significant decrease in IL-13, IL-17 and IFN (Fig. 6C), reflecting decreased histopathology and mucus hypersecretion.
Figure 6. Adoptive transfer of BMDC from L. johnsonii-supplemented mice or those treated with plasma from supplemented animals have reduced RSV-induced pathology and altered immune responses.
RSV-infected BMDC grown from L. johnsonii-supplemented mice were transferred into the airways of naïve mice followed by RSV infection 7 days later. After an additional 8 days of infection the lungs were harvested for histologic (A) and PCR (B) analysis. In a separate study using the same protocols lung draining lymph nodes (LDLN) were restimulated by RSV for 48 h for cytokine analysis (C). In a second set of studies BMDC from wildtype mice were treated with plasma from PBS or L. johnsonii-supplemented mice and infected with RSV, followed by instillation into the airways of naïve mice 24 hrs later. Seven days later the animals were infected with RSV and harvested 8 days later, similar to the experiment in Figure 6A. The histologic assessment demonstrated reduced inflammation and mucus staining by PAS (D). The mRNA expression of muc5ac and clca3 (gob5) was assessed by QPCR (E). In a separate study, RSV-restimulated LDLN supernatants were assessed by bioplex analysis after 48 hrs of culture (F). Data represent mean ± SE from 5 mice/group. *P<0.05. ***P<0.001.
Since we were able to recapitulate the BMDC altered phenotype with plasma from L. johnsonii supplemented mice in vitro, we also compared BMDC incubated with plasma from L. johnsonii- or PBS-supplemented animals that were then infected with RSV and instilled into the airways in a similar fashion (Supplemental Figure 1B). These data showed a comparable phenotype as those BMDC from the L. johnsonii supplemented animals. That is, a decrease in inflammation and mucus in mice sensitized with BMDC incubated with plasma from L. johnsonii-compared to PBS-supplemented animals was detected by histology (Fig. 6D). There was also a decrease in the mucus associated genes mclca3 (gob5) and muc5ac, although only muc5ac reached significance (Fig. 6E). The reduction in the cytokines in the L. johnsonii plasma group was significant with IL-4, IL-13, and IFN (Fig. 6F) again supporting the pathologic responses. Altogether, these experiments indicate that supplementation with L. johnsonii alters the metabolic profile of plasma and has an immune modulating effect on DC function that extends to progenitor cell populations in the bone marrow.
Discussion
The present study describes significant systemic metabolic reprogramming initiated by oral supplementation of a single gut bacterial species, L. johnsonii, which coincided with protection against RSV-induced immunopathology. Our previous investigations have demonstrated correlations with microbial exposures in infants during early life in house dust related to indoor/outdoor pet ownership and development of childhood atopy 27-30, and that dust from homes with pets, attenuates development of allergic disease in mice 10. Lactobacillus johnsonii, isolated from the cecum of the latter mice, protected against both allergen- and RSV-induced pulmonary disease when used as a gastrointestinal supplement 10. While the reduction in immunopathology corresponded to the increased RSV clearance, the L. johnsonii supplementation facilitated significant changes to the T cell activation in the lymph nodes that we have shown to represent lung immune responses in this model 10. In the present studies, significantly enriched metabolites in the L. johnsonii-supplemented mice during RSV infection included a range of amino acid, lysolipids, purine, inositol, sterol, and bile metabolites. A profound difference was also observed in concentrations of fatty acids with known immunomodulatory effects, such as the anti-inflammatory omega-3 polyunsaturated fatty acids (ω-3 PUFA), eicosapentaenoate (EPA; 20:5n3), docosapentaenoate (n6 DPA; 22:5n6) and docosahexaenoate (DHA; 22:6n3). L. johnsonii does not biosynthesize many of these lipid metabolites, suggesting that microbial species co-enriched with L. johnsonii are necessary for this protective effect. Indeed, our previous studies indicated that L. johnsonii supplementation promoted the expansion of other bacteria with the predicted genetic capacity to synthesize many of the metabolites that were enriched in this study 10. Additionally, increases in a broad range of microbial- and/or mammalian-derived lipids, amino acids, and peptides with immunomodulatory potential, support the growing concept that metabolic signaling may represent an important mechanism by which the microbiome and host interact 31. Because supplementation of the gastrointestinal tract with L. johnsonii leads to significantly decreased airway disease, these findings strengthen recent evidence in the field that the gut microbiome influences immune responses that extends beyond the gastrointestinal tract 11, 15, 32, 33.
Recent data have begun to build mechanistic evidence suggesting how the gut microbiome may regulate inflammation and immune responses. A link between diet and microbiome for regulation of immune responses has been established with dietary fiber and Clostridium species leading to short chain fatty acids (SCFA) production and development of Treg cells 34-37. Seminal studies examining the function of SCFA produced by Clostridium species demonstrated their role for inducing Treg cells protecting mice from colitis and reducing IgE-mediated responses 38-42. These latter results were largely recapitulated using supplementation with specific SCFA 43, 44. In addition, SCFA as well as ω-3 PUFA directly impact adhesion molecule expression and inflammatory cytokine production by altering inflammasome activation via inhibition of NALP3 45-48 and NF-kB activation pathways 49, 50. In the present studies mucosal Treg cell numbers were increased prior to RSV infection in the L. johnsonii supplemented mice, and may provide a basis for a regulated environment to alter the intensity of the immune responses during RSV infection. Treg cell functional alteration has been identified with probiotic supplementation 38, 39, 51 and important for regulation of the immunopathology during RSV infection 52, 53. Our studies demonstrated that BMDC from L. johnsonii supplemented mice had an altered innate immune profile upon RSV challenge suggesting that the modified response was imprinted in progenitor cell populations. We observed little change in progenitor cell percentages in L. johnsonii-supplemented mice prior to infection, even though the response of BMDC to RSV infection and TLR stimulation was altered. Dendritic cells are also able to maintain and establish immunological tolerance 54. Thus, capacity of microbial metabolites to modify progenitor cell populations may facilitate the generation of Treg cells in mucosal tissue.
The concept that L. johnsonii supplementation generated a systemic effect, was supported using plasma from supplemented mice on unaltered BMDC to recapitulate the responses in BMDC from L. johnsonii supplemented mice. Although BMDC may not fully represent tissue DC populations since some are CD11c+ macrophages 55, the responses elicited in vitro and in vivo by BMDC from supplemented animals reflected the responses observed in whole animals. Numerous publications have suggested that Lactobacillus supplementation may impact DC directly 51, 56-63, while others demonstrated that ω-3 PUFA has a direct and lasting effect on DC function 18, 64. This latter immune effect was exhibited in the DC transfer experiments using BMDC grown from supplemented mice or DC treated with plasma from supplemented mice to alter the RSV challenge response with reduced pathology and mucus hypersecretion. The data indicate that alteration of immune responses is dependent upon the L. johnsonii supplementation and contained within the plasma. Thus, we hypothesize that the modified metabolite profile that accompanied L. johnsonii supplementation had a systemic effect altering bone marrow progenitors leading to changes in multiple immune cell populations.
G Protein-coupled receptors (GPCRs) have been identified that mediate the immune response changes to lipids, including GPR41, GPR43, and GPR109A that bind SCFAs as well as GPR120 and PPARγ that bind ω-3 fatty acids 65, 66. These receptors are found on immune cells as well as colonic epithelial cells and implicated in regulation of inflammatory cell activity, Treg cell development, and maintenance of epithelial barrier function 67. One of the metabolite classes that were increased after supplementation with L. johnsonii was the ω-3 fatty acid family that bind PPARγ and GPR120 and have been implicated as protective metabolites for asthma, IBD, as well as cardiovascular disease. While GPR120 and PPARγ signal pathways induced by the ω-3 FA have been directly shown to influence immune cell reactivity, other ω-3 FA breakdown products, including the resolvins, maresins, protectins, prostaglandins, etc., appear to be more potent anti-inflammatory mediators 68, 69. These latter compounds have their own set of GPCRs including ChemR23, BLT, ALX/FPR2 and GPR that have the ability to reduce intensity of the immune responses in disease models. It is likely the combination of metabolites identified in supplemented animals contribute to a balanced and appropriate immune response.
Altogether, these studies outline a potential mechanism for immune alteration by manipulating the microbiome and facilitating the availability of a complex and broad profile of microbial and mammalian-derived immunomodulatory metabolites. These findings expand our perspective by describing the diversity of metabolic reprogramming with supplementation of a single gut species that collectively may play a significant immunomodulatory role by influencing DC and T-cell function. Further investigations should aim at deciphering this complexity in RSV infection and other inflammatory diseases toward developing microbial- and metabolite-based therapies.
Materials and Methods
Generation of Lactobacillus johnsonii supplements for murine studies
Supplements were generated using MRS broth inoculated with L. johnsonii from a glycerol stock prior to static overnight culture at 37°C. Stationary phase cells (OD600 = 0.89) were centrifuged at 4,000 rpm for 15 minutes at 4°C and resuspended in 60 ml of a 50:50 solution of MRS broth:50% glycerol. Batches of 500 μl were snap frozen in liquid nitrogen and stored at −80 °C until used in murine studies. Viable cell count of the glycerol stock was 2.7 × 108 CFU per vial. For murine studies, tubes were defrosted on ice, centrifuged at 14,000 rpm for at 4°C, and washed twice in sterile saline to remove glycerol. The cells were resuspended in 700 μl of sterile saline. Each mouse received 100 μl (1 × 107 CFU) of resuspended L. johnsonii. The remaining suspension was plated to confirm that viable L. johnsonii cell counts were stable.
Airway Epithelial Cell Culture
Airway epithelial cell cultures were prepared from lungs of PBS or L. johnsonii-supplemented mice prior to RSV infection by digestion with Dispase (BD Biosciences). Dispersed lungs were filtered through 25 uM nylon mesh. Immune cells were depleted using biotinylated anti-CD45 antibodies and streptavidin-conjugated Dynabeads (Thermo Fisher Scientific). Cells were plated on 10 cm tissue culture dishes and adherence-purified in DMEM-based complete media, followed by 4 day culture in fibronectin-coated wells.
Respiratory Syncytial Virus
Our laboratory utilizes antigenic subgroup A, Line 19 RSV, originally obtained from a sick infant at the University of Michigan. This isolate has been shown in animal models to mimic human infection by eliciting airway mucus production upon intratracheal inoculation with 1 × 105 pfu RSV. Animals were infected after 7 days of supplementation with L. johnsonii (1 × 107 CFU/mouse/day) or PBS as described above.
Culture and stimulation of lymph node cells
Mediastinal lymph nodes were dispersed using 18-gauge needles and enzymatically, via incubation with 1 mg/ml Collagenase A (Roche, Indianapolis, IN) and DNase I (Sigma-Aldrich, St. Louis, MO) in RPMI 1640 with 10% Fetal Calf Serum. Following red blood cell lysis, cells were passed through a 40 μm strainer and counted with a Z2 Beckman Coulter particle counter. Suspensions of total lymph node cells were cultured in complete medium and re-stimulated with RSV (MOI-1.0) for 48 hours. Levels of T-helper cytokines, IL-4, IL-5, IL-13, IFNγ and IL-17 were determined in culture supernatants using a Bio-Plex® assay (Bio-Rad).
Quantitative RT-PCR
RNA was isolated from cell cultures and lung tissue using TRIzol, according to manufacturer's instructions (Invitrogen). 5μg of RNA was then reverse-transcribed to determine cytokine gene expression using pre-developed TaqMan Gene Expression Assay primer/probe sets and analyzed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Transcription levels of Muc5ac, Gob5, (day 8 post infection) and RSV-G, −F, and –N proteins (days 2, 4 and 8 post infection) were assessed using custom primers as previously described 10. Gene expression of Muc5ac and Gob5 was normalized using GAPDH expression as an internal control, and fold change values were calculated relative to an uninfected, or wild-type control group assigned an arbitrary value of 1. The viral protein mRNA expression is presented relative to a control gene GAPDH as a ratio of RSV G protein expression/GAPDH.
Cytokine Assay
Cytokines from supernatants of in vitro culture assays were measured using BioPlex System and Suspension Array Technology (Bio-Rad).
Dendritic Cell Isolation and T cell culture
Bone marrow-derived dendritic cells (BMDCs) were cultured in complete media, from whole bone marrow in the presence of 20ng/ml GM-CSF (R&D Systems, Minneapolis, MN) for 10 days when ~95% of the cells are CD11c+ myeloid cells. Dendritic cells were used for RSV infection at a multiplicity of infection (MOI) of 1.0 in 24 well tissue culture dishes and harvested as described in the results section. In some experiments the BMDC were incubated 16 h with DHA (50 uM) or butyrate (1 uM), concentrations previously described to alter cellular function 70, 71.
For DC-T cell co-culture experiments, RSV-responsive CD4+ T cells were obtained from mediastinal lymph nodes (MLN) of RSV-infected mice, 8 days post-infection. Cells were purified using a magnetic column negative-selection protocol to isolate CD4+ T cells (Miltenyi Biotec; >98% pure). For RSV-reactive T cell co-cultures, DCs were infected with MOI of 1.0 RSV for 2 hours. The DC were washed and placed into culture with isolated T cells from lung draining lymph nodes of RSV infected mice at a 1:10 ratio of DC to T cells. Culture supernatants were harvested at 48 hours post-co culture and analyzed using a custom BioRad Bioplex.
Plasma incubation of DC
Plasma from 7 day L. johnsonii-supplemented or unsupplemented mice was harvested and frozen in aliquots at −80F. After growing BMDC from wildtype unsupplemented mice for 10 days, 2% plasma was used to incubate the BMDC 16 h prior to infection with RSV (MOI-1.0). In the DC studies the cells and/or supernatant were harvested at the indicated time point. For T cell co-culture studies the DC were incubated with RSV and plasma for overnight prior to combining with isolated T cells from lymph nodes of RSV infected mice at a ratio of 1:10 DC to T cells. The supernatants from the co-culture were then harvested after 48 hrs. and assayed for cytokines by Bioplex analysis.
Flow Cytometry
Cells were isolated from the right lungs and mediastinal LNs by digestion in 200Ng/ml Liberase TM (Roche Applied Science, Indianapolis, IN) and 200U/ml DNase I (Sigma-Aldrich) at 37C. After lysing RBCs, FcR-blocking was used to limit nonspecific staining. Cells were stained with Live/Dead Fixable Yellow (Invitrogen), followed by fluorescent antibodies for 30 min. Total number of cells for each population in individual lungs was calculated using gating percentage multiplied by total number of cells in each lung preparation. Analysis was performed using FlowJo software (TreeStar, Ashland OR).
Metabolomic analysis
Plasma samples were isolated from mice at identified stages of supplementation and or RSV infection as described in the Results section. Animals were bled as a terminal bleed prior to necropsy into an EDTA solution to inhibit coagulation and plasma was separated by centrifugation. Isolated plasma was aliquoted into 200 ul samples and frozen at -80C in sterile screw top freezer tubes. A single aliquot of each sample was prepared in to dry ice and shipped to Metabolon, Inc. (Durham, NC) for analysis of the metabolic profile using mass spectrometry.
Airway sensitization by DC transfer and RSV Challenge Model
Bone marrow-derived DC were grown from animals supplemented with PBS or L. johnsonii for one week as above. The BMDC were then infected with RSV overnight, washed thoroughly free of any external virus, resuspended in saline and injected into the airway of naïve mice 24, 25. In a second set of studies BMDC were grown from naïve mice then incubated with 2% plasma from PBS or L. johnsonii supplemented mice for 16h prior to RSV infection. After overnight RSV infection the DC were washed and instilled into the airways of naïve mice. After a one-week sensitization period the animals were infected with an RSV challenge followed by harvest at 8 days post-infection as outlined in Supplemental Figure 1B.
Statistics
Data were analyzed and graphed using GraphPad Prism software. Statistical significance for tissue culture and in vivo analyses was determined by one-way ANOVA and Bonferroni post-test to obtain p values. Metabolites exhibiting significantly different concentrations were identified using Welch's t-test.
Supplementary Material
Acknowledgements
This work was supported by NIH/NIAID grant funding (PO1AI1089473) and a research grant from Johnson & Johnson as well as support from the Mary H. Weiser Food Allergy Center at the University of Michigan.
Footnotes
Author Contribution
The experiments were designed by NWL, SVL, WF, and SJ. Experiments were performed by WF, KL, SJ, AR, JP, KRB, and HT. Manuscript was written by NWL, SVL, WF, KL, KEF, HAB, EZ, DRO and CCJ. Data analysis was performed by NWL, SVL, WF, KEF, SJ, AML, and KL. All authors participated in editing the manuscript.
References
- 1.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16(5):386–392. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–1052. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 3.Marra F, Lynd L, Coombes M, Richardson K, Legal M, Fitzgerald JM, et al. Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. 2006;129(3):610–618. doi: 10.1378/chest.129.3.610. [DOI] [PubMed] [Google Scholar]
- 4.Crane J. Asthma, atopy, antibiotics and the bowel. Mediators Inflamm. 2001;10(6):304–305. doi: 10.1080/09629350152700975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattes J, Karmaus W. The use of antibiotics in the first year of life and development of asthma: which comes first? Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1999;29(6):729–732. doi: 10.1046/j.1365-2222.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 6.Modi SRCJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest. 2014;124(10):4212–4218. doi: 10.1172/JCI72333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalliomäki M1KP, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107(1):129–134. doi: 10.1067/mai.2001.111237. [DOI] [PubMed] [Google Scholar]
- 8.Penders JTC, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth CohortStudy. Gut. 2007;56(5):661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch SV, Boushey HA. The microbiome and development of allergic disease. Current opinion in allergy and clinical immunology. 2016;16(2):165–171. doi: 10.1097/ACI.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):805–810. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 12.Stier MT, Bloodworth MH, Toki S, Newcomb DC, Goleniewska K, Boyd KL, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. The Journal of allergy and clinical immunology. 2016;138(3):814–824. e811. doi: 10.1016/j.jaci.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saravia J, You D, Shrestha B, Jaligama S, Siefker D, Lee GI, et al. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS pathogens. 2015;11(10):e1005217. doi: 10.1371/journal.ppat.1005217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reed M, Morris SH, Owczarczyk AB, Lukacs NW. Deficiency of autophagy protein Map1-LC3b mediates IL-17-dependent lung pathology during respiratory viral infection via ER stress-associated IL-1. Mucosal immunology. 2015;8(5):1118–1130. doi: 10.1038/mi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science translational medicine. 2015;7(307):307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 16.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161(1):146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nature immunology. 2013;14(7):676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draper E, Reynolds CM, Canavan M, Mills KH, Loscher CE, Roche HM. Omega-3 fatty acids attenuate dendritic cell function via NF-kappaB independent of PPARgamma. The Journal of nutritional biochemistry. 2011;22(8):784–790. doi: 10.1016/j.jnutbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Kong W, Yen JH, Vassiliou E, Adhikary S, Toscano MG, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids in health and disease. 2010;9:12. doi: 10.1186/1476-511X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplowitz N, Aw TY, Ookhtens M. The regulation of hepatic glutathione. Annual review of pharmacology and toxicology. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 21.Maskrey BH, Megson IL, Rossi AG, Whitfield PD. Emerging importance of omega-3 fatty acids in the innate immune response: molecular mechanisms and lipidomic strategies for their analysis. Molecular nutrition & food research. 2013;57(8):1390–1400. doi: 10.1002/mnfr.201200723. [DOI] [PubMed] [Google Scholar]
- 22.Teague H, Rockett BD, Harris M, Brown DA, Shaikh SR. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology. 2013;139(3):386–394. doi: 10.1111/imm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flock MR, Rogers CJ, Prabhu KS, Kris-Etherton PM. Immunometabolic role of long-chain omega-3 fatty acids in obesity-induced inflammation. Diabetes/metabolism research and reviews. 2013;29(6):431–445. doi: 10.1002/dmrr.2414. [DOI] [PubMed] [Google Scholar]
- 24.Reed M, Morris SH, Jang S, Mukherjee S, Yue Z, Lukacs NW. Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. Journal of immunology. 2013;191(5):2526–2537. doi: 10.4049/jimmunol.1300477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang S, Smit J, Kallal LE, Lukacs NW. Respiratory syncytial virus infection modifies and accelerates pulmonary disease via DC activation and migration. Journal of leukocyte biology. 2013;94(1):5–15. doi: 10.1189/jlb.0412195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ptaschinski C, Mukherjee S, Moore ML, Albert M, Helin K, Kunkel SL, et al. RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis In Vivo. PLoS pathogens. 2015;11(6):e1004978. doi: 10.1371/journal.ppat.1004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholas C, Wegienka G, Havstad S, Zoratti E, Ownby D, Johnson CC. Dog characteristics and allergen levels in the home. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;105(3):228–233. doi: 10.1016/j.anai.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegienka G, Johnson CC, Havstad S, Ownby DR, Zoratti EM. Indoor pet exposure and the outcomes of total IgE and sensitization at age 18 years. The Journal of allergy and clinical immunology. 2010;126(2):274–279. 279, e271–275. doi: 10.1016/j.jaci.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegienka G, Havstad S, Zoratti EM, Woodcroft KJ, Bobbitt KR, Ownby DR, et al. Regulatory T cells in prenatal blood samples: variability with pet exposure and sensitization. Journal of reproductive immunology. 2009;81(1):74–81. doi: 10.1016/j.jri.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aichbhaumik N, Zoratti EM, Strickler R, Wegienka G, Ownby DR, Havstad S, et al. Prenatal exposure to household pets influences fetal immunoglobulin E production. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38(11):1787–1794. doi: 10.1111/j.1365-2222.2008.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Frontiers in immunology. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arrieta MC, Finlay B. The intestinal microbiota and allergic asthma. The Journal of infection. 2014;69(Suppl 1):S53–55. doi: 10.1016/j.jinf.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends in immunology. 2015;36(1):3–12. doi: 10.1016/j.it.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosiewicz MM, Dryden GW, Chhabra A, Alard P. Relationship between gut microbiota and development of T cell associated disease. FEBS letters. 2014;588(22):4195–4206. doi: 10.1016/j.febslet.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Geuking MB, McCoy KD, Macpherson AJ. Metabolites from intestinal microbes shape Treg. Cell research. 2013;23(12):1339–1340. doi: 10.1038/cr.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narushima S, Sugiura Y, Oshima K, Atarashi K, Hattori M, Suematsu M, et al. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut microbes. 2014;5(3):333–339. doi: 10.4161/gmic.28572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 40.Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Current opinion in immunology. 2012;24(4):392–397. doi: 10.1016/j.coi.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Li YN, Huang F, Liu L, Qiao HM, Li Y, Cheng HJ. Effect of oral feeding with Clostridium leptum on regulatory T-cell responses and allergic airway inflammation in mice. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2012;109(3):201–207. doi: 10.1016/j.anai.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. Commensal bacteria protect against food allergen sensitization. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(36):13145–13150. doi: 10.1073/pnas.1412008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 44.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1beta in human monocytes through TLR2 activation; modulation by dietary fatty acids. Journal of immunology. 2013;191(8):4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, et al. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-kappaB activation and enhancing autophagy. PloS one. 2014;9(6):e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legrand-Poels S, Esser N, L'Homme L, Scheen A, Paquot N, Piette J. Free fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetes. Biochemical pharmacology. 2014;92(1):131–141. doi: 10.1016/j.bcp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-kappaB pathway. Journal of cellular biochemistry. 2008;103(5):1452–1463. doi: 10.1002/jcb.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World journal of gastroenterology : WJG. 2007;13(20):2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, Capel TM, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. The Journal of allergy and clinical immunology. 2005;115(6):1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Durant LR, Makris S, Voorburg CM, Loebbermann J, Johansson C, Openshaw PJ. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. Journal of virology. 2013;87(20):10946–10954. doi: 10.1128/JVI.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fulton RB, Meyerholz DK, Varga SM. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. Journal of immunology. 2010;185(4):2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Advances in immunology. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity. 2015;42(6):1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 56.Wiese M, Eljaszewicz A, Helmin-Basa A, Andryszczyk M, Motyl I, Wieczynska J, et al. Lactic acid bacteria strains exert immunostimulatory effect on H. pylori-induced dendritic cells. Journal of immunology research. 2015;2015:106743. doi: 10.1155/2015/106743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amar Y, Rizzello V, Cavaliere R, Campana S, De Pasquale C, Barberi C, et al. Divergent signaling pathways regulate IL-12 production induced by different species of Lactobacilli in human dendritic cells. Immunology letters. 2015;166(1):6–12. doi: 10.1016/j.imlet.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Elawadli I, Brisbin JT, Mallard BA, Griffiths MW, Corredig M, Sharif S. Differential effects of lactobacilli on activation and maturation of mouse dendritic cells. Beneficial microbes. 2014;5(3):323–334. doi: 10.3920/BM2013.0066. [DOI] [PubMed] [Google Scholar]
- 59.von Ossowski I, Pietila TE, Rintahaka J, Nummenmaa E, Makinen VM, Reunanen J, et al. Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PloS one. 2013;8(5):e64416. doi: 10.1371/journal.pone.0064416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macho Fernandez E, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60(8):1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 61.Livingston M, Loach D, Wilson M, Tannock GW, Baird M. Gut commensal Lactobacillus reuteri 100-23 stimulates an immunoregulatory response. Immunology and cell biology. 2010;88(1):99–102. doi: 10.1038/icb.2009.71. [DOI] [PubMed] [Google Scholar]
- 62.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53(11):1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. Journal of immunology. 2002;168(1):171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 64.Chang PK, Khatchadourian A, McKinney RA, Maysinger D. Docosahexaenoic acid (DHA): a modulator of microglia activity and dendritic spine morphology. Journal of neuroinflammation. 2015;12:34. doi: 10.1186/s12974-015-0244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et biophysica acta. 2015;1851(4):469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40(6):833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 67.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature communications. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 68.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in immunology. 2015 doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annual review of physiology. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain, behavior, and immunity. 2011;25(5):872–882. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berndt BE, Zhang M, Owyang SY, Cole TS, Wang TW, Luther J, et al. Butyrate increases IL-23 production by stimulated dendritic cells. American journal of physiology Gastrointestinal and liver physiology. 2012;303(12):G1384–1392. doi: 10.1152/ajpgi.00540.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.