Abstract
Folic acid supplementation confers modest benefit in schizophrenia, but its effectiveness is influenced by common genetic variants in the folate pathway that hinder conversion to its active form. We examined physiologic and clinical effects of L-methylfolate, the fully reduced and bioactive form of folate, in schizophrenia. In this randomized, double-blind trial, outpatients with schizophrenia (n=55) received L-methylfolate 15 mg or placebo for 12 weeks. Patients were maintained on stable doses of antipsychotic medications. The pre-defined primary outcome was change in plasma methylfolate at 12 weeks. Secondary outcomes included change in symptoms (PANSS, SANS, CDSS), cognition (MATRICS composite) and three complementary MRI measures (working memory-related activation, resting connectivity, cortical thickness). Primary, mixed model, intent-to-treat analyses covaried for six genetic variants in the folate pathway previously associated with symptom severity and/or response to folate supplementation. Analyses were repeated without covariates to evaluate dependence on genotype. Compared to placebo, L-methylfolate increased plasma methylfolate levels (d=1.00, p=.0009) and improved PANSS Total (d=.61, p=.03) as well as PANSS Negative and General Psychopathology subscales. While PANSS Total and General Psychopathology changes were influenced by genotype, significant PANSS Negative changes occurred regardless of genotype. No treatment differences were seen in other symptom rating scales or cognitive composite scores. Patients receiving L-methylfolate exhibited convergent changes in ventromedial prefrontal physiology, including increased task-induced deactivation, altered limbic connectivity, and increased cortical thickness. In conclusion, L-methylfolate supplementation was associated with salutary physiologic changes and selective symptomatic improvement in this study of schizophrenia patients, warranting larger clinical trials.
Keywords: schizophrenia, folate, MRI, randomized clinical trial, negative symptoms, genetics
Introduction
Evidence from clinical, genetic, and brain imaging studies has implicated altered folic acid metabolism in schizophrenia. Patients with schizophrenia frequently exhibit reduced blood folate levels (1, 2), which have in turn associated with more severe negative symptoms (3). This relationship is especially pronounced in patients who carry low-functioning genetic variants, such as MTHFR 677C>T, in the folate pathway (4, 5). The MTHFR 677T variant has also been associated with altered activation in prefrontal networks during executive function tasks (6, 7).
Folic acid supplementation has been proposed as an adjunctive treatment for schizophrenia, with the idea that correcting underlying metabolic deficiencies could improve clinical outcomes. Three previous randomized, placebo-controlled clinical trials of folic acid supplements have suggested efficacy, although results have been modest and difficult to generalize. An initial cross-over study of schizophrenia patients treated with a multivitamin that included folic acid indicated improvement in Positive and Negative Syndrome Scale (PANSS) total score, although enrollment was limited to patients with elevated baseline homocysteine levels (8). Two subsequent studies of folic acid with or without vitamin B12 indicated small but significant effects on negative symptoms, but the effects were limited to patients who carried certain genetic variants such as MTHFR 677T (9, 10).
Conversion of folic acid to its fully active form, L-methylfolate, requires the action of MTHFR and other enzymes in the folate pathway (Figure S1). The efficacy of folic acid may be reduced for individuals who carry low-functioning variants in these enzymes, which have been associated with altered blood folate levels both at baseline and following folic acid treatment (10, 11). Conversely, L-methylfolate bypasses several of these enzymes. Compared to folic acid, L-methylfolate may reduce variation in the physiologic and clinical responses to supplementation that would otherwise occur due to the presence of low-functioning genetic variants in certain individuals.
The present study, a randomized, double-blind, parallel arm, placebo-controlled clinical trial, evaluated biochemical, physiologic, and clinical effects of 12 weeks of 15 mg oral L-methylfolate supplementation in medicated outpatients with schizophrenia. The primary endpoint was change in blood methylfolate level; secondary endpoints included change in symptoms, cognition, and structural and functional MRI indices relevant to schizophrenia. We also explored the dependence of outcomes on common, functional genetic variants in six folate-related enzymes, each of which had been associated with variation in negative symptoms and/or response to folate supplementation in other work published during the course of the trial (4, 10, 12).
Methods and Materials
Study Design
This was a single-site, randomized, double-blind, placebo-controlled, parallel-group, 12-week trial of supplementation with L-methylfolate conducted at an urban community mental health clinic in Boston from May 2009 to May 2014. The double-blind portion of the trial was immediately followed by an open-label 12-week extension where all participants received L-methylfolate, primarily to assess safety outcomes. Following a screening visit, eligible participants completed a two-week single-blind placebo lead-in phase, after which clinical inclusion criteria were re-assessed (baseline visit). Study procedures were approved by the Partners HealthCare Human Research Committee. Enrollment continued until the pre-specified endpoint was reached (50 Week 12 completers).
Participants
Eligible participants had a diagnosis of schizophrenia, as confirmed with the Structured Clinical Interview for DSM-IV-TR, and provided written informed consent. Please refer to Supplementary Methods for inclusion and exclusion criteria and randomization procedures.
Study Visits
Subjects returned to the clinic for follow-up visits at Weeks 2, 4, 6, 8, 10, 12, and 24 following the Baseline visit. Medication was dispensed and interval adverse events were recorded at each visit. Blood for plasma methylfolate, homocysteine, methionine, and B12 levels was drawn at Baseline and Weeks 2, 8, 12, and 24. Clinical measurements, including the PANSS, Scale for Assessment of Negative Symptoms (SANS), and Calgary Depression Scale for Schizophrenia (CDSS) were collected at Baseline and Weeks 2, 8, 12, and 24. Subjects also completed the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) battery at Baseline and at Weeks 6, 12, and 24. Blood was collected for genotyping at the baseline visit.
MRI
Additional details of MRI acquisition, stimulus presentation, and pre-processing methods are described in Supplementary Methods. Briefly, eligible patients underwent MRI scans at 3T (Siemens TIM Trio with a 12 channel quadrature head coil) within a two-week window before the baseline visit and again within two weeks after the Week 12 visit. Pre- and post-treatment scans were identical except for specific working memory stimuli.
A high resolution T1 image was obtained to measure cortical thickness and for co-registration of functional scans. Resting state functional MRI (fMRI) data were acquired during a standard six minute sequence (13). Patients were instructed to keep their eyes open and lay as still as possible.
Task-based fMRI was conducted during performance of a version of the Sternberg Item Recognition Paradigm (SIRP), which evaluated working memory function at four different loads (1, 3, 5, or 7 consonants; Figure S2). Stimuli were presented with Eprime 1.1 software. The SIRP has been associated with linear increases in task-positive (e.g., frontoparietal) network activation, and in task-negative (e.g., limbic/default) network deactivation as a function of working memory load (14–16), and with minimal learning effects (17). Prior to scanning, subjects performed a practice run to ensure they understood the task.
Genotype and Blood Chemistry
DNA extracted from whole blood samples was genotyped (Sequenom MassARRAY) for six variants within folate-related genes: MTHFR (rs1801133), MTR (rs1805087), FOLH1 (rs202676), COMT (rs4680), DHFR (rs2618372), and GCH1 (rs8007267). These variants were selected because they previously associated with symptom severity in schizophrenia (4, 5), with response to folic acid supplementation in schizophrenia (9, 10), or with response to L-methylfolate supplementation in depression (12). Figure S2 depicts the location of each marker within the folate pathway.
Liquid chromatography with tandem mass spectroscopy measured plasma methylfolate (18), homocysteine (19), and methionine (20). Plasma vitamin B12 levels were measured using the SimulTRAC-SNB radioassay kit (MP Diagnostics, Orangeburg, NY).
Outcomes
Primary analysis focused on the double-blind part of the trial. A biochemical measure, change in plasma methylfolate, was the pre-defined primary endpoint in this first dedicated study of L-methylfolate in schizophrenia patients. Genetic alteration of enzymes situated both upstream and downstream of where methylfolate enters into the one carbon cycle have been implicated in schizophrenia (4). This, coupled with the well-known problem of treatment non-adherence in schizophrenia, could complicate examination of other clinical or physiologic endpoints without documented changes in plasma levels. Secondary endpoints included changes in clinical measures that had previously been responsive to folic acid (SANS, excluding the Attention Subscale and Inappropriate Affect item (21); PANSS), a single global measure of cognitive change (MATRICS composite score), and changes in plasma homocysteine, methionine, and vitamin B12 levels. Change in depression score (CDSS) was included to rule out confounding effects of improved mood on other clinical outcomes, given the previously reported improvement in depression with 15 mg L-methylfolate (22). Finally, we included three complementary MRI measures – working memory-related activation, cortical thickness, and ventromedial prefrontal cortex (vmPFC) connectivity – that have consistently been found to differ between schizophrenia patients and healthy individuals (14, 15, 23, 24), to determine whether L-methylfolate restored more normal brain physiology and structure.
Statistical Analysis
The target sample size of 50 completers provided power of 80% to detect an effect size of 0.8 between groups with alpha (two-tailed) set at 0.05 and allowing for a 10% drop-out rate. Within- and between-group differences for change in blood chemistry and clinical measures were assessed using linear mixed model analysis with unstructured variance-covariance structure (SAS v9.2) that included all randomized participants (intent-to-treat) and was centered at week 12. The dependent variable was difference from baseline, and baseline values and week of measurement were included as covariates. To test for the dependence of main effects on genotype, parallel analyses were conducted with and without the simultaneous entry of genotypes into the model as covariates, with two levels per genotype (homozygous for the major allele versus heterozygous + homozygous for the minor allele). Variables that demonstrated significant differences between treatment groups were subject to additional analyses that included covariates for any demographic or clinical variables that differed significantly at baseline between treatment groups. Exploratory analyses evaluated specific genotype × treatment interactions among measures showing significant treatment effects.
Additional details of MRI statistical analysis are described in the Supplementary Methods section. Briefly, for the task-based fMRI analysis, we focused on recall (probe presentation and response) time points and used a general linear model to determine at each voxel the extent to which linear activation was observed as load was parametrically increased from one to seven items (25). Parameter estimates and residual errors were computed for each subject, at each time point, and then subtracted to create a difference map for each subject. Difference maps were entered into a random-effects group analysis to determine whether treatment-related changes differed between treatment groups. The same general approach was used to evaluate treatment-related changes in cortical thickness: first-level analysis created subtraction maps for each subject, and second-level analysis contrasted cortical thickness changes between treatment groups. To correct for multiple comparisons, 10,000 Monte Carlo simulations of synthesized white Gaussian noise were conducted across the brain volume (for task-based fMRI) or surface (for cortical thickness) to determine whether clusters of a certain size at a certain threshold (p<.05) would be found by chance (cluster-wise probability). These methods set the corrected overall probability level to 0.05 (two-tailed).
For resting-state connectivity, we examined correlation in blood oxygen level dependent signal time course between seed regions in the left and right ventromedial prefrontal cortex (vmPFC) and the orbitofrontal cortex (OFC). The vmPFC seeds, 6 mm spheres (centered at MNI coordinates x=−7, y=32, z=−13 and x=5, y=22, z=−8), were generated in a previous study (25) of 40 schizophrenia patients (24 overlapping with baseline scans in the current sample) and 40 demographically matched control subjects based on the maximal difference between patients and controls on SIRP-induced deactivation during correct performance; in that analysis, patients demonstrated abnormally increased connectivity between both vmPFC seeds and lateral OFC (lOFC). Here, using the same within-subject approach described above, we determined whether pre- to post-treatment vmPFC to OFC connectivity changes differed between treatment groups. Correction for multiple comparisons within the OFC was implemented using AlphaSim software, setting the corrected clusterwise alpha at 0.05.
Post hoc analyses of relationships among significant clinical, biochemical, and imaging findings were corrected using the false discovery rate (Supplementary Methods).
Results
Patients
Between May 2009 and September 2013, 78 patients were screened, 61 were eligible, and 55 were randomized to L-methylfolate (n=29) or placebo (n=26). Enrollment continued until 50 subjects completed the week 12 assessment. Five subjects discontinued the study before week 12; of these, four were lost to follow-up and one was terminated due to hospitalization for increased psychosis (91% completion rate; Figure S3). 44 subjects completed the 12-week open-label extension. Treatment groups did not differ at baseline for any clinical, cognitive, biochemical, or genotype measure (Table 1), excepting anticonvulsant use, which was more common in the L-methylfolate group. Genotypes were in Hardy Weinberg equilibrium, with the exception of GCH1 (χ2=4.00, p=.045), which reflected previously described differences in allele frequency between racial groups (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=8007267).
Table 1.
Baseline demographic, clinical, biochemical, and genotype measures
| Measure | L-Methylfolate | Placebo | Statistics | p-value |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age, years | 46.3 (9.2) | 44.7 (12.9) | t=0.54 | n.s. |
| Male, % | 82.8 | 73.1 | χ2=0.75 | n.s. |
| Caucasian, % | 58.6 | 69.2 | χ2=0.67 | n.s. |
| Duration of illness, years | 19.4 (10.3) | 21.4 (12.3) | t=0.63 | n.s. |
| Schooling, years | 13.2 (2.3) | 12.2 (1.7) | t=1.96 | n.s. |
| Current smoker, % | 48.3 | 38.5 | χ2=0.54 | n.s. |
| MEDICATION USE | ||||
| Typical antipsychotics, % | 31.0 | 15.4 | χ2=1.86 | n.s. |
| Atypical antipsychotics, % | 79.3 | 96.2 | χ2=3.50 | n.s. |
| Antidepressants, % | 41.4 | 46.2 | χ2=0.13 | n.s. |
| Anticonvulsants, % | 44.8 | 15.4 | χ2=5.66 | p=0.02 |
| SYMPTOMS/COGNITION | ||||
| PANSS total | 74.5 (12.6) | 79.7 (15.9) | t=1.36 | n.s. |
| PANSS positive | 18.0 (5.1) | 19.7 (6.0) | t=1.13 | n.s. |
| PANSS negative | 20.8 (5.4) | 22.3 (4.4) | t=1.10 | n.s. |
| PANSS general | 35.6 (6.1) | 37.7 (8.5) | t=1.05 | n.s. |
| SANS total | 32.7 (15.0) | 36.3 (10.1) | t=1.03 | n.s. |
| CDSS total | 3.0 (2.4) | 2.3 (2.6) | t=1.02 | n.s. |
| MATRICS composite total | 30.6 (15.6) | 26.7 (14.9) | t=0.94 | n.s. |
| BLOOD CHEMISTRIES | ||||
| Plasma methylfolate, nmol/L | 40.7 (28.1) | 46.1 (29.4) | t=0.69 | n.s. |
| Plasma homocysteine, μmol/L | 17.8 (6.5) | 17.2 (5.4) | t=0.36 | n.s. |
| Plasma methionine, μmol/L | 31.6 (15.5) | 28.5 (12.9) | t=0.79 | n.s. |
| Plasma B12, pmol/L | 543 (279) | 448 (215) | t=1.39 | n.s. |
| GENOTYPES | ||||
| MTHFR rs1801133 CC/T-carrier, % | 46.4/53.6 | 46.2/53.8 | χ2=0.00 | n.s. |
| MTR rs1805087 AA/G-carrier, % | 67.9/32.1 | 53.8/46.2 | χ2=1.11 | n.s. |
| FOLH1 rs202676 TT/C-carrier, % | 42.9/57.1 | 57.7/42.3 | χ2=1.19 | n.s. |
| COMT rs4680 GG/A-carrier, % | 32.1/67.9 | 23.1/76.9 | χ2=0.55 | n.s. |
| DHFR rs2618372 CC/A-carrier, % | 63.0/37.0 | 56.0/44.0 | χ2=0.26 | n.s. |
| GCH1 rs8007267 CC/T-carrier, % | 48.1/51.9 | 56.0/44.0 | χ2=0.32 | n.s. |
Values given as mean (standard deviation) unless otherwise noted. PANSS=Positive and Negative Syndrome Scale, SANS=Scale for Assessment of Negative Symptoms, CDSS=Calgary Depression Scale for Schizophrenia, MATRICS=Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery.
Biochemistry
When including genotypes (Table 2), plasma methylfolate levels increased significantly in the L-methylfolate group (mean change=450 nmol/L, 95% C.I.=265 to 635, p<.0001) but not the placebo group, yielding a significant between-group difference (mean difference=446 nmol/L, 95% C.I.=197 to 695, p=.0009, Cohen’s d=1.00; see Table 2). Plasma homocysteine levels fell within the L-methylfolate group but the decrease was not significantly different than the change in the placebo group. Plasma methionine levels decreased slightly in both groups but changes did not reach significance. Plasma B12 levels did not change in either group. Inclusion of anticonvulsant use as a covariate did not affect within- and between-group changes in plasma methylfolate levels, which remained significant (Table S1). Changes in biochemistry were not substantially different when genotypes were removed from the model (Table 3).
Table 2.
Change from Baseline to Week 12 for biochemical, clinical, and cognitive measures, adjusted for genotypes
| Measure | df | L-Methylfolate | Placebo | Difference |
|---|---|---|---|---|
| BLOOD CHEMISTRIES | ||||
| Plasma methylfolate, nmol/L | 36 | 450 (265 to 635), p<.0001 | 5 (−163 to 172), p=.96 | 446 (197 to 695), p=.0009 |
| Plasma homocysteine, μmol/L | 36 | −3.9 (−7.4 to −0.4), p=.03 | −0.9 (−4.1 to 2.2), p=.55 | −3.0 (−7.6 to 1.7), p=.21 |
| Plasma methionine, μmol/L | 36 | −4.3 (−12.6 to 4.0), p=.91 | −4.1 (−9.5 to 1.4), p=.31 | −0.2 (−10.6 to 9.6), p=.92 |
| Plasma B12, pmol/L | 36 | −57 (−139 to 25), p=.17 | −2 (−78 to 75), p=.97 | −55 (−167 to 56), p=.32 |
| SYMPTOMS/COGNITION | ||||
| PANSS total | 37 | −7.0 (−11.1 to −3.0), p=.001 | −0.9 (−4.5 to 2.7), p=.62 | −6.1 (−11.6 to −0.6), p=.03 |
| PANSS positive | 37 | −1.5 (−3.3 to 0.3), p=.10 | −1.3 (−2.9 to 0.4), p=.13 | −0.2 (−2.8 to 2.3), p=.84 |
| PANSS negative | 37 | −1.9 (−3.7 to −0.1), p=.04 | 1.1 (−0.6 to 2.8), p=.19 | −3.0 (−5.4 to −0.5), p=.02 |
| PANSS general | 37 | −4.5 (−6.6 to −2.3), p=.0001 | −0.1 (−2.1 to 1.8), p=.88 | −4.3 (−7.2 to −1.4), p=.004 |
| SANS total | 37 | 1.5 (−2.6 to 5.6), p=.46 | 2.2 (−1.6 to 6.0), p=.25 | −0.7 (−6.3 to 4.9), p=.80 |
| CDSS total | 37 | 0.3 (−1.1 to 1.6), p=.68 | −0.4 (−1.6 to 0.8), p=.49 | 0.7 (−1.1 to 2.5), p=.44 |
| MATRICS composite total | 34 | 1.2 (−1.5 to 4.0), p=.37 | 0.2 (−2.4 to 2.8), p=.90 | 1.1 (−2.7 to 4.9), p=.57 |
Values given as mean (95% confidence interval). df=degrees of freedom, PANSS=Positive and Negative Syndrome Scale, SANS=Scale for Assessment of Negative Symptoms, CDSS=Calgary Depression Scale for Schizophrenia, MATRICS=Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery.
Table 3.
Change from Baseline to Week 12 for biochemical, clinical, and cognitive measures, not adjusted for genotypes
| Measure | df | L-Methylfolate | Placebo | Difference |
|---|---|---|---|---|
| BLOOD CHEMISTRIES | ||||
| Plasma methylfolate, nmol/L | 51 | 582 (400 to 765), p<.0001 | −1 (−183 to 181), p=.99 | 583 (326 to 841), p<.0001 |
| Plasma homocysteine, μmol/L | 51 | −2.8 (−5.5 to −0.2), p=.03 | 0.6 (−2.0 to 3.2), p=.64 | −3.5 (−7.2 to 0.2), p=.07 |
| Plasma methionine, μmol/L | 51 | −3.4 (−10.7 to 4.0), p=.36 | −4.0 (−10.0 to 2.0), p=.16 | 0.6 (−8.6 to 9.9), p=.89 |
| Plasma B12, pmol/L | 51 | −26 (−86 to 34), p=.38 | 8 (−53 to 69), p=.79 | −34 (−120 to 52), p=.43 |
| SYMPTOMS/COGNITION | ||||
| PANSS total | 52 | −4.7 (−8.2 to −1.3), p=.008 | −1.5 (−5.0 to 1.9), p=.38 | −3.2 (−8.1 to 1.7), p=.19 |
| PANSS positive | 52 | −1.2 (−2.4 to 0.1), p=.07 | −1.5 (−2.8 to −0.2), p=.02 | 0.3 (−1.5 to 2.1), p=.71 |
| PANSS negative | 52 | −1.3 (−2.6 to 0.0), p=.05 | 0.9 (−0.4 to 2.2), p=.19 | −2.1 (−4.0 to −0.3), p=.02 |
| PANSS general | 52 | −2.5 (−4.7 to −0.4), p=.02 | −0.6 (−2.8 to 1.6), p=.60 | −2.0 (−5.1 to 1.1), p=.21 |
| SANS total | 52 | −0.5 (−3.5 to 2.5), p=.73 | 2.4 (−0.6 to 5.4), p=.11 | −2.9 (−7.1 to 1.3), p=.17 |
| CDSS total | 52 | −0.0 (−1.0 to 0.9), p=.95 | −0.7 (−1.7 to 0.2), p=.12 | 0.7 (−0.6 to 2.0), p=.29 |
| MATRICS composite total | 48 | 1.1 (−1.0 to 3.1), p=.31 | −0.4 (−2.6 to 1.8), p=.70 | 1.5 (−1.5 to 4.5), p=.33 |
Values given as mean (95% confidence interval). df=degrees of freedom, PANSS=Positive and Negative Syndrome Scale, SANS=Scale for Assessment of Negative Symptoms, CDSS=Calgary Depression Scale for Schizophrenia, MATRICS=Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery.
Changes in Symptoms and Cognition
When including genotype covariates (Table 2), PANSS total score declined significantly in the L-methylfolate group compared to baseline (mean change=−7.0 points, 95% C.I. −11.1 to −3.0, p=.001) and changed significantly more than in the placebo group (mean difference=−6.1 points, 95% C.I. −11.6 to −0.6, p=.03, Cohen’s d=0.63). Statistically significant improvement was also evident for the PANSS negative (d=0.68) and general psychopathology (d=0.84) subscales (Table 2). Significant between-group differences persisted when including anticonvulsant use as a covariate (Table S1). No significant within- or between-group changes were observed for PANSS positive, SANS, CDRS, or MATRICS composite scores (Table 2). When genotype covariates were excluded, change in methylfolate and PANSS negative continued to differ between treatment groups, but between-group differences for changes in PANSS total and general psychopathology became non-significant (Table 3).
Interactions of Significant Variables with Genotype
For plasma methylfolate and PANSS negative, no significant treatment × genotype interactions were observed, suggesting that treatment effects occurred independent of genotype. For PANSS total and general psychopathology, significant treatment × genotype interactions were observed only for the MTR 2756A>G variant, where improvement was seen only among patients who received L-methylfolate and who were homozygous for the 2756G allele (Table S2).
Changes in MRI Measures
A total of 35 patients (20 L-methylfolate, 1/20 left-handed; and 15 placebo, 2/15 left-handed) had usable pre- and post-treatment scans for structural and resting-state MRI analyses. Of these, 25 patients (13 L-methylfolate, 0/13 left-handed; and 12 placebo, 0/12 left-handed) met criteria for inclusion in the task-based MRI analysis based on above-chance performance and minimal head motion. Head motion did not differ between groups (Table S3).
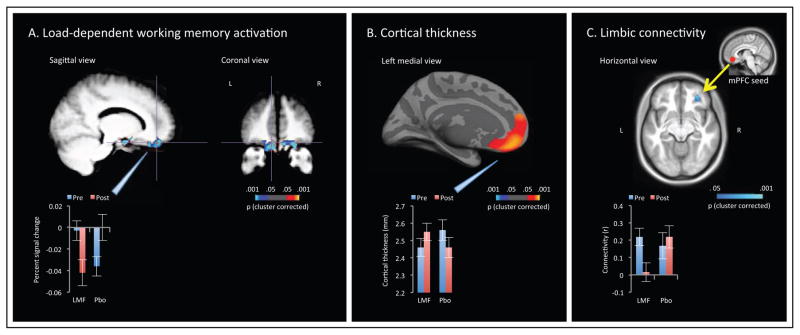
Performance on the working memory task did not differ between groups at baseline, and did not change significantly over time in either group (Table S4). Omnibus voxel-wise comparison of pre- and post-treatment scans in the L-methylfolate and placebo groups indicated a single, large cluster encompassing bilateral medial orbitofrontal cortex (mOFC), lateral orbitofrontal cortex (lOFC), and nucleus accumbens, with a significant time × treatment interaction following whole-brain correction for multiple comparisons (Table S5). The mOFC normally deactivates during working memory tasks, although blunted deactivation is observed in patients with schizophrenia and unaffected siblings (26). Here, patients receiving L-methylfolate exhibited potentiated deactivation following treatment, while the reverse pattern was evident in patients receiving placebo (Figure 1a).
Figure 1.
Time × treatment effects on MRI measures. Bar graphs indicate mean and standard error for extracted values within significant clusters. LMF=L-methylfolate, PBO=placebo. (a) Working memory load-dependent activation/deactivation during the Sternberg Item Recognition Paradigm. Visualized cluster survived whole-volume correction for multiple comparisons. (b) Cortical thickness. Visualized cluster survived surface-wide correction for multiple comparisons. (c) Functional connectivity between a seed region in the left ventromedial prefrontal cortex (vmPFC) and the entire orbitofrontal cortex (OFC). Visualized cluster survived small-volume correction within the OFC.
Omnibus vertex-wise comparison of structural MRI scans indicated two clusters with significant time × treatment interactive effects on cortical thickness, following surface-wide correction for multiple comparisons (Table S6). The larger cluster encompassed the left medial prefrontal cortex, with the largest effect seen in the mOFC, reflecting pre- to post-treatment cortical thickness increases in the L-methylfolate group but not the placebo group (Figure 1b). The smaller cluster, located in the right interior and middle temporal gyri, was characterized by the inverse pattern.
Finally, a focused functional connectivity analysis of resting-state MRI scans was then performed, examining connectivity between seed regions in the left and right vmPFC and the entire orbitofrontal cortex. Pre-to post-treatment connectivity differed between groups between the left vmPFC and a single cluster within the right lOFC; patients receiving L-methylfolate exhibited a pre-to-post treatment reduction in connectivity between these limbic regions that was not evident among patients receiving placebo (Figure 1c, Table S7). A similar pattern was observed using the right vmPFC seed, which demonstrated treatment-related reduction in connectivity to right lOFC at trend level. A third cluster, reflecting left vmPFC connectivity to left lOFC, displayed the inverse pattern. Exploratory whole-brain analyses found no additional clusters that reached significance.
All significant imaging findings persisted when analyses were adjusted for genotype (Table S8).
Adverse Events
Treatment-emergent adverse events were mild and did not differ significantly between the L-methylfolate and placebo groups (Table S9). One subject in each treatment group was hospitalized for worsening symptoms during the double-blind phase.
Supplementary Data
Please refer to Supplementary Methods, Results and Tables S10–S14 for post hoc analyses of negative symptom variance; post hoc analyses relating significant clinical and imaging changes to each other as well as to methylfolate levels; and results from the 12-week open-label extension.
Discussion
Supplementation with 15 mg daily L-methylfolate was associated with improvements in several key biochemical, clinical, and brain imaging measures in this randomized clinical trial. In the setting of significant increases in blood methylfolate levels, patients who received L-methylfolate exhibited reductions in PANSS total, general psychopathology, and negative scores that were of moderate-to-large effect size, although modest in absolute change. Patients in the L-methylfolate group also exhibited convergent changes in medial prefrontal structure and function that suggest a partial normalization of limbic dysfunction.
In the only previous randomized trial of methylfolate in schizophrenia, Godfrey and colleagues (27) reported a significant decrease on a 4-point scale of global clinical outcome in patients who received 15 mg daily methylfolate for 6 months. That smaller study involved 17 patients, each of whom had substantial deficits in red blood cell folate (<200 μg/L) at the time of randomization. The same trial also included 24 patients with depression, who also demonstrated significant improvement on the same global scale. A more recent placebo-controlled study of L-methylfolate augmentation of SSRIs in depression indicated a significant benefit (response rate, change in depression score) at a dose of 15 mg after 30 days (22).
To minimize the possibility that changes in schizophrenia symptoms might reflect improvement in depression, the present study excluded patients with active depression, and changes in depression score were monitored with the CDSS. There were no significant differences between treatment groups on change in CDSS scores, suggesting that the observed improvements in PANSS scores did not reflect L-methylfolate related improvement in depression.
Several differences emerge when contrasting the present results to previous studies of folic acid supplementation in schizophrenia. Both Hill et al. (9) and Roffman et al. (10) reported improvement in negative symptoms, as measured with the SANS, after 12 or 16 weeks of treatment with 2 mg folic acid; however, in the Hill et al. study, improvement occurred only in patients who carried the MTHFR 677T allele, and in the Roffman et al. study, SANS improvement was only significant after including MTHFR and other genotypes into the model. Here, L-methylfolate was associated with significant improvement in PANSS negative score regardless of genotype, although significant differences were not seen for the SANS. While PANSS negative and SANS scores correlated strongly, and both scales saw greater improvement in expressive (rather than social amotivation) symptoms, significantly greater variance in SANS ratings may have hampered detection of treatment effects in this relatively small sample.
Further, as in Levine and colleagues’ (8) study of vitamin supplementation in schizophrenia (which included 2 mg folic acid, but studied only patients with elevated homocysteine), PANSS total scores improved significantly, albeit modestly, following active treatment. Here, though, improvement in both PANSS total and PANSS general psychopathology scores were dependent on genotype, and specifically on a non-synonymous variant in MTR that has been previously associated with altered homocysteine metabolism (28). MTR, which generates one-carbon moieties through the remethylation of homocysteine to methionine, is situated immediately downstream of where L-methylfolate enters into the folate metabolic pathway (Figure S2). It remains unclear why changes in these specific PANSS measures, and not in PANSS negative, should be dependent on MTR genotype, although notably the same variant also influenced response to L-methylfolate in depression, and in the same direction (more improvement in G-allele carriers) (12). It is also notable that genetic variants that occur upstream of where L-methylfolate enters into the folate pathway (e.g., FOLH1, MTHFR) did not influence treatment response for any measure showing a significant between-group difference. This pattern suggests that functional genetic variants in these enzymes may not affect treatment response to L-methylfolate in the way that they do for folic acid, which enters into the pathway further upstream (9, 10). That said, the lack of significant treatment effects on homocysteine or methionine suggests that metabolic dysfunction downstream of where methylfolate enters into the one carbon cycle – potentially due to variants that were not included in the model – may ultimately influence efficacy of L-methylfolate in some patients.
The present study also included pre- and post-treatment MRI measures to provide structural and functional brain correlates of treatment response. Altered activation of the dorsolateral prefrontal cortex (dlPFC) and other regions within the frontoparietal control network (FPCN) during working memory performance and other executive function tasks have been well documented in schizophrenia, and have also been related to MTHFR 677C>T genotype (6, 7). More recently, impaired deactivation of task-negative regions, such as the medial prefrontal cortex (mPFC) has also been described in schizophrenia (16, 26, 29). A recent study of 40 schizophrenia patients and 40 demographically matched controls conducted by our group indicated that while altered FPCN activation in schizophrenia may largely reflect between-group differences in task performance, impaired deactivation of vmPFC (including mOFC) appears to be a trait difference that distinguishes patients from controls regardless of task performance (25). The same study found differences in resting-state connectivity within the vmPFC, characterized by increased connectivity to lOFC in patients. This pattern was consistent with other recent work demonstrating hyperconnectivity of limbic regions in schizophrenia (30, 31).
Following treatment, patients who received L-methylfolate demonstrated a significantly greater potentiation of medial and lateral OFC deactivation during working memory compared to those who received placebo. This was the only significant finding to emerge from our whole-brain fMRI analysis following correction for multiple comparisons. L-methylfolate was also associated with changes in resting-state connectivity between vmPFC and lOFC that were mostly consistent with dampened limbic hyperconnectivity. Finally, L-methylfolate was also associated with an increase in cortical thickness throughout the mPFC. Collectively, these findings suggest a partial restoration of mPFC structure and function following L-methylfolate treatment. Lack of improvement on MATRICS or working memory indices may suggest a dissociation of MRI and cognitive results. Alternatively, MRI changes may be a more sensitive measure of treatment-induced neuroplasticity; if so, cognitive improvement occurring downstream of neuroplastic changes may be detectable in a larger sample. Structural and functional changes also did not correlate with symptom change, although again this relatively small sample was potentially underpowered to observe such a relationship.
Similarly, changes in blood methylfolate level did not correlate with changes in clinical and brain imaging measures that differed significantly between treatment groups. While this pattern could also reflect insufficient power, an alternative possibility is that blood and central nervous system (CNS) methylfolate levels do not correlate, especially at higher concentrations. This pattern has been demonstrated for unmethylated folate, where CNS levels plateau due to tight regulation by transporters at the blood brain barrier (32). Here, clinical and MRI changes may have reflected rise in methylfolate up to, but not beyond, CNS saturation.
While the results of this study are promising with regard to the use of supplemental L-methylfolate in schizophrenia, several limitations should be acknowledged. With a single site, it is unclear whether the present results would generalize to a larger population. Statistically significant clinical improvements were still modest, although relatively small rating changes have been described as clinically meaningful in other samples with moderately severe baseline symptoms (33, 34). The small sample increases the risk for Type II error, particularly in regard to specific genotype interaction results, which should be regarded as exploratory. With regard to Type I error, we did not include a formal correction for multiple comparisons across secondary clinical outcome measures, although several outcomes of interest (SANS, PANSS) were not independent, and another (CDSS) was included to rule out confounding effects of improvement in depression. Treatment groups differed in use of anticonvulsants, which can affect folate metabolism (35, 36), although significant clinical effects persisted after adjustment for anticonvulsant status. Finally, between-group differences could potentially reflect differences in dietary folate intake, which was not measured due to the poor reliability of nutritional questionnaires for B vitamins (37); however, that the groups maintained comparable blood levels of vitamin B12, which is frequently found in the same fortified food products as folic acid (38, 39), argues against this concern.
Although replication in larger samples will be necessary to address these limitations, the present results demonstrate potential for L-methylfolate supplementation to improve both clinical and neurophysiologic outcomes in schizophrenia. Further, they suggest that L-methylfolate-related improvement in negative symptoms, a particularly disabling syndrome in schizophrenia, may be less dependent on common genetic variants in the folate pathway compared to other folate-based interventions.
Supplementary Material
Acknowledgments
We are grateful to the study participants and their families. We also thank Lisa Raeke, Leah Briggs, and Claire Oppenheim for administrative support and Franklin Huntington for assistance with the manuscript. The study was funded by Pamlab and NIMH (R01MH101425, S10RR023043, S10RR023401, K24MH094614). The funders of the study had no role in study design, patient recruitment, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication. Data were presented in part at the American College of Neuropsychopharmacology Annual Meeting, Phoenix, AZ, December 2014; and the American Society of Clinical Psychopharmacology Annual Meeting, Miami Beach, FL, June 2015.
Footnotes
Financial Disclosures
Dr. Roffman reports receiving grant support for this study from Pamlab, and has served as a consultant for Pamlab. Dr. Bottiglieri reports receiving grant support for this study from Pamlab. Dr. Smoller is an unpaid member of the Scientific Advisory Board of PsyBrain, Inc. Dr. Henderson reports personal fees from Otsuka Phamaceutical, McLean Hospital, and Global CME; and grants from Novartis, Forum, and Reckitt Benckiser; all outside the submitted work. Dr. Goff reports receiving grant support for this study from Pamlab. Drs. Goff and Roffman have a US patent application 13/885,337, “Treating schizophrenia”, that relates to the use of folic acid to treat schizophrenia. The remaining authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Herran A, Garcia-Unzueta MT, Amado JA, Lopez-Cordovilla JJ, Diez-Manrique JF, Vazquez-Barquero JL. Folate levels in psychiatric outpatients. Psychiatry Clin Neurosci. 1999;53:531–533. doi: 10.1046/j.1440-1819.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 2.Koren G, Cohn T, Chitayat D, Kapur B, Remington G, Reid DM, et al. Use of atypical antipsychotics during pregnancy and the risk of neural tube defects in infants. Am J Psychiatry. 2002;159:136–137. doi: 10.1176/appi.ajp.159.1.136. [DOI] [PubMed] [Google Scholar]
- 3.Goff DC, Bottiglieri T, Arning E, Shih V, Freudenreich O, Evins AE, et al. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161:1705–1708. doi: 10.1176/appi.ajp.161.9.1705. [DOI] [PubMed] [Google Scholar]
- 4.Roffman JL, Brohawn DG, Nitenson AZ, Macklin EA, Smoller JW, Goff DC. Genetic variation throughout the folate metabolic pathway influences negative symptom severity in schizophrenia. Schizophr Bull. 2013;39:330–338. doi: 10.1093/schbul/sbr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roffman JL, Weiss AP, Purcell S, Caffalette CA, Freudenreich O, Henderson DC, et al. Contribution of methylenetetrahyrdofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, et al. MTHFR 677C --> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val --> Met. Proc Natl Acad Sci U S A. 2008;105:17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roffman JL, Nitenson AZ, Agam Y, Isom M, Friedman JS, Dyckman KA, et al. A hypomethylating variant of MTHFR, 677C>T, blunts the neural response to errors in patients with schizophrenia and healthy individuals. PLoS One. 2011;6:e25253. doi: 10.1371/journal.pone.0025253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine J, Stahl Z, Sela BA, Ruderman V, Shumaico O, Babushkin I, et al. Homocysteine-Reducing Strategies Improve Symptoms in Chronic Schizophrenic Patients with Hyperhomocysteinemia. Biol Psychiatry. 2006;60:265–269. doi: 10.1016/j.biopsych.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Hill M, Shannahan K, Jasinski S, Mackin EA, Raeke L, Roffman JL, et al. Folate supplementation in schizophrenia: a possible role for MTHFR genotype. Schizophr Res. 2011;127:41–45. doi: 10.1016/j.schres.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Roffman JL, Lamberti JS, Achtyes E, Macklin EA, Galendez GC, Raeke LH, et al. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. 2013;70:481–489. doi: 10.1001/jamapsychiatry.2013.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin X, Li J, Cui Y, Liu Z, Zhao Z, Ge J, et al. MTHFR C677T and MTR A2756G polymorphisms and the homocysteine lowering efficacy of different doses of folic acid in hypertensive Chinese adults. Nutr J. 2012;11:2. doi: 10.1186/1475-2891-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papakostas GI, Shelton RC, Zajecka JM, Bottiglieri T, Roffman J, Cassiello C, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75:855–863. doi: 10.4088/JCP.13m08947. [DOI] [PubMed] [Google Scholar]
- 13.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The Organization of the Human Cerebral Cortex Estimated By Functional Connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryer SL, Woods SW, Kiehl KA, Calhoun VD, Pearlson GD, Roach BJ, et al. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front Psychiatry. 2013;4:92. doi: 10.3389/fpsyt.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristofferson MW. Effects of practice on character-classification performance. Can J Psychiatry. 1972;26:54–60. [Google Scholar]
- 18.Arning E, Bottiglieri T. Quantitation of 5-Methyltetrahydrofolate in Cerebrospinal Fluid Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Methods Mol Biol. 2016;1378:175–182. doi: 10.1007/978-1-4939-3182-8_19. [DOI] [PubMed] [Google Scholar]
- 19.Ducros V, Belva-Besnet H, Casetta B, Favier A. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin Chem Lab Med. 2006;44:987–990. doi: 10.1515/CCLM.2006.178. [DOI] [PubMed] [Google Scholar]
- 20.Butler LM, Arning E, Wang R, Bottiglieri T, Govindarajan S, Gao YT, et al. Prediagnostic levels of serum one-carbon metabolites and risk of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:1884–1893. doi: 10.1158/1055-9965.EPI-13-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- 23.Fan FM, Tan SP, Yang FD, Tan YL, Zhao YL, Chen N, et al. Ventral medial prefrontal functional connectivity and emotion regulation in chronic schizophrenia: a pilot study. Neurosci Bull. 2013;29:59–74. doi: 10.1007/s12264-013-1300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimol LM, Nesvag R, Hagler DJ, Jr, Bergmann O, Fennema-Notestine C, Hartberg CB, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Eryilmaz H, Tanner AS, Ho NF, Nitenson AZ, Silverstein NJ, Petruzzi LJ, et al. Disrupted working memory circuitry in schizophrenia: Disentangling fMRI markers of core pathology vs other aspects of impaired performance. Neuropsychopharmacology. 2016;41:2411–2420. doi: 10.1038/npp.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landin-Romero R, McKenna PJ, Salgado-Pineda P, Sarro S, Aguirre C, Sarri C, et al. Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol Med. 2014:1–11. doi: 10.1017/S0033291714002426. [DOI] [PubMed] [Google Scholar]
- 27.Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- 28.Sharp L, Little J. Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: a HuGE review. Am J Epidemiol. 2004;159:423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 29.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 30.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 31.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obeid R, Kostopoulos P, Knapp JP, Kasoha M, Becker G, Fassbender K, et al. Biomarkers of folate and vitamin B12 are related in blood and cerebrospinal fluid. Clin Chem. 2007;53:326–333. doi: 10.1373/clinchem.2006.076448. [DOI] [PubMed] [Google Scholar]
- 33.Hermes ED, Sokoloff D, Stroup TS, Rosenheck RA. Minimum clinically important difference in the Positive and Negative Syndrome Scale with data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) J Clin Psychiatry. 2012;73:526–532. doi: 10.4088/JCP.11m07162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leucht S, Kane JM, Etschel E, Kissling W, Hamann J, Engel RR. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacology. 2006;31:2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 35.Lambie DG, Johnson RH. Drugs and folate metabolism. Drugs. 1985;30:145–155. doi: 10.2165/00003495-198530020-00003. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DP, Van Dyke DC, Willhite LA, Stumbo PJ, Berg MJ. Phenytoin-folic acid interaction. Ann Pharmacother. 1995;29:726–735. doi: 10.1177/106002809502907-816. [DOI] [PubMed] [Google Scholar]
- 37.Henriquez-Sanchez P, Sanchez-Villegas A, Doreste-Alonso J, Ortiz-Andrellucchi A, Pfrimer K, Serra-Majem L. Dietary assessment methods for micronutrient intake: a systematic review on vitamins. Br J Nutr. 2009;102(Suppl 1):S10–37. doi: 10.1017/S0007114509993126. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr. 2005;24:266–274. doi: 10.1080/07315724.2005.10719474. [DOI] [PubMed] [Google Scholar]
- 39.Winkels RM, Brouwer IA, Clarke R, Katan MB, Verhoef P. Bread cofortified with folic acid and vitamin B-12 improves the folate and vitamin B-12 status of healthy older people: a randomized controlled trial. Am J Clin Nutr. 2008;88:348–355. doi: 10.1093/ajcn/88.2.348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.