Introduction
Obesity related sub-acute chronic inflammation contributes to type 2 diabetes and atherosclerosis 1,2. Multiple studies target inflammation to reduce dysglycemia and/or coronary heart disease (CHD) using diverse anti-inflammatory approaches. Several show salsalate, a pro-drug of salicylate, improves glycemia in type 2 diabetes, obesity, or impaired glucose tolerance. Durability of glycemic efficacy and safety of salsalate remains less well understood.
Higher fasting glucose and hemoglobin A1c (HbA1c), even within normal ranges, associate with increased risk for incident diabetes, ischemic- and all-cause mortality 3,4 supporting potential importance of lowering glucose in persons with established heart disease irrespective of the baseline glycemic status. This study aimed to evaluate effects of salsalate compared to placebo over 30 months on glycemia assessed by fasting glucose, HbA1c, and glycated serum protein (GSP), in overweight statin-treated persons with CHD, but without diabetes.
Research Design and Methods
This report details a pre-specified secondary endpoint in the Targeting INflammation using SALsalate in CardioVascular Disease study (TINSAL-CVD, ClinicalTrials.gov Identifier: NCT00624923), a double-blinded, randomized (1:1), placebo-controlled, parallel clinical trial. Study design, inclusion details for obese statin-treated persons with stable CHD, exclusion criteria, randomization and follow-up are available 5(Supplement). Participants with established diabetes at baseline were excluded from analysis a priori due to potential confounding from change in concomitant diabetes medications 6, and insufficient numbers with diabetes in the parent trial for adequate power to evaluate this sub-population. Primary outcomes were change in HbA1c, fasting glucose, and GSP over 30 months.
Results
Baseline Characteristics
Participants were generally Caucasian males, with mean age 60±7 years, BMI 31.4±3.0 kg/m2, fasting glucose 92.8±11.0 mg/dL, and HbA1c 5.8±0.3%. Baseline characteristics were similar between groups (sTable 1). Of the 192 trial participants without diabetes, 147 (77%) completed the study. The proportion who completed the trial tended lower in salsalate compared with placebo (P=0.088), reasons are provided in sFigure1. Expected visits were 99% completed. No participants were unmasked during the trial.
Glycemia
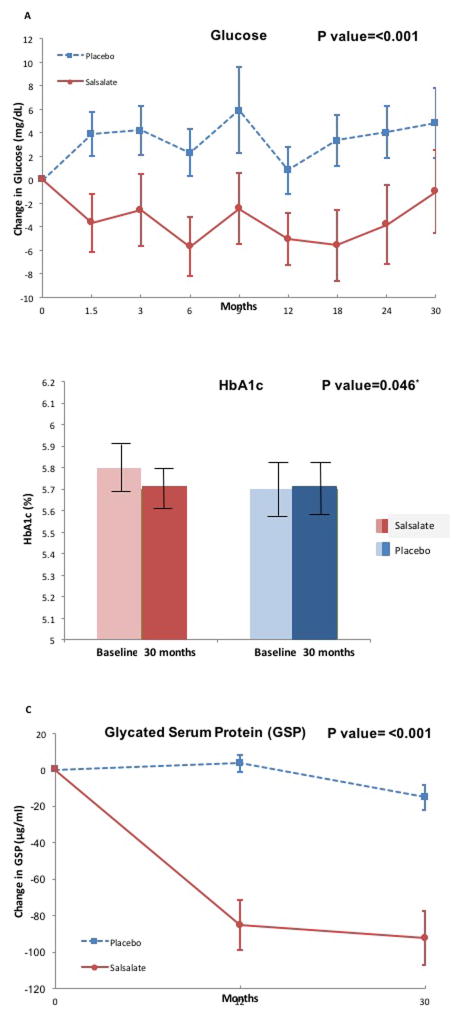
Fasting glucose decreased 6% in salsalate compared to placebo groups (P<0.001) over 30 months. Fasting glucose declined −2.8 mg/dL (95%Confidence Interval (CI): −1.5 to −3.9) within the salsalate group, and increased 2.9 mg/dL (95%CI: 1.7 to 4.2) within the placebo group (Figure-1A, sTable-2). The absolute difference in absolute mean change in HbA1c between salsalate and placebo was −0.107% (95%CI: −0.210 to −0.002, P=0.046). HbA1c tended lower −0.1% (95%CI: −0.2 to 0.0) within the salsalate group but remained unchanged 0.0% (95%CI: 0.0 to 0.1) within the placebo group (Figure-1B). GSP concentrations were 37% lower in salsalate compared to placebo groups (P<0.001) with a mean change of −87.5 μg/mL (95%CI: −96.1 to −79.0) within the salsalate group but unchanged (−5.7 μg/mL (95%CI: −13.9 to 2.6) in placebo (Figure-1C).
Figure 1.
Comparison of change from baseline in [A] fasting plasma glucose concentrations, [B] HbA1c and [C] glycated serum protein (GSP) between the salsalate and placebo treatment groups are shown. In [A] and [C] salsalate is shown in the red solid line, and placebo in the blue dotted line. In [B] baseline are shown in pale color while 30 month values are shown in darker color. (P values for difference in change in fasting glucose, HbA1c and GSP over 30 months between salsalate and placebo groups)
Glucose levels may drop more in those with prediabetes manifesting mild elevation compared to those with normal baseline levels. Thus we evaluated the potential interaction between prediabetes and glucose lowering. Presence of baseline prediabetes was associated with greater reduction in fasting glucose compared to those without when prediabetes was added to the repeated measures analysis with linear mixed models (Pinteraction=0.018)(Figure-2).
Figure 2.
Change in fasting glucose over 30 months in patients with and without prediabetes at baseline
Other measures of efficacy and safety
Adiponectin increased 25% more with salsalate compared to placebo (P<0.001). hsCRP decreased in both groups, with no difference in change between groups (P=0.885)(sTable-2). Total white blood cell (WBC) count decreased in salsalate by 11% compared to placebo (P<0.001), whereas hematocrit increased 1.6% (P=0.012).
Triglycerides trended 10% lower in salsalate compared with placebo (P=0.057). There was no difference in change for total cholesterol, LDL-cholesterol or HDL-cholesterol (P=0.169, P=0.111 and P=0.175, respectively) between groups.
Increased urine albumin-to-creatinine ratio (ACR) 16.7 mcg/mg (95%CI: 6.4 to 27.1, P<0.001) was seen in salsalate compared with placebo. Change in estimated glomerular filtration rate (eGFR) was similar between treatment groups (P=0.278) with decline in both (−3.7% versus −2.6%, salsalate versus placebo, respectively).
Adverse events
No episodes of symptomatic hypoglycemia were reported in either group. Serious adverse events were balanced between groups (sTable-3). More salsalate-treated patients reported new onset or worsening of tinnitus compared to placebo (35% versus 16%, P=0.005). Numerically more patients experienced atrial arrhythmias with salsalate than placebo (8% versus. 2%, P=0.103). Non-serious adverse events occurring in >5% of participants and numerically more with salsalate are provided (sTable-4).
Discussion
We demonstrate salsalate improves fasting glucose, HbA1c, and glycated serum protein compared to placebo, and effects are sustained over 30 months. Our findings are consistent with previous reports showing salsalate improves glycemia (sTable-5) but now provide the largest number of non-diabetic patients followed for the longest duration of time, furnishing new information on durability of glycemic improvement and safety in a population at high risk of developing type 2 diabetes.
Fasting glucose lowering occurred within 6 weeks and was sustained. The percentage decrease in fasting glucose (−6%) is similar to most shorter-duration studies (sTable-5). We also demonstrate a greater magnitude reduction in fasting glucose with salsalate in patients with prediabetes compared to those with normal glycemia. Changes in fasting glucose and hence basal hepatic glucose production, even without effect on post-prandial glucose concentrations (sTable-5) may impact HbA1c 7. Post-prandial glycemia was not assessed.
Although one might not anticipate HbA1c lowering within the nondiabetic range, an absolute 0.1% decrease in salsalate- compared to placebo-assigned participants was seen, from the normative mean baseline of 5.8%. In contrast, others report no changes in HbA1c over 12 weeks or have not reported on HbA1c change (sTable-5). In context, a dipeptidyl peptidase-4 inhibitor (linagliptin) reduced HbA1c by 0.16% in a 2 year efficacy study of patients with T2D and starting HbA1c of 7.7% 8.
Several mechanisms may explain glycemic improvements observed with salsalate (reviewed in 9). Salicylates exert anti-inflammatory effects through multiple cellular mechanisms including effects to stimulate adenosine monophosphate–activated protein kinase (AMPK), inhibition of NF-κB or transcription factors in addition to NF-κB, cellular kinases, mitochondrial dehydrogenases, or 11-hydroxysteroid dehydrogenase type 1 in adipose tissue. Relative contributions of these potential mechanisms in humans in vivo cannot be distinguished.
Anti-inflammatory effects are clinically manifest by lowered leukocyte counts, and increased adiponectin, consistent with prior reports (sTable-5). Increased adiponectin may be an informative biomarker for reduced risk for type 2 diabetes and cardiovascular disease 10,11. The durability of these anti-inflammatory effects is consistent with the improved glycemia, but does not establish causality. While shorter studies have demonstrated lowered CRP in overweight persons, CRP reductions are not seen in other studies, and were not seen in this study (sTable-5). Statins decrease CRP, and salsalate had no additional effect on CRP, whereas salsalate lowered WBC counts and statins do not. This suggests distinct mechanisms without interactions between the responsible pathways.
Total cholesterol, LDL-cholesterol and HDL-cholesterol did not differ between salsalate and placebo groups. Effects of salsalate on LDL-cholesterol are inconsistent, increasing in some studies and remaining unchanged in others (sTable-5). All our patients were on statins, and it is possible statins may attenuate possible lipid changes by salsalate. Prior studies demonstrated salsalate lowers triglycerides in type 2 diabetes and persons without diabetes (sTable-5). Salsalate’s effects on hepatic markers were inconclusive, with increased AST, and lower ALT and bilirubin. These findings could be spurious as safety assessments were not corrected for multiple testing.
Increased albuminuria limits our enthusiasm to recommend salsalate for type 2 diabetes prevention, and has been reported as a reversible phenomenon with salsalate in type 2 diabetes (sTable-5). As with prior studies, we did not find a difference in renal filtration with salsalate. The mechanism for increased albumin excretion remains unclear. Salsalate appears to have lower risk for acute kidney injury compared to other nonsteroidal anti-inflammatory agents, but is not free of risk 12. While increased albuminuria is a risk factor for coronary events, we did not see increased cardiovascular event rates in this high-risk group of patients with established cardiovascular disease. However, trial duration and number of patients studied were insufficient to determine long-term risk–benefit of salsalate with regard to cardio-renal safety. Alternative therapies including metformin and lifestyle can safely reduce risk of progression to diabetes, as seen in the Diabetes Prevention Program.
Limitations of our study include the relatively small number of patients and short trial duration, which restricts assessments of diabetes prevention and of cardio-renal outcomes. The study population was largely male and Caucasian, limiting generalizability.
Conclusion
Changes in renal function and associated long-term cardiovascular safety require further evaluation before salsalate can be recommended as a potential diabetes prevention strategy but the findings of this study support further study of anti-inflammatory approaches for improving glycemic control.
Supplementary Material
CONSORT diagram
Supplemental Table 1: Baseline demographic and clinical characteristics by treatment group
Supplemental Table 2: Baseline and change from baseline over 30 months in laboratory safety and efficacy parameters by treatment group.
Supplemental Table 3: Serious adverse events by treatment group
Supplemental Table 4: Non-serious Adverse Events in >5% of study cohort and numerically more in salsalate group
Supplemental Table 5: Comparison of previous studies reporting on the effects of salsalate on glycemia, inflammatory markers, low-density lipoprotein and triglycerides
Acknowledgments
This work was supported by grants P50HL083813 from the National Heart, Lung and Blood Institute, grant P30DK03836 from the National Institute of Diabetes and Digestive and Kidney Diseases from the National Institutes of Health, and by the Joslin Clinical Research Center and it’s philanthropic donors. Caraco Pharmaceutical and Amneal Pharmaceutical provided salsalate and identical placebo. Boston Heart Diagnostics provided assay determinations describe in this article free of charge in an anonymous and masked fashion.
References
- 1.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. The Journal of clinical investigation. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Brewer N, Wright CS, Travier N, et al. A New Zealand linkage study examining the associations between A1C concentration and mortality. Diabetes care. 2008;31(6):1144–1149. doi: 10.2337/dc07-2374. [DOI] [PubMed] [Google Scholar]
- 4.Lawes CM, Parag V, Bennett DA, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes care. 2004;27(12):2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 5.Hauser TH, Salastekar N, Schaefer EJ, et al. Effect of Targeting Inflammation With Salsalate: The TINSAL-CVD Randomized Clinical Trial on Progression of Coronary Plaque in Overweight and Obese Patients Using Statins. JAMA Cardiol. 2016;1(4):413–423. doi: 10.1001/jamacardio.2016.0605. [DOI] [PubMed] [Google Scholar]
- 6.Goldfine AB, Fonseca V, Jablonski KA, et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Annals of internal medicine. 2013;159(1):1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastyr EJ, 3rd, Stuart CA, Brodows RG, et al. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. IOEZ Study Group. Diabetes care. 2000;23(9):1236–1241. doi: 10.2337/diacare.23.9.1236. [DOI] [PubMed] [Google Scholar]
- 8.Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–483. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 9.Goldfine AB, Shoelson SE. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. The Journal of clinical investigation. 2017;127(1):83–93. doi: 10.1172/JCI88884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2009;302(2):179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- 11.Rothenbacher D, Brenner H, Marz W, Koenig W. Adiponectin, risk of coronary heart disease and correlations with cardiovascular risk markers. Eur Heart J. 2005;26(16):1640–1646. doi: 10.1093/eurheartj/ehi340. [DOI] [PubMed] [Google Scholar]
- 12.Lafrance JP, Miller DR. Selective and non-selective non-steroidal anti-inflammatory drugs and the risk of acute kidney injury. Pharmacoepidemiol Drug Saf. 2009;18(10):923–931. doi: 10.1002/pds.1798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT diagram
Supplemental Table 1: Baseline demographic and clinical characteristics by treatment group
Supplemental Table 2: Baseline and change from baseline over 30 months in laboratory safety and efficacy parameters by treatment group.
Supplemental Table 3: Serious adverse events by treatment group
Supplemental Table 4: Non-serious Adverse Events in >5% of study cohort and numerically more in salsalate group
Supplemental Table 5: Comparison of previous studies reporting on the effects of salsalate on glycemia, inflammatory markers, low-density lipoprotein and triglycerides