The kidney is a major site for hypertensive target organ damage which is second only to diabetic nephropathy as a primary etiology for end stage renal disease (ESRD). Moreover, the presence of chronic kidney disease (CKD), including that due to hypertension (HTN), has been shown to be a strong independent risk factor for adverse cardiovascular outcomes. 1 Nevertheless, major aspects of clinical hypertensive renal disease remain poorly understood such as the marked differences in individual susceptibility to hypertensive renal damage and/or the apparent variable renoprotective effectiveness of antihypertensive classes. Using experimental models to achieve clearer insights into such phenomenon through an integrated team approach has been a major focus of our laboratory. This review provides an overview of the collective contributions of this investigative team to address these unresolved issues of clinical hypertensive renal damage (see acknowledgments).
Clinical Patterns of Susceptibility to Hypertension-Induced Renal Damage (HIRD)
One of the most striking of such clinical observations is the fact that although hypertension (HTN) is a major population risk for ESRD, this is primarily due to the huge prevalence of HTN in the general population. The individual risk is surprisingly small (< 0.5%). 2,3 This is because the renal pathology typically observed in the vast majority of individuals with essential HTN is that of benign nephrosclerosis described as an accelerated aging of the renal vasculature. 3–5 It is characterized by a very slowly progressive thickening and sclerosis of the renal resistance vessels, while the glomerular capillaries are largely spared. Some ischemic glomerular loss does occur but is limited and happens slowly over decades. Given that significant reductions in renal function and ESRD only develop after large losses of glomerular filtration surface area have occurred, it is perhaps not too surprising that ESRD occurs infrequently in essential HTN. In fact, except for some genetically susceptible groups such as African-Americans, the only individuals with essential HTN who develop sufficient HIRD to cause ESRD are those in whom the HTN becomes very severe and results in the development of malignant nephrosclerosis. 3–9 The renal pathology in such individuals is characterized by acute disruptive injury and fibrinoid necrosis of small arteries, arterioles and glomerular capillaries, with prominent glomerular ischemia due to upstream vascular injury. Acute renal failure develops over days and despite treatment is often followed by CKD or even ESRD.
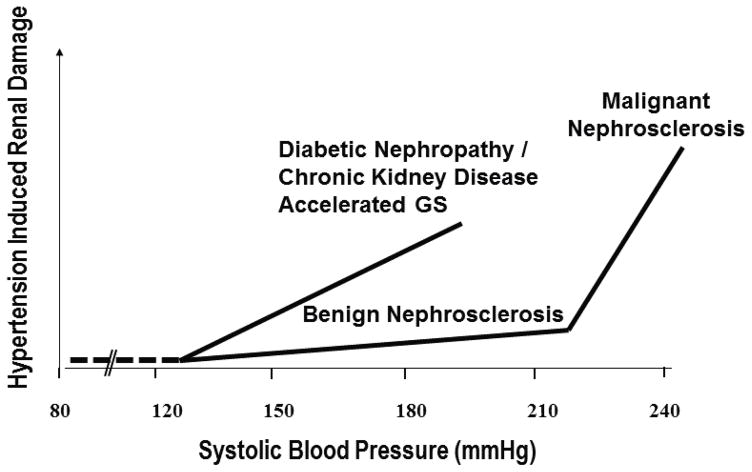
In contrast to the relative resistance to HIRD in individuals with essential HTN in the absence of genetic predisposition and/or malignant nephrosclerosis, individuals with CKD and/or diabetes seem to exhibit a much greater susceptibility to the adverse renal effects of even moderate HTN. 3–9 And, in contradistinction to the largely vascular pathology of benign and malignant nephrosclerosis, the site of HIRD in CKD states is predominantly glomerular, with a pattern of accelerated segmental or global glomerulosclerosis (GS) often superimposed on the intrinsic phenotype of the underlying renal disease. 4–9 Given that any increase in physical pressure (BP) within the intrarenal vasculature, if of sufficient magnitude and regardless of cause, is expected to result in barotrauma and local vascular injury as happens in malignant HTN, 10,11 we have proposed that susceptibility to HIRD should be quantitatively assessed in terms of BP threshold for HIRD and the slope of the relationship between BP and HIRD (the increase in HIRD for a given increase in BP). 5–9,12 The application of such a concept to the differences in susceptibility to HIRD between essential HTN vs. CKD is schematically illustrated in Fig. 1.
Fig. 1.
Schematic illustration of differences in susceptibility to hypertension-induced renal damage (HIRD) between patients with essential hypertension (benign and malignant nephrosclerosis) and those with diabetic and non-diabetic CKD with respect to BP thresholds and slopes of the relationship between BP and HIRD (Reproduced from Ref. 5 with permission).
Experimental Models of HTN and CKD Replicate Patterns of Clinical susceptibility to HIRD
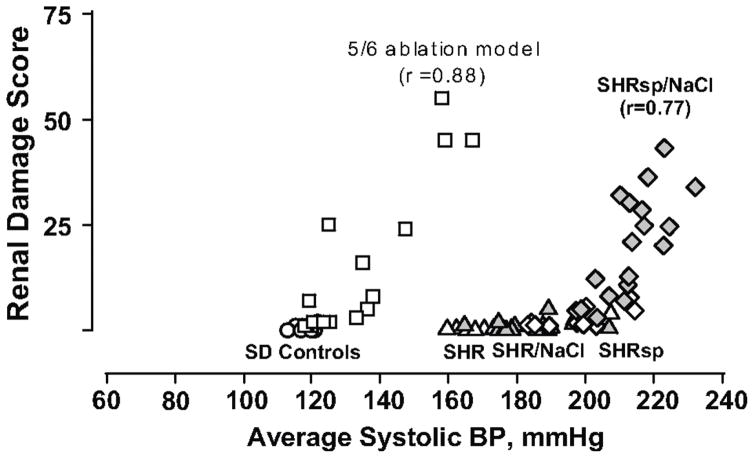
That experimental models of HTN exhibit considerable differences in the development and severity of renal damage has long been recognized. However, investigations to carefully define and quantitate such differences have been limited. Moreover, the isolated tail-cuff BP measurement methodology that has been utilized for such investigations is inherently inadequate, given the intrinsic biologic lability of BP with frequent large fluctuations particularly in hypertensive states. 13 In recognition of these considerations, our laboratory pioneered the use of the then recently developed methodology for chronic BP radiotelemetry to quantitate BP/HIRD relationships in experimental models of HTN. 14–19 Such results in models of non-malignant (spontaneously hypertensive rat, SHR) and malignant hypertension (stroke prone SHR, SHRsp) and progressive CKD (5/6 renal ablation) are shown in Fig. 2. As can be noted, these BP/HIRD relationships replicate the clinical patterns of HIRD susceptibility schematically illustrated in Fig. 1 with respect to the BP threshold for HIRD and the slopes of the relationship between BP and HIRD. The pathologic patterns are also similar. Both the SHR and SHRsp show minimal histologic injury and/or proteinuria despite substantial hypertension. Salt supplementation increases the BP in both but more so in the salt sensitive SHRsp such that the critical threshold for malignant nephrosclerosis is exceeded. By contrast, GS and proteinuria develop at a much lower BP threshold in the CKD model and increase linearly with increasing BP. Vascular injury is usually not observed except in severely hypertensive animals.
Fig. 2.
Quantitative relationships between radiotelemetrically measured systolic BP and renal injury in rats with intact autoregulation (normotensive Sprague-Dawley controls [SD, circles]; spontaneously hypertensive rat [SHR, triangles]; stroke-prone SHR [SHRsp; diamonds]; SHR [gray triangles] and SHRsp [gray diamonds] placed on a high salt diet) and in the 5/6 renal ablation model of CKD [squares], with impaired autoregulation. The renal damage score represents a composite of vascular and glomerular damage scores in the SHRsp and % GS in the 5/6 ablation model (reproduced with permission from Ref. 6, based on data from Ref. 14,18). Minimal injury is seen in SHR with intact autoregulation despite severe hypertension. Injury in the salt-sensitive SHRsp administered a high NaCl diet occurs at blood pressures that exceed the autoregulatory capacity while the 5/6 renal ablation model, with impaired autoregulation, exhibits a much lower BP threshold for hypertensive injury than normal or SHR kidneys.
Pathophysiologic Basis for the Differences in HIRD Susceptibility
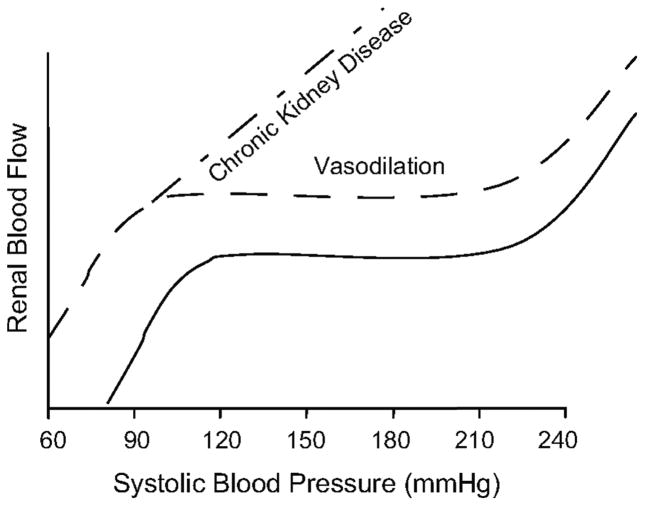
Based on the a-priori concept that an exposure to elevated pressures is a prerequisite for a vascular segment to develop hypertensive barotrauma-mediated injury, we have postulated that such large differences in HIRD susceptibility are best explained when considered in the context of physical BP transmission to the renal microvasculature. 5–9,12,14–23 Normally, episodic or sustained increases in BP result in proportionate autoregulatory vasoconstriction of the preglomerular resistance vessels, mainly the afferent arteriole, such that renal blood flow (RBF) is maintained relatively constant within this autoregulatory (AR) range 7–9,24 (Fig. 3). Because these resistance changes are essentially confined to the preglomerular vasculature, glomerular capillary pressure (PGC) and glomerular filtration rate (GFR) are also maintained constant. Thus the glomerular capillaries are protected from barotrauma as long as autoregulation is intact and BP is within the autoregulatory range. Given that such is the case in the vast majority of patients with essential HTN, they usually do not exhibit glomerular injury and proteinuria. However, the resistance arteries and arterioles exposed to the increased pressures develop the slowly progressive pathology of benign nephrosclerosis. But, if the HTN becomes very severe and exceeds the threshold for autoregulation and disruptive vascular injury, malignant nephrosclerosis develops.
Fig 3.
a) Summary of renal autoregulatory patterns obtained in rats with 1) intact kidneys and normal autoregulation (——), 2) vasodilated vascular bed and normal autoregulation as after uninephrectomy) ( – – – ) and 3) vasodilated vascular bed and impaired autoregulation (i.e., 5/6 renal ablation model of CKD) (– · – · –). (Reprinted with permission from reference 6).
By contrast, the impairment of renal AR associated with severe reductions in renal mass (≥ 75%) (Fig 3) allows even moderate hypertension to be more freely transmitted to the glomerular capillaries with resultant barotrauma and progressive GS. In this context, it needs to be emphasized that these adverse effects of impaired renal autoregulation on HIRD susceptibility only occur in a vasodilated vascular bed. Vasodilation alone with preserved autoregulation such as observed after uninephrectomy, 21 only modestly increases the susceptibility to hypertensive injury (Fig. 3). However, when combined with impaired AR, it greatly amplifies the effects on HIRD susceptibility. By contrast, with a vasoconstricted vasculature, impaired AR (inability to vasodilate) may primarily increase the susceptibility to ischemic renal parenchymal injury due to a reduced capacity to maintain renal perfusion when BP falls. 5,6
Validation of the Concept of Autoregulatory Protection in a Malignant Nephrosclerosis Model
If the concept is valid that when AR is intact, malignant nephrosclerosis only develops when severe HTN exceeds the critical ceiling for vascular injury and autoregulatory protection, then two predictions follow: (i) If BP is prevented from reaching this threshold with any antihypertensive class, HIRD should be prevented and (ii) even after malignant nephrosclerosis has developed, modest reductions in BP to below this threshold into the AR range should lead to healing and repair of the already developed HIRD lesions, despite the continued HTN. Both predictions were tested using BP radiotelemetry in the salt-supplemented SHRsp with preserved AR. 22,23 The control untreated rats only received 1% NaCl as their drinking water for 4 weeks while the treated groups also received one of three antihypertensive regimens of Hydralazine + Hydrochlorothiazide, Enalapril, or Amlodipine. Severe HTN and HIRD developed in the untreated SHRsp but were prevented by all three antihypertensive classes (Fig. S1a in the On-Line Only Supplement). 23
The second prediction was also tested using this model. As in the previous study SHRsp rats received 1% NaCl to drink and after 4 weeks when substantial proteinuria had already developed, the right kidney was removed for HIRD quantitation. Following nephrectomy, the rats continued to receive 1% NaCl with Hydralazine and Hydrochlorothiazide at dosages that kept the BP between 160–180 mmHg. As shown in Fig. S1b & S1c, reducing the BP to below the critical threshold and into the normal AR range resulted in a dramatic reduction in proteinuria within the first week and a striking resolution of the histologic injury by 2–3 weeks despite the continued substantial hypertension. 23 It is important to emphasize that this reversal of HIRD was observed without the use of regimens such as RAS blockade, aldosterone antagonists and/or endothelin receptor blockade that have been claimed to mediate such reversal through specific BP-independent mechanisms. Of note, similar recovery had been achieved with other antihypertensive agents before RAS blockade was available in patients with malignant nephrosclerosis requiring dialysis. 25 These data clearly demonstrate the barotrauma-mediated pathogenesis of HIRD in malignant nephrosclerosis and the striking capacity for its spontaneous and rapid repair, if new injury is prevented. It merits emphasis however, that while malignant nephrosclerosis can be prevented by modest BP reductions, the long-term risk for benign nephrosclerosis and more importantly, for other target organ damage continues with such sub-optimally controlled HTN, emphasizing the importance of adequate BP control.
Evidence that Physical BP Transmission is the Predominant Determinant of HIRD (the Angiotensin II Infusion Model)
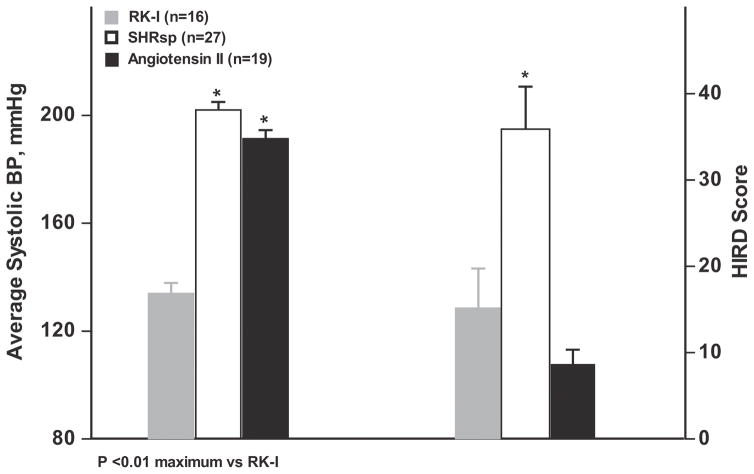
Theoretically, the severity of HIRD is expected to be a function of (i) the severity of HTN (ii) the degree to which the systemic HTN is transmitted to the renal microvasculature which in turn depends on (a) the ambient tone of the preglomerular vasculature (b) its ability to autoregulate and (iii) the BP independent mechanisms that may modulate/alter the local tissue injury response to a given degree of pressure exposure. 5,9,12 These latter BP-independent pathways have been the focus of much investigation and although a large number of such mechanisms have been postulated, 26,27 the relative quantitative contributions of BP-dependent and BP-independent pathways have not been rigorously assessed. A striking illustration of this phenomenon is provided by one of the most frequently used models of experimental HTN which is produced by the infusion of relatively large doses of exogenous Angiotensin II (Ang II). 12,28 Ang II is widely believed to promote renal damage through a plethora of BP-independent deleterious pathways that include inflammation, immune activation, oxidative stress, aberrant oxygen utilization, hypoxia and profibrotic signaling. 12,26,27,29 However, elegant studies using a chronic BP servocontrol methodology in Ang II infused rats have shown that while BP-independent mechanisms can cause significant renal damage, BP dependent mechanisms account for the majority of renal injury. 31–33 Nevertheless, the degree of renal injury observed in Ang II infusion models appears to be surprisingly modest. 12,30–33 Fig. 4 illustrates this strikingly limited HIRD despite very severe HTN in the angiotensin II infusion model when compared to either the SHRsp model of malignant nephrosclerosis or the 5/6 ablation model of CKD. Recent studies from our laboratory analyzing the pressure flow relationships in conscious Ang II infused hypertensive rats have provided an explanation for the discordance between the abundance of Ang II mediated pathogenic mechanisms and the paucity of actual histologic injury. 12 In addition to renal vasoconstriction which per se would be expected to reduce physical BP transmission, a potentiation of autoregulatory responses likely mediated by an interaction with the myogenic mechanism was also seen, further reducing renal microvascular pressure transmission and enhancing the resistance to HIRD. Thus, the protection conferred by the preglomerular vasoconstriction and the reduction of physical BP transmission by Ang II is apparently sufficient to counteract its direct deleterious effects in this model. While this Ang II infusion model does provide important insights into the predominant importance of physical BP transmission in the pathogenesis of HIRD, its clinical relevance may be limited as if it does not replicate CKD states which are usually characterized by normal, suppressed or only mildly elevated plasma renin levels and presumed increased local renal RAS activation (vide-infra).
Fig. 4.
A comparison of the qualitative relationship between BP and renal damage in the continuous exogenous Ang II infusion model of HTN (300–500 ng/kg/min for 4 weeks) 12 with the salt-supplemented stroke prone spontaneously hypertensive rat (SHRsp) model of malignant HTN 23 and the hypertensive 5/6 renal ablation model of CKD in Sprague-Dawley rats. 14 The average systolic BP (final 4 wks) and renal damage scores for each of the three models are shown. As can be seen, the rats with Ang II-induced HTN develop very limited HIRD despite an average systolic BP that is as high as the SHRsp. Moreover, in contrast to the strong correlations between BP and HIRD in both the SHRsp and 5/6 ablation models shown in Fig. 2, the Ang II-infused rats exhibit a much weaker correlation and a flatter slope (increase in HIRD/mmHg increase in average systolic BP) (r2 = 0.27, slope 0.28±0.11; p < 0.025). By comparison, the slope value for SHRsp were 1.13±0.24 and 1.3±0.15 for the 5/6 ablation model, p< 0.01 for both model vs. the Ang II model). (Adapted with permission from the cited references).
Role of Impaired Autoregulation and HIRD in the Progression of CKD
CKD regardless of etiology tends to progress even when the initiating disease is no longer active. The mechanisms responsible for this seemingly autonomous progression have been extensively investigated using the CKD model of 5/6 renal ablation as it replicates the phenomenon of initially normal remnant nephrons undergoing compensatory hypertrophy of size and function followed by proteinuria, HTN, GS and progressive nephron loss. 5,6,9,34 The most widely accepted pathogenesis construct is the maladaptive “hyperfiltration theory” proposed by Brenner and colleagues. 34 It postulates that “the elevated single nephron glomerular filtration rate (SNGFR) common to these pathophysiologic conditions is usually caused by increases in the glomerular capillary plasma flow rate (QA) and mean glomerular capillary hydraulic pressure (PGC). The increases in PGC are ascribed to the greater adaptive dilation of the afferent than efferent arteriole. This relative efferent vasoconstriction was attributed to the tonic vasoconstrictive effects of Ang II as angiotensin-converting enzyme (ACE) inhibitors were shown to reduce PGC and ameliorate glomerulosclerosis. Similar hyperfiltration, increased PGC, GS and response to RAS blockade were described in experimental diabetes. 34 It bears emphasis that the PGC elevations are believed to be intrinsic to the hyperfiltration process per se and “that ---systemic hypertension is not required for glomerular capillary hyperfiltration and hypertension”. 34
By contrast, studies from our laboratory have instead suggested a central role for an enhanced glomerular transmission of systemic HTN in the pathogenesis of increased PGC and GS. 5–9 Consistent with such concepts, the severity of progressive GS after 5/6 renal ablation in inbred rodent strains with differing genetic susceptibilities to HTN was noted to be directly proportional to the severity of systemic HTN in the same rat strains. 5–9,35 To exclude the possible role of BP-independent differences in genetic susceptibility to GS, we compared the course of normotensive and hypertensive remnant kidney models in the same Sprague-Dawley rat strain. 16 The conventional hypertensive 5/6 renal ablation model is produced by combining right uninephrectomy with infarction of ~2/3 of the mass of the left kidney. However, if surgical resection rather than infarction is used to produce equivalent renal mass reduction, the severity of HTN is greatly reduced. As depicted in Fig. S2a & S2b, significant HTN, proteinuria and GS only developed in the hypertensive infarction but not in the normotensive surgical excision model. However, kidney weights, glomerular volumes, RBF, GFR and renal AR were comparable after hypertensive and normotensive renal mass reduction. 16 Additional micropuncture studies showed that comparable increases in remnant kidney GFR, SNGFR, and glomerular volumes are achieved with only modest ~5 mmHg increases in PGC when 5/6 renal mass reduction is performed in rats that do not develop subsequent HTN either because of genetic resistance 36 or when surgical excision rather than infarction is used to reduce renal mass. 37 (Table S1) These data contradict the concept that glomerular hyperfiltration per se is intrinsically injurious in the absence of glomerular HTN or that glomerular HTN is necessary for hyperfiltration. We have further suggested hyperfiltration in normotensive models of reduced renal mass is accomplished without significant PGC increases through coordinated increases in glomerular filtration surface area (hypertrophy) and increases in single nephron plasma flow through proportionate afferent and efferent vasodilation 9,38 similar to that seen after uninephrectomy in kidney donors or during pregnancy. 39,40 In a sense, it is like growing new glomerular capillaries in situ. If there are increases in PGC, they are small and not likely to be pathogenic. In fact, increases in PGC are not even very effective in increasing SNGFR due to the inverse relationship between PGC and the ultrafiltration coefficient, Kf. 9,41,42 We have therefore interpreted the glomerular HTN in hyperfiltration states to be a consequence of superimposed transmission of coexistent systemic HTN rather than intrinsic to hyperfiltration per se (Fig. S3). 5–9,35–38
We have also suggested that the contribution of angiotensin II-mediated efferent constriction to glomerular HTN in these models has likely been greatly overestimated during micropuncture studies due to renin release and efferent constriction triggered by anesthesia, surgery and neurohormonal activation (vide-infra). 5,6,9,43 In this context, it may also be important to distinguish between the effects of Ang II on the renal microcirculation during states of low perfusion pressure and reduced macula densa flow (renal artery stenosis and hypovolemia) vs. hypertensive states with increased macula densa salt delivery (CKD states/models). 7,29 Low macula densa flow not only stimulates renin release but also concurrent PGE2 release, which attenuates afferent but not efferent arteriolar responses to Ang II. This results in more selective efferent constriction and a context appropriate preferential preservation of GFR and PGC. Hence, the sensitivity to NSAID induced acute renal failure in such settings. Although any efferent vasoconstriction has the potential to contribute to glomerular HTN in hypertensive states, it is unlikely that an Ang II mediated selective efferent constriction is a feature of the volume replete and hypertensive clinical CKD states. Nevertheless, independent of the potential efferent arteriolar effects, RAS activation is also widely believed to play a major role in the progression of CKD through a plethora of BP-independent non-hemodynamic pathways. However, the evidence for such pathways is much less definitive than claimed. The fact that RAS activation by a low salt diet in vivo does not activate the deleterious pathways that are initiated by angiotensin II in in vitro systems also indicates a context appropriate regulation of these signaling pathways in vivo. 29 And, given that many of the deleterious downstream pathways can also be activated by HTN, much of the in vivo evidence indicating a specific role for RAS that has been obtained in hypertensive rodent models is primarily based on the claims that RAS blockade provides BP-independent benefits that are not observed with other antihypertensives. However, such evidence is severely compromised by the limitations of the tail-cuff BP measurements that have been used to support such interpretations (vide-infra). 5,6,9,13–17,43,44
Renal Autoregulation, Glomerular BP Transmission, Antihypertensives, and CKD Progression
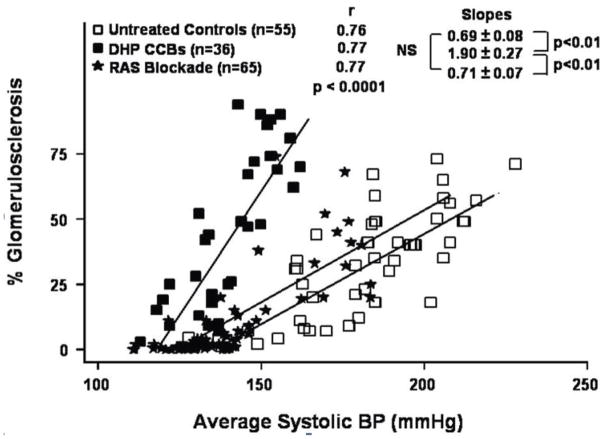
The concept that renal autoregulatory capacity and glomerular BP transmission are the predominant determinants of GS and CKD progression is additionally supported by studies of antihypertensive agents in the 5/6 ablation model. To illustrate, Calcium Channel Blockers (CCBs) particularly dihydropyridine CCBs predictably impair renal autoregulation given the central role of Ca2+ entry through voltage gated Ca2+ channels in the activation of the myogenic mechanism. 7–9,17,24 And although the mechanisms responsible for the impaired renal autoregulation in CKD models remain obscure, CCBs cause a further impairment of renal autoregulation (Fig. S4), reduce the BP threshold for GS and increase the slope of the relationship between BP and GS such that greater GS is observed in CCB treated animals at any given level of HTN as compared to untreated controls (Fig. 5). 5–9,17 By contrast, substitution of a low (8%) protein diet for the standard 24% protein diet, preserves autoregulatory capacity after 5/6 renal ablation and predictably increases the BP threshold for GS, reduces the slope of relationship between BP and GS and substantially ameliorates GS despite continued HTN. 20,45 However, if the low protein fed rats are also given CCBs, the protection against renal autoregulatory impairment and GS are both abolished. 45 Conversely, antihypertensive agents that are neutral with respect to autoregulatory capacity such as RAS blockade (Fig. S4) would be expected to provide renoprotection proportional to the achieved BP reductions in the absence of other BP-independent beneficial effects. Indeed, when direct BP radiotelemetry has been employed, little evidence of BP-independent protection by RAS blockade is seen, as illustrated in Figure 5 using 3 different doses of ACE inhibitors and ARBs. 43 Indeed, contrary to the earlier reports using tail-cuff BP measurements, when BP radiotelemetry was used to compare the renoprotective effectiveness of the ACE inhibitor enalapril vs. low or high dose triple therapy regimens, the protection was found to be proportionate to the achieved BP reductions with all regimens. 15 This apparent discrepancy with the interpretations of the clinical trial data are discussed in a subsequent section (Clinical Parallels and Implications).
Fig. 5.
Compilation of data obtained in our laboratory which illustrates the quantitative relationships between BP and glomerulosclerosis (GS) in rats with 5/6 renal ablation who were left untreated or received either dehydropyridine (DHP) calcium channel blockers or RAS blockade. The deleterious effects of calcium channel blockers on GS as compared to untreated or RAS blockade treated rats are evident. (Reproduced from Ref. 6 with permission).
Efferent Arteriolar Resistance, Nitric Oxide and HIRD Susceptibility
Ambient efferent arteriolar resistance is an important physical determinant of PGC. However, its contribution to HIRD has been difficult to establish. As noted earlier and discussed in greater detail elsewhere, there are reasons to question the interpretations of efferent vasoconstriction based on micropuncture data under anesthesia and/or changes in filtration fraction. 5–9,38 Nevertheless, there is accumulating evidence that in states of endothelial dysfunction, relative efferent constriction may indeed contribute to the pathogenesis of HIRD. 46,47 There is topographic evidence of significant expression of nitric oxide synthases (NOS) 1 & 3 in the efferent arteriolar endothelial cells. 48 It has been suggested that these cells may act as shear stress sensors with the released NO serving an important efferent arteriolar vasodilatory protective function in states of glomerular HTN. 47,48 An impairment of efferent arteriolar NO production during endothelial dysfunction states such as diabetes and obesity may thereby promote exaggerated PGC elevations in such states and contribute to increased susceptibility to GS. 47,49 Indeed, when HTN induced by NOS inhibition was compared to that induced by Ang II infusion in the rat, greater renal damage was observed in the NOS inhibition model despite a lesser severity of HTN. 12
Clinical Parallels and Implications
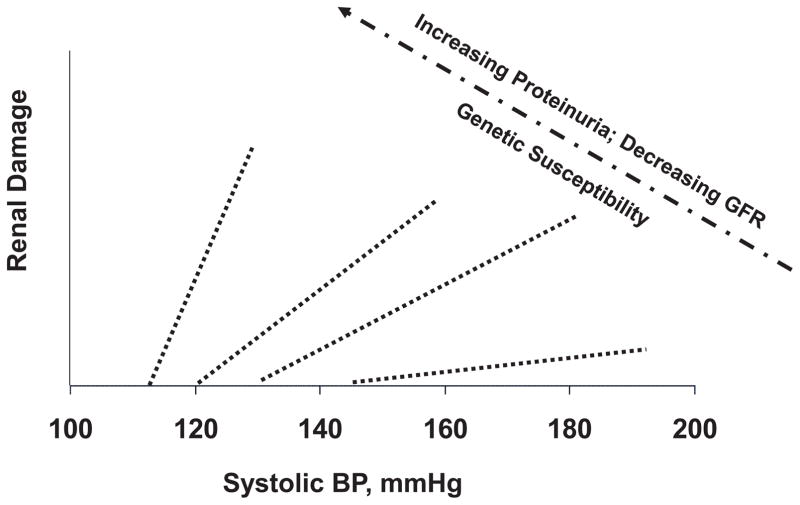
The validity of these concepts/insights derived from animal models have generally been borne out by the clinical data. 5–9,35 To illustrate, an impairment of GFR autoregulation similar to that in CKD models, has been observed in patients with diabetic and non-diabetic nephropathies. 50,51 Similarly, as would be predicted from the experimental data, substantially greater success has been achieved clinically with antihypertensive therapy in preventing malignant nephrosclerosis than in slowing CKD progression. 5–9,23 Moreover, the adverse impact of BP on proteinuria has been recently shown to increase with progressive reduction in GFR in humans as would be predicted by the data from CKD models. 52 The benefits from a lower BP target in patients with proteinuric renal disease and the lesser effectiveness of CCBs in preventing CKD progression in such patients, are also consistent with the experimental data. 6,42,53 By contrast, the experimental results with RAS blockade shown in Fig. 5 seem to be at odds with the clinical trial data and the general consensus that is reflected in the relevant Clinical Guidelines. However, the discrepancy may be more apparent than real. While the clinical trials have indeed clearly shown superior renoprotection with RAS blockade, the evidence that this is due to BP-independent mechanisms is less compelling than claimed as discussed and reviewed in greater detail elsewhere. 5–9,35,44 To illustrate, the original interpretations of the landmark trial of diabetic nephropathy conducted by the Collaborative Study Group claiming specific BP-independent protection by captopril are likely seriously compromised by the disproportionately greater randomization of the nephrotic patients at the highest risk of renal endpoints into the placebo group. Similarly, claims of superior renoprotection by RAS blockade in clinical trials where CCBs have been used in the comparator groups may in fact reflect the inferiority of CCBs based regimens due to adverse effects on glomerular BP transmission. Likewise, interpretations of BP-independent superiority of RAS blockade in clinical trials are confounded by the fact that systolic BP has been typically ~2–6 mmHg lower in RAS blockade groups than in the comparator groups. The post-hoc statistical adjustments for BP differences that have been used to justify such conclusions are quite problematic due to the phenomenon of effect modification schematically illustrated in Fig. 6. Unlike experimental models in which all of the animals in a group are expected to exhibit similar susceptibilities to HIRD, clinical trial populations are more heterogenous with varying degrees of HIRD susceptibility. And, most of the end points are likely to occur in the individual or subgroups with the greatest susceptibility to renal damage in whom small differences in BP are likely to have a greater impact than in the less susceptible individuals. Such differences in slopes of relationship between BP and renal damage may also help explain why the benefits of BP reduction may not be easily demonstrable in clinical trials conducted in non-proteinuric and slowly progressive CKD.
Fig. 6.
A schematic illustration of the potential differences in individual susceptibility to hypertensive renal damage. These differences are indicated by the differences in the BP threshold for renal (glomerular) damage and the slope of the relationships between BP and glomerular injury. (Reproduced from Ref. 6 with permission).
In any event, independent of the claims of BP-independent benefits, there is other compelling rationale for RAS blockade being the preferred antihypertensive strategy for CKD patients. 5,6,9,44 HTN in most CKD patients is primarily volume dependent with relative but perhaps incomplete RAS suppression. Therefore, adequate and sustained BP reductions cannot be achieved without effective diuresis. Because effective diuresis activates RAS, combining diuretics with RAS blockade is very effective antihypertensive therapy in CKD. Additionally, RAS blockade counteracts the tendency to potassium and magnesium wasting that occurs with such diuretic use. Therefore, the antihypertensive synergy of diuretics and RAS blockade, and their antagonism of each other’s adverse effects makes this combination an effective and logical initial antihypertensive regimen for CKD patients.
Work in Progress (Renal Microvascular BP Transmission in Real Time)
As the foregoing discussion indicates, a great deal of progress has been achieved in gaining insights into the pathophysiology of HIRD. However, several aspects of the dynamics of BP transmission in real-time remain poorly defined, including the potentially separate roles of the myogenic and tubuloglomerular feedback (TGF) mechanisms in mediating the protective vs. regulatory functions of the renal autoregulatory response. 7,8 Given that systolic BP may have the greatest potential for target organ damage, the rapid activation kinetics of the afferent arteriolar myogenic response and its potential triggering by the systolic rather than the mean BP are consistent with the protective function of autoregulation primarily being mediated by the myogenic mechanism. 7–9 However, the response is not instantaneous. The fact that BP continuously fluctuates at multiple frequencies rather than shifting from one steady state to another, indicates that although the conventional steady-state assessments of renal AR under anesthesia have provided valuable qualitative insights, they may nevertheless be inadequate to provide a complete assessment of the real-time mechanics of BP transmission in the conscious state. Our lab is currently interrogating simultaneous BP and RBF recordings in conscious animals to more completely define the dynamics of renal autoregulatory responses and glomerular BP transmission. 7–9,21,22
Supplementary Material
Acknowledgments
I am honored to be selected to give the Arthur C. Corcoran Memorial Lecture and wish to express my sincere gratitude to the number of collaborators, and research assistants that have worked in our lab and to Martha Prado for her secretarial and administrative assistance. First and foremost, I would like to express my appreciation for my mentor and long time collaborator, Dr. Anil Bidani for his great intellect, unwavering support, and the use of ‘strong inference’ to challenge accepted dogma by identifying what in retrospect becomes the obvious. I would also like to thank Drs. Paul Churchill, Rodger Loutzenhiser, Ted Kurtz, Geoffrey Williamson, Aaron Polichnowski, Manjeri Venkatachalam, and Maria Picken for their friendships, collaboration and contributions to the research presented. I additionally thank Drs. Leon Moore, Gabby Navar, Will Cupples, and Bill Arendshorst for the long and challenging after hours discussions at National meetings that served to hone and advance our ideas. Of course, I would be remiss to not thank the NIH and Veteran Affairs Merit Review for funding our labs. And last, but not least, I wish to thank Dr. Jane Reckelhoff for nominating me for this distinguished lectureship.
Source of Funding
National Institutes of Health - DK61653; DK40426
Veteran Affairs Merit Review
Footnotes
Disclosure
None
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risk of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarron C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 3.Kopp JB. Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens. 2013;22:266–272. doi: 10.1097/MNH.0b013e3283600f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson JL. Renal Disease caused by hypertension. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 937–990. Sixth. II. [Google Scholar]
- 5.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage: implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 6.Griffin KA, Bidani AK. Progression of renal disease: the renoprotective specificity of renin angiotensin system blockade. Clin J Am Soc Nephrol (invited review) 2006;1:1054–1065. doi: 10.2215/CJN.02231205. [DOI] [PubMed] [Google Scholar]
- 7.Loutzenhiser R, Griffin KA, Williamson G, Bidani AK. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–398. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidani AK, Polichnowski AJ, Loutzenhiser R, Griffin KA. Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens. 2013;22:1–9. doi: 10.1097/MNH.0b013e32835b36c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrom FB. The hypertensive vascular crisis: an experimental study [Heinemann Monograph] London: Pitman Press; 1969. [Google Scholar]
- 11.Hill GS. Studies on the pathogenesis of hypertensive vascular disease. Effects of high-pressure intra-arterial injections in rats. Circ Res. 1970;27:657–668. doi: 10.1161/01.res.27.5.657. [DOI] [PubMed] [Google Scholar]
- 12.Polichnowski AJ, Griffin KA, Picken MM, Licea-Vargas H, Long J, Williamson GA, Bidani AK. Hemodynamic basis for the limited renal injury in rats with Angiotensin II-induced hypertension. Am J Physiol. 2015;308:F252–F260. doi: 10.1152/ajprenal.00596.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. AHA Scientific Statement. Recommendation for blood pressure measurements in humans and animals. Part 2: Blood pressure measurements in experimental animals. Hypertension. 2005;45:299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- 14.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric BP monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–F398. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 15.Griffin KA, Picken M, Bidani AK. Radiotelemetric BP monitoring, antihypertensives and glomeruloprotection in remnant kidney model. Kidney Int. 1994;46:1010–1018. doi: 10.1038/ki.1994.361. [DOI] [PubMed] [Google Scholar]
- 16.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical determinant of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 17.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens. 2001;14:311–320. doi: 10.1016/s0895-7061(00)01282-6. [DOI] [PubMed] [Google Scholar]
- 19.Griffin KA, Abu-Amarah I, Picken M, Bidani AK. Renoprotection by ACE inhibition or aldosterone blockade is blood pressure dependent. Hypertension. 2003;41:201–206. doi: 10.1161/01.hyp.0000049881.25304.73. [DOI] [PubMed] [Google Scholar]
- 20.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:1003–1010. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 21.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. ‘Step’ vs ‘Dynamic’ autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol. 2003;285:F113–F120. doi: 10.1152/ajprenal.00012.2003. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol. 2005;289:F305–F313. doi: 10.1152/ajprenal.00349.2004. [DOI] [PubMed] [Google Scholar]
- 23.Griffin KA, Polichnowski A, Litbarg N, Picken M, Venkatachalam MA, Bidani AK. Critical blood pressure threshold dependence of hypertensive injury and repair in a malignant nephrosclerosis model. Hypertension. 2014;64(4):801–807. doi: 10.1161/HYPERTENSIONAHA.114.03609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamdani BH, Lim VS, Mahurkar SC, Katz AI, Dunes G. Recovery from prolonged renal failure in patients with accelerated hypertension. N Engl J Med. 1974;291:1343–1344. doi: 10.1056/NEJM197412192912509. [DOI] [PubMed] [Google Scholar]
- 26.Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med. 2006;57:365–380. doi: 10.1146/annurev.med.57.121304.131342. [DOI] [PubMed] [Google Scholar]
- 27.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 28.Galis ZS, Thrasher T, Reid DM, Stanley DV, Oh YS. Investing in high blood pressure research: a national institutes of health perspective. Hypertension. 2013;61:757–761. doi: 10.1161/HYPERTENSIONAHA.111.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin KA, Bidani AK. Angiotensin II type 2 receptor in chronic kidney disease: the good side of angiotensin II? Kidney Int (Commentary) 2009;75:1006–1008. doi: 10.1038/ki.2009.59. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 31.Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004;43:752–759. doi: 10.1161/01.HYP.0000120971.49659.6a. [DOI] [PubMed] [Google Scholar]
- 32.Polichnowski AJ, Cowley AW., Jr Pressure-induced renal injury in angiotensin II versus norepinephrine-induced hypertensive rats. Hypertension. 2009;54:1269–1277. doi: 10.1161/HYPERTENSIONAHA.109.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polichnowski AJ, Jin C, Yang C, Cowley AW., Jr Role of renal perfusion pressure versus angiotensin II on renal oxidative stress in angiotensin II-induced hypertensive rats. Hypertension. 2010;55:1425–1430. doi: 10.1161/HYPERTENSIONAHA.110.151332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 35.Griffin KA, Bidani AK. Hypertensive renal damage: insights from animal models and clinical relevance. Current Hypertension Reports. 2004;6:145–153. doi: 10.1007/s11906-004-0091-8. [DOI] [PubMed] [Google Scholar]
- 36.Bidani AK, Mitchell KD, Schwartz MM, Navar LG, Lewis EJ. Absence of progressive glomerular injury in a normotensive rat remnant kidney model. Kidney Int. 1990;38:28–38. doi: 10.1038/ki.1990.163. [DOI] [PubMed] [Google Scholar]
- 37.Griffin KA, Picken MM, Churchill M, Churchill P, Bidani AK. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol. 2000;11:497–506. doi: 10.1681/ASN.V113497. [DOI] [PubMed] [Google Scholar]
- 38.Griffin KA, Kramer H, Bidani AK. Adverse renal consequences of obesity. Am J Physiol. 2008;94:F685–F696. doi: 10.1152/ajprenal.00324.2007. [DOI] [PubMed] [Google Scholar]
- 39.Baylis C, Wilson CB. Sex and the single kidney. Am J Kidney Dis. 1989;13:290–298. doi: 10.1016/s0272-6386(89)80035-6. [DOI] [PubMed] [Google Scholar]
- 40.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest. 2015;125:1311–1318. doi: 10.1172/JCI78885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arendshorst WJ, Gottschalk CW. Glomerular ultrafiltration dynamics: euvolemic and plasma volume-expanded rats. Am J Physiol. 1980;239:F171–F186. doi: 10.1152/ajprenal.1980.239.2.F171. [DOI] [PubMed] [Google Scholar]
- 42.Tucker BJ, Blantz RC. Effects of glomerular filtration dynamics on the glomerular permeability coefficient. Am J Physiol. 1981;240:F245–F254. doi: 10.1152/ajprenal.1981.240.3.F245. [DOI] [PubMed] [Google Scholar]
- 43.Bidani AK, Picken MM, Bakris G, Griffin KA. Lack of evidence of BP independent protection by renin-angiotensin system blockade after renal ablation. Kidney Int. 2000;57:1651–1661. doi: 10.1046/j.1523-1755.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 44.Bidani AK, Griffin KA. The benefits of renin-angiotensin blockade in hypertension are dependent on blood-pressure lowering. Nature Clin Prac Nephrol (viewpoint) 2006;2:542–543.42. doi: 10.1038/ncpneph0299. [DOI] [PubMed] [Google Scholar]
- 45.Griffin KA, Picken M, Giobbie-Hurder A, Bidani AK. Low protein diet mediated renoprotection in remnant kidneys: renal autoregulatory vs hypertrophic mechanisms. Kidney Int. 2003;63:607–616. doi: 10.1046/j.1523-1755.2003.00759.x. [DOI] [PubMed] [Google Scholar]
- 46.Baylis C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr Opin Nephrol Hypertens. 2012;21:1–6. doi: 10.1097/MNH.0b013e32834d54ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin K, Polichnowski A, Licea-Vargas H, Picken M, Long J, Williamson G, Bidani A. Large BP-dependent and -independent differences in susceptibility to nephropathy after nitric oxide inhibition in Sprague-Dawley rats from two major suppliers. Am J Physiol. 2012;302:F173–F182. doi: 10.1152/ajprenal.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachmann S, Bosse HM, Mundel P. Topography of nitric oxide synthesis by localizing constitutive NO synthesis in mammalian kidney. Am J Physiol. 1995;268:F885–F898. doi: 10.1152/ajprenal.1995.268.5.F885. [DOI] [PubMed] [Google Scholar]
- 49.Polichnowski AJ, Licea-Vargas H, Picken MM, Long J, Bisla R, Williamson GA, Bidani AK, Griffin KA. Glomerulosclerosis in the diet induced obesity model correlates with sensitivity to nitric oxide inhibition but not glomerular filtration or hypertrophy. Am J Physiol. 2015;309:F791–F799. doi: 10.1152/ajprenal.00211.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen PK, Hansen HP, Parving HH. Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int. 1997;52:1369–1374. doi: 10.1038/ki.1997.463. [DOI] [PubMed] [Google Scholar]
- 51.Christensen PK, Hommel EE. Impaired autoregulation of the glomerular filtration rate in patients with nondiabetic nephropathy. Kidney Int. 1999;56:1517–1523. doi: 10.1046/j.1523-1755.1999.00676.x. [DOI] [PubMed] [Google Scholar]
- 52.Fotheringham J, Odudu A, McKane W, Ellam T. Modification of the relationship between blood pressure and renal albumin permeability by impaired excretory function and diabetes. Hypertension. 2015;65:510–516. doi: 10.1161/HYPERTENSIONAHA.114.04656. [DOI] [PubMed] [Google Scholar]
- 53.Upadhyay A, Earley A, Haynes SM, Uhlig K. Systemic review: Blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Int Med. 2011;154:541–548. doi: 10.7326/0003-4819-154-8-201104190-00335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.