Abstract
Psychopharmacology research has amassed substantial evidence for similarities between synthetic cathinones and other commonly abused psychostimulants. Few studies have utilized drug discrimination methods to investigate synthetic cathinones, and the precise neurochemical substrates underlying their interoceptive effects have not been examined. The present study assessed the involvement of D1 and D2 dopaminergic receptors in the stimulus effects of MDPV and mephedrone in rats trained to discriminate d-amphetamine. Eight male Sprague-Dawley rats were trained to discriminate 0.5 mg/kg d-amphetamine (AMPH) from saline. Dose-response curves were then generated with AMPH (0.0 – 1.0 mg/kg), MDPV (0.0 – 1.0 mg/kg), and mephedrone (MEPH) (0.0 – 2.0 mg/kg). Subsequently, Sch 39166 (0.3 mg/kg) and haloperidol (0.5 mg/kg) were administered in combination with select doses of MDPV and MEPH. Both MDPV and MEPH produced full substitution for AMPH. Sch 39166 produced a downward shift in the MDPV and MEPH dose response curves and haloperidol produced similar results with MDPV. These preliminary findings indicate MDPV and MEPH produce interoceptive stimuli that are similar to those produced by d-amphetamine and that D1 and D2 dopamine receptors contribute to these effects. Additional studies are warranted to investigate the contribution of other receptor mechanisms involved in the interoceptive stimuli produced by synthetic cathinones.
Keywords: Synthetic cathinones, methylenedioxypyrovalerone, MDPV, 4‐methylmethcathinone, 4-MMC, mephedrone, dopamine, drug discrimination, rats
Introduction
In the early to mid-2000s, synthetic cathinone derivatives were introduced as alternatives to illicit psychostimulants in the United States and the United Kingdom (Goodnough and Zezima, 2011; Winstock and Ramsey, 2010). The popularity of synthetic cathinones soon gave rise to significant public health concerns, indicated by numerous incident reports from law enforcement and hospital emergency rooms. In 2011, the United States Drug Enforcement Administration added synthetic cathinones to the Schedule I list of controlled substances (Drug Enforcement Administration, 2011). Despite their illicit status, these substances are still abused recreationally (Ashrafioun et al., 2016) and new derivatives continue to be introduced to the illicit drug market.
Methylone, 3,4-methylenedioxypyrovalerone (MDPV), and 4-methylmethcathinone (mephedrone) were among the first generation of synthetic cathinones (Valente et al., 2014). As such, most of the extant preclinical literature has focused on these substances. A growing body of research supports the legal restriction of these substances, as their pharmacology and abuse liability are comparable to other abused psychostimulants (Cameron et al., 2013; Baumann et al, 2012). However, only a few published studies have examined the interoceptive effects of MDPV or mephedrone using animal models of drug discrimination (Gannon et al., 2016; Fantegrossi et al., 2013; Gatch et al., 2013; Varner et al., 2013). To date, no published studies have examined the specific receptor mechanisms contributing to the interoceptive stimulus effects of these substances. The aim of the present study was to assess the involvement of D1 and D2 dopaminergic receptors in the stimulus effects of MDPV and mephedrone in rats trained to discriminate amphetamine.
Materials and Methods
Subjects
Eight adult male Sprague-Dawley rats (350 – 450g) were housed individually in polycarbonate cages with Teklad corncob bedding (#7097, Envigo, Madison, Wisconsin, USA). The animal facilities were maintained at a (20±2°C) and humidity (50±5%) under a 12:12 light/dark cycle, (lights on from 0700 to 1900). Subjects were given ad libitum access to water in the home cages and commercial rodent diet (LabDiet® 5001, PMI Nutrition Int. LLC, Brentwood, Missouri, USA) was restricted to maintain 85-90% of free-feeding weights. All procedures were reviewed and approved by the Western Michigan University Institutional Animal Care and Use Committee and were in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council of the National Academies, 2011) and EU Directive 2010/63/EU.
Apparatus
Training and testing were conducted in eight sound-attenuated operant conditioning chambers (ENV-001, Med Associates Inc., St. Albans, Vermont, USA) and experiments were controlled using Med-PC software (version IV, Med Associates Inc.). Subjects received 45 mg Dustless Precision Pellets® (Product# F0021, Bio-Serv Inc., Flemington, New Jersey, USA) as reinforcers during training sessions.
Drugs
d-Amphetamine-hemisulfate (Sigma-Aldrich Corp., St. Louis, Missouri, USA), Sch 39166 (Schering-Plough, Kenilworth, New Jersey, USA), 3,4-methylenedioxypyrovalerone-hydrochloride and mephedrone-hydrochloride (National Institute on Drug Abuse, Bethesda, Maryland, USA), were each dissolved in 0.9% sodium chloride. Haloperidol (Sigma-Aldrich Corp., St. Louis, Missouri, USA) was first dissolved in a few drops of 0.1 M HCl before being added to sterile water and then pH adjusted with 0.1 M NaOH. All drugs were administered via intraperitoneal (ip) injections at a constant volume of 1 ml/kg body weight. All doses were calculated based on the weights of solid compound.
Procedures
Preliminary training, discrimination training, and stimulus generalization testing procedures were identical to those used in previous studies in our laboratory and described elsewhere (Harvey and Baker, 2016). Rats were trained to reliably discriminate 0.5 mg/kg d- amphetamine (AMPH) from vehicle (saline) under a fixed ratio 20 (FR 20) schedule of food reinforcement. Dose-response curves were then generated with the following compounds: AMPH (0.0 – 1.0 mg/kg), MDPV (0.0 – 1.0 mg/kg), and mephedrone (MEPH) (0.0 – 2.0 mg/kg). All doses were administered 10 minutes pre-session. In addition, doses of the selective D1 receptor antagonist Sch 39166 (0.3 mg/kg, I.P. 30 min) or the high affinity D2 receptor antagonist haloperidol (0.5 mg/kg, I.P. 60 min) were given in combination with select doses of MDPV (0.0, 0.0625, 0.25, & 0.5 mg/kg) and MEPH (0.0, 0.5, 1.0, 2.0 mg/kg).
Data Analysis
Dose-response curves were graphed for each test compound alone and in combination with each antagonist, with the mean percentage of drug-appropriate lever responses (±SEM) as well as the mean (±SEM) response rate (lever presses per second) plotted as a function of test dose. Substitution test results were included for any subject that emitted at least ten responses on either lever during a test session. Response rate was included for all tests regardless of the number of responses made. For test compounds that displayed full substitution (> 80% drug- appropriate responding), ED50 values were calculated via straight-line regression of the linear portion of each dose-response curve. A one-way repeated-measures ANOVA was conducted on response rates obtained during test sessions with compound. Graphical and statistical analyses were conducted using GraphPad Prism (version 6, GraphPad Software, Inc., La Jolla, California, USA).
Results
Stimulus Generalization
Dose response curves generated from stimulus generalization tests with AMPH, MDPV, and MEPH, and stimulus antagonism tests with MDPV and MEPH in combination with Sch 39166 (0.3 mg/kg) or haloperidol (0.5 mg/kg) are displayed in figure 1. AMPH produced a dose- dependent increase in AMPH-lever responding with full substitution at the 1.0 mg/kg dose. The ED50 value for AMPH was calculated at 0.28 mg/kg (95% CI [0.003 – 0.47 mg/kg]). A one-way repeated-measures (RM) ANOVA showed a statistically significant effect of AMPH dose on response rate (F4, 28 = 2.86, p < .05). Dunnett's multiple comparison tests indicated that 1.0 mg/kg AMPH significantly reduced response rate compared to saline (p < .05).
Figure 1.
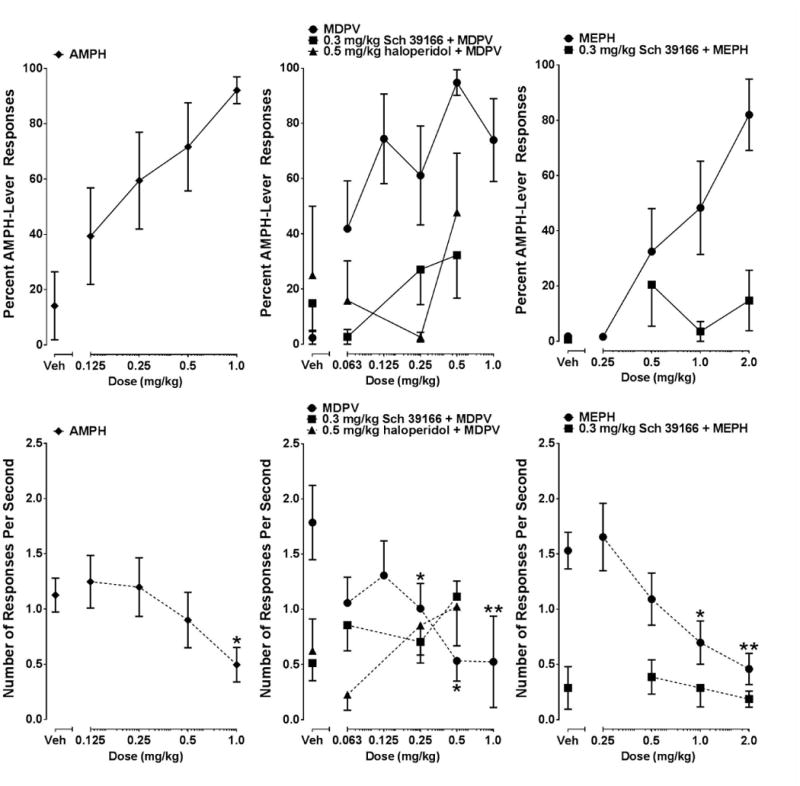
Dose-response curves determined from stimulus generalization tests with AMPH, MDPV, and MEPH and stimulus antagonism tests with 0.3 mg/kg Sch 39166 or 0.5 mg/kg haloperidol in combination with selected doses of MDPV or MEPH in rats trained to discriminate 0.5 mg/kg AMPH from saline (n=8). Graphs in the upper panel depict percentage of responses on the AMPH-appropriate lever. Graphs in the lower panel depict response rate. Individual points represent group means (± SEM). Significant Dunnett's multiple comparison tests on response rate between selected doses and vehicle are represented by * (p < .05) or ** (p < .01).
MDPV produced a dose-dependent increase in AMPH-appropriate responding and fully substituted at the 0.50 mg/kg dose. The ED50 for MDPV was estimated at 0.15 mg/kg (95% CI [0.04 – 0.25 mg/kg]). A one-way RM ANOVA indicated a statistically significant overall effect of MDPV dose on response rate (F5, 35 = 3.78, p < .01). Dunnett's multiple comparisons found that response rates were significantly decreased following 0.25 mg/kg (p < .05), 0.50 mg/kg (p < .05), and 1.0 mg/kg (p < .01) MDPV compared to response rates following saline injections.
MEPH yielded dose-dependent increases in AMPH-lever responding and produced full substitution at 2.0 mg/kg. The ED50 for MEPH was 1.15 mg/kg (95% CI [0.83 – 1.60 mg/kg]). A one-way RM ANOVA revealed a statistically significant effect of MEPH dose on response rate (F4, 28 = 9.73, p < .001). Dunnett's multiple comparison tests indicated 1.0 mg/kg (p < .05) and 2.0 mg/kg (p < .01) MEPH significantly lowered response rate compared to saline.
Stimulus Antagonism
Sch 39166 (0.3 mg/kg) produced a marked downward shift in the dose response curves for both MDPV and MEPH at all doses tested, with haloperidol (0.5 mg/kg) producing similar results with doses of MDPV. Specifically, doses of MDPV (0.5 mg/kg) and MEPH (2.0 mg/kg) that previously produced full substitution failed to do so when administered in combination with Sch 39166 or haloperidol.
One-way RM ANOVA tests did not find any significant effects on response rates following administration of either antagonist with MDPV or MEPH compared to rates following the antagonists alone. However, paired t-tests did find that Sch 39166 administered alone significantly reduced response rate compared to vehicle alone (t(7) = 4.19, p < .01), as did haloperidol (t(6) = 2.49, p < .05).
Discussion
The primary aim of this study was to evaluate the contribution of D1 and D2 dopamine receptors to the discriminative stimulus effects of MDPV and MEPH in rats trained to discriminate the closely-related and well-characterized psychostimulant, d-amphetamine. The current results that MDPV and MEPH produced full substitution for AMPH are consistent with previous reports that these substances share similar discriminative stimulus effects with cocaine and methamphetamine (Gannon et al, 2016; Gatch et al., 2013; Varner et al, 2013).
Neurochemical studies indicate that MDPV exerts its effects primarily through the uptake blockade of the dopamine transporter (DAT), with only very weak effects on monoamine release. In contrast, mephedrone is a non-selective releaser at DAT, as well as the serotonin transporter (SERT) and the norepinephrine transporter (NET) (Baumann et al., 2013; Eshleman et al., 2013). The direct involvement of dopaminergic actions in MDPV's effects is supported by the present findings that MDPV substitution for AMPH was attenuated by both the D1 receptor antagonist Sch 39166 and the D2 antagonist haloperidol. Interestingly, Sch 39166 also attenuated MEPH substitution. Previous findings from our laboratory showed that MEPH fully substituted in rats trained to discriminate MDMA (a potent serotonin releaser) from saline (Harvey and Baker, 2016). Considered together, these results may suggest that MEPH produces interoceptive stimulus effects through both dopaminergic (i.e. amphetamine-like) and serotonergic (i.e. MDMA-like) mechanisms. It should be noted, however, that Sch 39166 also significantly reduced response rates, indicating that it may have non-specific global effects that interfered with the discrimination of the test drugs.
The current study did not assess Sch 39166 or haloperidol for antagonism of d‐amphetamine, precluding a direct comparison of their effects on MDPV or MEPH stimulus generalization to their effects on the training stimulus. However, previous reports indicate potent blockade of the d-amphetamine discriminative cue by these antagonists (Callahan et al., 1991; Exner et al., 1989; Powell and Holtzman, 2000; West et al., 1995). Considered together with previous findings, the current results strongly indicate MDPV and MEPH stimulus generalization for d-amphetamine can be attributed to their actions on dopamine receptors.
In summary, these results indicate that MDPV and MEPH produce interoceptive stimuli similar to those produced by the potent stimulant, d-amphetamine. These stimulant-like effects appear to be at least in part mediated by both the D1 and the D2 dopamine receptors. However, additional antagonism tests are needed to determine if these effects persist at antagonist doses that do not produce significant reductions in overall response rate. Further, given that MEPH has been found to influence both serotonergic as well as dopaminergic activities in the brain, future tests with 5-HT antagonists are warranted in order to determine the extent to which each of these components contribute to its overall discriminative stimulus properties.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (R15DA038295). The National Institute on Drug Abuse drug control supply program provided the MDPV and mephedrone used in this study.
References
- Ashrafioun L, Bonadio FA, Baik KD, Bradbury SL, Carhart VL, Cross NA, et al. Patterns of use, acute subjective experiences, and motivations for using synthetic cathinones (“bath salts”) in recreational users. J of Psychoact Drugs. 2016;48:336–343. doi: 10.1080/02791072.2016.1229875. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. European J of Pharmacol. 2013;698(1):1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacol. 2012;38(4):552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan PM, Appel JB, Cunningham KA. Dopamine D1 and D2 mediation of the discriminative stimulus properties of d-amphetamine and cocaine. Psychopharmacology. 1991;103(1):50–55. doi: 10.1007/BF02244073. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, Felice L, Glennon R. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacol. 2013;227(3):493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration (DEA) Federal Register. Vol. 76. Washington, DC: U.S. Government Printing Office; 2011. Schedules of controlled substances: Temporary placement of three synthetic cathinones into schedule I; p. 204. [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85(12):1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner M, Furmidge LJ, White FJ, Clark D. Inhibitory effects of partial D2 dopamine receptor agonists on the d-amphetamine discriminative cue. Behav Pharmacol. 1989;1(2):101–111. [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’constituent 3, 4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacol. 2013;38(4):563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: Drug discrimination, locomotor activity, and thermoregulation. J of Pharmacol and Experiment Therap. 2016;356(3):615–623. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch M, Taylor C, Forster M. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24(5-6):437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnough A, Zezima K. An alarming new stimulant, legal in many states. The New York Times; 2011. Jul 16, [Accessed 7 Nov 2014]. http://www.nytimes.com/2011/07/17/us/17salts.html. [Google Scholar]
- Harvey EL, Baker LE. Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine MDMA mixture. Psychopharmacol. 2016;233(4):673–680. doi: 10.1007/s00213-015-4142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guide for the care and use of laboratory animals. Washington, D. C.: The National Academies Press; 2011. [Google Scholar]
- Powell KR, Holtzman SG. Modulation of the discriminative stimulus effects of d-amphetamine by mu and kappa opioids in squirrel monkeys. Pharmacol, Biochem, Behav. 2000;65(1):43–51. doi: 10.1016/s0091-3057(99)00183-5. [DOI] [PubMed] [Google Scholar]
- Valente MJ, Pinho PG, Bastos MD, Carvalho F, Carvalho M. Khat and synthetic cathinones: A review. Arch of Toxicol. 2014;88(1):15–45. doi: 10.1007/s00204-013-1163-9. [DOI] [PubMed] [Google Scholar]
- Varner K, Daigle K, Weed P, Lewis P, Mahne S, Sankaranarayanan A, et al. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacol. 2013;225(3):675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West WB, Van Groll BJ, Appel JB. Stimulus effects of d-amphetamine II: DA, NE, and 5-HT mechanisms. Pharmacology Biochemistry and Behavior. 1995;51(1):69–76. doi: 10.1016/0091-3057(94)00361-l. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Ramsey JD. Legal highs and the challenges for policy makers. Addict. 2010;105(10):1685–1687. doi: 10.1111/j.1360-0443.2010.03163.x. [DOI] [PubMed] [Google Scholar]


