Abstract
Background
In premature children, erythropoiesis stimulating agents (ESAs) may improve developmental outcome. It is not clear which of several potential mechanisms are responsible for this improvement. High resolution MRI and diffusion tensor imaging characterize brain structure and white matter organization, offering possible insight into the long-term effect of ESAs on brain development.
Design/Methods
MRI scans were performed at 3.5 to 4 years of age on former preterm infants treated with ESAs or placebo, and on healthy term controls. Mean cortical thickness, surface area and fractional anisotropy (FA) were compared across study groups, and correlated with general IQ measures.
Results
Univariate analysis found no significant effect of ESAs on cortical thickness (p = .366), surface area (p = .940) or FA (p = .150); however, there was a greater increase in FA among ESA treated girls. Group analysis found significant correlations between FA and Full Scale IQ (p = .044) and Verbal IQ (p = .036), although there was no significant relationship between Full Scale IQ and FA among just the preterm children.
Conclusions
ESA treatment may have a preferential effect on white matter development in girls, although factors other than just whole brain FA are involved in mediating cognitive outcome.
Introduction
Prematurity affects 15 million infants annually (1). Although survivability has improved in recent decades, premature infants still face major long-term sequelae including cerebral palsy, visual problems and intellectual disability. Outcome for infants born weighing less than 1000 grams is particularly worrisome, with 71% of survivors experiencing some degree of neurodevelopmental impairment (2). Reducing this disease burden is an important public health issue throughout the world.
Recent work suggests that erythropoiesis stimulating agents (ESAs) such as erythropoietin (Epo) and Darbepoetin (Darbe) may provide neuroprotection after premature birth. ESAs are essential for normal brain development and augment a variety of potential neuroprotective mechanisms including promoting neurogenesis and angiogenesis (3–4) and inhibiting apoptotic, excitotoxic and oxidative injury to neurons and oligodendroglia (5–11). ESAs may improve neurologic outcome of term infants with hypoxic ischemic encephalopathy (12), and appear safe in premature infants as well (13), decreasing major morbidity (14) and possibly improving cognitive outcome at 18–22 months (15), 3.5–4 years (16) and at 10 years (17), though some trials reporting no difference have been published (18–19).
While accumulating evidence supports a role for ESAs in the management of premature children, it is not clear which of several different mechanisms of action are responsible for beneficial effects in this clinical population. It would be helpful if dosing decisions or patient selection could target specific neuronal mechanisms. MRI might provide this information. High resolution MRI and diffusion tensor imaging (DTI) demonstrate significant consequences of premature birth (20–30). Although recent neuroimaging reports suggest that ESAs given in the first days of life to premature infants reduce brain injury at term (31–32), we are aware of no studies that characterize neuroimaging at later ages, or that integrate neuroimaging and cognitive outcome in children treated with ESAs.
Our recent work demonstrated improved cognitive outcome at 3.5 to 4 years of age in premature children treated with ESAs (16). Here we report results of DTI and high resolution MRI in this cohort. Our hypothesis was that ESAs would improve cortical structure and white matter organization reflected by a trend toward normalizing surface area, cortical thickness, and fractional anisotropy. Furthermore, we predicted that these anticipated anatomic effects of ESAs would contribute to better cognitive outcomes.
Methods
Participants
This investigation is part of a larger ongoing study of developmental follow up after prematurity being conducted at the University of New Mexico. The initial study (NCT 00334737) enrolled preterm infants 500–1,250 grams birth weight at ≤48 hours of age. Infants were randomized to one of three groups: Epo, 400 units/kg, given three times a week; Darbe, 10 ug/kg, given once a week, with sham dosing two other times per week; or placebo, consisting of three sham doses per week. Dosing continued until 35 completed weeks gestation. Details of methods and developmental outcome at 2 years have been published (15).
Children enrolled in the initial study were eligible for the BRITE (BRain Imaging and Developmental Follow-up of Infants Treated with Erythropoietin; NCT 01207778) follow up study, performed at the University of Utah and the University of New Mexico. In addition, healthy children previously born term (TC) without hospital complications were enrolled at the New Mexico site. Institutional Review Boards approved the study at both sites.
Developmental Assessments
Developmental assessments were acquired at 3.5–4 years of age using the Wechsler Preschool and Primary Scale of Intelligence - III (WPPSI-III) (33) administered by certified examiners (JL, MS) masked to the treatment group. The WPPSI-III is standardized and normed for children 2 years, 6 months through 7 years of age, and is a widely used scale of general cognitive abilities. Demographic measures were collected from parents. Results of developmental assessments at 3.5–4 years have been published (16).
Statistical Analysis
Rather than evaluate a very large number of specific regions with less statistical power, we focused on “global” neuroimaging variables, one each for cortical surface area, cortical thickness, and fractional anisotropy. Univariate analyses of treatment effects were conducted for each global imaging variable, and significant findings were followed up with more detailed analyses. As noted below, site of imaging acquisition and gender were included as covariates in all analyses. Statistical analyses were conducted across all three groups (placebo, ESA, term controls) for descriptive purposes; our hypotheses of beneficial effects of ESA on the three brain variables were tested through three univariate general linear models (GLM). As each hypothesis was pursued through a single analysis, correction for multiple comparisons was not necessary.
MRI Data Acquisition
Structural T1 imagines were obtained from Magnetization Prepared Rapid Gradient Echo sequences at both sites using a 3 tesla MRI scanner, and analyzed using the FreeSurfer data processing program as previously described (25). FreeSurfer provides separate measures of volume, surface area and cortical thickness for each anatomic region. In analyses reported below we focus on total cortical surface area and mean cortical thickness.
Diffusion images were acquired in New Mexico using a 30 gradient direction coil and 2 mm slice thickness, and at the Utah site using a 24 gradient direction coil with 3.4 mm slice thickness. Data were processed using the FSL software package (www.fmrib.ox.ac.uk/fsl). Fractional anisotropy (FA) images were calculated and normalized to a template using a nonlinear registration algorithm (fnirt/FSL). A 50 region Johns Hopkins atlas was used to calculate mean FA values over 50 atlas defined regions (34).
Results
Participants
77 participants were followed as part of the BRITE study (16), of whom 67 provided at least some adequate imaging datasets at 3.5 to 4 years of age. The 10 scan failures were due to excessive movement. All 21 former preterm Utah subjects and 14 former preterm New Mexico subjects who did not fall asleep naturally were sedated with chloral hydrate. Because initial developmental testing revealed no significant differences between the Epo and Darbe groups, these children were combined into a single ESA treated group. The final cohort consisted of 11 premature children treated with placebo (UNM male/female = 2/3, Utah male/female = 4/2), 33 premature children treated with ESAs (UNM male/female = 9/7, Utah male/female = 9/8), and 23 term born healthy control children. This was a subset of the same cohort of children who underwent full developmental assessment (reported in Ohls et al 2016).
Demographic data
Basic demographic information on each group is provided in Table 1. There were no significant differences between placebo and ESA groups in terms of age at testing, gestational birth age or gender. Principal Components Analysis (PCA) was used to reduce seven demographic variables into a “Socioeconomic Composite” (SEC) and a “Family Stress Composite” (FSC). Higher scores on the SEC indicated greater income and education, and higher scores on the FSC indicated more family moves, more children in the home and younger maternal age. The placebo group had higher scores than ESA groups on FSC (p=0.018).
Table 1.
Group characteristics for participants contributing imaging and cognitive data.
| Placebo (N = 11) | ESA (N =33) | Term (N = 23) | Placebo vs. ESA | ESA vs. Term | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p | p | |
| Age at Testing (mos.) | 48.55 | 3.04 | 48.91 | 3.83 | 45.09 | 2.13 | .77 | <.001 |
| Gestational age at birth (weeks) | 27.88 | 1.39 | 27.22 | 1.69 | 39.04 | 1.38 | .25 | <.001 |
| Gender (M/F) | 6/5 | 18/15 | 10/13 | .99 | .41 | |||
| Full Scale IQ | 78.73 | 21.02 | 92.21 | 16.17 | 102.30 | 13.00 | .03 | .016 |
| Income | 3.73 | 1.74 | 4.76 | 2.14 | 5.00 | 1.66 | .16 | .64 |
| Maternal Education | 4.27 | 1.10 | 4.82 | 1.24 | 5.36 | 1.36 | .20 | .13 |
| Maternal age | 24.09 | 3.83 | 27.82 | 6.68 | 29.45 | 7.46 | .09 | .40 |
| Number family moves | 2.73 | 1.90 | 1.33 | 1.32 | 1.23 | 1.44 | .01 | .78 |
| Number children under 6 | 2.36 | 1.43 | 1.55 | .67 | 1.68 | .78 | .01 | .49 |
| Ethnicity: Hispanic/Anglo | 4/7 | 13/20 | 14/9 | .86 | .11 | |||
| Primary Language: English/Spanish | 11/0 | 28/5 | 20/3 | .82 | ||||
| Socioeconomic Composite: SEC | −.12 | .71 | −.02 | 1.09 | .05 | .23 | .77 | .81 |
| Family Stress Composite: FSC | .99 | 1.08 | −.10 | .78 | −.20 | 1.00 | .001 | .65 |
Note: independent samples t-tests and chi-square analyses compared groups (though the chi square involving placebo group vs. ESA could not be calculated for primary language because one entry was zero). Higher SEC indicates greater income and maternal education. Higher FSC corresponds to more family moves, more young children in the home and younger mothers.
Imaging data
Global imaging data are summarized in Table 2. Total surface area, mean cortical thickness, and fractional anisotropy (FA) are indicated. To reduce the set of 50 FA values for statistical analyses, we used Principal Components Analysis and identified a single factor (termed “PC FA”) with an eigenvalue greater than one, capturing 69.49% of total variance. In Table 2 FA values were expressed as T-scores (mean = 50 and SD = 10). Because this was a 2-site imaging study, we evaluated whether imaging data was affected by scanner/site; univariate t-tests demonstrated that imaging site indeed did have a significant effect on mean cortical thickness (p < .001) and PC FA (p < .001), and therefore we covaried site in all statistical analyses.
Table 2.
Global Neuroimaging Variables
| Placebo | ESA | Term | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Mean Cortical Thickness (mm) | 10 | 2.94 | .20 | 32 | 2.87 | .22 | 23 | 3.01 | .08 |
| Cortical Surface Area (mm2) | 10 | 159930.70 | 20085.42 | 32 | 161542.47 | 21385.91 | 23 | 160055.13 | 13614.81 |
| PC FA | 11 | 42.18 | 9.38 | 32 | 45.49 | 9.62 | 18 | 58.80 | 5.35 |
Note: Only high quality data are reported; some participants did not provide data for each variable. PC FA is the first principal component of fractional anisotropy values for 50 white matter tracts, expressed as a T-score (mean = 50, SD = 10). See text for statistical data.
Group differences in global imaging parameters
Separate univariate GLM analyses were conducted for each imaging variable. Fixed effects were gender, group (placebo, ESA, term) and site of image acquisition (NM vs. UT), while the two demographic factors served as covariates. Our initial analysis included all three groups. There was no significant effect of group for mean cortical thickness (F(2, 54) = 1.917, p = .157, partial eta squared = .066), or cortical surface area (F(2, 50) = .844, p = .436, partial eta squared = .033). However, there were significant differences in mean PC FA across the three groups (F(2, 50) = 6.547, p = .003, partial eta squared = .208), with the term group showing greatest PC FA. In a follow up analysis, the term group PC FA was significantly greater than the ESA treated preterm group (F(1, 41) = 8.408, p = .006, partial eta squared = .170; see Table 2).
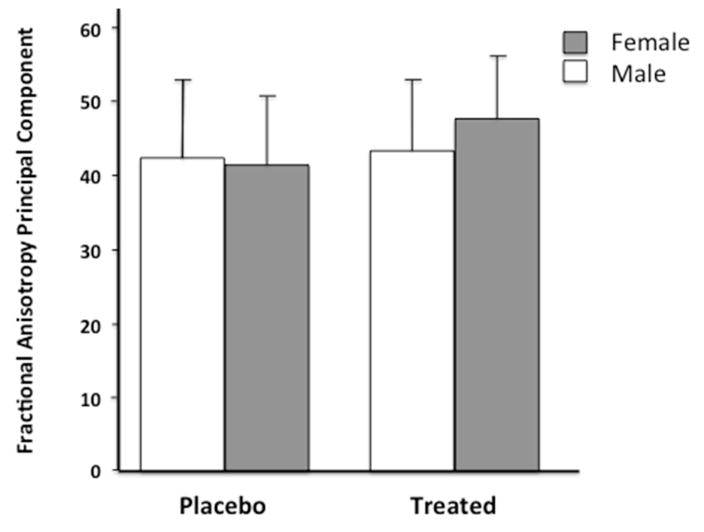
Our second set of analyses evaluated the effect of ESAs on just the preterm children (ESA treated vs placebo). There was no significant group effect for mean cortical thickness (F(1, 34) = .838, p = .366, partial eta squared = .024), surface area (F(1, 34) = .006, p = .940, partial eta squared = .000), or PC FA (F(1, 35) = 2.171, p = .150, partial eta squared = .058). However, a gender by group interaction was found (F(1,35) = 5.738, p = .022, partial eta squared = .141). Figure 1 shows that ESA treatment increased PC FA in females more than males.
Figure 1. ESA Treatment Effect On Fractional Anisotropy By Gender.
Interaction of gender and group (Placebo, Treated) on global fractional anisotropy of white matter tracts. Fractional anisotropy principal component is expressed as a T score (mean for entire sample, including term, was 50, SD =10).
Specific white matter tracts in females
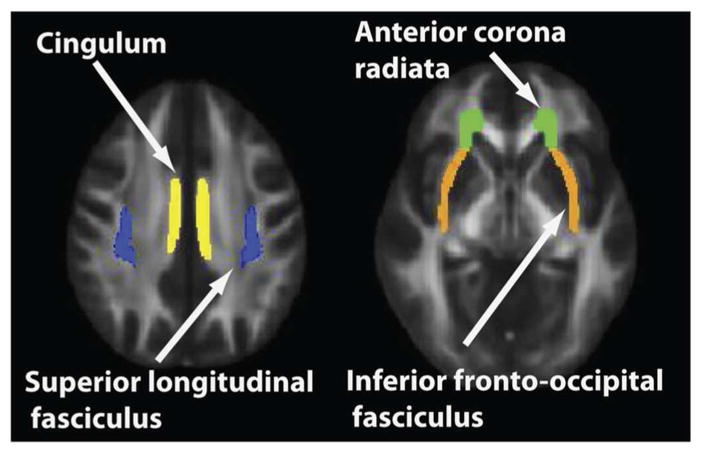
We identified white matter tracts most affected by ESA treatment in females. Partial correlations were obtained between group (placebo vs. ESA) and each tract (averaging left and right hemisphere values for bilateral tracts to reduce the number of correlations examined), controlling for site, SEC and FSC. Those tracts showing the greatest increase in PC FA with treatment were: cingulum (r = .687, p = .003), anterior corona radiate (r = .628, p = .009), superior longitudinal fasciculus (r = .606, p = .013), and the inferior fronto-occipital fasciculus (r = .543, p = .030). Figure 2 identifies the location of these fiber tracts most affected by ESA treatment in girls.
Figure 2. Fiber Tracts Most Affected By ESA Treatment In Girls.
The four fiber tracts most affected by ESA treatment in girls were cingulum, anterior corona radiata, superior longitudinal fasciculus, and inferior fronto-occipital fasciculus. Mean FA was calculated over each of these tracts as identified above.
Neuroimaging and cognition
Our prior publications on BRITE participants reported the beneficial effects of ESAs for Full Scale IQ (16) (see also Table 1). Given the above results regarding the effects of ESAs on PC FA, we examined relationships of PC FA with Full Scale IQ, and also with the two components of Full Scale IQ -- Verbal IQ and Performance IQ. All analyses controlled for site, gender, test age and the two demographic variables. Across all participants in the three groups, the partial correlation of PC FA with FSIQ was r = .273, p = .044; for VIQ, r = .284, p = .036; for PIQ, r = .219, p = .108. Among just the children born preterm (placebo plus ESA) the partial correlations were: for FSIQ r = .014, p = .933; for VIQ, r = .109, p = .515; for PIQ, r = −.021, p = .902). Thus, although across all groups FSIQ and VIQ had a significant relationship with PC FA, there was no relationship among just the preterm children (placebo and ESA), suggesting that for this group, the beneficial effects of ESAs on IQ were not directly mediated by PC FA.
Discussion
This is the first study to evaluate neuroimaging at 4 years of age in premature children treated with ESAs. We previously found that early ESA treatment improves cognitive outcome. Our current results suggest that ESAs may improve white matter development, albeit only in females. Although greater PC FA was associated with higher IQ scores when all subjects were pooled together, there was no relationship when the preterm group was analyzed separately, suggesting that in preterm children, the beneficial effects of ESAs on IQ is driven by factors other than just a general improvement in white matter structure.
Preclinical work identifies multiple cellular actions of ESAs that may contribute to neuroprotection (for review, see Wu 2015) (35). Because these mechanisms affect changes in both gray and white matter, a single neuroimaging approach is unlikely to capture the complexity of an in vivo response to ESA intervention. Recent animal studies suggest that diffusion tensor imaging and MR spectroscopy may be more sensitive to ESA treatment effects than a general measure of cortical volume (36–39). This is consistent with our findings of no difference in mean cortical thickness or surface area between treatment groups, but with a trend toward increasing fractional anisotropy from untreated former preterm children (lowest), to ESA treated former preterm children, to normal term born children (see Table 2).
In addition to demonstrating a trend toward improving white matter FA in ESA treated children, we found an unexpected gender effect – ESAs increased FA in females more than males. The reason for this gender difference is not clear. This was not due to site because as noted above, our analyses included site, gender and treatment group. FA is a measure of water diffusion, ranging from 0 to 1, with higher FA values in white matter generally felt to reflect greater axonal density, diameter or myelination. Thus, FA closer to 1 presumably reflects greater white matter integrity. There is a suggestion in the preclinical literature of a gender effect – Wen et al (40) published a study of stroke in neonatal rats demonstrating that females benefit from ESA treatment more than males, however this has not been replicated to our knowledge.
Interestingly, a gender effect has been noted in transfusion studies evaluating liberal vs restrictive blood transfusion criteria. In a long-term study from the University of Iowa, liberally using blood transfusions in preterm children was associated with adverse neurocognitive outcome and reduced brain volume, worse in females (41–42). The authors noted that erythropoietin levels were higher in infants receiving fewer blood transfusions, leading to a hypothesis that perhaps liberal use of blood transfusions delays the body’s natural erythropoietin response which reduces the accompanying neuroprotection associated with erythropoietin. Thus, the findings of McCoy et al and our data are consistent with what may be a greater female sensitivity to erythropoietin levels in premature infants. Further studies are required to replicate these findings and characterize the mechanism of action for any gender effect.
Recently O’Gorman et al reported an ESA-related increase in FA of several white matter tracts, but without a gender effect (32). Differences between our results and O’Gorman et al could be related to study design. O’Gorman used high dose Epo (3000 IU/kg) given 3 times over the first 2 days of life, whereas we treated with lower doses for a much longer period of time (through 35 completed weeks of gestation). Also, although our studies had comparable numbers of DTI datasets, we had fewer untreated subjects (32 treated/11 untreated) than the O’Gorman study (24 treated/34 untreated). Finally, a major difference was age at imaging. Both studies employed a 3 tesla MRI scanner, however O’Gorman et al scanned at term, whereas our group performed MRI assessments much later, at 3.5 to 4 years of age. Longitudinal studies will be required to determine whether there is an age-related gender effect after ESA treatment.
Although we found that ESA treatment improved cognitive outcome and, separately, was associated with a trend toward increasing PC FA, the two findings were not statistically related. Thus our hypothesis of a causal link between treatment, brain and IQ was not substantiated. The hypothesis was based on an assumption that ESA treatment affects all areas of the brain, which might be expected to improve global FA and thus increase IQ. This was a reasonable assumption, as a number of authors have already reported that global FA (43–44) and regional FA (45) are related to cognitive outcome in premature children. However, these were not treatment studies, and whether ESA therapy affects the FA/IQ relationship is not known.
After identifying a potential treatment effect in females, an exploratory analysis was performed just in girls that identified 4 fiber tracts with significant increases in FA related to ESA treatment. The superior longitudinal fasciculus (arcuate fasciculus) was one of these tracts. Others have reported reduced FA in the arcuate fasciculus as a result of prematurity, correlating with language skills (44). This raises the question of whether ESA neuroprotection might preferentially affect specific fiber tracts such as the arcuate fasciculus that might be at greater risk of injury related to prematurity.
Our study has several limitations. Low subject numbers limited our approach to data analysis as we were unable to evaluate correlations between multiple brain regions and developmental outcome. Also, this was a two site study, and because we found that scanner differences significantly affected data, all analyses were controlled for site. In addition, we report here results from a single time point. Age-related changes in cortical structure and white matter development have been reported in healthy populations, and recent work suggests that in at least one region, the corpus callosum, diffusion abnormalities present at term are less apparent by 7 years of age. It is not known whether similar accelerated imaging recovery occurs after ESA treatment.
Clearly, adequately powered, longitudinal studies will be necessary to disentangle the effects of age on regional brain development and cognitive outcome after ESA therapy. Our study is a step in this process. We note the importance of controlling for imaging site variables, and identify a potential gender effect of ESA therapy on white matter development. Although we were unable to identify a specific imaging variable associated with improved cognitive outcome, our findings suggest that white matter integrity, perhaps of specific tracts, may be more important than gray matter structure in mediating the ESA treatment effect.
Acknowledgments
The authors wish to thank the research coordinators and bedside nurses involved in the original randomized study. In addition, we are indebted to the parents of our subjects for providing inspiration for this work, and the US taxpayers for funding the National Institutes of Health and this study.
Statement of Financial Support: Supported by grants from the NIH NICHD (R01 HD059856), the University of New Mexico Clinical Translational Science Center (UL1 TR000041), the University of Utah Center for Clinical and Translational Sciences (UL1TR001067), and the University of New Mexico Department of Pediatrics.
Footnotes
Disclosure: The authors have no financial relationships or possible conflicts relevant to this article to disclose.
Clinical Trial Registration: Registered on ClinicalTrials.org: NCT# 01207778; NCT# 00334737; IND #100138
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson EA, De Luca CR, Doyle LW, et al. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053–e1061. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- 3.Tsai PT, Ohab JJ, Kertesz N, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26(4):1269–74. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwai M, Cao G, Yin W, et al. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38(10):2795–803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 5.Dzietko M, Felderhoff-Mueser U, Sifringer M, et al. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15(2):177–87. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Zacharias R, Schmidt M, Kny J, et al. Dose-dependent effects of erythropoietin in propofol anesthetized neonatal rats. Brain Res. 2010 Jul 9;1343:14–9. doi: 10.1016/j.brainres.2010.04.081. [DOI] [PubMed] [Google Scholar]
- 7.Wei L, Han BH, Li Y, Keogh CL, et al. Cell death mechanism and protective effect of erythropoietin after focal ischemia in the whisker-barrel cortex of neonatal rats. J Pharmacol Exp Ther. 2006 Apr;317(1):109–16. doi: 10.1124/jpet.105.094391. [DOI] [PubMed] [Google Scholar]
- 8.Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate. 2001;79(3–4):228–35. doi: 10.1159/000047097. [DOI] [PubMed] [Google Scholar]
- 9.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–9. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 10.Lewczuk P, Hasselblatt M, Kamrowski-Kruck H, et al. Survival of hippocampal neurons in culture upon hypoxia: effect of erythropoietin. Neuroreport. 2000;11(16):3485–8. doi: 10.1097/00001756-200011090-00017. [DOI] [PubMed] [Google Scholar]
- 11.Lan KM, Tien LT, Cai Z, et al. Erythropoietin Ameliorates Neonatal Hypoxia-Ischemia-Induced Neurobehavioral Deficits, Neuroinflammation, and Hippocampal Injury in the Juvenile Rat. Int J Mol Sci. 2016;17(3):289. doi: 10.3390/ijms17030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmahdy H, El-Mashad AR, El-Bahrawy H, et al. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125(5):e1135–42. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- 13.Fauchère JC, Koller BM, Tschopp A, et al. Swiss Erythropoietin Neuroprotection Trial Group. Safety of early high-dose recombinant erythropoietin for neuroprotection in very preterm infants. J Pediatr. 2015;167(1):52–7. doi: 10.1016/j.jpeds.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Song J, Sun H, Xu F, Kang W, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol. 2016 Jul;80(1):24–34. doi: 10.1002/ana.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohls RK, Kamath-Rayne BD, Christensen RD, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133(6):1023–30. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohls RK, Cannon DC, Phillips J, et al. Preschool Assessment of Preterm Infants Treated With Darbepoetin and Erythropoietin. Pediatrics. 2016;137(3):e20153859. doi: 10.1542/peds.2015-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67(5):657–666. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 18.Newton NR, Leonard CH, Piecuch RE, Phibbs RH. Neurodevelopmental outcome of prematurely born children treated with recombinant human erythropoietin in infancy. J Perinatol. 1999;19(6 Pt 1):403–6. doi: 10.1038/sj.jp.7200244. [DOI] [PubMed] [Google Scholar]
- 19.Luciano RI, Fracchiolla A, Ricci DA, et al. Are high cumulative doses of erythropoietin neuroprotective in preterm infants? A two year follow-up report. Ital J Pediatr. 2015;41:64. doi: 10.1186/s13052-015-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boardman JP, Counsell SJ, Rueckert D, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. NeuroImage. 2006;32(1):70–8. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 21.Bjuland KJ, Rimol LM, Løhaugen GC, Skranes J. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur J Paediatr Neurol. 2014;18(5):578–90. doi: 10.1016/j.ejpn.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Ajayi-Obe M, Saeed N, Cowan FM, et al. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356(9236):1162–3. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- 23.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3(8):e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips JP, Ruhl D, Montague E, et al. Anterior cingulate and frontal lobe white matter spectroscopy in early childhood of former very LBW premature infants. Pediatr Res. 2011;69(3):224–9. doi: 10.1203/PDR.0b013e3182091d52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips JP, Montague EQ, Aragon M, et al. Prematurity affects cortical maturation in early childhood. Pediatr Neurol. 2011;45(4):213–9. doi: 10.1016/j.pediatrneurol.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunewaldt KH, Fjørtoft T, Bjuland KJ, et al. Follow-up at age 10years in ELBW children—Functional outcome, brain morphology and results from motor assessments in infancy. Early Hum Dev. 2014;90(10):571–8. doi: 10.1016/j.earlhumdev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Frye RE, Malmberg B, Swank P, et al. Preterm birth and maternal responsiveness during childhood are associated with brain morphology in adolescence. J Int Neuropsychol Soc. 2010;16(5):784–94. doi: 10.1017/S1355617710000585. [DOI] [PubMed] [Google Scholar]
- 28.Skranes J, Løhaugen GC, Martinussen M, et al. Cortical surface area and IQ in very-low-birth-weight (VLBW) young adults. Cortex. 2013;49(8):2264–71. doi: 10.1016/j.cortex.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Sølsnes AE, Sripada K, Yendiki A, et al. Limited microstructural and connectivity deficits despite subcortical volume reductions in school-aged children born preterm with very low birth weight. NeuroImage. 2016;130:24–34. doi: 10.1016/j.neuroimage.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 30.Anjari M, Srinivasan L, Allsop JM, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35(3):1021–7. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 31.Leuchter RH, Gui L, Poncet A, et al. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. JAMA. 2014;312(8):817–824. doi: 10.1001/jama.2014.9645. [DOI] [PubMed] [Google Scholar]
- 32.O’Gorman RL, Bucher HU, Held U, et al. Swiss EPO Neuroprotection Trial Group. Tract-based spatial statistics to assess the neuroprotective effect of early erythropoietin on white matter development in preterm infants. Brain. 2015;138(pt 2):388–397. doi: 10.1093/brain/awu363. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- 34.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu YW, Gonzalez FF. Erythropoietin: a novel therapy for hypoxic–ischaemic encephalopathy? Dev Med Child Neurol. 2015;57(S3):34–9. doi: 10.1111/dmcn.12730. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Jiang Q, Ding G, et al. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke. 2009;40(3):936–41. doi: 10.1161/STROKEAHA.108.527713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding G, Jiang Q, Li L, et al. Cerebral tissue repair and atrophy after embolic stroke in rat: a magnetic resonance imaging study of erythropoietin therapy. J Neurosci Res. 2010;88(14):3206–14. doi: 10.1002/jnr.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van De Looij Y, Chatagner A, Quairiaux C, et al. Multi-modal assessment of long-term erythropoietin treatment after neonatal hypoxic-ischemic injury in rat brain. PLoS One. 2014;9(4):e95643. doi: 10.1371/journal.pone.0095643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Traudt CM, McPherson RJ, Bauer LA, et al. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev Neurosci. 2013;35(6):491–503. doi: 10.1159/000355460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen TC, Rogido M, Peng H, et al. Gender differences in long-term beneficial effects of erythropoietin given after neonatal stroke in postnatal day-7 rats. Neuroscience. 2006;139(3):803–11. doi: 10.1016/j.neuroscience.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 41.McCoy TE, Conrad AL, Richman LC, et al. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychology. 2011;17(4):347–367. doi: 10.1080/09297049.2010.544647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCoy TE, Conrad AL, Richman LC, et al. The Relationship Between Brain Structure and Cognition in Transfused Preterm Children at School Age. Developmental Neuropsychology. 2014;39(3):226–232. doi: 10.1080/87565641.2013.874428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yung A, Poon G, Qiu DQ, et al. White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr Res. 2007;61(6):732–6. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]
- 44.Feldman HM, Lee ES, Yeatman JD, Yeom KW. Language and reading skills in school-aged children and adolescents born preterm are associated with white matter properties on diffusion tensor imaging. Neuropsychologia. 2012;50(14):3348–62. doi: 10.1016/j.neuropsychologia.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullen KM, Vohr BR, Katz KH, et al. Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage. 2011;54(4):2563–70. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]