Abstract
Objective
Herpesvirus shedding in the oral cavity was analyzed to determine if presence in the oral compartment correlates with systemic changes in HIV-associated immune deficiency as measured by CD4+ counts, plasma HIV viral load (VL) and presence of AIDS-defining events.
Design
A5254 is a multicenter, cross-sectional, single-visit study to evaluate oral complications of HIV/AIDS and determine the association between clinical appearance, herpesvirus shedding and immune status as ascertained by CD4 count and HIV viral load. 307 HIV infected individuals were evaluated and throat wash collected.
Methods
Fisher’s exact test and Kruskal-Wallis test were used to assess the association between presence of herpes viruses and the state of immunodeficiency as stratified by a combination of CD4+ count and HIV VL. Relationship between pathogens and HIV VL in plasma was modeled by logistic regression.
Results
The presence of cytomegalovirus CMV and Herpes Simplex Virus-1 (HSV-1) in throat wash was associated with decreased CD4 counts. By contrast Kaposi Sarcoma-associated herpes virus (KSHV) and EBV were similarly detectable across all levels of CD4 counts. One unit increase in log10(HIV VL) was associated with 1.31 times higher odds of detecting CMV in throat wash when controlling for oral candidiasis, CD4 count, and sites (95% CI 1.04 –1.65, P=0.02).
Conclusions
Oral CMV shedding was significantly higher in highly immunocompromised HIV+ participants. Our finding supports the recommendations to start antiretroviral therapy (ART) independent of CD4 count as this may have the added benefit to lower the risk of herpes virus transmission among persons infected with HIV and their partners.
Keywords: Kaposi Sarcoma, Oral Hairy Leukoplakia, Epstein-Barr virus, KSHV, clinical trial, HIV, Kaposi sarcoma-associated herpesvirus, Cytomegalovirus, EBV, CMV, Herpesviruses, HIV, AIDS
Introduction
Combined clinical observations and pathologic evaluations represent the standard to diagnose oral complications of immunodeficiency and are used to make treatment decisions. By comparison, the treatment of human immunodeficiency virus (HIV) relies heavily on HIV plasma viral load (VL). Current recommendations are to initiate combination antiretroviral therapy (ART) as early as possible. Starting ART independently of CD4 count is justified, in part, in order to prevent HIV transmission [1]. It depends on an extensive health care infrastructure to supply ART and to provide for early detection of HIV. In populations with limited access to ART, Kaposi Sarcoma (KS), oral hairy leukoplakia (OHL), and oral candidiasis (OC) signify advanced HIV disease. This study asked whether the presence of herpes simplex virus (HSV-1), Cytomegalovirus (CMV), Epstein-Barr Virus (EBV), or Kaposi’s sarcoma-associated herpesvirus (KSHV) in the throat wash of persons with HIV infection correlated with immune status as measured by CD4 count and plasma HIV viral load. If this was true, monitoring the levels of oral pathogens could help in the diagnosis of deteriorating immune status. Since sampling the oral cavity is minimally invasive compared to blood sampling and can be done by self-collection this may present an option to reach underserved populations and to improve HIV care in resource limited settings.
Observing cutaneous KS is part of the definition of AIDS (reviewed in [2]). KS disease is associated with declining immune status in the setting of untreated HIV infection. In HIV-negative transplant recipients KS is a complication of iatrogenic immune suppression and the relative risk of KS in transplant recipients is estimated at 200 fold compared to the general population [3–5]. Oral KS in addition to cutaneous or systemic KS is considered an indicator of advanced KS disease (T1); it is associated with poor outcome in response to cytotoxic therapy. Oral KS may benefit from intralesional application of cytotoxic agents [6]. Aspects of the AIDS KS epidemic in US have changed since the introduction of ART. One third of KS now develops in patients on effective ART with plasma HIV VL below the limits of detection [7, 8]. The decline of KS incidence as observed concurrent to the introduction of ART has leveled off since 2010 and KS remains the single most prevalent cancer occurring in persons with HIV today [9, 10]. KSHV is the etiological agent of KS. All cases of KS are associated with prior or concurrent KSHV infection regardless of HIV status. KSHV is detected in saliva and an inhibitor of KSHV viral replication (ganciclovir) reduces oral shedding and lifetime KS risk [11–17]; whether or not established KS lesions respond to inhibitors of viral replication is unclear [18, 19].
OHL is an AIDS defining condition [20]. EBV is the etiological agent of OHL and all cases of OHL are associated with concurrent permissive EBV lytic replication in the lesion [21, 22]. OHL has also been found in HIV-negative, EBV-positive organ transplant patients [23]. It tends to resolve upon immune reconstitution in the setting of HIV infection and responds to acyclovir, an inhibitor of the EBV DNA polymerase [24]. EBV is detected at high levels in saliva and an inhibitor of EBV viral replication (acyclovir) reduces oral shedding [25–27].
OC was the most common oral complication of HIV/AIDS prior to the introduction of ART (reviewed in [28, 29]); it still is in many low and middle-income countries. OC can be caused by multiple Candida species in the setting of HIV [30]. OC is associated with severe immune suppression and extremely low CD4+ cell counts. Systemic therapy is indicated though studies suggest that local agents are efficacious as well [31, 32].
The primary objective of the ACTG A5254 trial was to describe the prevalence of oral lesions in people with HIV infection and to test the hypothesis that after training non-professional oral health specialists can accurately diagnose oral lesion frequently found in this population. This work has been published, as have the details of the clinical data and socioeconomic characteristics of the study population [33]. As oral complications of HIV/AIDS were previously detected at lower CD4+ counts, this cohort was geared towards enrollment of persons with low CD4 counts. This design allowed evaluation of associations between different pathogens in the oral cavity in participants with defined immunologic and HIV plasma VL status.
Methods
Study Design
Detailed study design, patient population and sample collection were previously published [33]. In brief, ACTG A5254 is a cross-sectional study that enrolled HIV-1-infected adults 18 years or older with or without prior ART from five locations in the US and one location in Haiti between 2009 and 2012. Institutional Review Boards or Ethics Committees of each participating institution approved the study, and each patient gave written informed consent.
As previously reported [33] there were 128 (42%) cases of OC, 39 (13%) cases of OHL, and 31 (10%) cases of oral KS in the A5254 cohort. OC was most common in stratum A (A: 66%, B: 18%, and C/D: 14%, P value for the Fisher’s exact test across strata < 0.001). OHL seemed less common in stratum B (A: 24 (15%); B: 5 (5%); C/D 10 (17%), p value for the Fisher’s exact test across strata < 0.05). Oral KS was most common in stratum A (A: 29 (18%); B: 2 (2%); C/D (0%), p value for the Fisher’s exact test across strata < 0.001). HSV-1 and CMV oral lesions could not be discerned from oral lesions of non-viral origin. In sum, OC and oral KS were more common in participants with lower CD4+ cell count overall and within the context of immunodeficiency associated higher HIV VL (comparing stratum A and B).
Throat wash Collection
A 5-minute unstimulated whole saliva (UWS) flow rate was recorded, and collected [34]. A 1-minute oral rinse/throat wash using 10 mL of sterile saline was collected afterwards. Both saliva and throat wash specimens were frozen in aliquots at −80°C at the site laboratory, and banked. Only soluble throat wash was used for the current study.
Candida analysis
Before the throat wash was processed further 2.5 mL sterile saline was aliquoted and processed for Candida detection by culture. A culture was defined as positive, and confirming the clinical diagnosis of OC, among individuals with clinical features of OC and a number of colony forming units (CFUs).
CD4+ count and plasma HIV VL
Phlebotomy was performed at the time of the visit and CD4+ cell count and plasma HIV-1 VL were measured. Plasma HIV-1 VL was measured using the Abbott real-time HIV-1 quantitative PCR Assay on 0.6–1.0 ml input volume (sensitivity of 40 copies/mL, linear range 1.6 log copies/mL to 7.0 log copies/mL, specificity >99% as reported by the manufacturer).
Throat wash herpesvirus load (CMV, EBV, KSHV, HSV-1)
Real-time qPCR was used to detect multiple herpesviruses as described previously [29]. The assay measures KSHV, CMV, EBV, HSV-1 independently. This was important, since we expected multiple herpesviruses be present at widely differing levels in the same sample, which may have impact the sensitivity of multiplexed PCR. Herpesvirus load were categorized either as present or absent (based on endpoint result regardless of viral load) and as copies/ml if >500 copies/ml, which was the lower limit of the linear range for this assay.
Data Availability
Analyses were performed by the Statistical Data Analysis Center for the ACTG, which is located within the Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, MA. This is where the datasets are stored. Consistent with NIH regulations and ACTG policy, the data is publically available. Requests for data are to be submitted to sdac.data@sdac.harvard.edu. Data are de-identified prior to distribution. Additional analyses were performed at the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, NC.
Statistical procedures
Sample characteristics were summarized using proportions for categorical variables, and mean, median with 1st and 3rd quantiles (Q1 and Q3) for continuous variables. The results were presented for all the strata, and for each CD4+ cell count / plasma HIV-1 viral load stratum separately (strata C and D were combined). The frequency of OC was confirmed by a positive culture (≥ 1 CFU/mL) was computed and the difference across strata using the Fisher’s exact test was explored. CD4+ cell count and log10 (plasma HIV-1 VL) was compared among participants across strata using the Kruskal-Wallis test. Log10 (VL) of individual herpesviruses in saliva was also calculated and summarized across strata for participants with detectable herpesviruses. The frequency of virus-detectable cases was computed by CD4+ cell count/plasma HIV-1 VL stratum, and the difference across strata was compared using the Fisher’s exact test. Logistic regression was used to model the relationship between the presence of herpesvirus oral shedding and HIV-1 VL in plasma controlling for CD4+ cell count, oral candidiasis and sites. A detailed description of the methods of diagnosis of oral manifestations of disease in this study has been reported previously [33]. The model yielded adjusted odds ratios with 95% confidence intervals.
Results
Oral disease was associated with severe immunodeficiency
The salient features of the A5254 study design were as follows: A5254 was a single time-point, cross sectional study, which enrolled its first participant in October 2009 and its last participant in September 2012 [33]. All participants were evaluated by oral health specialists, and throat wash samples were taken. Participants were initially enrolled across four strata. We combined participants for two strata (C and D) with CD4 counts > 200 cells/mm3 regardless of HIV VL in order to obtain a more balanced design (Table 1). Stratum A encompassed N=157 participants with a CD4+ cell count ≤ 200 cells/mm3 and plasma HIV-1 VL > 1,000 copies/ml. Of these, 99 (63%) were enrolled in Haiti and 58 (37%) in the continental US. 88% of participants in stratum A were black, non-Latino. The proportion of black, non-Latino was significantly larger than 60% and 47% in stratum B and C/D, respectively (P value for the Fisher’s exact test across strata < 0.001). The majority (93%) of participants from Haiti were enrolled only in stratum A. Stratum B encompassed N=91 participants with CD4+ cell count ≤ 200 cells/mm3 and a plasma HIV-1 VL ≤ 1,000 copies/mL. Stratum C/D encompassed N=59 participants with CD4+ cell count ≥ 200 cells/mm3 regardless of HIV plasma VL. The majority of participants were male in stratum B (85%) and C/D (68%), significantly larger than 55% in stratum A (P value for the Fisher’s exact test across strata < 0.001). The median age was 44 (Q1 – Q3: 38–51) with participants in stratum B being somewhat older compared to stratum A and C/D.
Table 1. Demographics and baseline characteristics.
Cohort Characteristics (non-viral). IV drug history and history of an AIDS-defining illness were recorded, but did not differ significantly among the three strata.
| HIV VL | >1000 | ≤1000 | any | |||
|---|---|---|---|---|---|---|
| CD4 | ≤200 | ≤200 | CD4 >200 | |||
| Characteristic | A (N=157) | B (N=91) | C/D (N=59) | Total (N=307) | P-Value | |
| Sex | M | 87 (55%) | 77 (85%) | 40 (68%) | 204 (66%) | <.001* |
| F | 70 (45%) | 14 (15%) | 19 (32%) | 103 (34%) | ||
| Location | US | 58 (37%) | 83 (91%) | 59 (100%) | 200 | <.001* |
| Haiti | 99 (63%) | 8 (9%) | 0 (0%) | 107 | ||
| Race/Ethnicity | White non-Latino | 8 (5%) | 28 (31%) | 13 (22%) | 49 (16%) | <.001* |
| Black non-Latino | 138 (88%) | 55 (60%) | 28 (47%) | 221 (72%) | ||
| Latino (regardless of race) | 7 (4%) | 8 (9%) | 15 (25%) | 30 (10%) | ||
| Other | 4 (3%) | 0 (0%) | 3 (5%) | 7 (2%) | ||
| Oral Candidiasis | No | 51 (32%) | 72 (79%) | 51 (86%) | 174 (57%) | <.001* |
| Yes | 104 (66%) | 16 (18%) | 8 (14%) | 128 (42%) | ||
| Missing | 2 (1%) | 3 (3%) | 0 (0%) | 5 (2%) | ||
| Oral Hairy Leukoplakia | No | 133 (85%) | 83 (91%) | 49 (83%) | 265 (86%) | .047* |
| Yes | 24 (15%) | 5 (5%) | 10 (17%) | 39 (13%) | ||
| Missing | 0 (0%) | 3 (3%) | 0 (0%) | 3 (1%) | ||
| Kaposi Sarcoma | No | 128 (82%) | 86 (95%) | 59 (100%) | 273 (89%) | <.001* |
| Yes | 29 (18%) | 2 (2%) | 0 (0%) | 31 (10%) | ||
| Missing | 0 (0%) | 3 (3%) | 0 (0%) | 3 (1%) | ||
| Currently on ART? | No | 75 (48%) | 2 (2%) | 22 (37%) | 99 (32%) | <.001* |
| Yes | 82 (52%) | 89 (98%) | 37 (63%) | 208 (68%) | ||
| Age at study entry | N | 157 | 91 | 59 | 307 | <.001** |
| Median | 42 | 48 | 43 | 44 | ||
| Q1, Q3 | 37, 50 | 43, 54 | 34, 49 | 38, 51 | ||
| CD4 (cells/μL) | N | 157 | 88 | 59 | 304 | <.001** |
| Missing | 0 | 3 | 0 | 3 | ||
| Median | 93 | 142 | 525 | 138 | ||
| Q1, Q3 | 38, 143 | 100, 172 | 339, 657 | 71, 189 | ||
| Log10[HIV RNA(cp/ml)] | N | 157 | 88 | 59 | 304 | <.001** |
| Missing No. | 0 | 3 | 0 | 3 | ||
| Median | 5.12 | 1.70 | 1.88 | 4.40 | ||
| Q1, Q3 | 4.70, 5.53 | 1.60, 1.89 | 1.68, 4.43 | 1.70, 5.18 | ||
| Colony Unit Count (CFU/ml) | N | 155 | 88 | 59 | 302 | <.001** |
| Missing | 2 | 3 | 0 | 5 | ||
| Median | 1,660 | 100 | 200 | 720 | ||
| Q1, Q3 | 400, 2,800 | 1, 1,223 | 1, 980 | 8, 2,360 |
Fisher’s Exact Test
Kruskal-Wallis Test
Ninety-nine (32%) participants were not on combination antiretroviral therapy (ART) at study entry. Almost all patients in stratum B were on ART. 52% of patients in stratum A also reported being on ART, yet >90% of those did not recount a prior AIDS defining illness (data not shown). Presumably this reflects participants, who only recently started on ART.
Immune status associated shedding in the oral cavity differs among different herpesviruses
HSV-1 was only detectable in stratum A participants (8%) (Table 2). The median (Q1, Q3) of HSV-1 VL (Log10 copies/mL) for all virus positive samples was 4.25 (3.90, 4.40). CMV was detectable in throat wash of 50 % of stratum A participants, 15% in stratum B, and 10% in stratum C/D. There was a significant association between the presence of CMV and stratum (p < 0.001). The median (Q1, Q3) of detectable CMV VL (Log10 copies/mL) was 3.60 (3.40, 3.90). KSHV, the etiologic agent of KS, was detectable in throat wash of 3% of stratum A participants, 4% of stratum B participants, and 7% of stratum C/D participants. There was no association between the presence of KSHV and stratum. Overall the median (Q1, Q3) of detectable KSHV VL (Log10 copies/mL) was 3.35 (3.20, 3.70). EBV was detectable in throat wash of 87% of stratum A participants, 77% of stratum B participants, and 83% of stratum C/D participants. There was no significant association between the presence of EBV and stratum. Overall the median (Q1, Q3) of detectable EBV VL (Log10 copies/mL) was 4.60 (4, 5). We found no statistically significant evidence for an association of EBV VL or CMV VL with candida burden.
Table 2.
Herpesvirus loads by stratum
| Characteristic | A (N=157) | B (N=91) | C/D (N=59) | Total (N=307) | P-Valuea | |
|---|---|---|---|---|---|---|
| CMV (Log10 cps/ml) of detectable | N (%b) | 78 (50%) | 14 (15%) | 6 (10%) | 98 (32%) | ≤ 0.001 |
| Median | 3.65 | 3.50 | 3.35 | 3.60 | ||
| Q1, Q3 | 3.50, 3.90 | 3.30, 3.80 | 3.20, 3.60 | 3.40, 3.90 | ||
| EBV (Log10 cps/ml) of detectable | N (%b) | 136 (87%) | 70 (77%) | 49 (83%) | 255 (83%) | 0.147 |
| Median | 4.70 | 4.20 | 4.50 | 4.60 | ||
| Q1, Q3 | 4.20, 5.10 | 3.80, 4.80 | 3.90, 4.94 | 4, 5 | ||
| HSV (Log10 cps/ml) of detectable | N (%b) | 12 (8%) | 0 (0%) | 0 (0%) | 12 (4%) | ≤ 0.001 |
| Median | 4.25 | n/a | n/a | 4.25 | ||
| Q1, Q3 | 3.90, 4.40 | n/a | n/a | 3.90, 4.40 | ||
| KSHV (Log10 cps/ml) of detectable | N (%b) | 4 (3%) | 4 (4%) | 4 (7%) | 12 (4%) | 0.327 |
| Median | 3.50 | 3.55 | 3.19 | 3.35 | ||
| Q1, Q3 | 3.35, 3.60 | 3.25, 3.95 | 3.15, 3.70 | 3.20, 3.70 |
Fisher’s Exact Test
percent of samples with detectable virus
The overall frequency of HSV-1 or KSHV positive samples was very low (4%), thus the study was inadequately powered to detect possible associations of HSV-1 or KSHV with candida burden, i.e., colony count units. EBV was detectable in 83% of samples and CMV in 32% and their VLs spanned a large range (Figure 1). Evidence for an association between EBV VL or CMV VL with candida burden was not statistically significant (P=0.98, P=0.60; data not shown). No significant association was found between EBV and CMV VL in the oral cavity (P=0.63; data not shown), suggesting that independent factors foster oral replication and shedding of these two herpesviruses.
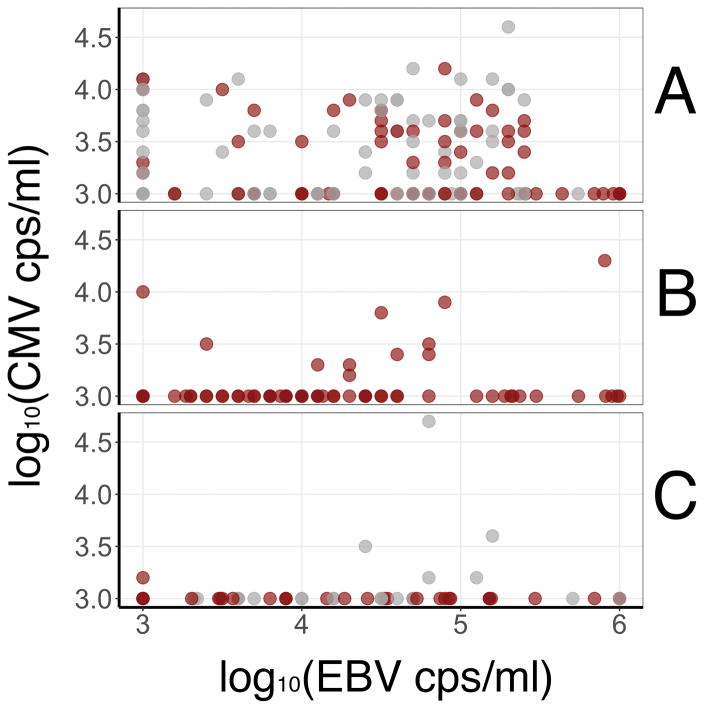
Figure 1.
Association between very high EBV and CMV levels in throat wash. The data are separated by stratum. The three panels represent the three study strata A, B, and C/D. The top panel shows the measurements for stratum A (high HIV VL, CD4 ≤ 200 cells/μl), the middle panel stratum B (low HIV VL, CD4 ≤ 200 cells/μl), and the bottom panel stratum C/D (CD4 > 200 cells/μl). Shown in each panel is log10 EBV copies/ml on the horizontal axis compared to log10 CMV copies/ml on the vertical axis. EBV is detectable at all strata across a wide range of VL. CMV is highly enriched in stratum A (top panel). This reflects the significant association with HIV viral load as described in Table 3. To aid visualization the threshold was set at 3 × log10 so that participants with detectable VL lower than 3 × log10 were coded as 3 × log10, and participants with detectable VL higher than 6 × log10 were coded as 6 × log10. Lastly, ART status is reflected in the color. Colored in gray are participants not on ART and shown in red are participants who self-reported being on ART, though we do not know the time of therapy initiation, nor did we detect a correlation between ART usage and CMV or EBV VL.
We used logistic regression to evaluate the relationship between CMV shedding in oral cavity and HIV plasma VL. (Table 3). The odds of detecting CMV was 1.31 times higher with one unit increase in log10(HIV VL) when controlling for OC, CD4 count, and sites (95% CI 1.04 –1.65, p=0.02), suggesting a significant association between detection of CMV in throat wash and HIV VL in plasma. The association between the presence of EBV in throat wash and log10 (HIV VL) in plasma was marginally significant with odds ratio 1.26 (95% CI 0.99 –1.61, P=0.06; Data not shown). CMV and HSV-1 were more often detected in stratum A than in stratum B, which had a similar level of immunodeficiency as ascertained by CD4+ count but suppressed HIV replication.
Table 3.
Logistic regression model exploring the association between detection of CMV in throat wash and HIV VL in plasma controlling for CD4 cell count, oral candidiasis, and sites.
| Independent variables | aOR (95% CI) | P value |
|---|---|---|
| Log10(HIV VL) | 1.31 (1.04, 1.65) | 0.02 |
| Oral candidiasis (yes vs. no) | 0.75 (0.37, 1.53) | 0.43 |
| Sqrt(CD4) | 0.95 (0.90, 1.01) | 0.13 |
| Sites (international vs. domestic) | 5.30 (2.56, 10.97) | <0.001 |
aOR = adjusted odds ratio; CI = confidence interval; Sqrt(CD4) = square root of CD4.
Discussion
Oral complications of HIV/AIDS manifest primarily in stages of advanced HIV disease. OHL was one of the first described clinical manifestations of AIDS and oral KS became widely recognized during the early AIDS epidemic in the US and Europe. CMV-associated diseases, in particular retinitis, pneumonia and encephalitis also are part of the clinical manifestations that signify end-stage AIDS, as is HSV-1 disease. The etiological agent for KS is KSHV and the etiological agent for OHL is EBV. CMV, EBV, KSHV, HSV-1 are present in oral secretions and transmitted via the oral route [12, 35–37]. CMV plasma VL is closely linked to immune suppression, whether by HIV or iatrogenic drug regimen [35, 38]. A close association between plasma VL and disease burden also holds true for EBV-associated post-transplant lymphoproliferative disease and some EBV-positive lymphoma [39, 40], but not always for KSHV and KS [41]. A5254 found that oral shedding of HSV-1 and CMV, but not of EBV and KSHV, were correlated with immune deficiency.
The VL of each of the four herpesviruses did not correlate with each other and not always with immune status. EBV was shed consistently independent of immune status, and independent of CMV VL (Figure 1). EBV oral VLs were much higher than those of the other herpesviruses consistent with prior work [25, 35, 37, 42]. Our single point detection rate for KSHV was low and not associated with immune status, whereas oral KS disease was. This lack of association may be the result of sampling limitations as outlined below or it may be due to the fact that for KSHV, like for other herpesviruses, the majority of shedding occurs in the absence of overt lesions. KSHV VLs in the oral cavity tended to fluctuate, necessitating the need to sample frequently over extended periods to detect virus [36, 43]. Longitudinal sampling of the same person and detailed biopsies may be needed to establish associations among the sporadically shed herpesviruses (HSV-1, KSHV), lesion phenotype, and clinical parameters. HSV-1 shedding and disease has been associated with overall immunodeficiency and more directly with HIV replication [35, 44]. In the A5254 trial, HSV-1 was only detected in the oral cavity of participants in stratum A, i.e. in the setting of high HIV VLs and CD4 < 200 cell/mm3, though we did not have enough events to establish a robust association. This may be due to the previously described episodic nature of HSV-1 shedding [43, 44].
CMV in the oral cavity was closely linked to immune suppression in the setting of HIV. This is consistent with prior studies [35, 38]. In addition, we found that CMV VL in the oral cavity was independently associated with HIV VL in the setting of immunodeficiency. This suggests a relation between CMV and HIV replication above and beyond immune status. It may be feasible to develop CMV saliva VL into a biomarker for immunodeficiency analogous to CMV plasma VL in transplant patients. Such an assay would be minimally invasive and lend itself repeat sampling as well as self-sampling.
This study had a number of limitations, such as the cross sectional nature of sample collection, which did not allow us to distinguish primary infection from reactivation. This design also does not allow us to make inferences about persistence. As the primary objective of A5254 was to evaluate oral examination by non-oral health specialists [33], we did not include a blood draw. Hence, we can only infer the underlying seroprevalence for each of the herpesviruses based on published reports in these populations. For CMV, seroprevalence in MSN, and in the general population has been consistently estimated at ≥80%, levels for EBV and HSV-1 are estimated at 90% and 67% in the general population globally and consistently higher among HIV+ [45, 46]. For KSHV the data are more variable [47–49]. Most recently, the ALLRT study estimates a seroprevalence of 38% in ACTG clinic attendants [50]. Lastly, a large proportion of patients in stratum A enrolled in Haiti, whereas few of the other strata enrolled at this location because of the state of HIV care on the island. This represents a potential confounder, which was included in the multivariable logistic regression model that demonstrated a direct association between CMV oral VL and HIV systemic VL.
In sum, we find that HIV-induced deficiency in the adaptive immune response, as ascertained here by CD4+ counts, does not elevate oral shedding among herpesviruses equally. These findings are consistent with a model in which local trigger/pathogen pairings synergize for herpesvirus replication, infection, reactivation, transmission, and disease. In case of CMV one of the triggers may be HIV replication itself. If we were to understand the forces that govern virus reactivation in the oral cavity, it would open up new avenues of intervention, prophylaxis, and diagnosis.
Acknowledgments
Funding
The project described was supported by Award Number AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Further analysis and assay development was supported by public health service grants DE018304 and DE023946 to DPD and AI068634 for the Statistical and Data Management Center for the AIDS Clinical Trials Group.
Dirk P. Dittmer designed the study and wrote the manuscript. Kristen Tamburro designed the assay, generated the data and conducted primary analysis. Huichao Chen designed and conducted the statistical analysis. Anthony Lee conducted the statistical analysis. Marcia K. Sanders designed the assay, generated the data and conducted primary analysis. Tischan A. Wade designed the assay, generated the data and conducted primary analysis. Sonia Napravnik conducted the statistical analysis. Jennifer Webster-Cyriaque designed the study. Mahmoud Ghannoum designed the study, generated primary data and analysis. Caroline H. Shiboski designed the study. Judith A. Aberg designed the study.
The list of sites for the A5254 protocol has been published [33] and was as follows: Jean William Pape, MD, Patrice Sévère, MD, Rode Secours, MD, Daphné Bernard, MD and Maria Linda Aristhomène, RN – Les Centres Gheskio (Gheskio-INLR) CRS (Site 30022), Caroline Shiboski, DDS, MPH, PhD, Sivappiriyai Veluppillai, DDS, Amanda Hutton Parrott, DPT, NP, and Jay Dwyer, RN, -UCSF AIDS CRS (Site 801) Judith A Aberg, MD, Karen Cavanagh, RN, Alexander Ross Kerr DDS, MSD, Sonal S Shah DDS, and Manley Lammarre RDH- New York University HIV/AIDS CRS (Site 401) Jennifer Webster-Cyriaque, DDS, PhD, Jonathan Oakes BA, Dirk P. Dittmer, Ph.D. and Lauren Patton DDS - Chapel Hill CRS (Site 3201), Jeffrey Lennox, MD, Dale Maddox, RN and David A. Reznik, DDDS- The Ponce de Leon Ctr. CRS (Site 5802) Emory University HIV/AIDS CTU Michael Lederman MD, Jane Baum RN, Mahmoud Ghannoum, PhD, Nancy Isham, and Richard Jurevic - Case CRS (Site 2501)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittmer DP, Damania B. Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest. 2016;126(9):3165–3175. doi: 10.1172/JCI84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grulich AE, Vajdic CM. The epidemiology of cancers in human immunodeficiency virus infection and after organ transplantation. Semin Oncol. 2015;42(2):247–257. doi: 10.1053/j.seminoncol.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 4.Pellet C, Chevret S, Frances C, Euvrard S, Hurault M, Legendre C, et al. Prognostic value of quantitative Kaposi sarcoma--associated herpesvirus load in posttransplantation Kaposi sarcoma. J Infect Dis. 2002;186(1):110–113. doi: 10.1086/341088. [DOI] [PubMed] [Google Scholar]
- 5.Unemori P, Leslie KS, Hunt PW, Sinclair E, Epling L, Mitsuyasu R, et al. Immunosenescence is associated with presence of Kaposi’s sarcoma in antiretroviral treated HIV infection. AIDS. 2013;27(11):1735–1742. doi: 10.1097/QAD.0b013e3283601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein JB, Scully C. Intralesional vinblastine for oral Kaposi sarcoma in HIV infection. Lancet. 1989;2(8671):1100–1101. doi: 10.1016/s0140-6736(89)91114-8. [DOI] [PubMed] [Google Scholar]
- 7.Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357(13):1352–1353. doi: 10.1056/NEJMc070508. [DOI] [PubMed] [Google Scholar]
- 8.Krown SE, Lee JY, Dittmer DP Consortium AM. More on HIV-associated Kaposi’s sarcoma. N Engl J Med. 2008;358(5):535–536. doi: 10.1056/NEJMc072994. author reply 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverberg MJ, Lau B, Achenbach CJ, Jing Y, Althoff KN, D’Souza G, et al. Cumulative Incidence of Cancer Among Persons With HIV in North America: A Cohort Study. Ann Intern Med. 2015;163(7):507–518. doi: 10.7326/M14-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;107(4) doi: 10.1093/jnci/dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, et al. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198(1):23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phipps W, Saracino M, Selke S, Huang ML, Jaoko W, Mandaliya K, et al. Oral HHV-8 replication among women in Mombasa, Kenya. J Med Virol. 2014;86(10):1759–1765. doi: 10.1002/jmv.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira J, Huang ML, Koelle DM, Corey L. Transmissible Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi’s sarcoma. J Virol. 1997;71(9):7083–7087. doi: 10.1128/jvi.71.9.7083-7087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koelle DM, Huang ML, Chandran B, Vieira J, Piepkorn M, Corey L. Frequent detection of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus-infected men: clinical and immunologic correlates. J Infect Dis. 1997;176(1):94–102. doi: 10.1086/514045. [DOI] [PubMed] [Google Scholar]
- 15.Boldogh I, Szaniszlo P, Bresnahan WA, Flaitz CM, Nichols MC, Albrecht T. Kaposi’s sarcoma herpesvirus-like DNA sequences in the saliva of individuals infected with human immunodeficiency virus. Clin Infect Dis. 1996;23(2):406–407. doi: 10.1093/clinids/23.2.406. [DOI] [PubMed] [Google Scholar]
- 16.Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 17.Lampinen TM, Kulasingam S, Min J, Borok M, Gwanzura L, Lamb J, et al. Detection of Kaposi’s sarcoma-associated herpesvirus in oral and genital secretions of Zimbabwean women. J Infect Dis. 2000;181(5):1785–1790. doi: 10.1086/315426. [DOI] [PubMed] [Google Scholar]
- 18.Krown SE, Dittmer DP, Cesarman E. Pilot study of oral valganciclovir therapy in patients with classic Kaposi sarcoma. J Infect Dis. 2011;203(8):1082–1086. doi: 10.1093/infdis/jiq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340(14):1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease C. Oral viral lesion (hairy leukoplakia) associated with acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1985;34(36):549–550. [PubMed] [Google Scholar]
- 21.Greenspan JS, Greenspan D, Lennette ET, Abrams DI, Conant MA, Petersen V, et al. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313(25):1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 22.Webster-Cyriaque J, Middeldorp J, Raab-Traub N. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J Virol. 2000;74(16):7610–7618. doi: 10.1128/jvi.74.16.7610-7618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itin P, Rufli T, Rudlinger R, Cathomas G, Huser B, Podvinec M, et al. Oral hairy leukoplakia in a HIV-negative renal transplant patient: a marker for immunosuppression? Dermatologica. 1988;177(2):126–128. doi: 10.1159/000248529. [DOI] [PubMed] [Google Scholar]
- 24.Resnick L, Herbst JS, Ablashi DV, Atherton S, Frank B, Rosen L, et al. Regression of oral hairy leukoplakia after orally administered acyclovir therapy. JAMA. 1988;259(3):384–388. [PubMed] [Google Scholar]
- 25.Miller CS, Avdiushko SA, Kryscio RJ, Danaher RJ, Jacob RJ. Effect of prophylactic valacyclovir on the presence of human herpesvirus DNA in saliva of healthy individuals after dental treatment. J Clin Microbiol. 2005;43(5):2173–2180. doi: 10.1128/JCM.43.5.2173-2180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbulaiteye SM, Walters M, Engels EA, Bakaki PM, Ndugwa CM, Owor AM, et al. High levels of Epstein-Barr virus DNA in saliva and peripheral blood from Ugandan mother-child pairs. J Infect Dis. 2006;193(3):422–426. doi: 10.1086/499277. [DOI] [PubMed] [Google Scholar]
- 27.Lucht E, Biberfeld P, Linde A. Epstein-Barr virus (EBV) DNA in saliva and EBV serology of HIV-1-infected persons with and without hairy leukoplakia. J Infect. 1995;31(3):189–194. doi: 10.1016/s0163-4453(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 28.Leigh JE, Shetty K, Fidel PL., Jr Oral opportunistic infections in HIV-positive individuals: review and role of mucosal immunity. AIDS Patient Care STDS. 2004;18(8):443–456. doi: 10.1089/1087291041703665. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson MA, Ditmer DP, Sinclair E, Martin JN, Deeks SG, Hunt P, et al. Human herpesvirus replication and abnormal CD8+ T cell activation and low CD4+ T cell counts in antiretroviral-suppressed HIV-infected patients. PLoS One. 2009;4(4):e5277. doi: 10.1371/journal.pone.0005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukherjee PK, Chandra J, Retuerto M, Sikaroodi M, Brown RE, Jurevic R, et al. Oral mycobiome analysis of HIV-infected patients: identification of Pichia as an antagonist of opportunistic fungi. PLoS Pathog. 2014;10(3):e1003996. doi: 10.1371/journal.ppat.1003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powderly WG, Gallant JE, Ghannoum MA, Mayer KH, Navarro EE, Perfect JR. Oropharyngeal candidiasis in patients with HIV: suggested guidelines for therapy. AIDS Res Hum Retroviruses. 1999;15(18):1619–1623. doi: 10.1089/088922299309658. [DOI] [PubMed] [Google Scholar]
- 32.Jurevic RJ, Traboulsi RS, Mukherjee PK, Salata RA, Ghannoum MA Oral HIVARAMFg. Identification of gentian violet concentration that does not stain oral mucosa, possesses anti-candidal activity and is well tolerated. Eur J Clin Microbiol Infect Dis. 2011;30(5):629–633. doi: 10.1007/s10096-010-1131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiboski CH, Chen H, Secours R, Lee A, Webster-Cyriaque J, Ghannoum M, et al. High Accuracy of Common HIV-Related Oral Disease Diagnoses by Non-Oral Health Specialists in the AIDS Clinical Trial Group. PLoS One. 2015;10(7):e0131001. doi: 10.1371/journal.pone.0131001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71(7):1363–1369. doi: 10.1177/00220345920710070301. [DOI] [PubMed] [Google Scholar]
- 35.Miller CS, Berger JR, Mootoor Y, Avdiushko SA, Zhu H, Kryscio RJ. High prevalence of multiple human herpesviruses in saliva from human immunodeficiency virus-infected persons in the era of highly active antiretroviral therapy. J Clin Microbiol. 2006;44(7):2409–2415. doi: 10.1128/JCM.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang ML, et al. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J Infect Dis. 2007;195(1):30–36. doi: 10.1086/509621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster-Cyriaque J, Edwards RH, Quinlivan EB, Patton L, Wohl D, Raab-Traub N. Epstein-Barr virus and human herpesvirus 8 prevalence in human immunodeficiency virus-associated oral mucosal lesions. J Infect Dis. 1997;175(6):1324–1332. doi: 10.1086/516463. [DOI] [PubMed] [Google Scholar]
- 38.Fidouh-Houhou N, Duval X, Bissuel F, Bourbonneux V, Flandre P, Ecobichon JL, et al. Salivary cytomegalovirus (CMV) shedding, glycoprotein B genotype distribution, and CMV disease in human immunodeficiency virus-seropositive patients. Clin Infect Dis. 2001;33(8):1406–1411. doi: 10.1086/322630. [DOI] [PubMed] [Google Scholar]
- 39.Pratesi C, Zanussi S, Tedeschi R, Bortolin MT, Talamini R, Rupolo M, et al. gamma-Herpesvirus load as surrogate marker of early death in HIV-1 lymphoma patients submitted to high dose chemotherapy and autologous peripheral blood stem cell transplantation. PLoS One. 2015;10(2):e0116887. doi: 10.1371/journal.pone.0116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westmoreland KD, Montgomery ND, Stanley CC, El-Mallawany NK, Wasswa P, van der Gronde T, et al. Plasma Epstein-Barr virus DNA for pediatric Burkitt lymphoma diagnosis, prognosis and response assessment in Malawi. Int J Cancer. 2017;140(11):2509–2516. doi: 10.1002/ijc.30682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosseinipour MC, Sweet KM, Xiong J, Namarika D, Mwafongo A, Nyirenda M, et al. Viral profiling identifies multiple subtypes of Kaposi’s sarcoma. MBio. 2014;5(5):e01633–01614. doi: 10.1128/mBio.01633-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn JA, Rudy BJ, Xu J, Kapogiannis B, Secord E, Gillison M. Prevalence and risk factors for oral DNA tumor viruses in HIV-infected youth. J Med Virol. 2016 doi: 10.1002/jmv.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gantt S, Orem J, Krantz EM, Morrow RA, Selke S, Huang ML, et al. Prospective Characterization of the Risk Factors for Transmission and Symptoms of Primary Human Herpesvirus Infections Among Ugandan Infants. J Infect Dis. 2016;214(1):36–44. doi: 10.1093/infdis/jiw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis. 2002;186(12):1718–1725. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 45.Remis RS, Liu J, Loutfy MR, Tharao W, Rebbapragada A, Huibner S, et al. Prevalence of Sexually Transmitted Viral and Bacterial Infections in HIV-Positive and HIV-Negative Men Who Have Sex with Men in Toronto. PLoS One. 2016;11(7):e0158090. doi: 10.1371/journal.pone.0158090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2(8):918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 48.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338(14):948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 49.Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, et al. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996;335(4):233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 50.Labo N, Miley W, Benson CA, Campbell TB, Whitby D. Epidemiology of Kaposi’s sarcoma-associated herpesvirus in HIV-1-infected US persons in the era of combination antiretroviral therapy. AIDS. 2015;29(10):1217–1225. doi: 10.1097/QAD.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Analyses were performed by the Statistical Data Analysis Center for the ACTG, which is located within the Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, MA. This is where the datasets are stored. Consistent with NIH regulations and ACTG policy, the data is publically available. Requests for data are to be submitted to sdac.data@sdac.harvard.edu. Data are de-identified prior to distribution. Additional analyses were performed at the University of North Carolina Lineberger Comprehensive Cancer Center, Chapel Hill, NC.