Abstract
The goal of the present study was to explore the protective effects of mTORC1 inhibition by rapamycin on salt-induced hypertension and kidney injury in Dahl salt-sensitive (SS) rats. We have previously demonstrated that H2O2 is elevated in the kidneys of SS rats. The present study showed a significant upregulation of renal mTORC1 activity in the SS rats fed a 4.0% NaCl for 3 days. Additionally, renal interstitial infusion of H2O2 into salt-resistant Sprague Dawley (SD) rats for 3 days was also found to stimulate mTORC1 activity independent of a rise of arterial pressure (BP). Together, these data indicate that the salt-induced increases of renal H2O2 in SS rats activated the mTORC1 pathway. Daily administration of rapamycin (i.p., 1.5 mg/Kg/day) for 21 days reduced salt-induced hypertension from 176.0 ± 9.0 to 153.0 ± 12.0 mmHg in SS rats but had no effect on BP salt-sensitivity in SD treated rats. Compared to vehicle, rapamycin reduced albumin excretion rate in SS rats from 190.0 ± 35.0 to 37.0 ± 5.0 mg/day and reduced the renal infiltration of T lymphocytes (CD3+) and macrophages (ED1+) in the cortex and medulla. Renal hypertrophy and cell proliferation was also reduced in rapamycin treated SS rats. We conclude that enhancement of intrarenal H2O2 with a 4.0% NaCl diet stimulates the mTORC1 pathway which is necessary for the full development of the salt-induced hypertension and kidney injury in the SS rat.
Keywords: mTORC1 signaling, salt-sensitive hypertension, H2O2, immune cells, renal injury
Introduction
Salt-sensitive hypertension is characterized by increases in blood pressure in response to elevations of dietary salt intake and by immune responses which play a key role in the pathophysiology of disease1, 2. Renal inflammation is a common feature of experimental and clinical hypertension, with lymphocytes and macrophages localizing to regions of renal injury2, 3. In the commonly used Dahl salt-sensitive (SS) rat, it has been shown that renal inflammation amplifies the hypertension and renal damage4, 5, similar to observations obtained from hypertensive patients1, 6. It has also been recognized that renal hypertrophy and cell proliferation are importantly associated with hypertension and kidney injury in SS rats7, 8.
The present study focused on the role of the mammalian target of rapamycin complex 1 (mTORC1) in blood pressure salt-sensitivity and renal injury in SS rats. mTOR is a serine/threonine kinase with two structurally and functionally distinct multiprotein complexes, mTORC1 and mTORC2 which correspond to two major branches of the overall pathway. mTORC1 is composed of mTOR and several subunits (notably Raptor) and serves as a master growth regulator that senses and integrates diverse cues such as insulin/IGF, amino acids and energy stress and thus, regulate several cellular processes such as cell proliferation, hypertrophy and immune cells proliferation9. mTORC1 controls protein synthesis by phosphorylating and activating the ribosomal protein S6 kinase (S6K)36. S6K phosphorylates C-terminal serine sites at the position of S235 and S236 of ribosomal protein S6 and pS6S235/236/S6 is measured as a surrogate marker of mTORC1 activity10. Phosphorylation of AKT at serine473 has been shown to be directly activated by mTORC2 and pAKTS473/AKT is the canonical readout of mTORC2 activity11.
The rich body of literature reporting the effect of the mTORC1 inhibitor rapamycin in blocking cell proliferation in cancer27, reducing ventricular hypertrophy12 and attenuating immune responses in the kidney transplantation field13. Interestingly, given the recognized immune/inflammation components of hypertension, it is remarkable that this pathway has not been meaningfully explored in this disease12, 14. The present study therefore explored the role of mTORC1 in the development of salt-induced hypertension and kidney injury in SS rats. The specific mTORC1 inhibitor rapamycin was used which significantly attenuated hypertension, kidney injury, immune cells infiltration, renal hypertrophy and cell proliferation in SS rats.
Methods Summary
Experiments were performed with male Dahl salt-sensitive and Sprague-Dawley rats. Rapamycin (i.p., 1.5 mg/Kg/day) was chronically administered to 10 weeks old SS and SD rats fed a 0.4% NaCl diet for 4 days and 21 days after switching to a 4.0% NaCl diet. As we have described15, radiotelemetry catheters and transmitters were surgically implanted for recording 24hrs/day blood pressure and heart rate. Daily body weight was measured to adjust the daily dose of rapamycin. On the final day of the 4.0% NaCl diet period, rats were placed in a metabolic cage for a 24 hr urine collection. As described previously16, unilaterally nephrectomized SD rats were prepared with a renal interstitial catheter as well as a femoral arterial catheter to infuse H2O2 (347 nmol·kg−1·min−1) for 3 days with the rats fed a 0.4% salt diet. Immunohistochemistry, TUNEL, Western blot, renal tubular injury and glomerular score were performed as we have described7, 15, 17. Detailed experimental methods and description of antibodies are available in the online-only Data Supplement.
Statistical Methods
Data are presented as mean values ± standard error. A two-way analysis of variance (ANOVA) for repeated measures test were used for blood pressure analysis. Students’t-test was used to compare between the two treatments. Paired t-test was used to compare the effect of treatments on body weight of SS rats fed a 4.0% NaCl diet. p<0.05 was considered significant.
Results
Upregulation of renal mTORC1 activity by 4.0% NaCl diet and H2O2 in vivo
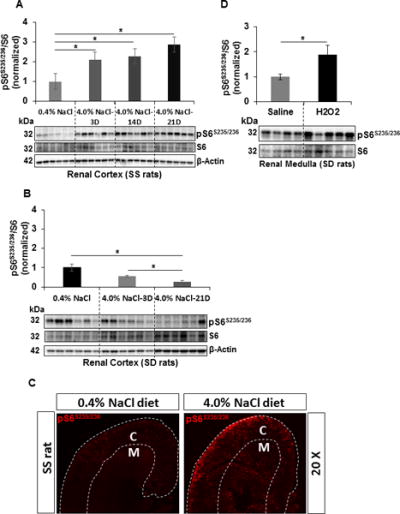
The cortical pS6S235/236/S6 was measured in the kidneys of SS and in salt-resistant SD rats which were fed a 4.0% NaCl diet for 3, 14 and 21 days and compared with 0.4% NaCl control diet by Western blot and densitometry. The pS6S235/236/S6 was increased significantly in the cortex of SS rats fed a 4.0% NaCl diet as early as day 3, rose further by day 14 and reached to the maximum at day 21 compared to 0.4% fed rats (Figure 1A). SD rats, in contrast, exhibited a progressive reduction in the cortical pS6S235/236/S6 which was significantly suppressed by day 21 of the 4.0% NaCl diet (Figure 1B). Immunohistochemical analysis qualitatively confirmed the elevation of pS6S235/236 in the cortex and medulla of SS rats fed a 4.0% NaCl for 21 days (Figure 1C). By immunohistochemistry, it was found that pS6S235/236 was ubiquitously present in the kidneys of SS rats fed a 0.4% NaCl diet which includes glomerulus, proximal convoluted tubules, medullary thick ascending limb, distal convoluted tubules, inter-medullary collecting duct, interstitium space and blood vessel (Figure S1).
Figure 1.
A&B, Western blot analysis of pS6S235/236 and S6 protein level in the renal cortex tissue homogenates prepared from SS and SD rats fed on a 0.4% NaCl diet (n=6) or 4.0% NaCl diet (n=6) for 3, 14 and 21 days. Densitometry was used to determine the mTORC1 activity by measuring the ratio of pS6S235/236 and S6. Values represent pS6S235/236/S6 normalized to the 0.4% NaCl fed SS or SD rats. C, Representative photomicrograph of the immunohistochemical profile of pS6 level in the renal cortex and medulla of SS rats fed a 0.4% NaCl or 4.0% NaCl diet. White discontinuous lines designate the boundaries of the cortex (C) and medulla (M) D, pS6S235/236/S6 was measured in the outer medulla of SD rats with renal interstitial infusion of H2O2 for 3 days. Values represent pS6S235/236/S6 normalized to the saline-treated SD rats. Saline (n=4) and H2O2 (n=5). * p<0.05
We have previously demonstrated that SS rats exhibit elevated levels of renal interstitial H2O2 and additionally salt-sensitivity was mimicked in salt-resistant SS.13BN rats by chronic renal interstitial infusion of H2O2 (347 nmol/kg/min) comparable to those observed in 4.0% NaCl fed SS rats16. In the present studies, this same dose of H2O2 was infused continuously for 3 days into the renal interstitium of unilaterally nephrectomized SD rats maintained on 0.4% NaCl diet to determine if these elevations would stimulate the mTORC1 activity. Figure 1D shows significant increases of pS6S235/236/S6 in the medulla of these SD rats compared to saline infused control rats indicating that H2O2 serves as an important upstream mediator of the mTORC1 pathway in vivo.
Effect of rapamycin on MAP, albumin and protein excretion rate, and glomerular injury
As summarized in Figure 2, the daily mean 24 hr arterial pressure (MAP) of SS rats treated with rapamycin (i.p.,1.5 mg/kg/day) averaged 128.0 ± 2.0 mmHg during the 4 days prior to drug treatment and rose to 139.0 ± 2.0 mmHg by the end of the first four days of drug treatment (P<0.05) maintained on 0.4% NaCl diet. Vehicle treated SS rats which averaged 126 ± 2 mmHg during the four days control period exhibited no change in MAP throughout this same period. MAP of vehicle treated rats increased to 153.0 ± 2.0 mmHg by day 10 of the 4.0% NaCl diet and then rose progressively to levels averaging 176.0 ± 3.0 mmHg on day 21 of 4.0% NaCl. In contrast, MAP of rapamycin treated rats rose only slightly during the first 10 days after switching to the 4.0% NaCl diet and thereafter increased only gradually to an average MAP of 153.0 ± 4.0 mmHg by day 21, values significantly less (P<0.05) than the vehicle treated rats. Rapamycin nearly abolished diurnal variations of MAP, diastolic blood pressure (DBP) and systolic blood pressure (SBP), changes that were apparent even during the first day of treatment shown in Figure S2A–C. Heart rate (HR) diurnal variations reduced slowly and sustained throughout the study period in the treated rats (Figure S2D).
Figure 2.
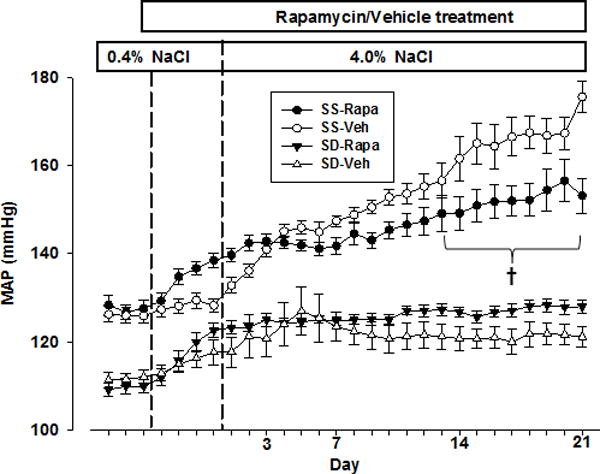
Mean arterial pressure (MAP) was measured with rats fed a 0.4% NaCl diet for 7 days the final 4 days of which rats were treated with rapamycin or vehicle prior to switching to 4.0% NaCl diet for 21 days. Close circles represent rapamycin (n=9) and open circles represent vehicle (n=7) treated SS rats. Close triangles represent rapamycin (n=6) and open triangles represent vehicle (n=6) treated SD rats. † Significant difference between vehicle and rapamycin treated SS rats (p<0.05) as determined using a two-way analysis of variance (ANOVA) for repeated measures; Holm-Sidak post hoc.
During the study, the animals were closely monitored noting appearance of eyes and fur, grooming, appetite, behavior, body weight and activity. Rapamycin treated SS rats did not exhibit a gain of body weight averaging 307 ± 4 g at control and 302 ± 8 g post 3 week period of treatment and was also less than the vehicle treated rats (347± 8 versus 302 ± 8 g; P<0.05). Neither the average total food intake nor the steady-state levels of Na+ excretion differed significantly between rapamycin and vehicle treated SS rats during 4.0% NaCl diet period (Figure S3A&B). Both rapamycin and vehicle treated rats appeared active and healthy throughout the study based on the above mentioned assessed parameters of well-being.
Sprague Dawley rats were studied in the same manner. As with SS rats, MAP was increased on average nearly 10 mmHg by day 4 of rapamycin treatment during the period of 0.4% NaCl diet (Figure 2). However, a similar rise of MAP was observed in vehicle treated SD rats suggesting a vehicle dependent response. In contrast to SS rats, rapamycin treated SD rats exhibited a gain in the body weight from 304 ± 8 to 324 ± 11 g (P<0.05) during the 21 days of the 4.0% NaCl diet although neither daily food intake nor steady-state Na+ excretion was altered (Figure S3C&D). However, when compared to vehicle treated SD rats, body weight of rapamycin treated rats was lower at the end of study (324 ± 11 versus 365 ± 2 g; P<0.05). As seen in SS rats, rapamycin abolished the diurnal variations of MAP, SBP, DBP but had little effect upon diurnal variations of HR in SD rats (Figure S2E–H). Albumin excretion rate was unaltered between the vehicle (26.6 ± 23.6 mg/day) and rapamycin-treated (11.7 ± 2.3 mg/day) SD rats.
Also seen in Figure 3A, treatment with rapamycin decreased the kidney injury in SS rats as reflected by albumin excretion rate (UalbV) from 190.0 ± 35.0 to 37.0 ± 5.0 mg/day compared to vehicle treated rats. Similar responses were observed as determined with urine protein excretion rate (Figure 3B). Protection from renal tubular injury in rapamycin treated rats was evident based on the significant reduction of tubular protein casts within the outer medulla (Figure 3C). Importantly, the glomerular injury index was significantly reduced in the kidney of rapamycin treated rats compared to vehicle treated SS rats (Figure 3D).
Figure 3.
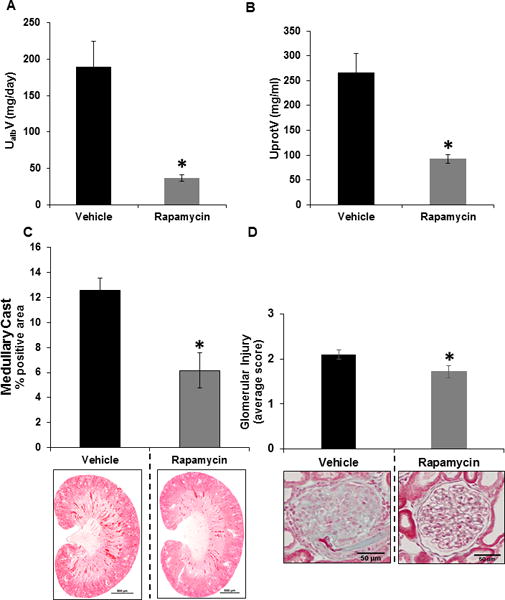
A&B, Albumin and protein were measured in urine samples collected for 24 hours on the last day of the 4.0 % NaCl for the determination of urinary excretion rates. Albumin (UalbV) and protein excretion (UprotV) rates were measured in SS rats treated with vehicle (n=7) or rapamycin (n=9). C, Summary of the outer medullary tubular injury (percentage of tubular cast positive region) and below graph shown here are the representative sections of trichrome-stained kidney from vehicle (n=7) or rapamycin (n=9) treated rats. D, Quantification of glomeruli injury in the cortex of vehicle (n=6) or rapamycin (n=6) treated SS rats. * p<0.05 Vs vehicle.
Rapamycin specifically inhibited renal mTORC1 activity
Rapamycin treated rats exhibited approximately 2-fold reduction in the level of pS6S235/236/S6 in both cortex and outer medulla relative to vehicle treated rats (Figure 4A). These reductions were consistent with the immunohistochemical analysis of pS6 S235/236 in both cortex and medulla of these kidneys represented in Figure 4B. In contrast, pAKTS473/AKT remained unaffected in both cortex and outer medulla of these rats kidney (Figure 4C). These data indicate that the rapamycin specifically inhibited the renal mTORC1 activity and that the protective effect upon salt-induced hypertension and renal injury observed with rapamycin treatment was independent of any possible off target inhibition of renal mTORC2 activity.
Figure 4.
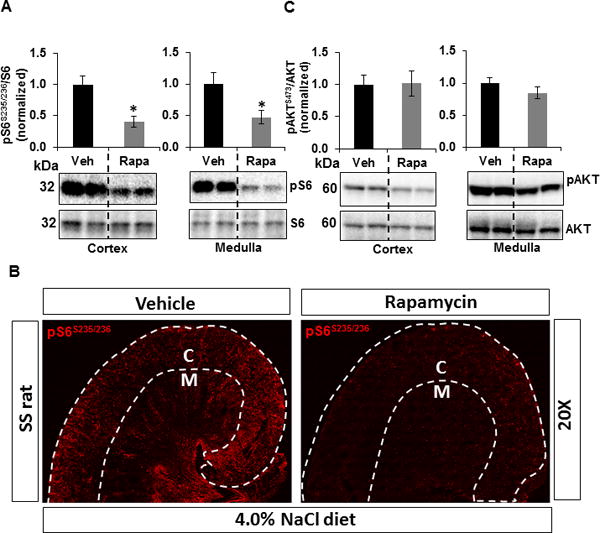
Western blots of pS6S235/236 and pAKTS473 were normalized with total endogenous S6 and AKT proteins, respectively. Graphs represent pS6S235/236/S6 or pAKTS473/AKT normalized to the vehicle treated SS rats. Corresponding representative immunoblots are shown below each graph. A, The level of pS6S235/236/S6 and B, Representative photomicrograph of the immunohistochemical profile of pS6S235/236 level in the renal cortex and medulla of SS rats treated with vehicle (n=7) or rapamycin (n=9) fed a 4.0% NaCl diet. White discontinuous lines designate the boundaries of the cortex (C) and medulla (M) C, pAKTS473/AKT in the cortex and outer medulla was measured in these rats kidney. * p<0.05 Vs vehicle.
mTORC1 inhibition reduced the immune cells infiltration into kidney
Renal infiltration of T lymphocytes (CD3+ cells) was significantly reduced in the cortex of rapamycin treated SS rats from an average of 192.0 ± 78.0 in the vehicle control to 80.0 ± 14.0 CD3+ cells/mm2. Similarly, CD3+ cells in the outer medulla of rapamycin treated rats averaged 71.0 ± 16.0 compared to 237.0 ± 70.0 cells/mm2 in the vehicle treated rats (Figure 5A). Rapamycin treatment also reduced macrophage (ED1+ cells) infiltration in the SS rats kidney with the cortex showing reductions from 605.0 ± 150.0 to 254.0 ± 83.0 and outer medulla from 484.0 ± 275.0 to 204.0 ± 85.0 ED1+ cells/mm2 (Figure 5B). Moreover, both T lymphocyte and macrophage markers (CD3 and CD68, respectively) were found to be co-localized with pS6S235/236 in the kidney of SS rats fed a 4.0% NaCl diet for 21 days (Figure S4).
Figure 5.
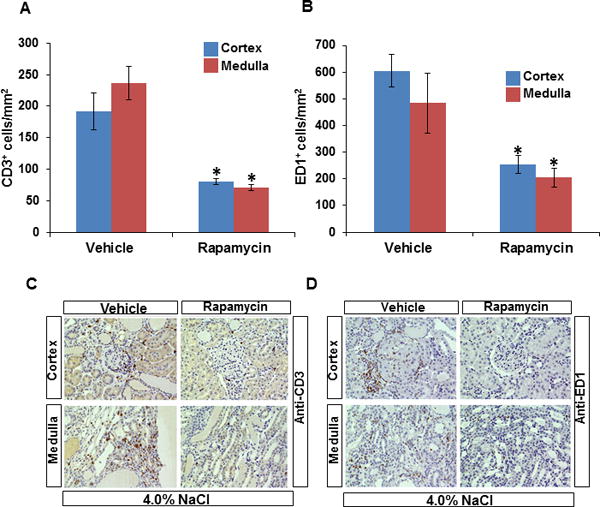
Quantification of T lymphocytes (CD3+) and macrophages (ED1+) cells per mm2 of kidneys of SS rats treated with vehicle (n=7) or rapamycin (n=9) after 21 days of the 4.0% NaCl diet. A, CD3+ cells/mm2 in the renal cortex and outer medulla B, ED1+ cells/mm2 in the renal cortex and outer medulla C, representative kidney sections illustrating the immunohistochemical localization of T cells and macrophage cells (D) in the renal cortex and outer medulla. Blue and brown column on bar graph represent cortex and outer medulla, respectively. * p<0.05 Vs vehicle.
Rapamycin reduced the renal hypertrophy and cell proliferation independent of apoptosis
The ratio of kidney weight to body weight was determined to obtain hypertrophy index and p27 (Cyclin dependent kinase inhibitor) and Ki67 were used as a marker to assess hypertrophic and proliferative cells, respectively. As summarized in Figure 6A, rapamycin lowered the hypertrophy index by 28.0% (P<0.05) compared to vehicle treated SS rats. Consistent with this observation, rapamycin reduced the percentage of p27 labeled cells by 50% in both cortex and outer medulla. (Figure 6B & Figure S5). The immunohistochemical analysis of Ki67 labeled cells in the kidney demonstrated that rapamycin significantly reduced the percentage of Ki67 labeled cells in the cortex from 11.0 ± 4.0 to 3.0 ± 1.0 and in the outer medulla from 9.0 ± 2.0 to 2.0 ± 1.0 compared to vehicle treated rats (Figure 6C & Figure S6). To assess the contribution of apoptosis to the observed reduction of proliferative cells, TUNEL immunohistochemical analysis for apoptosis was performed. As shown in Figure 6D & Figure S7, although the outer medulla exhibited greater levels of apoptosis than the cortex, neither rapamycin nor vehicle treatment had any effect in either region.
Figure 6.
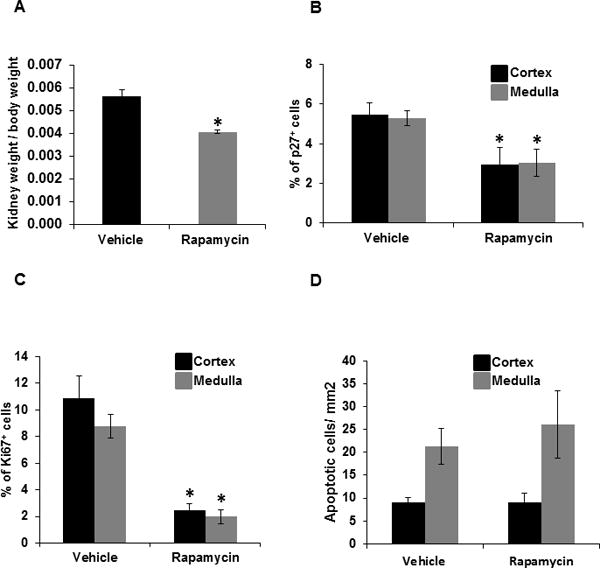
A, hypertrophic index was determined by measuring the ratio of right kidney weight (gm) and total body weight (gm) of the SS rats treated with vehicle (n=7) or rapamycin (n=9) maintained on the 4.0% NaCl diet. B, graph represents the percentage of p27+ cells (hypertrophy), C, percentage of Ki67+ cells (cell proliferation) D, quantification of apoptotic cells/mm2 (TUNEL assay) in the renal cortex and outer medulla of SS rats treated with vehicle (n=6) or rapamycin (n=6) maintained on the 4.0% NaCl diet. Blue and brown column on bar graph represent cortex and outer medulla, respectively. * p<0.05 Vs vehicle.
Discussion
The present studies found that the inhibition of mTORC1 pathway with rapamycin significantly attenuated the salt-induced hypertension and effectively protected against renal injury in SS rats. mTORC1 is ubiquitously expressed in the kidney as reflected in Figure 1C and Figure S1 and this signaling pathway has been studied in several human diseases such as diabetes where it appears to play a role in podocyte function and diabetic nephropathy in humans and mice18, 19. Although there have been remarkably few studies examining the effects of mTORC1 inhibition in hypertension, an upregulation of PI3K/Akt/mTOR pathway has been observed in a DOCA-salt model of hypertension20 and in Han:SPRD rats with polycystic kidney disease21.
In the present study, an elevation of mTORC1 activity in the kidneys of SS rats fed a 4.0% NaCl diet was reflected by significant increases of pS6S235/236/S6 (Figure 1A). In the SS rats, high salt intake is known to increase in the production of reactive oxygen species (ROS) including superoxide O•2−22 and H2O216 in the kidney from NADPH oxidases and mitochondria15,23. There is growing recognition that H2O2 is not simply a by-product of metabolism but it acts as a secondary messenger with active roles in signaling processes23. S6 kinase activity levels have been found to be stimulated by H2O2 treatment of mouse epidermal JB6 cells in a dose- and time-dependent manner24. The present chronic infusion studies importantly demonstrated the in vivo relevance of H2O2 stimulation of the mTORC1 pathway in the kidney. The amount of H2O2 delivered into the renal interstitium of SD rats in the present study produces tissue concentrations similar to those found in SS rats fed a high salt diet16. Since these chronic elevations of H2O2 elevated the renal tissue activity of pS6S235/236/S6, it appears that the elevations of renal H2O2 observed in the SS rats fed the 4.0% NaCl diet would serve as a secondary messenger to upregulate mTORC1 activity and dysregulation of mTORC1-mediated cellular processes.
We have observed a moderate initial rise of blood pressure during the four days of rapamycin treatment while both SS and SD rats received a 0.4% NaCl diet prior to switching to the 4.0% NaCl diet, a response also reported in Wistar rats treated with rapamycin for 7 weeks25. The mechanism(s) responsible for this rise of pressure remain unclear. Central neural actions of rapamycin are suggested by observed alterations of circadian rhythms. Cao et al (2010) recently reported that the mTORC1 pathway serves as a potent and selective regulator of light-evoked protein translation and suprachiasmatic nucleus clock entrainment26. Consistent with their observations, we found that the diurnal blood pressure rhythm was nearly abolished in rapamycin treated SS and SD rats which was apparent as early as 12 hours after start of drug treatment.
Immune cell participation in salt-induced hypertension and kidney injury
The most important observation of the present study is that the malignant phase of the hypertension was prevented by rapamycin treatment of SS rats fed a 4.0% NaCl diet. Among the most important mechanisms to consider when inhibiting the mTORC1 pathway are those related to both the innate (macrophages) and adaptive immune cells (T lymphocytes) that reside in the kidney and which are known to amplify the disease process in both SS rats and humans2. Phospho-S6 was co-localized with T lymphocytes and macrophages in our study suggesting that protective effects of rapamycin were a consequence of actions on both infiltrated immune cells and glomeruli/tubules mTORC1. Treatment with the immunosuppressant tacrolimus has been shown to attenuate salt-induced hypertension in SS rats by preventing immune cells infiltration4. However, tacrolimus as other similar compounds such as cyclosporine, are nephrotoxic as a consequence of vasoconstriction of the afferent and efferent glomerular arterioles27. In contrast, rapamycin appears to exhibit minimal vasomotor renal side effects as observed with chronic treatment of spontaneously hypertensive rats (SHR) in which no changes of glomerular filtration or renal blood flow were observed28.
In vehicle treated SS rats, upon switching to the 4.0% NaCl diet there was a rapid initial rise of blood pressure followed by a more dramatic rise of pressure after 10–14 days which is known to be accompanied by increased infiltration of immune cells into the kidney and culminating in ‘malignant hypertension’4,5. Both pharmacological and genetic approaches that reduce renal immune cells infiltration attenuate this secondary phase of salt-induced hypertension and renal injury in SS rats but not the primary phase5, 29. The rapamycin treated SS rats in the present study bear similarities to the phenotype observed in SS rats in which CD247 was globally knocked out (a T lymphocytes knockout rat model) and in SS rats treated with tacrolimus4, 30.
As seen in SSCD247−/− and tacrolimus treated SS rats4, rapamycin treated SS rats significantly reduced renal T cells infiltration (Figure 5A), a protective effect that has also been observed in Wistar rats with reduced renal mass31. Interestingly, increased proteinuria has been observed with chronic treatment with everolimus, a derivative of rapamycin32, 33. In the present study, rapamycin treated rats exhibited 5-fold reduction of daily albumin excretion which was an even greater response than that found in SSCD247−/− rats30 and tacrolimus treated rats4, which exhibited only a 3-fold reduction of daily albumin excretion. This greater protective effect could be explained by other mTORC1-mediated cellular processes which are important for the secondary phase of salt-sensitive hypertension. For example, the proto-oncogene cancer Osaka thyroid oncogene positively controls cap-dependent mRNA translation of Tnf and Il6 through mTORC1 in mouse macrophages34. IL-6 is a proinflammatory cytokine produced by various cells including macrophages, T-lymphocytes, endothelial cells, and vascular smooth muscle cells35. Chronic treatment of anti-IL-6 antibody reduced salt-sensitivity and renal macrophage accumulation in SS rats3. Rapamycin inhibition of IL-6 expression could therefore also account for some of the anti-inflammatory effects of rapamycin36. These actions would be consistent with our observation that rapamycin treated rats exhibited a significant reduction of macrophage infiltration into the kidney (Figure 6B). In this study, rapamycin was administrated systemically so the involvement of other organs and circulating immune cells in the observed responses cannot be ruled out. Nevertheless, present studies provide a strong association of renal mTORC1 activity and immune cell infiltration in salt-induced kidney injury in SS rats.
Rapamycin protects kidney injury by inhibiting renal hypertrophy and cell proliferation
We have recently reported the increased proliferative cells in the medullary thick ascending limbs of Henle in the SS rats on a high salt diet7. The development of hypertension is commonly associated with kidney hypertrophy in rodents8, 37. Rapamycin treatment to spontaneous hypertensive rats (SHRs) exhibited attenuation in cardiac hypertrophy although in that study renal hypertrophy was not studied12. In the present study, rapamycin inhibited renal hypertrophy and reduced cell proliferation (Ki67) in a 4.0% NaCl diet fed SS rats and this was associated with a significant inhibition of mTORC1 activity, each being novel findings. Although, others have observed enhancement of apoptosis by rapamycin38 this was not observed in our rapamycin treated SS rats. Prolonged rapamycin treatment has also been found to reduce mTORC2 activity in cancer cell lines39, which could have a direct effect on apoptosis pathways, but such effects were not apparent in present study and is consistent with our observation that renal mTORC2 activity (pAKTS473/AKT) was unchanged by rapamycin treatment.
Perspectives
The present study indicates that mTORC1 activity is upregulated in response to 4.0% NaCl diet feeding and H2O2 and that contributes significantly to hypertension and kidney injury in SS rats. The mTORC1 inhibitor rapamycin attenuated blood pressure salt-sensitivity and greatly reduced kidney injury and hypertrophy as reflected by reduction of immune cells infiltration and reduced cell proliferation. Although rapamycin is undoubtedly not the drug of choice to treat hypertension given the dose limiting side effects in human studies40, 41, the present studies have revealed the importance of the mTORC1 pathway in a widely used model of hypertension. New TOR kinase (TORK) inhibitors are currently being developed that should be explored as potentially novel therapeutic approaches for the treatment of hypertension and associated renal injury.
Supplementary Material
Novelty and Significance.
What is new?
The first study to recognize the association of mTORC1 pathway and dietary salt intake in SS rat model.
The study represents the first to demonstrate the selective inhibition of mTORC1 with rapamycin has protective effect on salt-induced hypertension and kidney injury in SS rats.
What is relevant
mTORC1 signaling contributes importantly in salt-induced hypertension and kidney injury in a clinically relevant model of hypertension.
Chronic treatment of mTORC1 inhibitor rapamycin attenuated the salt-induced hypertension and kidney injury in SS rats.
Summary
Selective inhibition of mTORC1 with rapamycin significantly protects the salt-induced hypertension and renal injury by the preventing microalbuminuia, glomerular injury, immune cells infiltration, cell proliferation and renal hypertrophy. It will now be important to study the association of mTOR complex 2 and dietary salt intake on hypertension.
Acknowledgments
We thank Matthew Hoffman for radiotelemetry implantation and tissue collection.
Source of Funding
This work was supported by National Institutes of Health grants P01 HL116264 and P01 HL082798 (A.W. Cowley, Jr).
Footnotes
Disclosures
None
References
- 1.Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol. 1958;34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 2.Ebringer A, Doyle AE. Raised serum IgG levels in hypertension. Br Med J. 1970;2:146–148. doi: 10.1136/bmj.2.5702.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2016;311:F555–561. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300:F734–742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol. 2014;307:F499–508. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughson MD, Gobe GC, Hoy WE, Manning RD, Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Yang C, Stingo FC, Ahn KW, Liu P, Vannucci M, Laud PW, Skelton M, O’Connor P, Kurth T, Ryan RP, Moreno C, Tsaih SW, Patone G, Hummel O, Jacob HJ, Liang M, Cowley AW., Jr Increased proliferative cells in the medullary thick ascending limb of the loop of Henle in the Dahl salt-sensitive rat. Hypertension. 2013;61:208–215. doi: 10.1161/HYPERTENSIONAHA.112.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57:269–274. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Bandi HR, Hofsteenge J, Bussian BM, Thomas G. Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem. 1991;266:22770–22775. [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Soesanto W, Lin HY, Hu E, Lefler S, Litwin SE, Sena S, Abel ED, Symons JD, Jalili T. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension. 2009;54:1321–1327. doi: 10.1161/HYPERTENSIONAHA.109.138818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 2013;17:599–606. doi: 10.1016/j.cmet.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowley AW, Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the Importance of Nox4 in Production of Hypertension in Dahl Salt-Sensitive Rats. Hypertension. 2016;67:440–450. doi: 10.1161/HYPERTENSIONAHA.115.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor NE, Cowley AW., Jr Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1573–1579. doi: 10.1152/ajpregu.00525.2005. [DOI] [PubMed] [Google Scholar]
- 17.Cowley AW, Jr, Yang C, Kumar V, Lazar J, Jacob H, Geurts AM, Liu P, Dayton A, Kurth T, Liang M. Pappa2 is linked to salt-sensitive hypertension in Dahl S rats. Physiol Genomics. 2016;48:62–72. doi: 10.1152/physiolgenomics.00097.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godel M, Hartleben B, Herbach N, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma SK, Choi JS, Joo SY, Kim HY, Kim CS, Bae EH, Lee JU, Kim SW. Activation of the Renal PI3K/Akt/mTOR Signaling Pathway in a DOCA-Salt Model of Hypertension. Chonnam Med J. 2012;48:150–154. doi: 10.4068/cmj.2012.48.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belibi F, Ravichandran K, Zafar I, He Z, Edelstein CL. mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol. 2011;300:F236–244. doi: 10.1152/ajprenal.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori T, O’Connor PM, Abe M, Cowley AW., Jr Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension. 2007;49:1336–1341. doi: 10.1161/HYPERTENSIONAHA.106.085811. [DOI] [PubMed] [Google Scholar]
- 23.Cowley AW, Jr, Abe M, Mori T, O’Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol. 2015;308:F179–197. doi: 10.1152/ajprenal.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae GU, Seo DW, Kwon HK, Lee HY, Hong S, Lee ZW, Ha KS, Lee HW, Han JW. Hydrogen peroxide activates p70(S6k) signaling pathway. J Biol Chem. 1999;274:32596–32602. doi: 10.1074/jbc.274.46.32596. [DOI] [PubMed] [Google Scholar]
- 25.Reis F, Parada B, Teixeira de Lemos E, Garrido P, Dias A, Piloto N, Baptista S, Sereno J, Eufrasio P, Costa E, Rocha-Pereira P, Santos-Silva A, Figueiredo A, Mota A, Teixeira F. Hypertension induced by immunosuppressive drugs: a comparative analysis between sirolimus and cyclosporine. Transplant Proc. 2009;41:868–873. doi: 10.1016/j.transproceed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Cao R, Obrietan K. mTOR Signaling and Entrainment of the Mammalian Circadian Clock. Mol Cell Pharmacol. 2010;2:125–130. doi: 10.4255/mcpharmacol.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 28.DiJoseph JF, Mihatsch MJ, Sehgal SN. Renal effects of rapamycin in the spontaneously hypertensive rat. Transpl Int. 1994;7:83–88. doi: 10.1007/BF00336467. [DOI] [PubMed] [Google Scholar]
- 29.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, PhysGen Knockout P. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diekmann F, Rovira J, Carreras J, Arellano EM, Banon-Maneus E, Ramirez-Bajo MJ, Gutierrez-Dalmau A, Brunet M, Campistol JM. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J Am Soc Nephrol. 2007;18:2653–2660. doi: 10.1681/ASN.2007010087. [DOI] [PubMed] [Google Scholar]
- 32.Daniel C, Ziswiler R, Frey B, Pfister M, Marti HP. Proinflammatory effects in experimental mesangial proliferative glomerulonephritis of the immunosuppressive agent SDZ RAD, a rapamycin derivative. Exp Nephrol. 2000;8:52–62. doi: 10.1159/000020648. [DOI] [PubMed] [Google Scholar]
- 33.Kirsch AH, Riegelbauer V, Tagwerker A, Rudnicki M, Rosenkranz AR, Eller K. The mTOR-inhibitor rapamycin mediates proteinuria in nephrotoxic serum nephritis by activating the innate immune response. Am J Physiol Renal Physiol. 2012;303:F569–575. doi: 10.1152/ajprenal.00180.2012. [DOI] [PubMed] [Google Scholar]
- 34.Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, Martineau Y, Larsson O, Rong L, Svitkin YV, Makrigiannis AP, Bell JC, Sonenberg N. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 35.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 36.Weichhart T, Hengstschlager M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo DF, Chenier I, Lavoie JL, Chan JS, Hamet P, Tremblay J, Chen XM, Wang DH, Inagami T. Development of hypertension and kidney hypertrophy in transgenic mice overexpressing ARAP1 gene in the kidney. Hypertension. 2006;48:453–459. doi: 10.1161/01.HYP.0000230664.32874.52. [DOI] [PubMed] [Google Scholar]
- 38.Avellino R, Romano S, Parasole R, Bisogni R, Lamberti A, Poggi V, Venuta S, Romano MF. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400–1406. doi: 10.1182/blood-2005-03-0929. [DOI] [PubMed] [Google Scholar]
- 39.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 40.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 41.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wuthrich RP. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.