Abstract
In recent years, bioactive compounds are in high demand in the pharmaceuticals and naturopathy, due to their health benefits to human and plants. Microorganisms synthesize these compounds and some enzymes either alone or in association with plants. Microbes residing inside the plant tissues, known as endophytes, also produce an array of these compounds. Endophytic actinomycetes act as a promising resource of biotechnologically valuable bioactive compounds and secondary metabolites. Endophytic Streptomyces sp. produced some novel antibiotics which are effective against multi-drug-resistant bacteria Antimicrobial agents produced by endophytes are eco-friendly, toxic to pathogens and do not harm the human. Endophytic inoculation of the plants modulates the synthesis of bioactive compounds with high pharmaceutical properties besides promoting growth of the plants. Hydrolases, the extracellular enzymes, produced by endophytic bacteria, help the plants to establish systemic resistance against pathogens invasion. Phytohormones produced by endophytes play an essential role in plant development and drought resistance management. The high diversity of endophytes and their adaptation to various environmental stresses seem to be an untapped source of new secondary metabolites. The present review summarizes the role of endophytic bacteria in synthesis and modulation of bioactive compounds.
Keywords: Antimicrobial agents, Bioactive compounds, Endophytic bacteria, Modulation, Phytohormones, Secondary metabolites
Introduction
The “bioactive” or “biologically active” compounds are extra-nutritional constituents present in small quantities in lipid-rich foods and plant products (Cammack et al. 2006). These compounds are mostly produced by plants and microbes, and have broad pharmaceutical properties including anti-cancer, cardiovascular, anti-lipidemic, anti-hypertensive, anti-glycaemic, antithrombotic, anti-atherogenic and anti-diabetic (Puri et al. 2005; Chang et al. 2013; Atanasov et al. 2015; Villaescusa et al. 2015). Nowadays, bioactive compounds are used as preferred synthetic medicines for various diseases with very few side effects (Chang et al. 2013).
The endophytic fungi, bacteria and actinomycetes play a significant role in the production of bioactive compounds. Bioactive compounds like alkaloids, steroids, terpenoids, peptides, polyketones, flavonoids, quinols and phenols, and the natural insecticide azadirachtin produced by endophytic bacteria (Li et al. 2008; Kusari et al. 2012; Molina et al. 2012) have agricultural, industrial and medical applications (Hallmann et al. 1997; Kobayashi and Palumbo 2000; Zinniel et al. 2002).
Endophytic microbes spend most of their life cycle within the plant tissues without causing any visible damage to the host plant. Many endophytes also secrete specialized metabolites or biologically active compounds (Liarzi et al. 2016). Endophytic bacteria are also having the potential due to their ability to produce plant growth hormones, phosphate solubilization, nutrient acquisition and fixation of N2 (Glick 2012).
Synthesis of bioactive compounds by endophytic microbes
The bioactive compounds synthesized by endophytes that help the host plant to develop systemic resistance against pathogens are also used in the pharmaceutical industries as antibiotics, anti-cancer, anti-viral, anti-diabetic and other bioactive compounds (Guo et al. 2008).
Endophytic fungi
Endophytic fungi are one of the major potential sources for the production of valuable bioactive compounds (Dreyfuss and Chapela 1994). Pestalotiopsis neglecta BAB-5510, an endophytic fungus of Cupressus torulosa, is considered to be a promising source of phenols, flavonoids, terpenoids, alkaloids, tannins, carbohydrates and saponin (Sharma et al. 2016), while Gilmaniella sp. AL12, an endophytic fungus, can stimulate Atractylodes lancea to produce volatile oils such as β-caryophyllene, zingiberene, caryophyllene oxide, β-sesquiphellandrene, hinesol, β-eudesmol and atractylone (Chen et al. 2016). Among the bioactive compounds, the antioxidants are the major compounds, frequently in discussions for good health and preventing diseases (Perucka and Materska 2003; Sanatombi and Sharma 2008; Al Othman et al. 2011). Capsaicin, a bioactive compound, abundantly found in red and chili peppers has been used as a medicine to remedy pain and as anti-cancerous agent for various human cancers. Alternaria alternata, an endophytic fungus isolated from Capsicum annum, produces capsaicin (Devari et al. 2014; Clark and Lee 2016). The endophytic organisms also produced various enzymes having pharmaceutical potential. Eurotium sp. an endophytic fungus, isolated from rhizomes of Curcuma longa produced asparaginase, an important anti-cancer enzyme (Jalgaonwala and Mahajan 2014). Endophytic fungus Huperzia serrata strains, L10Q37 and LQ2F02, also showed anti-acetylcholinesterase activity (Zhejian et al. 2015). Talaromyces pinophilus, an endophytic fungus isolated from strawberry tree (Arbutus unedo), produced siderophore ferrirubin, platelet-aggregation inhibitor herquline B and the antibiotic 3-O-methylfunicone. This strain also exhibited toxic effects against the pea aphid Acyrthosiphon pisum (Homoptera aphidiidae) (Vinale et al. 2017). The endophytic fungus Fusarium oxysporum 162 produced nematode antagonistic compounds, 4-hydroxybenzoic acid, indole-3-acetic acid (IAA) and gibepyrone D (Liu et al. 2016; Bogner et al. 2017).
Endophytic actinomycetes
Endophytic actinomycetes appear to be the promising source of bioactive agents, which can also be exploited for protection of crops and production of therapeutic agents (Balagurunathan and Radhakrishnan 2010; Prashith-Kekuda 2016). To date, more than 140 actinomycetes genera have been described and only a few of them produced majority of the known essential antibiotics (Jensen et al. 2005; Bull and Stach 2007; Pimentel-Elardo et al. 2010). Actinomycetes produce different types of secondary metabolites; many of these possess biological activities and have the potential to develop as therapeutic agents. Marine actinomycetes are underexploited source of novel secondary metabolites (Lam 2006). Streptomyces rochei CH1, an endophytic actinomycete of Cinnamomum sp., showed significant antibacterial activity against various test pathogens like Aeromonas caviae, Vibrio parahemolyticus and Pseudomonas aeruginosa (Roy and Banerjee 2015). Streptomyces cyaneofuscatus (KY287599) showed broad spectrum antimicrobial activities against Escherichia coli MTCC 739, P. aeruginosa MTCC 2453, Micrococcus luteus NCIM 2170, Staphylococcus aureus MTCC and yeast pathogen Candida albicans MTCC 3017. Streptomyces KX852460 also showed anti-fungal activity against Rhizoctonia solani AG-3 KX852461, the causative agent of target spot disease of tobacco leaf (Ahsan et al. 2017; Zothanpuia et al. 2017).
Endophytic bacteria
Majority of the endophytic bacteria showed beneficial effects like enhancement of biological N2-fixation, production of phytohormones, solubilization of phosphate and inhibition of ethylene (C2H2) biosynthesis in response to biotic and abiotic stresses and have bio-control activity. More than 300 endophytic actinobacteria and bacteria belonging to the genera Streptomyces, Nocardiopsis, Brevibacterium, Microbacterium, Tsukamurella, Arthrobacter, Brachybacterium, Nocardia, Rhodococcus, Kocuria, Nocardioides, and Pseudonocardia were isolated from different tissues of Dracaena cochinchinensis Lour. (a traditional Chinese medicine known as dragon’s blood). Of these, 17 strains having antimicrobial and anthracyclines-producing activities also showed anti-fungal and cytotoxic activities against two human cancer cell lines, MCF-7 and Hep G2 (Dudeja and Giri 2014; Salam et al. 2017).
Mode of entry and establishment of endophytic bacteria in the plant
Endophytic bacteria were found in various environments which include tropic, temperate, aquatic, xerophytic, deserts, Antarctic, geothermal soils, rainforests, mangrove swamps and also coastal forests (Strobel et al. 2002; Suryanarayanan and Murali 2006). Endophyte–plant interaction is controlled by the genes of both organisms and modulated by the environmental conditions (Battistoni et al. 2005; Rosenblueth and Romero 2006). Obligate bacterial endophytes are strictly dependent on their host plant for their growth while facultative endophytes are biphasic alternating between plants and the soil.
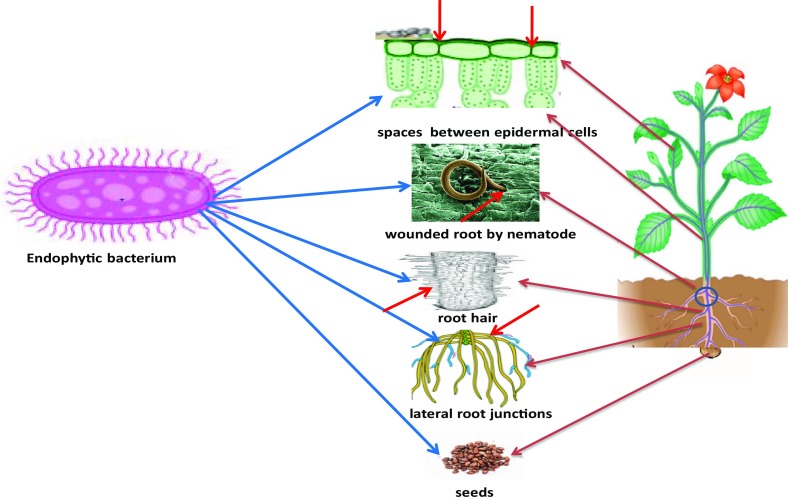
Endophytes are transmitted through the seeds or recruited from the soil rhizosphere. They enter the host plant through the cracks formed in the lateral root junction or wound caused by microbial or nematodes phytopathogens (Chi et al. 2005) and quickly spread to the endorhizosphere. Entry of endophytic bacteria in the plant roots also occurs through root hairs and spaces between epidermal cells (Hardoim et al. 2008). In the invasive process, the degradation of plant cell envelop occurs by enzymatic activity, such as production of endoglucanases, pectinases and cellulases which assist them to colonize endorhizosphere. Endoglucanases loosen larger cellulose fiber and may help in entry to the plant. In addition, exoglucanases may also help in the colonization process (Reinhold-Hurek and Hurek 2011). Some strains of Streptomyces have been reported to produce hydrolytic cell wall-degrading enzymes such as chitinases, cellulases, hemicellulases, amylases and glucanases along with lignin-degrading enzymes. Endophytic Streptosporangium sp. from maize produced glucoamylase. The cell wall degrading enzymes, endogluconase and polygalacturonase, seem to be required for the infection of Vitis vinifera by Burkholderia sp. (Compant et al. 2005) (Fig. 1).
Fig. 1.
Routes of entry of endophytic bacteria
Bioactive compounds synthesized by endophytic bacteria
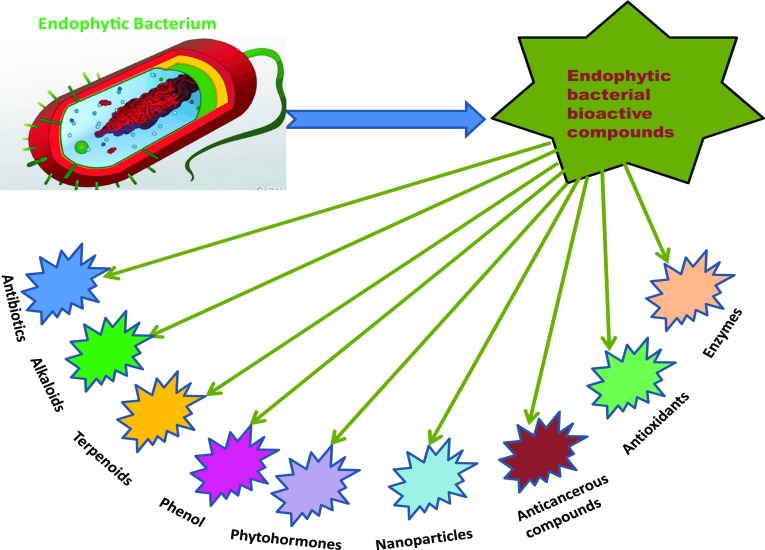
Bacterial endophytes have several potential applications in pharmaceutical and drug discovery (Strobel 2006; Guo et al. 2008). Endophytes associated with ethnomedicinal plants serve as a potential source of natural products for application in oxidative stress and as new bioactive agents (Nongkhlaw and Joshi 2015). The antimicrobial agents counteract the multi-drug resistance (MDR) in pathogenic microbes. Many microorganisms of agricultural concern have also acquired resistance to the commonly used antimicrobial compounds and the interest in natural methods of pathogen control through new, eco-friendly agents has been increasing day by day. Amines and amides are the common metabolites from endophytes that are toxic to insects but not mammals. Endophytes also produce extracellular hydrolyases such as cellulases, proteinase, lipases and esterases to establish resistance against plant invasions (Tan and Zou 2001). Endophytic bacteria associated with Hypericum perforatum and Ziziphora capitata belong to Arthrobacter, Achromobacter, Bacillus, Enterobacter, Erwinia, Pseudomonas, Pantoea, Serratia, and Stenotrophomonas. H. perforatum with antibacterial activity supported colonization of more bacteria with antagonistic activity, as compared to Z. capitata. Theses isolates were able to control tomato root rot caused by F. oxysporum (Egamberdieva et al. 2017) (Fig. 2).
Fig. 2.
Bioactive compounds synthesized by endophytic bacteria
Secondary metabolites
Secondary metabolites, though not essential for growth of an organism, play an adaptive role in functioning as the defense compound or the signaling molecule during ecological interactions and environmental stresses. Endophytic microorganisms produce low-molecular weight secondary metabolites that include antimicrobial compounds, phytohormones, or their precursors, vitamins like B12 (Ivanova et al. 2006) and B1 (Mercado and Bakker 2007), bioprotectants (Trotsenko and Khmelenina 2002). Several secondary metabolites are alkaloids, steroids, terpenoids, peptides, polyketones, flavonoids, quinols and phenols. These compounds also have important role in therapeutic applications such as anti-cancer, antioxidant, antimicrobial, anti-inflammatory, and immunosuppressive agents (Korkina 2007).
Secondary metabolites synthesis
Some endophytic bacteria modulate the production of secondary metabolites. Microbial secondary metabolites are synthesized from only a few precursors of primary metabolism with a relatively small number (Demain and Fang 2000). Endophytes synthesize secondary metabolites via a variety of pathways, e.g., polyketide, isoprenoid or amino acid derivation (Jalgaonwala 2013). However, the biosynthetic pathways are responsible for the production of both primary and secondary metabolites (Nicolaou et al. 2011) (Fig. 3).
Fig. 3.
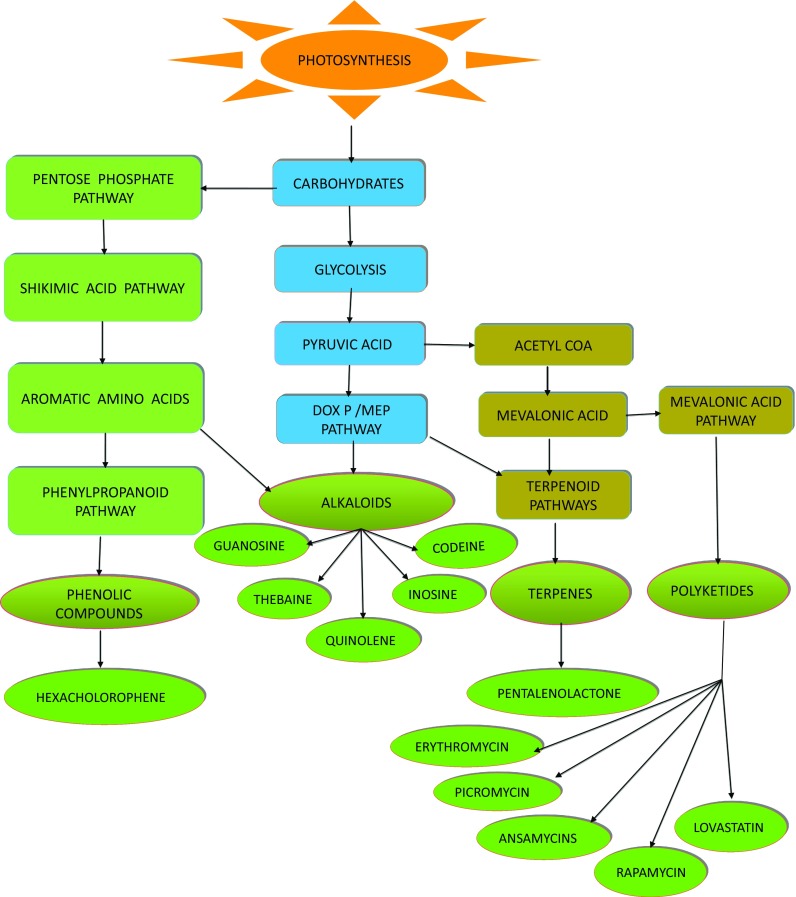
Pathways for the synthesis of some secondary metabolites
The high species diversity of endophytes and their adaption to various environments could be considered a rich and almost un-trapped source of new secondary metabolites for pharmaceutical or agricultural applications (Bacon and White 2000). Nature has provided a broad spectrum of structurally diverse secondary metabolites (Verpoorte 1998; Maier et al. 1999). These metabolites act as biofilm, toxins, virulence factors (Raaijmakers and Mazzola 2012) and interfering agents with the hormone signaling in plants (Lopez et al. 2008; Glick 2012). The production and modulation of auxins and C2H2 by endophytic bacteria–plant interactions play an essential role in plant development and drought stress management (Hardoim et al. 2008; Glick 2012).
Terpenoids
Terpenes are derived biosynthetically from isoprene units. Approximately, 50,000 terpenoid metabolites including monoterpenes, sesquiterpenes, and di-terpenes representing nearly 400 distinct structural families have been isolated from plants, fungi and bacteria. In contrast, a relatively minor fraction of these widely occurring metabolites has been identified in prokaryotes (Yamada et al. 2015). Among the numerous volatiles, the most common Streptomyces terpenoids, geosmin, 2-methylisoborneol, tricyclic α, β-unsaturated ketone and albaflavenone are the well-known volatile odoriferous microbial metabolites. The sesquiterpenoid antibiotic, pentalenolactone is the common metabolite isolated from more than 30 species of Streptomyces (Takahashi et al. 1983). Streptomyces exfoliatus UC5319 produced pentalenene synthase (ADO85594); the first characterized, cloned, and sequenced terpene synthase from Streptomyces (Lesburg et al. 1997). The endophytic bacterium Pseudomonas fluorescens ALEB7B can switch on the generation of reactive oxygen species (ROS) in A. lancea (a Chinese medicinal plant that contains oxygenous sesquiterpenoids) that leads to increased oxygenous sesquiterpenoid content (Zhou et al. 2015).
Alkaloids
Alkaloids, the low-molecular-weight, nitrogen-containing compounds, are important in pharmaceutical industries because of their high biological activities. Most alkaloids are derived from amines produced by the decarboxylation of amino acids, such as histidine, lysine, ornithine, tryptophan and tyrosine. Endophytes produce alkaloids as the secondary metabolites that have diverse biological potential as anti-fungal, anti-cancer, and anti-viral agents (Silva et al. 2007). Endophytic Bacillus cereus, Aranicola proteolyticus, Serratia liquefaciens, Bacillus thuringiensis, and Bacillus licheniformis isolated from Pinellia ternata have the ability to produce alkaloids (guanosine and inosine) in fermentation broth similar to their host plant (Liu et al. 2015). The capsular endophyte Acinetobacter SB1B in Opium poppy unregulated the expression of key genes for the benzylisoquinoline alkaloid (BIA) biosynthesis except thebaine and codeine. In contrast, Marmoricola sp. SM3B, another endophyte, could up-regulate the biosynthesis of both thebaine and codeine. Acinetobactor and Marmoricola sp. as microbial inoculants modulated the alkaloid producing genes in Opium poppy (Pandey et al. 2016).
Phenols
Among the different dietary bioactive compounds, polyphenols constitute an interesting group as some possess important biological activities including antioxidant, anti-carcinogenic or antimicrobial activities. They have been reported to lower the risks of many chronic diseases, including cancer, cardiovascular diseases, chronic inflammation, and many degenerative diseases. Phenolic compounds may be synthesized by the shikimate pathway (Kyselova 2011; Valdes et al. 2015; Carvalho et al. 2016; Lai Thi Ngoc Ha 2016). Dietary polyphenols may contribute to the maintenance of intestinal health by preserving the gut microbial balance through stimulation of the growth of beneficial bacteria (i.e., lactobacilli and bifidobacteria) and inhibition of pathogenic bacteria, exerting probiotic like effect (Duenas et al. 2015).
Endophytic bacterial isolates P. fluorescens Endo2 and Endo35 on inoculation induced systemic resistance against dry root rot of black gram (Vigna mungo L.) caused by Macrophomina phaseolina under glasshouse conditions. The bacterized black gram plants inoculated with dry root rot pathogen showed increased activities of peroxidase (PO), polyphenol oxidase (PPO), phenylalanine ammonia lyase (PAL) in addition to accumulation of phenolics and lignin (Karthikeyan et al. 2005). Inoculation of Talh tree (Acacia gerrardii Benth.) with endophytic bacterium B. subtilis (BERA 71) in combination with arbuscular mycorrhizal fungi (AMF) (Rhizophagus intraradices; Claroideoglomus etunicatum and Funneliformis mosseae) not only induced the acquired systemic resistance in plant but turned out to be potentially beneficial in ameliorating the deleterious impact of salinity on plant metabolism by modulating the osmoregulatory system (glycine, betaine, proline and phenols) and antioxidant enzymes system (Hashem et al. 2016).
Phytohormones and defense enzymes
Phytohormones are signal molecules that coordinate cellular activities and control plant growth and development. They play crucial roles in regulating plant responses to various stresses at extremely low concentrations. Bacterial endophytes and plants interactions result in the production and modulation of plant hormones (Lopez et al. 2008; Glick 2012). IAA is the most common, naturally occurring, plant hormone of the auxins class. The endophytic B. cereus (ECL1), B. thuringiensis (ECL2), Bacillus sp. (ECL3), Bacillus pumilis (ECL4), Pseudomonas putida (ECL5), and Clavibacter michiganensis (ECL6) isolated from C. longa L. produced IAA (Javid et al. 2011; Kumar et al. 2016). Pseudomonas, Agrobacterium and Bacillus isolated from the root of Cassia tora produced phytohormones and solubilized tricalcium phosphate (Kumar et al. 2015). Endophytic Artherobacter EZB-4 and Bacillus EZB-8 isolated from the pepper plant (Capsicum annum L.) produced IAA and increased the plant biomass. These endophytic bacteria also caused a significant reduction in up or down-regulation of the stress inducible genes (CaACCO and CaLTPI) compared to the gene expression in non-inoculated plants. Both the strains reduced osmotic and drought stresses (Sziderics et al. 2007). Achromobacter piechaudi E6S facilitated the plant growth by producting IAA, 1-aminocyclopropane-1-carboxylate (ACC) deaminase and solubilizing phosphate (Ma et al. 2016).
Abscisic acid (ABA) is considered as a plant stress hormone and is responsible for many kinds of stresses including water, salt, and cold temperatures. Drought is one of the stresses limiting crop production throughout the world. It is the single most devastating environmental constraint which hampers crop productivity more than any other stress (Farooq et al. 2012; Lambers et al. 2008). ABA and gibberellins (GA) produced by endophyte Azospirillum lipoferum alleviated the drought stress in maize (Cohen et al. 2009). Endophytic B. amyloliquefaciens SB-9 isolated from grapewine secreted high level of melatonin and produced three intermediates of melatonin biosynthetic pathway namely 5-hydroxytryptophan, serotonin and N-acetylserotonin. Serratia marcescens UPM39B3, an endophytic strain, induced the production of peroxidase, polyphenol oxidase, phenylalanine and ammonia lyase, besides total soluble phenols and lignothioglycolic acid in banana plantlets. Colonization of B. amyloliquefaciens SB-9 was also able to counteract the adverse effect of salt and drought-induced stresses by reducing the production of malondialdehyde (MDA) and reactive oxygen species (ROS) in grapewine roots (Jiao et al. 2016).
Besides phytohormones, salicylic acid (SA) is a critical plant hormone involved in various processes, such as seed germination, root initiation, stomatal closure, floral induction, and thermogenesis, besides the tolerance of plant to biotic and abiotic stresses. Endophytic bacteria enhanced the growth of sunflower seedlings under water stress, through the production of SA which also inhibited the growth of pathogenic fungi (Forchetti et al. 2010; Klessig et al. 2016).
Endophytic bacteria induced modulation of secondary metabolites production
Endophytic bacteria can be applied using different delivery techniques depending on need and growth stages of the plant. The seed treatment and foliar spray with bacterial endophytes seem to be economical because of lower cost. However, our knowledge of how bacterial endophytes enter and colonize plants is limited. Some endophytic plant growth promoting bacteria (PGPB) used as microbial inoculants enhanced the synthesis of biologically active compounds in the host plant. Turmeric rhizome contains a number of phenolic compounds, curcuminoids and sesquiterpenoids. Inoculation of turmeric rhizomes with endophytic Azotobactor chroococcum CL13 enhanced the production of curcumin (Kumar et al. 2014). Bacterial prodigiosins and their synthetic derivatives are effective pro-apoptotic agents against various cancer cell lines, with multiple cellular targets including multi-drug resistant cells with little or no toxicity towards normal cell lines. S. marcescens KC-1, an endophytic bacterial strain, secretes red pigment prodigiosins (Darshan and Manonmani 2015; Khanam and Chandra 2015).
Endophytic induction of secondary metabolites may be more widespread in aromatic and medicinal plants. The quality and quantity of flavor in strawberries are influenced by plant-associated methylobacteria (Verginer et al. 2010). Stenotrophomonas maltophilia (N5-18) delivered through foliar spray significantly enhanced the photosynthetic efficiency, total alkaloid and morphine content coupled with the decrease in thebaine content in Opium poppy (Pappaver sominiferum). The increase in capsule biomass and alkaloid content was followed by the consequent increase in productivity of P. sominiferum (Bonilla et al. 2014). Inoculation of Catharanthus roseus explants with endophytic Staphylococcus sciuri and Micrococcus sp. significantly enhanced the vindoline, ajmalicine and serpentine production (Tiwari et al. 2013).
Bioactive compounds produced by microbes are more convenient than plants
Bioactive compounds produced by the plants exhibit strong physiological activities. However, their production suffers from various problems including heterogeneous quality and insufficient level of productivity. Microorganisms produce primary and secondary metabolites under controlled environmental conditions assuring their maximum efficiency in uniform and high quality (Sato and Kumagai 2013). The association of plant with endophytic microorganisms has been proved as a source of materials and products with high medicinal potential as compared to the plants alone. Bioactive compounds such as secondary metabolites and enzymes synthesized by microorganisms have been extensively used as food and food supplements (Subbulakshmi et al. 2012; Mitsuhashi 2014; Wendisch 2014), pharmaceuticals (Elander 2003; Endo 2010), biofuels (Geddes et al. 2011), biopesticides (Waldron et al. 2001; Yoon et al. 2004) and detergents (Shaligram and Singhal 2010), as well as in the manufacturing process of these industrial products (Kirk et al. 2002; Merino and Cherry 2007). Metabolites and enzymes production methods have been improved since the time of their first importance was realized. B. subtilis and Lactobacillus plantarum enhanced the content of bioactive compounds (soluble phenolic compound content and antioxidant activity) in kidney bean extracts after fermentation (Limona et al. 2015).
Antimicrobial compounds
Antimicrobial metabolites produced from the endophytes are the bioactive natural compounds (Guo et al. 2008). Endophytes have developed a resistance mechanism to control pathogenic intrusion by producing secondary metabolites (Tan and Zou 2001). Many antimicrobial compounds produced by endophytes belong to several structural classes such as peptides, alkaloids, steroids, quinines, terpenoids, phenols and flavonoids (Yu et al. 2010). The novel antimicrobial metabolites from endophytes are now becoming the alternative option to overcome the increasing levels of drug resistance (Ferlay et al. 2010; Taechowisan et al. 2012). A large number of endophytic actinomycetes were isolated from 26 medicinal plants from Panxi plateau with the huge spectrum of antimicrobial activity, being the valuable reservoirs of novel bioactive compounds (Zhao et al. 2011). Endophytic Streptomyces sp. TQR12-4 isolated from Elite Citrus nobilis fruit showed antimicrobial activity and inhibited test pathogens Colletotrichum truncatum, Geotrichum candidum, F. oxysporum and F. udum (Hong-Thao et al. 2016).
Nanopartcles might play a significant role as antimicrobial agents. Nanoparticles have made a significant impact on the treatment of various types of cancer. Endophytic bacteria synthesize various nanoparticles which emerges as a novel field in the research area of pharmaceutical engineering (Sunkar and Nachiyar 2012). Silver nanoparticles have antibacterial properties and act as anti-viral agent against HIV-1, hepatitis B virus, respiratory syncytial virus and herpes simplex virus (Sun et al. 2005; Taylor et al. 2005; Lu et al. 2008; Baram-Pinto et al. 2009). Endophytic strains of Bacillus sp. isolated from the medicinal plants Adhatoda beddomei (Malabar nut) and Garcinia xanthochymus (Egg tree) synthesized silver nanoparticles (AgNPs) by reduction of silver nitrate (AgNO3) (Pissuwan et al. 2006; Kitov et al. 2008; Sunkar and Nachiyar 2012).
Anti-cancerous compounds
Several bioactive compounds produced by endophytes have been identified as anti-cancer agents (Firakova et al. 2007). Endophytic bacterial strain, EML-CAP3 isolated from C. annuum L. (red pepper) leaf, showed potent anti-angiogenic activity. This endophytic bacterial strain produced lipophilic peptides which inhibited the proliferation of human umbilical vein endothelial cells and also exhibited anti-angiogenic potential in tumor progression (Jung et al. 2015). Ginseng (Panax ginseng) is known for its ginsenosides that have anti-cancerous property. The transformed Paenibacillus polymyxa, an endophytic bacterium of Ginseng leaf, showed high ginsenoside concentration. This endophytic bacterial strain on inoculation to Ginseng plants through foliar applications combined with irrigation enhanced plant growth and the concentration of ginsenosides (Gao et al. 2015). Morphological abnormalities in the cells induced by exopolysaccharides (EPS) are the anti-tumoral mechanisms of action associated with the mitochondrial dysfunction of the treated cells. Bacillus serves a source of first discovered anti-tumoral EPS, a natural product of high therapeutic value for cancer treatment as a new anti-cancer agent (Chen et al. 2013). l-Asparaginase catalyzes the conversion of l-asparagine necessary for the function of some neoplastic cells, such as lymphoblasts. l-Asparaginase introduced to the multi-drug chemotherapy in children and adults with acute lymphoblastic leukemia resulted in significant improvement and complete remission in majority of the patients (Jakubas et al. 2008). Endophytic B. licheniformis, B. pseudomycoides and Paenibacillus denitriformis showed efficient production of l-asparaginase (Joshi and Kulkarni 2016).
Antibiotics
Antibiotics are natural compounds produced by microorganisms as secondary metabolites to kill or inhibit other microorganisms. They played an important role in twentieth century for the treatment of infectious diseases. Till now, pharmaceutical industries have primarily targeted the drugs from soil organisms, bacteria or fungi. Among the bacteria, actinobacteria are particularly noteworthy, as they produce antibiotics. Streptomyces sp. are fruitful organisms, producing ~80% of the total antibiotics (Sathiyaseelan and Stella 2011; Thenmozhi and Krishnan 2011). At least seven thousand different secondary metabolites have been discovered from Streptomyces isolates (Berdy 2005). Streptomyces synthesizes antibiotics, fungicides, modulators of the immune response, and effectors of plant growth (Hopwood 2007). Antimicrobial drugs produced by some endophytic bacteria are listed in Table 1. Endophytic Streptomyces sp. LJK109 isolated from Alpinia galangal root produces 3-methylcarbazoles which is major anti-inflammatory component and also suppresses macrophage production of the inflammatory mediators NO, PGE2, TNF-α, IL-1β, IL-6 and IL-10 in a dose-dependent manner (Taechowisan et al. 2012) (Table 1).
Table 1.
Antibiotics and drugs produced by some endophytic bacteria
| Compounds | Endophytic bacteria | Biological activity | References |
|---|---|---|---|
| Ecomycin | Pseudomonas viridiflava | Anti-fungal | Miller et al. (1998) |
| Bacilysocin | B. subtilis 168 | Anti-fungal | Tamehiro et al. (2002) |
| Nystatin | Streptomyces noursei | Anti-fungal | Fjaervik and Zotchev (2005) |
| KB425796-A | Paenibacillus sp. 530603 | Anti-fungal | Kai et al. (2013) |
| Bacillomycin | B. subtilis, B.amyloliquefaciens | Anti-fungal, Hemolytic | Aranda et al. (2005) |
| Munumbicin | Streptomyces NRRL 30562 | Antibacterial | Castillo et al. (2002) |
| Harmaomycin | Streptomyces sp. | Antibacterial | Bae et al. (2015) |
| Subtilin | B. subtilis | Antibacterial | Stein (2005) |
| Tetracyclin | Streptomyces remosus and S. aureofaciens | Antibacterial | Mark et al. (2001) |
| Bacteriocins | B. subtilis | Antibacterial | Sansinenea and Ortiz (2011) |
| Amicoumacin | B. subtilis | Antibacterial, anti-inflammatory | Pinchuka et al. (2002) |
| Artemisinin | Pseudonocardia sp. | Anti-malarial | Li et al. (2012) |
| Coronamycin | Streptomyces sp. | Anti-malarial | Ezra et al. (2004) |
| Spectinomycin | Streptomyces spectabilis | Anti-tuberculosis | Barry (2014) |
| Treponemycin | Streptomyces Strain MS-6-6 | Anti-tuberculous | Mahmoud et al. (2015) |
| Androprostamines | Streptomyces sp. MK932-CF8 | Anti-prostate cancer | Yamazaki et al. (2015) |
| Camptothecine | Lysinibacillus sp. and B. cereus | Anti-cancer | Singh et al. (2013) |
| Indolocarbazoles | Streptomyces sp. | Anti-cancer | Dong et al. (2014) |
| Doxorubicin | Streptomyces sp. | Treatment of Breast cancers and tumors | Brayfield (2013) |
| Anthracyclin | Streptomyces sp. YIM66403 | Antitumor | Wei et al. (2015) |
| Daptomycin | Streptomyces roseoporous | Bacterial infections of skin and underlying tissues | Miao (2005) |
| Monensin | Streptomyces cinnamonensis | Prevent coccidiosis | Lowicki and Nski (2013) |
| Mytomycin C | Streptomyces caespitosus and S. lavendulae | Chemotherapeutic agent | Danshiitsoodol et al. (2006) |
| Saadamycin | Streptomyces sp. Hedaya48, | Anti dermatophyte | Gendy and Bondkly (2010) |
| Strepturidin | Streptomyces albus DSM 40763 | Immunotherapy | Pesic et al. (2014) |
| Thaxtomin A | Streptomyces scabies | Cellulose synthesis inhibitor | Francis et al. (2015) |
| Xiamycin | Streptomyces sp. | Anti HIV activity | Ding et al. (2010) |
| β-exotoxin | B. thuringiensis | Insecticidal | Espinasse et al. (2002) |
| Albaflavenol B | Streptomyces sp. | As sesquiterpene | Raju et al. (2015) |
The majority of endophytic bacteria produce different kinds of antibiotics. Ecomycin, pseudomycins and kakadumycins are some of the novel antibiotics produced by endophytic bacteria (Christina et al. 2013). Pseudomonas viridiflava, an epiphyte or endophyte of the leaves of many grasses, produced ecomycin, which is used for the treatment of respiratory and urinary tract infections, skin, eye and gut Infections. The structure of ecomycin incorporates some unusual amino acids such as homoserine and beta-hydroxyaspartic acid, besides common amino acids alanine, serine, threonine and glycine (Miller et al. 1998). Naphthomycin K, a chlorine-containing ansamycin, showed cytotoxicity against P388 and A-549 cell lines. Endophytic Streptomyces sp. CS synthesized 24-demethylbafilomycin C1, a member of bafilomycin, which targets both autophagy and apoptosis pathways in pediatric B cell acute lymphoblastic leukemia (Li et al. 2010; Qin et al. 2011; Yuan et al. 2015). Endophytic Streptomyces sp. isolated from Aucuba japonica and Cryptomeria japonica produced two new novobiocin analogs and cedarmycins, respectively. A new naphthoquinone antibiotic, alnumycin was also isolated from the endophytic Streptomyces sp. from Alnus glutinosa. Streptomyces sp. NRRL30562, an endophyte of snake vine plant, produced new peptide antibiotic, munumbicins A-D40 with a broad spectrum activity against several human diseases, phytopathogenic fungi and bacteria. Endophytic Streptomyces sp. NRRL30566 isolated from a fern-leaved grevillea (Grevillea pteridifolia) tree produced kakadumycin which is chemically related to echinomycin (Castillo et al. 2003).
Future prospective
As our understanding of endophytic bacteria continues to grow, the potential to exploit their unique characteristics of bioactive compound synthesis alone or with plants is also increasing day by day. The plant benefits enhanced by combined application of beneficial microorganisms in the form of bio-fertilizer have become an alternative tool for organic farming. Exploitation of endophytic bacteria as a plant growth-promoting agent further necessitates our ability to understand and utilize bacterial endophytes in agriculture under integrated bio-fertilizer technology programme. How endophytes modulate the physiology of plant and its metabolism and how they use the intermediary substances of primary and secondary metabolism as nutrition and precursor to produce either novel compounds or enhance the existing important secondary metabolites are still largely unknown.
Acknowledgements
This work was supported by DST-Inspire (to Monika Singh), University Grant Commission (CAS in Botany), DST-FIST, New Delhi, India. Authors thank Prof. Madhoolika Agrawal, Head, Department of Botany, Banaras Hindu University, for providing facilities.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Ahsan T, Chen J, Zhao X, Irfan M, Wu Y. Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Expr. 2017;7:54. doi: 10.1186/s13568-017-0351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Othman ZA, Ahmed YB, Habila MA, Ghafar AA. Determination of capsaicin and dihydrocapsaicin in capsicum fruit samples using high performance liquid chromatography. Molecules. 2011;16:8919–8929. doi: 10.3390/molecules16108919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda FJ, Teruel JA, Ortiz A. Further aspects on the haemolytic activity of the antibiotic lipopeptide iturin A. Biochim Biophys Acta. 2005;1713:51–56. doi: 10.1016/j.bbamem.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon CW, White JF. Microbial endophytes. New York: Marcel Dekker Inc.; 2000. [Google Scholar]
- Bae M, Chung B, Oh KB, Shin J, Oh DC. Hormaomycins B and C: new antibiotic cyclic depsipeptides from a marine mudflat-derived Streptomyces sp. Mar Drugs. 2015;13:5187–5200. doi: 10.3390/md13085187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagurunathan R, Radhakrishnan M. Biotechnological, genetic engineering and nanotechnological potential of actinomycetes. In: Maheshwari DK, Dubey RC, Saravanamurthu R, editors. Industrial exploitation of microorganisms. 1. New Delhi: I.K. International Publishing House Pvt. Ltd; 2010. pp. 302–436. [Google Scholar]
- Baram-Pinto D, Shukla S, Perkas N, Gedanken A, Sarid R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug Chem. 2009;20:1497–1502. doi: 10.1021/bc900215b. [DOI] [PubMed] [Google Scholar]
- Barry CE. Tuberculosis drug discovery goes au naturel. Nature. 2014;27:436–437. doi: 10.1038/506436a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistoni F, Bartels D, Kaiser O, Reamon-Buettner MS, Hurek T, Reinhold Hurek B. Physical map of the Azoarcus sp. strain BH72 genome based on a bacterial artificial chromosome library as a platform for genome sequencing and functional analysis. FEMS Microbiol Lett. 2005;249:233–240. doi: 10.1016/j.femsle.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo) 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- Bogner CW, Kamdem RST, Sichtermann G, Mattheaus C, Heolscher D, Popp J, Proksch P, Grundler FMW, Schouten A. Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microb Biotechnol. 2017;10:175–188. doi: 10.1111/1751-7915.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla A, Sarria ALF, Algar E, Munoz Ledesma FJ, Solano BR, Fernandes JB, Gutierrez Manero FJ. Microbe associated molecular patterns from rhizosphere bacteria trigger germination and Papaver somniferum metabolism under greenhouse conditions. Plant Physiol Biochem. 2014;74:133–140. doi: 10.1016/j.plaphy.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Brayfield A (ed) (2013) Doxorubicin. Martindale: the complete drug reference. Pharmaceut Press. Retrieved 15 April 2014
- Bull AT, Stach JE. Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol. 2007;15:491–499. doi: 10.1016/j.tim.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Cammack R, Atwood T, Campell P, Parish H, Smith A, Vella F, Stirling J. Oxford dictionary of biochemistry and molecular biology. 2. Oxford: Oxford University Press; 2006. pp. 74–75. [Google Scholar]
- Carvalho PLN, Silva EO, Paula DAC, Luiz JHH, Ikegaki M. Importance and implications of the production of phenolic secondary metabolites by endophytic fungi: a mini-review. Mini Rev Med Chem. 2016;16:259–271. doi: 10.2174/1389557515666151016123923. [DOI] [PubMed] [Google Scholar]
- Castillo UF, Strobel GA, Ford EJ, Hess WM, Porter H, Jensen JB, Albert H, Robinson R, Condron MA, Teplow DB, et al. Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscans. Microbiol. 2002;148:2675–2685. doi: 10.1099/00221287-148-9-2675. [DOI] [PubMed] [Google Scholar]
- Castillo U, Harper JK, Strobel GA, Sears J, Alesi K, Ford E, Lin J, Hunter M, Maranta M, Ge H, et al. Kakadumycins, novel antibiotics from Streptomyces sp. NRRL 30566 an endophyte of Grevillea pteridifolia. FEMS Microbiol Lett. 2003;224:183–190. doi: 10.1016/S0378-1097(03)00426-9. [DOI] [PubMed] [Google Scholar]
- Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Base Complement Altern Med. 2013;2013:378657. doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Ren CG, Zhou T, Wei YJ, Dai CC. A novel exopolysaccharide elicitor from endophytic fungus Gilmaniella sp. AL12 on volatile oils accumulation in Atractylodes lancea. Sci Rep. 2016;6:34735. doi: 10.1038/srep34735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Yuan Q, Shan LT, Lin MA, Cheng DQ, Li CY. Anti-tumor activity of bacterial exopolysaccharides from the endophyte Bacillus amyloliquefaciens sp. isolated from Ophiopogon japonicas. Oncol Lett. 2013;5:1787–1792. doi: 10.3892/ol.2013.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi F, Shen S, Cheng H, Jing Y, Yanni Y, Dazzo F. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol. 2005;71:7271–7278. doi: 10.1128/AEM.71.11.7271-7278.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christina A, Christapher V, Bhore SJ. Endophytic bacteria as a source of novel antibiotics: an overview. Pharmacogn Rev. 2013;7:11–16. doi: 10.4103/0973-7847.112833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Lee S. Anti-cancer properties of capsaicin against human cancer. Anticancer Res. 2016;36:837–844. [PubMed] [Google Scholar]
- Cohen AC, Travaglia CN, Bottini R, Piccoli PN. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany. 2009;87:455–462. doi: 10.1139/B09-023. [DOI] [Google Scholar]
- Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Aitbarka E. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. Strain PsJN. Appl Environ Microbiol. 2005;71:1685–1693. doi: 10.1128/AEM.71.4.1685-1693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danshiitsoodol N, Pinho CA, Matoba Y, Kumagai T, Sugiyama M. The mitomycin C (MMC)-binding protein from MMC-producing microorganisms protects from the lethal effect of bleomycin: crystallographic analysis to elucidate the binding mode of the antibiotic to the protein. J Mol Biol. 2006;360:398–408. doi: 10.1016/j.jmb.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Darshan N, Manonmani HK. Prodigiosin and its potential applications. J Food Sci Technol. 2015;52:5393–5407. doi: 10.1007/s13197-015-1740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Fang A. The natural functions of secondary metabolites. Adv Biochem Eng. 2000;69:1–39. doi: 10.1007/3-540-44964-7_1. [DOI] [PubMed] [Google Scholar]
- Devari S, Jaglan S, Kumar M, Deshidi R, Guru S, Bhushan S, et al. Capsaicin production by Alternaria alternata, an endophytic fungus from Capsicum annum, LC–ESI–MS/MS analysis. Phytochemistry. 2014;98:183–189. doi: 10.1016/j.phytochem.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Ding L, Munch J, Goerls H, Maier A, Fiebig HH, Line WH, et al. Xiamycin, a pentacyclic indolo sesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg Med Chem Lett. 2010;20:6685–6687. doi: 10.1016/j.bmcl.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Dong BX, Ye WW, Han Y, Deng ZX, Hong K. Natural products from mangrove actinomycetes. Mar Drugs. 2014;12:2590–2613. doi: 10.3390/md12084326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss MM, Chapela IH. Potential of fungi in the diversity of novel, low molecular weight pharmaceuticals. In: Gullo VP, editor. The discovery of Natural Products with therapeutic Potential. Butterworth- Heinemann: Butterworth- Heinemann; 1994. pp. 49–80. [Google Scholar]
- Dudeja SS, Giri R. Beneficial properties, colonization, establishment and molecular diversity of endophytic bacteria in legumes and non leglumes. Afr J Micrbiol Res. 2014;8:1562–1572. doi: 10.5897/AJMR2013.6541. [DOI] [Google Scholar]
- Duenas M, Gonzalez IM, Cueva C, Giron AJ, Patán FS, Buelga CS, et al. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015 doi: 10.1155/2015/850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol. 2017;8:199. doi: 10.3389/fmicb.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elander RP. Industrial production of betalactam antibiotics. Appl Microbiol Biotechnol. 2003;61:385–392. doi: 10.1007/s00253-003-1274-y. [DOI] [PubMed] [Google Scholar]
- Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:484–493. doi: 10.2183/pjab.86.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinasse S, Gohar M, Lereclus D, Sanchis V. An ABC transporter from Bacillus thuringiensis is essential for beta-exotoxin I production. J Bacteriol. 2002;184:5848–5854. doi: 10.1128/JB.184.21.5848-5854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezra D, Castillo UF, Strobel GA, Hess WM, Porter H, Jensen JM, et al. Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp. (MSU-2110) endophytic on Monstera sp. Microbiol. 2004;150:785–793. doi: 10.1099/mic.0.26645-0. [DOI] [PubMed] [Google Scholar]
- Farooq M, Hussain M, Wahid A, Siddique KHM. Drought stress in plants: an overview. In: Aroca R, editor. Plant responses to drought stress. Berlin: Springer; 2012. pp. 1–5. [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Cancer incidence and mortality worldwide: IARC cancer base No. 10 Lyon, France: International Agency for Research on Cancer. Globocan 2008 v2.0
- Firakova S, Sturdikova M, Muckova M. Bioactive secondary metabolites produced by microorganisms associated with plants. Biologia. 2007;62:251–257. doi: 10.2478/s11756-007-0044-1. [DOI] [Google Scholar]
- Fjaervik E, Zotchev SB. Biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei. Appl Microbiol Biotechnol. 2005;67:436–443. doi: 10.1007/s00253-004-1802-4. [DOI] [PubMed] [Google Scholar]
- Forchetti G, Masciarelli O, Izaguirre MJ, Alemano S, Alvarez D, Abdala G. Endophytic bacteria improve seedling growth of sunflower under water stress, produce salicylic acid, and inhibit growth of pathogenic fungi. Curr Microbiol. 2010;61:485–493. doi: 10.1007/s00284-010-9642-1. [DOI] [PubMed] [Google Scholar]
- Francis IM, Jourdan S, Fanara S, Loria R, Rigali S. The cellobiose sensor CebR is the gatekeeper of Streptomyces scabies pathogenicity. mBio. 2015;6:02018-14. doi: 10.1128/mBio.02018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Lu Q, Pu Zang P, Li X, Ji Q, He Z, et al. An endophytic bacterium isolated from Panax ginseng CA Meyer enhances growth, reduces morbidity, and stimulates ginsenoside biosynthesis. Phytochem Lett. 2015;11:132–138. doi: 10.1016/j.phytol.2014.12.007. [DOI] [Google Scholar]
- Geddes CC, Nieves IU, Ingram LO. Advances in ethanol production. Curr Opin Biotechnol. 2011;22:312–319. doi: 10.1016/j.copbio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Gendy MM, Bondkly AM. Production and genetic improvement of a novel antimycotic agent, saadamycin, against dermatophytes and other clinical fungi from endophytic Streptomyces sp. Hedaya 48. J Ind Microbiol Biotechnol. 2010;37:831–841. doi: 10.1007/s10295-010-0729-2. [DOI] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012 doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Wang Y, Sun X, Tang K. Bioactive natural products from endophytes: a review. Prikl Biokhim Mikrobiol. 2008;44:153–158. [PubMed] [Google Scholar]
- Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43:895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- Hardoim PR, Overbeek LS, Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008;16:463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Hashem A, Abd-Allah EF, Alqarawi AA, Al-Huqail AA, Shah MA. Induction of osmoregulation and modulation of salt stress in Acacia gerrardii Benth. by arbuscular mycorrhizal fungi and Bacillus subtilis (BERA 71) BioMed Res Int. 2016 doi: 10.1155/2016/6294098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Thao PH, Mai-Linh NV, Hong-Lien NT, Hieu NV. Biological characteristics and antimicrobial activity of endophytic Streptomyces sp. TQR12-4 isolated from elite Citrus nobilis cultivar Ham Yen of Vietnam. Int J Microbiol. 2016;7207818:1–7. doi: 10.1155/2016/7207818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood DA. Streptomyces in nature and medicine. The antibiotic makers. New York: Oxford University Press Inc; 2007. [Google Scholar]
- Ivanova EG, Fedorov DN, Doronina NV, Trotsenko YA. Production of vitamin B12 in aerobic methylotrophic bacteria. Microbiology. 2006;75:494–496. doi: 10.1134/S0026261706040217. [DOI] [PubMed] [Google Scholar]
- Jakubas BP, Kulis MK, Giebel S, Cioch M, Czyż A, Maranda EL, et al. Use of L-asparaginase in acute lymphoblastic leukemia: recommendations of the Polish Adult Leukemia Group. Pol Arch Med Wewn. 2008;118:664–669. [PubMed] [Google Scholar]
- Jalgaonwala RE (2013) Bioprospecting for microbial endophytes and their natural products (Ph.D Thesis). North Maharastra University, Jalgaon, Maharastra, India
- Jalgaonwala RE, Mahajan RT. Production of anticancer enzyme asparaginase from endophytic Eurotium sp. isolated from rhizomes of Curcuma longa. Eur J Exp Biol. 2014;4:36–43. [Google Scholar]
- Javid MG, Sorooshzadeh A, Moradi F, Sanavy SAM, Allahdadi I. The role of phytohormones in alleviating salt stress in crop plants. AJCS. 2011;5:726–734. [Google Scholar]
- Jensen PR, Mincer TJ, Williams PG, Fenical W. Marine actinomycete diversity and natural product discovery. Antonie Leeuwenhoek. 2005;87:43–48. doi: 10.1007/s10482-004-6540-1. [DOI] [PubMed] [Google Scholar]
- Jiao J, Ma Y, Chen S, Liu C, Song Y, Qin Y, et al. Melatonin producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front Plant Sci. 2016;7:1387. doi: 10.3389/fpls.2016.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi RD, Kulkarni NS. Optimization studies on l-asparaginase production from endophytic bacteria. Int J Appl Res. 2016;2:624–629. [Google Scholar]
- Jung HJ, Yonghyo K, Hyang BL, Kwon HJ. Antiangiogenic activity of the lipophilic antimicrobial peptides from an endophytic bacterial strain isolated from Red Pepper leaf. Mol Cells. 2015;38:273–278. doi: 10.14348/molcells.2015.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai H, Yamashita M, Takase S, Hashimoto M, Muramatsu H, Nakamura I, et al. KB425796-A, a novel antifungal antibiotic produced by Paenibacillus sp. 530603. J Antibiot. 2013;66:465–471. doi: 10.1038/ja.2013.63. [DOI] [PubMed] [Google Scholar]
- Karthikeyan M, Bhaskaran R, Radhika K, Mathiyazhagan S, Jayakumar V, Sandosskumar R, et al. Endophytic Pseudomonas fluorescens Endo2 and Endo35 induce resistance in black gram (Vigna mungo L. Hepper) to the pathogen Macrophomina phaseolina. J Plant Interact. 2005;1:135–143. doi: 10.1080/17429140600997309. [DOI] [Google Scholar]
- Khanam B, Chandra R. Isolation and identification of endophytic bacteria producing bright red pigment from the dye yielding plant Beta vulgaris L. Int J Pharm Pharm Sci. 2015;7:220–224. [Google Scholar]
- Kirk O, Borchert TV, Fuglsang CC. Industrial enzyme applications. Curr Opin Biotechnol. 2002;13:345–351. doi: 10.1016/S0958-1669(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Kitov PI, Mulvey GL, Griener TP, Lipinski T, Solomon D, Paszkiewicz E, et al. In vivo supra molecular templating enhances the activity of multivalent ligands: a potential therapeutic against the Escherichia coli O157 AB5 toxins. Proc Natl Acad Sci USA. 2008;105:16837–16842. doi: 10.1073/pnas.0804919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Tian M, Choi HW. Multiple targets of salicylic acid and its derivatives in plants and animals. Front Immunol. 2016;7:206. doi: 10.3389/fimmu.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi DY, Palumbo JD. Bacterial endophytes and their effects on plants and uses in agriculture. In: Bacon CW, White JF, editors. Microbial endophytes. NY: Marcel Dekker; 2000. pp. 199–233. [Google Scholar]
- Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25. [PubMed] [Google Scholar]
- Kumar A, Singh R, Giri DD, Singh PK, Pandey KD. Effect of Azotobacter chroococcum CL13 inoculation on growth and curcumin content of turmeric (Curcuma longa L.) Int J Curr Microbiol Appl Sci. 2014;3:275–283. [Google Scholar]
- Kumar V, Kumar A, Pandey KD, Roy BK. Isolation and characterization of bacterial endophytes from the roots of Cassia tora L. Ann Microbiol. 2015;65:1391–1399. doi: 10.1007/s13213-014-0977-x. [DOI] [Google Scholar]
- Kumar A, Singh R, Yadav A, Giri DD, Singh PK, Pandey KD. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016;6:60. doi: 10.1007/s13205-016-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusari S, Verma VC, Lamshoeft M, Spiteller M. An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J Microbiol Biotechnol. 2012;28:1287–1294. doi: 10.1007/s11274-011-0876-2. [DOI] [PubMed] [Google Scholar]
- Kyselova Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip Toxicol. 2011;4:173–183. doi: 10.2478/v10102-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Thi Ngoc Ha Phenolic compounds and human health benefits. Vietnam J Agri Sci. 2016;14:1107–1118. [Google Scholar]
- Lam KS. Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol. 2006;9:245–251. doi: 10.1016/j.mib.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Lambers H, Chapin FS, Pons TL. Plant physiological ecology. New York: Springer; 2008. [Google Scholar]
- Lesburg CA, Zhai G, Cane DE, Christianson DW. Crystal structure of pentalenene synthase: mechanistic insights on terpenoid cyclization reactions in biology. Science. 1997;277:1820–1824. doi: 10.1126/science.277.5333.1820. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao GZ, Chen HH, Wang HB, Qin S, et al. Anti-tumour and antimicrobial activities of endophytic streptomycetes from pharmaceutical plants in rainforest. Lett Appl Microbiol. 2008;47:574–580. doi: 10.1111/j.1472-765X.2008.02470.x. [DOI] [PubMed] [Google Scholar]
- Li J, Lu C, Shen Y. Macrolides of the bafilomycin family produced by Streptomyces sp. C S J Antibiot. 2010;63:595–599. doi: 10.1038/ja.2010.95. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao GZ, Varma A, Qin S, Xiong Z, Huang HY, et al. An endophytic Pseudonocardia species induces the production of artemisinin in Artemisia annua. PLoS One. 2012;7:e51410. doi: 10.1371/journal.pone.0051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liarzi O, Bucki P, Braun Miyara S, Ezra D. Bioactive volatiles from an endophytic Daldinia cf. concentrica isolate affect the viability of the plant parasitic nematode Meloidogyne javanica. PLoS One. 2016;11:e0168437. doi: 10.1371/journal.pone.0168437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limona RI, Penas E, Torino MI, Villaluengaa CM, Duenas M, Frias J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015;172:343–352. doi: 10.1016/j.foodchem.2014.09.084. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu W, Liang Z. Endophytic bacteria from Pinellia ternata, a new source of purine alkaloids and bacterial manure. Pharm Biol. 2015;5:1545–1548. doi: 10.3109/13880209.2015.1016580. [DOI] [PubMed] [Google Scholar]
- Liu G, Lai D, Liu ZQ, Zhou L, Liu LZ. Identification of nematicidal constituents of Notopterygium incisum rhizomes against Bursaphelenchus xylophilus and Meloidogyne incognita. Molecules. 2016;21:1276. doi: 10.3390/molecules21101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MA, Bannenberg G, Castresana C. Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol. 2008;11:420–427. doi: 10.1016/j.pbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lowicki D, Nski AH. Structure and antimicrobial properties of Monensin A and its derivatives: summary of the achievements. Bio Med Res Int. 2013 doi: 10.1155/2013/742149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Sun RW, Chen R, Hui HK, Ho CM, Luk JM, Lau GK, Che CM. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13:253–262. [PubMed] [Google Scholar]
- Ma Y, Zhang C, Oliveira RS, Freitas H, Luo Y. Bioaugmentation with endophytic bacterium E6S homologous to Achromobacter piechaudii enhances metal rhizoaccumulation in host Sedum plumbizincicola. Front Plant Sci. 2016;4:75. doi: 10.3389/fpls.2016.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AY, Abdallah HM, El-Halawani MA, Jiman-Fatani AAM. Anti-tuberculous activity of Treponemycin produced by a Streptomyces strain MS-6-6 isolated from Saudi Arabia. Molecules. 2015;20:2576–2590. doi: 10.3390/molecules20022576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Maul C, Zerlin M, Grabley S, Thiericke R. Biomolecular chemical screening: a novel screening approach for the discovery of biologically active secondary metabolites. II. Application studies with pure metabolite. J Antibiot (Tokyo) 1999;52:952–959. doi: 10.7164/antibiotics.52.952. [DOI] [PubMed] [Google Scholar]
- Mark N, Greenwald RA, Hillen W, Nelson ML. Tetracyclin in biology, chemistry and medicine. Basel: Birkhauser; 2001. p. 8. [Google Scholar]
- Mercado BJ, Bakker PA. Interaction between plants and beneficial Pseudomonas sp., exploiting bacterial traits for crop protection. Antonie Leeuwenhok. 2007;92:367–389. doi: 10.1007/s10482-007-9167-1. [DOI] [PubMed] [Google Scholar]
- Merino ST, Cherry J. Progress and challenges in enzyme development for biomass utilization. Adv Biochem Eng Biotechnol. 2007;108:95–120. doi: 10.1007/10_2007_066. [DOI] [PubMed] [Google Scholar]
- Miao V. Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry. Microbiology. 2005;151:1507–1523. doi: 10.1099/mic.0.27757-0. [DOI] [PubMed] [Google Scholar]
- Miller RV, Miller CM, Kinney DG, Redgrave B, Sears J, Condron M, et al. Ecomycins, unique antimycotics from Pseudomonas viridiflava. J Appl Microbiol. 1998;84:937–944. doi: 10.1046/j.1365-2672.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S. Current topics in the biotechnological production of essential amino acids, functional amino acids, and dipeptides. Curr Opin Biotechnol. 2014;26:38–44. doi: 10.1016/j.copbio.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Molina G, Pimentel MR, Bertucci TCP, Pastore GM. Application of fungal endophytes in biotechnological processes. Chem Eng Trans. 2012;27:289–294. [Google Scholar]
- Nicolaou KC, Chen JS, Corey EJ. Classics in total synthesis, further targets, strategies, methods III. Weinheim: Wiley; 2011. pp. 1–770. [Google Scholar]
- Nongkhlaw FMW, Joshi SR. Investigation on the bioactivity of culturable endophytic and epiphytic bacteria associated with ethnomedicinal plants. J Infect Dev Ctries. 2015;9:954–961. doi: 10.3855/jidc.4967. [DOI] [PubMed] [Google Scholar]
- Pandey SS, Singh S, Babu CSV, Shanker K, Shrivastava NK, Kalra A. Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta. 2016;243:1097–1114. doi: 10.1007/s00425-016-2467-9. [DOI] [PubMed] [Google Scholar]
- Perucka I, Materska M. Antioxidant activity and content of capsaicinoids isolated from paprika fruits. Polish J Food Nutr Sci. 2003;12:15–18. [Google Scholar]
- Pesic A, Steinhaus B, Kemper S, Nachtigall J, Kutzner HJ, Höfle G, Sussmuth RD. Isolation and structure elucidation of the nucleoside antibiotic strepturidin from Streptomyces albus DSM 40763. J Antibiot. 2014;67:471–477. doi: 10.1038/ja.2014.16. [DOI] [PubMed] [Google Scholar]
- Pimentel-Elardo SM, Kozytska S, Bugni TS, Ireland CM, Moll H, Hentschel U. Anti-parastic compounds from Streptomyces sp. strains isolated from Mediterranean Sponges. Mar Drugs. 2010;23:373–380. doi: 10.3390/md8020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchuka IV, Bressollier P, Sorokulova IB, Verneuil B, Urdaci MC. Amicoumacin antibiotic production and genetic diversity of Bacillus subtilis strains isolated from different habitats. Res Microbiol. 2002;153:269–276. doi: 10.1016/S0923-2508(02)01320-7. [DOI] [PubMed] [Google Scholar]
- Pissuwan D, Valenzuela SM, Cortie MB. Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol. 2006;24:62–67. doi: 10.1016/j.tibtech.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Prashith-Kekuda TR. Isolation, characterization and antimicrobial potential of endophytic actinomycetes. Int J Curr Microbiol Appl Sci. 2016;5:100–116. doi: 10.20546/ijcmas.2016.507.008. [DOI] [Google Scholar]
- Puri SC, Verma V, Amna T, Qazi GN, Spiteller M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J Nat Prod. 2005;68:1717–1719. doi: 10.1021/np0502802. [DOI] [PubMed] [Google Scholar]
- Qin S, Xing K, Hong JJ, Xu LL, Li WJ. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol. 2011;89:457–473. doi: 10.1007/s00253-010-2923-6. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JM, Mazzola M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu Rev Phytopathol. 2012;50:403–424. doi: 10.1146/annurev-phyto-081211-172908. [DOI] [PubMed] [Google Scholar]
- Raju R, Gromyko O, Fedorenko V, Luzketsky A, Muller R, Albaflavenol B. Albaflavenol B, a new sesquiterpene isolated from the terrestrial actinomycete, Streptomyces sp. J Antibiot. 2015;68:286–288. doi: 10.1038/ja.2014.138. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B, Hurek T. Living inside plants: bacterial endophytes. Curr Opin Plant Biol. 2011;14:435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Rosenblueth M, Romero EM. Bacterial endophytes and their interactions with hosts. APS. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- Roy S, Banerjee D. Bioactive endophytic actinomycetes of Cinnamomum sp. isolation, identification, activity guided purification and process optimization of active metabolite. Am J Microbiol. 2015;6:4–13. [Google Scholar]
- Salam N, Khieu T, Liu M, Vu T, Chu-Ky S, Quach N, Phi Q, Rao MPN, Fontana A et al (2017) Endophytic actinobacteria associated with Dracaena cochinchinensis Lour.: Isolation, diversity, and their cytotoxic activities. BioMed Res Int. doi:10.1155/2017/1308563(Article ID 1308563) [DOI] [PMC free article] [PubMed]
- Sanatombi K, Sharma GJ. Capsaicin content and pungency of different capsicum sp. cultivars. Not Bot Hortic Agrobot Cluj. 2008;36:89–90. [Google Scholar]
- Sansinenea E, Ortiz A. Secondary metabolites of soil Bacillus spp. Biotechnol Lett. 2011;33:1523–1538. doi: 10.1007/s10529-011-0617-5. [DOI] [PubMed] [Google Scholar]
- Sathiyaseelan K, Stella D. Isolation, identification and antimicrobial activity of marine actinomycetes isolated from Parangipettai. Recent Res Sci Technol. 2011;3:74–77. [Google Scholar]
- Sato F, Kumagai H. Microbial production of isoquinoline alkaloids as plant secondary metabolites based on metabolic engineering research. Proc Jpn Acad Ser B. 2013;89:165–181. doi: 10.2183/pjab.89.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaligram NS, Singhal RS. Surfactin—a review on biosynthesis, fermentation, purification and applications. Food Technol Biotechnol. 2010;48:119–134. [Google Scholar]
- Sharma D, Pramanik A, Agrawal PK. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D.Don. 3 Biotech. 2016;6:210. doi: 10.1007/s13205-016-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GH, Teles LH, Zanardi LM, Young MC, Eberlin MN, Hadad R, et al. Cadinane sesquiterpenoids of Phomopsis cassiae an endophytic fungus associated with Cassia spectabilis (Leguminosae) Phytochemistry. 2007;67:1964–1969. doi: 10.1016/j.phytochem.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Singh S, Bindu H, Raghu J, Suma HK, Manjunatha BL, Kumara PM, et al. Isolation of endophytic bacteria producing the anti-cancer alkaloid camptothecine from Miqueliadentata Bedd. (Icacinaceae) Phytomedicine. 2013;20:913–917. doi: 10.1016/j.phymed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Strobel G. Harnessing endophytes for industrial microbiology. Curr Opin Microbiol. 2006;9:240–244. doi: 10.1016/j.mib.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Strobel G, Ford WJ, Harper JK, Arif AM, Grant DM, Peter F, Chau RM. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora possessing antifungal and antioxidant activities. Phytochemistry. 2002;60:179–183. doi: 10.1016/S0031-9422(02)00062-6. [DOI] [PubMed] [Google Scholar]
- Subbulakshmi GK, Thalavaipandian A, Bagyalakshmi RV, Rajendran A. Bioactive endophytic fungal isolates of Biota orientalis (L) Endl., Pinus excelsaWall. and Thuja occidentalis L. Int J Adv Life Sci. 2012;4:9–15. [Google Scholar]
- Sun RW, Chen R, Chung NP, Ho CM, Lin CL, Che CM. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem Commun (Camb) 2005;40:5059–5061. doi: 10.1039/b510984a. [DOI] [PubMed] [Google Scholar]
- Sunkar S, Nachiyar CV. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed. 2012;2:953–959. doi: 10.1016/S2221-1691(13)60006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan TS, Murali TS. Incidence of Leptosphaerulina crassiasca in symptomless leaves of peanut in southern India. J Basic Microbiol. 2006;46:1003–1006. doi: 10.1002/jobm.200510126. [DOI] [PubMed] [Google Scholar]
- Sziderics AH, Rasche F, Trognitz F, Sessitch A, Wilhelem E. Bacterial endophyte contribute to abiotic stress adaptation in pepper plants (Capsicum annum L.) Can J Microbiol. 2007;53:1195–1202. doi: 10.1139/W07-082. [DOI] [PubMed] [Google Scholar]
- Taechowisan T, Chanaphat S, Ruensamran W, Phutdhawong WS. Anti-inflammatory effect of 3-methylcarbazoles on RAW 2647 cells stimulated with LPS, polyinosinic-polycytidylic acid and Pam3CSK. Adv Microbiol. 2012;2:98–103. doi: 10.4236/aim.2012.22013. [DOI] [Google Scholar]
- Takahashi S, Takeuchi M, Arai M, Seto H, Otake N. Studies on biosynthesis of pentalenolactone. V isolation of deoxypentalenylglucuron. J Antibiot (Tokyo) 1983;36:226–228. doi: 10.7164/antibiotics.36.226. [DOI] [PubMed] [Google Scholar]
- Tamehiro N, Okamot-Hosova Y, Okamoto S, Ubukata M, Hamada M, Naganawa H, et al. Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicrob Agents Chemother. 2002;46:315–320. doi: 10.1128/AAC.46.2.315-320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep. 2001;18:448–459. doi: 10.1039/b100918o. [DOI] [PubMed] [Google Scholar]
- Taylor PL, Omotoso O, Wiskel JB, Mitlin D, Burrell RE. Impact of heat on nanocrystalline silver dressings. Part II: physical properties. Biomaterials. 2005;26:7230–7240. doi: 10.1016/j.biomaterials.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Thenmozhi M, Krishnan K. Anti-Aspergillus activity of Streptmyces sp. VITSTK7 isolated from bay of Bengal coast of Puducherry, India. J Nat Environ Sci. 2011;2:1–8. [Google Scholar]
- Tiwari R, Awasthi A, Mall M, Shukla AK, Srinivas KS, Syamasundar KV, Kalra A. Bacterial endophyte mediated enhancement of in planta content of key terpenoid indole alkaloids and growth parameters of Catharanthus roseus. Ind Crops Prod. 2013;43:306–310. doi: 10.1016/j.indcrop.2012.07.045. [DOI] [Google Scholar]
- Trotsenko YA, Khmelenina VN. Biology of extremophilic and extremotolerant methanotrophs. Arch Microbiol. 2002;177:123–131. doi: 10.1007/s00203-001-0368-0. [DOI] [PubMed] [Google Scholar]
- Valdes L, Cuervo A, Salazar N, Ruas-Madiedo P, Gueimonde M, Gonzalez S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food Funct. 2015;6:2424–2439. doi: 10.1039/C5FO00322A. [DOI] [PubMed] [Google Scholar]
- Verginer M, Siegmund B, Cardinale M, Muller H, Choi Y, Maıguez CB, et al. Monitoring the plant epiphyte Methylobacterium extorquens DSM 21961 by real-time PCR and its influence on the strawberry flavor. FEMS Microbiol Ecol. 2010;74:136–145. doi: 10.1111/j.1574-6941.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- Verpoorte R. Exploration of nature’s chemo diversity: the role of secondary metabolites as leads in drug development. Drug Discov Today. 1998;3:232–238. doi: 10.1016/S1359-6446(97)01167-7. [DOI] [Google Scholar]
- Villaescusa BP, Rangel-Huerta OD, Aguilera CM, Gil A. A systematic review of the efficacy of bioactive compounds in cardiovascular disease: carbohydrates, active lipids and nitrogen compounds. Ann Nutr Metab. 2015;66:168–181. doi: 10.1159/000430960. [DOI] [PubMed] [Google Scholar]
- Vinale F, Nicoletti R, Lacatena F, Marra R, Sacco A, Lombardi N, D’Errico G, Digilio MC, Lorito M, Woo SL. Secondary metabolites from the endophytic fungus Talaromyces pinophilus. Nat Prod Res. 2017;31:1778–1785. doi: 10.1080/14786419.2017.1290624. [DOI] [PubMed] [Google Scholar]
- Waldron C, Matsushima P, Rosteck PR, Broughton MC, Turner J, Madduri K. Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa. Chem Biol. 2001;8:487–499. doi: 10.1016/S1074-5521(01)00029-1. [DOI] [PubMed] [Google Scholar]
- Wei L, Xueqiong Y, Yabin Y, Lixing Z, Lihua X, Zhongtao D. A new anthracycline from endophytic Streptomyces sp. YIM66403. J Antibiot. 2015;68:216–219. doi: 10.1038/ja.2014.128. [DOI] [PubMed] [Google Scholar]
- Wendisch VF. Microbial production of amino acids and derived chemicals: synthetic biology approaches to strain development. Curr Opin Biotechnol. 2014;30:51–58. doi: 10.1016/j.copbio.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kuzuyama T, Komatsu M, Shinya K, Omura S, Cane DE, et al. Terpene synthases are widely distributed in bacteria. Proc Natl Acad Sci USA. 2015;112:857–862. doi: 10.1073/pnas.1422108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Someno T, Igarashi M, Kinoshita N, Hatano M, Kawada M, et al. Androprostamines A and B, the new anti-prostate cancer agents produced by Streptomyces sp. MK932-CF8. J Antibiot. 2015;68:279–285. doi: 10.1038/ja.2014.135. [DOI] [PubMed] [Google Scholar]
- Yoon YJ, Kim ES, Hwang YS, Choi CY. Avermectin: biochemical and molecular basis of its biosynthesis and regulation. Appl Microbiol Biotechnol. 2004;63:626–634. doi: 10.1007/s00253-003-1491-4. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhang L, Li L, Zheng C, Guo L, Li W, et al. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res. 2010;165:437–449. doi: 10.1016/j.micres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Yuan N, Song L, Zhang S, Lin W, Cao Y, Xu F, Fang Y, Wang Z, Zhang H, Li X. Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica. 2015;100:345–356. doi: 10.3324/haematol.2014.113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Penttinen P, Guan T, Xiao J, Chen Q, Xu J, et al. The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi Plateau, China. Curr Microbiol. 2011;62:182–190. doi: 10.1007/s00284-010-9685-3. [DOI] [PubMed] [Google Scholar]
- Zhejian W, Zhao M, Lili W, Chengchen T, Zhibi H, GuiXin C, Wankui L. Active anti-acetylcholinesterase component of secondary metabolites produced by the endophytic fungi of Huperzia serrata. Electron J Biotechnol. 2015;18:399–405. doi: 10.1016/j.ejbt.2015.08.005. [DOI] [Google Scholar]
- Zhou JY, Yuan J, Li X, Ning YF, Dai CC. Endophytic bacterium triggered reactive oxygen species directly increase oxygenous sesquiterpenoid content and diversity in Atractylodes lancea. Appl Environ Microbiol. 2015;82:1577–1585. doi: 10.1128/AEM.03434-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinniel DK, Lambrecht P, Harris NB, Feng Z, Kuczmarski D, Higley P. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol. 2002;68:2198–2208. doi: 10.1128/AEM.68.5.2198-2208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zothanpuia Passari AK, Chandra P, Leo VV, Mishra VK, Kumar B, Singh BP. Production of potent antimicrobial compounds from Streptomyces cyaneofuscatus associated with fresh water sediment. Front Microbiol. 2017;8:68. doi: 10.3389/fmicb.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]