Abstract
Background
The factors that contribute to the abnormal knee kinematics after anterior cruciate ligament (ACL) injury and ACL reconstruction remain unclear. Bone shape has been implicated in the development of hip and knee osteoarthritis, although there is little knowledge about the effects of bone shape on knee kinematics after ACL injury and after ACL reconstruction.
Questions/questions
(1) What is the relationship between bony morphology with alterations in knee kinematics after ACL injury? (2) Are baseline bone shape features related to abnormal knee kinematics at 12 months after ACL reconstruction?
Methods
Thirty-eight patients (29 ± 8 years, 21 men) were prospectively followed after acute ACL injury and before ligamentous reconstruction. Patients were excluded if there was a history of prior knee ligamentous injury, a history of inflammatory arthritis, associated meniscal tears that would require repair, or any prior knee surgery on either the injured or contralateral side. In total, 54 patients were recruited with 42 (78%) patients completing 1-year followup and four patients excluded as a result of incomplete or unusable imaging data. MR images were obtained for the bilateral knees at two time points 1 year apart for both the injured (after injury but before reconstruction and 1 year after reconstruction) and contralateral uninjured knees. Kinematic MRI was performed with the knee loaded with 25% of total body weight, and static images were obtained in full extension and in 30° of flexion. The side-to-side difference (SSD) between tibial position in the extended and flexed positions was determined for each patient. Twenty shape features, referred to as modes, for the tibia and femur each were extracted independently from presurgery scans with the principal component analysis-based statistical shape modeling algorithm. Spearman rank correlations were used to evaluate the relationship between the SSD in tibial position and bone shape features with significance defined as p < 0.05. Each of the shape features (referred to as the bone and mode number such as Femur 18 for the 18th unique femoral bone shape) associated with differences in tibial position was then investigated by modeling the mean shape ± 3 SDs.
Results
Two of the 20 specific femur bone shape features (Femur 10, Femur 18) and two of the 20 specific tibial bone shape features (Tibia 19, Tibia 20) were associated with an increasingly anterior SSD in the tibial position for the patients with ACL injury before surgical treatment. The shape features described by these modes include the superoinferior height of the medial femoral condyle (Femur 18; ρ = 0.33, p = 0.040); the length of the anterior aspect of the lateral tibial plateau (Tibia 20; ρ = −0.35, p = 0.034); the sphericity of the medial femoral condyle (Femur 10; ρ = −0.52, p < 0.001); and tibial slope (Tibia 19; ρ = 0.34; p = 0.036). One year after surgical treatment, there were two of 20 femoral shape features that were associated with SSD in the tibial position in extension (Femur 10, Femur 18), one of 20 femoral shape features associated with SSD in the tibial position in flexion (Femur 10), and three of 20 tibial shape features associated with SSD in the tibial position in flexion (Tibia 2, Tibia 4, Tibia 19). The shape features described by these modes include the sphericity of the medial femoral condyle (Femur 10; ρ = −0.38, p = 0.020); the superoinferior height of the medial femoral condyle (Femur 18; ρ = 0.34, p = 0.035); the height of the medial tibial plateau (Tibia 2; ρ = −0.32, p = 0.048); the AP length of the lateral tibial plateau (Tibia 4; ρ = −0.37, p = 0.021); and tibial slope (Tibia 19; ρ = 0.34, p = 0.038).
Conclusions
We have observed multiple bone shape features in the tibia and the femur that may be associated with abnormal knee kinematics after ACL injury and ACL reconstruction. Future directions of research will include the influence of bony morphology on clinical symptoms of instability in patients with and without ACL reconstruction and the long-term evaluation of these shape factors to better determine specific contributions to posttraumatic arthritis and graft failure.
Level of Evidence
Level II, therapeutic study.
Keywords: Anterior Cruciate Ligament, Anterior Cruciate Ligament Reconstruction, Tibial Plateau, Anterior Cruciate Ligament Injury, Tibial Slope
Introduction
Anterior cruciate ligament (ACL) injuries are commonly seen in athletic populations with an estimated incidence exceeding 200,000 injuries in the United States each year and over 120,000 ACL reconstructions in 2006 [7, 19]. These injuries result in decreased ability to participate in cutting and pivoting activities and also lead to an increased rate in the development of osteoarthritis [17]. Regardless of whether patients are treated with surgical reconstruction, posttraumatic osteoarthritis is observed in approximately 35% of patients at 10 years after ACL injury [9]. The factors contributing to the development of arthritis are multifactorial and include the alteration in knee kinematics [3]. Prior in vivo functional studies have demonstrated that the tibia in an ACL-deficient knee is positioned more anteriorly relative to a knee with an intact ACL, leading to a shift in tibiofemoral cartilage loading that has been hypothesized to contribute to the development of posttraumatic arthritis [2, 3]. In vivo research has also shown that postsurgical knee kinematics are altered, although the specific reasons for these changes even after ligamentous reconstruction remain unclear [11, 14, 24]. Additionally, correlations have been observed between postsurgical abnormalities in knee kinematics and early degenerative changes in the biochemical composition of the articular cartilage of the knee [1, 27].
Research into the pathogenesis of idiopathic osteoarthritis of the hip and knee has recently implicated bone shape as a potential risk factor [4, 13, 18, 20]. Statistical shape modeling (SSM) is a powerful method to impartially understand specific unique bony shape features [13]. The essence of SSM lays in the description of each shape with a set of anatomically matched landmarks and to extraction of modes of variation from the average shape through the analysis of the principal components. Although the two-dimensional application on plain radiographs is well established and the identification of anatomically matched coordinates can be performed manually by medical experts, the implementation of the same technique in three dimensions (3-D) imposes a fully automatic method to solve the matching task [18, 20]. Recently, we described a new method for the extraction of 3-D MRI-based SSM based on local curvature and regularized by the use of spectral coordinates and applied this method in knees with ACL injuries [22]. That study showed that specific aspects of the bone shape such as intercondylar notch width and medial tibia slope could potentially predispose certain subjects to ACL injuries. Additionally, bone shape features were observed as predictors of changes in cartilage matrix composition, assessed through T1 and T2 relaxation times at 12 months after surgical reconstruction [23]. However, to our knowledge, no studies have specifically investigated the relationship between bone shape and knee kinematics after ACL injury and reconstruction.
Accordingly, we sought to answer two questions with this study: (1) What is the relationship between bony morphology with alterations in knee kinematics after ACL injury? (2) Are baseline bone shape features related to abnormal knee kinematics at 12 months after ACL reconstruction?
Patients and Methods
A prospective cohort study was performed to follow 38 patients (29 ± 8 years, 21 men) after acute ACL injury and before ligamentous reconstruction. All patients were enrolled in the study within 6 months of sustaining an acute, isolated ACL injury. A total of 54 subjects were recruited between July 2011 and February 2014 with 42 patients (78%) completing 1-year followup. Four patients were excluded as a result of motion artifact or incomplete imaging acquisition. Patients were excluded if there was a history of prior knee ligamentous injury, a history of inflammatory arthritis, associated meniscal tears that would require repair, or any prior knee surgery on either the injured or contralateral side. For each patient, the contralateral, uninjured knee served as an internal control for comparison. All procedures were approved by the Committee on Human Research, and all patients provided informed consent to participate.
MRI was obtained for the bilateral knees at two time points: a baseline scan was completed before surgical reconstruction and a followup scan was obtained 1 year after surgical reconstruction. Kinematic scans were completed at each time point in accordance with previously described procedures by reconstructing static images obtained by scanning the knee in two positions [8, 15, 27]. Both knees for all patients were scanned. First, the uninjured knee was scanned in full extension while loaded with 25% of total body weight through a pulley system to simulate standing. Next, the uninjured knee was scanned in approximately 30° of flexion while loaded with 25% of total body weight. The injured knee was then scanned with the same loading protocol in both extension and flexion.
All images were acquired using a 3-T MRI scanner (GE Healthcare, Milwaukee, WI, USA) with an eight-channel knee coil (Invivo Inc, Gainesville, FL, USA). Sagittal T2 fast spin-echo (FSE) images were used for both extraction of the bone shape feature and knee kinematics (Fig. 1). MRI sequence parameters included: TR/TE = 4000/49.3-ms slice thickness of 1.5 mm, spacing of 1.5 mm, field of view 16 cm, 512 × 512 matrix size, and echo-train length = 9.
Fig. 1.
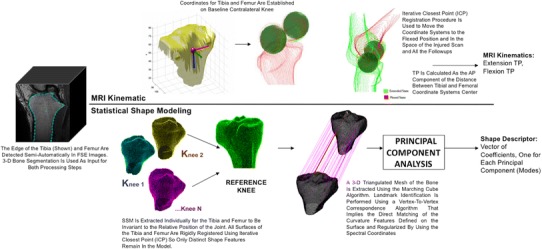
A schematic for data processing is displayed, including semiautomatic bone segmentation on FSE images, kinematic measurements, and SSM.
All patients with an ACL injury underwent surgical reconstruction by one of four fellowship-trained sports medicine orthopaedic surgeons (CBM, BF, CA, NC). All ACL reconstructions were performed with a soft tissue graft (hamstring autograft [27 patients; 71%] or allograft hamstring or posterior tibialis tendon [11 patients; 29%] with suspensory fixation on the femoral side and interference screw fixation on the tibial side. All reconstructions were performed with independent drilling of the femoral and tibial tunnels. After reconstruction, all patients were prescribed a standardized postoperative physical therapy program with immediate weightbearing with crutches and a hinged knee brace for 6 weeks, return to running at 4 months, and return to sports at 6 to 8 months after surgery.
Image processing was performed with a custom software program developed in-house using Matlab language (Matlab, Natick, MA, USA) software (Fig. 1). The femur and tibia were semiautomatically segmented for all knees in both extension and flexion. The segmentation algorithm was edge-based coupled with spatial constraints and Bezier spline interpolation. Kinematic measurements were determined according to our previously described and validated method [8, 14–16, 27]. Briefly, the femoral condyles in the extended contralateral knees at baseline time points were modeled as spheres, and a coordinate system was determined based on the axis between the center of the medial and lateral condyles and the long axis of the femur. A tibial-based axis was determined based on the posterior edge of the medial and lateral tibial plateaus and the long axis of the tibia. The position of the tibia relative to the femur was then determined based on the position of the origin of these axes. Iterative closest point registration was then used to move the established coordinate system on the space of the flexed contralateral knee, injured extended and flexed, and all the followup scans. The intraclass correlation coefficients for this measurement are 0.95 for repeated scans and 0.98 for multiple segmentations [16].
The AP position of the tibia relative to the femur was chosen as the primary measurement. To control for differences within patients, the alignment was compared with the contralateral side to determine the side-to-side difference (SSD) in tibial position. A positive value for this measurement represents a more anterior position, whereas a negative value represents a more posterior position. The AP difference in tibial position was selected for this study given prior work highlighting associations of this parameter with subjective instability [5, 6] and degenerative cartilage changes [27] and our reproducibility of this measurement on sagittal images [16].
SSM was performed using the semiautomatic segmentations of the femur and the tibia. A 3-D cloud of points was obtained and transformed in a triangulated mesh for each segmentation; a reference was chosen using an iterative process aimed to minimize the global registration error. Two separate models were extracted for the femur and tibia bones. All the meshes were rigidly aligned to make the model invariant to the knee positioning during the scan. All the vertices of the reference were then mapped on the reference using a fully automatic landmark-matching algorithm previously described and evaluated [22, 23]. A total of 8120 and 11,222 landmarks was identified for the tibia and the femur, respectively. Principal component analysis was performed to calculate the most important modes of variation of all the surfaces from the mean surface. Each mode contains a unique and complex shape that varies across the data set. Twenty modes that describe more than 90% of the total variation in this data set were considered for the description of femur and tibia bone shapes, equaling 40 total shape modes. Each shape feature will be referred to as the bone and mode number (eg, Femur 18 represents the 18th unique shape feature for the femur from our data set) and represents one independent shape for that specific bone.
Statistical Analysis
The relationship between the SSD in extension and flexion and femur and tibia shape modes was determined using Spearman rank correlations. Correlations were determined for the patients with ACL injuries at the baseline time point and 1 year after surgical reconstruction. Statistical significance was defined as p < 0.05.
Physical Interpretation of Shape Modes
After the significant shape features for both the femur and tibia were determined through the Spearman rank correlations, the physical interpretation of these significant shape modes was determined. An average femur and tibia were modeled independently based on the average value of all 20 modes specific to that bone. Next, the specific significant shape features were adjusted to ± 3 SDs and physical models were visualized for each of these models [10]. This model was inspected from all vantage points to understand the physical meaning of the shape mode variation.
Results
Two of the 20 specific femur bone shape features (Femur 10, Femur 18) and two of the 20 specific tibial bone shape features (Tibia 19, Tibia 20) were associated with the SSD in tibial position for the patients with ACL injury before surgical treatment (Table 1). Femur 18 (Fig. 2A) describes the height of the medial femoral condyle. An increasing height of the medial femoral condyle was associated with a more anteriorly positioned tibia relative to the contralateral knee (ρ = 0.33, p = 0.040) in the extended position. Femur 10 (Fig. 2B) describes the sphericity of the medial femoral condyle. A more spherically shaped medial femoral condyle was associated with a more anteriorly positioned tibia relative to the contralateral knee (ρ = −0.52, p < 0.001) in the flexed position (Fig. 3). Tibia 20 (Fig. 2C) described the length of the anterior aspect of the lateral tibial plateau. A shorter anterolateral plateau was associated with a more anteriorly positioned tibia in the extended position (ρ = −0.35, p = 0.034). Tibia 19 (Fig. 2D) described the height of the anteromedial tibial plateau. An increased height of the anteromedial tibial plateau was associated with a more anteriorly positioned tibia relative to the contralateral knee (ρ = 0.34, p = 0.036) in the flexed position.
Table 1.
Correlations of specific bone shape features with an increasingly anteriorly positioned tibia relative to the contralateral knee after injury and after ACL reconstruction
| Mode* | Spearman’s ρ | p value | Shape feature | Sign definition |
|---|---|---|---|---|
| After ACL injury: side-to-side difference of tibial position with knee in extension | ||||
| Femur 18 | 0.33 | 0.040 | SI height of MFC | + = increased height of MFC |
| Tibia 20 | −0.35 | 0.034 | Length of anterior aspect of lateral tibial plateau | − = shorter anterolateral plateau |
| After ACL injury: side-to-side difference of tibial position with knee in flexion | ||||
| Femur 10 | −0.52 | 0.0007 | Sphericity of MFC | − = more spherical MFC |
| Tibia 19 | 0.34 | 0.036 | Tibial slope—height of anteromedial tibial plateau | + = increased height of anteromedial tibial plateau |
| One year after ACL reconstruction: side-to-side difference of tibial position with knee in extension | ||||
| Femur 10 | −0.38 | 0.020 | Sphericity of MFC | − = more spherical MFC |
| Femur 18 | 0.34 | 0.035 | SI height of MFC | + = increased height of MFC |
| One year after ACL reconstruction: side-to-side difference of tibial position with knee in flexion | ||||
| Femur 10 | −0.46 | 0.004 | Sphericity of MFC | − = more spherical MFC |
| Tibia 2 | −0.32 | 0.048 | SI height of medial tibial plateau | − = shorter medial plateau |
| Tibia 4 | −0.37 | 0.021 | AP length of lateral plateau | − = longer lateral plateau |
| Tibia 19 | 0.34 | 0.038 | Tibial slope—height of anteromedial tibial plateau | + = increased height of anteromedial tibial plateau |
*The “Mode” refers to one of the specific unique bone shape feature identified through statistical shape modeling and is written as the bone plus the mode number (eg, Femur 18 refers to the 18th unique shape feature of the femur); ACL = anterior cruciate ligament; MFC = medial femoral condyle; ML = mediolateral; SI = superoinferior.
Fig. 2A–D.
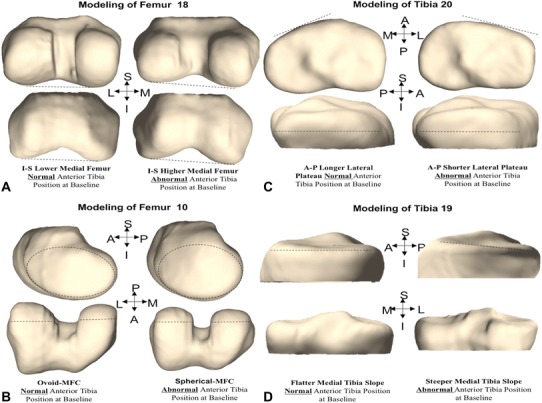
A representation is shown for each of the four unique shape features defined through principal component analysis that were correlated with abnormal knee kinematics after ACL injury but before surgical reconstruction, including: (A) the height of the medial femoral condyle (MFC), represented by Femur 18; (B) the sphericity of the medial femoral condyle, represented by Femur 10; (C) the length of the lateral tibial plateau, represented by Tibia 20; (D) tibial slope, represented by Tibia 19. S = superior; I = inferior; L = lateral; M = medial; A = anterior; P = posterior.
Fig. 3.
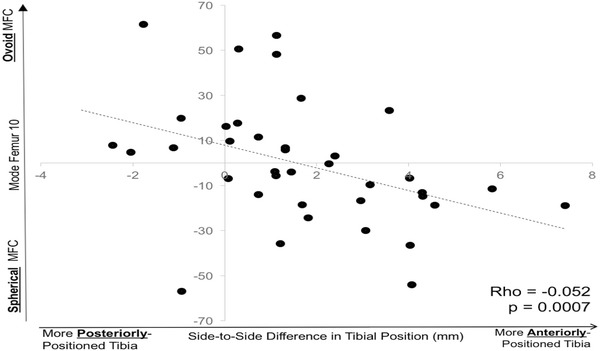
There was a significant relationship between the SSD in the tibial position in flexion after ACL injury and the sphericity of the medial femoral condyle, represented by Femur 10. A more spherical medial femoral condyle (more negative value on the y-axis) was correlated with a more anteriorly positioned tibia (more positive value on the x-axis).
One year after surgical treatment, there were two of 20 femoral shape features that were associated with SSD in the tibial position in extension (Femur 10, Femur 18), one of 20 femoral shape features associated with SSD in the tibial position in flexion (Femur 10), and three of 20 tibial shape features associated with SSD in the tibial position in flexion (Tibia 2, Tibia 4, Tibia 19). In both extension (ρ = −0.38, p = 0.020) and flexion (ρ = −0.46, p = 0.004), a more spherical medial femoral condyle shape (Femur 10) was associated with greater SSD in the tibial position. Increased height of the medial femoral condyle (Femur 18) was associated with greater SSD in the tibial position in the extended position only (ρ = 0.34, p = 0.035). Tibia 2 (Fig. 4A) describes the height of the medial tibial plateau. A shorter medial plateau in the superoinferior direction was associated with a greater SSD in the tibial position in the flexed position (ρ = −0.32, p = 0.048). Tibia 4 describes the AP length of the lateral tibial plateau. A longer lateral plateau in the AP dimension was associated with a greater SSD in the tibial position in flexion (ρ = −0.37, p = 0.021). Tibia 19 (Fig. 4B), which describes the tibial slope through the height of the lateromedial tibial plateau, was associated with a greater SSD in the tibial position in flexion with an increased height of the anteromedial tibial plateau associated with a more anteriorly positioned tibia (ρ = 0.34, p = 0.038).
Fig. 4A–B.
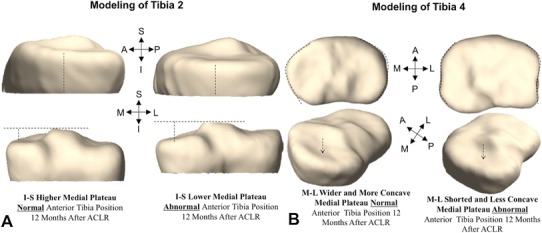
A representation is shown for two additional shape features that were correlated with abnormal knee kinematics at 1 year after ACL reconstruction, including: (A) the height of the medial tibial plateau, represented by Tibia 2; (B) the width of the tibial plateau and concavity of the medial plateau, represented by Tibia 4. S = superior; I = inferior; L = lateral; M = medial; A = anterior; P = posterior; ACLR = ACL reconstruction.
Discussion
Bone shape features have been associated with the development of idiopathic osteoarthritis in the knee and hip. After ACL injury, there are certain patients who can successfully return to activities with nonoperative treatment, whereas others remain quite limited and unstable. Increased SSDs in the tibial position in the sagittal plane have been associated with functionally unstable knees, whereas smaller SSDs in the tibial position have been reported in patients who can functionally tolerate an ACL-deficient knee [5, 6]. Alterations in knee kinematics have also been implicated in the development of posttraumatic arthritis, although the association between knee kinematics and bone shape has not yet been explored. The purpose of this study was to investigate the relationship between bone shape features and knee kinematics after ACL injury and after ACL reconstruction. We have observed four shape features associated with abnormal knee kinematics after ACL injury: the height of the medial femoral condyle, the sphericity of the medial femoral condyle, the length of the lateral tibial plateau, and the slope of the medial tibial plateau. There were five shape features associated with persistently abnormal knee kinematics after ACL reconstruction: the height of the medial femoral condyle, the sphericity of the medial femoral condyle, the height of the medial tibial plateau, the length of the lateral tibial plateau, and the slope of the medial tibial plateau.
The results of this study must be interpreted with an understanding of its limitations. First, the relationships observed are correlations and are not able to establish a causal relationship between bone shape and altered kinematics. With the results available, we also cannot exclude the possibility that other bone shape features not found to be associated with anterior tibial translation here are important in post-ACL injury and reconstruction knee kinematics. Larger epidemiologic studies incorporating an evaluation of bone shape features would provide further clarification on all potential bone shapes that may influence knee kinematics. Second, patients were observed 1 year after treatment. This time point is after full return to activities, and we would not expect much ongoing change in these measurements, although these results may differ at later time points after ACL reconstruction. Although the kinematic MR methods described here allow for advanced evaluation of joint function, this remains a static measurement and only assesses the knee up to 30° of flexion. This evaluation simulates a standing position and initiation of squatting but does not provide insight on deeper knee flexion. Future work could use other in vivo kinematic assessments such as multiplanar fluoroscopy to further evaluate kinematic changes through a full functional ROM and include coronal plane and rotational kinematic measurements. There are numerous other factors that were not controlled in this study such as patient age, muscle function, rehabilitation participation, surgical techniques, and others, although these factors likely have some contribution to knee kinematics after injury and after ACL reconstruction. The relative contribution of each of these factors may be clarified in future larger multivariate analyses, and the current clinical implications of these findings remain unclear.
First, we investigated the relationship between bone shape and knee kinematics after ACL injury. Previous studies have highlighted the importance of tibial slope on knee kinematics. Giffin et al. [12], in a cadaveric study, showed an increasing tibial slope resulted in an increasingly anteriorly positioned tibia. A shift in the slope of the tibia from 8.8° to 13.2° resulted in up to 3.6 mm of anterior translation. Voos et al. [25] evaluated anterior tibial translation during Lachman and pivot shift testing of cadaveric specimens. Tibial osteotomies were performed to change the posterior slope, and a 5° increase in posterior slope was associated with increased anterior tibial translation during the pivot shift test. These prior findings are like what we have observed in the relationship between increasing height of the anteromedial tibial plateau (Tibia 19) and abnormal knee kinematics in this cohort. Although tibial factors have been implicated previously in abnormal knee kinematics, the role of femoral shape features has not been described. From our results, variability in the medial femoral condyle shape appears to be associated with abnormal knee kinematics. Future large controlled studies are needed to truly define the role that bone shape has on post-ACL injury knee kinematics. Understanding patient-specific shape features that are associated with normal or abnormal post-ACL injury knee kinematics may eventually allow for clarification of which patients benefit most from ACL reconstruction.
The second question we sought to answer involved the relationship between bone shape features and postsurgical knee kinematics. Bone shape has been associated with both the progression of osteoarthritis and graft failure [21, 26]. Neogi et al. [21] followed a group of subjects in the Osteoarthritis Initiative and demonstrated that 3-D bone feature vectors could predict incident osteoarthritis over a 12-month period. Gregory et al. [13] also described imaging shape-based markers as predictive of hip osteoarthritis. Our group has previously described a relationship between knee bone shape and articular cartilage degeneration after ACL reconstruction in this same cohort of patients and using the same model making the results of this study directly comparable [23]. Specifically, both the height of the medial tibial plateau and the superoinferior height of the medial femoral condyle, which were also associated here with abnormal knee kinematics after reconstruction, were identified as contributing to progression of early articular cartilage degeneration after ACL reconstruction. Webb et al. [26] reported increasing posterior tibial slope to be a risk factor for both ACL graft failure and contralateral ACL injury. Patients with posterior slope above 12° had a 59% increase in risk of graft failure. Although our cohort had no patients with graft failure, our followup of 1 year was shorter than the 66-month mean time to failure in Webb et al.’s cohort. Persistent abnormalities in tibial position after surgical reconstruction may contribute to degenerative changes and risk of failure. Bone shape may therefore be an important factor in identifying patients who are at risk of persistent abnormal knee kinematics after ACL reconstruction and possibly a contributing factor in the development of postinjury osteoarthritis and graft failure although the results here are simply initial associations and not definitive confirmation of this relationship that may be clarified with future studies. Future studies on risk factors for posttraumatic arthritis and ACL reconstruction failure should include bone shape as a possible contributor to these conditions. If shape features are identified as independent risk factors, clinicians should then investigate if supplemental surgical procedures such as osteotomies or supplemental extraarticular ligamentous reconstructions or different rehabilitation programs are beneficial for this subset of patients.
In conclusion, we have observed two femoral and four tibial shape features that are associated with abnormal knee kinematics both after ACL injury and after ACL reconstruction. Future directions of research will include the influence of bony morphology on clinical symptoms of instability in patients with and without ACL reconstruction and the long-term evaluation of these shape factors to better determine specific contributions to posttraumatic arthritis and graft failure. Bone shape features are possibly one of many factors that may influence knee kinematics after ACL injury and after surgical reconstruction. Future larger clinical studies should include bone shape as a variable in multivariate investigations of factors determining knee kinematics after ACL injury and after ACL reconstruction, and modeling studies can investigate how alterations in these shape features may impact tibiofemoral alignment and forces on the reconstructed graft. Knowledge of these relationships could allow clinicians to better counsel patients on the role of ACL reconstruction and refining surgical and rehabilitation methods for patients particularly at risk for postreconstruction abnormalities.
Acknowledgments
We thank Brian Feeley MD, Christina Allen MD, and Nicolas Colyvas MD, for enrolling patients in this study.
Footnotes
Funding provided by NIH/NIAMS P50 060752 (XL) and the AOSSM Genzyme Cartilage Initiative (CBM).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Amano K, Pedoia V, Su F, Souza RB, Li X, Ma CB. Persistent biomechanical alterations after ACL reconstruction are associated with early cartilage matrix changes detected by quantitative MR. Orthop J Sports Med. 2016;4:2325967116644421. doi: 10.1177/2325967116644421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38:293–298. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/B:ABME.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 4.Baker-LePain JC, Lane NE. Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol. 2010;22:538–543. doi: 10.1097/BOR.0b013e32833d20ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrance PJ, Williams GN, Snyder-Mackler L, Buchanan TS. Altered knee kinematics in ACL-deficient non-copers: a comparison using dynamic MRI. J Orthop Res. 2006;24:132–140. doi: 10.1002/jor.20016. [DOI] [PubMed] [Google Scholar]
- 6.Barrance PJ, Williams GN, Snyder-Mackler L, Buchanan TS. Do ACL-injured copers exhibit differences in knee kinematics? An MRI study. Clin Orthop Relat Res. 2007;454:74–80. doi: 10.1097/BLO.0b013e31802bab0d. [DOI] [PubMed] [Google Scholar]
- 7.Brophy RH, Wright RW, Matava MJ. Cost analysis of converting from single-bundle to double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37:683–687. doi: 10.1177/0363546508328121. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter RD, Majumdar S, Ma CB. Magnetic resonance imaging of 3-dimensional in vivo tibiofemoral kinematics in anterior cruciate ligament-reconstructed knees. Arthroscopy. 2009;25:760–766. doi: 10.1016/j.arthro.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Chalmers PN, Mall NA, Moric M, Sherman SL, Paletta GP, Cole BJ, Bach BR. Does ACL reconstruction alter natural history? A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96:292–300. doi: 10.2106/JBJS.L.01713. [DOI] [PubMed] [Google Scholar]
- 10.Cootes TF, Taylor CJ, Cooper DH, Graham J. Active shape models–their training and application. Computer Vision and Image Understanding. 1995;61:38–59. doi: 10.1006/cviu.1995.1004. [DOI] [Google Scholar]
- 11.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 12.Giffin JR, Vogrin TM, Zantop T. Woo SL-Y, Harner CD. Effects of increasing tibial slope on the biomechanics of the knee. Am J Sports Med. 2004;32:376–382. doi: 10.1177/0363546503258880. [DOI] [PubMed] [Google Scholar]
- 13.Gregory JS, Waarsing JH, Day J, Pols HA, Reijman M, Weinans H, Aspden RM. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis Rheum. 2007;56:3634–3643. doi: 10.1002/art.22982. [DOI] [PubMed] [Google Scholar]
- 14.Haughom B, Schairer W, Souza RB, Carpenter D, Ma CB, Li X. Abnormal tibiofemoral kinematics following ACL reconstruction are associated with early cartilage matrix degeneration measured by MRI T1rho. Knee. 2012;19:482–487. doi: 10.1016/j.knee.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lansdown DA, Allen C, Zaid M, Wu S, Subburaj K, Souza R, Feeley BT, Li X, Ma CB. A comprehensive in vivo kinematic, quantitative MRI and functional evaluation following ACL reconstruction—a comparison between mini-two incision and anteromedial portal femoral tunnel drilling. Knee. 2015;22:547–553. doi: 10.1016/j.knee.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lansdown DA, Zaid M, Pedoia V, Subburaj K, Souza R, Benjamin C, Li X. Reproducibility measurements of three methods for calculating in vivo MR-based knee kinematics. J Magn Reson Imaging. 2015;42:533–538. doi: 10.1002/jmri.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohmander LS, Östenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 18.Lynch JA, Parimi N, Chaganti RK, Nevitt MC, Lane NE. The association of proximal femoral shape and incident radiographic hip OA in elderly women. Osteoarthritis Cartilage. 2009;17:1313–1318. doi: 10.1016/j.joca.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mall NA, Chalmers PN, Moric M, Tanaka MJ, Cole BJ, Bach BR, Jr, Paletta GA., Jr Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42:2363–2370. doi: 10.1177/0363546514542796. [DOI] [PubMed] [Google Scholar]
- 20.Nelson AE, Golightly YM, Renner JB, Schwartz TA, Liu F, Lynch JA, Gregory JS, Aspden RM, Lane NE, Jordan JM. Variations in hip shape are associated with radiographic knee osteoarthritis: cross-sectional and longitudinal analyses of the Johnston County Osteoarthritis Project. J Rheumatol. 2016;43:405–410. doi: 10.3899/jrheum.150559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neogi T, Bowes MA, Niu J, De Souza KM, Vincent GR, Goggins J, Zhang Y, Felson DT. Magnetic resonance imaging-based three-dimensional bone shape of the knee predicts onset of knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Rheum. 2013;65:2048–2058. doi: 10.1002/art.37987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedoia V, Lansdown DA, Zaid M, McCulloch CE, Souza R, Ma CB, Li X. Three-dimensional MRI-based statistical shape model and application to a cohort of knees with acute ACL injury. Osteoarthritis Cartilage. 2015;23:1695–1703. doi: 10.1016/j.joca.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedoia V, Su F, Amano K, Li Q, McCulloch CE, Souza RB, Link TM, Ma BC, Li X. Analysis of the articular cartilage T1ρ and T2 relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. J Orthop Res. 2017;35:707–717. doi: 10.1002/jor.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 25.Voos JE, Suero EM, Citak M, Petrigliano FP, Bosscher MRF, Citak M, Wickiewicz TL, Pearle AD. Effect of tibial slope on the stability of the anterior cruciate ligament–deficient knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:1626–1631. doi: 10.1007/s00167-011-1823-3. [DOI] [PubMed] [Google Scholar]
- 26.Webb JM, Salmon LJ, Leclerc E, Pinczewski LA, Roe JP. Posterior tibial slope and further anterior cruciate ligament injuries in the anterior cruciate ligament-reconstructed patient. Am J Sports Med. 2013;41:2800–2804. doi: 10.1177/0363546513503288. [DOI] [PubMed] [Google Scholar]
- 27.Zaid M, Lansdown D, Su F, Pedoia V, Tufts L, Rizzo S, Souza RB, Li X, Ma CB. Abnormal tibial position is correlated to early degenerative changes one year following ACL reconstruction. J Orthop Res. 2015;33:1079–1086. doi: 10.1002/jor.22867. [DOI] [PMC free article] [PubMed] [Google Scholar]


