Abstract
Background
When anterior cruciate ligament (ACL) reconstruction fails, a revision procedure may be performed to improve knee function, correct instability, and allow return to activities. The results of revision ACL reconstruction have been reported to produce good but inferior patient-reported and objective outcomes compared with primary ACL reconstruction, but the degree to which this is the case varies widely among published studies and may be influenced by heterogeneity of patients, techniques, and endpoints assessed. For those reasons, a systematic review may provide important insights.
Questions/purposes
In a systematic review, we asked: (1) What is the proportion of revision ACL reconstruction cumulative failures defined as rerupture or objective failure using prespecified clinical criteria at mean followup of at least 5 years? (2) What are the most common complications of revision ACL reconstruction?
Methods
A systematic review was performed by searching PubMed/Medline, EMBASE, and CENTRAL. We included studies that reported the clinical evaluation of revision ACL reconstruction with Lachman test, pivot shift test, side-to-side difference with KT-1000/2000 arthrometer, and with a mean followup of at least 5 years. We excluded studies that incompletely reported these outcomes, that reported only reruptures, or that were not in the English language. Extracted data included the number of graft reruptures and objective clinical failure, defined as a knee that met one of the following endpoints: Lachman test Grade II to III, pivot shift Grade II to III, KT-1000/2000 > 5-mm difference, or International Knee Documentation Committee Grade C or D. For each study, we determined the proportion of patients who had experienced a rupture of the revision ACL graft as well as the proportion of patients who met one or more of our clinical failure endpoints. Those proportions were summed for each study to generate a percentage of patients who met our definition of cumulative failure. Complications and reoperations were recorded but not pooled as a result of inconsistency of reporting and heterogeneity of populations across the included studies. Of the 663 screened studies, 15 articles were included in the systematic review. Because one study reported two separate groups of patients with different treatments, 16 case series were considered in the evaluation.
Results
The proportion of reruptures (range, 0%–25%) was > 5% in only four of 16 series and > 10% in only one of them. The objective clinical failures (range, 0%–82%) was > 5% in 15 of 16 series and > 10% in 12 of them. The proportion exceeded 20% in five of 16 series. The cumulative failures (range, 0%–83%) was > 5% in all except one series and > 10% in 12 of 16 series; five series had a cumulative failure proportion > 20%. The most frequent complications were knee stiffness and anterior knee pain, whereas reoperations were primarily débridement and meniscectomies.
Conclusions
Considering rerupture alone as a failure endpoint in patients who have undergone revision ACL reconstruction likely underestimates the real failure rate, because the percentage of failures noticeably increases when objective criteria are also considered. Whether patient-reported and subjective scores evaluating knee function, level of activity, satisfaction, and pain might also contribute to the definition of failure may be the focus of future studies.
Level of Evidence
Level IV, therapeutic study.
Keywords: Anterior Cruciate Ligament, Anterior Cruciate Ligament Reconstruction, Pivot Shift, International Knee Documentation Committee, International Knee Documentation Committee Score
Introduction
As many as 8% of patients undergoing ACL reconstruction will undergo a subsequent revision procedure [1, 13, 17], with as many as 13,000 patients undergoing revision ACL reconstruction each year in the United States alone [18]. Recent studies have shown that revision ACL reconstructions have less favorable patient-reported and objective outcomes when compared with primary ACL reconstructions [10, 30]. The most recent meta-analysis comparing revision and primary ACL reconstructions demonstrated lower Lysholm and International Knee Documentation Committee (IKDC) scores in revision ACL reconstructions [10]. Revisions also showed less rotatory stability despite similar AP stability [10]. However, interestingly, several large studies have reported the proportion of repeated revision ACL reconstructions to be 2.0% to 5.4% [15, 17, 24, 26], which is comparable to those reported for revision of primary ACL reconstructions (1.7%–7.7%) [15, 24]. In a recent case series, Schlumberger et al. [24] reported no difference in the risk of graft ruptures between primary and revision ACL reconstructions, which was 3.0% and 2.0%, respectively. These findings are not consistent with other reports of worse patient-reported and objective outcomes of revision ACL reconstructions [10].
Even the definition of what constitutes a failed revision ACL reconstruction is controversial. After considering multiple objective criteria, including KT-1000 (MEDmetric Corp, San Diego, CA, USA) measurements and pivot shift grades, the proportion of revision ACL reconstruction failure was reported as 13.7% in a recent systematic review by Wright et al. [30]. However, when different failure criteria such as swelling, pain, or knee stiffness are applied, the proportion has been reported as high as 25% [3, 19]. This discrepancy, partly caused by the heterogeneity of revision ACL patient demographics and surgical characteristics, highlights the need for a comprehensive definition of failure, because differing definitions have led to inconsistent reporting. It may also be true that patients are unwilling to undergo a second revision procedure even in the setting of symptomatic instability. A comprehensive definition should include the proportion of reruptures as well as consideration of validated clinical measures such as the IKDC score, KT-1000 measurements, Lachman grades, and pivot shift grades.
In an attempt to gain a better understanding of this issue, we performed a systematic review to answer the following questions: (1) What is the proportion of revision ACL reconstruction cumulative failures defined as either rerupture or objective failure using prespecified clinical criteria at mean followup of at least 5 years? (2) What are the most common complications of revision ACL reconstruction?
Materials and Methods
A systematic review was performed in accordance with the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [20].
Search Strategy and Selection Criteria
We searched PubMed/Medline, EMBASE, and CENTRAL using the terms “anterior cruciate ligament” OR “ACL” combined with the Boolean operator AND with “revision” OR “rerupture” terms. The search was supplemented by independently searching through the reference lists of the included articles and the ePublication lists of leading orthopaedic and sports medicine journals, including Clinical Orthopaedics and Related Research ®, American Journal of Sports Medicine, British Journal of Sports Medicine, Knee Surgery Sports Traumatology Arthroscopy, Arthroscopy, Journal of Bone and Joint Surgery American and British versions, and The Knee. All studies that evaluated outcomes after revision ACL reconstruction were selected and the full texts were obtained. Biomechanical, in vitro and in vivo studies, review articles, surgical techniques, case reports, letters to the editor, editorials, and conference abstracts were excluded. Studies published after January 6, 2016, were also excluded.
Two authors (AG, GMMM) independently reviewed the title and abstract of each article identified in the literature search. When eligibility was unclear from the title and abstract, the full text of the article was obtained and evaluated for eligibility. Any disagreements were resolved by consensus discussion between the two independent reviewers. A third reviewer (SZ) was consulted if the disagreement could not be resolved. The full text of the selected articles was screened for the following inclusion criteria: (1) prospective or retrospective study design of revision ACL reconstruction isolated or combined with osteotomy, meniscal, cartilage, or other ligament surgery; (2) clinical evaluation with Lachman test, pivot shift test, side-to-side difference with KT-1000/2000 arthrometer, with or without data regarding the proportion of reruptures, complications, and reoperations; and (3) mean followup of at least 5 years.
Articles were excluded based on the following exclusion criteria: (1) incomplete clinical evaluation (for example, presenting only mean values of KT-1000/2000 with no mention of number of patients with < 3 mm, 3–5 mm, and > 5-mm side-to-side difference, clinical grading presented as pooled grade and not separate grades); (2) only proportion of reruptures or complications or reoperations reported without clinical details; and (3) studies not reported in English.
No restrictions were imposed for demographic characteristics, surgical technique, or graft choice for both primary and revision ACL reconstructions.
The initial search produced 663 articles. After reviewing all abstracts, 67 articles were found to be eligible for initial screening according to the inclusion and exclusion criteria. Among these, 19 articles presented a mean followup of at least 5 years and were therefore included in the preliminary analysis. Three articles were excluded for only reporting the proportion of reruptures without clinical evaluation and one article was excluded for reporting clinical outcomes in a manner that did not allow data extraction. This left 15 studies to be included in the present systematic review [2–4, 6–9, 12, 14, 16, 17, 19, 22, 23, 27] (Fig. 1). However, because one study [19] described two separate groups of patients receiving different treatments and analyzed separately, each with proper outcomes, 16 case series were considered in this systematic review.
Fig. 1.
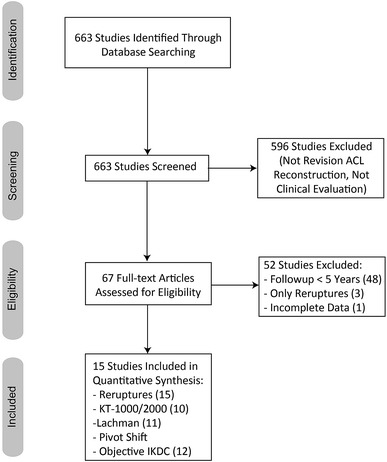
This figure displays the PRISMA flowchart of the included and excluded studies.
Assessment of Study Type and Quality
A modification of the Centre for Evidence-Based Medicine (OCEBM) Level of Evidence Table for the orthopaedic literature was used by two reviewers (AG, GMMM) to independently assess the included studies and assign a level of evidence (from I to IV) [29]. The methodological quality of the included studies was assessed using the Methodological Index for Nonrandomised Studies (MINORS) checklist [25]. According to the MINORS checklist, noncomparative studies were evaluated with eight items, each rated from 0 to 2 points, comprising study aim, consecutive patients, prospective design, appropriate endpoints, unbiased assessment, followup, dropout rate, and sample size calculation. Four additional items, including adequate control group, contemporary groups, baseline group equivalence, and adequate statistical analysis, were used for comparative studies. Therefore, the maximum possible scores were 16 and 24 for noncomparative and comparative studies, respectively. Any discrepancies regarding study quality were discussed between reviewers and the senior author (SZ) was consulted for any disagreements.
No randomized controlled trials were included. Four of 15 studies were Level III as a result of the presence of a control group of primary ACL reconstruction [9, 14, 27] or different grafts [19] (Table 1). The areas of best performance based on the MINORS checklist were the statement of a clear aim and use of a followup time deemed appropriate to accurately measure the failures (5 years mean followup), which were used in all studies. The areas of worst performance were the prospective calculation of study size and having a reported loss-to-followup rate < 5%, which were found in only one and five of the 15 included studies, respectively. The majority (12 of 15 studies) had a clearly retrospective study design.
Table 1.
Demographic details from the included studies
| Study | Year | Study design | Level of evidence | Patients treated | Patients at followup | Followup rate (%) | Followup after revision ACL-R (years) | Mean age at revision ACL-R (years) | Mean time from 1° ACL-R to Revision ACL (months) |
|---|---|---|---|---|---|---|---|---|---|
| O’Neill [22] | 2004 | Prospective | IV | 50 | 48 28 M/20 F |
96% | 7.5 (2–13) |
33 (13–57) |
60 (7–276) |
| Grossman et al. [12] | 2005 | Retrospective | IV | 35 | 29 22 M/7 F |
83% | 5.6 | 30.2 | 56 (9–192) |
| Thomas et al. [27] | 2005 | Prospective | III | 49 | 49 37 M/12 F |
100% | 6.2 (3–11) |
35.4 | NA |
| Ferretti et al. [7] | 2006 | Retrospective | IV | 30 | 30 | 100% | 5.0 (2–8) |
34 (21–39) |
60 (12–132) |
| Lidén et al. [16] | 2006 | Retrospective | IV | 14 | 12 | 86% | 9.6 (8.5–10.6) |
28 (23–39) |
64 (15–132) |
| Salmon et al. [23] | 2006 | Prospective | IV | 57 | 50 | 88% | 7.4 (5.0–9.1) |
27 (15–39) |
36 (2–132) |
| Battaglia et al. [3] | 2007 | Retrospective | IV | 95 | 63 36 M/27 F |
66% | 6.1 (3.0–13.2) |
31 (18–60) |
61 (3–316) |
| Diamantopoulos et al. [6] | 2008 | Retrospective | IV | 148 | 107 64 M/43 F |
72% | 6.0 ± 1.7 | 39 ± 9 | 60 ± 54 |
| Ahn et al. [2] | 2011 | Retrospective | IV | 50 | 41 | 82% | 6.3 (3.5–9.8) |
32 (21–55) |
59 (6–216) |
| Lind et al. [17] | 2012 | Retrospective | IV | 168 | 128 64 M/64 F |
76% | 5.9 (2.0–9.0) |
31 | 58 (0–311) |
| Mayr et al. [19] | 2012 | Retrospective | III | 17 (autografts) | 15 9 M/6 F |
88% | 5.7 ± 0.6 | 30 ± 9 | NA |
| 17 (allografts) | 14 | 82% | 5.8 ± 0.5 | 35 ± 13 | NA | ||||
| Franceschi et al. [8] | 2013 | Retrospective | IV | 30 | 30 19 M/11 F |
100% | 6.8 (5–9) |
29.1 (19–42) |
34 |
| Gifstad et al. [9] | 2013 | Retrospective | III | 69 | 56 25 M/31 F |
81% | 7.5 (2.8–13.2) |
26.5 | 38 (5–117) |
| Kievit et al. [14] | 2013 | Retrospective | III | 30 | 25 18 M/7 F |
86% | 5.3 (2.3–12.2) |
34.6 | NA |
| Chougule et al. [4] | 2015 | Retrospective | IV | 20 | 20 14 M/6 F |
100% | 6.0 | 33.7 | NA |
Values are mean ± SD or numbers with ranges in parentheses; ACL-R = anterior cruciate ligament reconstruction; ACL = anterior cruciate ligament; M = male; F = female; NA = not applicable.
The median MINORS score for the noncomparative studies was 9 (range, 6–11) out of a maximum score of 16, and the median score for comparative studies was 15 (range, 12–19) out of a maximum score of 24 (Table 2).
Table 2.
Methodological Index for Nonrandomised Studies checklist
| Study | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Followup period appropriate to the aim of the study | Loss to followup < 5% | Prospective calculation of the study size | An adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analysis | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Neill [22] | 2 | 1 | 2 | 1 | 0 | 2 | 2 | 0 | NA | NA | NA | NA | 10/16 |
| Grossman et al. [12] | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 6/16 |
| Thomas et al. [27] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 19/24 |
| Ferretti et al. [7] | 2 | 2 | 0 | 2 | 0 | 2 | 2 | 0 | NA | NA | NA | NA | 10/16 |
| Lidén et al. [16] | 2 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | NA | NA | NA | NA | 8/16 |
| Salmon et al. [23] | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 10/16 |
| Battaglia et al. [3] | 2 | 2 | 0 | 1 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 7/16 |
| Diamantopoulos et al. [6] | 2 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | NA | NA | NA | NA | 7/16 |
| Ahn et al. [2] | 2 | 1 | 0 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | NA | 9/16 |
| Lind et al. [17] | 2 | 1 | 0 | 2 | 2 | 2 | 0 | 0 | NA | NA | NA | NA | 9/16 |
| Mayr et al. [19] | 2 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 1 | 2 | 2 | 14/24 |
| Franceschi et al. [8] | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 0 | NA | NA | NA | NA | 11/16 |
| Gifstad et al. [9] | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 2 | 2 | 12/24 |
| Kievit et al. [14] | 2 | 1 | 0 | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 2 | 2 | 16/24 |
| Chougule et al. [4] | 2 | 1 | 0 | 2 | 1 | 2 | 2 | 0 | NA | NA | NA | NA | 10/16 |
The items were scored 0 (not reported), 1 (reported but inadequate), 2 (reported and adequate); NA = not applicable.
Patient demographic details including age at revision ACL reconstruction, sex, and length of followup were extracted to provide an overview of the population. Surgical details obtained included the cause of primary ACL reconstruction failure, type of graft, and the surgical techniques used for revision, including one- or two-stage, femoral tunnel drilling, femoral tunnel insertion, type of femoral and tibial fixation, medial and lateral meniscal lesion or defect, and abnormal cartilage.
Endpoints of Interest
First, we determined the proportion of patients reported to have had a graft rupture by extracting the number of graft reruptures from each article. Rerupture was defined, according to the different authors, as repeated ACL revision resulting from trauma [16, 23], graft rupture with persistent subjective instability [4], catastrophic failure [3], or reasons not reported because performed elsewhere respect to the revision procedure [7, 17]. Scheduled repeated revision resulting from traumatic injury or graft rupture [12, 27] was also accounted.
Then, we used prespecified criteria to define patients whose reconstructions had clinically failed based on instability or poor knee scores. Specifically, to meet our criterion of objective clinical failure, a patient needed to have had a Lachman Grade II or III, pivot shift Grade II or III, KT-1000/2000 measurements > 5 mm, or an overall objective IKDC score of C or D.
Finally, we summed the proportions of knees that had graft rerupture with those that had achieved an objective clinical failure endpoint to calculate the risk of cumulative failure according to Crawford et al. [5]. The number of the case series that presented an endpoint to be > 5% and > 10% were reported. The > 20% cutoff was used only if reported in more than two series of patients.
Overall, all the series reported evaluations utilizing the KT-1000/2000 arthrometer. However, only 10 of 16 series indicated the number of patients in each category, thus allowing determination of failures. Twelve series reported the use of Lachman testing, 13 series the result of pivot shift testing, and 13 series provided objective IKDC scores.
Finally, complications and reoperations as reported in each study were also extracted.
Patients Analyzed
A total of 879 patients were treated with revision ACL reconstruction in the 16 included case series (Table 1). Based on the reported data, revision ACL reconstruction was performed in patients with a mean age ranging from 26.5 years to 39 years (range, 13–60 years) with 12 of 16 studies (73%) presenting a mean age > 30 years; when sex was reported, females were the majority in only one series [9]. The mean followup ranged from 5.0 years to 9.6 years (range, 2.0–13.2 years), whereas the mean time from primary ACL reconstruction to the revision procedure was within an interval of 34 to 64 months (range, 0–316 months).
All the series reported first-time revision ACL reconstructions. The main cause of failure of primary ACL reconstruction was traumatic in seven series [7, 9, 12, 14, 17, 22, 23] and inadequate tunnel placement in four series [6, 16, 19, 27]. For primary ACL reconstructions, six series exclusively used autografts (three bone-patellar tendon-bone, one hamstrings, and two mixed autografts). Nine series used variable grafts (autografts, allografts, synthetic grafts), performed ACL repair, or performed isolated extraarticular reconstruction. One series did not report the type of graft used for primary ACL reconstruction. For revision ACL reconstructions, eight series used exclusively autografts (two bone-patellar tendon-bone, two hamstrings, and four mixed autografts), three used exclusively allografts, whereas five used a mix of autografts and allografts. All the studies used a single-bundle technique and a combined lateral augmentation in only one series [8]. A staged procedure was used systematically in two case series [8, 27], whereas the majority of the studies adopted a one-stage approach. The technique for femoral tunnel drilling was reported for 14 of 16 series. Most series (seven of 16), especially those published after 2010, explicitly referred to a transtibial technique, whereas the older series (two of 16) reported the use of a more general “endoscopic technique” [3, 16]. One series [23] explicitly utilized the anteromedial portal for tunnel creation, and one (7%) [7] used a two-incision technique. Two series used both two-incision and endoscopic techniques based on a single case each [12, 22]. Conversely, the femoral tunnel position was clearly reported only for three studies: two at 11 o’clock [2, 22] and one at 10 o’clock [6]. When reported (12 of 16 series), graft fixation was obtained predominantly with interference screws for both femoral and tibial sides. A meniscal lesion or defect was reported ranging from 10% to 86% for the medial meniscus and from 7% to 56% for the lateral meniscus. A certain degree of focal or diffuse cartilage damage in the medial, lateral, or patellofemoral compartment was reported ranging from 7% to 92% (Table 3).
Table 3.
Surgical details
| Study | Year | Single/multiple revision ACL-R | Cause of 1° ACL-R failure | Graft for 1° ACL-R | Graft for revision ACL-R | Stages | Femoral tunnel | Insertion | Fixation | Medial meniscal defect | Lateral meniscal defect | Abnormal cartilage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Neill [22] | 2004 | 28 (100%) first revision | 38 (79%) trauma | 24 (50%) BPTB autografts | 23 (48%) ipsilateral BPTB autografts | 1-stage | 2-incision | 11 o’clock | F: Screw | 60% | 56% | NA |
| 10 (21%) unknown | 17 (35%) HS autografts | 25 (52%) ipsilateral HS autografts | Endoscopic | T: Screw | ||||||||
| 6 (12%) ITB autografts | ||||||||||||
| 1 (2%) peroneus bervis | ||||||||||||
| Grossman et al. [12] | 2005 | 29 (100%) first revision | 14 (48%) trauma | 22 (76%) BPTB autografts | 22 (76%) BPTB allografts | 1-stage | 2-incision | NA | F: NA | 22% | 7% | 59% |
| 10 (34%) tunnel error | 3 (10%) HS autografts | 6 (21%) contralateral BPTB autografts | Endoscopic | T: NA | ||||||||
| 5 (17%) biologic | 3 (10%) synthetic | 1 (4%) Achilles allografts | ||||||||||
| 1 (4%) BPTB allografts | ||||||||||||
| Thomas et al. [27] | 2005 | 49 (100%) first revision | 49 (100%) tunnel error | 30 (61%) BPTB autografts | 38 (78%) ipsilateral HS autografts | 2-stage | NA | NA | F: Screw/Rigidfix | 86% | 53% | 92% |
| 15 (31%) synthetic | 11 (22%) ipsilateral BPTB autografts | T: Screw/Intrafix | ||||||||||
| 4 (8%) HS autografts | ||||||||||||
| Ferretti et al. [7] | 2006 | 30 (100%) first revision | 14 (47%) trauma | 26 (87%) BPTB autografts | 30 (100%) Ipsilateral HS autografts | 1-stage | 2-incision | NA | F: Swingbridge | 10% | 20% | 7% |
| 10 (33%) tunnel error | 4 (13%) synthetic | T: Evolgate/staples | ||||||||||
| 4 (13%) synthetic graft failure | ||||||||||||
| 2 (7%) unknown | ||||||||||||
| Lidén et al. [16] | 2006 | 14 (100%) first revision | 12 (86%) tunnel error | 14 (100%) BPTB autografts | 14 (100%) reharvested BPTB autografts | 1-stage | Endoscopic | NA | F: Screw | 75% | 42% | 42% |
| 2 (14%) trauma | T: Screw | |||||||||||
| Salmon et al. [23] | 2006 | 57 (100%) first revision | 29 (55%) trauma | 21 (47%) BPTB autografts | 26 (46%) contralateral HS autografts | 1-stage | AM portal | NA | F: Screw | NA | NA | 53% |
| 10 (22%) tunnel error | 21 (47%) HS autografts | 30 (53%) ipsilateral HS autografts | T: Screw | |||||||||
| 5 (11%) biologic | 3 (6%) synthetic | 1 (1%) HS allografts | ||||||||||
| 1 (2%) fixation failure | ||||||||||||
| *data of 45 patients | ||||||||||||
| Battaglia et al. [3] | 2007 | 63 (100%) first revision | NA | 41 (65%) BPTB autografts | 19 (30%) ipsilateral BPTB autografts | 1-stage | Endoscopic | NA | F: NA | 41% | 2% | NA |
| 9 (14%) HS autografts | 10 (16%) ipsilateral HS autografts | T: NA | ||||||||||
| 3 (5%) extraarticular | 1 (2%) reharvested BPTB autografts | |||||||||||
| 8 (12%) allografts | 1 (2%) contralateral BPTB autografts | |||||||||||
| 2 (3%) synthetic | 20 (32%) allografts | |||||||||||
| Diamantopoulos et al. [6] | 2008 | 107 (100%) first revision | 68 (63%) tunnel error | 64 (60%) BPTB autografts | 45 (42%) ipsilateral HS autografts | 1-stage (94%) | AM portal | 10 o’clock | F: No hardware | 48% | 11% | 64% |
| 26 (24%) trauma | 27 (25%) HS autografts | 41 (38%) ipsilateral BPTB autografts | 2-stage (6%) | T: No hardware | ||||||||
| 4 (4%) biologic | 14 (13%) synthetic | 21 (20%) ipsilateral QT autografts | ||||||||||
| 6 (5%) synthetic graft failure | 2 (2%) Allograftsgrafts | |||||||||||
| 3 (3%) PLC instability | ||||||||||||
| Ahn et al. [2] | 2011 | 41 (100%) first revision | NA | 11 (27%) BPTB autografts | 18 (44%) BPTB allografts | 1-stage (98%) | Transtibial | 11 o’clock | F: Cross-pin | 42% | 20% | NA |
| 10 (25%) Achilles allografts | 14 (34%) ipsilateral HS autografts | 2-stage (2%) | T: Screw | |||||||||
| 7 (18%) BPTB allografts | 9 (22%) Achilles allografts | |||||||||||
| 5 (12%) synthetic | ||||||||||||
| 4 (10%) Achilles autografts | ||||||||||||
| 3 (8%) HS autografts | ||||||||||||
| Lind et al. [17] | 2012 | 128 (100%) first revision | 38 (30%) trauma | 64 (50%) BPTB autografts | 36 (28%) ipsilateral BPTB autografts | 1-stage (89%) | NA | NA | F: NA | 30% | 13% | 59% |
| 41 (32%) tunnel error | 52 (41%) HS autografts | 52 (41%) ipsilateral HS autografts | 2-stage (11%) | T: NA | ||||||||
| 4 (3%) biologic | 8 (6%) synthetic | 40 (31%) allografts | ||||||||||
| 9 (7%) LCL injury | 4 (3%) other grafts | |||||||||||
| 6 (5%) other causes | ||||||||||||
| 31 (24%) unknown cause | ||||||||||||
| Mayr et al. [19] | 2012 | 17 (100%) first revision | 10 (58%) tunnel error | 17 (100%) BPTB autografts | 17 (100%) BPTB allografts | 1-stage | Transtibial | NA | F: Screw | NA | NA | NA |
| 6 (35%) trauma | T: Screw | |||||||||||
| 17 (100%) first revision | 9 (52%) tunnel error | 17 (100%) HS autografts | 17 (100%) ipsilateral BPTB autografts | 1-stage | Transtibial | NA | F: Screw | NA | NA | NA | ||
| 5 (29%) trauma | T: Screw | |||||||||||
| Franceschi et al. [8] | 2013 | 30 (100%) first revision | NA | 30 (100%) BPTB autografts | 30 (100%) ipsilateral HS autografts | 2-stage | Transtibial | NA | F: Screw | 33% | 27% | 40% |
| T: Screw | ||||||||||||
| Gifstad et al. [9] | 2013 | 56 (100%) first revision | 23 (41%) trauma | 54 (96%) BPTB autografts | 22 (39%) contralateral BPTB autografts | 1-stage (98%) | Transtibial | NA | F: NA | NA | NA | NA |
| 1 (2%) infection | 2 (4%) HS autografts | 25 (45%) ipsilateral HS autografts | 2-stage (2%) | T: NA | ||||||||
| 32 (57%) unknown | 9 (16%) ipsilateral BPTB autografts | |||||||||||
| Kievit et al. [14] | 2013 | 25 (100%) first revision | 14 (56%) trauma | NA | 12 (48%) PT allografts | 1-stage | Transtibial | NA | F: Endobutton | 56% | 32% | NA |
| 11 (44%) other | 11(44%) Achilles allografts | T: Staples | ||||||||||
| 2 (8%) BPTB allografts | ||||||||||||
| Chougule et al. [4] | 2015 | 20 (100%) first revision | NA | 11 (55%) HS autografts | 20 (100%) HS allografts | 1-stage (95%) | Transtibial | NA | F: Endobutton | NA | NA | NA |
| 6 (30%) BPTB autografts | 2-stage (5%) | T: Staples | ||||||||||
| 2 (10%) synthetic | ||||||||||||
| 1 (5%) allografts | ||||||||||||
ACL-R = anterior cruciate ligament reconstruction; NA = not applicable; PLC = posterolateral corner; LCL = lateral collateral ligament; BPTB = bone-patellar tendon-bone; HS = hamstrings; ITB = iliotibial band; PT = posterior tibialis; AM = anteromedial; F = femur; T = tibia.
Results
Proportion of Grafts Reaching a Failure Endpoint
The cumulative failure ranged from 0% to 83% with proportions of failures > 5% in all except one series and > 10% in 12 series; five series had a cumulative failure > 20% (Fig. 2).
Fig. 2.
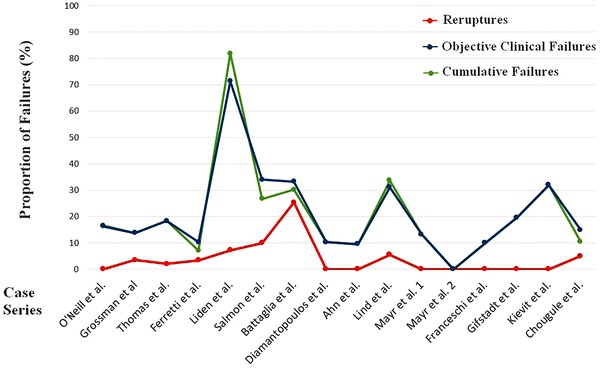
This figure displays a graphic representation of the proportion of reruptures (red line), objective clinical failures (blue line), and cumulative failure (green line) of the different studies. The proportions (from 0% to 100%) are displayed in the vertical line, whereas the case series are displayed in chronological order of publication in the horizontal line.
Overall, eight of the 16 series reported at least one graft rerupture (Table 4) with an incidence ranging from 0% to 25%; in eight of the 16 series, no reruptures were reported, whereas only four of the 16 studies reported reruptures in > 5% of patients with one series exceeding 10%. As a result of a followup rate ranging between 66% and 100%, the proportion of graft reruptures was determined in a subset of 716 patients.
Table 4.
Clinical outcomes, complications, and reoperations
| Study | Year | Objective IKDC | KT-1000/2000 | Lachman | Pivot shift | Complications | Reoperations | Reruptures |
|---|---|---|---|---|---|---|---|---|
| O’Neill [22] | 2004 | 20 (42%) A | 10 (21%) 0 mm | NA | NA | 1 (2%) wound infection | 5 (10%) meniscectomies | No |
| 20 (42%) B | 22 (46%) 0–2 mm | |||||||
| 6 (12%) C | 3 (6%) 2–3 mm | |||||||
| 2 (4%) D | 10 (21%) 3–5 mm | |||||||
| 3 (6%) > 5 mm | ||||||||
| Grossman et al. [12] | 2005 | 17 (58%) A | Mean 2.8 mm (total) | 28 (97%) negative | 27 (93%) negative | 2 (7%) mild contralateral harvest site pain | 1 (3%) rerevision (scheduled) | 1 (3%) graft rerupture |
| 8 (28%) B | Mean 3.2 mm (allograft) | 0 (0%) Grade I | 2 (7%) Grade I | |||||
| 4 (14%) C | Mean 1.3 mm (autograft) | 1 (3%) Grade II | 0 (0%) Grade II | |||||
| 0 (0%) D | 0 (0%) Grade III | 0 (0%) Grade III | ||||||
| Thomas et al. [27] | 2005 | 12 (24%) A | Mean 1.9 ± 2.4 mm | NA | 43 (88%) negative | No | 1 (2%) rerevision (scheduled) | 1 (2%) graft rerupture |
| 28 (57%) B | 46 (94%) 0–2 mm | 5 (10%) Grade I | 3 (6%) meniscus/cartilage débridement | |||||
| 8 (16%) C | 1 (2%) 3–4 mm | 1 (2%) Grade II | ||||||
| 1 (2%) D | 2 (4%) > 5 mm | 0 (0%) Grade III | ||||||
| Ferretti et al. [7]* | 2006 | 15 (53%) A | Mean 2.5 ± 1.8 mm | 22 (79%) negative | 15 (54%) negative | NA | 1 (3%) rerevision | 1 (3%) graft rerupture |
| 11 (39%) B | (0;6) | 6 (21%) Grade I | 11 (39%) Grade I | 1 (3%) screw removal | ||||
| 2 (7%) C | 20 (71%) 0–3 mm | 0 (0%) Grade II | 2 (7%) Grade II | |||||
| 0 (0%) D | 6 (21%) 3–5 mm | 0 (0%) Grade III | 0 (0%) Grade III | |||||
| 2 (7%) > 5 mm | ||||||||
| Lidén et al. [16]† | 2006 | 0 (0%) A | Mean 2 mm | NA | NA | 1 (7%) patellar fracture | 1 (8%) patellar fracture ORIF | 1 (7%) graft rerupture |
| 2 (18%) B | (−4; 7.5) | 1 (7%) PT rupture | 1 (8%) PT suture | |||||
| 3 (27%) C | ||||||||
| 6 (55%) D | ||||||||
| Salmon et al. [23]‡,§ | 2006 | 33 (73%) A/B | Mean 2.5 mm | 24 (53%) negative | 31 (69%) negative | 4 (8%) meniscal lesion | 3 (6%) rerevision | 5 (10%) graft rerupture |
| 12 (27%) C/D | (−1;4) | 20 (45%) Grade I | 14 (31%) Grade I | 10 (22%) mild donor site morbibity | 4 (8%) meniscectomies | |||
| 17 (50%) < 3 mm | 1 (2%) Grade II | 0 (0%) Grade II | 3 (7%) moderate donor site morbidity | |||||
| 17 (50%) 3–5 mm | 0 (0%) Grade III | 0 (0%) Grade III | 2 (4%) extension deficit | |||||
| 0 (0%) > 5 mm | 20 (44%) kneeling pain | |||||||
| Battaglia et al. [3]‖ | 2007 | NA | Mean 3.9 ± 2.4 mm | 44 (70%) negative/Grade I | NA | NA | 16 (25%) rerevision | 16 (25%) graft rerupture |
| 30 (48%) < 3 mm | 19 (30%) Grade II | |||||||
| 20 (31%) 3–5 mm | ||||||||
| 10 (21%) > 5 mm | ||||||||
| Diamantopoulos et al. [6] | 2008 | 79 (74%) A | Mean 0.9 ± 1.1 mm | 91 (85%) negative | 79 (74%) negative | NA | NA | No |
| 17 (16%) B | 9 (8%) Grade I | 17 (16%) Grade I | ||||||
| 11 (10%) C | 7 (7%) Grade II–III | 11 (10%) Grade II | ||||||
| 0 (0%) D | 0 (0%) Grade III | |||||||
| Ahn et al. [2] | 2011 | 13 (32%) A | Mean 2.0 mm | 27 (66%) negative | 29 (71%) negative | NA | NA | No |
| 24 (59%) B | (0; 5) | 14 (34%) Grade I | 12 (29%) Grade I | |||||
| 3 (7%) C | 0 (0%) Grade II | 0 (0%) Grade II | ||||||
| 1 (2%) D | 0 (0%) Grade III | 0 (0%) Grade III | ||||||
| Lind et al. [17]¶ | 2012 | 5 (5%) A | Mean 2.5 mm | NA | 40 (41%) negative | 13 (10%) meniscus lesion | 7 (6%) rerevision | 7 (5%) graft rerupture |
| 57 (59%) B | 120 (92%) < 5 mm | 47 (48%) Grade I | 2 (2%) ROM loss | 13 (10%) meniscectomy | ||||
| 28 (29%) C | 8 (6%) > 5 mm | 18 (18%) Grade II | 1 (1%) superficial infection | 2 (2%) arthrolysis | ||||
| 5 (5%) D | data of 98 patients | 0 (0%) Grade III | 1 (1%) wound débridement | |||||
| data of 98 patients | data of 98 patients | 2 (2%) TKA (pain) | ||||||
| 15 (12%) hardware removal | ||||||||
| Mayr et al. [19] | 2012 | 2 (13%) A | Mean 2.1 ± 2.3 mm | 7 (47%) negative | 12 (86%) negative | 6 (40%) 3°–5° extension deficit | NA | No |
| 12 (80%) B | 6 (40%) < 3 mm | 6 (40%) Grade I | 2 (14%) Grade I | 5 (33%) flexion deficit | ||||
| 1 (17%) C | 9 (60%) 3–5 mm | 2 (13%) Grade II | 0 (0%) Grade II | 6 (40%) donor site pain | ||||
| 0 (0%) D | 0 (0%) > 5 mm | 0 (0%) Grade III | 0 (0%) Grade III | |||||
| 6 (43%) A | Mean 2.1 ± 1.5 mm | 11 (79%) negative | 14 (100%) negative | 3 (21%) 3°–5° extension deficit | NA | No | ||
| 8 (57%) B | 9 (64%) < 3 mm | 3 (21%) Grade I | 0 (0%) Grade I | 7 (50%) flexion deficit | ||||
| 0 (0%) C | 5 (36%) 3–5 mm | 0 (0%) Grade II | 0 (0%) Grade II | 8 (57%) donor site pain | ||||
| 0 (0%) D | 0 (0%) > 5 mm | 0 (0%) Grade III | 0 (0%) Grade III | |||||
| Franceschi et al. [8] | 2013 | 27 (90%) A/B | Mean 3.1 mm | 24 (80%) negative | 25 (83%) negative | 1 (3%) hypoesthesia | No | No |
| 3 (10%) C | (1.3; 3.8) | 6 (20%) Grade I | 5 (17%) Grade I | |||||
| 0 (0%) D | 0 (0%) Grade II | 0 (0%) Grade II | ||||||
| 0 (0%) Grade III | 0 (0%) Grade III | |||||||
| Gifstad et al. [9]** | 2013 | NA | Mean 3.3 ± 2.7 mm | 48 (86%) negative | 45 (80%) negative-Grade I | 5 (9%) > 5° extension deficit | No | No |
| 28 (52%) < 3 mm | 7 (12%) Grade I | 11 (20%) Grade II–III | 6 (11%) > 5° flexion deficit | |||||
| 17 (23%) 3–5 mm | 1 (2%) Grade II | |||||||
| 8 (15%) > 5 mm | 0 (0%) Grade III | |||||||
| data of 53 patients | ||||||||
| Kievit et al. [14] | 2013 | 17 (68%) A/B | Mean 3.0 mm | 2 (8%) < 3 mm | 8 (32%) negative | No | No | No |
| 8 (32%) C/D | (−6; 11) | 15 (60%) 3–5 mm | 13 (52%) Grade I | |||||
| 7 (28%) 5–10 mm | 4 (16%) Grade II | |||||||
| 1 (4%) > 10 mm | 0 (0%) Grade III | |||||||
| Chougule et al. [4]†† | 2015 | NA | 15 (80%) < 3 mm | 13 (70%) Grade I | 14 (75%) negative | 1 (5%) tibial cyst | 1 (5%) rerevision | 1 (5%) graft rerupture |
| 3 (15%) 3–5 mm | 4 (20%) Grade II | 4 (20%) minor | 1 (5%) hardware and cyst removal | |||||
| 1 (5%) > 5 mm | 2 (10%) Grade III | 1 (5%) moderate | ||||||
* One patient not objectively evaluated (only phone interview), one not objectively evaluated because they had rerevision elsewhere; †1 patient not objectively evaluated because they had rerevision; ‡5 patients not objectively evaluated because they had graft failure; §11 patients not evaluated with KT-1000/2000 because they had contralateral ACL injury or reconstruction; ‖3 patients not evaluated with KT-1000/2000 because they had contralateral ACL reconstruction; ¶30 patients not objectively evaluated (only phone interview); **3 patients not evaluated with KT-1000/2000 because they had contralateral ACL injury; ††1 patient not objectively evaluated they because had rerevision; IKDC = International Knee Documentation Committee; NA = not applicable; PT = patellar tendon; ORIF = open reduction and internal fixation; ACL = anterior cruciate ligament.
According to KT-1000/2000 evaluation, failure ranged from 0% to 17%; six of 10 series had failures in > 5% of patients with only two of them having failures in > 10% of patients. According to the Lachman test, failure ranged from 0% to 32% with a proportion of failures > 5% in six of 12 series, of which five were > 10%. According to the pivot shift maneuver, failure ranged from 0% to 20% with a proportion of failures > 5% in six of 13 series, of which four were > 10%. According to the objective IKDC scale, failure ranged from 0% to 82%; a proportion of failure > 5% was reported in 12 of 13 series and > 10% in eight of them.
Combining the outcomes within each study, the predefined objective clinical failure ranged from 0% to 82%; proportions > 5% and > 10% were reported in 15 of 16 series and 12 of 16 series, respectively. The proportion exceeded 20% in five of 16 series. Because clinical evaluation was not performed in all of the patients investigated for a rerupture, the objective clinical failure was therefore available for a subset of 678 patients (Table 5).
Table 5.
Failures of revision ACL reconstruction
| Study | Year | Reruptures | Objective IKDC overall Grade C or D | Abonormal KT-1000/2000 | Abnormal Lachman | Abnormal pivot shift | Objective clinical failures | Cumulative failures | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Neill [22] | 2004 | 0/48 | (0%) | 8/48 | (17%) | 3/48 | (6%) | NA | NA | NA | NA | 8/48 | (17%) | 8/48 | (16%) |
| Grossman et al. [12] | 2005 | 1/29 | (3%) | 4/29 | (14%) | NA | NA | 1/29 | (3%) | 0/29 | (0%) | 4/29 | (14%) | 4/29 | (14%) |
| Thomas et al. [27] | 2005 | 1/49 | (2%) | 9/49 | (18%) | 2/49 | (4%) | NA | NA | 1/49 | (2%) | 9/49 | (18%) | 9/49 | (18%) |
| Ferretti et al. [7] | 2006 | 1/30 | (3%) | 2/28* | (7%) | 2/28* | (7%) | 0/28* | (0%) | 1/28* | (7%) | 2/28* | (7%) | 3/30 | (10%) |
| Lidén et al. [16] | 2006 | 1/12 | (8%) | 9/11† | (82%) | NA | NA | NA | NA | NA | NA | 9/11† | (82%) | 10/12 | (83%) |
| Salmon et al. [23] | 2006 | 5/50 | (10%) | 12/45‡ | (27%) | 0/34‡,§ | (0%) | 1/45‡ | (2%) | 0/45‡ | (0%) | 12/45‡ | (27%) | 17/50 | (34%) |
| Battaglia et al. [3] | 2007 | 16/63 | (25%) | NA | NA | 10/60‖ | (17%) | 19/63 | (30%) | NA | NA | 19/63 | (30%) | 21/63 | (33%) |
| Diamantopoulos et al. [6] | 2008 | 0/107 | (0%) | 11/107 | (11%) | NA | NA | 7/107 | (6%) | 11/107 | (11%) | 11/107 | (11%) | 11/107 | (11%) |
| Ahn et al. [2] | 2011 | 0/41 | (0%) | 4/41 | (10%) | NA | NA | 0/41 | (0%) | 0/41 | (0%) | 4/41 | (10%) | 4/41 | (10%) |
| Lind et al. [17] | 2012 | 7/128 | (6%) | 33/98¶ | (34%) | 8/98¶ | (8%) | NA | NA | 18/98¶ | (18%) | 33/98¶ | (34%) | 40/128 | (31%) |
| Mayr et al. [19] | 2012 | 0/15 | (0%) | 1/15 | (7%) | 0/15 | (0%) | 2/15 | (13%) | 0/15 | (0%) | 2/15 | (13%) | 2/15 | (13%) |
| 0/14 | (0%) | 0/14 | (0%) | 0/14 | (0%) | 0/14 | (0%) | 0/14 | (0%) | 0/14 | (0%) | 0/14 | (0%) | ||
| Franceschi et al. [8] | 2013 | 0/30 | (0%) | 3/30 | (10%) | NA | NA | 0/30 | (0%) | 0/30 | (0%) | 3/30 | (10%) | 3/30 | (10%) |
| Gifstad et al. [9] | 2013 | 0/56 | (0%) | NA | NA | 8/53** | (15%) | 1/56 | (18%) | 11/56 | (20%) | 11/56 | (20%) | 11/56 | (20%) |
| Kievit et al. [14] | 2013 | 0/25 | (0%) | 8/25 | (32%) | NA | NA | 8/25 | (32%) | 4/25 | (16%) | 8/25 | (32%) | 8/25 | (32%) |
| Chougule et al. [4] | 2015 | 1/20 | (5%) | NA | NA | 1/19†† | (6%) | 2/19†† | (11%) | 1/19†† | (6%) | 2/19†† | (11%) | 3/20 | (15%) |
* One patient not objectively evaluated (only phone interview), 1 not objectively evaluated because they had rerevision elsewhere; †1 patient not objectively evaluated because they had rerevision; ‡5 patients not objectively evaluated they because had graft failure; §11 patients not evaluated with KT-1000/2000 because they had contralateral ACL injury or reconstruction; ‖3 patients not evaluated with KT-1000/2000 because they had contralateral ACL reconstruction; ¶30 patients not objectively evaluated (only phone interview); ** 3 patients not evaluated with KT-1000/2000 because they had contralateral ACL injury; ††1 patient not objectively evaluated because they had rerevision; ACL = anterior cruciate ligament; IKDC = International Knee Documentation Committee; NA = not applicable.
Complications
Overall, nine studies reported at least one complication. Major complications such as patellar fracture and patellar tendon rupture were reported in only one series when bone-patellar tendon-bone harvesting was performed. Two series reported a wound or superficial infection. Five of 16 series reported some degree of ROM loss, extension deficit, or flexion deficit, ranging from 2% to 50%. Donor site morbidity (pain or hypoesthesia) was reported in five series, ranging from 7% to 57%.
Overall, nine studies reported at least one reoperation; excluding repeated revisions, three series reported hardware removal in 5% to 12% of patients, and four series reported arthroscopic treatment for débridement or meniscectomies in 6% to 10% of patients. Arthrolysis was performed in only 2% of patients from a single series.
Discussion
As a result of the large number of primary ACL reconstructions and a risk of failure of those reconstructions approaching 8% [1, 5, 10, 13, 18], revision ACL reconstruction represents a topic of ongoing interest. However, because much of what we know comes from small and heterogeneous case series [30], the risk of reruptures, persistent instability, and poor knee scores remains somewhat ill defined. The present study found that the risk of graft rerupture at mean 5 years after surgery was < 5% in 12 of the 16 case series of revision ACL reconstruction in this systematic review (from 0% to 25%). However, when additionally considering the sum of reruptures and objective clinical failures, here termed cumulative failure, the proportion of failed revision ACL reconstruction rose to more than 20% in one-third of the included series (from 0% to 83%).
The present study has, however, some important limitations, similar to other studies of this design [5]. Differential losses to followup may have produced selection bias, because the total number of patients evaluated for reruptures (716) was slightly larger than the patients evaluated objectively (678). Nevertheless, the latter still represents almost 95% of the number of patients evaluated for reruptures, which minimizes the likelihood of bias. Also, as a result of strict inclusion criteria and the paucity of studies available, our cumulative failure incidence did not account for other functional or subjective outcome measures such as Lysholm, Tegner, or subjective IKDC scores. These were excluded from the current study as a result of inconsistent reporting. Also, there was variability in laxity measurements (instrumented versus manual) and blinding of the examiner, which could have caused detection bias. Finally, the presence of high heterogeneity regarding patients’ demographic characteristics and surgical techniques does not facilitate comparison within the different studies and the generalizability of the findings. Of note, there were also inconsistencies in the reporting of important surgical variables such as femoral tunnel drilling technique and location.
The proportion of reruptures after revision ACL reconstruction was < 5% in the majority of the studies in this review and 0% in eight of 16 series. These results do not substantially differ from those of registries and high-volume centers. The proportion of reruptures in the 1205 patients from the Multicenter ACL Revision Study at 2 years (MARS) [26] and in the 448 patients from a single institution reported by Schlumberger et al. [24] were 3.3% and 2%, respectively. Notably, the latter authors did not find a difference in this outcome compared with 2467 primary ACL reconstructions performed in the same institution (2.0% versus 3.0%) [24].
The objective clinical failure of revision ACL reconstruction reported in the present study was instead generally higher than the one reported in the review for primary ACL reconstruction by Crawford et al. (14 studies including almost 3000 patients) despite their followup of > 10 years [5]. These findings are in keeping with a recent meta-analysis of controlled studies comparing the outcomes of primary versus revision ACL reconstruction, in which revision reconstructions were twice as likely to be classified as abnormal according to the objective IKDC criteria [10]. The increased incidence of abnormal knees could be the result of the morbidity of having multiple operations. Studies have shown increased presence of meniscal pathology in revision ACL procedures [21, 27, 28], which may lead to higher degrees of osteoarthritis. In fact, it has been estimated that up to 60% of patients undergoing revision ACL reconstructions will develop knee osteoarthritis in approximately 6 years [11]. This is nearly double the risk compared with primary reconstructions [10]. Certainly the swelling and stiffness caused by a degenerative knee condition could account for worse IKDC scores as well. In addition, revision operations are often associated with multiple graft harvests, increased stiffness, and increased iatrogenic joint damage that all contribute to increased morbidity.
Despite a proportion of reruptures < 5% in 12 of 16 series, the results are decidedly worse when considering cumulative failures, here defined as reruptures and objective clinical failures combined. In fact, 12 of 16 series of patients in our systematic review reported a cumulative failure incidence > 10%, and five of 16 series reported this proportion to exceed 20%. Wright et al. [30] in a systematic review in 2012 reported a failure of the revision procedure in 13.7% of the 863 analyzed patients; however, this study did not include abnormal objective IKDC as an objective failure mode. Moreover, the authors of that study did not separately report the proportion of reruptures or the number of patients considered abnormal for each parameter nor was there a restriction based on mean followup. This could explain the generally lower failures compared with the present study. In the present systematic review, the objective clinical failure included an overall objective IKDC score of C or D in accordance with the criteria set by Crawford et al. [5]. The latter authors, evaluating the failures of primary ACL reconstruction with a minimum 10-year followup [5], showed that the proportion of failures almost doubled (6.2%–11.9%) after adding other objective failure criteria to reruptures as overall criteria for failure. Like in the present study, the authors highlighted the underestimation of primary ACL reconstruction failure when only reruptures are considered. Therefore, we suggest a comprehensive definition of failure based on rerupture and objective outcomes such as AP laxity, pivot shift, and objective IKDC score. Whether patient-reported and subjective scores evaluating knee function, level of activity, satisfaction, and pain could contribute to the definition of failure should be the matter of future studies.
As a result of inconsistencies in defining and reporting reoperations and complications, it was not possible to pool complication results in a systematic manner without incurring bias. However, we observed only two major complications in these series: one patellar fracture and one patellar tendon rupture after bone-patellar tendon-bone harvesting [16]. The authors advised against harvesting a bone-patellar tendon-bone autograft in a revision setting. ROM deficit and donor site pathology were reported in up to 50% and 57%, respectively, in some series [19, 23]. As previously mentioned, these parameters could certainly contribute to a high number of abnormal knees on IKDC scoring.
The results of the present systematic review show that generally < 5% of patients who undergo revision ACL reconstruction experience graft rerupture at a mean followup of at least 5 years. However, this likely represents an underestimation of the real proportion of failures when additionally considering the objective clinical failure criteria used in this review. A comprehensive definition of ACL reconstruction failure should be used to report outcomes to allow realistic expectations and eventual comparison among different series and reports. Care should be used when counseling patients regarding expectations after revision ACL reconstruction, because this systematic review indicates less than satisfactory objective clinical outcomes with abnormal knees in up to one-third of patients.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This work was performed at the Department of Orthopaedic Surgery, Duke Sports Sciences Institute, Duke University Medical Center, Durham, NC, USA.
References
- 1.Ahldén M, Samuelsson K, Sernert N, Forssblad M, Karlsson J, Kartus J. The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. Am J Sports Med. 2012;40:2230–2235. doi: 10.1177/0363546512457348. [DOI] [PubMed] [Google Scholar]
- 2.Ahn JH, Lee YS, Chang MJ, Yim HS. Analysis of revision anterior cruciate ligament reconstruction according to the combined injury, degenerative change, and MRI findings. Knee. 2011;18:382–386. doi: 10.1016/j.knee.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia MJ, Cordasco FA, Hannafin JA, Rodeo SA, O’Brien SJ, Altchek DW, Cavanaugh J, Wickiewicz TL, Warren RF. Results of revision anterior cruciate ligament surgery. Am J Sports Med. 2007;35:2057–2066. doi: 10.1177/0363546507307391. [DOI] [PubMed] [Google Scholar]
- 4.Chougule S, Tselentakis G, Stefan S, Stefanakis G. Revision of failed anterior cruciate ligament reconstruction with quadrupled semitendinosus allograft: intermediate-term outcome. Eur J Orthop Surg Traumatol. 2015;25:515–523. doi: 10.1007/s00590-014-1549-2. [DOI] [PubMed] [Google Scholar]
- 5.Crawford SN, Waterman MBR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29:1566–1571. doi: 10.1016/j.arthro.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36:851–860. doi: 10.1177/0363546507312381. [DOI] [PubMed] [Google Scholar]
- 7.Ferretti A, Conteduca F, Monaco E, De Carli A, D’Arrigo C. Revision anterior cruciate ligament reconstruction with doubled semitendinosus and gracilis tendons and lateral extra-articular reconstruction. J Bone Joint Surg Am. 2006;88:2373–2379. doi: 10.2106/JBJS.F.00064. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi F, Papalia R, Del Buono A, Zampogna B, Diaz Balzani L, Maffulli N, Denaro V. Two-stage procedure in anterior cruciate ligament revision surgery: a five-year follow-up prospective study. Int Orthop. 2013;37:1369–1374. doi: 10.1007/s00264-013-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gifstad T, Drogset J, Viset A, Grøntvedt T, Hortemo G. Inferior results after revision ACL reconstructions: a comparison with primary ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2013;21:2011–2018. doi: 10.1007/s00167-012-2336-4. [DOI] [PubMed] [Google Scholar]
- 10.Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50:716–724. doi: 10.1136/bjsports-2015-094948. [DOI] [PubMed] [Google Scholar]
- 11.Grassi A, Zaffagnini S, Marcheggiani Muccioli GM, Roberti Di Sarsina T, Urrizola Barrientos F, Marcacci M. Revision anterior cruciate ligament reconstruction does not prevent progression in one out of five patients of osteoarthritis: a meta-analysis of prevalence and progression of osteoarthritis. J ISAKOS. 2016;1:16–24. doi: 10.1136/jisakos-2015-000029. [DOI] [Google Scholar]
- 12.Grossman MG, ElAttrache NS, Shields CL, Glousman RE. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21:418–423. doi: 10.1016/j.arthro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Hettrich CM, Dunn WR, Reinke EK, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41:1534–1540. doi: 10.1177/0363546513490277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kievit AJ, Jonkers FJ, Barentsz JH, Blankevoort L. A cross-sectional study comparing the rates of osteoarthritis, laxity, and quality of life in primary and revision anterior cruciate ligament reconstructions. Arthroscopy. 2013;29:898. doi: 10.1016/j.arthro.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Leroux T, Wasserstein D, Dwyer T, Ogilvie-Harris DJ, Marks PH, Bach BR, Townley JB, Mahomed N, Chanhal J. The epidemiology of revision anterior cruciate ligament reconstruction in Ontario. Canada. Am J Sports Med. 2014;42:2666–2672. doi: 10.1177/0363546514548165. [DOI] [PubMed] [Google Scholar]
- 16.Lidén M, Ejerhed L, Sernert N, Bovaller Å, Karlsson J, Kartus J. The course of the patellar tendon after reharvesting its central third for ACL revision surgery: a long-term clinical and radiographic study. Knee Surg Sports Traumatol Arthrosc. 2006;14:1130–1138. doi: 10.1007/s00167-006-0167-x. [DOI] [PubMed] [Google Scholar]
- 17.Lind M, Lund B, Faunø P, Said S, Miller L, Christiansen S. Medium to long-term follow-up after ACL revision. Knee Surg Sports Traumatol Arthrosc. 2012;20:166–172. doi: 10.1007/s00167-011-1629-3. [DOI] [PubMed] [Google Scholar]
- 18.Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91:2321–2328. doi: 10.2106/JBJS.H.00539. [DOI] [PubMed] [Google Scholar]
- 19.Mayr H, Willkomm D, Stoehr A, Schettle M, Suedkamp N, Bernstein A, Hube R. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132:867–874. doi: 10.1007/s00402-012-1481-z. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niki Y, Matsumoto H, Enomoto H, Toyama Y, Suda Y. Single-stage anterior cruciate ligament revision with bone–patellar tendon–bone: a case-control series of revision of failed synthetic anterior cruciate ligament reconstructions. Arthroscopy. 2010;26:1058–1065. doi: 10.1016/j.arthro.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 22.O’Neill DB. Revision arthroscopically assisted anterior cruciate ligament reconstruction with previously unharvested ipsilateral autografts. Am J Sports Med. 2004;32:1833–1841. doi: 10.1177/0363546504264585. [DOI] [PubMed] [Google Scholar]
- 23.Salmon LJ, Pinczewski LA, Russell VJ, Refshauge K. Revision anterior cruciate ligament reconstruction with hamstring tendon autograft: 5- to 9-year follow-up. Am J Sports Med. 2006;34:1604–1614. doi: 10.1177/0363546506288015. [DOI] [PubMed] [Google Scholar]
- 24.Schlumberger M, Schuster P, Schulz M, Immendörfer M, Mayer P, Bartholomä J, Richter J. Traumatic graft rupture after primary and revision anterior cruciate ligament reconstruction: retrospective analysis of incidence and risk factors in 2915 cases. Knee Surg Sports Traumatol Arthrosc. 2015 Sep 26. [Epub ahead of print] [DOI] [PubMed]
- 25.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 26.The MARS Group MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2014;42:2301–2310. doi: 10.1177/0363546514549005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas NP, Kankate R, Wandless F, Pandit H. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;33:1701–1709. doi: 10.1177/0363546505276759. [DOI] [PubMed] [Google Scholar]
- 28.Trojani C, Sbihi A, Djian P, Potel J-F, Hulet C, Jouve F, Bussiere C, Ehkirch FP, Burdin G, Dubrana F, Beaufils P, Franceschi JP, Chassing V, Colombet P, Neyret P. Causes for failure of ACL reconstruction and influence of meniscectomies after revision. Knee Surg Sports Traumatol Arthrosc. 2011;19:196. doi: 10.1007/s00167-010-1201-6. [DOI] [PubMed] [Google Scholar]
- 29.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the journal. J Bone Joint Surg Am. 2003;85:1–3. doi: 10.2106/00004623-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Wright RW, Gill CS, Chen L, Brophy RH, Matava MJ, Smith MV, Mall NA. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94:531–536. doi: 10.2106/JBJS.K.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]


