Abstract
Background
There are several options for reconstruction of proximal humerus resections after wide resection for malignant tumors in children. The clavicula pro humero technique is a biologic option that has been used in the past, but there are only scant case reports and small series that comment on the results of the procedure. Because the longevity of children mandates a reconstruction with potential longevity not likely to be achieved by other techniques, the clavicula pro humero technique may be a potential option in selected patients.
Questions/purposes
(1) How successful is the clavicula pro humero procedure in achieving local tumor control? (2) What is the frequency of nonunion? (3) What are the complications of the procedure? (4) What scores do patients achieve (on the Musculoskeletal Tumor Society (MSTS) and the Toronto Extremity Salvage Score (TESS) after this procedure?
Methods
Four university hospitals performed the clavicula pro humero technique in eight children aged 8 to 18 years between June 2006 and February 2014. During that period, general indications for this approach included all reconstructions of the proximal humerus for malignant tumors in children older than 8 years. All patients were followed for a mean of 40 months (range, 25–86 months); one patient was lost to followup before 2 years. The tumor resections removed the rotator cuff muscles in all patients, glenohumeral joint in five, and deltoid muscle in three. The median length of the bone defect after resection was 20 cm (range, 7–25 cm). It was reduced to 9 cm (range, 0–17 cm) or 27% (range, 0%–64%) of the total humerus length after clavicular rotation. Direct osteosynthesis (one patient), induced membrane technique (one patient), or vascularized fibular autograft (six patients) was used to complete the defect after rotation of the clavicle if necessary. Presence of union (defined as bone healing before 10 months, as assessed by disappearance of the osteotomy on AP and lateral view radiographs), and complications were determined by chart review performed by a surgeon not involved in patient care. Function assessed by the MSTS and the TESS scores were determined by the patients with their families.
Results
None of the patients had tumor recurrence. One patient died of pulmonary metastases before the 2-year followup. Proximal and distal bone unions were achieved before 10 months without an additional surgical procedure in two and six of seven patients, respectively. Fourteen local complications occurred resulting in nine revision operations. The main complication was aseptic proximal pseudarthrosis (five patients); other complications included one proximal junction fracture, one clavicle fracture complicated by clavicle osteolysis, one distal junction fracture, one necrosis of the skin paddle of the fibular autograft, one glenoclavicular ossification, and one distal pseudarthrosis complicated by a fracture of this distal junction. Function, as assessed by the MSTS score, was a median of 23 of 30 (range, 11–27). The median TESS score was 82% (range, 75%–92%). Shoulder ROM (median; range) in abduction, front elevation, and external and internal rotations were 70°(30°–90°), 75°(30°–85°), 10°(0°–20°), and 80°(80°–100°), respectively. Three of the seven patients reported dissatisfaction with the cosmetic appearance.
Conclusions
The clavicula pro humero technique achieved oncologic local control after resection and reconstruction of proximal humerus tumors in children. Although union times are approximately 2 years and some patients underwent augmentation with other grafts, it eventually provides a solid, painless, biologic, and stable reconstruction and creates a mobile acromioclavicular joint and generally good function. Nonunion of the proximal junction is the main complication of this technique. We cannot directly compare this technique with other reconstruction options, and longer followup is needed, but this may be a useful reconstruction option to consider in select pediatric patients with sarcomas of the proximal humerus.
Level of Evidence
Level IV, therapeutic study.
Introduction
The proximal humerus is the third-most-common location of osteosarcoma after the distal femoral metaphysis and the proximal tibial metaphysis [36]. Osteosarcoma occurs most commonly in children and adolescents, and is the most-common primary malignant tumor in the pediatric age group excluding hematopoietic tumors [36]. The survival of patients has improved to approximately 70% since the 1970s owing to the development of chemotherapy and because reconstructive surgery is no longer “limb-salvage surgery” but has become “functional reconstructive surgery” [3, 31]. Amputation of the upper limb is very disfiguring and provides limited function and a disappointing cosmetic appearance, regardless whether the patient uses an orthosis. However, the choice of reconstructive procedure after extensive resection of a malignant tumor removing muscular or skeletal architecture of the glenohumeral joint remains controversial [17, 22, 30, 37]. In addition to removing the proximal humerus and sometimes part of the scapula, tumor invasion often results in sacrifice of stabilizing elements of the shoulder such as the rotator cuff, all or part of the deltoid muscle, and the axillary nerve [13, 30].
Traditional methods of proximal humeral reconstruction, including arthrodesis or endoprosthesis, have advantages and disadvantages, especially in children. These methods call for making a choice between stability and strength or mobility of the shoulder [17]. The goal of these reconstructions is to achieve a stable shoulder to maintain maximum function of the elbow and hand. In 1992, Winkelmann [38] reported on the clavicula pro humero technique in this location. This method, first described by Sulamaa [34] in 1963 in patients with phocomelia, uses the ipsilateral clavicle to reconstruct the proximal humerus. The clavicle is cut into its medial third and returned through a lateral pivot point corresponding to the acromioclavicular joint (Fig. 1A), allowing verticalization of the clavicular segment (Fig. 1B). Osteosynthesis of the distal humeral segment then could be performed directly or through an interposed graft if a bone defect persists after clavicle rotation. This technique allows for maintenance of upper limb length, with growth potential of the lateral growth plate of the clavicle, stability of the “new shoulder,” and its mobility. The largest series of which we are aware included four patients [6]. That being so, we felt it important to evaluate the technique in a larger group.
Fig. 1A–B.
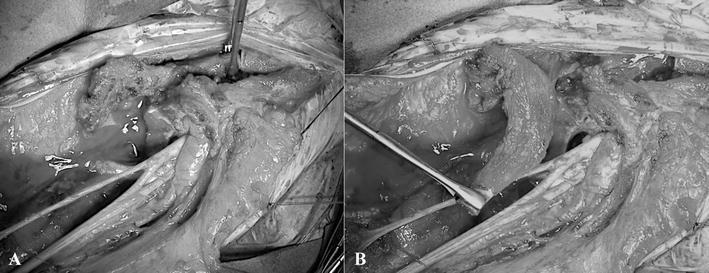
These intraoperative views show (A) the clavicle in anatomic position and (B) its verticalization through a lateral pivot point corresponding to the acromioclavicular joint after clavicle osteotomy on its medial third.
We asked: (1) How successful is the procedure in achieving local tumor control? (2) What is the frequency of nonunion? (3) What are the complications of the procedure? (4) What is the function of patients after this procedure?
Patients and Methods
We report a retrospective multicenter series of reconstructions of the proximal humerus, using the clavicula pro humero technique described by Sulamaa [34], after tumor resections of osteosarcoma in children, at four university hospitals between June 2006 and February 2014.
All patients with an osteoblastic osteosarcoma of the proximal humerus confirmed by biopsy and between 8 to 18 years old (mean, 13 years; SD, 3.5 years) were treated by the clavicula pro humero technique using various interposition systems if the residual bone defect was substantial. These eight patients (two boys, six girls) were included for descriptions of the technique and the different interposition systems. The nondominant arm was involved in four of the eight patients. The lesion was discovered during assessment of mechanical pain of the shoulder present for a median of 2 months, with a history of trauma in 50% of the patients. In one patient the diagnosis was made because of shoulder pain for 2 months owing to a pathologic fracture of the greater tuberosity. In two other patients, a pathologic fracture led to the diagnosis. In one patient, an asymptomatic proximal humeral osteosarcoma was discovered during staging of a femoral osteosarcoma. One of these eight patients died early after surgery owing to the general pathologic features of his tumor, making analysis of his functional results impossible; this patient has been included in the description of the reconstruction but excluded from the results. The median radiologic and clinical followup was 32 months (range, 25–86 months).
Resection
The median bony tumor invasion was 60% (range, 25%–90%) of the length of the humerus. The tumor extended to involve the glenoid in five patients. Patients were staged with chest CT scan, total body PET scan, and humerus MRI. Metastatic lesions to the lung were noted in two of eight patients. All patients received neoadjuvant chemotherapy using a protocol described by Piperno-Neumann et al. [25]. Pathologic specimens were assessed postresection; there were five good responders with less than 5% viable cells and three poor responders with 30%, 25%, and 40% viable cells after neoadjuvant chemotherapy according to the Huvos histologic classification as described by Juergens et al. [16].
Tumor resection removed the glenohumeral joint in five patients. Proximally, we used a deltopectoral surgical approach that extended horizontally along the clavicle in all patients. Distally, we used an anteromedial incision in five patients, and an anterolateral approach in three.
The biopsy tract was resected with the tumor in continuity with the resected specimen. No arthrotomy was used in the five patients who underwent glenohumeral joint resection. The extent of the muscle resection (Table 1) was classified according to the system proposed by Malawer et al. [18] and the Musculoskeletal Tumor Society (MSTS) system [13]. The rotator cuff muscles were sacrificed in all patients whereas the deltoid muscle was sacrificed in three of the eight patients. The axillary nerve was sacrificed in two patients, the musculocutaneous nerve was divided and sutured to the axillary nerve in one patient, and the radial nerve was resected with the specimen and reconstructed in one patient with a sural nerve graft.
Table 1.
Extent of muscle resection
| Patient number | Surgical approach | Glenohumeral joint resection | Rotator cuff | Deltoid | Long biceps | Triceps | Pectoral | Coracobrachial, latissimus dorsi, teres major |
Nerve | Malawer type | MSTS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Anteromedial | No | Complete resection | P1 | RB | RT | RP | RL | – | IB | S3,4,5 |
| 2 | Anteromedial | Yes | Complete resection | P1 | Complete resection | RT | RP | RL | – | VB | S2,3,4,5 |
| 3 | Anteromedial | Yes | Complete resection | Complete resection | Complete resection | Complete resection | RP | Complete resection | Partial musculocutaneous | VB | S2,3,4,5 |
| 4 | Anteromedial | Yes | Complete resection | Complete resection | RB | Complete resection | RP | Complete resection | Radial | VB | S2,3,4,5,E1 |
| 5 | Anteroateral | Yes | Complete resection | Complete resection | RB | Complete resection | RP | Complete resection | Axillary | VB | S2,3,4,5 |
| 6 | Anteroateral | No | Complete resection | P2 | RB | RT | RP | RL | Axillary | IB | S3,4,5 |
| 7 | Anteroateral | Yes | Complete resection | P3 | RB | Complete resection | RP | Complete resection | – | VA | S2,3,4,5 |
| 8 | Anteromedial | No | Complete resection | P3 | Complete resection | RT | RP | RL | – | IA | S3,4 |
Malawer = Malawer classification of the of shoulder girdle resections [18]; MSTS = Musculoskeletal Tumor Society; P = partial resection; R = reinsertion; P1 = intact posterior bundle; P2 = resection of ¾; P3 = resection of ¼; RB = reinsertion long biceps on short biceps muscle; RT = reinsertion triceps on deltoid muscle; RP = reinsertion pectoral muscle on clavicle; RL = reinsertion latissimus dorsi on acromion; I = intraarticular proximal humeral resection; V = extraarticular humeral and glenoid resection; A = absence of resection of abductors; B = resection of abductors; MSTS = Musculoskeletal Tumor Society classification of skeletal resections about the shoulder girdle [13]: S2 = glenoid resection; 3 = head of the humerus; 4 = proximal metaphysis of the humerus; 5 = shaft of the humerus; E1 = distal metaphysis of the humerus.
Reconstruction
The median length of the bone defect after resection was 20 cm (range, 7–25 cm). After osteotomy and verticalization of the lateral part of the clavicle, bleeding of the clavicular extremity was present in all patients, confirming that the clavicle was still viable after rotation. The median length of the clavicle used was 9 cm (range, 7–12 cm). The median length of the bone defect after clavicular osteotomy and rotation of the lateral clavicular segment was 9 cm (range, 0–17 cm), or 28% (range, 0%–64%) of the total length of the humerus. The fixation techniques used for the two segments, the clavicle and distal part of the humerus, were variable: an induced membrane technique was used in one patient to fill a 12-cm bone defect (Fig. 2) [19], a vascularized fibular autograft bone was used in six patients (Fig. 3), and a direct osteosynthesis without interposition material was used in one patient (Fig. 4) (Table 2). All osteosyntheses were made using a bone plate with compression of the osteotomy areas.
Fig. 2A–D.
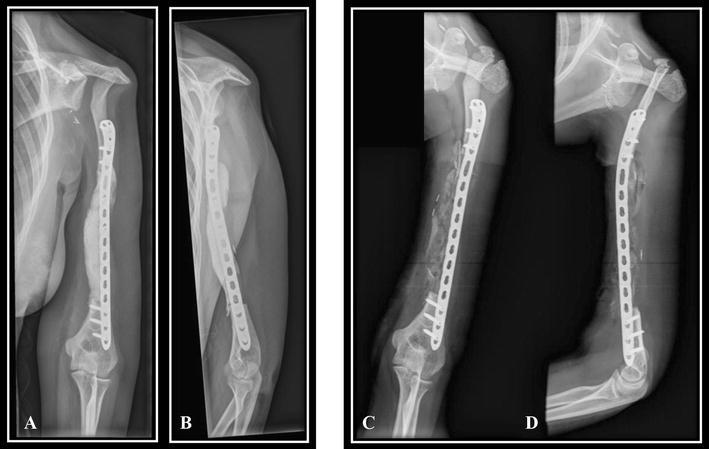
In Patient 1, the reconstruction used an induced membrane technique [19] after resection of 70% of the total length of the humerus and rotation of the clavicle. This technique needs two steps shown on these postoperative radiographs: Step 1 is filling the gap with bone cement to induce a membrane as shown on these (A) lateral and (B) AP radiographs; and Step 2 is iliac bone grafting after removal of the cement, as shown on these (C) AP and (D) lateral radiographs.
Fig. 3A–B.

In Patient 3, the reconstruction used vascularized fibular autograft after resection of 60% of the total length of the humerus and rotation of the clavicle as shown on these (A) lateral and (B) AP radiographs.
Fig. 4.
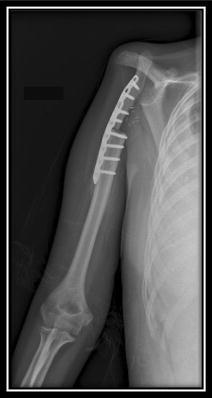
In Patient 8, the reconstruction did not use interposition material after resection of 7 cm of the total length of the humerus and rotation of the clavicle because the length of the clavicle was sufficient to fill the bone defect.
Table 2.
Characteristics of the reconstruction
| Patient number | Length of the bone defect (cm) | Length of clavicular segment (cm) | Interposition material | Associated procedures |
|---|---|---|---|---|
| 1 | 23 | 11 | Induced membrane technique | None |
| 2 | 17 | 12 | VF, 7 cm | None |
| 3 | 24 | 11 | VF, 13 cm | Nerve suture: musculocutaneous |
| 4 | 24 | 7 | VF, 17 cm | Nerve autograft: sural on radial |
| 5 | 14 | 9 | VF, 5 cm | None |
| 6 | 25 | 8 | VF, 17 cm | None |
| 7 | 12 | 7.5 | VF, 5 cm | None |
| 8 | 7 | 7 | None | None |
VF = vascularized fibular autograft.
Aftercare
After surgery, a simple shoulder splint or simple arm sling immobilized the surgically treated upper limb during 3 weeks in all patients except one who wore it for 3 months because he was afraid to remove it. Passive and active rehabilitation was started between 1 and 3 months postoperatively. The surgical procedure and its aftercare have not modified the timing of the chemotherapy protocol that continued from the 15th postoperative day in all patients: no complication delayed this medical treatment.
We evaluated AP and lateral view radiographs at various intervals postoperatively. The time to proximal and distal bone nonunion was defined by observation of the osteotomy after 10 months followup. We chose 10 months, which is longer than the usual time defined for pseudarthrosis, because these patients were being treated with chemotherapy which increases the time to bony healing. Complications such as infection, frame breakage, bone healing issues, fracture, and others, and their management for seven of the eight patients were determined by chart review performed by a surgeon (DB) not involved in patient care. One patient (Patient 8) died of metastases 3 months after surgery. At the final followup, a surgeon not involved in patient care (DB) determined ROM of the shoulder (abduction, internal rotation, external rotation, front elevation), and of the elbow (flexion and extension). Function of the upper limb according to the MSTS and TESS scores was determined by the patient with his or her family [9, 14].
This study was performed in accordance with national ethical guidelines from the committee for clinical research in humans and the Declaration of Helsinki revised in 2000.
Results
None of the patients had local tumor recurrence. One patient died of pulmonary metastases, absent at initial diagnosis (Patient 8) at 3 months followup, making analysis of bone union, complications, and his functional results impossible.
Bone healing in the proximal osteosynthesis site was achieved before 10 months in two of the seven patients. In one patient, consolidation was noted before 6 months followup. Among these seven patients, five had a proximal pseudarthrosis (Fig. 5), and four of these patients were treated with revision surgery. Consolidation was obtained in these patients 3, 2, 19, and 3 months after resection of the pseudarthrosis and bone grafting. The fifth patient with a proximal pseudarthrosis refused additional surgery. Six of the seven patients achieved bone healing at the distal site before 10 months. The only patient with distal pseudarthrosis was treated with a resection and bone grafting of the nonunion zone. The osteosynthesis device was removed in one patient 6 years after surgery.
Fig. 5A–B.
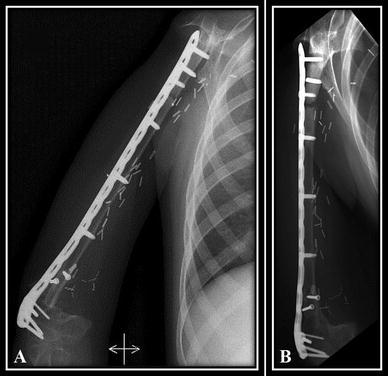
Five of our patients had aseptic proximal junction pseudarthrosis as shown on the (A) AP and (B) lateral radiographs of the shoulder for Patient 4.
We recorded 14 local complications in five of the seven patients (Table 3), including the five proximal pseudarthroses and the distal pseudarthrosis described above. Nine of the complications were treated surgically. Among these complications were one cosmetic revision surgery for a skin paddle used to monitor the vascularized fibular graft (Patient 3); one deep infection at 4 years followup treated successfully with débridement, retention of the hardware, and antibiotics; one progressive proximal clavicular osteolysis visible on radiographs 6 months after concomitant surgical treatment of a proximal nonunion and a clavicular fracture (Fig. 6); one fracture of the distal junction (vascularized fibula/humerus) during surgical treatment of a proximal pseudarthrosis; and one glenoclavicular arthrodesis (Fig. 7).
Table 3.
Results of consolidation and complications
| Patient number | Radiologic followup (months) | Proximal consolidation (months) | Distal consolidation (months) | Complications | Delay before complications (months) | Revision surgery |
|---|---|---|---|---|---|---|
| 1 | 31 | 15 | 6 | Proximal pseudarthrosis | 10 | None |
| Clavicle fracture | 12 | Resection, bone grafting, osteosynthesis | ||||
| Proximal clavicle osteolysis | 18 | None | ||||
| 2 | 38 | 12 | 9 | Proximal pseudarthrosis | 10 | Resection, bone grafting, osteosynthesis |
| Distal junction fracture | 10 | Osteosynthesis of the fracture by plate | ||||
| 3 | 30 | 2 | 2 | None | None | Skin paddle monitoring of vascularized fibular flap excision for esthetic reason |
| 4 | 86 | 52 | 8 | Proximal pseudarthrosis | 10 | None |
| Proximal posttraumatic frame breakage | 33 | Resection, bone grafting, osteosynthesis | ||||
| Failure to heal | 76 | Osteosynthesis removal | ||||
| 5 | 32 | None | 3 | Tight proximal pseudarthrosis | 18 | None |
| 6 | 25 | 17 | 3 | None | None | None |
| 7 | 38 | 18 | 18 | Necrosis of the skin paddle flap | 2 | None |
| Glenoclavicular ossification | 2 | None | ||||
| Proximal and distal pseudarthrosis with | 15 | Resection, bone grafting, osteosynthesis | ||||
| frame breakage |
Fig. 6A–B.
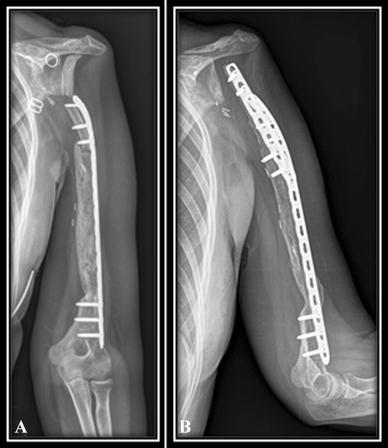
Osteolysis of the proximal part of the rotated clavicle of Patient 1 (reconstruction by clavicula pro humero technique and the induced membrane technique) appeared at 6 months after posttraumatic fracture, as shown on these (A) AP and (B) lateral radiographs.
Fig. 7.
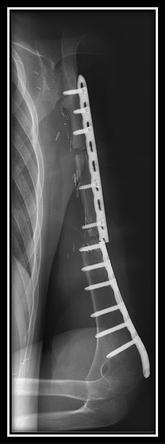
In Patient 7 an unexpected glenoclavicular ossification occurred at 2 months after reconstruction of the proximal humerus by clavicula pro humero technique.
The median MSTS upper limb rating system score, performed in seven patients with a median clinical followup of 40 months (range, 25–86 months), was 23 of 30 (range, 11–27; 77%) (Table 4). The median TESS score was 82% (range, 75–92) (Table 4). Concerning active shoulder motion after the clavicula pro humero technique (Fig. 8A), the median abduction was 70° (range, 30°–90°) (Fig. 8B); front elevation was 75° (range, 30°–85°) (Fig. 8C), with achievable “hand to mouth function” (Fig. 8D); external rotation was 10° (range, 0°–20°) (Fig. 8E); and internal rotation was 80° (range, 80°–100°) (Fig. 8F), with achievable “hand to contralateral shoulder” (Fig. 8G) and “hand to lumbar area” functions; and possible use of a keyboard (Fig. 8H). Elbow flexion varied from 110° to 160° with a median of 140°, and a 20°-extension deficit in one patient. The primary reason for dissatisfaction was the appearance of the shoulder for three of the seven patients.
Table 4.
Results for function
| Patient number | Clinical followup (months) | MSTS (/30) | TESS (%) | ABD (°) | R (°) | IR (°) | Front elevation (°) | Elbow ROM* (°) | Patient dissatisfaction |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | 19 | 81 | 30 | 20 | 90-T4 | 80 | 135/0 | No |
| 2 | 38 | 11 | 75 | 90 | 20 | 80-T7 | 75 | 140/0 | Cosmetic appearance |
| 3 | 30 | 20 | 77 | 90 | 20 | 80-T7 | 75 | 140/0 | No |
| 4 | 86 | 23 | 82 | 70 | 0 | 80-L4 | 70 | 110/−20 | Cosmetic appearance |
| 5 | 32 | 26 | 87 | 30 | 0 | 80-T4 | 30 | 135/0 | Brushing his hair, cosmetic appearance |
| 6 | 25 | 27 | 92 | 50 | 10 | 100-T7 | 40 | 140/0 | No |
| 7 | 38 | 26 | 85 | 85 | 15 | 90-L4 | 90 | 160/0 | Fear of a humerus fracture therefore sports restriction |
Fig. 8A–H.

Clavicula pro humero reconstruction of the shoulder shows (A) the at-rest position, (B) active abduction, (C) forward flexion, (D) hand-to-mouth function, (E) external rotation, (F) internal rotation, (G) hand-to-contralateral shoulder position, and (H) use of a keyboard.
Discussion
The choice of the technique in proximal humerus reconstruction after oncologic resection in children is complicated because of the extent of muscle resection, the need for stable reconstruction, and the goal of maximum mobility and sustainability, introducing the concept of fully biological reconstruction of the growing limb for young patients with the potential for long-term survival. Many techniques such as arthrodesis [4, 15, 24], vascularized fibular epiphysis transfer [20, 23], and endoprostheses [1, 2, 5, 10–12, 26, 29, 37] have been described in the adult population and some of these studies include pediatric patients, but the small number of patients makes comparison of different techniques difficult. There is no apparent functional benefit of one type of reconstruction compared with the others that have been described in the adult population [17, 20, 23, 30]. We showed that the clavicula pro humero technique is an alternative that provides shoulder stability, and likely a durable reconstruction option, for pediatric patients with shoulder reconstruction without local tumor recurrence in our series.
The main limitation of our study was the small number of patients owing to the low frequency of this disorder, but it is an exclusively pediatric series and the largest series of clavicula pro humero in children, of which we are aware. A second major limitation was the inclusion of eight patients with different tumoral extension which was treated by an extraarticular resection in five patients and sacrifice of the deltoid and axillary nerves in three patients. When the glenoid and abductor apparatus are preserved, other reconstructive techniques may be used successfully in children after proximal humerus resection. After extraarticular resection in children, a clavicula pro humero procedure may provide an advantage in stability and function. Function can be different when the abductor apparatus is retained or sacrificed and the results should be compared with those for shoulder arthrodesis with bone grafts. Unfortunately the small numbers of patients do not allow such analysis. The followup is another limitation. The followup is short, a median of 3.3 years, and does not allow us to comment on the durability of these results in terms of growth and wear of the acromioclavicular joint. Kitagawa et al. [17] reported breakage of the acromioclavicular ligament after the clavicula pro humero procedure in a 64-year-old patient. In our patients, different muscle and nerve resections were performed and the patients were treated using different reconstruction methods such as neurorraphy or nerve graft (Table 1). This variability creates an inhomogeneous population. We also used several types of bone augmentation such as vascularized fibular grafts and a membrane technique. This heterogeneity of approach makes it difficult to generalize this technique for this site; however, common to all patients was the use of the acromioclavicular joint to stabilize the shoulder.
We obtained local tumor control in all of our patients, without recurrence at last followup of 3 years on average. This suggests that this technique provides adequate local control and is similar to local control reported by Calvert et al. [6] for four patients and by Nishida et al. [21] for two patients. Kitagawa et al. [17] described recurrence for one patient among 15 claviculo pro humero reconstructions in children and adults. Reports of other types of reconstructions have noted local recurrence in 0% to 7% of patients for endoprosthetic reconstructions [12, 26] or arthrodesis [15, 24]. We cannot conclude that this technique offers better tumor control because larger numbers of patients and longer followup are necessary to make this assertion, but our results are encouraging.
In our patients, proximal nonunion was noted in five of seven patients and distal nonunion was noted in one patient with adequate followup. Five of these patients were treated with revision surgery and bone grafting. Nonunion in patients who undergo reconstructive arthrodesis procedures is not rare, with reported rates ranging from 4% to 12% [7, 8, 27, 28, 32, 33]. The main complication with this technique is the pseudarthrosis at the proximal junction of the clavicle and the interposition device [6, 30]. In a comparative series of different reconstruction techniques of the proximal humerus after tumoral resection, Rödl et al. [30] described the clavicula pro humero technique used in 15 patients, including adults and children without distinction. Among them, four patients had a proximal pseudarthrosis develop which led the authors to use a new plate adapted to the curvature of the clavicle [30]. In a pediatric series of four patients who underwent clavicula pro humero reconstructions, Calvert et al. [6] reported two proximal pseudarthroses with the use of nonvascularized fibular bone transfers. They described failure of the pseudarthrosis treatment using a nonvascularized bone graft for their first patient. A vascularized fibular bone graft in two other patients resulted in consolidation. This suggests that during major defects with addition of an interposition graft to augment the length of the clavicular graft, the vascularization of the distal end of the clavicle may be precarious.
The median functional MSTS score for our patients was 77% (23 of 30). Our results seem similar to scores reported in other studies using the same technique, which range from 70% to 87% [17, 21, 30, 35]. These series are mixed and have only one to three pediatric patients per series. Only Calvert et al. [6] described the clavicula pro humero technique performed in four children in their series of patients younger than 10 years and reported a MSTS score between 87% and 90%. However, they recognize that this score in children is not ideal, which is why we also used the TESS score. O’Connor et al. [22] reported a MSTS score of 66% for seven patients with an arthrodesis and 52% for an endoprosthesis.
The clavicula pro humero technique is a biological procedure used for reconstruction of the proximal humerus in children after wide resection for a malignant tumor with removal of the rotator cuff and all or part of the deltoid muscle. It provides a solid and stable mount, with creation of a moving acromioclavicular joint and ROM of the scapulothoracic joint. The stability allowed by the acromioclavicular ligaments, despite loss of the stabilizer muscle of the shoulder, allows use of the elbow and hand. If necessary, augmentation of humeral length by adding vascularized fibular autografts or the membrane technique may be used according to the patient’s age, the length of the clavicular segment, and the desired humeral length. The main complication of the clavicula pro humero technique is nonunion of the distal end of the clavicle (corresponding to the proximal junction of the reconstruction), even in patients with autologous vascularized fibular interposition. Initial results are favorable in terms of function and pain. Long-term results of maintenance of these reconstructions are still needed.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Children’s Hospital, CHU Nancy, Nancy, France.
References
- 1.Abdeen A, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1):188–196. doi: 10.2106/JBJS.J.00167. [DOI] [PubMed] [Google Scholar]
- 2.Abdeen A, Hoang BH, Athanasian EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91:2406–2415. doi: 10.2106/JBJS.H.00815. [DOI] [PubMed] [Google Scholar]
- 3.Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A, Angeles C, Menendez LR. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma. 2012;2012:704872. doi: 10.1155/2012/704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin SN, Ebeid WA. Shoulder reconstruction after tumor resection by pedicled scapular crest graft. Clin Orthop Relat Res. 2002;397:133–142. doi: 10.1097/00003086-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Black AW, Szabo RM, Titelman RM. Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J Shoulder Elbow Surg. 2007;16:525–533. doi: 10.1016/j.jse.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Calvert GT, Wright J, Agarwal J, Jones KB, Randall RL. Is claviculo pro humeri of value for limb salvage of pediatric proximal humerus sarcomas? Clin Orthop Relat Res. 2015;473:877–882. doi: 10.1007/s11999-014-3814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charnley J, Houston JK. Compression arthrodesis of the shoulder. J Bone Joint Surg Br. 1964;46:614–620. [PubMed] [Google Scholar]
- 8.Clawson RS, McKay DW. Arthrodesis in the presence of infection. Clin Orthop Relat Res. 1976;114:207–208. [PubMed] [Google Scholar]
- 9.Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5:508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 10.De Wilde L, Sys G, Julien Y, Van Ovost E, Poffyn B, Trouilloud P. The reversed Delta shoulder prosthesis in reconstruction of the proximal humerus after tumour resection. Acta Orthop Belg. 2003;69:495–500. [PubMed] [Google Scholar]
- 11.De Wilde LF, Plasschaert FS, Audenaert EA, Verdonk RC. Functional recovery after a reverse prosthesis for reconstruction of the proximal humerus in tumor surgery. Clin Orthop Relat Res. 2005;430:156–162. doi: 10.1097/01.blo.0000146741.83183.18. [DOI] [PubMed] [Google Scholar]
- 12.De Wilde LF, Van Ovost E, Uyttendaele D. Verdonk R [Results of an inverted shoulder prosthesis after resection for tumor of the proximal humerus][in French] Rev Chir Orthop Réparatrice Appar Mot. 2002;88:373–378. [PubMed] [Google Scholar]
- 13.Enneking W, Dunham W, Gebhardt M, Malawar M, Pritchard D. A system for the classification of skeletal resections. Chir Organi Mov. 1990;75(1 suppl):217–240. [PubMed] [Google Scholar]
- 14.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 15.Gao YH, Ketch LL, Eladoumikdachi F, Netscher DT. Upper limb salvage with microvascular bone transfer for major long-bone segmental tumor resections. Ann Plast Surg. 2001;47:240–246. doi: 10.1097/00000637-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Juergens H, Kosloff C, Nirenberg A, Mehta BM, Huvos AG, Rosen G. Prognostic factors in the response of primary osteogenic sarcoma to preoperative chemotherapy (high-dose methotrexate with citrovorum factor) Natl Cancer Inst Monogr. 1981;56:221–226. [PubMed] [Google Scholar]
- 17.Kitagawa Y, Thai DM, Choong PF. Reconstructions of the shoulder following tumour resection. J Orthop Surg (Hong Kong). 2007;15:201–206. doi: 10.1177/230949900701500216. [DOI] [PubMed] [Google Scholar]
- 18.Malawer MM, Meller I, Dunham WK. A new surgical classification system for shoulder-girdle resections: analysis of 38 patients. Clin Orthop Relat Res. 1991;267:33–44. [PubMed] [Google Scholar]
- 19.Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft [in French] Ann Chir Plast Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 20.Medrykowski F, Barbary S, Gibert N, Lascombes P, Dautel G. Vascularized proximal fibular epiphyseal transfer: two cases. Orthop Traumatol Surg Res. 2012;98:728–732. doi: 10.1016/j.otsr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Nishida Y, Tsukushi S, Yamada Y, Kamei Y, Toriyama K, Ishiguro N. Reconstruction of the proximal humerus after extensive extraarticular resection for osteosarcoma: a report of two cases with clavicula pro humero reconstruction. Oncol Rep. 2008;20:1105–1109. [PubMed] [Google Scholar]
- 22.O’Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle: intermediate reconstructive and functional results. J Bone Joint Surg Am. 1996;78:1872–1888. doi: 10.2106/00004623-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Onoda S, Sakuraba M, Asano T, Miyamoto S, Beppu Y, Chuman H, Kawai A, Nakatani F, Kimata Y. Use of vascularized free fibular head grafts for upper limb oncologic reconstruction. Plast Reconstr Surg. 2011;127:1244–1253. doi: 10.1097/PRS.0b013e318205f34b. [DOI] [PubMed] [Google Scholar]
- 24.Padiolleau G, Marchand JB, Odri GA, Hamel A, Gouin F. Scapulo-humeral arthrodesis using a pedicled scapular pillar graft following resection of the proximal humerus. Orthop Traumatol Surg Res. 2014;100:177–181. doi: 10.1016/j.otsr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Piperno-Neumann S, Le Deley MC, Rédini F, Pacquement H, Marec-Bérard P, Petit P, Brisse H, Lervat C, Gentet JC, Entz-Werlé N, Italiano A, Corradini N, Bompas E, Penel N, Tabone MD, Gomez-Brouchet A, Guinebretière JM, Mascard E, Gouin F, Chevance A, Bonnet N, Blay JY. Brugières L; Sarcoma Group of UNICANCER; French Society of Pediatric Oncology (SFCE); French Sarcoma Group (GSF-GETO). Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17:1070–1080. doi: 10.1016/S1470-2045(16)30096-1. [DOI] [PubMed] [Google Scholar]
- 26.Raiss P, Kinkel S, Sauter U, Bruckner T, Lehner B. Replacement of the proximal humerus with MUTARS tumor endoprostheses. Eur J Surg Oncol. 2010;36:371–377. doi: 10.1016/j.ejso.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Raunio P. The role of non-prosthetic surgery in the treatment of rheumatoid arthritis by fusions and auto-arthroplasties: current practice at the Rheumatism Foundation Hospital, Heinola. Ann Chir Gynaecol Suppl. 1985;198:96–102. [PubMed] [Google Scholar]
- 28.Richards RR, Waddell JP, Hudson AR. Shoulder arthrodesis for the treatment of brachial plexus palsy. Clin Orthop Relat Res. 1985;198:250–258. [PubMed] [Google Scholar]
- 29.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10:17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 30.Rödl RW, Gosheger G, Gebert C, Lindner N, Ozaki T, Winkelmann W. Reconstruction of the proximal humerus after wide resection of tumours. J Bone Joint Surg Br. 2002;84:1004–1008. doi: 10.1302/0301-620X.84B7.12989. [DOI] [PubMed] [Google Scholar]
- 31.Rosen G. Preoperative (neoadjuvant) chemotherapy for osteogenic sarcoma: a ten year experience. Orthopedics. 1985;8:659–664. doi: 10.3928/0147-7447-19850501-19. [DOI] [PubMed] [Google Scholar]
- 32.Rouholamin E, Woottow JR, Jamieson AM. Arthrodesis of the shoulder following brachial plexus injury. Injury. 1991;22:271–274. doi: 10.1016/0020-1383(91)90004-X. [DOI] [PubMed] [Google Scholar]
- 33.Stark MD, Benett JB, Tullo HS. Rigid internal fixation for shoulder arthrodesis. Orthopedics. 1991;14:849–855. doi: 10.3928/0147-7447-19910801-08. [DOI] [PubMed] [Google Scholar]
- 34.Sulamaa M. Upper extremity phocomelia: a contribution to its operative treatment. Clin Pediatr (Phila). 1963;2:251–257. doi: 10.1177/000992286300200511. [DOI] [PubMed] [Google Scholar]
- 35.Tsukushi S, Nishida Y, Takahashi M, Ishiguro N. Clavicula pro humero reconstruction after wide resection of the proximal humerus. Clin Orthop Relat Res. 2006;447:132–137. doi: 10.1097/01.blo.0000201169.80011.ff. [DOI] [PubMed] [Google Scholar]
- 36.Unni KK, Inwards CY. Osteosarcoma. In: Unni KK, Inwards CY, editors. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases. 6. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. pp. 122–157. [Google Scholar]
- 37.van de Sande MA, Dijkstra PD, Taminiau AH. Proximal humerus reconstruction after tumour resection: biological versus endoprosthetic reconstruction. Int Orthop. 2011;35:1375–1380. doi: 10.1007/s00264-010-1152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkelmann WW. Clavicula pro humero: a new surgical method for malignant tumors of the proximal humerus][in German. Z Orthop Ihre Grenzgeb. 1992;130:197–201. doi: 10.1055/s-2008-1040138. [DOI] [PubMed] [Google Scholar]


