Abstract
Introduction
Hypoglycemia and fear of hypoglycemia may contribute to basal insulin discontinuation, poor glycemic control, and increased healthcare burden in patients with type 2 diabetes (T2D). This study aimed to determine the impact of hypoglycemia soon after basal insulin initiation on treatment discontinuation and economic outcomes in patients with T2D.
Methods
Hypoglycemic events within 6 months of basal insulin initiation were identified using retrospective cohort data from patients with T2D, at least 18 years of age, initiated on basal insulin therapy in the Clinformatics™ Data Mart for Multiplan claims database from January 1, 2008, through August 31, 2012. Data were adjusted for baseline characteristics. Discontinuation was established for patients with 12-month follow-up data, while discontinuation risk was assessed in the extended analysis (6- to 24-month follow-up) by Cox regression analysis. Healthcare use and costs were determined.
Results
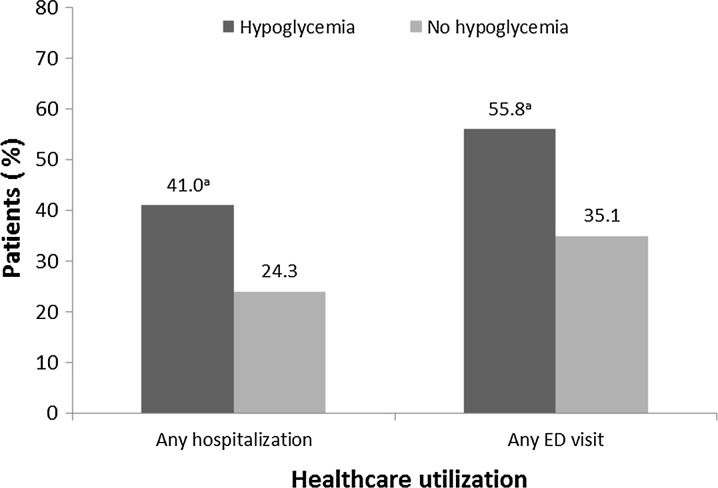
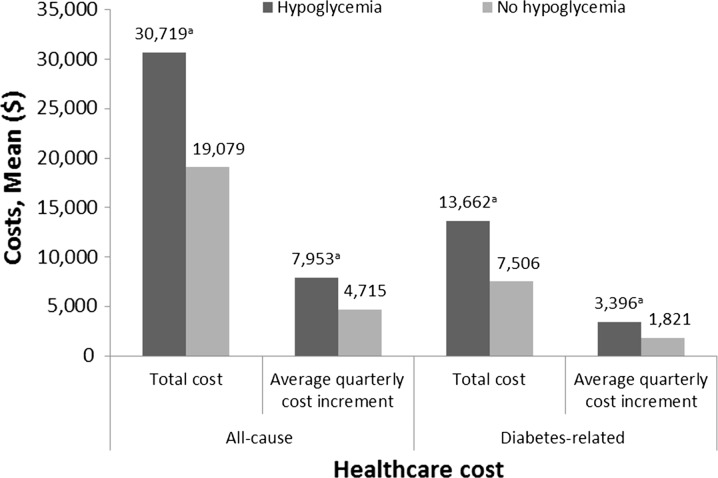
Of 55,608 patients, 4.5% experienced hypoglycemia within 6 months of basal insulin initiation. Patients with hypoglycemia were more likely to discontinue basal insulin within 12 months of initiation (79.0% vs. 74.2%; P < 0.001). Data, adjusted for baseline characteristics such as age, any baseline hypoglycemia, and use of oral antidiabetes drugs, showed that patients with hypoglycemia had a greater risk of discontinuation (hazard ratio 1.16; 95% confidence interval 1.03, 1.32; P = 0.0164), were more likely to have a hospitalization (41.0% vs. 24.3%; P < 0.001) or an ED visit (55.8% vs. 35.1%; P < 0.001), and had higher diabetes-related ($13,662 vs. $7506; P < 0.001) and all-cause ($30,719 vs. $19,079; P < 0.001) healthcare costs.
Conclusions
US patients with T2D who experienced hypoglycemia within 6 months of basal insulin initiation were more likely to discontinue treatment, accompanied by a greater healthcare burden.
Funding
Sanofi US, Inc.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-017-0592-x) contains supplementary material, which is available to authorized users.
Keywords: Basal insulin, Healthcare costs, Hypoglycemia, Initiation, Type 2 diabetes
Introduction
The major focus of disease management in patients with type 2 diabetes (T2D) is glycemic control, which might be initially attained through lifestyle modification and treatment with first-line oral metformin [1]. Owing to the progressive nature of this disease, patients with T2D eventually require therapy intensification to achieve and maintain glycemic control. The current position statement from the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) recommends the initiation of basal insulin, a second oral agent, or a glucagon-like peptide-1 receptor agonist in patients with T2D who do not achieve glycated hemoglobin (A1C) targets despite the use of non-insulin therapy at maximum tolerated dose over 3 months [2].
Five different basal insulins are currently available in the USA: intermediate-acting neutral protamine Hagedorn (NPH) insulin, the long-acting basal insulin analogues, such as insulin glargine 100 U/mL (Gla-100), the new insulin glargine 300 U/mL (Gla-300), insulin detemir, and insulin degludec [2–4]. The efficacy of each of these agents in lowering blood-glucose levels, however, is associated with adverse events, of which hypoglycemia is the most common. In patients with T2D, the frequency of hypoglycemia observed during early insulin use is generally equivalent to that in patients treated with sulfonylureas [5]. Compared with Gla-100 and insulin detemir, NPH insulin is associated with a greater risk of symptomatic hypoglycemia [6] and nocturnal hypoglycemia [1]. Among basal insulin analogues, the relative risk of hypoglycemia appears to be similar for Gla-100 and insulin detemir on the basis of data from clinical trials [6] and real-world observational studies [7, 8]. Compared to Gla-100, Gla-300 is associated with a lower incidence of a severe or confirmed nocturnal hypoglycemic episode (blood glucose ≤70 mg/dL [≤3.9 mmol/L]) [9, 10], and a lower occurrence of a severe or confirmed hypoglycemic event at any time of the day [9]. These results were reported throughout the clinical trial periods, including during the first 8 weeks of treatment when the greatest insulin dose titration occurs [9, 10].
Hypoglycemia and fear of hypoglycemia are associated with delayed insulin initiation [11, 12] and non-adherence to insulin treatment [13], both of which can compromise clinical outcomes. These delays occur despite the known benefits of early insulin initiation [14, 15]. For example, the UK Prospective Diabetes Study showed that timely insulin initiation is associated with better glycemic control and a lower risk of microvascular complications [14, 15]. Further, persistence to insulin treatment is linked to greater reductions in A1C from baseline [16]. Conversely, patients’ well-being and quality of life are negatively affected by hypoglycemia, which has potential clinical sequelae including dysrhythmias [17], accidents, falls and related fractures [18], and neurological symptoms, such as dizziness and confusion. Hypoglycemia also contributes to mortality in patients with T2D [19, 20]. Such clinical consequences can be particularly relevant in elderly patients [21].
There is also an economic burden associated with hypoglycemia due to increased healthcare resource use and costs. In one study, the mean costs of hypoglycemia-related hospital visits requiring medical assistance were estimated to be $19,345 per patient during 2004–2008 [22]. Among patients with newly diagnosed T2D, hypoglycemia was associated with additional costs of $71 per patient per month when initiating insulin compared with non-insulin antidiabetes medications [23]. Hypoglycemia is also associated with work disability [24], which can further impact patient-level costs. The objective of the present study was to evaluate, in patients with T2D who were newly initiated to basal insulin, the impact of hypoglycemia on healthcare resource use and costs, as well as insulin discontinuation, in the first few months immediately after initiation.
Methods
This is a retrospective cohort study that analyzed data from adult patients at least 18 years of age with T2D extracted from the Clinformatics™ Data Mart for Multiplan (IMPACT™; Waltham, MA, USA) claims database from January 1, 2007, through March 31, 2013. IMPACT™ is a managed care database that comprises about 50 US healthcare plans, and includes medical claims, pharmacy claims, enrollment data, and laboratory results for 107 million patients, of whom 73% had pharmacy benefits and 18% had laboratory results. Patients diagnosed with T2D were identified on the basis of at least one inpatient/emergency department (ED) medical claim or at least two ambulatory medical claims (≥30 days apart) indicating presence of T2D (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes: 250.x0 or 250.x2) at any time during the study period. For inclusion in the study, besides diagnosis of T2D, patients had to have initiated basal insulin within the identification period, and to have continuous enrollment for the study period. Patients were excluded who had any prior use of basal insulin, premixed insulin, or prandial insulin during the 12-month baseline period, or who were prescribed more than one type of basal insulin on the index date. Initiation of basal insulin therapy was defined as having no prescription for basal insulin at least 12 months before starting basal insulin treatment with Gla-100, insulin detemir, or NPH insulin, from January 1, 2008, through August 31, 2012. The date of the first insulin prescription was the index date. Each patient had a 12-month baseline period and at least a 6-month follow-up period after the index date of insulin initiation, with a maximum follow-up period of 24 months. The subset of patients with a 12-month follow-up period was used for the primary analysis, while all patients with follow-up data ranging from 6 to 24 months were used for an extended analysis. Analysis was replicated to evaluate the same outcomes using the Truven Health MarketScan™ (Ann Arbor, MI, USA) claims database. MarketScan™ is a US medical and drugs insurance claims database of approximately 138 million patients and their dependents insured by over 150 employer-sponsored plans.
Patient data were analyzed according to hypoglycemic events during the first 6 months of basal insulin use. Hypoglycemic events were identified by healthcare encounters using ICD-9-CM diagnosis codes 251.0-3 and 270.3 in any position on a medical claim, or diagnosis code 250.8x with no evidence of diagnosis codes 259.8, 272.7, 681.xx, 682.xx, 686.9x, 707.1-9, 709.3, 730.0-2, and 731.8 on the same date of service as the claim for 250.8x.
Outcomes
The primary outcomes measured were healthcare resource use and costs, and discontinuation from basal insulin treatment. Insulin discontinuation was defined as a gap of greater than 45 days in any insulin prescription coverage. Analyses of prescription gaps greater than 60 days or greater than 90 days were used to test the robustness of the results. Healthcare resource use included the number of hospitalization and ED visits, while healthcare costs included all-cause and diabetes-related costs.
Statistical Analyses
Study variables, including baseline characteristics and outcome measures, were analyzed descriptively, by comparing two cohorts. The “hypoglycemia” cohort included all patients who experienced hypoglycemic events in the first 6 months of basal insulin use, and the “no hypoglycemia” cohort included patients who did not experience hypoglycemic events in the first 6 months of basal insulin use. The number and percentage of patients were calculated for dichotomous and polychotomous variables. Means and standard deviations were calculated for continuous variables. Statistical tests of significance for differences between the cohorts were conducted, with Chi-square tests used to evaluate the statistical significance of differences in categorical variables, and Student t tests used for the means of continuous variables. The time to basal insulin discontinuation was adjusted for baseline characteristics and modeled using Cox proportional hazards multivariate regression analysis, with the presence or absence of hypoglycemia at the 6-month follow-up as the primary predictor. The impact of hypoglycemia during the first 6 months of basal insulin use on healthcare costs and use were adjusted for baseline patient characteristics and analyzed with a generalized linear model.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
A total of 71,470 patients who were initiated on treatment with basal insulin for the first time with follow-up periods ranging from 6 to 24 months were identified in the Clinformatics™ Data Mart for Multiplan (IMPACT™) claims database, and a subset of 55,608 patients with a 12-month follow-up was used for the primary analysis of this study (see Appendix 1 in the supplementary material). Among patients in that subset, 2495 (4.5%) experienced hypoglycemia within the first 6 months of basal insulin use and constituted the “hypoglycemia” cohort, with the remaining 53,113 patients constituting the “no hypoglycemia” cohort. Baseline patient characteristics were found to differ significantly between the two cohorts (P < 0.001; Tables 1, 2). Patients in the “hypoglycemia” cohort were likely to have a history of previous hypoglycemia and hospitalization, had more comorbidities, and a slight difference in age compared with those in the “no hypoglycemia” cohort (Table 1).
Table 1.
Demographic and clinical baseline characteristics for the subset of patients newly initiated on basal insulin with a 12-month follow-up
| Hypoglycemia cohort (n = 2495) | No hypoglycemia cohort (n = 53,113) | P value | |
|---|---|---|---|
| Age, mean (SD), years | 55.6 (13.2) | 54.2 (11.8) | <0.001 |
| ≥65 years [n (%)] | 585 (23.5) | 8692 (16.4) | <0.001 |
| Female [n (%)] | 1135 (45.5) | 23,786 (44.8) | 0.488 |
| CCI score, mean (SD) | 1.63 (2.11) | 1.01 (1.70) | <0.001 |
| Hypertension [n (%)] | 1866 (74.8) | 38,464 (72.4) | <0.010 |
| Hyperlipidemia [n (%)] | 1605 (64.3) | 36,446 (68.6) | <0.001 |
| Mental illness [n (%)] | 718 (28.8) | 11,823 (22.3) | <0.001 |
| Neuropathy [n (%)] | 691 (27.7) | 6621 (12.5) | <0.001 |
| Chronic obstructive pulmonary disease [n (%)] | 487 (19.5) | 8078 (15.2) | <0.001 |
| Renal disease [n (%)] | 438 (17.6) | 4763 (9.0) | <0.001 |
| Nephropathy [n (%)] | 280 (11.2) | 3194 (6.0) | <0.001 |
| Retinopathy [n (%)] | 417 (16.7) | 6464 (12.2) | <0.001 |
| Congestive heart failure [n (%)] | 413 (16.6) | 5054 (9.5) | <0.001 |
| Peripheral vascular disease [n (%)] | 373 (15.0) | 4281 (8.1) | <0.001 |
| Cerebrovascular disease [n (%)] | 338 (13.6) | 4259 (8.0) | <0.001 |
| Any baseline hypoglycemia [n (%)] | 691 (27.7) | 2402 (4.5) | <0.001 |
| Number of OADs, mean (SD) | 1.40 (1.26) | 1.65 (1.24) | <0.001 |
| Baseline SU use [n (%)] | 1148 (46.0) | 27,055 (50.9) | <0.001 |
CCI Charlson comorbidity index, OAD oral antidiabetes drug, SU sulfonylurea, SD standard deviation
Table 2.
Healthcare resource utilization and costs at baseline for the subset of patients newly initiated on basal insulin with a 12-month follow-up
| Hypoglycemia cohort (n = 2495) | No hypoglycemia cohort (n = 53,113) | P value | |
|---|---|---|---|
| Baseline all-cause healthcare utilization | |||
| Any hospitalization [n (%)] | 1101 (44.1) | 14,135 (26.6) | <0.001 |
| Any ED visit [n (%)] | 1312 (52.6) | 20,277 (38.2) | <0.001 |
| Baseline diabetes-related healthcare utilization | |||
| Any hospitalization [n (%)] | 993 (39.8) | 12,300 (23.2) | <0.001 |
| Any ED visit [n (%)] | 980 (39.3) | 13,624 (25.7) | <0.001 |
| Baseline all-cause healthcare costs | |||
| Total costs [mean (SD), $] | 33,301 (59,318) | 20,086 (44,771) | <0.001 |
| ED costs [mean (SD), $] | 987 (2596) | 628 (1708) | <0.001 |
| Baseline diabetes-related healthcare costs | |||
| Total costs [mean (SD), $] | 10,026 (19,913) | 5820 (12,464) | <0.001 |
| ED costs [mean (SD), $] | 425 (1441) | 257 (987) | <0.001 |
ED emergency department, SD standard deviation
Among patients with hypoglycemia, a greater proportion had their first hypoglycemia event diagnosed within the first month after starting basal insulin therapy (see Appendix 2 in the supplementary material). During the first 12 months after basal insulin initiation, patients in the “hypoglycemia” cohort were more likely to discontinue basal insulin within 12 months of initiation compared with patients in the “no hypoglycemia” cohort. Using a definition of discontinuation of a greater than 45-day gap in insulin prescription coverage, we observed that 79.0% of patients in the “hypoglycemia” cohort discontinued compared with 74.2% of patients in the “no hypoglycemia” cohort (P < 0.001). Despite lower insulin discontinuation percentages being observed when using prescription gaps of greater than 60 days or greater than 90 days, similar statistically significant results were consistently seen between cohorts and when considering pooled data of patients with follow-up periods between 6 and 24 months (data not shown). Cox proportional hazards analysis for patients with a 6- to 24-month follow-up showed a 16% greater risk of discontinuation (>45-day prescription gap) within 12 months of follow-up for patients who experienced hypoglycemia early after basal insulin initiation compared with those patients who did not (hazard ratio 1.16; 95% confidence interval 1.03, 1.32; P = 0.016).
Patients in the “hypoglycemia” cohort were more likely than those in the “no hypoglycemia” cohort to have any hospitalization (41.0% vs. 24.3%; P < 0.001) or any ED visit (55.8% vs. 35.1%; P < 0.001) (Fig. 1). Patients in the “hypoglycemia” cohort also experienced significantly greater total all-cause healthcare costs ($30,719 vs. $19,079 per year; P < 0.001), average quarterly all-cause healthcare costs, total diabetes-related healthcare costs ($13,662 vs. $7506 per year), and average quarterly diabetes-related healthcare costs (Fig. 2). Similar statistically significant results were seen in the extended analyses, which included follow-up data ranging from 6 to 24 months (data not shown).
Fig. 1.
Final model adjusted for baseline differences showing healthcare utilization associated with patients newly initiated on basal insulin—subset of patients with 12-month follow-up. Logistic regression was used to model any inpatient admission and any ED visit. a P < 0.001. ED emergency department
Fig. 2.
Final model adjusted for baseline differences showing healthcare costs utilization associated with patients newly initiated on basal insulin. Generalized linear model was used to analyze healthcare costs. a P < 0.001
Analysis that used information from the MarketScan™ database yielded similar results to the primary analysis confirming the association between hypoglycemia and discontinuation and healthcare costs. This database analysis identified 134,934 patients who initiated basal insulin therapy for whom 12-month follow-up data were available. Among these patients was a “hypoglycemia” cohort comprising 7371 (5.5%) patients newly started on basal insulin, who experienced hypoglycemic events that required medical assistance in the first 6 months following initiation (see Appendix 1 in the supplementary material). Results were similar for patients with follow-up periods of up to 24 months, as 9854 of 175,934 patients (5.6%) experienced hypoglycemia within the first 6 months of treatment with basal insulin.
On the basis of baseline characteristics analysis, patients in the “hypoglycemia” cohort with a 12-month follow-up, compared with those without hypoglycemia, were slightly older (59.9 years vs. 57.4 years), had a greater Charlson comorbidity index score (1.36 vs. 0.94), and had a history of previous hypoglycemia (32.3% vs. 4.0%) and hospitalization (42.5% vs. 27.9%).
Discontinuation of basal insulin within 12 months of initiation was greater in those patients in the “hypoglycemia” cohort than in the “no hypoglycemia” cohort (74.6% vs. 73.3%, respectively; P < 0.05) based on a greater than 45-day prescription gap. Again, similar results were found when considering greater than 60-day or greater than 90-day prescription gaps and among patients with follow-up periods ranging from 6 to 24 months (data not shown). In the 12-month follow-up, patients with T2D newly initiating basal insulin and experiencing hypoglycemia in the first 6 months, compared to those who did not, had higher rates of hospitalization (35.3% vs. 23.3%; P < 0.001) and ED visits (51.3% vs. 32.3%; P < 0.001), as well as greater annual total all-cause healthcare costs ($28,253 vs. $19,439; P < 0.001) and annual total diabetes-related healthcare costs ($13,458 vs. $7668; P < 0.001).
Discussion
In this real-world analysis of patients newly initiating basal insulin therapy for the management of T2D, approximately 5% experienced hypoglycemia that required medical assistance during the first 6 months after starting basal insulin therapy. Compared with those patients who did not experience hypoglycemia, the patients in the “hypoglycemia” cohort were more likely to have comorbid conditions, more often had experienced hypoglycemia before starting therapy with basal insulin, and were slightly older. Nevertheless, compared with patients without hypoglycemia, those with hypoglycemia were hospitalized more often and incurred greater annual diabetes-related healthcare costs even after controlling for differences in baseline differences that included healthcare costs. Patients with hypoglycemia were also more likely to discontinue basal insulin therapy than those patients who did not experience hypoglycemia soon after starting insulin. The robustness of these results was confirmed when consistent trends were observed on the basis of greater than 60-day or greater than 90-day prescription gap definitions of discontinuation, as reported with the primary greater than 45-day gap definition, as well as consistency between the primary and extended analyses, and also the analysis that used information from the unrelated MarketScan™ database.
It is increasingly recognized that clinical trials might not address issues related to real-world settings, as they operate in an idealized environment and assess limited, standardized populations [25]. To the best of our knowledge, this is the only real-world analysis to date of healthcare resource use and economic outcomes related to hypoglycemia occurring soon after patients newly initiated basal insulin. Prospective real-world studies may further complement the data. Discontinuation from basal insulin therapy soon after initiating the treatment has important consequences for patient management. Not only is continuation with insulin important for achieving treatment goals, but also greater insulin treatment persistence is linked to improved clinical outcomes and reduced healthcare utilization [16]. The impact of hypoglycemia on clinical and economic outcomes might be alleviated by the use of newer basal insulin analogues with lower risks of hypoglycemia, such as Gla-300 and insulin degludec, which are available in the US market or were recently approved by the US Food and Drug Administration, respectively [9, 10, 26, 27].
Recent studies have used the same or similar databases for investigating clinical and economic outcomes associated with basal insulin use, but have considered different subpopulations of US patients with T2D than the one used in this study. In a study of patients with uncontrolled glycemia (A1C ≥ 9.0%), insulin continuation was significantly associated with lower risks of all-cause and diabetes-related hospital readmissions [28]. In another study, hypoglycemia was found to be associated with an increased length of hospital stay and an increased in-hospital mortality rate [20]. A recent study that used Truven’s Health Analytics Commercial Claims and Encounters database identified greater costs over a 12-month period among patients with intermittent insulin use (early discontinuation and restart) compared with early discontinuation alone [29]. Finally, in another analysis that used the IMPACT™ database, hypoglycemia in patients with T2D treated with oral antidiabetes drugs only was associated with treatment discontinuation and significantly increased healthcare costs [30].
There are several limitations associated with the present study. This was a retrospective analysis of claims data, which are collected for billing purposes. As the detection of hypoglycemic events was based exclusively on ICD-9-CM diagnostic codes, any hypoglycemic events not severe enough to require medical intervention, and thus result in a healthcare claim, are excluded. This results in an underestimation of the overall incidence of hypoglycemia. Furthermore, the sample extracted from the Clinformatics™ Data Mart for Multiplan (IMPACT™) dataset might not be representative of all patients with T2D in the USA. A further issue with studies of this nature is that claims data analyses can be subject to selection bias or confounding, and coding errors. Specific to this study, discontinuation was measured using prescription orders. However, the presence of a claim for a filled prescription does not necessarily mean that the medication was taken as prescribed. Finally, specific data were not available, including the reason for discontinuation, the duration of T2D, and the insulin dose. Despite the limitations, comparable results found across the Clinformatics™ Data Mart for Multiplan (IMPACT™) and MarketScan™ databases support the consistency and robustness of this study.
Conclusion
This analysis shows that patients who experienced hypoglycemia shortly after basal insulin initiation were more likely to discontinue therapy and were associated with greater healthcare resource use and costs than patients with no hypoglycemia during the first 6 months following initiation. These data may help healthcare decision-makers in their clinical management of patients with T2D when starting new basal insulin analogues.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by Sanofi US, Inc. The authors received writing/editorial support in the preparation of this manuscript provided by Catarina Fernandes, PhD, of Excerpta Medica, funded by Sanofi US, Inc. Article processing charges and Open Access fees were funded by Sanofi US, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Parts of this work were presented in poster format at the American Diabetes Association (ADA) 75th Scientific Sessions on Sunday, June 7, 2015, and at the 51st Annual Meeting of the European Association for the Study of Diabetes (EASD) Annual Meeting on Wednesday, September 16, 2015.
Compliance with Ethical Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Disclosures
Mehul Dalal was an employee and stock/shareholder of Sanofi US, Inc. during the conduction of this study. Mahmood Kazemi was an employee and stock/shareholder of Sanofi US, Inc. during the conduction of this study. Fen Ye is an employee of Sanofi US, Inc. Lin Xie is an employee of STATinMED Research, under contract with Sanofi US, Inc.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/92E8F060311F4DD4.
Contributor Information
Mehul R. Dalal, Phone: +1 617-444-3350, Email: Mehul.dalal@takeda.com
Mahmood Kazemi, Phone: +1 510-239-2610.
Fen Ye, Phone: +1 908-981-6061.
Lin Xie, Phone: +1 734- 546-8661.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Sanofi US, Inc. Press Release. Sanofi receives FDA approval of once-daily basal insulin Toujeo®. February 25, 2015. http://www.news.sanofi.us/2015-02-25-Sanofi-Receives-FDA-Approval-of-Once-Daily-Basal-Insulin-Toujeo. Accessed July 13, 2015.
- 4.Novo Nordisk Inc. Press Release. Novo Nordisk receives FDA approval for Tresiba® (insulin degludec injection) for adults with type 1 and type 2 diabetes. September 25, 2015. http://press.novonordisk-us.com/2015-09-25-Novo-Nordisk-Receives-FDA-Approval-for-Tresiba-insulin-degludec-injection-for-Adults-with-Type-1-and-Type-2-Diabetes. Accessed January 19, 2016.
- 5.UK Hypoglycaemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 6.Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52:649–662. doi: 10.1007/s00592-014-0698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis KL, Tangirala M, Meyers JL, Wei W. Real-world comparative outcomes of US type 2 diabetes patients initiating analog basal insulin therapy. Curr Med Res Opin. 2013;29:1083–1091. doi: 10.1185/03007995.2013.811403. [DOI] [PubMed] [Google Scholar]
- 8.Levin P, Wei W, Miao R, et al. Therapeutically interchangeable? A study of real-world outcomes associated with switching basal insulin analogues among US patients with type 2 diabetes mellitus using electronic medical records data. Diabetes Obes Metab. 2015;17:245–253. doi: 10.1111/dom.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle MC, Yki-Järvinen H, Bolli GB, et al. One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension. Diabetes Obes Metab. 2015;17:835–842. doi: 10.1111/dom.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–1149. doi: 10.1111/dom.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krall J, Gabbay R, Zickmund S, Hamm ME, Williams KR, Siminerio L. Current perspectives on psychological insulin resistance: primary care provider and patient views. Diabetes Technol Ther. 2015;17:268–274. doi: 10.1089/dia.2014.0268. [DOI] [PubMed] [Google Scholar]
- 12.Edelman S, Pettus J. Challenges associated with insulin therapy in type 2 diabetes mellitus. Am J Med. 2014;127(10 Suppl.):S11–S16. doi: 10.1016/j.amjmed.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR, UK Prospective Diabetes Study Group Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–336. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 15.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 16.Wei W, Pan C, Xie L, Baser O. Real-world insulin treatment persistence among patients with type 2 diabetes. Endocr Pract. 2014;20:52–61. doi: 10.4158/EP13159.OR. [DOI] [PubMed] [Google Scholar]
- 17.Pistrosch F, Ganz X, Bornstein SR, Birkenfeld AL, Henkel E, Hanefeld M. Risk of and risk factors for hypoglycemia and associated arrhythmias in patients with type 2 diabetes and cardiovascular disease: a cohort study under real-world conditions. Acta Diabetol. 2015;52:889–895. doi: 10.1007/s00592-015-0727-y. [DOI] [PubMed] [Google Scholar]
- 18.Kachroo S, Kawabata H, Colilla S, et al. Association between hypoglycaemia and fall-related events in type 2 diabetes mellitus: analysis of a US commercial database. J Manag Care Spec Pharm. 2015;21:243–253. doi: 10.18553/jmcp.2015.21.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901. doi: 10.2337/dc11-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan HK, Flanagan D. The impact of hypoglycaemia on patients admitted to hospital with medical emergencies. Diabet Med. 2013;30:574–580. doi: 10.1111/dme.12123. [DOI] [PubMed] [Google Scholar]
- 21.Jaap AJ, Jones GC, McCrimmon RJ, Deary IJ, Frier BM. Perceived symptoms of hypoglycaemia in elderly type 2 diabetic patients treated with insulin. Diabet Med. 1998;15:398–401. doi: 10.1002/(SICI)1096-9136(199805)15:5<398::AID-DIA595>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Manag Care. 2011;17:673–680. [PubMed] [Google Scholar]
- 23.Bell K, Parasuraman S, Raju A, Shah M, Graham J, Denno M. Resource utilization and costs associated with using insulin therapy within a newly diagnosed type 2 diabetes mellitus population. J Manag Care Spec Pharm. 2015;21:220–228a. doi: 10.18553/jmcp.2015.21.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoads GG, Orsini LS, Crown W, Wang S, Getahun D, Zhang Q. Contribution of hypoglycemia to medical care expenditures and short-term disability in employees with diabetes. J Occup Environ Med. 2005;47:447–452. doi: 10.1097/01.jom.0000161727.03431.3e. [DOI] [PubMed] [Google Scholar]
- 25.Dreyer NA, Tunis SR, Berger M, Ollendorf D, Mattox P, Gliklich R. Why observational studies should be among the tools used in comparative effectiveness research. Health Aff (Millwood) 2010;29:1818–1825. doi: 10.1377/hlthaff.2010.0666. [DOI] [PubMed] [Google Scholar]
- 26.Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naïve patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35:2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3) Diabetes Obes Metab. 2015;17:386–394. doi: 10.1111/dom.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu EQ, Zhou S, Yu A, et al. Outcomes associated with post-discharge insulin continuity in US patients with type 2 diabetes mellitus initiating insulin in the hospital. Hosp Pract (1995) 1995;2012:40–48. doi: 10.3810/hp.2012.10.1002. [DOI] [PubMed] [Google Scholar]
- 29.Ascher-Svanum H, Lage MJ, Perez-Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5:225–242. doi: 10.1007/s13300-014-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bron M, Marynchenko M, Yang H, Yu AP, Wu EQ. Hypoglycemia, treatment discontinuation, and costs in patients with type 2 diabetes mellitus on oral antidiabetic drugs. Postgrad Med. 2012;124:124–132. doi: 10.3810/pgm.2012.01.2525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.