Abstract
Purpose
Patients with non-small cell lung cancer (NSCLC) brain metastases (BM) have poor clinical outcomes. We sought to determine if AXL–GAS6 expression can be used as independent prognostic biomarkers for NSCLC BM.
Methods
We retrospectively studied the medical records of 98 patients diagnosed with advanced metastatic NSCLC from December 2000 to June 2014. Out of a total of 98 patients with NSCLC metastases, 66 patients were identified to have brain metastases. The expressions of AXL and GAS6 were assessed by standard immunohistochemistry and correlated with clinicopathological factors and overall survival (OS) outcomes.
Results
The expression of AXL was positively associated with GAS6 expression (P < 0.001), and tumor differentiation (P = 0.014) in advanced NSCLC with metastases. AXL expression displayed no association with gender, age, smoking history, pathology, T stage, N stage, CEA, and LDH. In univariate analysis, both AXL and GAS6 were found to predict worse OS outcomes (AXL: HR 1.77, 95% CI 1.13–2.79, P = 0.01; GAS6: HR 1.80, 95% CI 1.14–2.84, P = 0.01). In the brain metastasis subgroup, the expression of AXL was positively associated with GAS6 expression (P < 0.001). Both AXL and GAS6 were found to predict worse BM-OS outcomes in univariate analysis (AXL: HR 2.19, 95% CI 1.33–4.10, P = 0.005; GAS6: HR 2.04, 95% CI 1.01–3.71, P = 0.019). In multivariate analysis, high co-expression of AXL/GAS6 was found to be an independent unfavorable risk factor for the overall study population (HR 2.33, 95% CI 1.40–3.87, P = 0.0011) and also in BM (HR 2.76, 95% CI 1.45–5.25, P = 0.001), predicting worse survival outcome.
Conclusions
AXL–GAS6 co-expression represents a potential independent prognostic biomarker for survival outcome in NSCLC BM patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00432-017-2408-4) contains supplementary material, which is available to authorized users.
Keywords: NSCLC, Brain metastasis, AXL, GAS6, EMT, Survival, Prognostic biomarker
Introduction
Lung cancer is a disease with high morbidity and is the leading cause of cancer mortality worldwide (Chen et al. 2016; Siegel et al. 2016). Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancer cases (Ettinger et al. 2012). Unfortunately, although lung cancer treatments have had considerable advances over recent years, most of the cases present at late stages and remain incurable. Prognosis of patients with advanced NSCLC remains very poor, especially in patients with NSCLC brain metastases (BM). Among all patients with NSCLC, nearly 20–40% will develop brain metastases during the course of disease and ultimately succumb to it (Barnholtz-Sloan et al. 2004; Mujoomdar et al. 2007). The median survival time of patients with NSCLC BM is approximately 9.3–19.1 months (Baek et al. 2016; Sperduto et al. 2010; Welsh et al. 2013). Hence, it is an unmet need to identify potential biomarkers in NSCLC patients with BM to provide better insights into the disease prognostication. Also, it is crucial to identify novel potential actionable targets in this disease group that can be translated into therapeutic benefits to impact the clinical outcome.
AXL (also known as tyrosine-protein kinase receptor UFO, TYRO7, and ARK) is a receptor tyrosine kinase belonging to the TAM (TYRO3/AXL/MER) family (O’Bryan et al. 1991). AXL has been reported to be overexpressed or ectopically expressed in a multitude of human cancers, including breast (Zhang et al. 2008), colon (Craven et al. 1995), lung (Ishikawa et al. 2013), hepatocellular carcinoma (He et al. 2010), and esophageal adenocarcinoma (Hector et al. 2010). Its natural ligand, growth arrest-specific gene 6 (GAS6), can bind to AXL receptor kinase to activate its catalytic kinase activity, which in turn leads to downstream activation of various oncogenic signaling pathways, regulating cell proliferation, invasion, survival, metastases, anti-apoptosis, and drug resistance (Linger et al. 2008). These biological effects are mainly mediated by GAS6/AXL-induced activation of MAPK/ERK and PI3K/AKT signaling pathways (Hasanbasic et al. 2004; Hong et al. 2008; Lee et al. 2002).
Overexpression of AXL has been observed in nearly 60% of NSCLC cell lines, and it is also found highly expressed among primary lung cancers (Shieh et al. 2005; Wimmel et al. 2001). AXL has been nominated as a potent epithelial–mesenchymal transition (EMT) inducer, and a potential molecular target for lung cancer therapy (Shieh et al. 2005; Vaughan et al. 2012). Genetic knockdown of AXL using siRNA can inhibit invasion of NSCLC cells. Importantly, induced AXL–GAS6 signaling has also been reported to mediate cancer drug resistance against both cytotoxic chemotherapeutics and targeted therapies in lung cancer (Zhang et al. 2012). Although the role of AXL–GAS6 expression and signaling effects in lung cancer have been reported in recent years (Ishikawa et al. 2013; Shieh et al. 2005; Wimmel et al. 2001; Zhang et al. 2012), the role of AXL–GAS6 in brain metastases from lung cancer and its potential prognostic importance have not been well clarified.
In this study, we conducted a retrospective analysis to evaluate AXL and GAS6 expression levels in NSCLC patients with BM by standard immunohistochemistry (IHC) and further interrogated its potential role in survival outcome prognosis.
Materials and methods
Patient characteristics
All tissues were collected from 98 patients who had received surgical resection from December 2000 to June 2014 at the Department of Surgery of Sun Yat-sen University (Guangzhou, China). Ninety one (91) patients had distant metastases at the time of diagnosis. The remaining seven patients were without metastases at initial diagnosis, but continued monitoring revealed that there was eventually metastatic disease. A subgroup of 66 patients had distant metastases to the brain, while the other 32 patients had distant metastases to other organs (liver, bone, or adrenal gland). The eligibility criteria are as follows: (1) All the patients had developed distant metastases. (2) All the pathological diagnoses of tumor tissues were confirmed to be NSCLC by anatomic pathologist. The cases were selected consecutively on the basis of availability of resection tissues and follow-up data. (3) Tumor stage was classified according to the 7th lung cancer TNM staging edition from International Association for the Study of Lung Cancer (IASLC).
Immunonhistochemical (IHC) staining
A total of 98 tumor tissue samples [51 samples from lung, 45 samples from other organs: brain (n = 35), adrenal gland (n = 5), kidney (n = 2), liver (n = 2), bone (n = 1)] were used in the IHC analysis. Formalin-fixed, paraffin-embedded tumor specimens were cut into 5-µm sections. The samples were deparaffinized in xylene and rehydrated in a series of graded ethanol after being baked at 60 °C for 2 h. Next, the samples were incubated with 3% hydrogen peroxide for 15 min to block endogenous peroxidase activity. The sections were microwaved in 1 mM ethylenediamine tetra-acetic acid (EDTA, pH 8.0) for 25 min for antigen retrieval, and then pre-incubated in 2.5% normal horse serum (VECTOR, #S-2012) for 30 min to block non-specific staining. The sections were incubated with the primary rabbit anti-human AXL monoclonal antibody (working dilution 1:200, Cell Signaling Technology, #8661) and goat anti-human GAS6 polyclonal antibody (working dilution 1:200, R&D Systems, #AF885-SP) overnight at 4 °C. Subsequently, the samples were incubated with secondary antibody (VECTOR, #MP-7500, #MP-7405) at room temperature for 1 h. All specimens were scored independently by two experienced pathologists who were blinded to the patients’ identity and clinical status. H-scores of percentage of positive tumor cells (0–100%) and dominant staining intensity (0, 1+, 2+, and 3+) of immunostaining were adopted for the expression data analysis. The final quantitation of each staining was determined by averaging the H-scores (% positive tumor cells × staining intensity) obtained from the two pathologists. AXL expression was classified as high or low based on whether the H-score was above or below the median cut-off score of 0.4, respectively. GAS6 expression was considered high if the score was above 0.3 as the median cut-off.
Follow-up
The last date of follow-up was on June 30, 2016. In all patients (47 females and 51 males), the follow-up period ranged from 3 to 67 months. All patients were followed up every 3 months in the first year and every 6 months thereafter. The follow-up protocol included physical examination, carcinoembryonic antigen (CEA) level, brain MRI, chest X-ray or CT, and abdominal ultrasonography. During the course of follow-up, 79 patients (80.6%) died of lung cancer-related causes. 19 patients were still alive at the time of the last follow-up report.
Statistical analysis
Overall survival (OS) was defined as the time from diagnosis of metastatic NSCLC to the death of the patient or last date of follow-up. Overall survival of the brain metastatic NSCLC subgroup (BM-OS) was calculated from the time of diagnosis of the first BM to the time of death of the patient or last date of follow-up. The SPSS software package (version 24.0, IBM, USA) and GraphPad Prism (version 6.0, GraphPad Software Inc, USA) were used for the statistical analysis. The Chi-square test was used to assess the correlation of AXL–GAS6 status with clinicopathological characteristics. Survival curves were generated using the Kaplan–Meier method, and differences between curves were assessed by the log-rank test. The Cox multivariate proportional hazards regression model was used to determine the independent risk factors that influence overall survival. P values less than 0.05 were considered to be statistically significant.
Results
AXL and GAS6 expression in NSCLC
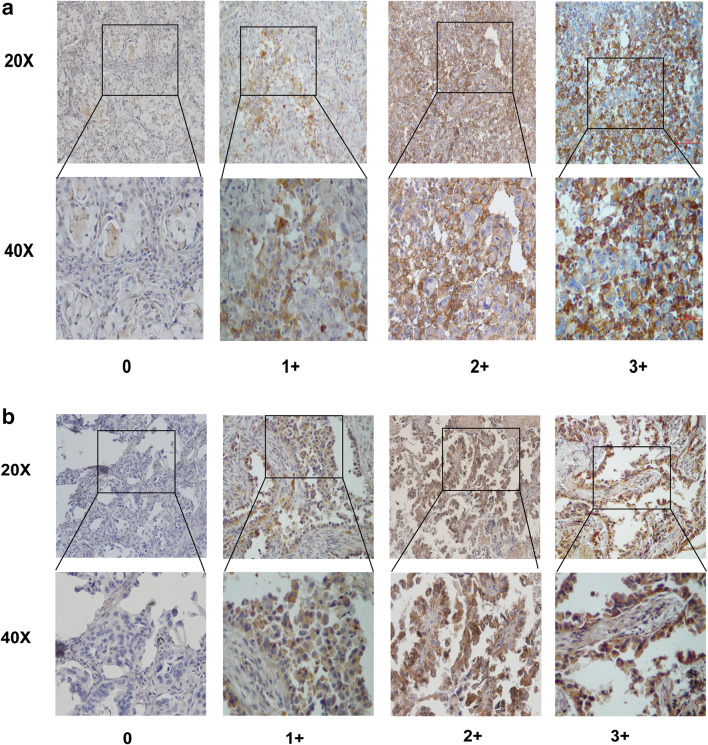
To elucidate the biological significance of AXL in lung cancer metastases, especially in NSCLC with brain metastases, we tested the expression of AXL and its ligand GAS6 in the selected 98 lung cancer specimens by immunohistochemical staining. The results showed that AXL and GAS6 expressions are primarily localized both at the cytoplasmic membrane and within the cytoplasm of tumor cells, respectively (Fig. 1). In the brain metastasis subgroup, high AXL and GAS6 expression was found in 36 of 66 (54.5%) and 37 of 66 (56.0%) patients, respectively. In the subgroup of lung cancer with metastases to organs other than brain, high AXL and GAS6 expression accounted for nine of 32 (28.1%) in both markers.
Fig. 1.
AXL and GAS6 expression in NSCLC tumor tissues. a Examples of tumoral staining intensity (0, 1+, 2+, and 3+) of AXL in immunohistochemistry (IHC) analysis. b Examples of tumoral staining intensity (0, 1+, 2+, and 3+) of GAS6 in immunohistochemistry (IHC) analysis
Correlation of AXL–GAS6 protein expression with clinicopathological parameters in lung cancer with metastases
To gain insight into the potential role of AXL in NSCLC with metastases, we correlated AXL expression status in the study cohort of 98 NSCLC patients with distant metastases with various clinical and pathological features. The expression of AXL was significantly correlated with tumor differentiation (P = 0.014). The association between AXL and GAS6 expressions was estimated using Spearman’s correlation with the scatterplot and fitted straight line and was found to have a positive correlation (R = 0.532, P < 0.001) (Figure S1). In contrast, AXL expression displayed no association with gender, age, smoking history, pathology, T stage, N stage, CEA, or LDH (all P > 0.05) (Table 1).
Table 1.
Correlation of AXL expression with clinicopathological parameters in lung cancer metastases
| Characteristics | Total | Low AXL (%) | High AXL (%) | P value |
|---|---|---|---|---|
| Gender | 0.56 | |||
| Male | 51 | 22 (43.1) | 29 (56.9) | |
| Female | 47 | 27 (57.4) | 20 (42.6) | |
| Age, years | 0.39 | |||
| ≤50 | 34 | 15 (44.1) | 19 (55.9) | |
| >50 | 64 | 34 (53.1) | 30 (46.9) | |
| Smoking history | 0.47 | |||
| Yes | 40 | 18 (45) | 22 (55) | |
| No | 58 | 31 (53.4) | 27 (46.6) | |
| Pathology | 0.18 | |||
| Adenocarcinoma | 88 | 46 (52.3) | 42 (47.7) | |
| Non-adeno | 10 | 3 (30) | 7 (70) | |
| Tumor differentiation | 0.014 | |||
| Poor | 53 | 20 (37.7) | 33 (62.3) | |
| Moderate and well | 45 | 29 (64.4) | 16 (35.6) | |
| T stage | 0.32 | |||
| 1/2 | 77 | 37 (48.1) | 40 (51.9) | |
| 3/4 | 21 | 14 (66.7) | 7 (33.3) | |
| N stage | 0.54 | |||
| 0/1 | 40 | 23 (57.5) | 17 (42.5) | |
| 2/3 | 58 | 27 (46.5) | 31 (53.5) | |
| CEA (ng/mL) | 0.22 | |||
| ≤5 | 46 | 20 (43.5) | 26 (56.5) | |
| >5 | 52 | 29 (55.8) | 23 (44.2) | |
| LDH (u/L) | 0.79 | |||
| ≤245 | 81 | 41 (50.6) | 40 (49.4) | |
| >245 | 17 | 8 (47.1) | 9 (52.9) | |
| GAS6 expression | <0.001 | |||
| Low | 49 | 37 (75.5) | 12 (24.5) | |
| High | 49 | 12 (24.5) | 37 (75.5) | |
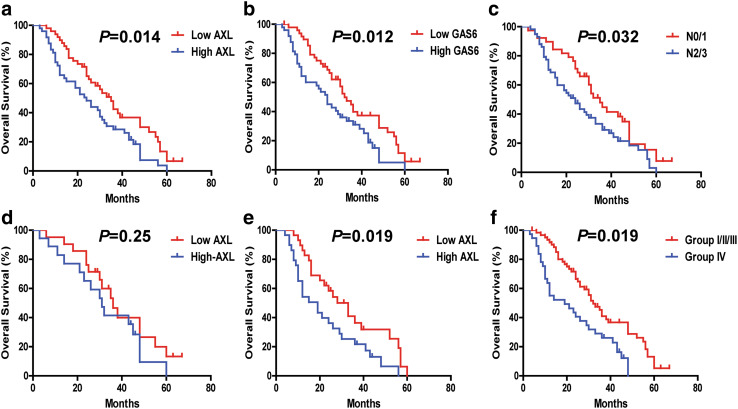
We next analyzed the correlation between AXL and traditional clinicopathological parameters with patients’ outcomes by univariate analysis. Significantly increased OS was observed for the NSCLC metastasis-positive patients with low AXL expression (AXLLow) (P = 0.014), low GAS6 (GAS6Low) (P = 0.012), N 0/1 (P = 0.032) (Fig. 2a–c), but not with other clinicopathological parameters (Figure S2, a–h). In addition, the median OS in the group with low levels of AXL (AXLLow) (n = 49) and in the group with high levels of AXL (AXLHigh) (n = 49) were 35 and 24 months, respectively. Furthermore, the 2-year OS rates of AXLLow vs AXLHigh were 65.1 and 48.3%, respectively (Table 2).
Fig. 2.
The correlation of NSCLC overall survival (OS) with different clinicopathological characteristics. a–c Survival curves were generated using the Kaplan–Meier method, and differences between survival curves were estimated by the log-rank test. AXL, GAS6, and nodal (N) stage have statistically significant correlation with OS differences. d, e The correlation between AXL expression and NSCLC OS in nodal (N) stage group. d N 0/1 stage subgroup (n = 40, P > 0.05). e N 2/3 stage subgroup (n = 58, P < 0.05). f Combined analysis of AXL and GAS6 co-expression and its correlation with NSCLC metastasis overall survival. The association of AXL/GAS6 co-expression with overall survival (log-rank P < 0.05) is shown here. Group I with AXLLow and GAS6Low (n = 37); Group II with AXLHigh and GAS6Low (n = 12); Group III with AXLLow and GAS6High (n = 12); Group IV, AXLHigh and GAS6High (n = 37)
Table 2.
Two-year overall survival (OS) rates of AXLLow and AXLHigh tumors in NSCLC metastasis and NSCLC BM groups
| NSCLC metastases | NSCLC brain metastases | |
|---|---|---|
| Median OS (months) | ||
| Low AXL (mo) | 35 | 48 |
| High AXL (mo) | 24 | 21 |
| 2-year survival rates | ||
| Low AXL (%) | 65.1 | 68.8 |
| High AXL (%) | 48.3 | 44.8 |
We also performed univariate and multivariate Cox model analyses to analyze whether AXL–GAS6 represent potential independent predictors for OS outcome in patients with NSCLC metastases. Both AXL and GAS6 were found to predict OS outcomes in our univariate analysis (AXL: HR 1.77, 95% CI 1.13–2.79, P = 0.01; GAS6: HR 1.80, 95% CI 1.14–2.84, P = 0.01) (Table 3). Combined AXL/GAS6 co-expression analysis yielded stronger predictor index with HR 2.20 (95% CI 1.36–3.56, P = 0.001) in the AXLHigh/GAS6High group. Multivariate analysis of AXL/GAS6 co-expression significantly correlated with OS (HR 2.33, 95% CI 1.40–3.87, P = 0.0011) (Table 4). Our results revealed that AXL/GAS6 co-expression and N stage (HR 1.81, 95% CI 1.13–2.90, P = 0.013) were independent poor predictors for OS in patients with NSCLC metastases (Tables 3, 4).
Table 3.
Univariate analysis of the correlation of AXL with overall survival in patients with NSCLC metastasis
| HR | 95% CI | P value | |
|---|---|---|---|
| Gender (male vs female) | 0.93 | 0.60–1.47 | 0.78 |
| Age, years (≤50 vs >50) | 1.08 | 0.68–1.74 | 0.76 |
| Smoking history (yes or no) | 1.18 | 0.78–1.78 | 0.44 |
| Pathology (adeno vs non-adeno) | 1.00 | 0.48–2.08 | 1.00 |
| Tumor differentiation (poor vs moderate and well) | 1.07 | 0.69–1.67 | 0.77 |
| T stage (T 3/4 vs T 1/2) | 1.23 | 0.46–1.42 | 0.44 |
| LDH (u/L) (≤245 vs >245) | 1.27 | 0.72–2.23 | 0.41 |
| CEA (ng/mL) (≤5 vs >5) | 1.53 | 0.98–2.41 | 0.07 |
| N stage (N 0/1 vs N 2/3) | 1.65 | 1.04–2.61 | 0.03 |
| AXL (high vs low) | 1.77 | 1.13–2.79 | 0.01 |
| GAS6 (high vs low) | 1.80 | 1.14–2.84 | 0.01 |
| AXL/GAS6 (IV vs I/II/III) | 2.20 | 1.36–3.56 | 0.001 |
Table 4.
Multivariate analysis of the correlation of AXL with overall survival in patients with NSCLC metastases
| HR | 95% CI | P value | |
|---|---|---|---|
| N stage (N 2/3 vs N 0/1) | 1.81 | 1.13–2.90 | 0.013 |
| AXL/GAS6 (IV vs I/II/III) | 2.33 | 1.40–3.87 | 0.0011 |
We also sought to determine whether the prognostic value of AXL changes among different N stages. The AXLHigh expression status maintained its prognostic value in predicting shorter OS in N 2/3 stage (n = 58) (P = 0.019), but not in N 0/1 stage (n = 40) (P = 0.25) (Fig. 2d, e).
High expression of AXL–GAS6 is significantly associated with poor prognosis in patients with NSCLC with BM
In the subgroup of patients with NSCLC with brain metastases, the high expression of AXL (AXLHigh) was significantly associated with GAS6 expression (P < 0.001), but not with tumor differentiation, gender, age, smoking history, pathology, T stage, N stage, CEA, and LDH (all P > 0.05) (Table 5).
Table 5.
Correlation of AXL expression with clinicopathological parameters in NSCLC brain metastases
| Characteristics | Total | Low AXL (%) | High AXL (%) | P value |
|---|---|---|---|---|
| Gender | 0.21 | |||
| Male | 34 | 18 (58.9) | 16 (41.1) | |
| Female | 32 | 12 (43.8) | 20 (56.2) | |
| Age (years) | 0.89 | |||
| ≤50 | 27 | 12 (48.2) | 15 (51.8) | |
| >50 | 39 | 18 (51.2) | 21 (48.8) | |
| Smoking history | 0.59 | |||
| Yes | 28 | 14 (53.6) | 14 (46.4) | |
| No | 38 | 16 (47.4) | 22 (52.6) | |
| Pathology | 0.85 | |||
| Adenocarcinoma | 62 | 28 (52.3) | 34 (47.7) | |
| Non-adeno | 4 | 2 (30) | 2 (70) | |
| Tumor differentiation | 0.83 | |||
| Poor | 35 | 13 (42.8) | 22 (57.2) | |
| Moderate and well | 31 | 17 (58.1) | 14 (41.9) | |
| T stage | 0.49 | |||
| 1/2 | 51 | 22 (45.1) | 29 (54.9) | |
| 3/4 | 15 | 8 (66.7) | 7 (33.3) | |
| N stage | 0.19 | |||
| 0/1 | 23 | 14 (60.9) | 9 (39.1) | |
| 2/3 | 43 | 19 (44.2) | 24 (55.8) | |
| CEA(ng/mL) | 0.087 | |||
| ≤5 | 34 | 12 (41.2) | 22 (58.8) | |
| >5 | 32 | 18 (59.4) | 14 (40.6) | |
| LDH(u/L) | 0.74 | |||
| ≤245 | 55 | 27 (49.1) | 28 (50.9) | |
| >245 | 11 | 6 (54.6) | 5 (45.4) | |
| GAS6 expression | <0.001 | |||
| Low | 29 | 20 (68.9) | 9 (31.1) | |
| High | 37 | 9 (24.4) | 28 (75.6) | |
| Tissue | 0.49 | |||
| Lung | 31 | 15 (54.9) | 16 (45.1) | |
| Brain | 35 | 14 (45.8) | 21 (54.2) | |
| Low AXL/GAS6 (%) | High AXL/GAS6 (%) | |||
|---|---|---|---|---|
| Tissue | ||||
| Lung | 21 | 12 (57.2) | 9 (42.8) | 0.021 |
| Brain | 26 | 7 (26.9) | 19 (73.1) | |
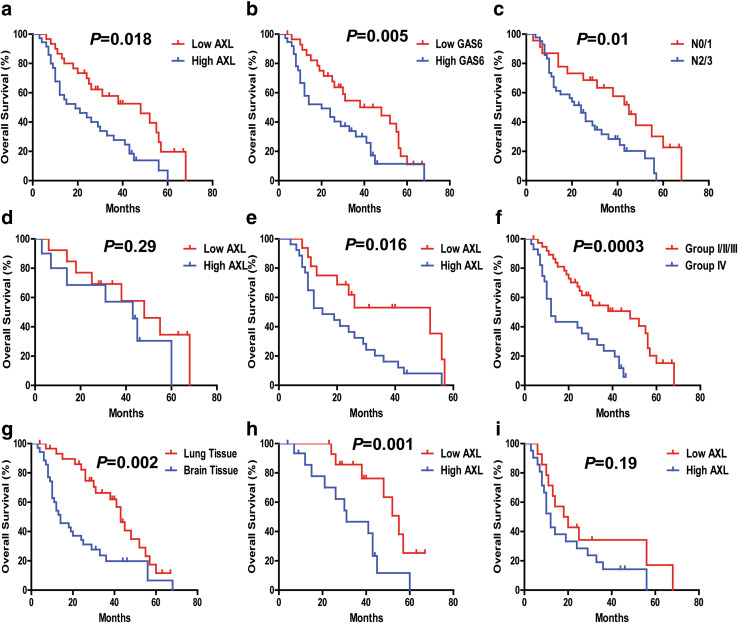
Significant OS disadvantages were observed for the NSCLC metastasis patients with high AXL expression (AXLHigh), high GAS6 expression (GAS6High), and N 2/3 stage (P < 0.01), compared with the AXLLow, GAS6Low, N 0/1 stage group, respectively (Fig. 3a–c). Median OS for AXLLow (n = 29) and AXLHigh (n = 37) were 48 and 21 months, respectively (P < 0.05). Furthermore, the 2-year OS rates of the AXLlow (n = 29) and AXLhigh were 68.8% and 44.8%, respectively (Table 2).
Fig. 3.
The correlation of NSCLC BM-OS with different clinicopathological characteristics. a–c AXL, GAS6, and nodal (N) stage have statistically significant correlation with BM-OS. d, e The correlation between AXL expression and NSCLC BM-OS in N 2/3 stage group (n = 43), P < 0.05. f Combined analysis of AXL and GAS6 co-expression and its correlation with NSCLC BM-OS. The association of AXL/GAS6 high co-expression with overall survival (log-rank P < 0.05) is shown here. Group I with AXLLow and GAS6Low (n = 20); Group II with AXLHigh and GAS6Low (n = 9); Group III with AXLLow and GAS6High (n = 9); Group IV, AXLHigh and GAS6High (n = 26). g–i The correlation between AXL expression and NSCLC BM-OS in the lung tissue subgroup and brain tissue subgroup. AXL expression carries a statistically significant BM-OS difference in lung tissue subgroup, and not in brain tissue subgroup
We used both univariate and multivariate Cox models to analyze whether AXL–GAS6 could be an independent predictor for BM-OS in patients with NSCLC with brain metastases. Our results in the univariate analysis revealed that high expression of AXL (AXLHigh) (HR 2.19, 95% CI 1.33–4.10, P = 0.005), and GAS6 (GAS6High) (HR 2.04, 95% CI 1.01–3.71, P = 0.019), and N stage (HR 2.08, 95% CI 1.24–3.82, P = 0.01) were predictors for poor OS in patients with NSCLC with BM (Table 6). In the multivariate Cox analysis model, combined AXL/GAS6 co-expression significantly predicts poor OS with HR 2.76 (95% CI 1.45–5.25, P = 0.001) in the AXLHigh/GAS6High group (Group IV vs I/II/III) (Table 7).
Table 6.
Univariate analysis of the correlation of AXL with overall survival in patients with NSCLC brain metastases
| HR | 95% CI | P value | |
|---|---|---|---|
| Gender (male vs female) | 1.39 | 0.78–2.50 | 0.26 |
| Age, years (>50 vs ≤50) | 1.21 | 0.46–1.51 | 0.54 |
| Smoking history (yes vs no) | 1.46 | 0.81–2.66 | 0.20 |
| Tumor differentiation (poor vs moderate and well) | 1.37 | 0.77–2.49 | 0.28 |
| T stage (T3/4 vs T1/2) | 1.02 | 0.53–1.96 | 0.95 |
| LDH (u/L) (>245 vs ≤245) | 1.26 | 0.57–2.78 | 0.56 |
| CEA (ng/mL) (>5 vs ≤5) | 1.32 | 0.74–2.35 | 0.34 |
| N stage (N2/3 vs N0/1) | 2.08 | 1.24–3.82 | 0.01 |
| AXL (high vs low) | 2.19 | 1.33–4.10 | 0.005 |
| GAS6 (high vs low) | 2.04 | 1.01–3.71 | 0.019 |
| AXL/GAS6 (IV vs I/II/III) | 2.44 | 1.44–4.17 | 0.001 |
Table 7.
Multivariate analysis of the correlation of AXL with overall survival in patients with NSCLC brain metastases
| HR | 95% CI | P value | |
|---|---|---|---|
| N stage (N2/3 vs N0/1) | 2.12 | 1.08–4.17 | 0.029 |
| AXL/GAS6 (IV vs I/II/III) | 2.76 | 1.45–5.25 | 0.001 |
We further sought to determine whether the prognostic value of AXL changes in different N stages of NSCLC BM patients. The high expression of AXL (AXLHigh) maintained its prognostic value in predicting shorter BM-OS in N 2/3 stage (P = 0.016) (Fig. 3e), not in N 0/1 stage (P = 0.29) (Fig. 3d).
Significant OS advantages were observed for the NSCLC BM in lung tissue subgroup compared with the brain tissue subgroup. The AXLHigh expression has prognostic value in predicting shorter OS in lung tissue group (n = 31) (P = 0.001), but not significantly so in the brain tissue group (n = 35) (P = 0.19) (Fig. 3g–i). In addition, the brain tissues comparing with the lung tissues was associated with significantly higher co-expression of AXL/GAS6 (AXLHigh/GAS6High) (P = 0.021), albeit not with just high expression of AXL alone (P = 0.49) (Table 5).
In the patient subgroup with NSCLC with other organ metastases (OM), the high expression of AXL/GAS6/N stage was not associated with OS (P > 0.05) (Figure S3, a–c).
Combination of AXL and GAS6 co-expression is associated with poor prognosis in advanced NSCLC with distant metastases especially in the BM group
We found that 37/49 (75.5%) patients with GAS6Low expression also had AXLLow expression (i.e., GAS6Low/AXLLow), while 75.5% (37/49) of GAS6High patient also had AXLHigh co-expression (GAS6High/AXLHigh) (Table 1). In the patient subgroup with NSCLC with brain metastases, 20/29 (68.9%) of GAS6Low patients were found also to have AXLLow expression (GAS6Low/AXLLow), while 28/37 (75.6%) of GAS6High patients also had AXLHigh co-expression (GAS6High/AXLHigh) (P < 0.001) (Table 5). Moreover, the co-expression relationship between AXL and GAS6 was further confirmed by IHC assays in serial sections of lung cancer metastasis tissues.
Univariate Cox model analysis indicated that GAS6 High and AXLHigh tumoral expression levels were significantly associated with shorter OS (P < 0.01) (Tables 3, 6). We further evaluated the potential prognostic value of AXL expression coupled with GAS6 co-expression levels for overall survival of NSCLC metastasis patients. According to AXL/GAS6 co-expression pattern, the cases were divided into four groups: Group I with AXLLow and GAS6Low; Group II with AXLHigh and GAS6Low, and Group III with AXLLow and GAS6High; and Group IV with AXLHigh and GAS6High. Comparing to all the other groups, in multivariate Cox model analysis, the group IV (GAS6High/AXLHigh) patients displayed the worst survival prognosis correlation, robustly predicting poor outcomes both in the overall NSCLC metastasis cohort group (P = 0.001) (Table 4; Fig. 2f, Figure S2, i) and also in the NSCLC BM cohort group (Table 7; Fig. 3f, Figure S2, q) (P = 0.001).
Discussion
AXL, a transforming protooncogene, was originally isolated from chronic myelogenous leukemia cells (O’Bryan et al. 1991). AXL has been reported to be ectopically expressed or overexpressed in a multitude of human cancers. GAS6 can bind to the extracellular domain of AXL leading to autophosphorylation of tyrosine residues on the intracellular tyrosine kinase domain of AXL, and induce activation of PI3K/AKT and MAPK/ ERK signaling pathways (Wu et al. 2014b). To the best of our knowledge, there are no reports to this date on the role of AXL–GAS6 in NSCLC BM, and the prognostic significance of AXL–GAS6 expression in lung cancer brain metastases remains undefined.
The epithelial–mesenchymal transition (EMT) process allows epithelial cells to lose their cell polarity and adopt mesenchymal-like phenotypes, thereby gaining the ability to migrate and invade surrounding tissue. EMT is correlated with the development of acquired resistance to chemotherapy (Fischer et al. 2015) and targeted therapy in a number of cancers (Shintani et al. 2016; Zhang et al. 2012). Previous studies reported by our group demonstrated that acquired epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-resistant EGFR-mutant NSCLC cells not only overexpressed AXL, but also demonstrated a concomitant EMT-associated transcriptional program involving upregulation of vimentin (Zhang et al. 2012). Our recent studies also supported the notion that early adaptive precision drug-resistant escape in EGFR-mutant NSCLC under EGFR inhibitor treatment is associated with a quiescence cell state under transcriptomic-metabolomic cellular reprogramming that has an enhanced EMT-ness, cancer stemness, and upregulated vimentin (Thiagarajan et al. 2016). The EMT signature and AXL might be predictive biomarkers of drug response-resistance profile and therapeutic targets in patients with NSCLC (Fischer et al. 2015; Thiagarajan et al. 2016; Wu et al. 2014a). As overexpression of AXL/GAS6 can contribute to tumor invasion, metastasis especially to the brain, and drug resistance against chemotherapy and targeted therapies in NSCLC, the AXL/GAS6 pathway can be promising therapeutic target for clinical inhibition. There are a number of ongoing cancer clinical trials aiming at inhibiting the activity of AXL kinase, such as using the novel small-molecule inhibitor R428 (BGB324) to potently block autophosphorylation of AXL (Wu et al. 2014b). Final clinical study results upon completion of the trials would provide insights into the potential significance of AXL inhibition in NSCLC and other human cancers. Also, novel GAS6- and AXL-targeting inhibitors would be of value to be developed as well as combination inhibitory strategy co-targeting the two molecules.
NSCLC BM patients often have poor prognosis, with reported median overall survival time being only 7 months (Sperduto et al. 2012; Zimmermann et al. 2014). The median survival time of patients with metastatic NSCLC to the brain with tumoral EGFR mutations is more favorable at the range of 19–26 months, likely as a reflection of the favorable predictive and prognostic indices for EGFR mutations per se (Jiang et al. 2016; Luo et al. 2014; Welsh et al. 2013). In our current study, the median BM-OS time for the AXLHigh group was 21 months and that for AXLLow was 48 months. We did not include EGFR and ALK genotype information in our study analysis; and there was no complete treatment information on all the study patients. While EGFR and ALK kinase inhibitors as standard-of-care first-line targeted therapy for EGFR-mutated/ALK-rearranged NSCLC patients began in 2004 and 2011, respectively, in the US, for China, the genotype-guided therapy started since 2006 and 2013, respectively (Cohen et al. 2005; Sasaki and Janne 2011). The tumor tissues included in our current study were collected from December 2000 onwards. Moreover, not all the EGFR-mutated/ALK-rearranged patients would have received targeted therapy during the study period in China. Furthermore, AXLHigh and GAS6High expressions are each an independent predictor for poor OS in advanced lung cancer especially in the BM patients, but not in the subgroup of patients with metastasis to other organs. In addition, AXLHigh expression level maintained its prognostic value in predicting shorter OS in N 2/3 stage, but not in N 0/1 stage. Of note, the metastatic brain tissues from lung primary was significantly associated with high co-expression of AXL/GAS6. AXL/GAS6 are known to be a pivotal signaling axis involved in EMT, which could conceivably be the underlying factor in leading to tumor progression and adverse prognosis in lung cancer BM. Our data support the finding that significant OS advantages were observed for the NSCLC BM in lung tissue subgroup compared with the brain tissue subgroup. We believe that there were confounding clinical factors in the NSCLC BM brain tissue subgroups inherent in the subgroup patients’ clinical conditions and scenarios as the basis of the decision of brain metastatic tissue craniotomy resection may contribute to the unfavorable outcome in this subgroup relative to the NSCLC BM lung tissue subgroup. These include factors such as symptomatic brain metastasis, and larger metastatic tumor size not amenable for gamma-knife radiosurgery in the brain tissue subgroup. In turn, these factors may also be correlated with the AXL–GAS6 expression as well. Moreover, the added potential perioperative risks may also contribute negatively to the brain tissue NSCLC BM subgroup patients’ survival outcome.
Based on our study results, we nominate GAS6High/AXLHigh as a potential molecular determinant in NSCLC patients more likely to form metastatic sites at the CNS brain compartment, and is associated with poor survival outcome prognosis. Further preclinical translational model system to test and validate this hypothesis would be of great value. Our laboratory is pursuing these studies further to advance the clinical–translational impact of our study results.
Our current study has several limitations. First, it is a retrospective study with its intrinsic associated limitations. Second, our cohort size is modest; although it consists of well-annotated and unique sample cohort set. Third, there can be inherent methodological limitations and variations in the performance and scoring of standard IHC study and results. To minimize bias and methodological variations, we have herein adopted rigorous standardized assay methods in our study and the results were scored by two blinded independent well-trained clinic pathologists. Furthermore, additional studies with larger clinical sample cohort size from different centers would be of value to further validate our results.
Our results reveal that AXL–GAS6 signal axis potentially has a key role in NSCLC tumor progression and survival prognosis; and AXL alone or in combination with GAS6 may serve as feasible biomarker for prognostic prediction in patients with metastatic NSCLC to the brain. Combined co-expression analysis of AXL with GAS6 may serve to identify the high-risk NSCLC BM patients, and could engender an attractive therapeutic approach to prevent or combat brain metastases in NSCLC in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81372274, 81461168028, and 81672276) and Natural Science Foundation of Guangdong Province, China (Nos. 2014A030313033 and 2014A050503037) and IDeA CTR support from National Institute of Health (NIH)/National Institute of General Medical Sciences (NIGMS), U54GM104942 (P.C.M. and S.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Patrick C. Ma is supported by WVU Cancer Institute, and the West Virginia Clinical and Translational Science Institute (WVCTSI). Xiaoliang Wu was supported by International Program for Ph.D. Candidates, Sun Yat-sen University and Guizhou Provincial People’s Hospital Youth Funding (No. GZSQN [2016]20).
Compliance with ethical standards
Conflict of interest
Patrick C. Ma is a member of Speaker’s Bureau of Merck and Pfizer, Advisory Board of Caris Center of Excellence Network for Precision Medicine (Caris Life Science) and received contracted clinical research support from Medimmune, Bristol-Myers Squibb, AstraZeneca, EpicentRx, Tesaro, PrECOG, LOXO Oncology, and XCovery.
Ethics approval
This study was approved by Sun Yat-sen University Cancer Center Institutional Review Board (IRB) and Human Ethics Committee (HEC).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Xiaoliang Wu, Wenjuan Ma, and Qianghua Zhou contribute equally to the manuscript.
Contributor Information
Zhenfeng Zhang, Phone: +86 20 34153532, Phone: +86 20 87343180, Email: zhangzhf@gzhmu.edu.cn.
Patrick C. Ma, Phone: 304-293-4229, Email: pcma@hsc.wvu.edu
References
- Baek MY et al (2016) Epidermal growth factor receptor mutation and pattern of brain metastasis in patients with non-small cell lung cancer. Korean J Intern Med. doi:10.3904/kjim.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22:2865–2872. doi:10.1200/JCO.2004.12.149 [DOI] [PubMed] [Google Scholar]
- Chen W et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66:115–132. doi:10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- Cohen MH, Johnson JR, Chen YF, Sridhara R, Pazdur R (2005) FDA drug approval summary: erlotinib (Tarceva) tablets. Oncologist 10:461–466. doi:10.1634/theoncologist.10-7-461 [DOI] [PubMed] [Google Scholar]
- Craven RJ et al (1995) Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer 60:791–797 [DOI] [PubMed] [Google Scholar]
- Ettinger DS et al (2012) Non-small cell lung cancer. J Natl Compr Canc Netw 10:1236–1271 [DOI] [PubMed] [Google Scholar]
- Fischer KR et al (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanbasic I, Cuerquis J, Varnum B, Blostein MD (2004) Intracellular signaling pathways involved in Gas6–Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol 287:H1207–H1213. doi:10.1152/ajpheart.00020.2004 [DOI] [PubMed] [Google Scholar]
- He L, Zhang J, Jiang L, Jin C, Zhao Y, Yang G, Jia L (2010) Differential expression of Axl in hepatocellular carcinoma and correlation with tumor lymphatic metastasis. Mol Carcinog 49:882–891. doi:10.1002/mc.20664 [DOI] [PubMed] [Google Scholar]
- Hector A et al (2010) The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther 10:1009–1018. doi:10.4161/cbt.10.10.13248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CC et al (2008) Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 268:314–324. doi:10.1016/j.canlet.2008.04.017 [DOI] [PubMed] [Google Scholar]
- Ishikawa M et al (2013) Higher expression of receptor tyrosine kinase Axl, and differential expression of its ligand, Gas6, predict poor survival in lung adenocarcinoma patients. Ann Surg Oncol 20(Suppl 3):S467–S476. doi:10.1245/s10434-012-2795-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T et al (2016) EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol 11:1718–1728. doi:10.1016/j.jtho.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Lee WP, Wen Y, Varnum B, Hung MC (2002) Akt is required for Axl–Gas6 signaling to protect cells from E1A-mediated apoptosis. Oncogene 21:329–336. doi:10.1038/sj.onc.1205066 [DOI] [PubMed] [Google Scholar]
- Linger RM, Keating AK, Earp HS, Graham DK (2008) TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 100:35–83. doi:10.1016/S0065-230X(08)00002-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D et al (2014) EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol 35:2437–2444. doi:10.1007/s13277-013-1323-9 [DOI] [PubMed] [Google Scholar]
- Mujoomdar A, Austin JH, Malhotra R, Powell CA, Pearson GD, Shiau MC, Raftopoulos H (2007) Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 242:882–888. doi:10.1148/radiol.2423051707 [DOI] [PubMed] [Google Scholar]
- O’Bryan JP et al (1991) axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 11:5016–5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Janne PA (2011) New strategies for treatment of ALK-rearranged non-small cell lung cancers. Clin Cancer Res 17:7213–7218. doi:10.1158/1078-0432.CCR-11-1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW (2005) Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia 7:1058–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, Okumura M (2016) IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol 11:1482–1492. doi:10.1016/j.jtho.2016.05.025 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30. doi:10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- Sperduto PW et al (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 77:655–661. doi:10.1016/j.ijrobp.2009.08.025 [DOI] [PubMed] [Google Scholar]
- Sperduto PW et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425. doi:10.1200/JCO.2011.38.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan PS et al (2016) Transcriptomic-metabolomic reprogramming in EGFR-mutant NSCLC early adaptive drug escape linking TGFbeta2-bioenergetics-mitochondrial priming. Oncotarget 7:82013–82027. doi:10.18632/oncotarget.13307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CA et al (2012) Gain-of-function activity of mutant p53 in lung cancer through up-regulation of receptor protein tyrosine kinase Axl genes. Cancer 3:491–502. doi:10.1177/1947601912462719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JW et al (2013) Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 31:895–902. doi:10.1200/JCO.2011.40.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M (2001) Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer 37:2264–2274 [DOI] [PubMed] [Google Scholar]
- Wu F, Li J, Jang C, Wang J, Xiong J (2014a) The role of Axl in drug resistance and epithelial-to-mesenchymal transition of non-small cell lung carcinoma. Int J Clin Exp Pathol 7:6653–6661 [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu X, Koul S, Lee CY, Zhang Z, Halmos B (2014b) AXL kinase as a novel target for cancer therapy. Oncotarget 5:9546–9563. doi:10.18632/oncotarget.2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YX et al (2008) AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res 68:1905–1915. doi:10.1158/0008-5472.CAN-07-2661 [DOI] [PubMed] [Google Scholar]
- Zhang Z et al (2012) Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 44:852–860. doi:10.1038/ng.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Dziadziuszko R, Peters S (2014) Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 40:716–722. doi:10.1016/j.ctrv.2014.03.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.