Abstract
Key points
Changes in extracellular osmolarity stimulate thirst and vasopressin secretion through a central osmoreceptor; however, central infusion of hypertonic NaCl produces a greater sympathoexcitatory and pressor response than infusion of hypertonic mannitol/sorbitol.
Neurons in the organum vasculosum of the lamina terminalis (OVLT) sense changes in extracellular osmolarity and NaCl.
In this study, we discovered that intracerebroventricular infusion or local OVLT injection of hypertonic NaCl increases lumbar sympathetic nerve activity, adrenal sympathetic nerve activity and arterial blood pressure whereas equi‐osmotic mannitol/sorbitol did not alter any variable.
In vitro whole‐cell recordings demonstrate the majority of OVLT neurons are responsive to hypertonic NaCl or mannitol. However, hypertonic NaCl stimulates a greater increase in discharge frequency than equi‐osmotic mannitol.
Intracarotid or intracerebroventricular infusion of hypertonic NaCl evokes a greater increase in OVLT neuronal discharge frequency than equi‐osmotic sorbitol.
Collectively, these novel data suggest that subsets of OVLT neurons respond differently to hypertonic NaCl versus osmolarity and subsequently regulate body fluid homeostasis. These responses probably reflect distinct cellular mechanisms underlying NaCl‐ versus osmo‐sensing.
Abstract
Systemic or central infusion of hypertonic NaCl and other osmolytes readily stimulate thirst and vasopressin secretion. In contrast, central infusion of hypertonic NaCl produces a greater increase in arterial blood pressure (ABP) than equi‐osmotic mannitol/sorbitol. Although these responses depend on neurons in the organum vasculosum of the lamina terminalis (OVLT), these observations suggest OVLT neurons may sense or respond differently to hypertonic NaCl versus osmolarity. The purpose of this study was to test this hypothesis in Sprague‐Dawley rats. First, intracerebroventricular (icv) infusion (5 μl/10 min) of 1.0 m NaCl produced a significantly greater increase in lumbar sympathetic nerve activity (SNA), adrenal SNA and ABP than equi‐osmotic sorbitol (2.0 osmol l−1). Second, OVLT microinjection (20 nl) of 1.0 m NaCl significantly raised lumbar SNA, adrenal SNA and ABP. Equi‐osmotic sorbitol did not alter any variable. Third, in vitro whole‐cell recordings demonstrate that 50% (18/36) of OVLT neurons display an increased discharge to both hypertonic NaCl (+7.5 mm) and mannitol (+15 mm). Of these neurons, 56% (10/18) displayed a greater discharge response to hypertonic NaCl vs mannitol. Fourth, in vivo single‐unit recordings revealed that intracarotid injection of hypertonic NaCl produced a concentration‐dependent increase in OVLT cell discharge, lumbar SNA and ABP. The responses to equi‐osmotic infusions of hypertonic sorbitol were significantly smaller. Lastly, icv infusion of 0.5 m NaCl produced significantly greater increases in OVLT discharge and ABP than icv infusion of equi‐osmotic sorbitol. Collectively, these findings indicate NaCl and osmotic stimuli produce different responses across OVLT neurons and may represent distinct cellular processes to regulate thirst, vasopressin secretion and autonomic function.
Keywords: blood pressure, osmoreceptor, sympathetic
Key points
Changes in extracellular osmolarity stimulate thirst and vasopressin secretion through a central osmoreceptor; however, central infusion of hypertonic NaCl produces a greater sympathoexcitatory and pressor response than infusion of hypertonic mannitol/sorbitol.
Neurons in the organum vasculosum of the lamina terminalis (OVLT) sense changes in extracellular osmolarity and NaCl.
In this study, we discovered that intracerebroventricular infusion or local OVLT injection of hypertonic NaCl increases lumbar sympathetic nerve activity, adrenal sympathetic nerve activity and arterial blood pressure whereas equi‐osmotic mannitol/sorbitol did not alter any variable.
In vitro whole‐cell recordings demonstrate the majority of OVLT neurons are responsive to hypertonic NaCl or mannitol. However, hypertonic NaCl stimulates a greater increase in discharge frequency than equi‐osmotic mannitol.
Intracarotid or intracerebroventricular infusion of hypertonic NaCl evokes a greater increase in OVLT neuronal discharge frequency than equi‐osmotic sorbitol.
Collectively, these novel data suggest that subsets of OVLT neurons respond differently to hypertonic NaCl versus osmolarity and subsequently regulate body fluid homeostasis. These responses probably reflect distinct cellular mechanisms underlying NaCl‐ versus osmo‐sensing.
Abbreviations
- ABP
arterial blood pressure
- aCSF
artificial cerebrospinal fluid
- AP
action potential
- ENaC
epithelial sodium channel
- icv
intracerebroventricular
- KRB
oxygenated Krebs buffer
- NMDG
N‐methyl‐d‐glucamine
- OVLT
organum vasculosum of the lamina terminalis
- PVN
paraventricular nucleus
- SFO
subfornical organ
- SNA
sympathetic nerve activity
- SON
supraoptic nucleus
- VP
vasopressin
Introduction
Extracellular fluid osmolality is a consistent and vital variable that contributes to body fluid homeostasis. Elevations in either plasma or cerebrospinal fluid (CSF) osmolality as low as 1–3% stimulate motivated behaviours, stimulate neuroendocrine function, and alter sympathetic nerve activity (SNA) and arterial blood pressure (ABP) to maintain an osmotic set point that preserves body fluid homeostasis (Verney, 1947; Bourque, 2008). A multitude of studies have established a causative relationship between increases in plasma or CSF osmolality and the genesis of behavioural thirst and vasopressin (VP) secretion, regardless of the osmotic solute (Dunn et al. 1973; Buggy et al. 1979; McKinley et al. 1980a, b; Thrasher et al. 1980a, b; Thompson et al. 1986). Curiously, the ability of hyperosmotic perturbations to increase ABP varies depending on the solute. Specifically, central infusion of hypertonic NaCl elevates ABP much more than hypertonic sugar osmolytes (i.e. sorbitol, mannitol) (Bunag & Miyajima, 1984; Tiruneh et al. 2013; Frithiof et al. 2014). This dichotomy has important implications regarding NaCl‐ versus osmosensory function as sodium and chloride are the major ubiquitous extracellular osmolytes to elicit osmoregulatory responses to osmotic challenges imposed by dehydration and high dietary NaCl intake.
The primary set of osmosensitive neurons is located within sensory circumventricular organs such as the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT) (Bourque, 2008; Kinsman et al. 2017a). The SFO and OVLT sit outside the blood–brain barrier and, as a consequence, are uniquely poised to detect alterations in plasma and CSF osmolality (Broadwell & Brightman, 1976; McKinley et al. 1983). Peripheral or central administration of hypertonic solutes activate OVLT and SFO neurons, as evidenced by elevated nuclear c‐Fos immunoreactivity (Oldfield et al. 1991, 1994; Solano‐Flores et al. 1993; Larsen & Mikkelsen, 1995; Taylor et al. 2008; Kinsman et al. 2014). Furthermore, hypertonic NaCl and sorbitol/mannitol independently elicit excitatory responses from OVLT and SFO neurons in vitro (Sayer et al. 1984; Sibbald et al. 1988; Vivas et al. 1990; Anderson et al. 2000; Ciura & Bourque, 2006; Kinsman et al. 2017b). Limited in vivo single unit recordings also indicate that peripheral or central hypertonic NaCl stimulates increases in OVLT and SFO discharge frequency (Gutman et al. 1988; Honda et al. 1990; Nation et al. 2016; Kinsman et al. 2017b). In turn, OVLT and SFO neurons activate downstream targets in thalamico‐cortical circuits to stimulate thirst (Denton et al. 1999; Hollis et al. 2008), in the supraoptic (SON) and paraventricular (PVN) nuclei to increase VP secretion, and in the PVN to alter SNA and ABP (Ferguson & Kasting, 1986; Gutman et al. 1988; Oldfield et al. 1994; Larsen & Mikkelsen, 1995; Richard & Bourque, 1995; Hochstenbach & Ciriello, 1996; Shi et al. 2008; Kinsman et al. 2017b). The functional significance of these cellular responses is highlighted by studies in which lesion or pharmacological inhibition of the SFO or OVLT attenuates thirst, VP secretion and pressor responses to hypertonic NaCl (Thrasher et al. 1982; Lind et al. 1984; Mangiapane et al. 1984; McKinley et al. 1999; Shi et al. 2007; Tiruneh et al. 2013; Kinsman et al. 2017b).
Although OVLT (and SFO) neurons sense changes in extracellular NaCl concentrations and osmolarity, previous studies have not assessed whether OVLT neurons respond differently to hypertonic NaCl versus mannitol/sorbitol. Differences in the response magnitudes or within distinct populations of OVLT neurons may explain the dichotomy in osmotic regulation of thirst and VP secretion versus NaCl‐driven changes in autonomic function and ABP. Furthermore, such findings may suggest distinct cellular mechanisms underlying NaCl‐ versus osmosensory function. Consequently, we conducted a series of novel in vivo neurophysiological experiments and in vitro whole cell patch‐clamp experiments to determine the extent by which hypertonic NaCl versus mannitol or sorbitol exerted differential effects on OVLT neurons, SNA and ABP.
Methods
Ethical approval
All experimental procedures conform to the National Institutes of Health Guide for the Care and use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Pennsylvania State College of Medicine (#46442) and the University of Pittsburgh (#16129731). Experiments were conducted in male Sprague‐Dawley rats (250–400 g, Charles River Laboratories, Wilmington, MA, USA) housed in a temperature‐controlled room (22 ± 1°C) with a 12 h dark/light cycle. Rats were fed standard chow (Teklad Global Diet 2018, Harlan, Indianapolis, IN, USA) and given access to deionized water ad libitum. Anaesthesia and euthanasia methods are described below for each experimental protocol. In all experiments, the level of anaesthesia was assessed by the lack of a withdrawal reflex to a foot pinch. The investigators conform to the ethical principles and animal ethics checklist required by The Journal of Physiology.
In vivo experiments
General procedures
Rats were initially anaesthetized with isoflurane (2–3% in 100% O2) and prepared for ABP measurements (brachial arterial catheter) and simultaneous recording of multiple sympathetic nerves (lumbar, renal, splanchnic and adrenal) as described previously (Ward et al. 2011; Simmonds et al. 2014; Steiner et al. 2014; Stocker et al. 2015; Kinsman et al. 2017b). Briefly, the lumbar sympathetic nerve was isolated through a ventral midline incision after gentle retraction of the vena cavae, isolated and placed on bipolar stainless steel electrodes, and insulated with KWIK‐SIL (World Precision Instruments, Sarasota, FL, USA). The renal, splanchnic and adrenal sympathetic nerves were isolated through a left retroperitoneal incision and gentle retraction of the kidney, placed on separate sets of bipolar electrodes, and insulated with KWIK‐SIL. Retractors were removed, and both incisions were closed with staples. An additional catheter was implanted in the femoral artery and jugular vein. Animals were artificially ventilated. (35–40 mmHg) and end‐tidal CO2 (3.5–4.5%) were measured continuously by a GEMINI Gas Respiratory Analyser (CWE, Inc., Ardmore, PA, USA) maintained at the respective levels by adjusting ventilation rate (60–80 bpm) or tidal volume (1 ml/100 g body weight). Body temperature was measured continuously via rectal probe (Sable Systems, Las Vegas, NV, USA) and maintained at 37 ± 0.5°C by a water‐circulating blanket. After rats were placed into a stereotaxic head frame, 26‐gauge cannulae were implanted into the left and right lateral ventricles (Simmonds et al. 2014). After all surgical procedures were completed, anaesthesia was replaced by inactin (120 mg kg−1, i.v.). Animals also received a continuous infusion of 0.75% NaCl and 0.25% glucose (0.5 ml h−1, i.v.). To gain access to the OVLT in a subset of experiments, the rat was placed supine into the stereotaxic frame. The ventral surface of the hypothalamus was visualized through a ventral midline approach by removal of the hard and soft palate as described previously (Leng & Dyball, 1991; Kinsman et al. 2017b). Experiments began at 60 min after all surgical procedures were completed.
Sympathetic and ABP responses during icv infusion of hypertonic NaCl vs sorbitol
Rats were prepared as described above for SNA recordings and brain cannulae in the lateral ventricle. Animals were pretreated with the VP antagonist Manning Compound (10 μg kg−1, i.v.) to prevent the vasconstrictive effects of VP and isolate sympathetically mediated changes in ABP. Then, icv infusion (5 μl for 10 min) of 1 m NaCl or 2 osmol l−1 sorbitol was performed in a randomized manner, and variables were recorded for an additional 60 min. We have previously reported that icv infusion of 1 m NaCl increases CSF [Na+] ∼4–5 mm (Stocker et al. 2015). Each animal received both icv infusions separated by >1 h when variables returned to baseline values for at least 30 min. Solutions were prepared in artificial CSF (aCSF) containing (in mm): 128 NaCl, 3 KCl, 1.2 CaCl2, 0.8 MgCl2, 3.4 glucose, 5 HEPES adjusted to pH 7.4. Animals were killed via an overdose of inactin (250 mg kg−1, i.v.) and cardiac transection. Variables were averaged in 1 min bins. The area under the curve was calculated as the average 10 min peak response.
OVLT‐specific effects of hypertonic NaCl vs sorbitol on SNA and ABP
Animals were prepared for SNA recordings and ventral hypothalamic exposure as described above. Initially, the OVLT was identified functionally by a sympathoexcitatory and pressor response (>5 mmHg) to microinjection of 1 m NaCl (20 nl over 60 s) using coordinates in reference to the rostral end of the optic chiasm: −0.6 to 1.2 mm caudal, 0.0 mm lateral and 1.3–1.6 mm depth relative to the ventral surface. The pipette was moved in 200 μm steps rostral‐caudally until a sympathoexcitatory response was observed and required up to two injections. The pipette was removed and rinsed with aCSF (5×). Then, hypertonic sorbitol (2 osmol l−1 dissolved in aCSF) or NaCl (1 m) was microinjected in a randomized manner separated by 45 min. Variables were recorded for an additional 45 min. Variables were averaged in 1 min bins. Responses were analysed and compared between a 1 min peak versus a 5 min baseline.
All microinjections were performed using single‐barrel glass micropipettes (20–30 μm outer diameter) connected to a picopump and monitored with an eyepiece reticule. Injection sites were marked by addition of rhodamine (sorbitol) or fluorescein isothiocyanate (NaCl) beads (0.2%, Molecular Probes, Carlsbad, CA, USA) to the respective solution. Animals were killed via exsanguination and transcardial perfusion with 4% paraformaldehyde. Brains were post‐fixed overnight, sectioned at 50 μm on a vibratome, and mounted to visualize injection sites with a Leica DM6000b epifluorescence microscope in Volocity 6.3 software (PerkinElmer, Waltham, MA, USA).
In vivo single‐unit responses of OVLT neurons to intracarotid infusion of hypertonic NaCl vs sorbitol
Rats were prepared as described above using a ventral approach to access the OVLT. Intracarotid infusion of hypertonic NaCl and sorbitol were performed via a non‐occlusive catheter (heat‐stretched PE‐10) inserted into the ascending pharyngeal artery and then advanced 1.5 mm past the carotid bifurcation and into the internal carotid artery. Single‐unit recordings were performed with glass electrodes (10–25 MΩ), filled with 4% neurobiotin (dissolved in 0.5% sodium acetate, pH 7.4) and an intracellular amplifier in bridge mode (Axoclamp 2B, Molecular Devices, Sunnyvale, CA, USA). The OVLT was probed for spontaneously active units in 2 μm steps. Once a unit was isolated, neuronal responses to intracarotid infusion (50 μl) of NaCl (0.15, 0.3 or 0.5 m) or sorbitol (1.0 osmol l−1) were tested in a randomized order and separated by >5 min. All solutions were prepared in isotonic saline and flushed through the intracarotid catheter with isotonic saline infusion (150 μl over 15 s). Assuming carotid blood flow is 5.5 ml min−1 in a rat (Guyton & Hartley, 1985), these infusions of 0.15, 0.3 and 0.5 m NaCl or 1.0 osmol l−1 sorbitol should acutely increase carotid blood osmolality by 0, 3 and 7%, respectively. All variables were averaged in 1 s bins. Discharge responses, SNA and mean ABP were compared between a 30 s baseline versus a 4 s peak response.
At the end of single‐unit recordings, cells were juxtacellularly labelled as described previously (Pinault, 1996; Stocker & Toney, 2005; Kinsman et al. 2017b) by applying current pulses (200 ms, 50% duty cycle) of increasing amplitude (1.0–8.0 nA) delivered through the recording electrode for 20–180 s of entrainment. Then, animals were perfused transcardially with 4% paraformaldehyde. Brains were post‐fixed overnight, sectioned at 50 μm on a vibratome and incubated with streptavidin AlexaFluor 488 or 594 to visualize filled cells (or recording sites) with a Leica DM6000b epifluorescence microscope in Volocity 6.3 software (PerkinElmer).
In vivo single‐unit responses to icv infusion of hypertonic NaCl vs sorbitol
A separate group of rats was prepared as described above including icv brain cannulas and an intracarotid catheter. The OVLT was exposed using a ventral approach. After a spontaneously active unit was identified, neuronal responses to intracarotid injection of 0.5 m NaCl (50 μl) were tested to identify an NaCl‐responsive OVLT neuron as described above. Then, neuronal responses to icv infusion (2.5 μl for 5 min) of 0.5 m NaCl and 1.0 osmol l−1 sorbitol (dissolved in aCSF) were tested in a randomized order separated by 30 min. A smaller infusion rate and concentration was used here versus other experiments to facilitate shorter response duration to permit sufficient time to test both icv infusion per unit. At the end of recordings, units were juxtacellularly labelled and brains harvested as described above. Discharge responses to icv infusions were compared between a 3 min baseline and 1 min peak discharge response.
In vitro electrophysiology
Whole‐cell patch‐clamp recordings of OVLT neurons were performed as described previously in our laboratory (Kinsman et al. 2017b). Briefly, rats were anaesthetized deeply with 5% isoflurane and decapitated. The brains were extracted rapidly into oxygenated (95% O2/5% CO2), ice‐cold N‐methyl‐d‐glucamine (NMDG)‐based aCSF (composition in mm): 98 NMDG, 2.5 KCl, 1.2 NaH2PO4, 20 HEPES, 91 HCl, 10 MgSO4, 0.5 CaCl2, 25 NaHCO3, 11 d‐glucose (pH 7.39 and 295 mosmol l−1). Coronal slices containing the OVLT were cut at 250 μm thickness on a vibratome with a sapphire blade (Delaware Diamond Knives, Wilmington, DE, USA). Slices were then incubated at 33 ± 1°C in oxygenated NMDG aCSF for 15 min, then transferred to oxygenated Krebs buffer (KRB; composition in mm): 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4 and 11 d‐glucose (pH 7.4 and 295 mosmol l−1) to incubate for an additional 90 min prior to cell recordings. Slices were continuously bathed in the slice chamber (500 μl) by KRB via a gravity‐fed perfusion system at 2–3 ml min−1 and warmed to 31 ± 0.5°C with an SF‐28 inline heater and TC‐324B temperature controller (Warner Instruments, Hamden, CT, USA).
Whole‐cell recordings were made with borosilicate patch‐pipettes pulled to resistance of 5–8 MΩ when filled with potassium gluconate intracellular solution (composition in mm): 128 potassium gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 HEPES, 1 EGTA, 4 MgATP, 2 Na2phosphocreatine and 0.3 NaGTP adjusted to pH 7.35 with KOH and osmolarity 280 ± 2 mosmol l−1. Data were acquired in Clampex 10.3 software with an Axopatch 200B amplifier (Molecular Devices) at a rate of 10 kHz, filtered at 2 kHz and digitized with a Digidata 1440A interface before being saved on a personal computer and analysed in Clampfit 10.7 (Molecular Devices) and Spike 2.0 software. Only neuronal recordings maintaining a series resistance (i.e. pipette + access resistance) <20 MΩ were considered of acceptable quality. Liquid junction potential was measured as −12.1 mV and was digitally corrected for post hoc in Clampfit 10.7.
In vitro OVLT neuron excitation by hypertonic NaCl vs mannitol
Current‐clamp recordings evaluated OVLT neurons that were spontaneously firing action potentials (AP) when held at approximately −55 ± 2 mV with current injection. Firing rates were recorded in response to baseline KRB (3–5 min, 295 mosmol l−1), +15 mosmol (3 min, +7.5 mm NaCl or +15 mm mannitol, 310 mosmol l−1) and wash‐out KRB (5–10 min, 295 mosmol l−1). Neuronal responses to both NaCl and mannitol were tested in a randomized sequence. Hypertonic solutions were prepared by adding NaCl or mannitol to KRB. All solution osmolarities were measured in triplicate by freezing point depression using a 3320 Micro Osmometer (Advanced Instruments, Norwood, MA, USA). OVLT neurons were classified as NaCl‐sensitive and/or mannitol‐sensitive by evidence of a 25% or greater increase in peak AP firing rate averaged over the final 1 min interval of each stimulus. Neurons were classified post hoc as dual responsive, NaCl responsive, mannitol responsive or non‐responsive by the response criteria stated above. Only one OVLT slice was obtained per rat and one or two OVLT neurons were recorded per slice.
In vitro OVLT neuron passive membrane properties
Current–voltage relationships were analysed in voltage clamp by holding OVLT neurons at −50 mV and applying 400 ms duration, 10 mV hyperpolarizing current steps from −50 to −120 mV. A linear regression was derived from the I–V relationship from −80 to −50 mV. Resting membrane potential was calculated as the x‐intercept of the regression equation. Input resistance was calculated from the difference in current between −60 and −70 mV holding potentials
Statistics
Analyses and graphs were prepared with SigmaPlot 11 (Systat Software, Chicago, IL, USA). All data are presented as mean ± SEM. In vivo experiments were analysed by one‐ or two‐way ANOVA. When significant F values were obtained, post hoc comparisons were analysed through paired or independent t tests with a layered Bonferroni correction.
For in vitro cell recordings, the proportion of OVLT neurons in each stimulus response category was compared between stimulus application sequences using a chi‐square test of independence. These AP firing rates were analysed within each response group by one‐way repeated‐measures ANOVA. When significant F values were obtained, layered Bonferroni paired t tests were performed to evaluate differences in AP firing rates from baseline in response to hypertonic NaCl versus mannitol. The change in AP firing rate compared to baseline was calculated for each neuron response to NaCl and mannitol, and compared within each response group using Wilcoxon signed rank tests. OVLT neuron passive membrane properties were compared between response groups by one‐way ANOVA. The delta AP firing rates to hypertonic NaCl versus mannitol were compared between OVLT neuron subgroups by two‐way ANOVA and layered Bonferroni independent t tests. P < 0.05 was considered statistically significant for all comparisons.
Results
Sympathetic and ABP responses during icv infusion of hypertonic NaCl vs sorbitol
An initial set of experiments evaluated the extent by which icv infusion of hypertonic NaCl produced a differential effect on SNA and ABP versus icv infusion of equi‐osmotic sorbitol. There were no differences between hypertonic NaCl and sorbitol trials for baseline mean ABP (89 ± 4 vs 85 ± 3 mmHg, respectively; P = 0.374) and heart rate (360 ± 12 vs 358 ± 10 bpm, respectively; P = 0.816). As previously reported (Simmonds et al. 2014; Stocker et al. 2015; Kinsman et al. 2017b), icv infusion of 1 m NaCl increased lumbar SNA, adrenal SNA and ABP (Fig. 1). On the other hand, icv infusion of equi‐osmotic 2.0 osmol l−1 sorbitol (dissolved in aCSF) did not alter any variable (Fig. 1). In fact, hypertonic NaCl produced significantly greater increases in lumbar SNA, adrenal SNA and mean ABP versus equi‐osmotic sorbitol. There were no statistical differences in renal SNA (P = 0.268), splanchnic SNA (P = 0.310) or heart rate (P = 0.188) between 1 m NaCl vs 2 osmol sorbitol (Fig. 1 B).
Figure 1. icv infusion of hypertonic NaCl versus sorbitol produces a significantly greater sympathoexcitatory response.
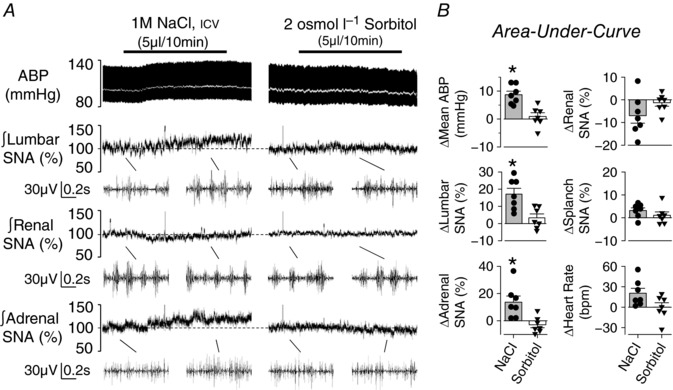
A, example of ABP, mean ABP (white line), and integrated lumbar, renal and adrenal SNA during icv infusion of (left) 1 m NaCl (5 μl per 10 min) or (right) 2 osmol l−1 sorbitol (5 μl per 10 min). Note, baseline and peak examples (0.8 s) of raw SNA for the respective nerve are provided below the integrated signal. B, mean ± SEM and individual data points representing average change or area under the curve (5–15 min) of the respective variable. * P < 0.05, NaCl vs sorbitol.
OVLT‐specific effects of hypertonic NaCl vs sorbitol on SNA and ABP
A second set of experiments was performed to assess whether local OVLT injection of hypertonic NaCl produced different effects on SNA and ABP versus equi‐osmotic sorbitol. There were no differences between hypertonic NaCl and sorbitol injections for baseline ABP (89 ± 7 vs 91 ± 6 mmHg, respectively; P = 0.421) and heart rate (425 ± 18 vs 428 ± 12 bpm, respectively; P = 0.780). As previously reported (Kinsman et al. 2017b), OVLT injection of 1 m NaCl promptly increased lumbar SNA, adrenal SNA, heart rate and mean ABP (Fig. 2 A, B). OVLT injection of equi‐osmotic sorbitol did not alter any variable. In fact, OVLT injection of 1 m NaCl evoked a significantly greater increase in lumbar SNA, adrenal SNA, heart rate and mean ABP than equi‐osmotic sorbitol (Fig. 2 A, B). There were no statistical differences in renal SNA (P = 0.161) or splanchnic SNA (P = 0.100) between treatments (Fig. 2 B). Histological verification of OVLT injection sites confirmed injection of NaCl and sorbitol were localized to overlapping regions of the OVLT (Fig. 2 C).
Figure 2. OVLT microinjection of hypertonic NaCl versus sorbitol produced a greater sympathoexcitatory response.
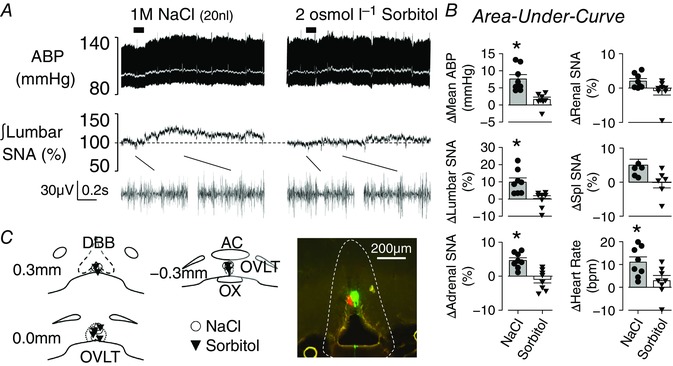
A, example of ABP, mean ABP (white line) and integrated lumbar SNA during OVLT microinjection (20 nl) of 1 m NaCl or 2 osmol l−1 sorbitol. Note, baseline and peak examples (0.8 s) of raw lumbar SNA are provided below the integrated signal. B, mean ± SEM and individual data points of the peak 2 min response. * P < 0.05 NaCl vs sorbitol. C, schematic illustration of OVLT injection sites. Digital images of representative injection sites marked with 0.2% fluorescein isothiocyanate (NaCl) or rhodamine (sorbitol) beads. [Color figure can be viewed at wileyonlinelibrary.com]
In vitro OVLT neuron excitation by hypertonic NaCl vs mannitol
In vitro experiments were then performed to investigate whether these divergent effects of NaCl versus osmolality on SNA and ABP reflect different responses of OVLT neurons to these stimuli. Whole‐cell patch‐clamp experiments were performed on OVLT neurons to assess neuronal response to bath application of hypertonic NaCl versus equi‐osmotic mannitol. A total of 36 neurons were recorded from 32 rats. Of these OVLT neurons, 50% (18/36) displayed an increase in AP firing rates to both hypertonic NaCl (1.26 ± 0.25 to 2.16 ± 0.36 Hz; P < 0.001) and mannitol (1.13 ± 0.25 Hz to 1.58 ± 0.28 Hz; P < 0.01; between osmotic treatments F 3,51 = 6.859, P < 0.01) and were deemed dual‐responsive (Fig. 3 Ai, Ci). Interestingly, these dual‐responsive OVLT neurons displayed a greater change in AP firing rate in response to hypertonic NaCl versus mannitol (0.90 ± 0.21 vs 0.45 ± 0.07 Hz; P < 0.01, Fig. 3 Di).
Figure 3. Hypertonic NaCl and mannitol produce different reponses in OVLT neurons in vitro .
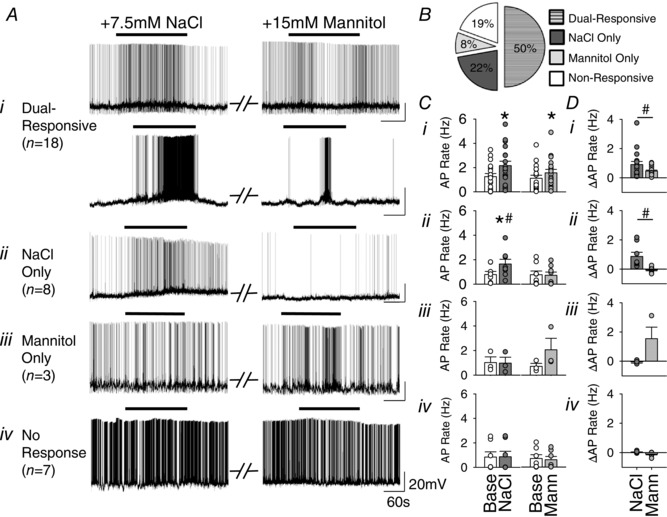
A, (i–iv) whole‐cell recording examples of OVLT neurons at baseline (approximately −55 mV) and in response to randomized bath application of +7.5 mm NaCl (left) and +15 mm mannitol (right) demonstrating four distinct response groups (total n = 36 neurons/32 rats). B, pie chart illustrating the proportion of OVLT neurons classified as dual‐responsive, NaCl only, mannitol only and non‐responsive. A 25% or greater increase in peak 60 s AP firing rate qualified responsiveness to a given hypertonic stimulus. C, (i–iv) peak 60 s AP firing rates in response to hypertonic NaCl and mannitol in four distinct response groups. D, (i–iv) Comparison of the change in AP firing rate from baseline elicited by hypertonic NaCl versus mannitol in four distinct groups. * P < 0.05, stimulus compared to baseline. # P < 0.05, NaCl compared to mannitol.
Smaller populations of OVLT neurons were responsive to either hypertonic NaCl or hypertonic mannitol. For example, 22% (8/36) of OVLT neurons displayed an increase in AP firing rate to hypertonic NaCl (0.79 ± 0.26 to 1.65 ± 0.39 Hz; P < 0.001) but not mannitol (0.77 ± 0.30 to 0.72 ± 0.27 Hz; P = 1.000, between osmotic treatments F 3,21 = 10.733, P < 0.001, Fig. 3 Aii, Cii, Dii). In fact, 3/8 of these NaCl‐sensitive OVLT neurons decreased AP firing rate in response to hypertonic mannitol (Fig. 3 Aii, Dii). Conversely, 8% (3/36) of OVLT neurons demonstrated a non‐significant increase in AP firing rate in response to mannitol (0.81 ± 0.28 to 2.35 ± 1.06 Hz; P = 0.189) but no response to hypertonic NaCl (1.02 ± 0.46 to 0.98 ± 0.48 Hz; P = 0.606; between osmotic treatments F 3,6 = 4.032, P = 0.069, Fig. 3 Aiii, Ciii, Diii). Finally, a small population of OVLT neurons (19%, 7/36) did not display a change in AP firing rates to either hypertonic NaCl (0.84 ± 0.43 to 0.86 ± 0.45 Hz; P = 0.352) or hypertonic mannitol (0.70 ± 0.31 to 0.60 ± 0.27 Hz; P = 0.121; between osmotic treatments F 3,18 = 1.662, P = 0.211; Fig. 3 Aiv, Civ, Div).
OVLT neuron responsiveness was not dictated by stimulus application order (χ2 = 2.3414, P = 0.505). In addition, no significant differences were observed between response groups on the basis of resting membrane potential (in mV; dual responsive: −50.5 ± 3.8; NaCl: −48.8 ± 7.0; mannitol: −47.0 ± 6.1; non‐responsive: −50.6 ± 5.6; P = 0.328) or input resistance (in MΩ; dual responsive: 1956 ± 392; NaCl: 1862 ± 410; mannitol: 2163 ± 292; non‐responsive: 2138 ± 451; P = 0.783).
A post hoc analysis of dual‐responsive OVLT neurons revealed two distinct populations by comparing the magnitude of the discharge response to hypertonic NaCl versus mannitol (ΔAP RateNaCl − ΔAP RateMann). This comparison stratified OVLT neurons into two groups based on whether the difference in response magnitude was less than or greater than 0.1 Hz (Fig. 4 D). This threshold for group stratification was less than the 25% change in AP firing rate observed in response to either hypertonic NaCl or mannitol. Group 1 dual‐responsive neurons (10/18) displayed a greater increase in AP firing rate in response to hypertonic NaCl versus mannitol (1.36 ± 0.31 vs 0.50 ± 0.09 Hz; P < 0.05; Fig. 4 Ai, Bi, C). On the other hand, the magnitude of the changes in AP firing rate of Group 2 dual‐responsive OVLT neurons (6/18) was not different between hypertonic NaCl versus hypertonic mannitol (0.32 ± 0.08 vs 0.27 ± 0.07 Hz, P = 1.00; Fig. 4 Aii, Bii, C). Two dual‐responsive neurons displayed a greater increase in AP firing rate in response to hypertonic mannitol than NaCl and were excluded from the above analysis. Curiously, the baseline firing rates were significantly greater among Group 1 versus Group 2 neurons (1.57 ± 0.32 vs 0.54 ± 0.26 Hz; P < 0.05, Fig. 4 B). Furthermore, the change in AP firing rates in response to NaCl was greater in Group 1 versus Group 2 dual‐responsive neurons (1.36 ± 0.31 vs 0.32 ± 0.08 Hz; P < 0.05; across both osmotic treatments between groups F 1,28 = 9.026, P = 0.006; Fig. 4 C).
Figure 4. Post hoc analysis of dual‐responsive OVLT neurons reveals two populations with differential responses to hypertonic NaCl versus mannitol.
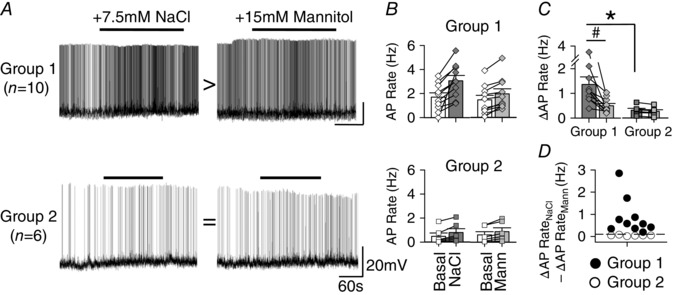
A, whole‐cell recording examples of OVLT neurons held at approximately −55 mV to illustrate two groups of OVLT dual‐responsive neurons. (top) Group 1 displayed a greater increase in AP firing rate in response to hypertonic NaCl vs mannitol. (bottom) Group 2 had similar increases in AP firing rates to hypertonic NaCl vs mannitol. B, peak 60 s AP firing rates in response to hypertonic NaCl and mannitol among Group 1 and Group 2. C, comparison of the change in AP firing rate from baseline elicited by NaCl vs mannitol shows significant difference in NaCl responses between groups. D, scatterplot illustrating the difference in discharge magnitudes to NaCl vs mannitol (ΔAP RateNaCl – ΔAP RateMann) of Group 1 (filled circles) versus Group 2 (open circles) neurons using a 0.1 Hz threshold (horizontal dashed line). # P < 0.05, NaCl compared to mannitol. * P < 0.05, NaCl compared between subgroups.
In vivo single‐unit responses of OVLT neurons to intracarotid infusion of hypertonic NaCl vs sorbitol
A fourth set of experiments was performed to assess in vivo discharge responses of OVLT neurons to hypertonic NaCl and sorbitol through an acute intracarotid injection. Baseline mean ABP and heart rate were 104 ± 3 mmHg and 397 ± 15 bpm, respectively. A total of 25 neurons were recorded from 13 rats. The majority of OVLT neurons (76%, 19/25) displayed a concentration‐dependent increase in cell discharge to intracarotid injection of 0.15, 0.3 and 0.5 m NaCl (Fig. 5 A, B). Intracarotid injection of NaCl also produced concentration‐dependent increases in lumbar SNA and mean ABP (Fig. 5 A, B). It is noteworthy that the changes in cell discharge preceded the changes in lumbar SNA and mean ABP as reflected by a shorter latency (7 ± 1 vs 12 ± 1 vs 14 ± 2 s, respectively; P < 0.05). Intracarotid injection of 1.0 osmol l−1 sorbitol dissolved in isotonic saline also increased cell discharge and mean ABP (Fig. 5). However, the magnitude of these responses was significantly less than those observed during intracarotid injection of 0.5 m NaCl (Fig. 5). Such differences were observed during analysis of absolute values (Fig. 5 B) or the change from baseline values (Fig. 5 C). It is noteworthy that a small population of OVLT neurons displayed a decrease (12%, 3/25) or no change (15%, 4/26) in cell discharge during intracarotid injection of 0.5 m NaCl and 1.0 osmol l−1 sorbitol (data not shown).
Figure 5. Intracarotid infusion of hypertonic NaCl produced a greater increase in OVLT neuronal discharge, ABP and lumbar SNA versus equi‐osmotic sorbitol.
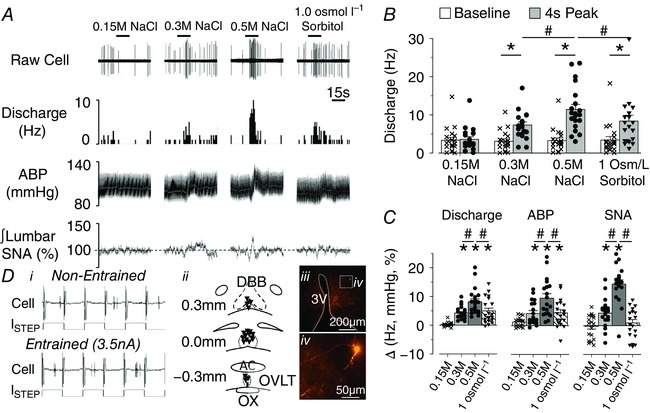
A, example of an in vivo single‐unit recording, rate‐meter histogram, ABP, mean ABP (white line) and integrated lumbar SNA during intracarotid infusion (50 μl over 15 s) of 0.15, 0.3 and 0.5 m NaCl or 1.0 osmol l−1 sorbitol. B, mean ± SEM and individual data points representing baseline and 4 s peak response in cell discharge. * P < 0.05, baseline vs 4 s peak; # P < 0.05, 0.5 m NaCl vs 0.3 m NaCl or 1.0 osmol l−1 sorbitol. C, mean + SEM and data points representing change in discharge, mean ABP or lumbar SNA. * P < 0.05 vs 0.15 m; # P < 0.05, 0.5 m NaCl vs 0.3 m NaCl or 1.0 osmol l−1 sorbitol. D, (i) example of cell discharge non‐entrained or entrained to the current pulses, (ii) schematic illustration of the anatomical location of recorded cells, and (iii, iv) low and high‐power digital image of a labelled cell visualized with streptavidin AlexaFluor 594. [Color figure can be viewed at wileyonlinelibrary.com]
At the end of experiments, recorded neurons were juxtacellularly labelled (Fig. 5 D). Figure 5 D illustrates an example of an OVLT neuron with activity not entrained to the current pules. After current pulses of increasing amplitude, cell discharge became entrained to the current pulses. Entrainment was maintained for 20–180 s depending on the neuron. Subsequent visualization of the neurobiotin indicated OVLT responsive neurons were distributed throughout the OVLT with processes that coursed along the 3rd ventricle (Fig. 5 Dii–iv).
In vivo single‐unit responses to icv infusion of hypertonic NaCl vs sorbitol
A final set of experiments were performed to assess neuronal responses in vivo to icv infusion of hypertonic NaCl versus sorbitol. There were no statistical differences between hypertonic NaCl versus sorbitol infusions for baseline mean ABP (113 ± 6 vs 113 ± 9 mmHg, P = 0.975, n = 6) or heart rate (407 ± 7 vs 403 ± 9 bpm, P = 0.391, n = 6). A total of six neurons were recorded from six rats that received both icv infusion of 0.5 m NaCl and 1.0 osmol l−1 sorbitol dissolved in aCSF. Initially, these cells were identified as NaCl‐responsive as intracarotid injection of 0.5 m NaCl increased cell discharge (2.8 ± 1.0 to 12.8 ± 3.8 Hz; P < 0.05). Intracarotid injection of sorbitol also appeared to increase cell discharge in these neurons but the effect was not statistically significant (2.7 ± 1.3 to 8.1 ± 2.0 Hz; P = 0.092 two‐tailed). The icv infusion of 0.5 m NaCl significantly increased cell discharge in these neurons from 2.4 ± 1.3 to 8.2 ± 2.7 Hz (Fig. 6 A, C). Although icv infusion of 1.0 osmol l−1 sorbitol also appeared to increased cell discharge from 2.6 ± 0.8 to 4.3 ± 1.6 Hz, the effect was not statistically significant (Fig. 6 A, C; P = 0.055, two‐tailed paired t test). In only one of six OVLT neurons was the increase in single‐unit discharge similar in response to 0.5 m NaCl and 1.0 osmol l−1 sorbitol (Fig. 6 B). Statistical analysis of the change in peak 2 min change in discharge for all neurons and mean ABP revealed a significantly greater increase in response to 0.5 m NaCl versus 1.0 osmol l−1 sorbitol (Fig. 6 D).
Figure 6. icv infusion of hypertonic NaCl produced a greater increase in OVLT neuronal discharge and ABP versus equi‐osmotic sorbitol.
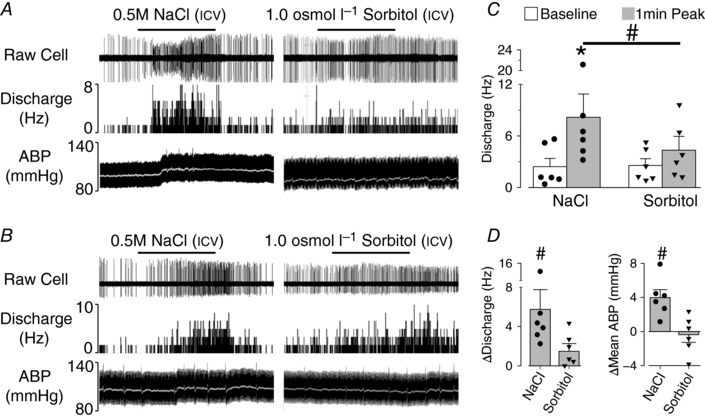
A, in vivo single‐unit recording of an OVLT neuron, rate‐meter histogram, ABP and mean ABP (white line) during icv infusion (2.5 μl/5 min) of 0.5 m NaCl or 1.0 osmol l−1 sorbitol; 0.5 m NaCl but not 1.0 osmol l−1 sorbitol increased OVLT discharge. B, OVLT single‐unit recording, rate‐meter histogram, ABP and mean ABP (white line) in which 0.5 m NaCl and 1.0 osmol l−1 sorbitol elicit a similar increase in cell discharge (n = 1). C, mean ± SEM baseline and peak cell discharge of all OVLT neurons during icv infusion of NaCl or sorbitol. D, mean ± SEM change in cell discharge and ABP. * P < 0.05 vs baseline, # P < 0.05 NaCl vs sorbitol.
Discussion
Evidence suggests hypertonic NaCl and osmolality stimulate similar increases in thirst and VP secretion but differentially affect SNA and ABP. Despite observations that NaCl‐ or osmosensing OVLT neurons mediate these effects, whether OVLT neurons differentially respond to hypertonic NaCl versus osmolarity was unknown previously. The current series of experiments provide several novel and insightful observations: (1) icv infusion of hypertonic NaCl produced significantly greater increases in lumbar SNA, adrenal SNA and ABP versus equi‐osmotic sorbitol; (2) local OVLT injection of hypertonic NaCl produced a greater increase in SNA and ABP than equi‐osmotic sorbitol; (3) in vitro whole‐cell recordings identified a population of OVLT neurons responsive to both hypertonic NaCl and mannitol but a subset of these neurons displayed a greater increase in AP firing rate to hypertonic NaCl versus mannitol; and (4) in vivo single‐unit recordings demonstrate intracarotid or icv infusion of hypertonic NaCl produced a significantly greater increase in OVLT neuronal discharge versus equi‐osmotic sorbitol. Taken together, these findings indicate hypertonic NaCl and osmolarity produce different cellular responses across OVLT neurons and raise the possibility that different cellular mechanisms may contribute to NaCl‐ versus osmosensing to regulate thirst, VP and autonomic function.
The prevailing theory indicates that a central osmoreceptor, located in the periventricular hypothalamus outside the blood–brain barrier, regulates thirst and VP secretion (Bourque, 2008; Kinsman et al. 2017a). Regardless of the osmotic solute, increased extracellular osmolality is known to stimulate thirst and VP secretion (Dunn et al. 1973; Buggy et al. 1979; McKinley et al. 1980a; Thrasher et al. 1980a,b; McKinley et al. 1980b; Thompson et al. 1986). However, central hypertonic NaCl increases ABP by a greater magnitude than hypertonic sugar osmolytes (Bunag & Miyajima, 1984; Tiruneh et al. 2013; Frithiof et al. 2014; Stocker et al. 2015). Our findings extend these observations as both icv and intracarotid infusion of hypertonic NaCl produced a greater increase in lumbar SNA and adrenal SNA versus equi‐osmotic sorbitol. These observations suggest distinct mechanisms underlie NaCl‐ versus osmo‐sensing.
OVLT neurons represent the primary set of central osmoreceptors and largely mediate thirst, VP secretion and sympathoexcitation to hypernatraemia (Bourque, 2008; Kinsman et al. 2017b). Immunocytochemical detection of Fos and single‐unit recordings have revealed the presence of OVLT neurons responsive to hypertonic NaCl or osmolarity (Oldfield et al. 1991; Larsen & Mikkelsen, 1995; Shi et al. 2008; Kinsman et al. 2014, 2017b). In vitro electrophysiological studies report OVLT neurons display increased AP firing rate or inward current responses to physiological increases in hypertonic mannitol (+10 mosmol l−1) (Ciura & Bourque 2006) or NaCl (+2.5 mm or +5 mosmol l−1) (Kinsman et al. 2017b). Since OVLT neurons largely mediate the sympathoexcitatory response to icv hypertonic NaCl in rats, we initially examined whether local application of hypertonic NaCl versus sorbitol produced a differential response on SNA and ABP. Indeed, OVLT injection of 1 m NaCl produced a greater increase in SNA and ABP than injection of equi‐osmotic sorbitol. This observation suggests that the cellular response of OVLT neurons may differ between hypertonic NaCl versus osmolarity.
To test this hypothesis, we performed a series of in vivo single‐unit and in vitro whole‐cell patch clamp recordings of OVLT neurons during acute increases in NaCl concentrations versus osmolarity. Our investigations indicate that the majority of OVLT neurons are responsive to both hypertonic NaCl and hypertonic sorbitol/mannitol. Interestingly, hypertonic NaCl evoked a greater discharge response in a subset of OVLT neurons in vitro versus equi‐osmotic mannitol. This finding was confirmed in vivo during intracarotid or icv infusion of hypertonic NaCl and sorbitol. Although it is difficult to measure osmolality in the OVLT during intracarotid or icv infusions, these manipulations alter extracellular CSF or carotid sodium concentration or osmolality ∼0–7% as described in the Methods and published previously (Stocker et al. 2015). A previous study reported a similar observation on osmosensitive neurons of the supraoptic nucleus (Voisin et al. 1999). In addition, a small proportion of OVLT neurons in vitro displayed an increase in AP firing rate to either hypertonic NaCl or mannitol alone. Since such neurons were infrequently encountered, it is uncertain whether such responses reflect a distinct population of OVLT neurons. Collectively, these in vivo single‐unit and in vitro whole‐cell recordings provide the first evidence that hypertonic NaCl and osmolarity may produce different cellular responses in OVLT neurons and raise the possibility that NaCl‐ and osmo‐sensing are mediated by distinct cellular processes.
One explanation is that divergent OVLT neuron responses to hypertonic NaCl versus sorbitol/mannitol could reflect distinct neural circuits that differentially regulate thirst and VP secretion versus SNA. Retrograde tracing studies indicate that OVLT neurons innervate several thalamic nuclei and other hypothalamic structures including the median preoptic nucleus, SFO, SON and PVN (Oldfield et al. 1994; Larsen & Mikkelsen, 1995; Hollis et al. 2008). However, a paucity of OVLT neurons project to multiple efferent targets (Weiss & Hatton, 1990). Whether separate NaCl‐ and osmo‐sensitive OVLT populations contribute to the coordinated regulation of thirst and VP versus autonomic activity remains unknown. Such a parsimonious explanation of these experimental results warrants future investigations to delineate the properties and sensitivity (NaCl vs osmolarity) of OVLT neurons projecting to these different efferent targets and the neurochemical phenotype of these OVLT neurons.
While our data indicate that the OVLT is a critical regulator of NaCl‐ and osmo‐receptor‐related functions, the SFO plays a complementary role in these responses. SFO neurons are also excited by either hypertonic NaCl or hypertonic mannitol (Sibbald et al. 1988; Anderson et al. 2000). Compared to OVLT lesions, SFO lesions have a lower impact on osmotically stimulated thirst but attenuate osmotically stimulated VP secretion (Simpson et al. 1978; Hosutt et al. 1981; Thrasher et al. 1982; Lind et al. 1984; Mangiapane et al. 1984). Moreover, pressor responses to hypertonic NaCl, but not mannitol, can be evoked by the SFO (Tiruneh et al. 2013).
Whether changes in Na+ versus NaCl concentrations underlie salt‐sensitive neural regulation of BP remains uncertain. Dietary NaCl loading in both salt‐sensitive humans and rodent models increases plasma or CSF NaCl concentrations by 3–5 mm to elevate SNA and ABP (Nakamura & Cowley, 1989; Kawano et al. 1992; Schmidlin et al. 2007). Broad lesions of the anteroventral region of the third ventricle, which includes the OVLT, attenuate or prevent the development of hypertension in these same salt‐sensitive models including the Dahl salt‐sensitive (Dahl‐S), DOCA‐salt and AngII‐salt (Berecek et al. 1982; Goto et al. 1982; Marson et al. 1983). Limited data are available regarding the specific contribution of OVLT neurons (Collister et al. 2013). In regard to an Na+ versus NaCl‐specific mechanism, Na+ supplementation with non‐Cl− anions does not raise BP in these models (Kotchen et al. 1983; Whitescarver et al. 1984; Passmore et al. 1985; Sato et al. 1991). Moreover, Cl− supplementation with glycine, instead of Na+, also fails to raise BP in the Dahl‐S strain (Whitescarver et al. 1986). Similarly, among humans with essential hypertension, NaCl loading raises BP, but Na+ supplementation with an alternative anion (e.g. citrate or phosphate) does not (Kurtz et al. 1987; Shore et al. 1988). In contrast, a cohort of salt‐sensitive normotensive black men showed changes in BP specific to Na+ intake (Schmidlin et al. 2007). Clearly, pathophysiologies in renal electrolyte handling contribute to salt‐sensitive hypertension; however, the specific neural mechanisms are not well described.
Classically, hyperosmotic stimuli (NaCl or saccharides) are considered to stimulate thirst as a consequence of cellular dehydration due to osmotic pressure (Fitzsimons, 1972). Admittedly, we assume that osmotically equivalent solutions of NaCl and sorbitol/mannitol exert osmotic pressures that generate equivalent tonicity. Sorbitol and mannitol are inert saccharide isomers to which mammalian cells are largely impermeable. Consequently, these solutes are hypertonic and cause cellular dehydration and volume contraction, which is known to excite OVLT neurons (Ciura et al. 2011). Neurotransmission does cause persistent flux of Na+ and Cl− between intracellular and extracellular compartments; however, the intracellular concentrations of Na+ and Cl− are generally preserved by the Na+/K+ ATPase and the K+–Cl− cotransporter 2, respectively. As a consequence, the elevated extracellular concentrations of these ions are anticipated to remain hypertonic, evoking a similar cellular dehydration to sorbitol/mannitol. However, methods do not exist to measure absolute intracellular concentrations of these ions in these experimental preparations. If the NaCl and sorbitol or mannitol solutions are of equivalent tonicity, what accounts for the different responses of OVLT neurons to NaCl versus sorbitol/mannitol?
To date, the identity of the vertebrate NaCl‐ or osmo‐receptor mechanism remains unclear (Kinsman et al. 2017a). In vitro electrophysiology evidence indicates the osmosensitivity of OVLT neurons is mediated by an N‐terminal variant of the TRPV1 channel (Ciura & Bourque, 2006). This channel conducts a non‐selective cation current in response to osmotic shrinkage of OVLT neurons caused by mannitol (Ciura et al. 2011). However, experiments using TRPV1−/− mice or rats suggest slightly attenuated or normal osmotically stimulated thirst and VP secretion (Ciura & Bourque, 2006; Taylor et al. 2008; Kinsman et al. 2014; Tucker & Stocker, 2016). Second, electroneutral transporters may also contribute to brain Na+ and/or Cl− sensing. Selective knockdown of Na–K–Cl co‐transporter in PVN and SON impairs osmoregulatory responses to dehydration by depolarizing E Cl– (Konopacka et al. 2015). A third possibility is the NaX channel. Within the rat median preoptic nucleus, hypertonic NaCl activates a leak sodium current through the NaX channel, which is functionally coupled to activity of membrane‐proximate α1 isoforms of the Na+/K+ ATPase (Berret et al. 2013). Within the mouse SFO, hypertonic sodium stimulates NaX‐expressing ependycytes to release lactate and metabolically activate GABAergic SFO interneurons (Shimizu et al. 2007). These neurons modulate activity of glutamatergic efferent projections from SFO to the ventral bed nucleus of the stria terminalis to regulate salt appetite (Matsuda et al. 2017). In OVLT, NaX channel expression is confined to neurons and ependycytes in rats, and ependycytes in mice (Nehme et al. 2012). However, NaX knockout mice vs wild‐type littermates show normal osmoregulatory thirst or VP secretion (Hiyama et al. 2004; Nagakura et al. 2010). A final candidate is the epithelial sodium channel (ENaC). Previous studies suggest that central infusion of benzamil, a non‐voltage‐gated sodium channel blocker, attenuates VP secretion and sympathoexcitatory responses to central hypernatraemia as well as several models of salt‐sensitive hypertension (Gomez‐Sanchez & Gomez‐Sanchez, 1995; Nishimura et al. 1998; Huang & Leenen, 2002). Benzamil blocks a number of non‐voltage‐dependent Na+ channels including the ENaC. ENaC α, β, and γ subunits are expressed throughout the lamina terminalis on both neurons and glia (Amin et al. 2005; Miller et al. 2013). Moreover, the αENaC subunit colocalizes with c‐Fos immunoreactive neurons throughout the SFO and OVLT in response to systemic hypernatraemia (Miller et al. 2013). Although evidence supports a potential role for each of these candidates, future experiments are needed to identify NaCl versus osmosensitive mechanisms within OVLT neurons.
In summary, we have provided the first evidence that the hypertonic NaCl versus osmolarity produce different cellular responses across OVLT neurons. The divergent responses or distinct populations of OVLT neurons may permit a differential regulation of body fluid homeostatic responses (thirst and VP versus SNA). However, the identity and cellular mechanisms that underlie NaCl‐ versus osmo‐sensing in OVLT neurons has not been identified and could represent a novel therapeutic target to selectively treat salt‐sensitive hypertension but preserve osmoregulatory responses.
Additional information
Competing interests
The authors have no competing interests or conflicts.
Author contributions
Conception or design of the work (BJK, KNB, SDS), acquisition, analysis, and interpretation of data for the work (BJK, SDS), and drafting the work or revising it critically for intellectual content (BJK, KNB, SDS). All authors (BJK, KNB, SDS) approved the final version of the manuscript, confirm that they are accountable for the accuracy and integrity of the findings, and confirm that they are the authors of the manuscript.
Funding
The research was supported by National Heart, Lung, and Blood Institute Grants R01 HL‐113270 (SDS), R01 HL‐128388 (SDS) and F30 HL131269 (BJK) and American Heart Association Established Investigator Award 12EIA8230000 (SDS) and Great Rivers Predoctoral Fellowship 14PRE1953001 (BJK). The authors also thank the imaging support of the Pittsburgh Centre for Kidney Research NIH P30DK079307.
Acknowledgements
The authors thank Sarah S. Simmonds for technical assistance.
Linked articles This article is highlighted by a Perspective by Antunes. To read this Perspective, visit https://doi.org/10.1113/JP274868.
This is an Editor's Choice article from the 15 September 2017 issue.
References
- Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS & Leenen FH (2005). Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289, R1787–1797. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Washburn DL & Ferguson AV (2000). Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience 100, 539–547. [DOI] [PubMed] [Google Scholar]
- Berecek KH, Barron KW, Webb RL & Brody MJ (1982). Vasopressin–central nervous system interactions in the development of DOCA hypertension. Hypertension 4, 131–137. [PubMed] [Google Scholar]
- Berret E, Nehme B, Henry M, Toth K, Drolet G & Mouginot D (2013). Regulation of central Na+ detection requires the cooperative action of the NaX channel and α1 isoform of Na+/K+‐ATPase in the Na+‐sensor neuronal population. J Neurosci 33, 3067–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW (2008). Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Broadwell RD & Brightman MW (1976). Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol 166, 257–283. [DOI] [PubMed] [Google Scholar]
- Buggy J, Hoffman WE, Phillips MI, Fisher AE & Johnson AK (1979). Osmosensitivity of rat third ventricle and interactions with angiotensin. Am J Physiol Regul Integr Comp Physiol 236, R75–82. [DOI] [PubMed] [Google Scholar]
- Bunag RD & Miyajima E (1984). Sympathetic hyperactivity elevates blood pressure during acute cerebroventricular infusions of hypertonic salt in rats. J Cardiovasc Pharmacol 6, 844–851. [DOI] [PubMed] [Google Scholar]
- Ciura S & Bourque CW (2006). Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26, 9069–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Liedtke W & Bourque CW (2011). Hypertonicity sensing in organum vasculosum lamina terminalis neurons: a mechanical process involving TRPV1 but not TRPV4. J Neurosci 31, 14669–14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collister JP, Olson MK, Nahey DB, Vieira AA & Osborn JW (2013). OVLT lesion decreases basal arterial pressure and the chronic hypertensive response to AngII in rats on a high‐salt diet. Physiol Rep 1, e00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Shade R, Zamarippa F, Egan G, Blair‐West J, McKinley M, Lancaster J & Fox P (1999). Neuroimaging of genesis and satiation of thirst and an interoceptor‐driven theory of origins of primary consciousness. Proc Natl Acad Sci USA 96, 5304–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FL, Brennan TJ, Nelson AE & Robertson GL (1973). The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest 52, 3212–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV & Kasting NW (1986). Electrical stimulation in subfornical organ increases plasma vasopressin concentrations in the conscious rat. Am J Physiol Regul Integr Comp Physiol 251, R425–428. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT (1972). Thirst. Physiol Rev 52, 468–561. [DOI] [PubMed] [Google Scholar]
- Frithiof R, Xing T, McKinley MJ, May CN & Ramchandra R (2014). Intracarotid hypertonic sodium chloride differentially modulates sympathetic nerve activity to the heart and kidney. Am J Physiol Regul Integr Comp Physiol 306, R567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Sanchez EP & Gomez‐Sanchez CE (1995). Effect of central infusion of benzamil on Dahl S rat hypertension. Am J Physiol Heart Circ Physiol 269, H1044–1047. [DOI] [PubMed] [Google Scholar]
- Goto A, Ganguli M, Tobian L, Johnson MA & Iwai J (1982). Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt‐sensitive rats. Am J Physiol Heart Circ Physiol 243, H614–618. [DOI] [PubMed] [Google Scholar]
- Gutman MB, Ciriello J & Mogenson GJ (1988). Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 254, R746–754. [DOI] [PubMed] [Google Scholar]
- Guyton JR & Hartley CJ (1985). Flow restriction of one carotid artery in juvenile rats inhibits growth of arterial diameter. Am J Physiol Heart Circ Physiol 248, H540–546. [DOI] [PubMed] [Google Scholar]
- Hiyama TY, Watanabe E, Okado H & Noda M (2004). The subfornical organ is the primary locus of sodium‐level sensing by Na(x) sodium channels for the control of salt‐intake behavior. J Neurosci 24, 9276–9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach SL & Ciriello J (1996). Effect of lesions of forebrain circumventricular organs on c‐fos expression in the central nervous system to plasma hypernatremia. Brain Res 713, 17–28. [DOI] [PubMed] [Google Scholar]
- Hollis JH, McKinley MJ, D'Souza M, Kampe J & Oldfield BJ (2008). The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: a neuroanatomical framework for the generation of thirst. Am J Physiol Regul Integr Comp Physiol 294, R1390–1401. [DOI] [PubMed] [Google Scholar]
- Honda K, Negoro H, Dyball RE, Higuchi T & Takano S (1990). The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol 431, 225–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosutt JA, Rowland N & Stricker EM (1981). Impaired drinking responses of rats with lesions on the subfornical organ. J Comp Physiol Psychol 95, 104–113. [DOI] [PubMed] [Google Scholar]
- Huang BS & Leenen FH (2002). Brain amiloride‐sensitive Phe‐Met‐Arg‐Phe‐NH2‐gated Na+ channels and Na+‐induced sympathoexcitation and hypertension. Hypertension 39, 557–561. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Yoshida K, Kawamura M, Yoshimi H, Ashida T, Abe H, Imanishi M, Kimura G, Kojima S, Kuramochi M & Omae T (1992). Sodium and noradrenaline in cerebrospinal fluid and blood in salt‐sensitive and non‐salt‐sensitive essential hypertension. Clin Exp Pharmacol Physiol 19, 235–241. [DOI] [PubMed] [Google Scholar]
- Kinsman B, Cowles J, Lay J, Simmonds SS, Browning KN & Stocker SD (2014). Osmoregulatory thirst in mice lacking the transient receptor potential vanilloid type 1 (TRPV1) and/or type 4 (TRPV4) receptor. Am J Physiol Regul Integr Comp Physiol 307, R1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman BJ, Nation HN & Stocker SD (2017a). Hypothalamic signaling in body fluid homeostasis and hypertension. Curr Hypertens Rep 19, 50. [DOI] [PubMed] [Google Scholar]
- Kinsman BJ, Simmonds SS, Browning KN & Stocker SD (2017b). Organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension 69, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopacka A, Qiu J, Yao ST, Greenwood MP, Greenwood M, Lancaster T, Inoue W, Mecawi AS, Vechiato FM, de Lima JB, Coletti R, Hoe SZ, Martin A, Lee J, Joseph M, Hindmarch C, Paton J, Antunes‐Rodrigues J, Bains J & Murphy D (2015). Osmoregulation requires brain expression of the renal Na–K–2Cl cotransporter NKCC2. J Neurosci 35, 5144–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchen TA, Luke RG, Ott CE, Galla JH & Whitescarver S (1983). Effect of chloride on renin and blood pressure responses to sodium chloride. Ann Intern Med 98, 817–822. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Al‐Bander HA & Morris RC, Jr (1987). “Salt‐sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med 317, 1043–1048. [DOI] [PubMed] [Google Scholar]
- Larsen PJ & Mikkelsen JD (1995). Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci 15, 2609–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G & Dyball REJ (1991). Functional identification of magnocellular neurones In Neuroendocrine Research Methods, ed. Greenstein B, pp. 769–791. Harwood Academic Publishers, Chur, Switzerland. [Google Scholar]
- Lind RW, Thunhorst RL & Johnson AK (1984). The subfornical organ and the integration of multiple factors in thirst. Physiol Behav 32, 69–74. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Thrasher TN, Keil LC, Simpson JB & Ganong WF (1984). Role for the subfornical organ in vasopressin release. Brain Res Bull 13, 43–47. [DOI] [PubMed] [Google Scholar]
- Marson O, Saragoca MA, Ribeiro AB, Bossolan D, Tufik S & Ramos OL (1983). Anteroventral third ventricle and renin‐angiotensin system interaction in the two‐kidney, one clip hypertensive rat. Hypertension 5, V90–93. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Hiyama TY, Niimura F, Matsusaka T, Fukamizu A, Kobayashi K, Kobayashi K & Noda M (2017). Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat Neurosci 20, 230–241. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Leksell L, Tarjan E & Weisinger RS (1980a). Evidence for cerebral sodium sensors involved in water drinking in sheep. Physiol Behav 25, 501–504. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Leventer M, Penschow J, Weisinger RS & Wright RD (1983). Morphology of the organum vasculosum of the lamina terminalis (OVLT) of the sheep. Brain Res Bull 11, 649–657. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Mathai ML, Pennington G, Rundgren M & Vivas L (1999). Effect of individual or combined ablation of the nuclear groups of the lamina terminalis on water drinking in sheep. Am J Physiol Regul Integr Comp Physiol 276, R673–683. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Olsson K, Fyhrquist F & Liljekvist E (1980b). Transient vasopressin release and thirst in response to prolonged intracerebroventricular infusions of hypertonic mannitol in saline. Acta Physiol Scand 109, 427–431. [DOI] [PubMed] [Google Scholar]
- Miller RL, Wang MH, Gray PA, Salkoff LB & Loewy AD (2013). ENaC‐expressing neurons in the sensory circumventricular organs become c‐Fos activated following systemic sodium changes. Am J Physiol Regul Integr Comp Physiol 305, R1141–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura A, Hiyama TY & Noda M (2010). Na(x)‐deficient mice show normal vasopressin response to dehydration. Neurosci Lett 472, 161–165. [DOI] [PubMed] [Google Scholar]
- Nakamura K & Cowley AW, Jr (1989). Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension 13, 243–249. [DOI] [PubMed] [Google Scholar]
- Nation HL, Nicoleau M, Kinsman BJ, Browning KN & Stocker SD (2016). DREADD‐induced activation of subfornical organ neurons stimulates thirst and salt appetite. J Neurophysiol 115, 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme B, Henry M, Mouginot D & Drolet G (2012). The expression pattern of the Na+ sensor, NaX in the hydromineral homeostatic network: a comparative study between the rat and mouse. Front Neuroanat 6, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Ohtsuka K, Nanbu A, Takahashi H & Yoshimura M (1998). Benzamil blockade of brain Na+ channels averts Na+‐induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 274, R635–644. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK & McKinley MJ (1994). Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience 60, 255–262. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Bicknell RJ, McAllen RM, Weisinger RS & McKinley MJ (1991). Intravenous hypertonic saline induces Fos immunoreactivity in neurons throughout the lamina terminalis. Brain Res 561, 151–156. [DOI] [PubMed] [Google Scholar]
- Passmore JC, Whitescarver SA, Ott CE & Kotchen TA (1985). Importance of chloride for deoxycorticosterone acetate‐salt hypertension in the rat. Hypertension 7, I115–120. [DOI] [PubMed] [Google Scholar]
- Pinault D (1996). A novel single‐cell staining procedure performed in vivo under electrophysiological control: morpho‐functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or neurobiotin. J Neurosci Methods 65, 113–136. [DOI] [PubMed] [Google Scholar]
- Richard D & Bourque CW (1995). Synaptic control of rat supraoptic neurones during osmotic stimulation of the organum vasculosum lamina terminalis in vitro . J Physiol 489, 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ogata E & Fujita T (1991). Role of chloride in angiotensin II‐induced salt‐sensitive hypertension. Hypertension 18, 622–629. [DOI] [PubMed] [Google Scholar]
- Sayer RJ, Hubbard JI & Sirett NE (1984). Rat organum vasculosum laminae terminalis in vitro: responses to transmitters. Am J Physiol Regul Integr Comp Physiol 247, R374–379. [DOI] [PubMed] [Google Scholar]
- Schmidlin O, Forman A, Sebastian A & Morris RC, Jr (2007). Sodium‐selective salt sensitivity: its occurrence in blacks. Hypertension 50, 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT & Toney GM (2008). Intra‐carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol 586, 5231–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Stocker SD & Toney GM (2007). Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol 293, R2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K & Noda M (2007). Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 54, 59–72. [DOI] [PubMed] [Google Scholar]
- Shore AC, Markandu ND & MacGregor GA (1988). A randomized crossover study to compare the blood pressure response to sodium loading with and without chloride in patients with essential hypertension. J Hypertens 6, 613–617. [DOI] [PubMed] [Google Scholar]
- Sibbald JR, Hubbard JI & Sirett NE (1988). Responses from osmosensitive neurons of the rat subfornical organ in vitro . Brain Res 461, 205–214. [DOI] [PubMed] [Google Scholar]
- Simmonds SS, Lay J & Stocker SD (2014). Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JB, Epstein AN & Camardo JS, Jr (1978). Localization of receptors for the dipsogenic action of angiotensin II in the subfornical organ of rat. J Comp Physiol Psychol 92, 581–601. [DOI] [PubMed] [Google Scholar]
- Solano‐Flores LP, Rosas‐Arellano MP & Ciriello J (1993). C‐fos expression in arcuate nucleus following intracerebroventricular hypertonic saline injections. Neurosci Lett 164, 217–220. [DOI] [PubMed] [Google Scholar]
- Steiner JL, Bardgett ME, Wolfgang L, Lang CH & Stocker SD (2014). Glucocorticoids attenuate the central sympathoexcitatory actions of insulin. J Neurophysiol 112, 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Lang SM, Simmonds SS, Wenner MM & Farquhar WB (2015). Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 66, 1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD & Toney GM (2005). Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568, 599–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AC, McCarthy JJ & Stocker SD (2008). Mice lacking the transient receptor vanilloid potential 1 channel display normal thirst responses and central Fos activation to hypernatremia. Am J Physiol Regul Integr Comp Physiol 294, R1285–1293. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Bland J, Burd J & Baylis PH (1986). The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clin Sci (Lond) 71, 651–656. [DOI] [PubMed] [Google Scholar]
- Thrasher TN, Brown CJ, Keil LC & Ramsay DJ (1980a). Thirst and vasopressin release in the dog: an osmoreceptor or sodium receptor mechanism? Am J Physiol 238, R333–339. [DOI] [PubMed] [Google Scholar]
- Thrasher TN, Jones RG, Keil LC, Brown CJ & Ramsay DJ (1980b). Drinking and vasopressin release during ventricular infusions of hypertonic solutions. Am J Physiol 238, R340–345. [DOI] [PubMed] [Google Scholar]
- Thrasher TN, Keil LC & Ramsay DJ (1982). Lesions of the organum vasculosum of the lamina terminalis (OVLT) attenuate osmotically‐induced drinking and vasopressin secretion in the dog. Endocrinology 110, 1837–1839. [DOI] [PubMed] [Google Scholar]
- Tiruneh MA, Huang BS & Leenen FH (2013). Role of angiotensin II type 1 receptors in the subfornical organ in the pressor responses to central sodium in rats. Brain Res 1527, 79–86. [DOI] [PubMed] [Google Scholar]
- Tucker AB & Stocker SD (2016). Hypernatremia‐induced vasopressin secretion is not altered in TRPV1–/– rats. Am J Physiol Regul Integr Comp Physiol 311, R451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney EB (1947). The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond B Biol Sci 135, 25–106. [PubMed] [Google Scholar]
- Vivas L, Chiaraviglio E & Carrer HF (1990). Rat organum vasculosum laminae terminalis in vitro: responses to changes in sodium concentration. Brain Res 519, 294–300. [DOI] [PubMed] [Google Scholar]
- Voisin DL, Chakfe Y & Bourque CW (1999). Coincident detection of CSF Na+ and osmotic pressure in osmoregulatory neurons of the supraoptic nucleus. Neuron 24, 453–460. [DOI] [PubMed] [Google Scholar]
- Ward KR, Bardgett JF, Wolfgang L & Stocker SD (2011). Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57, 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ML & Hatton GI (1990). Collateral input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Res Bull 24, 231–238. [DOI] [PubMed] [Google Scholar]
- Whitescarver SA, Holtzclaw BJ, Downs JH, Ott CE, Sowers JR & Kotchen TA (1986). Effect of dietary chloride on salt‐sensitive and renin‐dependent hypertension. Hypertension 8, 56–61. [DOI] [PubMed] [Google Scholar]
- Whitescarver SA, Ott CE, Jackson BA, Guthrie GP, Jr & Kotchen TA (1984). Salt‐sensitive hypertension: contribution of chloride. Science 223, 1430–1432. [DOI] [PubMed] [Google Scholar]


