Abstract
Key points
It has long been known that the somatosensory cortex gates sensory inputs from the contralateral side of the body. Here, we examined the contribution of the ipsilateral somatosensory cortex (iS1) to sensory gating during index finger voluntary activity.
The amplitude of the P25/N33, but not other somatosensory evoked potential (SSEP) components, was reduced during voluntary activity compared with rest. Interhemispheric inhibition between S1s and intracortical inhibition in the S1 modulated the amplitude of the P25/N33. Note that changes in interhemispheric inhibition between S1s correlated with changes in cortical circuits in the ipsilateral motor cortex.
Our findings suggest that cortical circuits, probably from somatosensory and motor cortex, contribute to sensory gating in the iS1 during voluntary activity in humans.
Abstract
An important principle in the organization of the somatosensory cortex is that it processes afferent information from the contralateral side of the body. The role of the ipsilateral somatosensory cortex (iS1) in sensory gating in humans remains largely unknown. Using electroencephalographic (EEG) recordings over the iS1 and electrical stimulation of the ulnar nerve at the wrist, we examined somatosensory evoked potentials (SSEPs; P14/N20, N20/P25 and P25/N33 components) and paired‐pulse SSEPs between S1s (interhemispheric inhibition) and within (intracortical inhibition) the iS1 at rest and during tonic index finger voluntary activity. We found that the amplitude of the P25/N33, but not other SSEP components, was reduced during voluntary activity compared with rest. Interhemispheric inhibition increased the amplitude of the P25/N33 and intracortical inhibition reduced the amplitude of the P25/N33, suggesting a cortical origin for this effect. The P25/N33 receives inputs from the motor cortex, so we also examined the contribution of distinct sets of cortical interneurons by testing the effect of ulnar nerve stimulation on motor‐evoked potentials (MEPs) elicited by transcranial magnetic stimulation over the ipsilateral motor cortex with the coil in the posterior–anterior (PA) and anterior–posterior (AP) orientation. Afferent input attenuated PA, but not AP, MEPs during voluntary activity compared with rest. Notably, changes in interhemispheric inhibition correlated with changes in PA MEPs. Our novel findings suggest that interhemispheric projections between S1s and intracortical circuits, probably from somatosensory and motor cortex, contribute to sensory gating in the iS1 during voluntary activity in humans.
Keywords: cortical interneurons, primary motor cortex, sensory cortex, somatosensory cortex, somatosensory evoked potentials, transcallosal pathway
Key points
It has long been known that the somatosensory cortex gates sensory inputs from the contralateral side of the body. Here, we examined the contribution of the ipsilateral somatosensory cortex (iS1) to sensory gating during index finger voluntary activity.
The amplitude of the P25/N33, but not other somatosensory evoked potential (SSEP) components, was reduced during voluntary activity compared with rest. Interhemispheric inhibition between S1s and intracortical inhibition in the S1 modulated the amplitude of the P25/N33. Note that changes in interhemispheric inhibition between S1s correlated with changes in cortical circuits in the ipsilateral motor cortex.
Our findings suggest that cortical circuits, probably from somatosensory and motor cortex, contribute to sensory gating in the iS1 during voluntary activity in humans.
Abbreviations
- AP
anterior–posterior
- CS
conditioned stimulus
- EMG
electromyography
- FDI
first dorsal interosseous
- iS1
ipsilateral somatosensory cortex
- ISI
inter‐stimulus interval
- M1
motor cortex
- MEP
motor‐evoked potential
- MSO
maximal stimulator output
- MVC
maximal voluntary contraction
- PA
posterior–anterior
- RMT
resting motor threshold
- SAI
short‐afferent inhibition
- SSEP
somatosensory evoked potential
- TMS
transcranial magnetic stimulation
- TS
test stimulus
Introduction
The somatosensory cortex gates sensory input from the contralateral side of the body contributing to filter irrelevant signals during a motor behaviour (for a review see Borich et al. 2015). Although much of the current literature reflects this long‐standing view, evidence also exists that activity in the ipsilateral somatosensory cortex (iS1) can be modulated by a stimuli applied to the ipsilateral limb. For example, electrical stimulation of a peripheral nerve or mechanical stimulation of the skin changes iS1 activity in non‐human primates (Iwamura et al. 2001; Lipton et al. 2006; Tommerdahl et al. 2006) and humans (Allison et al. 1989, 2000; Korvenoja et al. 1995; Hamzei et al. 2002; Stefanovic et al. 2004; Newton et al. 2005; Nihashi et al. 2005; Hlushchuk & Hari, 2006). Neuroimaging data in humans showed clusters of activation in the iS1 during a unilateral voluntary contraction (Allison et al. 2000; Nirkko et al. 2001; Hamzei et al. 2002; Stefanovic et al. 2004; Newton et al. 2005). Concurrent tactile stimulation of both hands often results in better recognition and identification patterns during a motor behaviour (Essick & Whitsel, 1988; Craig & Qian, 1997), highlighting the need for information from both S1s. Despite all this evidence, the role of the iS1 in sensory gating during voluntary activity and its mechanisms of action in humans remain largely unknown.
A critical question is which neural pathways might contribute to modulate the activity in the iS1 during a voluntary contraction by the ipsilateral limb. Evidence showed that regions within the iS1 receive transcallosal inputs from the contralateral hemisphere (for a review see Iwamura, 2000). In non‐human primates, area 2 of the primary somatosensory cortex has relatively dense interhemispheric connections (Pandya & Vignolo, 1969; Killackey et al. 1983). In agreement, electrophysiological studies in humans using paired‐pulse peripheral nerve electrical stimulation revealed the presence of interhemispheric inhibitory interactions between S1s (Ragert et al. 2011; Brodie et al. 2014). Neuroimaging studies also showed that median nerve stimulation (Nihashi et al. 2005) or a unilateral tactile stimulation to the fingers (Hlushchuk & Hari, 2006) changes the activity in area 2 of the primary somatosensory cortex in the ipsilateral hemisphere. GABAergic‐mediated cortical mechanisms play a role in modulating responses in the somatosensory cortex when using a paired‐pulse suppression paradigm (Stude et al. 2016), and transmission in GABAergic cortical circuits is modulated by sensory input (Tokimura et al. 2000; Ni et al. 2011). We hypothesized that interactions between interhemispheric projections between S1s and intracortical circuits contribute to gate sensory inputs in the iS1 during tonic voluntary activity. Electrophysiological (Perez & Cohen, 2008, 2009) and neuroimaging (Dettmers et al. 1995; van Duinen et al. 2008) studies also showed that a unilateral voluntary contraction, such as the one tested in this study, changes activity in the ipsilateral motor cortex (M1). The M1 has reciprocal connections to area 2 of the somatosensory cortex (Yumiya & Ghez, 1984). Thus, we also expected that interneuronal circuits within the M1 contribute to sensory gating in the iS1 during tonic voluntary activity.
Methods
Subjects
Eighteen right‐handed healthy volunteers (31.5 ± 11.2 years old, six females) participated in the study. All subjects gave informed consent to the experimental procedures, which were approved by the local ethics committee at the University of Miami. The study was performed in accordance with the Declaration of Helsinki.
Electromyographic (EMG) recordings
EMG was recorded bilaterally from the first dorsal interosseous muscle (FDI) through surface electrodes (Ag‐AgCl; 10 mm diameter) secured to the skin over the belly of each muscle. EMG signals were amplified and filtered (bandwidth 30–2000 Hz) with a bioamplifier (Neurolog System, Digitimer, Welwyn Garden City, UK) and then converted to digital data with a sampling rate of 10 kHz with an A/D converter (CED Micro 1401, Cambridge Electronic Design, Cambridge, UK) and stored on a computer for off‐line analysis.
Experimental paradigm
Subjects were seated in a custom chair with both arms flexed at the elbow by 90 deg with the forearm pronated and the wrist restrained by straps. The left and right index fingers were attached to custom two axis load cells (Honeywell), which measures the forces exerted by the subject. At the beginning of the experiment, subjects performed two or three brief maximal voluntary contractions (MVCs) for 3–5 s with the right index finger into abduction, separated by 60 s of rest. During maximal contractions, subjects were verbally encouraged to perform maximally and visual feedback was provided. The maximal forces were used to set targets for subsequent submaximal contractions. During testing, subjects were instructed to remain at rest with the left hand while the right hand was at rest or performed 30% or 70% of MVC into index finger abduction (Fig. 1 A). Custom software was written to acquire signals from load cells to display visual feedback corresponding to rest and MVC levels in real time (LabVIEW). Subjects were instructed to control a cursor on a computer monitor to a target line displaying the force target by performing index finger abduction. A familiarization trial was completed at the beginning of each experiment to ensure that subjects were able to complete the task. In addition, verbal feedback was provided to the subjects to ensure that the left hand remained at rest at all times. A total of 1.9 ± 3.9% trials in which mean rectified EMG activity in the left FDI was 2 SD above the resting baseline were excluded from further analysis. Physiological measurements included somatosensory‐evoked potentials (SSEPs) in the iS1, paired‐pulse SSEPs between S1s, paired‐pulse SSEP suppression within the iS1 and short‐afferent inhibition (SAI) in the ipsilateral M1.
Figure 1. Experimental setup.
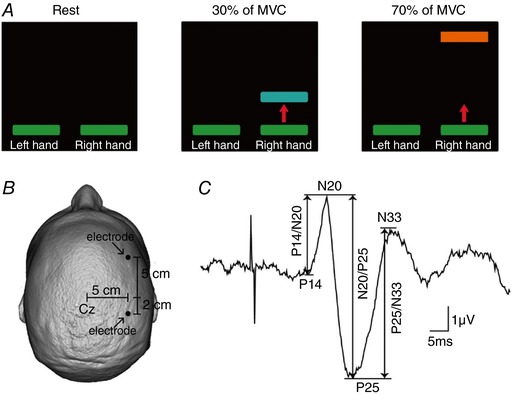
A, diagram showing the visual display presented to all subjects during testing of unilateral isometric index finger abduction. Subjects were instructed to remain at rest with the left hand while the right hand was at rest or performed 30% or 70% of maximal voluntary contraction (MVC) into index finger abduction. Colored bars represent the targets to which subjects needed to move a cursor. The distance between the bars represents the magnitude of force required to accomplish each task, normalized to the maximal index finger abduction determined in each subject. B, electrode positions for electroencephalographic (EEG) recordings. C, somatosensory evoked potential (SSEP) recorded over the ipsilateral (right) somatosensory cortex (iS1) following left ulnar nerve simulation for a representative subject. Waveform shows the average of 300 trials. The peak‐to‐peak amplitudes of all SSEP components (P14/N20, N20/P25 and P25/N33) were measured as shown by the arrows.
SSEPs
We recorded SSEPs from the right somatosensory cortex following electrical stimulation of the left ulnar nerve at the wrist (300 pulses at 5 Hz, 0.1 ms pulse duration), with pairs of adhesive Ag‐AgCl electrodes positioned 5 cm lateral, 5 cm anterior (frontal component) and 2 cm posterior (parietal component) to the vertex (Fig. 1 B). These locations correspond to regions anterior and posterior of the C3 area in the 10–20 system (Tsuji & Rothwell, 2002). The ground electrode was located on the forehead. The peak‐to‐peak amplitudes of all SSEP components (P14/N20, N20/P25 and P25/N33; Fig. 1 C) were tested while the right FDI was at rest or performed 30% or 70% of MVC into index finger abduction. At rest, the amplitude of the N20/P25 (4.7 ± 2.4 μV, P < 0.001) and P25/N33 (3.9 ± 1.6 μV, P < 0.001) was larger than the P14/N20 (1.4 ± 0.6 μV) SSEP component. Signals were amplified (gain 50k, bandwidth 3 Hz to 2 kHz) and the stimuli were delivered at an intensity of ∼10% of the maximal motor response (M‐max) across tasks (rest = 9.9 ± 4.3% of M‐max, 30% of MVC = 9.7 ± 4.6% of M‐max, 70% of MVC = 11.3 ± 6.3% of M‐max; P = 0.6).
Paired‐pulse SSEPs between S1s
A paired‐pulse SSEP paradigm was used to measure interhemispheric inhibition from the left to the right S1 (Ragert et al. 2011). Here, a conditioning electrical stimulus (CS) was given to the right ulnar nerve at the wrist at an intensity needed to elicit a response in the right FDI muscle of ∼10% of the M‐max across tasks (rest = 11.3 ± 4.9% of M‐max, 30% of MVC = 10.2 ± 4.6% of M‐max; P = 0.4). A test electrical stimulus (TS) was given to the left ulnar nerve at the wrist at an intensity needed to elicit a motor response in the left FDI muscle of ∼10% M‐max across tasks (rest = 12.8 ± 4.9% of M‐max, 30% of MVC = 11.4 ± 5.8% of M‐max; P = 0.2). The CS was delivered at an inter‐stimulus interval (ISI) of 15, 20, 25, 30, 35 and 40 ms before the TS in a randomized order. Because we did not find differences in the magnitude of gating in the P25/N33 component during 30% and 70% of MVC, interhemispheric inhibition was only measured during 30% of MVC. As in other studies the electrical stimuli to the left ulnar nerve did not result in a visible SSEP and/or changes in background EEG activity in the left S1 (Ragert et al. 2011). However, to eliminate the potential influence of an ipsilateral response from the CS on the contralateral response to the TS, the response from the CS alone condition was subtracted from the responses from each CS+TS condition (Ragert et al. 2011). At rest, a conditioning pulse attenuated the amplitude of the N20/P25 (ISIs of 20–35 ms, F 1,14 = 6.3, P = 0.02) and P25/N33 (ISIs of 15–30 ms, F 1,14 = 28.1, P < 0.001; Ragert et al. 2011; Brodie et al. 2014) but not the P14/N20 component (F 1,14 = 3.1, P = 0.1) compared with a test pulse. In an additional control experiment (n = 8), we recorded SSEPs from the left S1 following electrical stimulation of the right ulnar nerve at the wrist using an intensity of ∼10% of the M‐max across tasks (rest = 9.8 ± 3.6% of M‐max, 30% of MVC = 11.7 ± 4.6% of M‐max, 70% of MVC = 10.4 ± 4.1% of M‐max; P = 0.5) with and without a CS given to the left ulnar nerve at the wrist at an ISI of 25 ms in a randomized order. Paired‐pulse SSEPs between S1s were analyzed by expressing the size of the conditioned SSEP as a percentage of the size of the test SSEP [(conditioned SSEP × 100)/(test SSEP)]. The effect of interhemispheric inhibition was measured in each of the SSEP components. Three hundred test SSEPs and 300 conditioned SSEPs were tested in each condition.
Paired‐pulse SSEP suppression within the iS1
Paired‐pulse SSEP suppression was used to make inferences about the contribution of intracortical inhibitory mechanisms on SSEP components (Hoffken et al. 2013; Stude et al. 2016). Paired‐pulse SSEP suppression was measured in the iS1 using a paired‐pulse paradigm where repeated paired pulses were applied to the left ulnar nerve at the wrist at an ISI of 40 ms at rest and during 30% of MVC. The stimulation was given at an intensity needed to elicit a response in the left FDI muscle of ∼10% of the M‐max across conditions (rest = 9.4 ± 4.6% of M‐max, 30% of MVC = 10.2 ± 7.5% of M‐max; P = 0.6). Paired‐pulse SSEP suppression was calculated as a ratio of the amplitude of the second response (A2) and the amplitude of the first response (A1) measured in each of the SSEP components. Three hundred paired‐pulses were applied at each condition.
SAI
We used a previously established paired‐pulse paradigm to test SAI (Ni et al. 2011) at rest and during 30% of MVC with the right index finger. A CS was given to the left ulnar nerve at the wrist at an intensity needed to elicit a motor response in the left FDI muscle of ∼10% M‐max across tasks (rest = 9.4 ± 5.5% of M‐max, 30% of MVC = 9.7 ± 6.5% of M‐max; P = 0.6). A test magnetic stimulus (TS) was applied to the right hand representation of the M1 using a Magstim 200 magnetic stimulator (Magstim, Whitland, UK) through a figure‐of‐eight coil (loop diameter, 7 cm, type number, 16342) with a monophasic current waveform. During testing the transcranial magnetic stimulation (TMS) coil was firmly held to the head of the subject by a custom coil holder, with the head secured to a headrest by straps to restrict head movements. To further examine the effect of afferent input onto separate synaptic inputs to corticospinal neurons the TS was given with the TMS coil in two different orientations. Here, the TMS coil was held tangentially on the scalp at an angle of 45 deg to the midline with the handle pointing laterally and posteriorly [posterior–anterior (PA) induced current in the brain] or with the coil handle reversed around 180° to the PA currents [anterior–posterior (AP) induced current in the brain]. The resting motor threshold (RMT, determined as the minimum stimulus intensity required to elicit MEP > 50 μV peak‐to‐peak amplitude above the background EMG activity in at least 5 out of 10 consecutive trials in the relaxed muscle; Rothwell et al. 1999) was higher for MEPs elicited in the AP (61.7 ± 10.5% of the maximal stimulator output, MSO) compared with the PA (47.9 ± 8.0% of the MSO, P < 0.001; Federico & Perez, 2016) orientation. Also, MEP onset latencies (defined as the time when mean rectified EMG activity was > 2 SD of the mean resting EMG activity, measured 100 ms before the stimulus artifact) were longer for MEPs elicited in the AP compared with the PA orientation (rest: AP = 24.4 ± 1.9 ms, PA = 22.8 ± 1.4 ms, P < 0.001; 30% of MVC: AP = 24.0 ± 1.7 ms, PA = 22.8 ± 1.3 ms, P < 0.001). PA and AP current directions were used for the TS at an intensity needed to elicit a test MEP at rest of ∼1 mV (PA: rest = 1.1 ± 0.3 mV, 30% of MVC = 1.8 ± 1.4 mV, P = 0.04; AP: rest = 1.1 ± 0.9 mV, 30% of MVC = 1.7 ± 0.9 mV, P = 0.01) and ∼0.2 mV (PA: rest = 0.28 ± 0.1 mV, 30% of MVC = 0.66 ± 0.3 mV, P = 0.001; AP: rest = 0.21 ± 0.1 mV, 30% of MVC = 0.57 ± 0.5 mV, P = 0.02) across conditions because a smaller test MEP more selectively examines PA and AP inputs. The CS was delivered at ISIs of 15, 20, 25, 30, 35 and 40 ms before the TS in a randomized manner. At rest, a CS to the left ulnar nerve attenuated the amplitude of test MEPs in the PA (MEP of ∼1 mV: ISIs of 20 and 25 ms, F 1,14 = 6.8, P = 0.02; MEP of ∼0.2 mV: ISIs of 20, 25 and 40 ms, F 1,11 = 6.9, P = 0.02) and the AP orientation (MEP of ∼1 mV: ISIs between 20, 25, 30 ms, F 1,14 = 8.3, P = 0.01; MEP of ∼0.2 mV: ISIs of 20, 25, 30 and 40 ms, F 1,11 = 11.5, P = 0.01). MEPs in the left FDI increased while the other index finger performed 30% of MVC (Perez & Cohen, 2008), therefore, in a control experiment SAI was tested by adjusting the test MEP size to match MEP amplitudes produced at rest across tasks (PA: rest = 1.0 ± 0.4 mV, 30% of MVC = 1.2 ± 0.7 mV, P = 0.8; AP: rest = 1.1 ± 0.6 mV, 30% of MVC = 1.3 ± 0.8 mV, P = 0.3; n = 8). SAI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the test MEP [(conditioned MEP × 100)/(test MEP)]. Twenty test MEPs and 20 conditioned MEPs were tested at each ISI, coil orientation and task every 5 s separated by resting periods as needed. In an additional control experiment (n = 8), we also measured MEPs in the left FDI by stimulating the right ipsilateral M1 with the coil in the PA and AP orientation at an intensity needed to elicit a test MEP of ∼1 mV (PA = 1.09 ± 0.37 mV, AP = 1.01 ± 0.57 mV) and ∼0.2 mV (PA = 0.19 ± 0.05 mV, AP = 0.21 ± 0.05 mV) when the right index finger was at rest or performed 30% and 70% of MVC.
Data analysis
Normal distribution was tested by the Shapiro–Wilk's test and homogeneity of variances by the Levene's test of equality and Mauchly's test of sphericity. When a normal distribution could not be assumed, the data were log transformed. When sphericity could not be assumed, the Greenhouse–Geisser correction statistic was used. Repeated‐measures ANOVAs were performed to determine the effect of TASK (rest, 30% and 70% of MVC) and COMPONENT (P14/N20, N20/P25 and P25/N33) on the peak‐to‐peak amplitude of SSEPs in the iS1. We measured the effect of ISI (15, 20, 25, 30 and 40 ms) and CONDITION (rest and 30% of MVC) on paired‐pulse SSEPs between S1s (from the left to the right) on each SSEP component. The same analysis was used to determine the effect of ISI, CONDITION and COIL ORIENTATION (PA and AP) on SAI tested with a 1 mV and 0.2 mV test MEP size. We also measured the effect of TASK on paired‐pulse SSEPs between S1s (from the right to the left) on each SSEP component at an ISI of 25 ms. Paired t tests were used to compare the size of the test MEP and the effects of paired‐pulse SSEP suppression across conditions corrected for multiple comparisons as needed. We also measured the effect of TASK and SIDE (left and right) on mean EMG activity. Tukey post hoc analysis was used to test for significant comparisons. Pearson correlation analysis was used as needed corrected for multiple comparisons. Significance was set at P < 0.05. Group data are presented as the mean ± SD in the text.
Results
EMG
Figure 2 A and B gives examples of rectified EMG activity measured in the left and right FDI across tasks in a representative subject. Note that EMG activity increased during increasing levels of voluntary contraction with the right FDI while the left FDI remained at rest.
Figure 2. Electromyographic (EMG) activity.
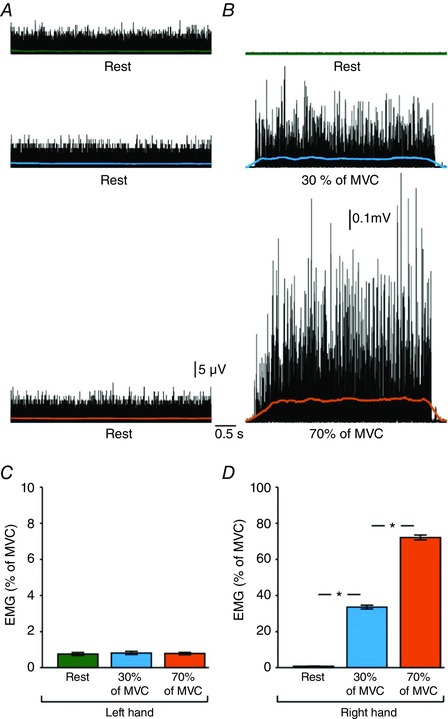
Rectified EMG activity in the first dorsal interosseous (FDI) muscle in a representative subject when the left hand remained at rest (A) while the right hand (B) remained at rest (green) or performed 30% (blue) and 70% (orange) of MVC. C and D, group data (n = 15) showing mean rectified EMG activity (expressed as % of MVC) in the left (C) and right (D) hand during unilateral index finger abduction with the right FDI. Error bars indicate SEMs. * P < 0.05, comparison between rest and contraction.
Repeated‐measures ANOVA showed a significant effect of TASK (F 2,28 = 1400.4, P < 0.001), SIDE (F 1,14 = 2872.8, P < 0.001) and in their interaction (F 2,28 = 1431.5, P < 0.001) on mean EMG activity in the FDI. In the right side, post hoc analysis showed that EMG activity increased at 70% (P < 0.001) and 30% (P < 0.001) of MVC compared with rest and it was higher at 70% compared with 30% of MVC (P < 0.001; Fig. 2 D). In the left side, EMG activity remained similar across tasks (F 2,28 = 0.4, P = 0.7; Fig. 2 C).
SSEPs
Figure 3 A illustrates raw SSEP traces in a representative subject recorded from the iS1 at rest (green), and during 30% (blue) and 70% (orange) of MVC with the right index finger. Note that in the iS1 the amplitude of the P25/N33, but not other SSEP components, decreased during 30% and 70% of MVC compared with rest.
Figure 3. Somatosensory evoked potentials (SSEPs).
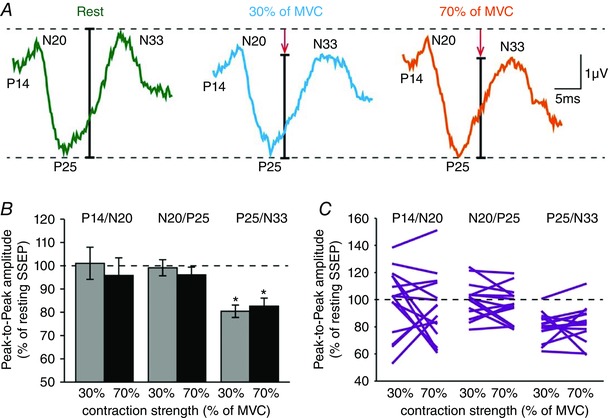
A, raw SSEP traces recorded from the iS1 in a representative subject when the right FDI was at rest (green) or performed 30% (blue) and 70% (orange) of MVC. Each waveform represents the average of 300 SSEPs. B, group data (n = 15) showing the amplitude of all SSEP components in the iS1 (expressed as % of resting SSEPs) during 30% (grey) and 70% (black) of MVC. The horizontal dotted line represents the amplitude of the SSEP components at rest. Data from individual subjects are also shown (C). Error bars indicate SEMs. * P < 0.05, comparison between rest and contraction.
Repeated‐measures ANOVA showed an effect of TASK (F 2,28 = 4.7, P = 0.01), COMPONENT (F 1.3,18.2 = 4.9, P = 0.01) and in their interaction (F 4,56 = 2.7, P = 0.04) on SSEPs amplitude. Post hoc analysis revealed that the amplitude of the P25/N33 (30% = 80.4 ± 10.3%, P < 0.001; 70% = 82.7 ± 13.3%, P < 0.001), but not the P14/N20 (30% = 101.0 ± 26.7%, P = 0.9; 70% = 95.8 ± 29.3%, P = 0.6) or N20/P25 (30% = 99.1 ± 13.4%, P = 0.8; 70% = 96.1 ± 12.7%, P = 0.3) components, was reduced during 30% and 70% of MVC compared with rest (Fig. 3 B) in the majority of subjects (14/15, most values for P25/N33 below the dotted line in Fig. 3 C). No changes were observed in the amplitude of the P25/N33 during 30% and 70% of MVC (P = 0.5).
Paired‐pulse SSEPs between S1s
Figure 4 A illustrates raw SSEP data showing the effect of a CS over the right ulnar nerve on an SSEP tested in the iS1 by stimulation of the left ulnar nerve in a representative subject. Note that a CS increased the amplitude of the P25/N33 in the iS1 during 30% of MVC compared with rest.
Figure 4. Paired‐pulse SSEPs between S1s.
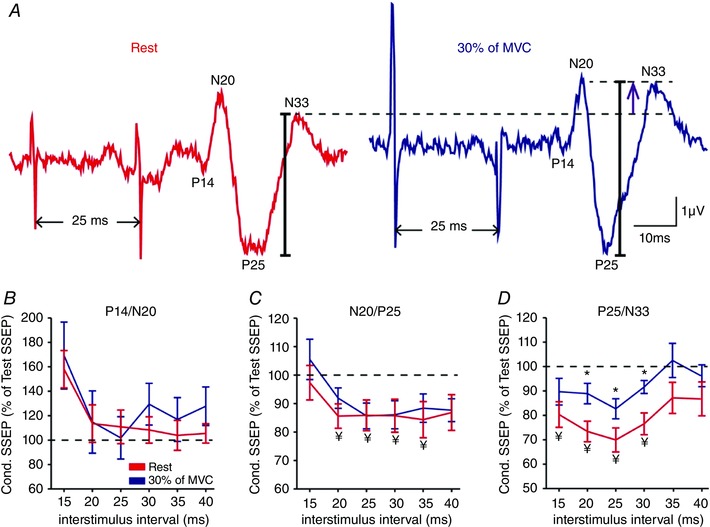
A, raw SSEP traces showing the effect of a conditioning stimulus over the right ulnar nerve on an SSEP tested in the iS1 by stimulation of the left ulnar nerve in a representative subject at rest (red) and during 30% (blue) of MVC at an inter‐stimulus interval (ISI) of 25 ms. B–D, group data (n = 15) showing the amplitude of the conditioned SSEP expressed as % of the test SSEP at rest (red line) and during 30% (blue line) of MVC at ISIs of 15, 20, 25, 30, 35 and 40 ms for the P14/N20 (B), N20/P25 (C) and P25/N33 (D) components. The horizontal dotted line represents the amplitude of the test SSEP at rest. Note that a conditioning stimulus increased the amplitude of the P25/N33 during 30% of MVC compared with rest, suggesting that interhemispheric inhibition between S1s was decreased. * P < 0.05, comparison between rest and contraction; ¥ P < 0.05, comparison between test SSEP and conditioned SSEP at rest.
Repeated‐measures ANOVA showed an effect of ISI (F 5,70 = 4.4, P = 0.002) and CONDITION (F 1,14 = 13.2, P = 0.003), but not their interaction (F 5,70 = 0.4, P = 0.8) on paired‐pulse SSEPs from the left to the right S1. Post hoc analysis showed that a CS resulted in a larger amplitude of the P25/N33 component measured in the iS1 at an ISI of 20 ms (rest = 73.4 ± 16.4%, 30% of MVC = 88.9 ± 16.3%, P = 0.005), 25 ms (rest = 69.9 ± 19.1%, 30% of MVC = 82.7 ± 16.0%, P = 0.009), and 30 ms (rest = 76.5 ± 17.2%, 30% of MVC = 91.6 ± 10.3%, P = 0.003; Fig. 4 D) during 30% of MVC compared with rest in the majority of subjects (20 ms: 14/15; 25 ms: 11/15; 30 ms: 12/15). Note that a CS did not change the amplitude of the P14/N20 (F 1,12 = 1.1, P = 0.3, Fig. 4 B) and N20/P25 (F 1,14 = 0.6, P = 0.5, Fig. 4 C) SSEP components measured in the iS1 during 30% of MVC compared with rest.
Repeated‐measures ANOVA also showed an effect of TASK (F 1,7 = 14.1, P = 0.007) on paired‐pulse SSEPs from the right to the left S1. Post hoc analysis showed that a CS decreased the amplitude of the P25/N33, but not the P14/N20 (F 1,7 = 0.1, P = 0.7) and N20/P25 (F 1,7 = 1.2, P = 0.3) components, at an ISI of 25 ms during 30% (rest = 93.2 ± 8.1%, 30% of MVC = 81.8 ± 13.8%, 7/8, P = 0.03) and 70% (rest = 94.4 ± 10.1%, 30% of MVC = 85.7 ± 12.3%, 7/8, P = 0.01) of MVC compared with rest in the majority of subjects.
Paired‐pulse SSEP suppression within the iS1
Figure 5 A illustrates raw data showing paired‐pulse SSEP suppression of the P25/N33 component in the iS1 in a representative subject. Note that paired‐pulse suppression of the P25/N33 was more pronounced during 30% of MVC compared with rest. We found that the A2/A1 ratio decreased during 30% of MVC compared with rest for the P25/N33 component in the majority of subjects [rest = 0.72 ± 0.20, 30% of MVC = 0.64 ± 0.21, (13/15), P = 0.002; Fig. 5 D]. Note that paired‐pulse SSEP suppression of the other SSEP components (P14/N20: P = 0.9, Fig. 5 B; N20/P25: P = 0.4, Fig. 5 C) remained similar during rest and 30% of MVC.
Figure 5. Paired‐pulse SSEP suppression within the iS1.
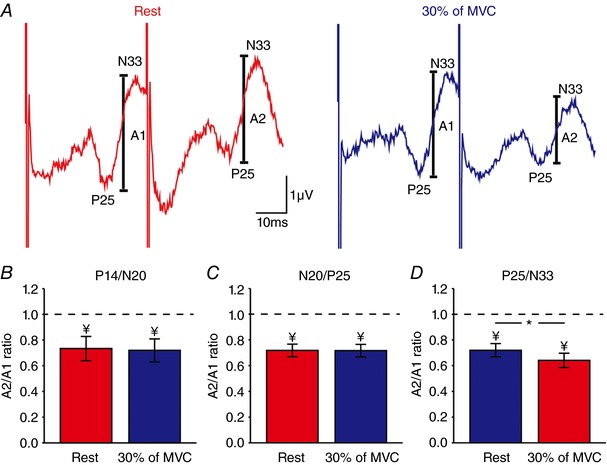
A, raw SSEP traces showing paired‐pulse SSEP suppression of the P25/N33 component in the iS1 in a representative subject at rest (red) and during 30% (blue) of MVC at an ISI of 40 ms. The amplitude of the P25/N33 component (A1 and A2, see Methods for details) is marked. Each waveform represents the average of 300 SSEPs. B–D, group data (n = 15) showing mean paired‐pulse ratios (A2/A1) at rest (red) and during 30% of MVC (blue) for the P14/N20 (B), N20/P25 (C) and P25/N33 (D) components. Note that paired‐pulse SSEP suppression of the P25/N33 but not other SSEP components was increased during 30% of MVC compared with rest, suggesting that intracortical inhibition in the iS1 was increased. * P < 0.05, comparison between rest and contraction; ¥ P < 0.05, comparison between testSSEP and conditioned SSEP at rest.
SAI
Figures 6 and 7 A and C illustrate examples of test and conditioned MEPs recorded from the left FDI of a representative subject. Note that the conditioned MEP was more suppressed during 30% of MVC compared with rest when the coil was in the PA compared with the AP orientation.
Figure 6. Short afferent inhibition (SAI) with a test MEP ∼1 mV.
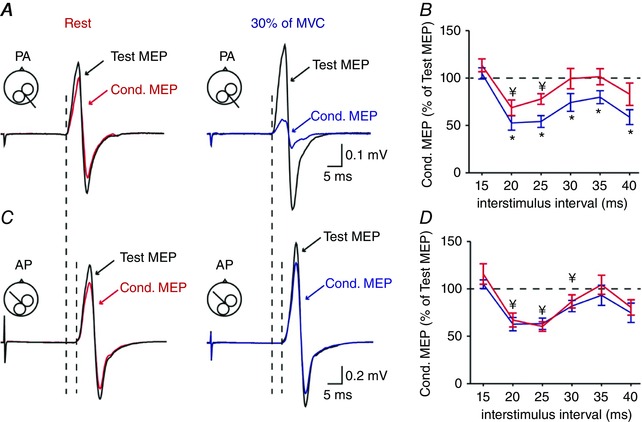
A C, SAI tested with the transcranial magnetic stimulation (TMS) coil in the PA (A) and AP (C) orientation over the right ipsilateral motor cortex (M1) in a representative subject at rest (left) and during 30% of MVC (right). Test motor evoked potentials (MEPs) are indicated by black traces and arrows at rest and during 30% of MVC. Conditioned (Cond.) MEPs are indicated by red and blue traces and arrows. Traces show the average of 20 MEPs. Note that the latencies of MEPs were longer in the AP compared with the PA orientation. B and D, group data (n = 15) showing the magnitude of the conditioned MEP expressed as % of the test MEP at rest (red) and during 30% (blue) of MVC at ISIs of 15, 20, 25, 30, 35 and 40 ms for PA (B) and AP (D) MEPs. The horizontal dotted line represents the size of the test MEP. Results show that SAI tested with the coil in the PA but not AP orientation increases during voluntary activity compared with rest. * P < 0.05, comparison between the test and conditioned MEP ratio at rest and contraction; ¥ P < 0.05, comparison between test MEP and conditioned MEP at rest.
Figure 7. SAI with a test MEP ∼0.2 mV.
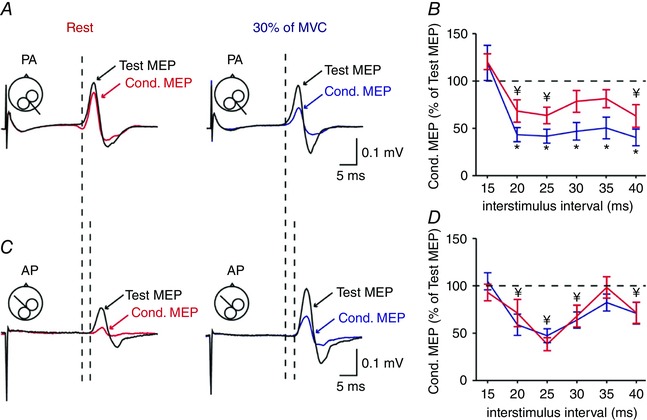
A and C, SAI was also tested with a test MEP of 0.2 mV with the TMS coil in the PA (A) and AP (C) orientation over the right ipsilateral M1. See data from a representative subject at rest (left) and during 30% of MVC (right). Test MEPs are indicated by black traces and arrows at rest. Conditioned (Cond.) MEPs are indicated by red and blue traces and arrows. Traces show the average of 20 MEPs. The latencies of AP MEPs were longer compared with PA MEPs. B and D, group data (n = 12) showing the magnitude of the conditioned MEP expressed as % of the test MEP at rest (red) and during 30% (blue) of MVC at ISIs of 15, 20, 25, 30, 35 and 40 ms for PA (B) and AP (D) MEPs. The horizontal dotted line represents the size of the test MEP. As for larger test MEPs, SAI tested with the coil in the PA but not AP orientation increases during voluntary activity compared with rest. * P < 0.05, comparison between the test and conditioned MEP ratio at rest and contraction; ¥ P < 0.05, comparison between test MEP and conditioned MEP at rest.
Repeated‐measures ANOVA revealed an effect of CONDITION (F 1,14 = 6.6, P = 0.02) and ISI (F 2.6,36.9 = 12.5, P < 0.001), but not COIL ORIENTATION (F 1,14 = 0.3, P = 0.6) or their interaction (F 5,70 = 1.1, P = 0.4) on SAI tested with a test MEP of ∼1 mV. Post hoc analysis showed that SAI increased during 30% of MVC compared with rest at ISIs of 20 ms (P = 0.02), 25 ms (P = 0.004), 30 ms (P = 0.006), 35 ms (P = 0.02) and 40 ms (P = 0.007) for the PA orientation (Fig. 6 B). In contrast, SAI remained similar during 30% of MVC and rest at all ISIs (F 1,14 = 0.6, P = 0.5, Fig. 6 D) in the AP orientation. Similar results were found when SAI was tested using a test MEP of ∼0.2 mV [CONDITION (F 1,11 = 6.2, P = 0.03), ISI (F 2.3,25.3 = 15.5, P < 0.001), but not COIL ORIENTATION (F 1,11 = 1.8, P = 0.2) or their interaction (F 5,55 = 0.4, P = 0.8) on SAI]. Here, SAI increased during 30% of MVC compared with rest at ISIs of 20 ms (P = 0.002), 25 ms (P = 0.01), 30 ms (P = 0.01), 35 ms (P < 0.001) and 40 ms (P = 0.01) in the PA (Fig. 7 B) but not AP (F 1,11 = 0.4, P = 0.5, Fig. 7 D) orientation. SAI increased during 30% of MVC compared with rest when adjusting the test MEP size to match MEP amplitudes across conditions (F 1,7 = 22.8, P = 0.002).
Figure 8 A and C illustrates MEPs elicited by stimulation of the ipsilateral M1 and SSEPs recorded from the iS1 at rest (green), and during 30% (blue) and 70% (orange) of MVC with the right index finger. Note that FDI MEPs increased during 30% (MEP of ∼1 mV: PA = 168.3 ± 69.5%, P = 0.008; AP = 142.5 ± 40.6%, P = 0.02; MEP of ∼0.2 mV: PA = 166.9 ± 44.3%, P = 0.004; AP = 164.5 ± 50.9%, P = 0.009) and 70% (MEP of ∼1 mV: PA = 198.3 ± 56.8%, P = 0.001; AP = 198.1 ± 72.5%, P = 0.006; MEP of ∼0.2 mV: PA = 212.7 ± 40.6%, P < 0.001; AP = 206.3 ± 64.4%, P = 0.002) of MVC compared with rest (Fig. 8 B). By contrast, the amplitude of the P25/N33 (30% = 80.2 ± 8.2%, P < 0.001; 70% = 82.5 ± 10.2%, P < 0.001; Fig. 8 D) but not the P14/N20 (30% = 106.2 ± 11.0%, P = 0.2; 70% = 100.6 ± 13.2%, P = 0.9) or N20/P25 (30% = 109.6 ± 25.6%, P = 0.3; 70% = 90.2 ± 38.4%, P = 0.5) components decreased during 30% and 70% of MVC compared with rest. No changes were observed in the amplitude of the P25/N33 during 30% and 70% of MVC (P = 0.7).
Figure 8. MEPs and SSEPs.
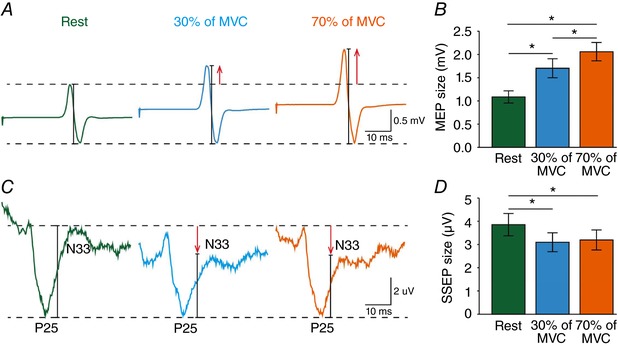
MEPs were also tested with the TMS coil in the PA and AP (not shown) orientation by stimulating the right ipsilateral M1 at rest (green) or at 30% (blue) and 70% (orange) of MVC. For comparisons between M1 and S1 responses during the same motor tasks, here we also added the SSEP data recorded from the iS1 in the same subjects. A and C, MEP (A) traces show the average of 20 MEPs and SSEP (C) traces show the average of 300 SSEPs. B and D, group data (n = 8) showing the magnitude of MEPs (B) and the P25/N33 SSEP component (D) across conditions (rest = green bars, 30% of MVC = blue bars, 70% of MVC = orange bars). * P < 0.05, comparison between rest and contraction.
Correlation analysis
A positive correlation was found between changes in the amplitude of the P25/N33 SSEP component and paired‐pulse SSEP suppression within the iS1 during 30% of MVC compared with rest (r = 0.64, P = 0.01; Fig. 9 A). Here, note that individuals with more attenuated P25/N33 were those with more pronounced intracortical inhibition of the P25/N33 in the iS1. We also found a negative correlation between changes in paired‐pulse SSEPs between S1s and SAI tested in the PA (r = 0.69, P = 0.004; Fig. 9 B), but not in the AP (r = 0.32, P = 0.24; Fig. 9 C), orientation during 30% of MVC compared with rest. This result indicated that individuals with less pronounced interhemispheric inhibition of the P25/N33 in the iS1 were those with larger suppression of MEPs in the PA orientation. No correlation was found between changes in MEPs elicited by stimulating the ipsilateral M1 and SSEPs measured in the iS1 (r = 0.24, P = 0.41).
Figure 9. Correlations.
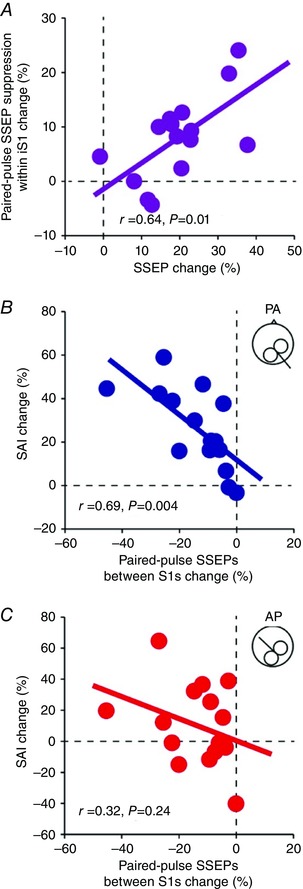
A, graph showing correlation between changes in the paired‐pulse SSEP suppression of the P25/N33 (rest – 30% of MVC) and the amplitude of the P25/N33 elicited by single electrical nerve stimulation (rest – 30% of MVC) in the iS1. Note that individuals with a more attenuated P25/N33 component also showed more intracortical inhibition in the P25/N33. B and C, graphs showing a correlation between the effect of conditioning stimulus over the right ulnar nerve on the P25/N33 in the iS1 (rest – 30% of MVC) and SAI (rest – 30% of MVC) tested with the coil in the PA (B) and AP (C) orientations. Note that individuals with larger suppression of the P25/N33 by interhemispheric inhibition also showed a larger suppression on MEP tested in the PA orientation in the ipsilateral M1.
Discussion
Our novel findings suggest that interhemispheric projections between S1s and intracortical circuits contribute to gate sensory inputs in the iS1 during voluntary activity in humans. We found that the amplitude of the P25/N33, but not other SSEP components, was reduced in the iS1 during voluntary activity compared with rest. Interhemispheric inhibition and intracortical inhibition in the S1 modulated the amplitude of the P25/N33, suggesting a cortical origin for this effect. The P25/N33 receives inputs from the M1, so we also examined sensorimotor interactions by testing SAI in the ipsilateral M1. SAI was attenuated when tested with the TMS coil in the PA but not AP orientation during voluntary activity compared with rest. Notably, changes in interhemispheric inhibition correlated with changes in SAI tested in the PA orientation. We propose that cortical circuits in somatosensory and M1 contribute to sensory gating in the iS1 during voluntary activity.
Gating of sensory input in the iS1 and its mechanisms of action
Extensive evidence in animals and humans showed that during voluntary activity sensory inputs are gated in the somatosensory cortex contralateral to a moving limb (Cohen & Starr, 1987; Jiang et al. 1990a,b; Schnitzler et al. 1995; Nakata et al. 2003; Wasaka et al. 2005; Ogata et al. 2009; Huttunen & Lauronen, 2012; Seki & Fetz, 2012; Kirimoto et al. 2014; Sugawara et al. 2016). Here, we report for the first time that the amplitude of SSEPs is also gated in the iSP during a unilateral voluntary contraction. Specifically, we found that the amplitude of the P25/N33, but not other SSEP components, decreases during voluntary activity compared with rest. This finding agrees with evidence showing that electrical stimulation of a peripheral nerve or a mechanical stimulation of the skin changes the activity in the iS1 in non‐human primates (Iwamura et al. 2001; Lipton et al. 2006; Tommerdahl et al. 2006) and humans (Allison et al. 1989; Korvenoja et al. 1995; Nihashi et al. 2005; Hlushchuk & Hari, 2006). This also agrees with neuroimaging data in humans showing some clusters of activation in the iS1 during a unilateral voluntary contraction (Allison et al. 2000; Nirkko et al. 2001; Hamzei et al. 2002; Stefanovic et al. 2004; Newton et al. 2005). Evidence showed that tactile stimulation of both hands often results in better recognition and identification patterns during a motor behaviour (Essick & Whitsel, 1988; Craig & Qian, 1997), highlighting the need for information from both S1s.
A critical question is which neuronal pathways contributed to sensory gating in the iS1 during a voluntary contraction. Evidence showed that different parts within the iS1 receive transcallosal inputs from the contralateral hemisphere (Iwamura, 2000; Iwamura et al. 2001). For example, in non‐human primates area 2 has relatively dense interhemispheric connections whereas areas 3b and 1 have only sparse connections (Pandya & Vignolo, 1969; Killackey et al. 1983). In agreement, electrophysiological studies in humans using paired‐pulse peripheral nerve electrical stimulation revealed the presence of interhemispheric inhibitory interactions between S1s (Ragert et al. 2011; Brodie et al. 2014). Neuroimaging studies also showed that electrical stimulation of the median nerve (Nihashi et al. 2005) or a unilateral tactile stimulation to the fingers (Hlushchuk & Hari, 2006) results in activation of area 2 and deactivation of area 3b and the M1 in both hemispheres. Thus, it is possible that transcallosal connections between S1s contributed to sensory gating in the iS1. This is supported by our findings showing that interhemispheric inhibition decreased the amplitude of the P25/N33, but not other SSEP components. Previous studies conducted at rest reported that interhemispheric inhibition between S1s suppresses, at least to some extent, the amplitude of earlier SSEP components in the iS1 (Ragert et al. 2011; Brodie et al. 2014), which provides a possible route for these bilateral effects. An intriguing question is why we found that during voluntary activity the later P25/N33 SSEP component was gated in the iS1. We first consider the possibility that this is related to its neural origin. The P14/N20 results from subcortical contributions and probably reflects the arrival of medial lemniscal signals to the thalamus (Desmedt & Cheron, 1981; Lee & Seyal, 1998). Whereas most studies agree that the N20/P25 reflects activation of area 3b (Allison et al. 1991; Forss et al. 1994; Huttunen et al. 2006) while the P25/N33 reflects activation of area 1 (Jones et al. 1978; Allison et al. 1991; Ishikawa et al. 2007). Area 2, which has relatively dense interhemispheric connections, communicates with area 1 (Allison et al. 1991) and has reciprocal connections with the M1 (Yumiya & Ghez, 1984). A possibility is that transcallosal connections between area 2 and interactions with area 1 and the M1 contributed to modulate the P25/N33 component. This is supported by neuroimaging (Dettmers et al. 1995; van Duinen et al. 2008) and electrophysiological studies (Muellbacher et al. 2000; Perez & Cohen, 2008, 2009) showing that activity in the ipsilateral M1 increases during increasing levels of tonic voluntary activity such as those tested in our study. Indeed, we found, as in previous studies (Perez & Cohen, 2008, 2009), that the size of MEPs in the ipsilateral M1 increased during 30% and 70% of MVC compared with rest. This may also explain the lack of changes in the N20/P25 component during voluntary activity. The N20/P25 component probably reflects activation of area 3b, an area that does not have direct connections with the M1 (Jones et al. 1978). It is also possible that the electrical stimuli applied to the left ulnar nerve affected the excitability of the left S1 and therefore influenced the right S1, which is the iS1 for our tasks. We found that interhemispheric inhibition from the right to the left S1 increased gating of the P25/N33 but not other SSEP components during 30% and 70% of MVC. Although possible, the lack of correlation between these changes and the magnitude of sensory gating in the right S1 and/or the magnitude of interhemispheric inhibition from the left to the right S1 suggest that it is less likely that this factor contributed to our results. Also, bidirectional interhemispheric inhibitory effects between S1s are continuously present during all tasks when selective gating was detected in the iS1.
The next question to address is which mechanisms within the iS1 contributed to the sensory gating in this region. Two of our results support the view that the reduced amplitude of the P25/N33 involved cortical mechanisms. First, we found paired‐pulse SSEP suppression tested in the iS1 reduced the amplitude of the P25/N33, but no other SSEP components, during voluntary activity compared with rest. Evidence showed that GABAergic‐mediated cortical mechanisms play a role in modulating responses in the somatosensory cortex tested using the paired‐pulse SSEP suppression paradigm (Hoffken et al. 2010; Stude et al. 2016). This is also supported by the correlation found between changes in the amplitude of the P25/N33 and the paired‐pulse SSEP suppression. Thus, individuals with more suppression of the P25/N33 also showed more intracortical inhibition in the iS1. Second, we found that SAI tested in the ipsilateral M1 increased during voluntary activity compared with rest when tested with the coil in the PA but not in the AP orientation. Studies in humans showed that different descending volleys can be elicited by a single TMS pulse depending on the current flow across the hand representation of the M1. TMS‐induced electric currents flowing from PA across the central sulcus preferentially evoke highly synchronized corticospinal activity whereas currents flowing from anterior to posterior preferentially evoke less synchronized and, in some cases, delayed corticospinal activity (Day et al. 1989; Sakai et al. 1997; Di Lazzaro et al. 2001). Therefore, it has been suggested that it is possible to activate two separate sets of synaptic inputs to corticospinal neurons (Di Lazzaro et al. 2012). Because PA inputs have lower threshold (Nakamura et al. 1996; Di Lazzaro et al. 1998) and are largely influenced by cortical mechanisms (Cirillo & Perez, 2015) compared with AP inputs, it might be easier to engage them in the ipsilateral M1 during voluntary activity. In non‐human primates, afferent information influences M1 activity via dense intracortical projections between the S1 and M1 (Goldring et al. 1970), so it is also possible that intracortical circuits contributing to the paired‐pulse SSEP suppression and PA MEPs interact and/or overlapped. Regardless of the precise nature of the cortical circuits contributing to these effects, our findings show that interactions between interhemispheric inhibition and intracortical circuits contributed to a more selective gating of the cortically mediated P25/N33 component during voluntary activity compared with rest.
It is interesting to note that during a voluntary contraction interhemispheric inhibition decreases and intracortical inhibition increases in the iS1. The modulation of these sensory‐mediated pathways in the iS1 is opposite to what has been reported in the ipsilateral M1 during similar motor tasks. Evidence showed that a unilateral voluntary contraction of similar magnitude to that in our study increases interhemispheric inhibition and decreases intracortical inhibition in the ipsilateral M1 (Perez & Cohen, 2008). Notably, we found that MEPs increased in the ipsilateral M1 while SSEPs decreased in the iS1 during the same motor tasks. This might reflect the complex interplay between pathways during a motor behaviour. Although our montage targeted the S1 (Tsuji & Rothwell, 2002) it is possible that SSEPs were influenced by signals from the M1. Evidence showed that regions of the hand M1 identified by electrical brain stimulation coincide with sites showing large N20 SSEP peaks (Fukuda et al. 2008), suggesting that the M1 and S1 areas involve overlapping neural networks. Thus, it is possible that the pronounced sensory gating in the iS1 and the increased corticospinal excitability in the ipsilateral M1 contribute to balance the activity of sensory and motor cortices during the same task. Our results might therefore be particularly relevant for individuals with motor disorders such as stroke where there is abnormal gating in somatosensory cortices (Staines et al. 2002; Borich et al. 2015) and an abnormal activation in the ipsilateral M1 during voluntary activity (Kokotilo et al. 2010).
Additional information
Competing interests
The authors declare that they have no competing financial interests.
Author contributions
This work was carried out at the University of Miami. YL and MAP conceived and designed the study. YL and MAP contributed to the data collection and analysis and interpreted the experimental results. The manuscript was drafted and revised by YL and MAP. Both authors approved the final version of the manuscript. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was funded by the National Institute of Neurological Disorders and Stroke ‐ National Institutes of Health (R01NS076589, R01 NS090622), the Department of Veterans Affairs (I01RX000815, I01RX001807) and the Craig H. Neilsen Foundation (454590).
References
- Allison T, McCarthy G, Wood CC, Williamson PD & Spencer DD (1989). Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long‐latency activity. J Neurophysiol 62, 711–722. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC & Jones SJ (1991). Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114, 2465–2503. [DOI] [PubMed] [Google Scholar]
- Allison JD, Meador KJ, Loring DW, Figueroa RE & Wright JC (2000). Functional MRI cerebral activation and deactivation during finger movement. Neurology 54, 135–142. [DOI] [PubMed] [Google Scholar]
- Borich MR, Brodie SM, Gray WA, Ionta S & Boyd LA (2015). Understanding the role of the primary somatosensory cortex: opportunities for rehabilitation. Neuropsychologia 79, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie SM, Villamayor A, Borich MR & Boyd LA (2014). Exploring the specific time course of interhemispheric inhibition between the human primary sensory cortices. J Neurophysiol 112, 1470–1476. [DOI] [PubMed] [Google Scholar]
- Cirillo J & Perez MA (2015). Subcortical contribution to late TMS‐induced I‐waves in intact humans. Front Integr Neurosci 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG & Starr A (1987). Localization, timing and specificity of gating of somatosensory evoked potentials during active movement in man. Brain 11, 451–467. [DOI] [PubMed] [Google Scholar]
- Craig JC & Qian X (1997). Tactile pattern perception by two fingers: temporal interference and response competition. Percept Psychophys 59, 252–265. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens De Noordhout A, Marsden CD, Nakashima K, Rothwell JC & Thompson PD (1989). Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412, 449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE & Cheron G (1981). Non‐cephalic reference recording of early somatosensory potentials to finger stimulation in adult or aging normal man: differentiation of widespread N18 and contralateral N20 from the prerolandic P22 and N30 components. Electroencephalogr Clin Neurophysiol 52, 553–570. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ & Frackowiak RSJ (1995). Relation between cerebral activity and force in motor areas of the human brain. J Neurophysiol 74, 802–815. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P & Rothwell JC (1998). Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res 119, 265–268. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P & Rothwell JC (2001). The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res 138, 268–273. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A & Pilato F (2012). I‐wave origin and modulation. Brain Stimul 5, 512–525. [DOI] [PubMed] [Google Scholar]
- Essick GK & Whitsel BL (1988). The capacity of human subjects to process directional information provided at two skin sites. Somatosens Mot Res 6, 1–20. [DOI] [PubMed] [Google Scholar]
- Federico P & Perez MA (2016). Distinct corticocortical contributions to human precision and power grip. Cereb Cortex, https://doi.org/10.1093/cercor/bhw291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss N, Hari R, Salmelin R, Ahonen A, Hämäläinen M, Kajola M, Knuutila J & Simola J (1994). Activation of the human posterior parietal cortex by median nerve stimulation. Exp Brain Res 99, 309–315. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT & Asano E (2008). Short‐latency median‐nerve somatosensory‐evoked potentials and induced gamma‐oscillations in humans. Brain 131, 1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S, Aras E & Weber PC (1970). Comparative study of sensory input to motor cortex in animals and man. Electroencephalogr Clin Neurophysiol 29, 537–550. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rzanny R, Liepert J, Büchel C & Weiller C (2002). Reduction of excitability ("inhibition") in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage 17, 490–496. [DOI] [PubMed] [Google Scholar]
- Hlushchuk Y, & Hari R (2006). Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci 26, 5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffken O, Lenz M, Tegenthoff M & Schwenkreis P (2010). Multichannel SEP‐recording after paired median nerve stimulation suggests origin of paired‐pulse inhibition rostral of the brainstem. Neurosci Lett 468, 308–311. [DOI] [PubMed] [Google Scholar]
- Hoffken O, Tannwitz J, Lenz M, Sczesny‐Kaiser M, Tegenthoff M & Schwenkreis P (2013). Influence of parameter settings on paired‐pulse‐suppression in somatosensory evoked potentials: a systematic analysis. Clin Neurophysiol 124, 574–580. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Komssi S & Lauronen L (2006). Spatial dynamics of population activities at S1 after median and ulnar nerve stimulation revisited: an MEG study. Neuroimage 32, 1024–1031. [DOI] [PubMed] [Google Scholar]
- Huttunen J & Lauronen L (2012). Intracortical modulation of somatosensory evoked fields during movement: evidence for selective suppression of postsynaptic inhibition. Brain Res 1459, 43–51. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Matsunaga K, Nakanishi R, Kawahira K, Murayama N, Tsuji S, Huang YZ & Rothwell JC (2007). Effect of theta burst stimulation over the human sensorimotor cortex on motor and somatosensory evoked potentials. Clin Neurophysiol 118, 1033–1043. [DOI] [PubMed] [Google Scholar]
- Iwamura Y (2000). Bilateral receptive field neurons and callosal connections in the somatosensory cortex. Philos Trans R Soc Lond B Biol Sci 355, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura Y, Taoka M & Iriki A (2001). Bilateral activity and callosal connections in the somatosensory cortex. Neuroscientist 7, 419–429. [DOI] [PubMed] [Google Scholar]
- Jiang W, Lamarre Y & Chapman CE (1990a). Modulation of cutaneous cortical evoked potentials during isometric and isotonic contractions in the monkey. Brain Res 536, 69–78. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chapman CE & Lamarre Y (1990b). Modulation of somatosensory evoked responses in the primary somatosensory cortex produced by intracortical microstimulation of the motor cortex in the monkey. Exp Brain Res 80, 333–344. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD & Hendry SH (1978). Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181, 291–347. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Gould HJ 3rd, Cusick CG, Pons TP & Kaas JH (1983). The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol 219, 384–419. [DOI] [PubMed] [Google Scholar]
- Kirimoto H, Tamaki H, Suzuki M, Matsumoto T, Sugawara K, Kojima S & Onishi H (2014). Sensorimotor modulation differs with load type during constant finger force or position. PLoS One 9, e108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokotilo KJ, Eng JJ, McKeown MJ & Boyd LA (2010). Greater activation of secondary motor areas is related to less arm use after stroke. Neurorehabil Neural Repair 24, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvenoja A, Wikström H, Huttunen J, Virtanen J, Laine P, Aronen HJ, Seppäläinen AM & Ilmoniemi RJ (1995). Activation of ipsilateral primary sensorimotor cortex by median nerve stimulation. NeuroReport 6, 2589–2593. [DOI] [PubMed] [Google Scholar]
- Lee EK & Seyal M (1998). Generators of short latency human somatosensory evoked potentials recorded over the spine and scalp. J Clin Neurophysiol 15, 227–234. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Fu KM, Branch CA & Schroeder CE (2006). Ipsilateral hand input to area 3b revealed by converging hemodynamic and electrophysiological analyses in macaque monkeys. J Neurosci 26, 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B & Hallett M (2000). Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111, 344–349. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y & Tsuji H (1996). Direct and indirect activation of human corticospinal neurons by transcranial magnetic and electrical stimulation. Neurosci Lett 210, 45–48. [DOI] [PubMed] [Google Scholar]
- Nakata H, Inui K, Wasaka T, Nishihira Y & Kakigi R (2003). Mechanisms of differences in gating effects on short‐and long‐latency somatosensory evoked potentials relating to movement. Brain Topogr 15, 211–222. [DOI] [PubMed] [Google Scholar]
- Newton JM, Sunderland A & Gowland PA (2005). fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. Neuroimage 24, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh IJ & Chen R (2011). Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol 105, 749–756. [DOI] [PubMed] [Google Scholar]
- Nihashi T, Naganawa S, Sato C, Kawai H, Nakamura T, Fukatsu H, Ishigaki T & Aoki I (2005). Contralateral and ipsilateral responses in primary somatosensory cortex following electrical median nerve stimulation–an fMRI study. Clin Neurophysiol 116, 842–848. [DOI] [PubMed] [Google Scholar]
- Nirkko AC, Ozdoba C, Redmond SM, Bürki M, Schroth G, Hess CW & Wiesendanger M (2001). Different ipsilateral representations for distal and proximal movements in the sensorimotor cortex: activation and deactivation patterns. Neuroimage 13, 825–835. [DOI] [PubMed] [Google Scholar]
- Ogata K, Okamoto T, Yamasaki T, Shigeto H & Tobimatsu S (2009). Pre‐movement gating of somatosensory‐evoked potentials by self‐initiated movements: the effects of ageing and its implication. Clin Neurophysiol 120, 1143–1148. [DOI] [PubMed] [Google Scholar]
- Pandya DN& Vignolo LA (1969). Interhemispheric projections of the parietal lobe in the rhesus monkey. Brain Res 15, 49–65. [DOI] [PubMed] [Google Scholar]
- Perez MA & Cohen LG (2008). Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28, 5631–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA & Cohen LG (2009). Scaling of motor cortical excitability during unimanual force generation. Cortex 45, 1065–1071. [DOI] [PubMed] [Google Scholar]
- Ragert P, Nierhaus T, Cohen LG & Villringer A (2011). Interhemispheric interactions between the human primary somatosensory cortices. PLoS One 6, e16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P & Paulus W (1999). Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52, 97–103. [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T & Kanazawa I (1997). Preferential activation of different I waves by transcranial magnetic stimulation with a figure‐of‐eight‐shaped coil. Exp Brain Res 113, 24–32. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Witte OW, Cheyne D, Haid G, Vrba J & Freund HJ (1995). Modulation of somatosensory evoked magnetic fields by sensory and motor interferences. Neuroreport 6, 1653–1658. [DOI] [PubMed] [Google Scholar]
- Seki K & Fetz EE (2012). Gating of sensory input at spinal and cortical levels during preparation and execution of voluntary movement. J Neurosci 32, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines WR, Black SE, Graham SJ & McIlroy WE (2002). Somatosensory gating and recovery from stroke involving the thalamus. Stroke 33, 2642–2651. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM & Pike GB (2004). Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22, 771–778. [DOI] [PubMed] [Google Scholar]
- Stude P, Lenz M, Höffken O, Tegenthoff M & Dinse H (2016). A single dose of lorazepam reduces paired‐pulse suppression of median nerve evoked somatosensory evoked potentials. Eur J Neurosci 43, 1156–1160. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Onishi H, Yamashiro K, Kotan S, Kojima S, Miyaguchi S, Tsubaki A, Kirimoto H, Tamaki H, Shirozu H & Kameyama S (2016). Effect of muscle contraction strength on gating of somatosensory magnetic fields. Exp Brain Res 234, 3389–3398. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P & Rothwell JC (2000). Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerdahl M, Simons SB, Chiu JS, Favorov O & Whitsel BL (2006). Ipsilateral input modifies the primary somatosensory cortex response to contralateral skin flutter. J Neurosci 26, 5970–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T & Rothwell JC (2002). Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J Physiol 540, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits NM & Zijdewind I (2008). Relation between muscle and brain activity during isometric contractions of the first dorsal interosseous muscle. Hum Brain Mapp 29, 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasaka T, Nakata H, Kida T & Kakigi R (2005). Changes in the centrifugal gating effect on somatosensory evoked potentials depending on the level of contractile force. Exp Brain Res 166, 118–125. [DOI] [PubMed] [Google Scholar]
- Yumiya H & Ghez C (1984). Specialized subregions in the cat motor cortex: anatomical demonstration of differential projections to rostral and caudal sectors. Exp Brain Res 53, 259–276. [DOI] [PubMed] [Google Scholar]


