Abstract
Tocotrienols, the unsaturated form of vitamin E, were reported to modulate platelet aggregation and thrombotic mechanisms in pre-clinical studies. Using a Food and Drug Administration (FDA)-approved cartridge-based measurement system, a randomised, double-blind, crossover and placebo-controlled trial involving 32 metabolic syndrome adults was conducted to investigate the effect of palm-based tocotrienols and tocopherol (PTT) mixture supplementation on platelet aggregation reactivity. The participants were supplemented with 200 mg (69% tocotrienols and 31% α-tocopherol) twice daily of PTT mixture or placebo capsules for 14 days in a random order. After 14 days, each intervention was accompanied by a postprandial study, in which participants consumed 200 mg PTT mixture or placebo capsule after a meal. Blood samples were collected on day 0, day 14 and during postprandial for the measurement of platelet aggregation reactivity. Subjects went through a 15-day washout period before commencement of subsequent intervention. Fasting platelet aggregation reactivity stimulated with adenosine diphosphate (ADP) did not show substantial changes after supplementation with PTT mixture compared to placebo (p = 0.393). Concomitantly, changes in postprandial platelet aggregation reactivity remained similar between PTT mixture and placebo interventions (p = 0.408). The results of this study highlight the lack of inhibitory effect on platelet aggregation after short-term supplementation of PTT mixture in participants with metabolic syndrome.
Introduction
Metabolic syndrome is associated with disrupted haemostasis balance indicated by higher platelet reactivity and hypercoagubility1–3. Changes in the regulation of thrombosis promote the development of cardiovascular diseases in individuals with metabolic syndrome, which has been classified as a real disease entity by Center for Disease Control4–6. Being a global public health concern, there are no clinical recommendations thus far to initiate antithrombotic therapy for individuals with metabolic syndrome. This advocates the need for investigations on supplements or nutraceuticals that have the potential to reduce the risk of cardiovascular diseases as preventive measures.
Platelet aggregation, a pathophysiologic process by which platelets adhere at the disrupted sites of vascular upon stimulation is critical for haemostatic plug formation7. It is well established that high platelet aggregation activity promotes formation of thrombus and disturbance of blood flow, leading to cardiovascular diseases7, 8. Tocotrienols, the unsaturated form of vitamin E, are principally found in several edible vegetable oils such as palm oil, rice bran oil and barley oil9, 10. There is documented evidence that tocotrienols exhibit antioxidant, neuroprotection, anticancer and antidiabetic attributes, which are beneficial to human’s health10, 11. In addition, both pre-clinical and clinical studies suggested possible modulation of mechanisms in platelet aggregation with tocotrienols12–18.
The inhibitory effect of tocotrienols in platelet aggregation was first reported by Qureshi et al.12, who showed that supplementation of tocotrienols-enriched diet reduced plasma thromboxane B2 in swine model. This finding was further corroborated by several animal models fed with tocotrienols-enriched diet13, 14. In a study using canine model, tocotrienols were found to cause marked reduction in ADP-stimulated platelet aggregation15. In hypercholesterolemic subjects, supplementation of tocotrienols significantly reduced plasma thromboxane B2 and platelet aggregation in response to ADP stimulation16–18. Despite evidence showing significant effect of tocotrienols in the modulation of platelet aggregation, several studies reported otherwise. Koba et al.19 and Watkins et al.20 showed that tocotrienols did not alter ADP-stimulated platelet aggregation in rat models. In human studies, Wahlqvist et al.21 reported no change in plasma thromboxane B2 and platelet aggregation stimulated by ADP and collagen after tocotrienol supplementation. In later studies, Tomeo et al.22 and Mensink et al.23 also failed to show that administration of tocotrienols could reduce collagen-induced platelet aggregation.
On the other hand, the saturated family of vitamin E, tocopherols, were reported to exhibit antithrombotic effect in several human trials. Oral administration of α-tocopherol at doses of 400 IU to 1200 IU resulted in attenuation of platelet aggregation via agonist dependent pattern24, 25. In vitro studies on tocopherols suggested modulation of pathways involving their antioxidant properties26, aminophospholipid translocase activity27 and protein kinase C-dependent mechanism28. Interestingly, tocopherol supplementation was also found to decrease ADP-induced platelet aggregation in type 1 diabetes mellitus patients29, 30.
Collectively, the discrepancy in the efficacy of PTT mixture in modulating platelet aggregation is likely to be affected by several factors. First being the dose of tocotrienols. In previous studies, the dose of tocotrienols supplemented ranged from 40 mg to 300 mg per day. Besides, covariate from the studied population and background dietary pattern might affect the study end point. Most importantly, measurement of platelet reactivity was not standardised across the studies. Light transmission aggregometry (LTA) and whole blood aggregometry were found to be the most common methods used. Though being a gold standard method for measurement of platelet aggregation, LTA has been reported with low reproducibility even when the assays were conducted in the same laboratory31. Further, platelet-rich plasma samples are needed for measurements using LTA. Several notable disadvantages of using platelet-rich plasma are 1) loss of hyperactive, hypoactive, or giant thrombocytes32, injury to platelets or platelets artefact activation could occur during platelet-rich plasma preparation33 and 2) measurement is in an artificial milieu lacking leukocytes and erythrocytes under relatively low shear conditions33, 34, which influence the results of platelet aggregation. While whole blood aggregometry is designed to circumvent the disadvantages of using platelet-rich plasma, it has been shown to give higher variability than LTA34. On the other hand, the concentration of stimulants used in the assays varies among research laboratories and was reported to affect data interpretation significantly. In view of these limitations that may contribute to inconsistent findings, attempt to use an improved instrument is desired for this study. VerifyNow instrument is a point of care instrument that has been designed to overcome the limitations of LTA utilising whole blood for analysis. It is a cartridge-based system able to minimise concentration and operator variability. Results interpretation using VerifyNow were reproducible and correlated reasonably well with LTA and other types of assays34–36. Hence, current study was designed to ascertain the effect of PTT mixture on platelet aggregation in metabolic syndrome subjects, specifically using VerifyNow instrument.
Materials and Methods
This single centre human trial was conducted in Malaysian Palm Oil Board, Malaysia. Ethical approval was obtained from Medical Research Ethics Committee of Universiti Putra Malaysia, identification number UPM/FPSK/100-9/2-MJKEtikaPen(FBSB_Nov(11)16). The study protocol was registered in ClinicalTrials.gov (NCT01631838) on 26 June 2012. The trial protocol was in compliance with Declaration of Helsinki and Malaysian Guidelines for Good Clinical Practice. Written informed consent was obtained from all participants prior to initiation of screening and trial procedures.
Participants
The participants were aged between 25 and 60 years old. Metabolic syndrome was defined according to the Clinical Practice Guidelines, Management of Type 2 Diabetes in Malaysia 200937. Participants were identified with waist circumference ≥90 cm in men and ≥80 cm in women and with any two of the following criteria: elevated triacylglycerol (>1.7 mmol/L), low high density lipoprotein (HDL) cholesterol (<1.0 mmol/L in men and 1.3 mmol/L in women), elevated blood pressure (≥130 mm Hg/≥85 mm Hg) or elevated fasting glucose (≥5.6 mmol/L to 7 mmol/L).
The exclusion criteria were as follows: 1) medical history of myocardial infarction, angina, ischemic attack, haemorrhagic stroke, deep vein thrombosis, coronary artery disease, bleeding disorder, or cancer, 2) significant hepatic or renal impairment, 3) fever, cold or infection during bleeding day, 4) fasting serum ferritin below 15 µg/L, 5) fasting haemoglobin below 11.5 g/dL in women and 12.5 g/dL in men, 6) smoker, 7) lactose intolerance, 8) pregnant or breast feeding, 9) alcohol drinker. Subjects were excluded if they were taking vitamin E supplements or medications for anticoagulant, antiplatelet, antihypertensive, glucose lowering, lipid lowering or corticosteroids.
Trial design
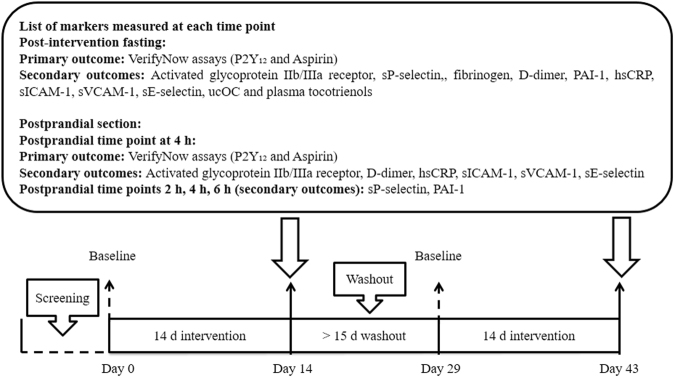
This was a randomised, double-blind, crossover and placebo-controlled trial consisting of two interventions, namely PTT mixture and placebo. Participants were randomised to start with any one of the two interventions using Latin square design generated in computer. Participants and investigators were blinded from the allocation of interventions. Each intervention involved a supplementation period of 14 days separated by a washout period of at least 15 days (Fig. 1). During PTT mixture intervention, participants consumed one capsule of 200 mg TocovidTM SupraBio TM (containing 61.52 mg α-tocotrienol, 112.80 mg γ-tocotrienol, 25.68 mg δ-tocotrienol and 61.07 mg α-tocopherol, batch no: 11901BBA) twice daily after breakfast and dinner. Similarly, during placebo intervention, participants consumed one placebo capsule (palm olein containing <1 mg vitamin E, batch no: 11902BBA) twice daily. Both types of capsules were similar in physical appearance and were supplied in colour coded bottles (Hovid Bhd., Malaysia). As vitamin E absorption could vary with the amount of fat intake, participants were instructed to consume 125 mL of full cream milk containing 4.3 g of fat together with the capsules. One day before study visit, participants were instructed to avoid strenuous exercise, consumption of alcohol, caffeine, and high fat dinner, and to fast after 10.00 pm.
Figure 1.
Study design. Abbreviation: hsCRP: high sensitivity C-reactive protein, PAI-1: plasminogen activator inhibitor type 1, sE-selectin: soluble E-selectin, sICAM-1: soluble intracellular adhesion molecules 1, sP-selectin: soluble P-selectin, sVCAM-1: soluble vascular cellular adhesion molecules 1, ucOC: undercarboxylated osteocalcin.
Blood samples were collected on day 0 (baseline fasting blood sample) and day 14 (post-intervention fasting blood sample). On postprandial day, participants were given a high fat breakfast consisting of one high fat muffin and a glass of 100 mL milkshake (providing approximately 828 kcal energy, 54 g fat, 73 g carbohydrate, and 12 g protein) before consuming one test capsule. Blood samples were collected at 2 h, 4 h, and 6 h (Fig. 1). Participants were allowed to sip water (<600 mL) but not allowed to eat over the postprandial period. Lunch was given to participants after blood sampling. Participants were advised to maintain their usual lifestyle and diet throughout the study period. They were requested to record any illness, medication used and study deviation during study period, and notify investigator at each study visit. Any usage of medications that could interfere with the results of this study was not allowed. If the medication were critical for participant’s health, subjects were dropped out.
Compliance measurement
Measurement of compliance was conducted via pill counting of returned bottles from the participants during post-intervention visit. Plasma tocotrienol levels were also measured using high performance liquid chromatography method as previously described by Che et al.38.
Primary outcome - VerifyNow assays
VerifyNow instrument (Accumetrics, Inc., California, USA) measures platelet aggregation reactivity based on the ability of activated platelets binding towards fibrinogen-coated beads in the presence of specific agonist. This rapid automated cartridge-based analyser was attached either with VerifyNow Aspirin cartridge or VerifyNow P2Y12 cartridge during assays39. As for the VerifyNow Aspirin cartridge, platelet aggregation in whole blood sample was induced by arachidonic acid, and the results were expressed as aspirin reactivity unit (ARU). Whereas in VerifyNow P2Y12 cartridge, ADP was used to stimulate platelet aggregation, and the results were expressed as P2Y12 reactivity unit (PRU). During analysis, whole blood samples were collected into a 2 mL 3.2% sodium citrate tubes and inverted five times. Subsequently, the samples were incubated at room temperature for 10 min and 30 min for VerifyNow P2Y12 and VerifyNow Aspirin assays, respectively.
Secondary outcomes – Blood, serum and plasma assays
Activated glycoprotein IIb/IIIa receptor, which is expressed only on the activated platelet surface, was determined using a method derived from Furman et al.40. Whole blood sample was collected into a tube contained 3.2% sodium citrate and processed within 30 min. A volume of 5 µL of anticoagulated whole blood was added into a polypropylene tubes containing 20 µM (DL-Isoser1)-thrombin receptor activating peptide-6 trifluoroacetate salt (thrombin mimic peptide, Bachem AG, Switzerland), fluorescein isothiocyanate conjugated PAC-1 (PAC-1-FITC) (Becton, Dickinson and Company, New Jersey, USA) and peridinin chlorophyll protein complex conjugated CD61 (CD61-PerCP) (Becton, Dickinson and Company, New Jersey, USA). Sample was gently swirled for mixing and incubated at room temperature for 20 min. The mixture was then fixed with 0.5% formalin at a pH of 7.4 (10 mmol/L HEPES buffer, 0.15 mM sodium chloride). The fixed sample was placed at 4 °C for at least 30 min before analysing with a BD FACSCalibur™ flow cytometer (Becton, Dickinson and Company, New Jersey, USA). Platelets were identified by CD61-PerCP, and data were obtained for 10,000 platelet events. The activated glycoprotein IIb/IIIa event was determined based on the mean fluorescence intensity (MFI) of PAC-1-FITC antibody binding on the dual parameter dot plot of PAC-1-FITC fluorescence displaying events from CD61 positive region.
Plasma plasminogen activator inhibitor type 1 (PAI-1) and D-dimer were determined using IMUBIND® plasma PAI-1 ELISA kit (Sekisui Diagnostics, LLC., USA) and IMUCLONE® D-dimer ELISA kit (Sekisui Diagnostics, LLC., USA), respectively. Plasma soluble P-selectin (sP-selectin), soluble E-selectin (sE-selectin), soluble intracellular adhesion molecule 1 (sICAM-1) and soluble vascular cellular adhesion molecule 1 (sVCAM-1) were analysed using Human sP-selectin/CD62P Immunoassay kit (R&D Systems, Inc., USA), Quantikine® Human sE-selectin/CD62E Immunoassay kit (R&D Systems, Inc., USA), Quantikine® Human sICAM-1/CD54 Immunoassay kit (R&D Systems, Inc., USA) and Quantikine® Human sVCAM-1 Immunoassay kit (R&D Systems, Inc., USA), respectively. Plasma undercarboxylated osteocalcin (ucOC) was measured using ucOC EIA kit (Takara Bio Inc., Japan). Assay of plasma fibrinogen was carried out using Sysmex automated haematology analyser XT-4000i (Sysmex Corporation, Kobe, Japan). Serum high sensitivity C-reactive protein (hsCRP) assay was performed using ADVIA® 2400 Clinical Chemistry System autoanalyser (Siemens AG, Munich, Germany).
Sample size calculation and statistical analysis
Sample size was calculated based on 95% power at P = 0.01 to detect a 8% of mean reduction in ADP stimulated platelet aggregation (estimated from Qureshi et al.16) based on fasting platelet aggregation units (PAU) in metabolic syndrome reported by Serebruany et al.1. All data were presented as means ± standard deviations (SDs). The normality of data distribution was examined using D’Agostino & Pearson omnibus test in GraphPad Prism software (Version5.01; GraphPad Software, Inc., California, USA). Data was assumed normally distributed when P > 0.05 based on 95% confidence interval. Logarithmic transformation was performed for several parameters as stated in the Results section, with data shown being original values. Differences between means were tested with Student’s paired t-test for data distributed normally. Non-parametric test (Wilcoxon Signed Ranks test) was used for the statistical analysis of data that were different from Gaussian distribution. Repeated measures generalised linear model with Bonferroni test was performed for postprandial PAI-1 and sP-selectin. All data was assumed significantly different at P < 0.05 based on the confidence interval of 95%. These statistical analyses were performed using IBM SPSS statistical software (version 20; SPSS, Inc., Illinois, USA).
Results
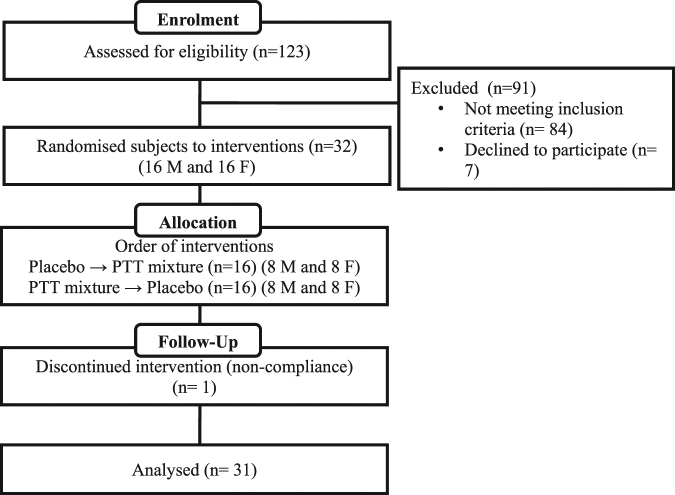
Figure 2 showed the consort diagram of this study. Recruitment for the study were carried out between early April 2012 and mid of July 2012. Out of 123 individuals who attended the screening, 32 metabolic syndrome subjects who met the participation criteria were recruited in this crossover study. One male volunteer had discontinued from the study due to non-compliance. Table 1 summarised the baseline characteristics of 31 subjects who completed the study and on whom data were available for the statistical analysis of primary outcomes.
Figure 2.
Consort diagram.
Table 1.
Baseline characteristics of study population.
| Men (n = 15) | Women (n = 16) | |
|---|---|---|
| Ethnicity | ||
| Malay | 9 (60.0%) | 14 (87.5%) |
| Chinese | 4 (26.7%) | 0 (0.0%) |
| Indian | 2 (13.3%) | 2 (12.5%) |
| Age (y) | 34 ± 8.2 | 41.6 ± 10.7 |
| Weight (kg) | 86.4 ± 14.6 | 73.4 ± 15.3 |
| BMI (kg/m2) | 29.4 ± 5.3 | 30.5 ± 5.4 |
| Waist circumference (cm) | 100.9 ± 9.4 | 96.5 ± 7.7 |
| Blood pressure (mm Hg) | ||
| SBP | 133.4 ± 7.8 | 133.2 ± 15.2 |
| DBP | 86.4 ± 7.6 | 85.2 ± 9.5 |
| Serum triacylglycerol (mmol/L) | 2.1 ± 0.7 | 1.6 ± 0.7 |
| Serum HDL cholesterol (mmol/L) | 1.0 ± 0.1 | 1.2 ± 0.1 |
| Fasting glucose (mmol/L) | 5.1 ± 0.5 | 5.1 ± 0.4 |
Abbreviation: BMI: body mass index, DBP: diastolic blood pressure, SBP: systolic blood pressure, HDL: high density lipoprotein.
No serious adverse events were reported throughout the study period. Subjects’ body weights were relatively stable recording approximately 0.01% changes. Compliance via pill counting showed an average of 99.5% (1.99 capsules/d) and 98.4% (1.97 capsules/d) compliance for PTT mixture and placebo interventions, respectively. Fasting plasma tocotrienol concentration in PTT mixture group (0.58 ± 0.50 µg/mL) was found to be significantly higher (p < 0.001) than placebo group (0.03 ± 0.06 µg/mL).
Primary outcome
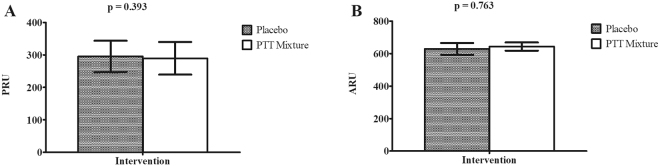
Following 14 days of supplementation, results of VerifyNow P2Y12 assay demonstrated no significant difference in ADP-induced platelet aggregation reactivity between PTT mixture and placebo interventions (290 ± 50 PRU vs 295 ± 48 PRU, p = 0.393) (Fig. 3 Panel A). Arachidonic acid induced platelet aggregation reactivity, as measured by VerifyNow Aspirin assay, were 631 ± 33 ARU and 628 ± 36 ARU in PTT mixture and placebo interventions respectively, with no significant difference among interventions (p = 0.763) (Fig. 3 Panel B).
Figure 3.
Post-intervention platelet aggregation reactivity results of PRU (A) and ARU (B). (n = 31; 15 males and 16 females). Intervention effect of PRU and ARU was examined using Student t-test. Abbreviation: ARU: Aspirin reactivity units, PRU: P2Y12 reactivity units.
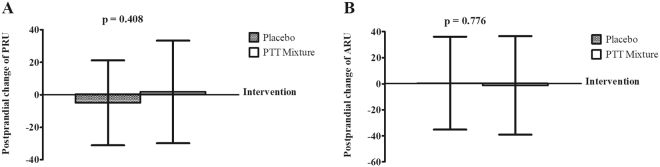
A postprandial study was carried out to examine the acute response of platelet aggregation reactivity following PTT mixture supplementation (Fig. 4). Changes in postprandial platelet aggregation induced by ADP were found to be 1.77 ± 31.59 PRU and −4.97 ± 26.11 PRU in PTT mixture and placebo interventions, respectively (Fig. 4 Panel A). As for platelet aggregation induced by arachidonic acid, postprandial changes did not differ significantly in PTT mixture and placebo interventions, i.e. −1.29 ± 37.77 ARU vs 0.45 ± 35.63 ARU, p = 0.776 (Fig. 4 Panel B).
Figure 4.
Postprandial change of platelet aggregation reactivity of PRU (A) and ARU (B). (n = 31; 15 males and 16 females). Intervention effect of PRU and ARU was examined using Student t-test and Wilcoxon Signed Rank test, respectively. Abbreviation: ARU: Aspirin reactivity units, PRU: P2Y12 reactivity units.
Secondary outcome
Post-intervention results for secondary outcome were summarised in Table 2. Measurements of glycoprotein IIb/IIIa receptor activation using flow cytometry method did not show significant difference between PTT mixture and placebo groups (12.37 ± 3.42 MFI vs 12.09 ± 3.53 MFI, p = 0.602). Plasma sP-selectin, a marker representing in vivo platelet activation status, was measured at 93.96 ± 67.53 ng/mL and 78.60 ± 43.97 ng/mL for PTT mixture and placebo interventions, respectively. A slight decrease in fasting D-dimer levels was noted in the PTT mixture group compared to placebo, although the difference did not reach statistical significance (p = 0.505). Additional thrombogenic markers including fibrinogen, PAI-1 and ucOC were found to be similar between the two intervention groups. Measurements of soluble inflammatory (hsCRP) and adhesion molecules (sE-selectin, sICAM-1 and sVCAM-1) did not show notable changes throughout the interventions.
Table 2.
Effect of PTT mixture and placebo supplementations for 14 days on platelet activation, haemostatic and inflammatory markers.
| PTT Mixture Intervention | Placebo Intervention | P | |
|---|---|---|---|
| Activated glycoprotein IIb/IIIa receptor (MFI) | 12.37 ± 3.42 | 12.09 ± 3.53 | 0.602 |
| sP-selectin (ng/mL) | 93.96 ± 67.53 | 78.60 ± 43.97 | 0.224 |
| Fibrinogen (g/L) | 3.00 ± 0.68 | 2.97 ± 0.69 | 0.865 |
| D-dimer (ng/mL) | 241.82 ± 170.72 | 287.80 ± 418.08 | 0.505 |
| PAI-1 (ng/mL) | 78.69 ± 33.91 | 80.11 ± 33.30 | 0.551 |
| ucOC (ng/mL) | 4.74 ± 2.10 | 4.90 ± 1.98 | 0.196 |
| hsCRP (mg/L) | 4.81 ± 4.86 | 4.94 ± 4.65 | 0.795 |
| sE-selectin (ng/mL) | 43.55 ± 15.02 | 43.45 ± 15.99 | 0.715 |
| sICAM-1 (ng/mL) | 220.20 ± 56.62 | 219.20 ± 63.68 | 0.629 |
| sVCAM-1 (ng/mL) | 554.60 ± 138.00 | 576.00 ± 149.00 | 0.210 |
Prior to statistical analysis, data for activated glycoprotein IIb/IIIa receptor, fibrinogen, sE-selectin, sICAM-1 and hsCRP were logarithmic transformed. Data for activated glycoprotein IIb/IIIa receptor, fibrinogen, PAI-1, sE-selectin, sICAM-1 and hsCRP were analysed using Student t-test while data for D-dimer, ucOC, sP-selectin and sVCAM-1 were analysed using Wilcoxon Signed rank test. Abbreviation: hsCRP: high sensitivity C-reactive protein, PAI-1: plasminogen activator inhibitor type 1, sE-selectin: soluble E-selectin, sICAM-1: soluble intracellular adhesion molecules 1, sP-selectin: soluble P-selectin, sVCAM-1: soluble vascular cellular adhesion molecules 1, ucOC: undercarboxylated osteocalcin.
Table 3 summarised the postprandial changes of secondary outcome. Minimal changes were observed in postprandial samples of glycoprotein IIb/IIIa receptor activation, D-dimer, hsCRP, sE-selectin and sVCAM-1 (p > 0.05). Plasma sICAM-1 concentration was significantly lowered in PTT mixture group compared to placebo group (−5.27 ± 15.28 ng/ml vs 2.66 ± 14.56, p = 0.046) during postprandial measurements. Figure 5 illustrated the postprandial responses of sP-selectin and PAI-1 after a high fat meal and test capsules. A significant time effect (p < 0.001) was observed for sP-selectin for both PTT mixture and placebo interventions. A drop in sP-selectin levels (64.6% and 56.5% from fasting levels in PTT mixture and placebo groups) was recorded at 2 h postprandial, followed by an increase at 4 h. However, no significant difference was observed when analysed for intervention × time effect (p = 0.282) (Fig. 5 Panel A). As illustrated in Fig. 5 Panel B, postprandial plasma PAI-1 decreased by 43.6% and 39.3% from fasting levels for PTT mixture and placebo interventions at 4 hr. Similar to the trend of sP-selectin, PAI-1 levels recorded a slight increase at 6 h postprandial. Thus, a significant difference was detected for time effect (P < 0.001). When analysed for intervention × time effect, no statistical significance was observed (p = 0.540).
Table 3.
Postprandial change (4 hour) of platelet function values, haemostatic and inflammatory markers.
| PTT Mixture Intervention | Placebo Intervention | P | |
|---|---|---|---|
| Activated glycoprotein IIb/IIIa receptor(MFI) | −1.59 ± 2.89 | −0.70 ± 2.92 | 0.298 |
| D-dimer (ng/mL) | 30.72 ± 73.42 | 15.08 ± 109.4 | 0.814 |
| hsCRP (mg/L) | 0.11 ± 0.68 | 0.21 ± 1.28 | 0.871 |
| sE-selectin (ng/mL) | −1.49 ± 2.36 | −0.79 ± 2.90 | 0.286 |
| sICAM-1 (ng/mL) | −5.27 ± 15.28* | 2.66 ± 14.56* | 0.046 |
| sVCAM-1 (ng/mL) | −19.92 ± 33.29 | −19.42 ± 43.11 | 0.952 |
Interventions effect of activated glycoprotein IIb/IIIa receptor and sVCAM-1 were analysed using Student t-test. While intervention effect of D-dimer, hsCRP, sE-selectin and sICAM-1 were analysed using Wilcoxon Signed Rank test. Abbreviation: hsCRP: high sensitivity C-reactive protein, sE-selectin: soluble E-selectin, sICAM-1: soluble intracellular adhesion molecules 1, sVCAM-1: soluble vascular cellular adhesion molecules.*Indicated p < 0.05 between interventions.
Figure 5.
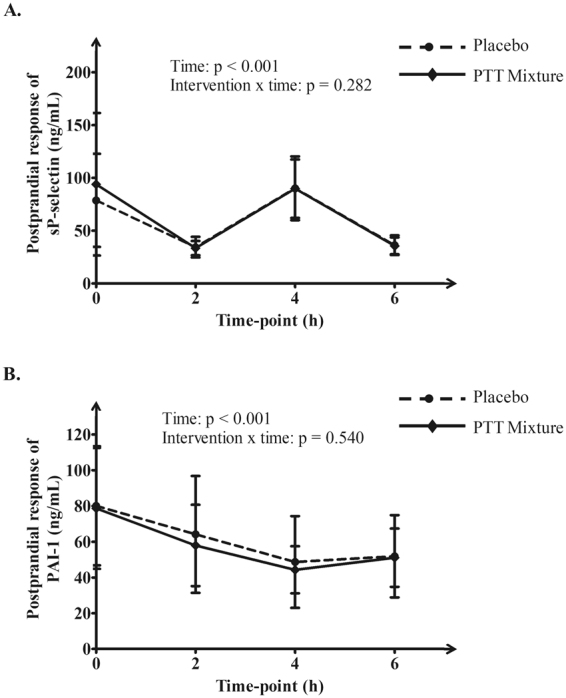
Postprandial response of plasma sP-selectin (A) and plasma PAI-1 (B). Only data for PAI-1 was logarithmic transformed during statistical analysis. The intervention and time effects were analysed using repeated measures generalised linear model. Abbreviation: PAI-1: plasminogen activator inhibitor type 1, sP-selectin: soluble P-selectin.
Discussion
ADP is a platelet agonist released from dense granules of activated platelets. It activates platelets through two purinegic receptors, which are P2Y12 and P2Y1 41. Several studies investigated the effects of tocotrienol supplementation on ADP-induced platelet aggregation, but these results are inconsistent. Qureshi et al.16 demonstrated significantly lower percentage of platelet aggregation in response to ADP after 4 weeks supplementation of 200 mg tocotrienol-rich fraction in hypercholesterolemic subjects. Pronounced inhibition effect on ADP induced platelet aggregation was also observed in both α-tocotrienol and tocotrienol-rich fraction treatments in their animal study using canine model15. On the contrary, in a human study conducted by Wahlqvist et al.21 where subjects were supplemented for 16 weeks with increasing doses of tocotrienol-rich fraction (60 to 240 mg/day) no significant reduction in platelets aggregability responded to ADP activation was reported. The variability results between these studies may be due to variation of methods and different concentrations of ADP used in platelet aggregation measurement using LTA. While Wahlqvist et al.21 investigated platelet aggregation using ADP concentrations ranging from 10 to 62.5 μM, Qureshi et al.16 used 5 and 20 μM of ADP as stimulants, correlating to up to 3-fold difference between the two studies. Unlike previous studies, VerifyNow instrument, a fully automated cartridge-based instrument that measures platelet aggregation at a standardised condition with its cartridge-based design and at a fixed concentration of ADP was used in current trial. In the VerifyNow P2Y12 cartridge, platelets are activated by ADP (20 μM) in the presence of prostaglandin E1 (22 nM)42. Prostaglandin E1 is an adenylyl cyclase stimulator, which inhibits calcium ion releases, thus suppresses the platelet activation induced via P2Y1 receptor to enhance specificity and sensitivity of ADP activation through P2Y12 receptor43. Results of current study suggested that tocotrienols did not modulate platelet aggregation through P2Y12 receptor following 14 days of PTT mixture supplementation in metabolic syndrome population. Similar observations were found during fasting and postprandial states.
Platelet aggregation can also be mediated through arachidonic acid signalling pathway. During platelet activation, arachidonic acid is released from phospholipids and converted to thromboxane A2 through a series of enzyme activities. Thromboxane A2 will then bind to thromboxane receptor to activate platelets44. In order to measure the effect from this pathway, a surrogate marker, thromboxane B2 was commonly determined being a stable metabolite derived from thromboxane A2. In two human studies, plasma and urine thromboxane B2 levels did not show significant changes after tocotrienol supplementation21, 22. In line with the results from these studies, PTT mixture supplementation for 14 days and postprandially did not exert any inhibitory effect on arachidonic acid induced platelet aggregation using VerifyNow system. However, marked reduction of serum or plasma thromboxane B2 after tocotrienol supplementation was found in studies conducted by Qureshi et al.17, 18. It is notable that diet control (American Heart Association Step 1 diet or National Cholesterol Education Program Step 1 diet) was implemented in the studies of Qureshi et al.17, 18, limiting the intake of fat, cholesterol and total calorie. It was postulated that a combinatorial effect on the inhibition of platelet aggregation might be observed with PTT mixture and diet control.
For secondary outcomes, platelet activation was monitored via thrombin mimic peptide agonist and plasma sP-selectin levels. Thrombin is a platelet agonist generated in the coagulation system which binds to protease-activated receptor-1 and protease-activated receptor-4 on platelet’s surface, subsequently triggering the cascade events of platelet aggregation44. An in vitro study with tocopherol showed a dose dependent involvement of aminophospholipid translocase activity induced by thrombin, which was responsible for the inhibitory effect in platelet aggregation27. In this study, thrombin-induced activation of glycoprotein IIb/IIIa receptors was measured using flow cytometry. Our results indicated no changes in platelet activation in whole blood samples from subjects taking PTT mixture. The results were correlated to a study conducted by Tomeo et al.22 where subjects with hyperlipidemia and carotid stenosis consumed tocotrienols in an increasing dose manner (224 mg/day – 336 mg/day) for 18 months. Measurements of platelet activation via platelet adenosine triphosphate release induced by thrombin did not show significant reduction. In the same study, changes in platelet aggregation induced by collagen were not significant in groups taking palm mixed tocotrienols and tocopherol compared to placebo. Similar data on collagen-induced platelet aggregation was reported in Mensink et al.23. Future study on collagen-induced platelet aggregation after tocotrienol supplementation is warranted to confirm its effect. As for sP-selectin, plasma levels were measured at fasting and postprandial states, being an indicator of in vivo platelet activation status. Although time dependent changes at 6-hour postprandial was observed, PTT mixture supplementation for 14 days did not modulate sP-selectin levels compared to placebo group. As for haemostatic markers, results obtained were in consistent with platelet aggregation and platelet activation. Biomarkers measured in this study include D-dimer, PAI-1 and fibrinogen. D-dimer is a biomarker that reflects activation of coagulation and fibrinolysis45, while PAI-1 is a regulator of D-dimer concentration, which inhibits the initiation of fibrinolysis process46. Fibrinogen is a precursor for fibrin formation and glycoprotein that bridging the activated platelets via activated glycoprotein IIb/IIIa receptors to promote platelet aggregation44. These findings were in accordance with results demonstrated by Mensink et al.23, suggesting the lack of influence from tocotrienols on the coagulation system. In Mensink et al.23, no changes were found in D-dimer, PAI-1, Factor VII, fibrinogen, fragment 1 + 2 and antithrombin III. On the other hand, plasma ucOC is a sensitive measure of vitamin K status47, in which vitamin K acts as an important cofactor in the coagulation cascade. Our results showed minimal changes in ucOC levels in both groups, indicating the lack of interaction between tocotrienols and the vitamin K cascade in affecting the coagulation status. As exploratory outcome, this study measured several plasma inflammatory markers at fasting and 4-hour postprandial. The results did not show improvement on inflammation status based on plasma concentrations of inflammatory markers, i.e. sE-selectin, sVCAM-1 and hsCRP. A slight reduction was observed in 4-hour postprandial levels of sICAM-1 in the PTT mixture group, although the difference was minimal with relatively large SD. However, soluble fractions of these biomarkers in the plasma were measured using ELISA method. Our results leave room for doubt on their expression profile on platelets especially for indicators of platelet function including P-selectin and ICAM-1. Future study using flow cytometry method is warranted.
From another point of view, there are several potential limitations in this study. First being the duration of study with supplementation period of 14 days. The supplementation period was proposed to be 14 days in this study in view that: i) physiological life span of blood platelets is about 7 to 10 days with a daily renewal rate of about 20% of total platelet count48, ii) maximal inhibition of platelet aggregation following oral administration of platelet aggregation inhibition agents occurred within 5 days49 and iii) concentration of tocotrienols in platelet was able to double after 10 days of tocotrienol (80 mg/d) supplementation50. However, taken from previous studies that tocotrienols were able to improve lipid profiles after 6 months supplementation51, the supplementation period of 14 days in this study might be relatively too short for tocotrienols to exhibit platelet-aggregating inhibitory effect. Further, the effect of tocotrienols in protection against white matter lesion was only noted after 2 years supplementation of palm-mixed tocotrienols52. Although not investigated in this study, measurement of tocotrienols incorporation into platelet fractions would have provided interesting perspective complementing measurement of tocotrienol levels in plasma. In vitro, Freedman et al.28 reported a dose and time dependent increment of α-tocopherol from 0.25 to 2.5 mM and 2 to 30 minutes, respectively. In these experiments, high levels of platelet α-tocopherol were correlated with lower extent of platelet aggregation. In a separate study, Hayes et al.50 conducted a short term supplementation study with palm-mixed tocotrienols and tocopherol. Similar trend was observed in plasma and platelet levels of tocotrienols after 10 days supplementation (80 mg tocotrienols and 64 mg α-tocopherol). The percentage of increment from baseline was higher in tocotrienol levels (272% in plasma and 96% in platelet) compared to tocopherol levels (69% in plasma and 35% in platelet). Secondly, it was unclear if the presence of tocopherol at 31% had a possible impact on the transport mechanism of tocotrienols. It was reported by Shibata et al.53 in a rat feeding trial that α-tocopherol at 50 mg/day attenuated the cholesterol-lowering effect of rice bran tocotrienols at 11 mg/day. The effect corroborated with a decrease in peripheral tissue concentrations of tocotrienols after coadministration with tocopherols, suggesting possible inhibitory mechanism on the absorption pathways. Nevertheless, the dose of α-tocopherol administered in this study (122 mg, equivalent to 91 IU per day) was lower than published results reporting at 400 to 1200 IU. Besides, several studies reported the lack of inhibitory effect on platelet aggregation with α-tocopherol compared to mixed tocopherols that contained high concentration of γ-tocopherol54, 55. In summary, this study implied that acute supplementation of PTT mixture at a dose of 400 mg/day did not affect the modulation of platelet aggregation, platelet activation, coagulation and inflammatory status in subjects with metabolic syndrome. All subjects well-tolerated the 2 weeks PTT mixture supplementation without any adverse events being reported. Our results implied the lack of clinically significant effect on platelet homeostasis with acute supplementation of PTT mixture. Information from this study would add value to health practitioners and this cohort intending to consume PTT mixture as supplements for a short period of time. Nevertheless, their long term effects on platelet function remained uncertain and should be warranted in future studies.
Acknowledgements
We thank the Director-General of Malaysian Palm Oil Board for permission to publish this paper; the volunteers for their participation in this trial as well as laboratory staffs in the Nutrition Unit and Genomic Unit for their kind assistance; and Hovid Bhd. for supplying TocovidTM SupraBio TM 200 mg and placebo capsules.
Author Contributions
J.Y.F., K.N., K.T.T., K.R.S., K.H.Y., P.M. and Y.L.G. were responsible for the trial design and establishment of the methods used. J.Y.F. and Y.L.G. organised and conducted the trial; Y.L.G. carried out the sample analyses; O.M.L., B.H.C., K.H.Y., K.T.T., J.Y.F., and Y.L.G. contributed to the statistical analysis and interpretation of data. J.Y.F., O.M.L., B.H.C., K.H.Y., K.N., K.T.T., K.R.S. and P.M. substantially advised on the trial. All authors had contributed to the writing of manuscript and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Serebruany VL, Malinin A, Ong S, Atar D. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. J. Thromb. Thrombolysis. 2008;25:207–213. doi: 10.1007/s11239-007-0047-3. [DOI] [PubMed] [Google Scholar]
- 2.Alessi MC, Juhan-Vague I. Metabolic syndrome, haemostasis and thrombosis. Thromb. Haemost. 2008;99:995–1000. doi: 10.1160/TH07-11-0682. [DOI] [PubMed] [Google Scholar]
- 3.Morange PE, Alessi MC. Thrombosis in central obesity and metabolic syndrome: mechanisms and epidemiology. Thromb. Haemost. 2013;110:669–680. doi: 10.1160/TH13-01-0075. [DOI] [PubMed] [Google Scholar]
- 4.Jang MJ, et al. Metabolic syndrome is associated with venous thromboembolism in the Korean population. Arterioscler. Thromb. Vasc. Biol. 2009;29:311–315. doi: 10.1161/ATVBAHA.109.184085. [DOI] [PubMed] [Google Scholar]
- 5.Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke. 2005;36:1366–1371. doi: 10.1161/01.STR.0000169945.75911.33. [DOI] [PubMed] [Google Scholar]
- 6.Lau DC, Yan H, Dhillon B. Metabolic syndrome: a marker of patients at high cardiovascular risk. Can. J. Cardiol. 2006;22(Suppl B):85B–90B. doi: 10.1016/S0828-282X(06)70992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sweeny JM, Gorog DA, Fuster V. Antiplatelet drug ‘resistance’. Part 1: mechanisms and clinical measurements. Nat. Rev. Cardiol. 2009;6:273–282. doi: 10.1038/nrcardio.2009.10. [DOI] [PubMed] [Google Scholar]
- 9.Goh SH, Choo YM, Ong SH. Minor constituents of palm oil. J. Am. Oil Chem. Soc. 1985;62:237–240. doi: 10.1007/BF02541384. [DOI] [Google Scholar]
- 10.Bardhan J, Chakraborty R, Raychaudhuri U. The 21st century form of vitamin E–tocotrienol. Curr. Pharm. Des. 2011;17:2196–2205. doi: 10.2174/138161211796957472. [DOI] [PubMed] [Google Scholar]
- 11.Budin SB, et al. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics (Sao Paulo) 2009;64:235–244. doi: 10.1590/S1807-59322009000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qureshi AA, et al. Dietary tocotrienols reduce concentrations of plasma cholesterol, apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs with inherited hyperlipidemias. Am. J. Clin. Nutr. 1991;53:1042S–1046S. doi: 10.1093/ajcn/53.4.1042S. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi AA, Peterson DM. The combined effects of novel tocotrienols and lovastatin on lipid metabolism in chickens. Atherosclerosis. 2001;156:39–47. doi: 10.1016/S0021-9150(00)00612-2. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AA, Peterson DM, Hasler-Rapacz JO, Rapacz J. Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J. Nutr. 2001;131:223–230. doi: 10.1093/jn/131.2.223. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AA, et al. Tocotrienols-induced inhibition of platelet thrombus formation and platelet aggregation in stenosed canine coronary arteries. Lipids Health Dis. 2011;10:58. doi: 10.1186/1476-511X-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi AA, et al. Lowering of serum cholesterol in hypercholesterolemic humans by tocotrienols (palmvitee) Am. J. Clin. Nutr. 1991;53:1021S–1026S. doi: 10.1093/ajcn/53.4.1021S. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi AA, et al. Response of hypercholesterolemic subjects to administration of tocotrienols. Lipids. 1995;30:1171–1177. doi: 10.1007/BF02536620. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AA, Bradlow BA, Salser WA, Brace LD. Novel tocotrienols of rice bran modulate cardiovascular disease risk parameters of hypercholesterolemic humans. J. Nutr. Biochem. 1997;8:290–298. doi: 10.1016/S0955-2863(97)89667-2. [DOI] [Google Scholar]
- 19.Koba K, Abe K, Ikeda I, Sugano M. Effects of alpha-tocopherol and tocotrienols on blood pressure and linoleic acid metabolism in the spontaneously hypertensive rat (SHR) Biosci. Biotechnol. Biochem. 1992;56:1420–1423. doi: 10.1271/bbb.56.1420. [DOI] [PubMed] [Google Scholar]
- 20.Watkins T, et al. gamma-Tocotrienol as a hypocholesterolemic and antioxidant agent in rats fed atherogenic diets. Lipids. 1993;28:1113–1118. doi: 10.1007/BF02537079. [DOI] [PubMed] [Google Scholar]
- 21.Wahlqvist ML, et al. Differential serum responses of tocopherols and tocotrienols during vitamin supplementation in hypercholesterolaemic individuals without change in coronary risk factors. Nutr. Res. 1992;12:S181–S201. doi: 10.1016/S0271-5317(05)80463-4. [DOI] [Google Scholar]
- 22.Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids. 1995;30:1179–1183. doi: 10.1007/BF02536621. [DOI] [PubMed] [Google Scholar]
- 23.Mensink RP, van Houwelingen AC, Kromhout D, Hornstra G. A vitamin E concentrate rich in tocotrienols had no effect on serum lipids, lipoproteins, or platelet function in men with mildly elevated serum lipid concentrations. Am. J. Clin. Nutr. 1999;69:213–219. doi: 10.1093/ajcn/69.2.213. [DOI] [PubMed] [Google Scholar]
- 24.Steiner M. Effect of alpha-tocopherol administration on platelet function in man. Thromb. Haemost. 1983;49:73–77. [PubMed] [Google Scholar]
- 25.Williams JC, et al. Dietary vitamin E supplementation inhibits thrombin-induced platelet aggregation, but not monocyte adhesiveness, in patients with hypercholesterolaemia. Int. J. Clin. Exp. Pathol. 1997;78:259–266. doi: 10.1046/j.1365-2613.1997.260359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F. Vitamin E inhibits collagen-induced platelet activation by blunting hydrogen peroxide. Arterioscler. Thromb. Vasc. Biol. 1999;19:2542–2547. doi: 10.1161/01.ATV.19.10.2542. [DOI] [PubMed] [Google Scholar]
- 27.Kim JE, Han M, Hanl KS, Kim HK. Vitamin E inhibition on platelet procoagulant activity: involvement of aminophospholipid translocase activity. Thromb. Res. 2011;127:435–442. doi: 10.1016/j.thromres.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Freedman JE, Farhat JH, Loscalzo J, Keaney JF. α-tocopherol inhibits aggregation of human platelets by a protein kinase C–dependent mechanism. Circulation. 1996;94:2434–2440. doi: 10.1161/01.CIR.94.10.2434. [DOI] [PubMed] [Google Scholar]
- 29.Colette C, Pares-Herbute N, Monnier LH, Cartry E. Platelet function in type I diabetes: effects of supplementation with large doses of vitamin E. Am. J. Clin. Nutr. 1988;47:256–261. doi: 10.1093/ajcn/47.2.256. [DOI] [PubMed] [Google Scholar]
- 30.Gisinger C, et al. Effect of vitamin E supplementation on platelet thromboxane A2 production in type I diabetic patients: double-blind crossover trial. Diabetes. 1988;37:1260–1264. doi: 10.2337/diab.37.9.1260. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson NS, et al. Assessment of platelet function assays. Am. Heart. J. 1998;135:S170–178. doi: 10.1016/S0002-8703(98)70245-5. [DOI] [PubMed] [Google Scholar]
- 32.Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. Approach to the assessment of platelet function: comparison between optical-based platelet-rich plasma and impedance-based whole blood platelet aggregation methods. Clin. Appl. Thromb. Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- 33.Harrison P, Frelinger AL, 3rd, Furman MI, Michelson AD. Measuring antiplatelet drug effects in the laboratory. Thromb. Res. 2007;120:323–336. doi: 10.1016/j.thromres.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Harrison P. Platelet function analysis. Blood Rev. 2005;19:111–123. doi: 10.1016/j.blre.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Saw, J. & Moliterno, D. J. Antiplatelet Agents in Platelet Function:Assessment, Diagnosis, and Treatment (eds Quinn, M. & Fitzgerald, D.) 335–367 (Humana Press, 2005).
- 36.Nielsen HL, et al. Aspirin response evaluated by the VerifyNow Aspirin System and light transmission aggregometry. Thromb. Res. 2008;123:267–273. doi: 10.1016/j.thromres.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Ministry of Health Malaysia. Clinical practice guideline: management of type 2 diabetes mellitus 36-37 (Ministry of Health Malaysia, 2009).
- 38.Che HL, et al. Validation of a HPLC/FLD method for quantification of tocotrienols in human plasma. Int. J. Anal. Chem. 2015;2015:357609. doi: 10.1155/2015/357609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lev EI, et al. Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation. J. Am. Coll. Cardiol. 2010;55:114–121. doi: 10.1016/j.jacc.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Furman MI, et al. Quantification of abciximab-induced platelet inhibition is assay dependent: a comparative study in patients undergoing percutaneous coronary intervention. Am. Heart. J. 2003;145:e6. doi: 10.1067/mhj.2003.116. [DOI] [PubMed] [Google Scholar]
- 41.Remijn JA, et al. Role of ADP receptor P2Y(12) in platelet adhesion and thrombus formation in flowing blood. Arterioscler Thromb. Vasc. Biol. 2002;22:686–691. doi: 10.1161/01.ATV.0000012805.49079.23. [DOI] [PubMed] [Google Scholar]
- 42.Steinhubl, S. R. The VerifyNow System A2 In Platelets (ed. Michelson, A. D.) 509–518 (Academic Press, 2007).
- 43.Kreutz RP, et al. Inhibition of platelet aggregation by prostaglandin E1 (PGE1) in diabetic patients during therapy with clopidogrel and aspirin. Platelets. 2013;24:145–150. doi: 10.3109/09537104.2012.661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker, R. C. Platelet biology the role of platelets in hemostasis, thrombosis and inflammation in Platelets in cardiovascular disease (ed. Bhatt, D. L.) 1–36 (Imperial College Press, 2008).
- 45.von Kanel R, Dimsdale JE. Fibrin D-dimer: a marker of psychosocial distress and its implications for research in stress-related coronary artery disease. Clin. Cardiol. 2003;26:164–168. doi: 10.1002/clc.4960260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler. Thromb. Vasc. Biol. 2006;26:2200–2207. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 47.Binkley NC, Krueger DC, Engelke JA, Foley AL, Suttie JW. Vitamin K supplementation reduces serum concentrations of under-gamma-carboxylated osteocalcin in healthy young and elderly adults. Am. J. Clin. Nutr. 2000;72:1523–1528. doi: 10.1093/ajcn/72.6.1523. [DOI] [PubMed] [Google Scholar]
- 48.Gawaz, M. Platelets and primary hemostasis in Blood platelets: physiology, pathophysiology, membrane receptors, antiplatelet principles, and therapy for atherothrombotic diseases (ed. Gawaz, M.) 4–24 (Theime Medical Publishers, 2001).
- 49.Lange RA, Hillis LD. Antiplatelet therapy for ischemic heart disease. N. Engl. J. Med. 2004;350:277–280. doi: 10.1056/NEJMe038191. [DOI] [PubMed] [Google Scholar]
- 50.Hayes KC, Pronczuk A, Liang JS. Differences in the plasma transport and tissue concentrations of tocopherols and tocotrienols: observations in humans and hamsters. Proc. Soc. Exp. Biol. Med. 1993;202:353–359. doi: 10.3181/00379727-202-43546. [DOI] [PubMed] [Google Scholar]
- 51.Yuen KH, Wong JW, Lim AB, Ng BH, Choy WP. Effect of mixed-tocotrienols in hypercholesterolemic subjects. Funct. Foods. Health Dis. 2011;3:106–117. [Google Scholar]
- 52.Gopalan Y, et al. Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke. 2014;45:1422–1428. doi: 10.1161/STROKEAHA.113.004449. [DOI] [PubMed] [Google Scholar]
- 53.Shibata A, Kawakami Y, Kimura T, Miyazawa T, Nakagawa K. α-tocopherol attenuates the riglyceride- and cholesterol-lowering effects of rice bran tocotrienol in rats Fed a western diet. J. Agric. Food Chem. 2016;64:5361–5366. doi: 10.1021/acs.jafc.6b02228. [DOI] [PubMed] [Google Scholar]
- 54.Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am. J. Clin. Nutr. 2003;77:700–706. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 55.Singh I, Turner AH, Sinclair AJ, Li D, Hawley JA. Effects of gamma-tocopherol supplementation on thrombotic risk factors. Asia Pac. J. Clin. Nutr. 2007;16:422–428. [PubMed] [Google Scholar]