Abstract
Primary motor cortex has been studied for more than a century, yet a consensus on its functional contribution to movement control is still out of reach. In particular, there remains controversy as to the level of control produced by motor cortex (“low-level” movement dynamics vs. “high-level” movement kinematics) and the role of sensory feedback. In this review, we present different perspectives on the two following questions: What does activity in motor cortex reflect? and How do planned motor commands interact with incoming sensory feedback during movement? The four authors each present their independent views on how they think the primary motor cortex (M1) controls movement. At the end, we present a dialogue in which the authors synthesize their views and suggest possibilities for moving the field forward. While there is not yet a consensus on the role of M1 or sensory feedback in the control of upper limb movements, such dialogues are essential to take us closer to one.
Keywords: primary motor cortex, motor control, sensory feedback, movement dynamics, movement kinematics
movement is perhaps the most critical function of nervous systems; without movement, no amount of sensory inputs, memories, and reasoning will aid survival (Wolpert 2011). It is thus crucial to understand how this output comes to be. More than a century has passed since Hitzig and Fritsch first discovered that electrical stimulation of primary motor cortex evokes movement in the motor periphery, yet a consensus on the functional contribution of primary motor cortex to movement control still has not been reached.
The motor control literature is packed with theoretical frameworks and experimental results, yet key controversies date to some of the earliest experiments in neuroscience. Shortly after Hitzig and Fritsch demonstrated that exciting the motor cortex in dog could evoke muscle twitches (Fritsch and Hitzig 1870), Ferrier demonstrated a similar phenomenon in monkey (Ferrier 1873). Using longer duration electrical stimulation, Ferrier was able to evoke body movements that were more complex and seemingly purposeful. Ferrier concluded that motor cortex controls complex features of movement rather than muscles per se, as Hitzig and Fritsch were suggesting.
The degree to which the cortical motor areas are involved with basic movement control was challenged by a landmark study by Lawrence and Kuypers (Lawrence and Kuypers 1968; Lemon et al. 2012). They showed that just a few days following bilateral pyramidotomy (i.e., severing the dominant output pathway of primary motor cortex and other motor cortical areas to the spinal cord), a monkey could perform many gross movements such as walking or even climbing in a manner similar to an intact monkey. However, on closer examination, the monkey exhibited serious deficits for fine hand movements, such as food manipulation, suggesting a role for primary motor cortex in fine movement control, and deficits in novel movements or those involving visuomotor complexity, such as reaching around a clear barrier to retrieve food.
Discussion of these same issues has continued into the modern days of recording neural activity of the motor cortex. In particular, the level of control produced by motor cortex has commanded much attention. Evarts (1968) was the first to show that cells in the motor cortex were sensitive to the force needed for a given movement, suggesting that “low-level” movement control signals are present in M1. Georgopoulos et al. (1982), on the other hand, demonstrated that the discharge frequency of cells varied in an orderly fashion with the direction of movement. They therefore suggested that motor cortex may produce a “high-level” movement representation for reach direction (i.e., movement kinematics) with overlapping cosine tuning curves and vector averaging. In this view, the details of muscle activation patterns are generated by subcortical or spinal processing. As time has gone on, others have argued for relatively direct muscle control (Ben Hamed et al. 2007), torque representation (Scott and Kalaska 1997), cooperative muscle “synergies” (d’Avella and Bizzi 2005), control via summing complex posture primitives (Graziano et al. 2002), pattern generation (Shenoy et al. 2013), and high-level coordinate transformations (Buneo and Andersen 2006).
Another critical question about motor control is the role of sensory feedback in the process. The presence of sensory feedback in motor cortex was established long ago (Evarts 1973; Fritsch and Hitzig 1870); however, its role is still a matter of debate (Scott 2008, 2016). Inspired by feedback control theories, some researchers have argued that feedback has an essential role in motor control (Phillips 1969; Scott 2004; Todorov and Jordan 2002). Others have suggested that this feedback is an accessory to preplanned movements, either to correct deviations from the original plan (Flash and Hogan 1985), to choose the appropriate pattern of muscle activity (Bioulac and Lamarre 1979; Cheung et al. 2005), or to update the plan for future movements when movement dynamics do not match expectations (Diedrichsen et al. 2005; Wolpert and Ghahramani 2000).
The field has, however, come to agree on one point: activity in motor cortex does not solely reflect muscle activity. The possibilities for the meaning of these “extra” signals are diverse, with ideas including feedback, high-level signals, cognitive inputs, other control signals, incidental activity of a pattern generator, and learning signals. The central question of this collection is to discuss the nature of this “dark matter”.
Understanding how primary motor cortex controls movement has serious practical implications. Motor disorders caused by stroke or spinal injuries are among the most debilitating and costly disorders for patients and society (Mozaffarian et al. 2016; Singh et al. 2014). Better understanding of the physiology of motor control could translate into the development of better treatments to rehabilitate these patients. For instance, understanding the rules that neural activity follows (Kao et al. 2016) and providing synthetic sensory feedback (Bensmaia and Miller 2014) have been demonstrated to increase the performance of brain-machine interfaces. For these reasons, the functions performed by motor cortex are a mystery that remains as urgent now as ever (Cherian et al. 2013; Churchland et al. 2010; Golub et al. 2014; Griffin et al. 2015; Hatsopoulos et al. 2007; Heming et al. 2016; Moran and Schwartz 1999; Todorov 2000).
In this review, we present four perspectives on these classical controversies, and discuss how these viewpoints might fit together. Paul Cheney first reviews what is known about M1 pyramidal tract neurons, arguing that these output neurons reflect a fine-grained control signal that is a context-dependent combination of central and sensory influences. Nicholas Hatsopoulos argues that M1 activity does not encode any single movement parameter, but rather is selective to a preferred movement fragment or trajectory, which is characterized by a combination of parameters evolving in time. Components of the preferred trajectory leading M1 activity reflect sensory feedback, whereas lagging components correspond to motoric drive. Matthew Kaufman argues that M1 should be thought of as a dynamical machine, whose goal is to produce the time-varying pattern of control signals needed to achieve the desired movement. This view implies that M1 does not directly code for movement parameters, but instead should be understood in terms of the rules that govern its pattern generation. Mohsen Omrani argues that sensory feedback is an essential part of motor control and therefore, to better understand the neural circuitry underlying motor control, we should pay more attention to the role of sensory feedback in this system. Finally, we present a discussion by the authors of how to reconcile these views, and lay out future directions to close in on a unified answer.
What Is Encoded in Motor Cortex Output? –P. Cheney
The question of what is encoded by motor cortex neurons seems relatively straightforward, but it continues to be asked, often precipitating lively discussion and differing conclusions (Fetz 1992). However, it now seems possible to provide some answers with a relatively high degree of confidence. The brief review that follows is an attempt to focus on conclusions that can be drawn from the considerable body of evidence that now exists relative to this question.
The question of encoding by motor cortex cells needs to be addressed on two levels. One concerns the cell’s spike train activity and with what it is best correlated. This is the dimension that has received most of the attention over the years. However, it is not only important to understand what is encoded in the signals the cell is transmitting, but it is also essential to know how the signal is distributed to motoneuron pools and muscles. Thus the second dimension of encoding concerns the cell’s muscle targets. Specifically, what is the set of muscles the cell influences? This has been termed the cell’s muscle field (Fetz et al. 1976; Fetz and Cheney 1978, 1979, 1980). How does this relate to the cell’s activity during movements that engage the target muscles?
Regarding the question of what is encoded in the activity of cortical cells, it is important not to overlook the fact that there are six cortical layers and a large diversity of different cell types with different central nervous system (CNS) targets (Brodal 1981). It is reasonable to expect that the signals carried by different neurons will vary depending on the functional role and CNS targets of the neurons. Although it is clear that relationships to virtually any motor parameter can be found by looking at a heterogeneous set of motor cortical neurons, the relationships narrow and become much clearer by reducing the set of neurons of interest to just those output neurons that can be demonstrated to have a linkage to motoneurons. This section will focus specifically on cortical cells with a demonstrable linkage to motoneurons. How are these neurons identified? Although the importance of identifying the synaptic targets of neurons under study is clear, it can represent a significant technical challenge for many experiments. However, the introduction of spike-triggered averaging of electromyographic (EMG) activity (SpTA) has provided a method for identifying cortical cells in awake behaving animals that have a synaptic linkage to motoneurons (Cheney and Fetz 1980, 1985; Fetz and Cheney 1980). Averages of EMG activity triggered from the spikes of some motor cortex cells show a short-latency transient increase corresponding to increases in the firing probability of the underlying motor units. We refer to these transient increases in EMG activity as postspike facilitation (PSpF) effects (Fetz and Cheney 1980). PSpF is interpreted as evidence of an underlying synaptic linkage to motoneurons of the muscles showing PSpF. In most cases this is likely to be mediated by a monosynaptic linkage, so cells showing PSpF are often referred to as corticomotoneuronal (CM) cells. The method’s utility is not limited to revealing excitatory linkages. It is also capable of revealing inhibitory linkages as transient troughs in averages of EMG activity of antagonist muscles (Bennett and Lemon 1996; Cheney et al. 1982; Griffin et al. 2015; Kasser and Cheney 1985; Schieber and Rivlis 2007). These effects are referred to as postspike suppression (PSpS) effects and have latencies that average ~4 ms longer than PSpF effects from the same cell (Kasser and Cheney 1985). PSpS is interpreted as evidence that the cortical cell has an inhibitory linkage to motoneurons. The longer latency and fact that these effects are weaker than PSpF effects is consistent with a less direct linkage involving interneurons, probably the Ia inhibitory interneurons, which are known to receive monosynaptic input from cortical neurons (Jankowska et al. 1976). Although we have no fully reliable way of identifying non-monosynaptic excitatory connections, the fact that PSpS can be detected suggests that non-monosynaptic PSpF is also detected with the SpTA method. Moreover, cells producing PSpF can readily be found on the rostral surface of M1 where linkages to motoneurons are largely non-monosynaptic (Rathelot and Strick 2009). Cells producing non-monosynaptic PSpF are clearly very important and may have properties similar in many respects to those producing monosynaptic PSpF, but an answer to this question remains for future investigation. The SpTA method has now been applied by numerous groups whose studies have contributed greatly to understanding the organization and function of brain descending systems (Baker and Lemon 1998; Belhaj-Saïf et al. 1996; Buys et al. 1986; Cheney et al. 1988; Davidson et al. 2007a, 2007b; Drew 1993; Fourment et al. 1991, 1995; Lemon et al. 1986; Maier et al. 1993; Mewes and Cheney 1991, 1994; Olivier et al. 1995; Porter and Lemon 1993; Schieber and Rivlis 2007; Sinkjaer et al. 1995).
We can now make the question of encoding more specific and restrict it to motor cortex output cells that can be shown to have a demonstrable synaptic linkage to motoneurons. For wrist movement related cortical output cells, Cheney and Fetz (1980) showed that over a large part of their firing range, the tonic activity of these cells was linearly related to wrist torque. In many cases, phasic activity was related to the rate of change of torque consistent with the work of others (Smith et al. 1975). These relationships held for both isometric and concentric tasks and reinforced the original findings of Evarts (1968) showing a clear relationship between firing rate and movement force. Relationships between the firing rate of CM cells and force reported by Maier et al. (1993) for a precision grip task were more complex. Whereas 11 of 33 cells analyzed had significant positive correlations with force, 6 CM cells showed negative correlations with force. Negative correlations with force seem counterintuitive. However, these might be functionally important for assisting with precise release of grip force. It is also important to note that the precision grip task used in this study required co-contraction of muscles and was biomechanically more complex than the simple wrist flexion and extension task used by Cheney and Fetz (1985), and this may have contributed to seemingly counterintuitive activity relationships.
Of course, movement direction and force covary for many tasks, which has led to ambiguity as to which is the primary parameter linked to the discharge of cortical output cells (Georgopoulos et al. 1982). It seems that this has been largely resolved by experiments that have dissociated movement direction and force (Kalaska et al. 1989). Although the subject of much controversy over the years, it now seems clear that for motor cortex output to motoneurons, a large part of neuronal discharge encodes muscle activity related parameters. In support of this conclusion, Morrow et al. (2007), using a center-out task, analyzed the relationship of M1 neuron activity to preferred direction based on muscle space vs. hand space. They found that the majority of M1 neurons had muscle-like properties. In a different study, work from Lee Miller’s laboratory has demonstrated that the activity of M1 neurons relates best to functional groups of muscles and that the activity of a relatively small number of cortical neurons (e.g., 20) contains “sufficient information to reconstruct the time course of EMG activity with considerable precision” (Morrow and Miller 2003). Several modeling studies also support encoding of muscle related parameters by motor cortex cells (Mussa-Ivaldi 1988; Todorov 2000). Most recently, Lillicrap and Scott (2013) showed that the bias of M1 neurons in favor of certain directions of hand movement and joint-torque loads arises naturally in a neural network model when detailed features of the musculoskeletal system such as limb geometry and dynamics as well as biarticular muscles are included. They propose a general principle: “neural activity in M1 commands muscle activity and is optimized for the physics of the motor effector.”
Because cortical output cells have a set of target muscles that can be identified with SpTA of EMG activity, it is natural to ask how closely the firing rate modulations of these cells covary with the activity of their target muscles. This potentially could be a more robust linkage than relationships to joint force, because the cell’s target muscles are, in most cases, only a partial contributor to movement force measured at the joint. Griffin et al. (2008) investigated this issue by recording EMG activity from 22–24 arm and hand muscles simultaneously with the activity of identified CM cells during a reach-to-grasp task that produced rich muscle activation patterns. At least one firing rate peak for nearly all (95%) CM cells tested matched a corresponding peak in the EMG activity of one of its target muscles. Nevertheless, significant disparities were common. However, correlations between the activity of small ensembles of CM cells sharing the same target muscle and the EMG activity of that muscle were relatively strong (r ≥ 0.8). These results support the view that CM output from M1 cortex encodes movement in a framework of muscle-based parameters, specifically muscle-activation patterns as reflected in EMG activity.
How can mismatches between the activity of CM cells and their target muscles be explained? A major factor is likely to be the multitude of inputs motoneurons receive. The motoneuron pool for a particular muscle receives convergent input not only from the recorded CM cell but also from a large number of additional CM cells. It is perhaps not surprising that the activity of any single CM cells does not correlate perfectly with the EMG activity of the target muscle. Additionally, there are multiple brain descending systems (Davidson and Buford 2004; Lemon 2008; Mewes and Cheney 1991, 1994; Miller et al. 1993; Miller and Houk 1995; Riddle et al. 2009), and it is reasonable to assume that motoneuron activity for any given movement is the result of summation of inputs from all these descending systems together with input from sensory afferents. Such a multitude of inputs to motoneurons would produce an expectation that the activity of any given single neuron should show broad positive covariation with target muscle activity, but with likely discrepancies in the presence and timing of specific EMG peaks. This is exactly what we see.
There is another very interesting category of discrepancy between CM cell and target muscle activity that strongly suggests specialization for specific categories of muscle activity. CM cells are not involved in all movements that engage their target muscles, but rather only a subset of these movements. The idea that CM cells are functionally tuned for particular movements or phases of movements has received substantial new support in an elegant series of experiments from Peter Strick and colleagues (Griffin et al. 2015). Using a wrist center-out task, they were able to dissociate movement direction from muscle EMG activity by having the monkey perform the task with the wrist supinated, pronated, or vertical. Movement direction in external coordinates remained the same for all these wrist orientations, but the muscle activity to achieve the movement varied substantially for each orientation. A previous study from Strick’s laboratory (Kakei et al. 1999) using this task and recording unidentified motor cortical cells showed that a large fraction of cells tested (32%) exhibited activity that was muscle-like; however, an even larger number of cells (50%) had activity that was related to the direction of wrist movement in space independent of the pattern of muscle activity underlying the movement. The existence of true direction-related cells in motor cortex is consistent with the findings of others (Kalaska et al. 1989). However, an important question is, how would the results change if the motor cortex cells were identified as CM cells? Griffin et al. (2015) have now taken advantage of the same movement paradigm as the previous study (Kakei et al. 1999) with the additional condition that motor cortex cells were identified as CM cells using spike-triggered averaging of EMG activity. Their results support the concept that “the different functional uses of muscles are represented by separate populations of CM cells.” Under different conditions, the same muscle might function as an agonist, synergist, fixator, or antagonist. Different CM cells were found to represent each of these functions. In this way, nearly all (19/20) were muscle-like, but for a particular category of function. Their study raises the question of whether this functional tuning is a categorical or continuous function. Their data suggest it maybe continuous, but they point out that broad tuning may mask a more categorical relationship.
The conclusions of Griffin et al. (2015) are consistent with several previous examples of functional dissociation between the activity of CM cells and their target muscles. Cheney and Fetz (1980) found CM cells that were reliably and robustly modulated during controlled ramp-and-hold movements but nearly inactive during ballistic movements of the same joint despite much greater activity of the cell’s target muscles, suggesting that some other system is involved in producing relatively uncontrolled ballistic movements. Another example of this type of specialization was reported by Fetz and Cheney (1987). They showed that CM cells can be strongly activated for alternating wrist movements involving a reciprocal pattern of flexor and extensor muscle activation, but inactivated and nearly silent for power grip movements that coactivated the wrist flexors and extensors. In this case, the activity of the CM cell can be understood in terms of its postspike effects. Because the cell in this case produced inhibition of the flexor muscles in addition to excitation of extensors, its activation during power grip would have been inconsistent with coactivation of flexors and extensors required for the power grip task. This suggests that the synaptic actions cells have on agonist and antagonist muscles is a major factor in their selection for involvement in particular movements. The cells with negative firing rate force relations reported by Muir and Lemon (1983) for a precision grip task requiring muscle co-contraction might also be explained in terms of reducing possible reciprocal inhibition. Yet another example of dissociation between the activity of CM cells and target muscles is a study of precision grip and power grip by Muir and Lemon (1983). CM cells discharged at a higher rate for precision grip than power grip despite the fact that the cell’s target muscles were often more active for power grip. In some cases, the CM cell showed no modulation for power grip. The specialization of CM cells for different categories of movement may explain why the same muscle is re-represented many times throughout multiple sites in motor cortex (Capaday et al. 2013; Lemon 1988, 1990; Park et al. 2001; Porter and Lemon 1993; Schieber and Hibbard 1993; Van Acker et al. 2014).
We can conclude from the evidence presented above that CM cells encode muscle-related parameters of movement such as EMG activity and muscle force. However, unlike the activity of α-motoneurons, the activity of CM cells is not rigidly coupled to the activity of its target muscles. Rather, CM cells show specialization for particular movements or categories of muscle activity.
As mentioned earlier, there is another dimension or level on which CM cell encoding occurs. Knowing what the activity encodes is one step, but the next step is knowing where the activity goes and what is encoded in the cell’s target muscles. An early question related to this issue was whether CM cells influence single muscles or multiple muscles. The answer to this question has come from the results of spike-triggered averaging (Fetz and Cheney 1980; Lemon et al. 1986; Schieber and Rivlis 2005) and intra-axonal labeling of pyramidal tract axons (Shinoda et al. 1981). Although some CM cells, particularly those involved with finger movements, have their terminations confined to motoneurons of single muscles, the majority distribute terminals to multiple synergist muscles involved in a task (Fetz and Cheney 1980; Lemon et al. 1986; Porter and Lemon 1993; Schieber and Rivlis 2007). Many CM cells not only facilitate multiple synergist muscles but also suppress the activity of antagonists (Kasser and Cheney 1985). Agonist muscles in this case are ones that coactivate with the CM cell. This simple pattern of synaptic organization represents a specialized functional muscle synergy operating at a single joint to produce movement in a particular direction where suppression of antagonist muscle activity is hard-wired into the connections of CM cells to facilitate the movement. This result raises the question of how divergent the terminations single CM cells might be. Do the target muscles of some CM cells include muscles at multiple forelimb joints? To address this question, McKiernan et al. (1998) recorded CM cells in relation to a reach-to-grasp task requiring different patterns of coactivation of muscles at the shoulder, elbow, wrist, forearm digit, and intrinsic hand muscles. They found that the terminations of CM cells engaged in this task were not confined to muscles at a single joint; rather, 45% of cells facilitated muscles involving at least one distal and one proximal joint. The synergies formed by the target muscles of CM cells are not random but show a strong bias in favor of combinations of muscles at adjacent joints (Park et al. 2004).
The final question to consider is how CM cells are organized in the cortex. There is evidence that corticospinal cells in layer 5 are not uniformly distributed. Rather, they show a patchy or clustered organization (Jones and Wise 1977). Is there any functional significance to this clustered organization? Cheney and Fetz (1985) were able to identify the muscle fields of neighboring CM cells. They showed that neighboring CM cells recorded simultaneously through the same electrode had muscles fields that were very similar in terms of both the muscles with PSpF as well as the strength of effects in those muscles. This result was strengthened further by showing that effects in stimulus-triggered averages obtained by applying microstimuli at the same cortical site produced profiles of effects on muscles that closely matched the effects in spike-triggered averages from the same site. As expected, the poststimulus facilitation effects were stronger than postspike effects consistent with activation of multiple output cells with stimulation. However, the fact that the distribution and magnitude profile of effects matched the postspike effects suggests that all the cells activated with stimulation must have a similar set of target muscles and even a common strength of synaptic input to the motoneurons of those muscles. Similar results have been reported by Lemon (1988).
It should be noted that not all studies have found a clustered organization of corticospinal neurons (Rathelot and Strick 2006). However, the organization proposed above does not require actual clustering of corticospinal neurons. It only requires that neighboring corticospinal neurons share a largely common set of target muscles (muscle synergy). These localized collections of corticospinal neurons representing different muscle synergies might then be viewed as the fundamental output modules of motor cortex. It should also be noted that this view of organization is not in conflict with findings from viral labeling of corticospinal neurons with monosynaptic linkages to a single muscle showing a widely dispersed organization (Rathelot and Strick 2006, 2009). This result would be expected because any single muscle would be re-represented many times in the cortex as components of output modules for many different muscle synergies.
In conclusion, what is encoded by motor cortex cells? The answer would be many different parameters if all motor cortex cells are considered. However, if cells with a synaptic linkage to motoneurons are identified, a more uniform answer emerges. Motor cortex output encodes intrinsic muscle-related parameters (force, EMG activity). The axons of corticospinal neurons distribute these signals at the spinal level not just to motoneurons of single muscles, but rather to combinations of motoneuron pools (muscles), which encode functional muscle synergies for single and multijoint coordinated movements. CM cells represent the full range of ways in which their target muscles participate in movements including roles as agonists, synergists, fixators, and antagonists (Griffin et al. 2015). CM cells show motor task specialization and only become recruited for tasks appropriate for their set of target muscles and only for particular categories of movement. In this way they differ from α-motoneurons. Cells with similar muscles fields are clustered together in the cortex. Cells influencing a particular muscle are re-represented many times across the cortex as components of numerous different functional muscle synergies.
Trajectory Representations in the Motor Cortex –N. G. Hatsopoulos
In the early 20th century, Leyton and Sherrington argued that the motor cortex (M1) “seems to possess, or to be in touch with, the small localized movements as separable units, and to supply great numbers of connecting processes between these, so as to associate them together in extremely varied combinations” (Leyton and Sherrington 1917). In today’s parlance, this view might be rephrased by stating that individual M1 neurons represent or “code for” elementary movements and can be combined via the intrinsic connectivity of motor cortex to generate the rich variety of complex motor behaviors that are observed in mammals, in general, and primates, more specifically. The key to this perspective is that M1 neurons encode temporally extensive movements. In contrast, most theories of M1 function over the past 50 years have focused on different time-independent movement parameters such as force, direction, velocity, position, speed, acceleration, or combinations of these that might be encoded in the firing rates of individual M1 neurons (Ashe and Georgopoulos 1994; Cabel et al. 2001; Cheney and Fetz 1980; Evarts 1968; Fu et al. 1993, 1995a; Georgopoulos et al. 1982, 1984; Hepp-Reymond et al. 1978; Kalaska et al. 1989; Kurata 1993; Moran and Schwartz 1999; Paninski et al. 2004; Smith et al. 1975; Stark et al. 2006; Taira et al. 1996).
Unfortunately, despite numerous studies examining a whole host of static movement parameters, no consensus has been reached as to what exactly M1 encodes. There are several possible reasons why the state of understanding of motor cortical function is in such a confused state. First, most movement parameters are highly correlated with each other during natural movement, so it becomes very challenging to tease apart which parameter, if any, is actually represented in the firing response of individual neurons. Unlike the study of sensory processing, where the stimulus is under the control of the experimenter, movements are generated by the organism, and movement parameters that characterize these movements are interdependent due to limitations in the behavioral paradigm, physical laws of motion, and regularities in biological motion (Lacquaniti et al. 1983; Viviani and Cenzato 1985; Viviani and McCollum 1983; Viviani and Terzuolo 1982). Second, the motor cortex is likely not a single monolithic structure representing one thing, but rather may have distinct functional properties in depth over its six layers and horizontally across its cortical sheet. For example, the caudal portion of the macaque motor cortex within the central sulcus possesses nearly all fast, direct corticomotoneuronal neurons that make direct connections with motor neurons controlling the distal as well as proximal limb (Rathelot and Strick 2006, 2009; see, however, Witham et al. 2016, which provides evidence that slower and weaker corticomotoneuronal neurons exist in rostral motor cortex on the precentral gyrus). Therefore, caudal motor cortex may represent lower level force or muscle activity, whereas rostral motor cortex may code for higher level kinematic features of movement. A third possibility is that the notion that M1 codes for static, Newtonian parameters of motion is misguided (Churchland et al. 2012).
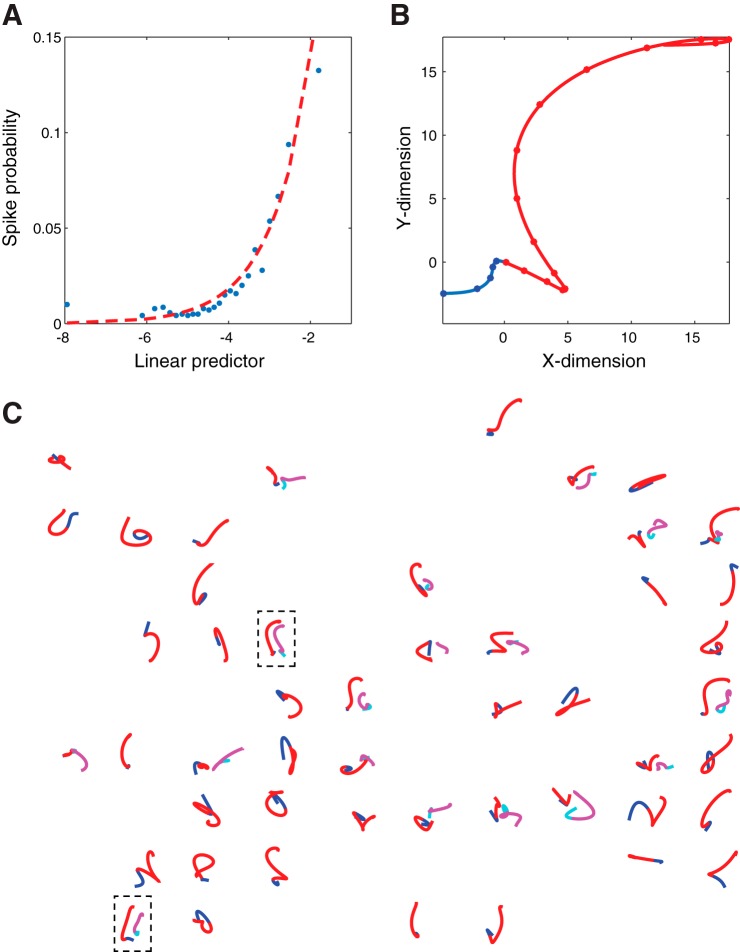
We have put forth a model of M1 function that resurrects the early viewpoint of Leyton and Sherrington in which we argue that M1 represents temporally extensive movement fragments or trajectories (Hatsopoulos et al. 2007). This model is motivated by the fact M1 activity is related to movement parameters such as direction at multiple time lags and the observation that preferred directions can vary at these different time lags. Although the model remains agnostic as to exactly whether these fragments are characterized kinematically or kinetically, it more accurately predicts the firing responses of M1 neurons than a static, parametric model such as one that assumes that neurons encode direction. Using a generalized linear model that assumes Poisson noise, we fit the mean spike count, μ(t), within the spike sampling time, t, to an exponential of the inner product between the preferred velocity trajectory, , and the normalized velocity trajectory of the hand, , fit the data (Fig. 1A):
| (1) |
where each velocity trajectory extends over a range of times before and after the spike sampling time, t, and γ is an offset parameter of the model. The spike sampling time, t, is centered on a small sampling window (e.g., 10 ms) such that the mean spike count is approximately equal to the probability of a spike occurring, P[spike(t)]. The normalized velocity trajectory, , is a sequence of hand velocities over a predefined time duration and is normalized by dividing by the L2-norm. We call the preferred velocity trajectory because the inner product in the exponent is maximized when the vector is aligned with it. Normalization mathematically describes the shape of the velocity trajectory but does not consider average speed and position, which are known to affect the firing of motor cortical neurons (Georgopoulos et al. 1984; Moran and Schwartz 1999). Therefore, the model in Eq. 1 can be expanded to include terms reflecting the average speed and average position of the trajectory sample. For simplicity, we limit our discussion of the model to two-dimensional reaching movements of the hand, although it has been shown to apply to more complex three-dimensional reaching and grasping movements (Saleh et al. 2010, 2012). By integrating the x- and y-components of in time, the preferred paths or “pathlets” for each neuron can be created (Fig. 1B). The vector, , is dimensionless but can be treated as if it had units of velocity because it is proportional to the normalized velocity trajectory that maximizes the mean spike count, and, therefore, its time integral be viewed as having units of position. The blue and red segments of the pathlet occur before or after the spike sampling time, respectively.
Fig. 1.
Trajectory encoding model used to predict spiking responses of single motor cortical neurons. A: the empirical spiking probability (blue dots) as well as the fitted model probability (red dashed line) as a function of the scalar linear predictor ( · + γ) for one motor cortical neuron. B: the preferred trajectory (“pathlet”) for the same neuron in A, generated by temporally integrating the coefficients of the fitted model (). The blue portion of each pathlet occurs before the spike sampling time, whereas the red portion occurs after the spike sampling time. C: a map of pathlets for all neurons recorded on the multielectrode array. Only pathlets with areas under the ROC curve > 0.55 are plotted. On some electrodes, more than one unit was isolated, and their pathlets are shown in cyan (before the sampling time) and magenta (after the sampling time). The dashed black boxes enclose electrodes with 2 recorded units whose pathlets are similar in shape.
Using receiver operating characteristic (ROC) analysis, we found that a 400-ms-long trajectory (−100 to +300 ms with respect to the spike sampling time) maximized the spike prediction accuracy. A 400-ms trajectory more accurately predicted the occurrence of a spike (or lack of a spike) even when compared with a brief 50-ms trajectory between +100 and +150 ms with respect to the spike sampling time (P < 0.01, sign test), which is often treated as the optimal lag between a motor cortical neuron’s firing and velocity (Moran and Schwartz 1999; Paninski et al. 2004). We also showed that a population vector decoding algorithm can more accurately predict movement direction when each neuron in the population possesses multiple preferred directions at different time leads/lags vs. an algorithm that considers only a single static preferred direction per neuron (Hatsopoulos et al. 2007).
Our model also has a number of other attractive features. First, several groups have observed that the preferred directions of individual M1 neurons shift in orientation over the course of reaching movements in a center-out task when measured at a finer timescale even before movement begins (Churchland and Shenoy 2007; Mason et al. 1998; Sergio and Kalaska 1998; Sergio et al. 2005;). This is an important phenomenon that calls into question the idea that motor cortical neurons possess a fixed preferred movement direction. Our trajectory encoding model can predict these systematic shifts in preferred direction by demonstrating that the path generated by temporal integration of these fine timescale preferred directions are similar to the shape of the preferred trajectory estimated by our model (Hatsopoulos et al. 2007). Second, the response profiles of M1 neurons have been noted to be complex in time and heterogeneous across neurons (Churchland and Shenoy 2007). Although it would be hard to explain why there are so many different complex response profiles if M1 neurons were only encoding direction, our trajectory model suggests that this heterogeneity is useful in generating a rich set of motion templates with complex shapes (Fig. 1C). Third, given the exponential nature of our model, we have shown how simultaneously firing neurons generate pathlet representations that are synthesized by mathematically adding the pathlet representations of the constituent neurons, thus creating a still richer set of motion templates (Hatsopoulos and Amit 2012). Finally, our model implicitly incorporates the effects of sensory feedback on the encoding properties of M1 neurons by constructing preferred movement fragments that extend in the future (motoric effects) as well as in the past (sensory effects) with respect to the neuron’s response.
Motor Cortex as a Dynamical Machine for Controlling Movement –M. T. Kaufman
Motor cortex presumably exists to control movement (Fetz 1992; Scott 2008). It has therefore long been argued that M1 is best understood as a pattern generator (Brown 1914) whose key function is to produce the coordinated, time-varying control signals needed to guide the spinal cord in causing a precise sequence of muscle contractions.
One possibility to understand this pattern generator is that we can describe each neuron in terms of its representation of high-level signals. A sizable branch of the literature argues that neural activity in motor and premotor cortex directly encodes high-level movement parameters such as hand velocity (Moran and Schwartz 1999) or position (Aflalo and Graziano 2006).
However, a preponderance of more recent evidence does not support the conclusion that high-level signals make up the strongest component of M1 activity. For example, neurons seemingly switch their relationships with hand velocity and other movement parameters over the course of a movement (Churchland and Shenoy 2007; Churchland et al. 2010; Crammond and Kalaska 2000; Fu et al. 1995b). Individual neurons in these areas do not even have consistent relationships with movement direction over hundreds of milliseconds (Hatsopoulos et al. 2007) or across postures (Scott and Kalaska 1997), despite the tight connection between M1 and the muscles (Dum and Strick 2002; Morrow and Miller 2003; Morrow et al. 2007). Moreover, neurons seemingly vary widely in how much the changes in their firing rates lead or lag muscle activity or hand velocity, both across neurons and for the same neuron over the course of a movement (Moran and Schwartz 1999). High-level representations may be present (Sanger 1994), but they cannot explain a large fraction of the activity (Aflalo and Graziano 2007). Why are the neuron-muscle relationships inconsistent? What are these other patterns of activity?
As an alternative approach, we can take a more mechanistic view and ask how the movement control signals come to be generated. That is, how does the neural population work together to produce the correct outputs (Briggman et al. 2005; Mazor and Laurent 2005)?
Considering that M1 is a biologically complex and highly recurrent network of neurons, perhaps we should expect a dynamical system whose individual units are unlikely to have external functional meanings (Fetz 1992). Fitting dynamical systems models is an approach to understanding these kinds of systems (Shenoy et al. 2011, 2013). This approach eschews trying to understand the response of each neuron considered separately, since neurons are simply cogs in a larger machine. Neurons are therefore expected to contain mixes of various command signals (Mussa-Ivaldi 1988; Sanger 1994), plus other activity that serves purely internal roles in helping to generate the output patterns. If this view is correct, it may not be productive to talk about what a single M1 neuron “codes”; neurons’ responses will correlate with virtually any external parameters or reference frame simply because these parameters correlate with the needed command signals and feedback.
Dynamical systems models explain a number of previously confusing aspects of M1 activity and have made a number of predictions tested by experiments. Four sets of tested predictions are described below.
First, despite the complexity of individual neurons’ responses, dynamical systems models predict that when activity is viewed at the population level, it is likely to be simple. In particular, the neural state (the set of firing rates across neurons) should follow consistent dynamical “rules” governing how that state changes as a function of what it was a moment ago. As predicted, for reaching movements, these rules are surprisingly simple and consistent across many different movement directions, distances, speeds, and curvatures. Specifically, the population largely acts like an oscillator: during movement, neurons’ firing rates oscillate at two frequencies, and firing rates maintain consistent phase relationships with one another across conditions (Churchland et al. 2012). These oscillations are well suited for motor cortex’s role as a generator of output patterns sent to the muscles: just as one can use a Fourier transform to decompose any time-varying pattern into a sum of sinusoids, these oscillations make an excellent basis for producing the time-varying muscle activity we observe during movement. Oscillators are such a parsimonious mechanism for generating these signals that when artificial neural networks are trained to recreate recorded muscle activities, these networks converge on solving the problem using oscillators, too (Sussillo et al. 2015). Knowledge of these dynamics has practical implications, as well: incorporating oscillatory dynamics into neural prosthetics algorithms improves performance (Kao et al. 2015). Thus, despite the confusing responses of individual neurons, the pattern of activity can be seen to be highly structured at the level of the population when viewed through a dynamical systems lens.
Second, because neurons in motor cortex can covary in numerous patterns (neural activity is ~10–20 dimensional), whereas muscles covary in fewer patterns (muscle activity is ~5 dimensional), dynamical systems models predict that not all of the neural patterns are needed to control the muscles. Thus only a limited number of the possible neural readouts will be “output-potent”; the other activity patterns should be “output-null” and presumably serve internal functions. As predicted, most of the activity when preparing movements, a time when muscle activity must remain constant, is in these output-null patterns, which are invisible from the vantage point of the muscles (Kaufman et al. 2014). This activity may nonetheless be important both for “seeding” the dynamics needed for movement (Churchland et al. 2010) and for supporting the oscillatory dynamical rules during the movement itself. Because the output-potent patterns pool over many neurons, and each neuron may contribute to multiple muscles, this idea may also explain how individual neurons can have seemingly inconsistent relationships with individual muscles (Druckmann and Chklovskii 2012; Schieber and Rivlis 2007).
Third, the dynamical systems view predicts that the neural state should evolve smoothly over time; the relevant time constants should match the movements being generated, which are much slower than the time constants of neural membranes (e.g., Bernacchia et al. 2011). This helps explain why firing rates tend to undergo smooth changes over time during normal reaches, during re-planning after a target is abruptly changed (Ames et al. 2014), or when an animal spontaneously changes its mind about which target to reach to (Kaufman et al. 2015). Additionally, this expectation of smoothness correctly predicts a reaction time benefit when the neural state happens to be “close” to movement-related states at the time of a go cue (Afshar et al. 2011).
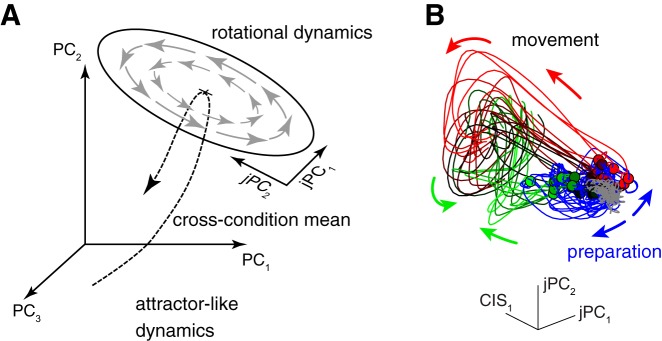
Finally, in order for the system to have smooth, simple dynamics, dynamical systems models predict that there should be a large and stereotyped change in firing rates just before movement activity begins. This change would serve the mechanistic role of bringing the neural state from a zone with stable dynamics (suited to keeping the arm still during preparation) to a zone with the oscillatory dynamics needed to drive movement (Fig. 2A; Churchland et al. 2012; Hennequin et al. 2014; Petreska et al. 2011; Sussillo et al. 2015). We recently reported such a “condition-invariant” signal, which comprises a surprisingly large fraction of the data variance (at least half) and is nearly identical across many different reaching movements (Fig. 2B; Kaufman et al. 2016).
Fig. 2.
Activity undergoes a large, condition-invariant change to a zone with oscillatory dynamics just before reaching movements in monkeys. A: illustration of the dynamical systems prediction that the neural state should be quite different during preparation vs. during movement. This is because there must be a large distance in firing rate space between the active, oscillatory dynamics needed to produce time-varying patterns during movement and the stable dynamics needed to keep the arm still during preparation. PC, principal component; jPC, component that captures rotational structure in the activity. [Reproduced with modification from Churchland et al. (2012).] B: recorded neural activity exhibits such a condition-invariant change, followed by oscillatory patterns. Each trace corresponds to a different reach, with trial-averaged activity plotted over time. Baseline activity is plotted in gray, preparatory activity in blue, and movement-epoch activity in shades of green and red. For the many different reaches tested, neural activity underwent a stereotyped translation in the dimension CIS1 (the first dimension of the condition-invariant signal). This was followed by rotations in state space, which correspond to oscillations in firing rates. [Reproduced with modification from Kaufman et al. (2016).]
Although this view has made a number of strong predictions about what M1 activity should look like, it also has room for compatibility with a number of other models. Whereas individual neurons contain confusing mixtures of signals, at the population level it may be possible to separate out the sorts of signals that researchers have looked for previously: muscle output, feedback, joint angles, high-level planning signals, learning signals, control policies, etc. These different signals could exist in different subspaces of all the possible activity patterns, in the same way that output-potent patterns are a subspace of all the possible activity patterns. The idea of dynamical systems modeling is helpful in part because it is unifying: it allows us to think about what different activity patterns might mean, how they might come to be, and what their effects will be. At the same time, it makes us consider that some of these signals might not mean much at all in themselves. Instead, they might simply be part of how the cortical pattern-generation machine functions.
Dynamical systems models thus provide insight into how a population of neurons transforms its inputs into the detailed, time-varying outputs needed to control the body. This approach also opens many new lines of questions. First, how are the needed movement-epoch dynamics learned? How similar are they across more diverse contexts (Kurata and Wise 1988; Perfiliev et al. 2010)? Second, is feedback vital to achieving the needed dynamics? Does the network act as an optimal feedback controller (a type of dynamical systems model; Todorov and Jordan 2002), capable of shaping its perturbation responses according to the task demands (Omrani et al. 2016; Pruszynski et al. 2014; Scott et al. 2015)? Third, how do networks of real, spiking neurons give rise to smooth network dynamics (Abbott et al. 2016; Litwin-Kumar and Doiron 2012)? Fourth, what are the roles of known biological structure, such as cell types, cortical layers, and connectivity? Inhibitory neurons, for example, are probably not simply gating outputs (Kaufman et al. 2010, 2013; Merchant et al. 2008). Instead, cell types likely play crucial dynamical roles such as shaping time constants and stability (Litwin-Kumar et al. 2016; Rajan and Abbott 2006) and in enabling plasticity (Chen et al. 2015). The notion of a population-level understanding is thus not in conflict with understanding the roles of neurons in the circuit; it simply acknowledges that individual neurons probably cannot be understood in isolation.
Sophisticated Feedback Processing for Motor Control –M. Omrani
Graceful control of body movement relies heavily on the integrity of the sensory feedback pathways. This integral role is evident from the observation that pure sensory deficits can often cause devastating motor consequences (Marsden 1998; Sarlegna et al. 2006), comparable in severity to those caused by defects in motor-related areas (e.g., stroke or cerebellar ataxia). Any damage to the integrity of the sensory feedback system inevitably causes motor deficits. For instance, as you might have experienced, it is extremely hard to whistle a tune after dental freezing; however, only the sensory branches of the trigeminal nerve are blocked through dental freezing, whereas the motor branches of the facial nerve are left intact. Loss of motor function following sensory deficit is commonly observed in patients with peripheral sensory neuropathy (e.g., Charcot-Marie-Tooth disease), to the extent that it can cause coordination loss similar to that observed in cerebellar diseases (i.e., “sensory ataxia”; see Riggins and England 2012). Interestingly, in these patients, the motor symptoms are aggravated when the patients shut their eyes, and are improved when visual feedback about the state of their body and the world surrounding them is available. These examples highlight the significance of sensory feedback in conserving the agility of our movements. Nevertheless, over the past few decades, the crucial role of sensory feedback for motor control has not received the attention it deserves.
Parallel somatosensory pathways to primary motor cortex.
There is an extensive network of direct and indirect pathways that deliver peripheral sensory information to the primary motor cortex. A major source of sensory feedback to M1 is primary somatosensory cortex (S1), but S1 is neither an exclusive source of feedback to M1, nor does it send a homogenous type of feedback to M1. For instance, whereas S1 ablation reduces the size of sensory evoked responses in M1, it neither abolishes nor changes the latency of these responses (Asanuma et al. 1980). Sensory information about proprioception, touch, and vibration is transferred from peripheral receptors, through large-diameter myelinated axons, to the dorsal root of the spinal cord. These fibers ascend through the dorsal column toward higher sensory areas. The ventral posterior nucleus (VP) of the thalamus is the main target of these cutaneous afferents (Kaas 2008). VP has a dense connection with primary somatosensory areas of the cortex (areas 3b and 1; Jones and Powell 1970; Padberg et al. 2009; Whitsel et al. 1978). On the other hand, areas 3a and 2 of cortex mostly receive sensory information from muscle spindles through the ventral lateral nucleus (VLp) and the ventral posterior superior nucleus (VPs) respectively. VLp contains neurons that respond to muscle manipulation and joint rotations, and has dense projections to motor and premotor areas in addition to area 3a (Asanuma et al. 1979; Fang et al. 2006). VLp also receives cerebellar projections from the dentate and interpositus nuclei of the cerebellum (Sakai et al. 1999; Stepniewska et al. 2003). VPs receives sensory information from muscle spindles and feeds projections to posterior parietal area 5, as well (Kaas 2008).
In addition to these direct feedback pathways, there are ample recurrent connections between these cortical areas, which could act as indirect feedback pathways to M1. M1 receives abundant direct sensory input from the periphery (through VLp; Asanuma et al. 1979; Fang et al. 2006). M1 also receives information from primary (areas 3a, 1 and 2) and secondary (area 5) somatosensory areas (Jones et al. 1978; Kalaska 1996; Lucier et al. 1975; Strick and Kim 1978; Zarzecki et al. 1978) as well as deep cerebellar nuclei (Strick 1983; Vilis et al. 1976). Proprioceptive information is transferred to cerebellum through spinocerebellar tracts (Yaginuma and Matsushita 1987) to the interpositus nucleus (IN). Interestingly, IN also receives somatotopically relevant information from primary cortical areas (i.e., S1 and M1) (Allen et al. 1977). Sensory information from IN is in turn passed to higher cortical areas, through thalamic nuclei (e.g., VLp). One of the areas receiving a major contribution from IN is area 5, which receives proprioceptive information through the anterior ventral (VA) thalamic nucleus (Sasaki et al. 1972, 1973, 1976) and feeds directly to M1. M1 also receives feedback from premotor areas [i.e., dorsal and ventral premotor areas (PMd and PMv) and the supplementary motor area (SMA)], which in turn receive input from the periphery through VLp and posterior parietal cortex. Many of these areas respond to mechanical perturbation, such as area 5 (Weber and He 2004) and premotor areas (Bauswein et al. 1991; Boudreau et al. 2001). These direct and indirect pathways demonstrate the diverse and intimate relation of M1 to peripheral somatosensory feedback. This relation becomes even more interesting knowing that almost all these areas receive reciprocal connections from M1 (Coulter and Jones 1977; Johnson et al. 1993, 1996). Note that this section is only focused on somatosensory feedback and does not discuss the rich feedback pathways from other sensory modalities such as auditory or visual systems, although they are undoubtedly quite important (Budinger and Scheich 2009; Miller and Vogt 1984).
Theoretical frameworks for integration of sensory feedback for motor control.
The first theoretical framework to explain the use of sensory feedback in motor control was servo-control (closed-loop control) theory, which was adapted from control engineering by physiologists such as Matthews (1933) and Merton (1950). According to this theory, to control a system, a desired state is defined for the controller, to which it constantly compares the current state to generate an error signal (i.e., sensory discrepancy). For instance, in the example of a thermostat, this error signal is the difference between the desired temperature and the current temperature. The output of the controller is proportional to the size of this error signal; therefore, bigger error entails bigger output to correct the error faster. The controller stops when there is no more difference between the desired and the current states (i.e., no sensory discrepancy). Merton suggested a similar mechanism for controlling joint movement. According to his theory, to move a joint from one position to the next, sensory discrepancy, induced by changing the excitability of the muscle spindles, was transformed into a change in efferent muscle activation (Eldred et al. 1953). In this scheme, the desired position is determined by the supraspinal areas through changing the gamma drive. The gamma drive (i.e., fusimotor system) changes the positional sensitivity of the muscle spindles by making them taut/slack, therefore changing its firing rate in any given position. The difference in the current activity of the spindle and a given base rate is in turn transformed to alpha drive through a spinal reflexive loop, changing the length of the extrafusal muscle fibers. This system could also counteract any peripheral perturbations, by correcting any imposed sensory discrepancy (the difference between the induced firing rate and the base firing rate of the muscle spindles) through the same spinal loop. Nevertheless, this theory necessitates the intra- and extrafusal drives to act independently, a notion disproved through later studies, showing a high correlation between the two efferent motor drives (i.e., alpha-gamma coactivation; Hagbarth and Vallbo 1968).
The initial experiments testing the servo-control hypothesis were mostly performed on a single joint (e.g., wrist; Hammond et al. 1956; and see Desmedt 1977 for a review), therefore dismissing the complications of controlling a multijointed system such as the arm. When a multijointed system is being controlled, torques applied to one joint cause movement not only in that joint but also in all the joints connected to it, a concept known as intersegmental dynamics (e.g., consider moving the head of a Wiggle snake by flicking its tail). Due to intersegmental dynamics, every time each controller (e.g., the elbow joint) compensates for the movement caused by another joint torque (e.g., torque on the shoulder), it will in turn cause extra movement back in all other connected joints. In a system equipped with noise-free sensors and instantaneous reaction time, a servo-controller could still effectively control this jointed system, but in a system bound with biological noise and delay in signal transmission, such a control scheme is destined for instability.
A more recent theory of feedback control, named optimal feedback control (OFC), overcomes this theoretical challenge by suggesting that instead of using the delayed sensory feedback, the system could use the best estimate of sensory feedback (“state estimation”) to generate motor output (Scott 2004; Todorov and Jordan 2002). Knowing the dynamics of the system being controlled, one could make a forward model to predict the sensory outcomes of an action before it happens (e.g., where would a car be in an hour driving 80 miles/h?). This sensory prediction could then be combined with the actual noisy and delayed sensory feedback, to provide the best estimation of the system state (Wolpert et al. 1995; Wolpert and Ghahramani 2000), which could be used for feedback control. For instance, when one intends to move the shoulder joint, the nervous system could predict that as a consequence the elbow joint would move, as well, and could stabilize the elbow joint even before the sensory feedback about its motion gets back to the brain (Gribble and Ostry 1999). The controller also considers the reliability of each source of information and accordingly weights them before combining them to generate state estimation (Körding and Wolpert 2004; Wolpert 2007).
A more exclusive feature of OFC is the concept of the minimum intervention principle. (Todorov and Jordan 2002). Although sensory feedback is necessary for successful movement in a variable environment, our corrective responses to an identical perturbation often depend on the behavior in which we are engaged. Consider shaving your face with a razor or carrying a few drinks across a busy bar. In both cases, you should be wary of any possible bumps and perturbations. However, your responses to someone bumping your arm would not be the same in the two situations. In the one case, you need to move the razor away from your face to avoid cuts, but in the other case, you need to pull your arms in tighter to avoid dropping any of the drinks. Not only can our corrective responses change across different behavioral contexts, but they can also change within the same behavioral context, demonstrating itself in the form of movement variability. This means that not all deviations in our actions from the original plan (whether caused by internal noise or external perturbations) are corrected to generate a stereotypical course of action. In fact, variability is an inherent feature of our day-to-day actions. Think of your handwriting, for instance: how you write slightly changes depending on what you write with (i.e., pencil vs. chalk), what you write on (i.e., paper vs. blackboard), the size of your writing, and your writing posture (horizontal vs. vertical). However, despite the considerable variability in writing any single letter, your handwriting is generally unique to you (Ghali et al. 2013). Variability is present even in our most precise actions. For instance, it has been shown that joint configuration in professional pistol shooters varies on a trial-by-trial basis, whereas their performance remains surprisingly unchanged (Scholz et al. 2000). OFC can nicely capture this flexible use of sensory feedback across tasks and the natural variability in our day-to-day behaviors by correcting the errors only if they affect the goal of the task; otherwise, they are ignored: hence the name, minimum intervention.
Physiological evidence of feedback control of movement.
In the attempt to unravel the physiological circuitry of feedback driven motor control, it is important to remember a few points. First, OFC is a behavioral model and therefore does not make any predictions about the neural substrate of motor control. As a normative model, it needs to have separate boxes (e.g., state estimator) to explain different steps in processing the behavior. However, in reality there is no reason for each step to be processed in different brain areas, or even in a single area. All those mentioned functions could be processed in a distributed, parallel structure. Therefore, trying to find functional counterparts for each module in the brain might not be a fruitful approach. Second, we are sure the brain does not solve optimal feedback equations, yet its behavioral output looks optimal. Therefore, it is helpful to test the concepts falling out of this theory, such as the minimum intervention principle, in trying to understand the system, rather than formally looking for OFC in the brain.
In testing feedback controllers, an informative approach is to apply mechanical perturbations to induce sensory discrepancy and study the system’s output. The same concept has been used to investigate feedback-processing circuitry in the brain across different behavioral contexts. Using this technique, researchers have found that rapid motor responses to mechanical perturbations (i.e., sensory-driven motor outputs) share attributes previously reserved for voluntary motor control, such as sensitivity to task instruction and target selection (Hammond et al. 1956; Pruszynski et al. 2008; Rothwell et al. 1980; Shemmell et al. 2009), decision making (Nashed et al. 2014; Selen et al. 2012), adaptation and learning (Ahmadi-Pajouh et al. 2012; Cluff and Scott 2013; Crevecoeur and Scott 2013), consideration of target properties and obstacles in the way (Nashed et al. 2012; Yang et al. 2011), mechanical properties of the limb and environment (Cluff and Scott 2013; Kurtzer et al. 2008, 2009; Shemmell et al. 2010; Weiler et al. 2015), timing constraints or task urgency (Cluff and Scott 2015; Crevecoeur et al. 2013; Omrani et al. 2013), and also knowledge about the gain scaling of muscle forces (Pruszynski et al. 2009). Given the extensive sophistication of these feedback responses, it is reasonable to assume that they are processed in the same cortical (and subcortical) areas as the voluntary responses (Scott 2004, 2008, 2012). Therefore, learning more about the underlying neural circuitry of feedback processing can also help us better understand voluntary control in the brain (Scott 2016).
Hitzig and Fritsch were first to suggest the abundance of sensory feedback in motor cortex and suggest a role for that in motor control (Fritsch and Hitzig 1870). Phillips (1969) theoretically formulated this involvement in a framework, suggesting that primary motor cortex forms part of a transcortical pathway contributing to rapid motor responses (long-latency reflexes), with its gain changing on the basis of the behavioral task. Several studies have since confirmed the contribution of M1 activity in rapidly responding to mechanical perturbations and demonstrating its response timing to be compatible with its role in the long-latency stretch response of the muscles (Cheney and Fetz 1984; Evarts and Tanji 1976; Herter et al. 2009; Picard and Smith 1992; Pruszynski et al. 2011). It also has been shown that perturbation-related activity in M1 can be altered by behavioral context before concomitant changes in the long-latency stretch response (Conrad et al. 1974; Evarts and Tanji 1976; Omrani et al. 2014; Pruszynski et al. 2014; Wolpaw 1980a, 1980b).
Seminal work by Evarts and Tanji (1976) demonstrated that M1 cells have a dual response to mechanical perturbations. In this study, monkeys were trained to rapidly push or pull a handle following a mechanical perturbation that either assisted or resisted the instructed action. They found that the initial cortical response (i.e., 20–40 ms following the perturbation) was tightly coupled to the applied perturbation; however, the later response (starting at ~50 ms) clearly reflected the instructed motor action. Pruszynski et al. (2014) found a similar pattern of responses in M1 when monkeys responded to mechanical perturbations by rapidly placing their hand into visual spatial targets while the perturbation either assisted or resisted their intended movement: a relatively invariant initial response followed by a modulated (target dependent) later response. This dual perturbation response of M1 cells is present whether the monkey is engaged in a postural control task or not (Omrani et al. 2014; see also Conrad et al. 1974; Wolpaw 1980a, 1980b). But what is the neural circuitry underlying such a dual perturbation response (i.e., an initial task-independent response followed by a later, task-dependent response) in M1?
It is generally believed that this dual response is driven by an initial task-independent input from the somatosensory cortex (Evarts 1973; Evarts and Fromm 1977, 1981), followed by task-dependent drive from the dentate nucleus of cerebellum (Hore and Vilis 1984; Meyer-Lohmann et al. 1975; Strick 1983; Vilis et al. 1976). However, given the sophistication of these rapid responses, we believe other cortical areas beyond the cerebellum also contribute to the M1 response to mechanical perturbations. We speculated that different sources of feedback, with different time delays and sensitivity to behavioral tasks, might be driving M1 activity, one driving the early task-independent response and the others driving the late task-dependent response. We therefore studied perturbation responses in a range of cortical regions associated with voluntary motor control [posterior parietal area 5 (A5), primary somatosensory area 2 (A2), primary somatosensory area 1 and 3 (S1), M1, and PMd] across three different behavioral contexts (i.e., engaged or not in a postural control task and target selection in response to perturbation; Omrani et al. 2016). We found sensory feedback to be rapidly transmitted to all these cortical regions within 25 ms of limb disturbance. Furthermore, sensory feedback was differentially modulated across these areas, depending on the behavioral task. When limb feedback was salient to an ongoing motor action (task engagement), neurons in parietal area 5 immediately (~25 ms) increased their response to limb disturbances, whereas neurons in other regions did not alter their response until 15–40 ms later (i.e., ~40–65 ms postperturbation). In contrast, initiation of motor actions, elicited by limb disturbance, to different targets (target selection) altered neural responses in primary motor cortex ~65 ms after the limb disturbance, and then in PMd, with no effect in parietal regions until 150 ms postperturbation (see Fig. 3). These results suggest that a highly distributed neural substrate is involved in processing sensory feedback and that each area plays a unique role in context-dependent modulation of feedback responses.
Fig. 3.
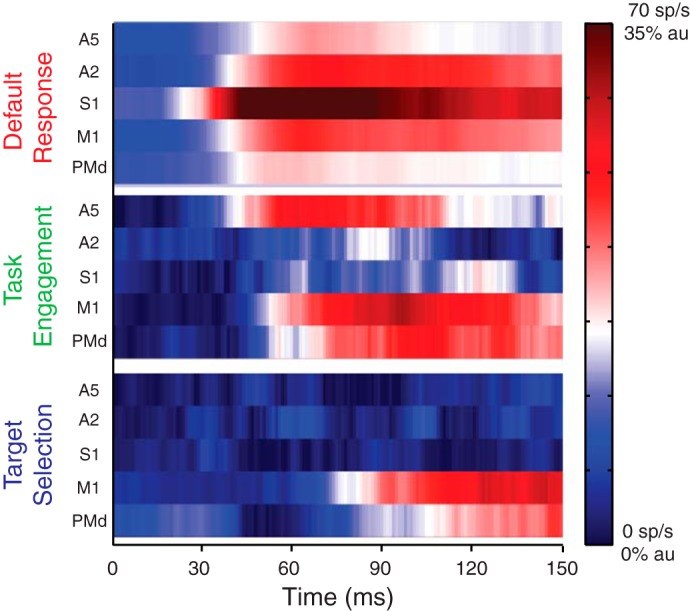
Context-dependent patterns across sensorimotor cortex. Default response (top) is represented by activity patterns when the animal is not engaged in a postural task [i.e., the Movie Task in the original experiment; see Omrani et al. (2016)]. Task engagement (middle) is represented by the differential signal between being engaged (i.e., the Posture Task in the original experiment) and not (i.e., the Movie Task) in a postural task. Target selection (bottom) is represented by differential activity between the targets the hand is pushed away from (i.e., OUT in the original experiment) and pushed into (i.e., IN in the original experiment). Activity is plotted using a color map. In the default response, population response is capped at 70 spikes/s. Differential signals are normalized to their maximum response in the posture task (au, arbitrary units). [Adapted from Omrani et al. (2016).]
Such distributed neural circuitry could potentially convey multiple modalities of sensory feedback to the primary motor cortex. For instance, although most studies focus on the role of muscle spindle afferents in eliciting perturbation-related activities (Lucier et al. 1975), the contribution of cutaneous afferents that signal skin stretch, caused by the perturbation, should not be forgotten. Nevertheless, the very fastest responses, even in area 3b, are likely due to muscle afferents (Heath et al. 1976). Neurons in S1 with cutaneous receptive fields are broadly tuned to the direction of movement during reaching (Cohen et al. 1994; Prud’homme et al. 1994), much like those in M1 with receptive fields from shoulder and elbow muscles (Scott and Kalaska 1997). Therefore, we expect both muscle and cutaneous afferents to be broadly tuned to loads applied to the shoulder and elbow and to contribute to feedback responses observed in the sensory and motor cortices. Modalities other than somatosensory, such as vision, most probably contribute to these feedback processing networks, as well (Franklin and Wolpert 2008), yet how multiple sources of sensory information interact is an important question to be further explored (Crevecoeur et al. 2016). Considering such rich representation of feedback from a variety of sources, it is not so strange that the motor cortex reflects multiple movement-related variables in its activity (Scott 2004; Todorov 2000).
In this section, I have aimed to establish the rich and intimate relation between sensory pathways and primary motor cortex, which is essential for a feedback controller. I have also argued that changing the behavioral goals of the task changes the activity in both sensory and motor cortices, reflecting task dependent sensory processing. Sensory evoked responses could be as flexible as voluntary actions, blurring the distinction between sensory evoked responses and voluntary control. Our neural findings suggest that multiple areas are involved with flexible processing of sensory feedback, with all their output converging at a common motor output: primary motor cortex. Although this evidence does not directly support the existence of an optimal feedback controller in the brain, OFC theory opens a window to better understand the relation between sensory feedback and motor output. I therefore suggest that to better understand the neural circuitry underlying motor control, we should pay more attention to the role of sensory feedback in motor cortex.
Discussion
P. D. Cheney (PDC): “Although the papers in this review seem to present contradictory points of view, in an open discussion the authors were able to reach consensus on a number of key issues. That conversation provided an outline for the authors to produce a synthesis, presented in discussion form below.”
Motor output, the better understood part.
PDC: “There was consensus that while the properties of M1 neurons in general are highly diverse, the properties of M1 output neurons with monosynaptic linkages to motoneurons [corticomotoneuronal (CM) cells] are much more uniform with more consistent relationships to movement parameters. There was agreement that, at this point, an overwhelming body of evidence exists to support control of low-level parameters (EMG, muscle force) by CM cell activity. The same is probably also true of cortical cells with less direct synaptic linkages. It is also accepted that CM cells are just one cell type in motor cortex and that the properties of the vast majority of cells are not as well understood. In future studies, significant progress in understanding the properties of other cells in motor cortex would benefit from establishing the synaptic targets of the cells under study. There was also discussion as to whether the properties of CM cells are in some way incompatible with the dynamic systems model or the trajectory model. There was agreement that no incompatibility exists and that these properties can be easily integrated into both models.
“Having agreed that the output from M1 cortex to muscles can be understood in terms of well-documented properties of CM cells, then it seems that a major question for future studies is how the activity of CM cells is generated by cortical, subcortical, and sensory afferent inputs.”
M1, not a monolithic structure.
N. G. Hatsopoulos (NGH): “So, we can all agree that M1 is not one thing. Horizontally across the cortical sheet, caudal M1 on the anterior bank of the central sulcus has physiological and anatomical properties distinct from rostral M1 on the precentral gyrus. Based on Strick’s rabies virus studies (Rathelot and Strick 2009), it appears that almost all CM cells reside in caudal M1. Rostral M1 acts on motor neurons indirectly via polysynaptic connections. This suggests that caudal M1 may be considered a direct controller of muscle activity. A recent study by Witham et al. (2016), however, provides evidence that slower and weaker corticomotoneuronal neurons exist in rostral motor cortex on the precentral gyrus. Nevertheless, even if there are CM cells in rostral motor cortex, their properties are different from those in caudal motor cortex. Moreover, caudal M1 tends to receive predominantly tactile sensory inputs from the periphery, whereas rostral M1 receives proprioceptive inputs (Tanji and Wise 1981). Vertically, only layer 5 neurons project down the spinal cord. However, layer 5 cells also project to other areas, including the striatum, the pontine nuclei, and the red nucleus. Therefore, it does not seem fair to generalize findings in one area of M1 to its function as a whole.”
Control as a unifying theme.
M. T. Kaufman (MTK): “I argue that the key perspective for understanding M1 is that it presumably exists to control the body. My choice of a unifying theme is the idea of ‘control.’ We would expect spinal projection neurons to carry mostly output and other control signals, but the many other cells in M1 may be needed to support production of these outputs. Producing a complex and appropriate output signal is a challenging optimization problem, even for computers, so it makes sense that there are all sorts of other signals floating around in motor cortex: they are needed as part of the pattern generator even if they are not output to the spinal cord. Sensory feedback will also surely be important for control, both because the muscle commands needed to accomplish a movement are heavily dependent on joint positions and because the system needs to be responsive to perturbations.”
NGH: “As to the question of whether M1 is a controller, I suggest that it is likely part of a much larger control system that includes other motor structures such as the red nucleus, basal ganglia, cerebellum, and other brain stem areas. However, control is only part of what it does. First, I suggest that it is critical for motor skill learning. The work of Ölveczky and colleagues (Kawai et al. 2015) shows that in rats, at least, M1 may act like a tutor to acquire new motor skills or sequences but is not necessary for executing the sequence once it has been learned. Second, it may be providing information to other brain areas via efference copy about the commands that are driving the motor system. It may also be providing motor plans (based on the fact that M1 exhibits planning activity during instructed delay tasks) that are executed by other areas. Third, it may be modulating instead of driving motor behavior. For example, we have seen that M1 exhibits rhythmic activity during chewing behavior. The actual chewing is being done by a central pattern generator in the brain stem, but M1 may be monitoring the chewing behavior and modulating the frequency of chewing, determining how hard to chew, as well as being responsible for starting and stopping the chewing. Also, the fact that motor cortex shows mirror-like responses, as we have shown during visual display of a cursor moving to targets, may imply that motor cortex is also involved in mental simulation of action and not only execution” (Tkach et al. 2007).
M. Omrani (MO): “Although I agree with the facts that the controller network probably comprises areas beyond M1, and that M1 probably has other roles (like tutoring), I strongly disagree with a ‘tutor only’ message. Whereas some movements, even seemingly complicated ones, are possible without M1 contribution (Lamarre et al. 1978; Lawrence and Kuypers 1968), in the absence of M1 function, or even feedback pathways upstream to M1 (Bioulac and Lamarre 1979; Brinkman et al. 1985; Conrad et al. 1974; Meyer-Lohmann et al. 1975), movement integrity gets completely disrupted when there is a disturbance to the movement, or simply a change in premovement limb configuration.
“It is also important to remember that the control signals in a system controlling a nonlinear plant, such as the arm, are not necessarily intuitive. By that I mean the complexity observed in the dynamics of neural activity in M1 might not be due to a complex pattern generator or intrinsic to M1 connectivity but could simply be a necessity for controlling a physical plant (Lillicrap and Scott 2013). For instance, a simple ballistic movement is achieved through a triphasic agonist/antagonist/agonist muscle pattern activation (Mustard and Lee 1987). This means that if there are cells in M1 directly controlling the muscle activity, they would show a similar pattern of triphasic change through the course of movement (Heming et al. 2016). That means M1 tuning property would dynamically change through the course of movement: cells initially tuned to movement direction (i.e., agonist cells) losing their tuning to that direction through the movement, and cells initially not tuned for that direction (i.e., antagonist cells) now becoming active in the later phase of the reach and therefore seemingly tuned for that direction. This phenomenon has been observed by multiple studies and could simply be explained by controlling movement dynamics (Churchland et al. 2010; Griffin et al. 2008; Morrow et al. 2007; Oby et al. 2013; Sergio and Kalaska 1998; Sergio et al. 2005; Suminski et al. 2015). This triphasic pattern could also explain the rotational dynamics of neurons observed through the reaching movement (Churchland et al. 2012), without the necessity for an underlying structure to specifically generate them (Michaels et al. 2016). These patterns become even more counterintuitive, considering that due to the structure of arm muscles, their physiological tuning properties do not match their anatomical tuning curves (Kurtzer et al. 2006). This means, for instance, that when we try to flex our elbow, due to the presence of biarticular muscles (e.g., biceps), the shoulder gets flexed along with the elbow. To oppose the shoulder flexion, shoulder extensors should get activated; therefore, physiologically, shoulder extensors are seemingly involved in elbow flexion. Now consider a shoulder-controlling cell in M1 which is now active during elbow flexion. While it would be hard to understand the function of this neuron from a kinematic/representative point of view, its function can reliably be explained through its role in movement control (Heming et al. 2016).”
MTK: “I agree with Dr. Omrani that M1 needs to produce a variety of complex signals in part because the muscles need complex activation patterns. However, I disagree that this captures all of the complexity in M1, due to empirical results from some of the same studies cited above. Churchland et al. (2010) showed that neural activity during movement contained many signals not present in the muscles (nor in movement kinematics). Churchland et al. (2012) went looking in the muscles for the rotational dynamics found in cortex, and they simply were not present, so we know that there is dynamical structure in M1 that is absent in the muscles.”
NGH: “If we are not so hung up about control as the sole function of M1, one question is how best to characterize the encoding properties of motor cortex just as we might examine the encoding of any brain area. My argument is that M1 is better characterized as encoding a temporally extensive movement trajectory that spans about ~400 ms as opposed to a static movement parameter such as velocity or direction. This duration is interesting because several groups have indicated that natural movement segments are around ~300–400 ms long. A study by Aflalo and Graziano (2007) decomposed seminaturalistic, unstructured arm movements of monkeys into movement segments that were about this length in duration. A recent study by Wiltschko et al. (2015) showed that natural three-dimensional movements of the mouse are composed of blocks that are about ~350 ms in duration. The trajectory model also acknowledges that M1 encodes both sensory and motor effects, because the preferred trajectory spans both time leads and lags with respect to the response of an M1 neuron.”
MO: “I totally agree that static movement parameters do not capture M1 activity through movement, but could not the temporal patterns be a by-product of control as I explained above? Physical movements have limitations and regularities (e.g., reaching movements usually take between 600 and 1,000 ms) which could produce those predominant temporal patterns observed by many groups.”
MTK: “I like Dr. Hatsopoulos’s point that M1 activity is temporally extensive and that it changes over the course of even rapid movements. It could well be that M1 is serving other important functions besides control, such as tutoring other brain areas. It is also important to look at whether M1 really does carry information about, say, only the next 300–400 ms, but that does not mean it is not primarily a controller. This might just tell us what the properties of the controller are.
“However, I think we should be careful about using the word ‘encode.’ There is an important distinction beyond semantics. Muscle activity carries time-varying information about movement, but I object to saying the muscles ‘code for’ movement. It is just not the right way of understanding that activity. Similarly, I argue that much of motor cortical activity is there to act as a control signal, or to assist in producing it. There is clearly possible room for other functions of M1, though.”
Reasons why so many different signals are present in M1.
NGH: “My reason for why there is such heterogeneity in responses in M1 is threefold. First, as I have stated, M1 has potentially many functional roles and layer 5 (and layer 6) cells send signals to many targets besides the spinal cord. Second, perhaps in line with Dr. Kaufman’s ideas, the other layers of the motor cortex may be generating the necessary dynamics onto layer 5 cells to generate the necessary control signals to be sent to the periphery. Third, the encoding model I put forth suggests that a large set of motion templates or basis set are represented in the millions of M1 neurons from which a particular movement can be built.”
MTK: “I think to check whether these ‘extra’ signals are really due to dynamics, we can take two approaches. First, with enough simultaneous recordings, we can get good estimates of activity on single trials. In principle, at least, we should then be able to check whether trial-by-trial deviations from the mean neural trajectory lead to the later deviations we would expect. Second, if we could causally perturb the neural state in a variety of different directions, we could again see whether the neural state follows the dynamical rules that we expect. However, neither of these tests solidly rules out whether that ‘extra’ activity is doing something else, as well. It is not obvious how to get more than good circumstantial evidence.
“It is also worth considering how the dynamics are generated. It could be that the rotations and other dynamics are completely intrinsic to M1 and PMd. Supporting this idea, the dynamics start quite early, roughly 125–150 ms before movement onset. However, as Dr. Hatsopoulos mentions, it could also be that these dynamics require the interplay of motor cortex with other brain structures: cerebellum, basal ganglia, cingulate motor areas, red nucleus, etc. It is also possible that during the late portion of the movement, some portion of the dynamics requires sensory feedback to unfold normally. These possibilities can be tested, by inactivating other brain structures or anesthetizing the sensory afferents during movement. Such an interaction would tell us about the source of the dynamics, even if they do not tell us whether they exist to act as a pattern generator or for some other reason.”
MO: “As I discussed earlier, there are multiple direct and indirect feedback pathways to M1. Each pathway relays different modalities of sensory feedback (e.g., cutaneous feedback from area 1, proprioceptive feedback from 3a or visual from area 7), which could be used to form a feedback policy based on that specific pathway. Therefore, it is natural to find so many different signals being represented in M1 (e.g., target position, direction of hand movement, hand speed, movement dynamics, etc.; see Scott 2004; Todorov 2000). Throughout the evolution of the mammalian brain, certain structures have been preserved: the primary and secondary sensory areas, and a small posterior parietal cortex plus primary motor cortex in eutherian mammals. With greater progression through the phylogenetic tree, extra brain areas and connections are added on top of these basic structures that could contribute to more sophisticated functionality (see Krubitzer 2009 for a review). It seems that higher animals gain extra motor functionality through such parallel augmentation mechanisms, rather than through restructuring of the whole network. For instance, Cebus monkeys have complex manual abilities, which their close relatives (e.g., titi monkeys) lack (Krubitzer 2009). Developmental comparison between these two species shows that Cebus monkeys have developed a cortical proprioceptive area (area 2) and an expanded posterior parietal cortical area 5, which seems to be responsible for the added functionality (Padberg et al. 2007). Considering such parallel augmented modular structure (Guillery and Sherman 2011), we can now expect the integrating output module to reflect the activity across all intermediary areas. This view could explain why multiple pathways exist that are sensitive to sensory information, and why some show task dependency while the older and shorter pathways (e.g., S1-M1) do not.
“If M1 is representing multiple feedback loops, rather than coding a specific aspect of movement, we should be able to change its activity by imposing sensory discrepancy in a specific modality (e.g., target jump for visual vs. mechanical perturbation for somatosensory). As predicted by optimal feedback control theory, when the task in hand changes, M1 activity should also change to best control the modality of sensory information that is important for that given task. We could also study how each pathway contributes to M1 activity by imposing discrepancies between different sources of sensory information (Crevecoeur et al. 2016) and study M1 responses in each case. Of course, more causal inferences could be made by changing the activity of each pathway independently, using optogenetic techniques, for instance, and evaluating how that changes M1 activity (Mathis et al. 2017).”
Sensory feedback and control theory.
MO: “OFC theory provides a nice platform to think about what the significance of sensory feedback might be in generating motor output. Concepts like the minimum intervention principle can nicely explain the structure of inherent variability in our motor performance, and the idea of state estimation can explain how current evidence is integrated with previous experience. Nevertheless, OFC is a behavioral model and therefore might be implemented any number of ways in the underlying neural circuitry. By building models of how neural circuits approximate OFC given the availability of different signals, it may provide insight into “why” the circuits are the way they are. For instance, Lillicrap and Scott (2013) showed that by applying optimal control theory to a simple network model driven by muscle spindle activity, many different known phenomena in M1 could be explained. For example, the distribution of preferred torque and movement directions were bimodal, much like M1 cells (Scott 2003); M1 activity resembles EMG activity, as explained earlier by Dr. Cheney (Heming et al. 2016); and this model exhibits the temporal evolution of tuning properties observed in M1 by Dr. Hatsopoulos’s group (Suminski et al. 2015). This model even exhibits the rotational dynamics demonstrated by Churchland et al. (2012) (Omrani M and Lillicrap TP, unpublished data).
“Although OFC cannot predict the underlying neural circuitry for motor control, it provides functional concepts which can be tested in examining the physiology of motor control. No one would argue that the nervous system solves the equations of OFC, but through evolution perhaps it has found ways to generate nearly optimal responses. It seems that this solution involves multiple feedback pathways, which are differentially sensitive to behavioral aspects of motor tasks. Using mechanical perturbation, I tried to pull apart the contribution of each pathway in M1 activity (see Fig. 3).”
This discussion is about primates.
MTK: “It is also important to be clear that we are all mostly talking about motor cortex in Old World primates. It is not settled whether motor cortex serves the same function in rodents, for example. Concretely, Kawai et al. (2015) makes abundantly clear that motor cortex can be heavily lesioned in rats, and yet complex behavior is retained, at least for complex proximal movements. However, Dr. Hatsopoulos is surely right that for the rodent, M1 seems to provide a strong learning signal: motor cortex lesions totally prevented learning in that study. Plus, as the work of Peter Strick has so nicely shown, primates have unique pathways and specializations of motor cortex that seem to be absent in other mammals. Therefore, our discussion mostly applies to the primate.”
Where should we go from here?
MO: “As we discussed here extensively, much of what we know about M1 is about its peripheral output, such as CM cells, or lacks target specification. Knowing that M1 might be involved in more than one function, it is essential to study the function of each neuron on the basis of its projection (i.e., distinguishing pyramidal tract neurons from thalamic or striatal projection neurons). The information each neuron type carries could provide clues into its role in motor planning, learning or execution (Suter et al. 2013). On a similar note, it is important to distinguish the function of microcircuitries present in different cortical layers and how they could change M1’s function (Li et al. 2015; Mao et al. 2011; Shepherd 2013). Another important aspect needing more attention is the role of interneurons in movement processing and M1 function (Chen et al. 2015; Guo et al. 2015). Finally, focused and cell-specific intervention techniques during behavior could help us differentiate the function of each cell group and how they participate in the process of motor planning and control (Azim et al. 2014; Bouvier et al. 2015; Bui et al. 2013; Fink et al. 2014; Yttri and Dudman 2016).”
Conclusion
PDC: “Although the goal of understanding brain function at the level of single neurons or even small populations of neurons might seem like a daunting, even hopeless task, the progress that has been made applying this approach to various brain systems is actually quite remarkable. Compared with motor function, brain sensory systems have been easier to investigate with this approach because of the greater ease in controlling and quantifying modalities of sensory input compared with movement. However, in this review we have sought to emphasize areas of consensus concerning progress in understanding the signals of individual neurons and how they relate to aspects of movement. On the basis of considerable accumulated evidence, it now seems appropriate to assign control of ‘low-level’ movement dynamics (muscle-related parameters) as one function of primary motor cortex. This function is clearly consistent with evidence from recordings of corticomotoneuronal cells but probably also applies to layer 5 output cells that contact motoneurons less directly through spinal interneurons. It is important to note that relationships to output might be complex and even seem counterintuitive without detailed information about agonist and antagonist muscle activity patterns coupled with knowledge of the cell’s target muscles. For instance, the triphasic pattern of agonist/antagonist/agonist EMG activation in rapid movements can create counterintuitive activity relationships. Beyond this low-level output function of motor cortex, the picture gets less well defined. Nevertheless, we might all agree that motor cortex is clearly not monolithic but is also involved in a variety of higher level control functions that depend heavily on sensory signals from the periphery for their robust and precise operation. These control functions include motor skill learning, motor planning, sensory guidance of manipulative movements, movement pattern modulation, starting and stopping low-level pattern generators operating largely at the spinal or brain stem level, mental simulation of movements, and others. Progress in understanding these more complex functions should be facilitated through the use of large-scale electrode array recording enabling real-time correlations between neural trajectories and movement trajectories. Although it may be tedious, future studies might also benefit from devoting greater effort to identifying the output targets of recorded neurons. Optogenetic techniques also offer a promising approach to manipulate the activity of neurons in ways that reveal function. At the same time, it may also be necessary to understand how populations of neurons cooperate, to produce a consistent net output even as individual neurons’ activity varies from trial to trial. Although many questions remain concerning the totality of motor cortex functions, substantial progress has occurred over the last few decades, paving the way for continued exciting progress in the future.”
GRANTS
M. Omrani was supported by CIHR Vanier Canada Graduate Scholarship. M. T. Kaufman was supported by Simons Foundation Fellowship 351178. N. G. Hatsopoulos was supported by National Institutes of Health (NIH) Grant R01 NS045853. P. D. Cheney was supported by NIH Grants NS051825 and NS39023, the Kathleen M. Osborn Endowment, and NIH Center Grant HD02528.
DISCLOSURES
N. G. Hatsopoulos serves as a consultant for BlackRock Microsystems, Inc., the company that sells the multielectrode arrays and acquisition system used in his work.
Other authors do not have any conflict of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
M.O., M.T.K., and N.G.H. prepared figures; M.O., M.T.K., N.G.H., and P.D.C. drafted manuscript; M.O., M.T.K., N.G.H., and P.D.C. edited and revised manuscript; M.O., M.T.K., N.G.H., and P.D.C. approved final version of manuscript.
ACKNOWLEDGMENTS
This review was inspired by a debate session, organized by M. Omrani at the 2013 annual meeting of the Society for Neural Control of Movement (NCM), titled “What have we learned from a century of studying primary motor cortex?” The debate panel included S. H. Scott, P. Cheney, M. M. Churchland, N. G. Hatsopoulos, and D. W. Moran. We thank all the panel members and the debate moderator, T. Drew, for presenting this great event. The final Discussion was written following a verbal discussion by the authors after reading one another’s contributions.
We thank J. Cunningham, D. O’Shea, E. Trautmann, and S. Stavisky for helpful comments on drafts.
REFERENCES
- Abbott LF, DePasquale B, Memmesheimer RM. Building functional networks of spiking model neurons. Nat Neurosci 19: 350–355, 2016. doi: 10.1038/nn.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflalo TN, Graziano MS. Partial tuning of motor cortex neurons to final posture in a free-moving paradigm. Proc Natl Acad Sci USA 103: 2909–2914, 2006. doi: 10.1073/pnas.0511139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aflalo TN, Graziano MS. Relationship between unconstrained arm movements and single-neuron firing in the macaque motor cortex. J Neurosci 27: 2760–2780, 2007. doi: 10.1523/JNEUROSCI.3147-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshar A, Santhanam G, Yu BM, Ryu SI, Sahani M, Shenoy KV. Single-trial neural correlates of arm movement preparation. Neuron 71: 555–564, 2011. doi: 10.1016/j.neuron.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi-Pajouh MA, Towhidkhah F, Shadmehr R. Preparing to reach: selecting an adaptive long-latency feedback controller. J Neurosci 32: 9537–9545, 2012. doi: 10.1523/JNEUROSCI.4275-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GI, Gilbert PF, Marini R, Schultz W, Yin TCT. Integration of cerebral and peripheral inputs by interpositus neurons in monkey. Exp Brain Res 27: 81–99, 1977. doi: 10.1007/BF00234827. [DOI] [PubMed] [Google Scholar]
- Ames KC, Ryu SI, Shenoy KV. Neural dynamics of reaching following incorrect or absent motor preparation. Neuron 81: 438–451, 2014. doi: 10.1016/j.neuron.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H, Larsen K, Yumiya H. Peripheral input pathways to the monkey motor cortex. Exp Brain Res 38: 349–355, 1980. doi: 10.1007/BF00236655. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Larsen KD, Zarzecki P. Peripheral input pathways projecting to the motor cortex in the cat. Brain Res 172: 197–208, 1979. doi: 10.1016/0006-8993(79)90532-8. [DOI] [PubMed] [Google Scholar]
- Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex 4: 590–600, 1994. doi: 10.1093/cercor/4.6.590. [DOI] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508: 357–363; advance online publication, 2014. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Lemon RN. Computer simulation of post-spike facilitation in spike-triggered averages of rectified EMG. J Neurophysiol 80: 1391–1406, 1998. [DOI] [PubMed] [Google Scholar]
- Bauswein E, Fromm C, Werner W, Ziemann U. Phasic and tonic responses of premotor and primary motor cortex neurons to torque changes. Exp Brain Res 86: 303–310, 1991. doi: 10.1007/BF00228953. [DOI] [PubMed] [Google Scholar]
- Belhaj-Saïf A, Fourment A, Maton B. Adaptation of the precentral cortical command to elbow muscle fatigue. Exp Brain Res 111: 405–416, 1996. doi: 10.1007/BF00228729. [DOI] [PubMed] [Google Scholar]
- Ben Hamed S, Schieber MH, Pouget A. Decoding M1 neurons during multiple finger movements. J Neurophysiol 98: 327–333, 2007. doi: 10.1152/jn.00760.2006. [DOI] [PubMed] [Google Scholar]
- Bennett KMB, Lemon RN. Corticomotoneuronal contribution to the fractionation of muscle activity during precision grip in the monkey. J Neurophysiol 75: 1826–1842, 1996. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Miller LE. Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nat Rev Neurosci 15: 313–325, 2014. doi: 10.1038/nrn3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchia A, Seo H, Lee D, Wang XJ. A reservoir of time constants for memory traces in cortical neurons. Nat Neurosci 14: 366–372, 2011. doi: 10.1038/nn.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bioulac B, Lamarre Y. Activity of postcentral cortical neurons of the monkey during conditioned movements of a deafferented limb. Brain Res 172: 427–437, 1979. doi: 10.1016/0006-8993(79)90576-6. [DOI] [PubMed] [Google Scholar]
- Boudreau MJ, Brochier T, Paré M, Smith AM. Activity in ventral and dorsal premotor cortex in response to predictable force-pulse perturbations in a precision grip task. J Neurophysiol 86: 1067–1078, 2001. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O. Descending command neurons in the brainstem that halt locomotion. Cell 163: 1191–1203, 2015. doi: 10.1016/j.cell.2015.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Abarbanel HD, Kristan WB Jr. Optical imaging of neuronal populations during decision-making. Science 307: 896–901, 2005. doi: 10.1126/science.1103736. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Colebatch JG, Porter R, York DH. Responses of precentral cells during cooling of post-central cortex in conscious monkeys. J Physiol 368: 611–625, 1985. doi: 10.1113/jphysiol.1985.sp015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal A. Neurological Anatomy in Relation to Clinical Medicine. Oxford: Oxford University Press, 1981. [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol 48: 18–46, 1914. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budinger E, Scheich H. Anatomical connections suitable for the direct processing of neuronal information of different modalities via the rodent primary auditory cortex. Hear Res 258: 16–27, 2009. doi: 10.1016/j.heares.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Bui TV, Akay T, Loubani O, Hnasko TS, Jessell TM, Brownstone RM. Circuits for grasping: spinal dI3 interneurons mediate cutaneous control of motor behavior. Neuron 78: 191–204, 2013. doi: 10.1016/j.neuron.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol 381: 529–549, 1986. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabel DW, Cisek P, Scott SH. Neural activity in primary motor cortex related to mechanical loads applied to the shoulder and elbow during a postural task. J Neurophysiol 86: 2102–2108, 2001. [DOI] [PubMed] [Google Scholar]
- Capaday C, Ethier C, Van Vreeswijk CV, Darling WG. On the functional organization and operational principles of the motor cortex. Front Neural Circuits 7: 66, 2013. doi: 10.3389/fncir.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SX, Kim AN, Peters AJ, Komiyama T. Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nat Neurosci 18: 1109–1115, 2015. doi: 10.1038/nn.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44: 773–791, 1980. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol 53: 786–804, 1985. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Kasser R, Holsapple J. Reciprocal effect of single corticomotoneuronal cells on wrist extensor and flexor muscle activity in the primate. Brain Res 247: 164–168, 1982. doi: 10.1016/0006-8993(82)91043-5. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Mewes K, Fetz EE. Encoding of motor parameters by corticomotoneuronal (CM) and rubromotoneuronal (RM) cells producing postspike facilitation of forelimb muscles in the behaving monkey. Behav Brain Res 28: 181–191, 1988. doi: 10.1016/0166-4328(88)90095-2. [DOI] [PubMed] [Google Scholar]
- Cherian A, Fernandes HL, Miller LE. Primary motor cortical discharge during force field adaptation reflects muscle-like dynamics. J Neurophysiol 110: 768–783, 2013. doi: 10.1152/jn.00109.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, d’Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 25: 6419–6434, 2005. doi: 10.1523/JNEUROSCI.4904-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV. Neural population dynamics during reaching. Nature 487: 51–56, 2012. doi: 10.1038/nature11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron 68: 387–400, 2010. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Temporal complexity and heterogeneity of single-neuron activity in premotor and motor cortex. J Neurophysiol 97: 4235–4257, 2007. doi: 10.1152/jn.00095.2007. [DOI] [PubMed] [Google Scholar]
- Cluff T, Scott SH. Rapid feedback responses correlate with reach adaptation and properties of novel upper limb loads. J Neurosci 33: 15903–15914, 2013. doi: 10.1523/JNEUROSCI.0263-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluff T, Scott SH. Apparent and actual trajectory control depend on the behavioral context in upper limb motor tasks. J Neurosci 35: 12465–12476, 2015. doi: 10.1523/JNEUROSCI.0902-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Prud’homme MJ, Kalaska JF. Tactile activity in primate primary somatosensory cortex during active arm movements: correlation with receptive field properties. J Neurophysiol 71: 161–172, 1994. [DOI] [PubMed] [Google Scholar]
- Conrad B, Matsunami K, Meyer-Lohmann J, Wiesendanger M, Brooks VB. Cortical load compensation during voluntary elbow movements. Brain Res 71: 507–514, 1974. doi: 10.1016/0006-8993(74)90994-9. [DOI] [PubMed] [Google Scholar]
- Coulter JD, Jones EG. Differential distribution of corticospinal projections from individual cytoarchitectonic fields in the monkey. Brain Res 129: 335–340, 1977. doi: 10.1016/0006-8993(77)90012-9. [DOI] [PubMed] [Google Scholar]
- Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: activity during the delay period and effect on pre-movement activity. J Neurophysiol 84: 986–1005, 2000. [DOI] [PubMed] [Google Scholar]
- Crevecoeur F, Kurtzer I, Bourke T, Scott SH. Feedback responses rapidly scale with the urgency to correct for external perturbations. J Neurophysiol 110: 1323–1332, 2013. doi: 10.1152/jn.00216.2013. [DOI] [PubMed] [Google Scholar]
- Crevecoeur F, Munoz DP, Scott SH. Dynamic multisensory integration: somatosensory speed trumps visual accuracy during feedback control. J Neurosci 36: 8598–8611, 2016. doi: 10.1523/JNEUROSCI.0184-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crevecoeur F, Scott SH. Priors engaged in long-latency responses to mechanical perturbations suggest a rapid update in state estimation. PLOS Comput Biol 9: e1003177, 2013. doi: 10.1371/journal.pcbi.1003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Avella A, Bizzi E. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci USA 102: 3076–3081, 2005. doi: 10.1073/pnas.0500199102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, O’Dell R, Chan V, Schieber MH. Comparing effects in spike-triggered averages of rectified EMG across different behaviors. J Neurosci Methods 163: 283–294, 2007a. doi: 10.1016/j.jneumeth.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007b. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, editor. Cerebral Motor Control in Man: Long Loop Mechanisms (1st ed.). Basel: Karger, 1977. [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci 25: 9919–9931, 2005. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. Motor cortical activity during voluntary gait modifications in the cat. I. Cells related to the forelimbs. J Neurophysiol 70: 179–199, 1993. [DOI] [PubMed] [Google Scholar]
- Druckmann S, Chklovskii DB. Neuronal circuits underlying persistent representations despite time varying activity. Curr Biol 22: 2095–2103, 2012. doi: 10.1016/j.cub.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682, 2002. doi: 10.1016/S0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Eldred E, Granit R, Merton PA. Supraspinal control of the muscle spindles and its significance. J Physiol 122: 498–523, 1953. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31: 14–27, 1968. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Motor cortex reflexes associated with learned movement. Science 179: 501–503, 1973. doi: 10.1126/science.179.4072.501. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C. Sensory responses in motor cortex neurons during precise motor control. Neurosci Lett 5: 267–272, 1977. doi: 10.1016/0304-3940(77)90077-5. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Fromm C. Transcortical reflexes and servo control of movement. Can J Physiol Pharmacol 59: 757–775, 1981. doi: 10.1139/y81-112. [DOI] [PubMed] [Google Scholar]
- Evarts EV, Tanji J. Reflex and intended responses in motor cortex pyramidal tract neurons of monkey. J Neurophysiol 39: 1069–1080, 1976. [DOI] [PubMed] [Google Scholar]
- Fang P-C, Stepniewska I, Kaas JH. The thalamic connections of motor, premotor, and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti). Neuroscience 143: 987–1020, 2006. doi: 10.1016/j.neuroscience.2006.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier D. Experimental researches in cerebral physiology and pathology. J Anat Physiol 8: 152–155, 1873. [PMC free article] [PubMed] [Google Scholar]
- Fetz EE. Are movement parameters recognizably coded in the activity of single neurons? Behav Brain Sci 15: 679–690, 1992. [Google Scholar]
- Fetz EE, Cheney PD. Muscle fields of primate corticomotoneuronal cells. J Physiol (Paris) 74: 239–245, 1978. [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Muscle fields and response properties of primate corticomotoneuronal cells. Prog Brain Res 50: 137–146, 1979. doi: 10.1016/S0079-6123(08)60814-6. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol 44: 751–772, 1980. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Functional relations between primate motor cortex cells and muscles: fixed and flexible. Ciba Found Symp 132: 98–117, 1987. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD, German DC. Corticomotoneuronal connections of precentral cells detected by postspike averages of EMG activity in behaving monkeys. Brain Res 114: 505–510, 1976. doi: 10.1016/0006-8993(76)90973-2. [DOI] [PubMed] [Google Scholar]
- Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. doi: 10.1038/nature13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flash T, Hogan N. The coordination of arm movements: an experimentally confirmed mathematical model. J Neurosci 5: 1688–1703, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourment A, Belhaj-Saïf A, Maton B. Functional linkages between motor cortical cells and elbow flexor muscles. Evidence for and characteristics of postspike facilitation. J Neurophysiol 74: 130–141, 1995. [DOI] [PubMed] [Google Scholar]
- Fourment A, Maton B, Saïf AB. Cortical post-spike facilitations in elbow muscles during isometric contraction. Neurosci Lett 125: 41–44, 1991. doi: 10.1016/0304-3940(91)90126-E. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Wolpert DM. Specificity of reflex adaptation for task-relevant variability. J Neurosci 28: 14165–14175, 2008. doi: 10.1523/JNEUROSCI.4406-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch G, Hitzig E. Über die elektrische Erregbarkeit des Grosshirns. Arch Anat Physiol Wissen 37: 300–332, 1870. [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ. Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol 73: 836–854, 1995a. [DOI] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J Neurophysiol 70: 2097–2116, 1993. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Caminiti R, Kalaska JF. Static spatial effects in motor cortex and area 5: quantitative relations in a two-dimensional space. Exp Brain Res 54: 446–454, 1984. doi: 10.1007/BF00235470. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2: 1527–1537, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali B, Thalanki Anantha N, Chan J, Chau T. Variability of grip kinetics during adult signature writing. PLoS One 8: e63216, 2013. doi: 10.1371/journal.pone.0063216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MD, Yu BM, Schwartz AB, Chase SM. Motor cortical control of movement speed with implications for brain-machine interface control. J Neurophysiol 112: 411–429, 2014. doi: 10.1152/jn.00391.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron 34: 841–851, 2002. doi: 10.1016/S0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Gribble PL, Ostry DJ. Compensation for interaction torques during single- and multijoint limb movement. J Neurophysiol 82: 2310–2326, 1999. [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hoffman DS, Strick PL. Corticomotoneuronal cells are “functionally tuned”. Science 350: 667–670, 2015. doi: 10.1126/science.aaa8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DM, Hudson HM, Belhaj-Saïf A, McKiernan BJ, Cheney PD. Do corticomotoneuronal cells predict target muscle EMG activity? J Neurophysiol 99: 1169–1986, 2008. doi: 10.1152/jn.00906.2007. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Branched thalamic afferents: what are the messages that they relay to the cortex? Brain Res Brain Res Rev 66: 205–219, 2011. doi: 10.1016/j.brainresrev.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Graves AR, Guo WW, Zheng J, Lee A, Rodríguez-González J, Li N, Macklin JJ, Phillips JW, Mensh BD, Branson K, Hantman AW. Cortex commands the performance of skilled movement. eLife 4: e10774, 2015. doi: 10.7554/eLife.10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Vallbo AB. Discharge characteristics of human muscle afferents during muscle stretch and contraction. Exp Neurol 22: 674–694, 1968. doi: 10.1016/0014-4886(68)90156-8. [DOI] [PubMed] [Google Scholar]
- Hammond PH, Merton PA, Sutton GG. Nervous gradation of muscular contraction. Br Med Bull 12: 214–218, 1956. doi: 10.1093/oxfordjournals.bmb.a069553. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos NG, Amit Y. Synthesizing complex movement fragment representations from motor cortical ensembles. J Physiol Paris 106: 112–119, 2012. doi: 10.1016/j.jphysparis.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsopoulos NG, Xu Q, Amit Y. Encoding of movement fragments in the motor cortex. J Neurosci 27: 5105–5114, 2007. doi: 10.1523/JNEUROSCI.3570-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Hore J, Phillips CG. Inputs from low threshold muscle and cutaneous afferents of hand and forearm to areas 3a and 3b of baboon’s cerebral cortex. J Physiol 257: 199–227, 1976. doi: 10.1113/jphysiol.1976.sp011364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heming EA, Lillicrap TP, Omrani M, Herter TM, Pruszynski JA, Scott SH. Primary motor cortex neurons classified in a postural task predict muscle activation patterns in a reaching task. J Neurophysiol 115: 2021–2032, 2016. doi: 10.1152/jn.00971.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin G, Vogels TP, Gerstner W. Optimal control of transient dynamics in balanced networks supports generation of complex movements. Neuron 82: 1394–1406, 2014. doi: 10.1016/j.neuron.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepp-Reymond MC, Wyss UR, Anner R. Neuronal coding of static force in the primate motor cortex. J Physiol (Paris) 74: 287–291, 1978. [PubMed] [Google Scholar]
- Herter TM, Korbel T, Scott SH. Comparison of neural responses in primary motor cortex to transient and continuous loads during posture. J Neurophysiol 101: 150–163, 2009. doi: 10.1152/jn.90230.2008. [DOI] [PubMed] [Google Scholar]
- Hore J, Vilis T. Loss of set in muscle responses to limb perturbations during cerebellar dysfunction. J Neurophysiol 51: 1137–1148, 1984. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Padel Y, Tanaka R. Disynaptic inhibition of spinal motoneurones from the motor cortex in the monkey. J Physiol 258: 467–487, 1976. doi: 10.1113/jphysiol.1976.sp011431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119, 1996. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Caminiti R. Cortical networks for visual reaching. Exp Brain Res 97: 361–365, 1993. doi: 10.1007/BF00228707. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH. Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181: 291–347, 1978. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TP. Connexions of the somatic sensory cortex of the rhesus monkey. 3. Thalamic connexions. Brain 93: 37–56, 1970. doi: 10.1093/brain/93.1.37. [DOI] [PubMed] [Google Scholar]
- Jones EG, Wise SP. Size, laminar and columnar distribution of efferent cells in the sensory-motor cortex of monkeys. J Comp Neurol 175: 391–438, 1977. doi: 10.1002/cne.901750403. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The evolution of the complex sensory and motor systems of the human brain. Brain Res Bull 75: 384–390, 2008. doi: 10.1016/j.brainresbull.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science 285: 2136–2139, 1999. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Kalaska JF. Parietal cortex area 5 and visuomotor behavior. Can J Physiol Pharmacol 74: 483–498, 1996. [PubMed] [Google Scholar]
- Kalaska JF, Cohen DA, Hyde ML, Prud’homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JC, Nuyujukian P, Ryu SI, Shenoy KV. A high-performance neural prosthesis incorporating discrete state selection with hidden Markov models. IEEE Trans Biomed Eng 64: 935–945, 2016. [DOI] [PubMed] [Google Scholar]
- Kao JC, Nuyujukian P, Ryu SI, Churchland MM, Cunningham JP, Shenoy KV. Single-trial dynamics of motor cortex and their applications to brain-machine interfaces. Nat Commun 6: 7759, 2015. doi: 10.1038/ncomms8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser RJ, Cheney PD. Characteristics of corticomotoneuronal postspike facilitation and reciprocal suppression of EMG activity in the monkey. J Neurophysiol 53: 959–978, 1985. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Seely JS, Sussillo D, Ryu SI, Shenoy KV, Churchland MM. The largest response component in motor cortex reflects movement timing but not movement type. eNeuro 3: ENEURO.0085-16.2016, 2016. doi: 10.1523/ENEURO.0085-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV. Cortical activity in the null space: permitting preparation without movement. Nat Neurosci 17: 440–448; advance online publication, 2014. doi: 10.1038/nn.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Ryu SI, Shenoy KV. Vacillation, indecision and hesitation in moment-by-moment decoding of monkey motor cortex. eLife 4: e04677, 2015. doi: 10.7554/eLife.04677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Santhanam G, Yu BM, Afshar A, Ryu SI, Shenoy KV. Roles of monkey premotor neuron classes in movement preparation and execution. J Neurophysiol 104: 799–810, 2010. doi: 10.1152/jn.00231.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Shenoy KV. The roles of monkey M1 neuron classes in movement preparation and execution. J Neurophysiol 110: 817–825, 2013. doi: 10.1152/jn.00892.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, Kampff AR, Ölveczky BP. Motor cortex is required for learning but not for executing a motor skill. Neuron 86: 800–812, 2015. doi: 10.1016/j.neuron.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding KP, Wolpert DM. Bayesian integration in sensorimotor learning. Nature 427: 244–247, 2004. doi: 10.1038/nature02169. [DOI] [PubMed] [Google Scholar]
- Krubitzer L. In search of a unifying theory of complex brain evolution. Ann N Y Acad Sci 1156: 44–67, 2009. doi: 10.1111/j.1749-6632.2009.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata K. Premotor cortex of monkeys: set- and movement-related activity reflecting amplitude and direction of wrist movements. J Neurophysiol 69: 187–200, 1993. [DOI] [PubMed] [Google Scholar]
- Kurata K, Wise SP. Premotor and supplementary motor cortex in rhesus monkeys: neuronal activity during externally- and internally-instructed motor tasks. Exp Brain Res 72: 237–248, 1988. doi: 10.1007/BF00250247. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Herter TM, Scott SH. Primate upper limb muscles exhibit activity patterns that differ from their anatomical action during a postural task. J Neurophysiol 95: 493–504, 2006. doi: 10.1152/jn.00706.2005. [DOI] [PubMed] [Google Scholar]
- Kurtzer I, Pruszynski JA, Scott SH. Long-latency responses during reaching account for the mechanical interaction between the shoulder and elbow joints. J Neurophysiol 102: 3004–3015, 2009. doi: 10.1152/jn.00453.2009. [DOI] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH. Long-latency reflexes of the human arm reflect an internal model of limb dynamics. Curr Biol 18: 449–453, 2008. doi: 10.1016/j.cub.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Terzuolo C, Viviani P. The law relating the kinematic and figural aspects of drawing movements. Acta Psychol (Amst) 54: 115–130, 1983. doi: 10.1016/0001-6918(83)90027-6. [DOI] [PubMed] [Google Scholar]
- Lamarre Y, Bioulac B, Jacks B. Activity of precentral neurones in conscious monkeys: effects of deafferentation and cerebellar ablation. J Physiol (Paris) 74: 253–264, 1978. [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon RN. The output map of the primate motor cortex. Trends Neurosci 11: 501–506, 1988. doi: 10.1016/0166-2236(88)90012-4. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Mapping the output functions of motor cortex. In: Signal and Sense: Local and Global Order in Perceptual Maps, edited by Edelman GM, Einer Gall W, and Cowan WM. New York: Wiley-Liss, 1990, p. 315–355. [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers (1968a, b) revisited: copies of the original filmed material from their classic papers in Brain. Brain 135: 2290–2295, 2012. doi: 10.1093/brain/aws037. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GW, Muir RB. Corticospinal facilitation of hand muscles during voluntary movement in the conscious monkey. J Physiol 381: 497–527, 1986. doi: 10.1113/jphysiol.1986.sp016341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton SS, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orangutan and gorilla. Q J Exp Physiol 11: 135–222, 1917. doi: 10.1113/expphysiol.1917.sp000240. [DOI] [Google Scholar]
- Li N, Chen T-W, Guo ZV, Gerfen CR, Svoboda K. A motor cortex circuit for motor planning and movement. Nature 519: 51–56, 2015. doi: 10.1038/nature14178. [DOI] [PubMed] [Google Scholar]
- Lillicrap TP, Scott SH. Preference distributions of primary motor cortex neurons reflect control solutions optimized for limb biomechanics. Neuron 77: 168–179, 2013. doi: 10.1016/j.neuron.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Litwin-Kumar A, Doiron B. Slow dynamics and high variability in balanced cortical networks with clustered connections. Nat Neurosci 15: 1498–1505, 2012. doi: 10.1038/nn.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin-Kumar A, Rosenbaum R, Doiron B. Inhibitory stabilization and visual coding in cortical circuits with multiple interneuron subtypes. J Neurophysiol 115: 1399–1409, 2016. doi: 10.1152/jn.00732.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucier GE, Rüegg DC, Wiesendanger M. Responses of neurones in motor cortex and in area 3A to controlled stretches of forelimb muscles in Cebus monkeys. J Physiol 251: 833–853, 1975. doi: 10.1113/jphysiol.1975.sp011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier MA, Bennett KM, Hepp-Reymond MC, Lemon RN. Contribution of the monkey corticomotoneuronal system to the control of force in precision grip. J Neurophysiol 69: 772–785, 1993. [DOI] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L, Svoboda K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72: 111–123, 2011. doi: 10.1016/j.neuron.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD. The apraxias are higher-order defects of sensorimotor integration. Novartis Found Symp 218: 308–325, 1998. [DOI] [PubMed] [Google Scholar]
- Mason CR, Johnson MT, Fu QG, Gomez JE, Ebner TJ. Temporal profile of the directional tuning of the discharge of dorsal premotor cortical cells. Neuroreport 9: 989–995, 1998. doi: 10.1097/00001756-199804200-00007. [DOI] [PubMed] [Google Scholar]
- Mathis MW, Mathis A, Uchida N. Somatosensory cortex plays an essential role in forelimb motor adaptation in mice. Neuron 93: 1493.e6–1503.e6, 2017. doi: 10.1016/j.neuron.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BH. Nerve endings in mammalian muscle. J Physiol 78: 1–53, 1933. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor O, Laurent G. Transient dynamics versus fixed points in odor representations by locust antennal lobe projection neurons. Neuron 48: 661–673, 2005. doi: 10.1016/j.neuron.2005.09.032. [DOI] [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80: 1961–1980, 1998. [DOI] [PubMed] [Google Scholar]
- Merchant H, Naselaris T, Georgopoulos AP. Dynamic sculpting of directional tuning in the primate motor cortex during three-dimensional reaching. J Neurosci 28: 9164–9172, 2008. doi: 10.1523/JNEUROSCI.1898-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Significance of the silent period of muscles. Nature 166: 733–734, 1950. doi: 10.1038/166733a0. [DOI] [PubMed] [Google Scholar]
- Mewes K, Cheney PD. Facilitation and suppression of wrist and digit muscles from single rubromotoneuronal cells in the awake monkey. J Neurophysiol 66: 1965–1977, 1991. [DOI] [PubMed] [Google Scholar]
- Mewes K, Cheney PD. Primate rubromotoneuronal cells: parametric relations and contribution to wrist movement. J Neurophysiol 72: 14–30, 1994. [DOI] [PubMed] [Google Scholar]
- Meyer-Lohmann J, Conrad B, Matsunami K, Brooks VB. Effects of dentate cooling on precentral unit activity following torque pulse injections into elbow movements. Brain Res 94: 237–251, 1975. doi: 10.1016/0006-8993(75)90059-1. [DOI] [PubMed] [Google Scholar]
- Michaels JA, Dann B, Scherberger H. Neural population dynamics during reaching are better explained by a dynamical system than representational tuning. PLOS Comput Biol 12: e1005175, 2016. doi: 10.1371/journal.pcbi.1005175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, Houk JC. Motor co-ordinates in primate red nucleus: preferential relation to muscle activation versus kinematic variables. J Physiol 488: 533–548, 1995. doi: 10.1113/jphysiol.1995.sp020988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LE, van Kan PL, Sinkjaer T, Andersen T, Harris GD, Houk JC. Correlation of primate red nucleus discharge with muscle activity during free-form arm movements. J Physiol 469: 213–243, 1993. doi: 10.1113/jphysiol.1993.sp019812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. Direct connections of rat visual cortex with sensory, motor, and association cortices. J Comp Neurol 226: 184–202, 1984. doi: 10.1002/cne.902260204. [DOI] [PubMed] [Google Scholar]
- Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol 82: 2676–2692, 1999. [DOI] [PubMed] [Google Scholar]
- Morrow MM, Jordan LR, Miller LE. Direct comparison of the task-dependent discharge of M1 in hand space and muscle space. J Neurophysiol 97: 1786–1798, 2007. doi: 10.1152/jn.00150.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow MM, Miller LE. Prediction of muscle activity by populations of sequentially recorded primary motor cortex neurons. J Neurophysiol 89: 2279–2288, 2003. doi: 10.1152/jn.00632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Muir RB, Lemon RN. Corticospinal neurons with a special role in precision grip. Brain Res 261: 312–316, 1983. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- Mussa-Ivaldi FA. Do neurons in the motor cortex encode movement direction? An alternative hypothesis. Neurosci Lett 91: 106–111, 1988. doi: 10.1016/0304-3940(88)90257-1. [DOI] [PubMed] [Google Scholar]
- Mustard BE, Lee RG. Relationship between EMG patterns and kinematic properties for flexion movements at the human wrist. Exp Brain Res 66: 247–256, 1987. doi: 10.1007/BF00243302. [DOI] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Influence of the behavioral goal and environmental obstacles on rapid feedback responses. J Neurophysiol 108: 999–1009, 2012. doi: 10.1152/jn.01089.2011. [DOI] [PubMed] [Google Scholar]
- Nashed JY, Crevecoeur F, Scott SH. Rapid online selection between multiple motor plans. J Neurosci 34: 1769–1780, 2014. doi: 10.1523/JNEUROSCI.3063-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oby ER, Ethier C, Miller LE. Movement representation in the primary motor cortex and its contribution to generalizable EMG predictions. J Neurophysiol 109: 666–678, 2013. doi: 10.1152/jn.00331.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier E, Grantyn A, Kitama T, Berthoz A. Post-spike facilitation of neck EMG by cat tectoreticulospinal neurones during orienting movements. J Physiol 482: 455–466, 1995. doi: 10.1113/jphysiol.1995.sp020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani M, Diedrichsen J, Scott SH. Rapid feedback corrections during a bimanual postural task. J Neurophysiol 109: 147–161, 2013. doi: 10.1152/jn.00669.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani M, Murnaghan CD, Pruszynski JA, Scott SH. Distributed task-specific processing of somatosensory feedback for voluntary motor control. eLife 5: e13141, 2016. doi: 10.7554/eLife.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani M, Pruszynski JA, Murnaghan CD, Scott SH. Perturbation-evoked responses in primary motor cortex are modulated by behavioral context. J Neurophysiol 112: 2985–3000, 2014. doi: 10.1152/jn.00270.2014. [DOI] [PubMed] [Google Scholar]
- Padberg J, Cerkevich C, Engle J, Rajan AT, Recanzone G, Kaas J, Krubitzer L. Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb Cortex 19: 2038–2064, 2009. doi: 10.1093/cercor/bhn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Franca JG, Cooke DF, Soares JG, Rosa MG, Fiorani M Jr, Gattass R, Krubitzer L. Parallel evolution of cortical areas involved in skilled hand use. J Neurosci 27: 10106–10115, 2007. doi: 10.1523/JNEUROSCI.2632-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paninski L, Fellows MR, Hatsopoulos NG, Donoghue JP. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. J Neurophysiol 91: 515–532, 2004. doi: 10.1152/jn.00587.2002. [DOI] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J Neurophysiol 92: 2968–2984, 2004. doi: 10.1152/jn.00649.2003. [DOI] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Gordon M, Cheney PD. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci 21: 2784–2792, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfiliev S, Isa T, Johnels B, Steg G, Wessberg J. Reflexive limb selection and control of reach direction to moving targets in cats, monkeys, and humans. J Neurophysiol 104: 2423–2432, 2010. doi: 10.1152/jn.01133.2009. [DOI] [PubMed] [Google Scholar]
- Petreska B, Yu BM, Cunningham JP, Santhanam G, Ryu SI, Shenoy KV, Sahani M. Dynamical segmentation of single trials from population neural data. In: Advances in Neural Information Processing Systems 24 (NIPS 2011), edited by Shawe-Taylor J, Zemel RS, Bartlett P, Pereira F, and Weinberger KQ, editors). Cambridge, MA: MIT Press, 2011, p. 756–764. [Google Scholar]
- Phillips CG. The Ferrier lecture, 1968. Motor apparatus of the baboon’s hand. Proc R Soc Lond B Biol Sci 173: 141–174, 1969. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Picard N, Smith AM. Primary motor cortical responses to perturbations of prehension in the monkey. J Neurophysiol 68: 1882–1894, 1992. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon, 1993. [Google Scholar]
- Prud’homme MJ, Cohen DA, Kalaska JF. Tactile activity in primate primary somatosensory cortex during active arm movements: cytoarchitectonic distribution. J Neurophysiol 71: 173–181, 1994. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH. Temporal evolution of “automatic gain-scaling”. J Neurophysiol 102: 992–1003, 2009. doi: 10.1152/jn.00085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH. Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478: 387–390, 2011. doi: 10.1038/nature10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol 100: 224–238, 2008. doi: 10.1152/jn.90262.2008. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Omrani M, Scott SH. Goal-dependent modulation of fast feedback responses in primary motor cortex. J Neurosci 34: 4608–4617, 2014. doi: 10.1523/JNEUROSCI.4520-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan K, Abbott LF. Eigenvalue spectra of random matrices for neural networks. Phys Rev Lett 97: 188104, 2006. doi: 10.1103/PhysRevLett.97.188104. [DOI] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA 103: 8257–8262, 2006. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106: 918–923, 2009. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins S, England JD. Ataxias related to sensory neuropathies. Handb Clin Neurol 103: 605–617, 2012. doi: 10.1016/B978-0-444-51892-7.00043-7. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature 286: 496–498, 1980. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J. Pallidal and cerebellar inputs to thalamocortical neurons projecting to the supplementary motor area in Macaca fuscata: a triple-labeling light microscopic study. Anat Embryol (Berl) 199: 9–19, 1999. doi: 10.1007/s004290050204. [DOI] [PubMed] [Google Scholar]
- Saleh M, Takahashi K, Amit Y, Hatsopoulos NG. Encoding of coordinated grasp trajectories in primary motor cortex. J Neurosci 30: 17079–17090, 2010. doi: 10.1523/JNEUROSCI.2558-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Takahashi K, Hatsopoulos NG. Encoding of coordinated reach and grasp trajectories in primary motor cortex. J Neurosci 32: 1220–1232, 2012. doi: 10.1523/JNEUROSCI.2438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD. Theoretical considerations for the analysis of population coding in motor cortex. Neural Comput 6: 29–37, 1994. doi: 10.1162/neco.1994.6.1.29. [DOI] [Google Scholar]
- Sarlegna FR, Gauthier GM, Bourdin C, Vercher JL, Blouin J. Internally driven control of reaching movements: a study on a proprioceptively deafferented subject. Brain Res Bull 69: 404–415, 2006. doi: 10.1016/j.brainresbull.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Kawaguchi S, Oka H, Sakai M, Mizuno N. Electrophysiological studies on the cerebellocerebral projections in monkeys. Exp Brain Res 24: 495–507, 1976. doi: 10.1007/BF00234966. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Matsuda Y, Kawaguchi S, Mizuno N. On the cerebello-thalamo-cerebral pathway for the parietal cortex. Exp Brain Res 16: 89–103, 1972. doi: 10.1007/BF00233376. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Matsuda Y, Mizuno N. Distribution of cerebellar-induced responses in the cerebral cortex. Exp Neurol 39: 342–354, 1973. doi: 10.1016/0014-4886(73)90236-7. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science 261: 489–492, 1993. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Rivlis G. A spectrum from pure post-spike effects to synchrony effects in spike-triggered averages of electromyographic activity during skilled finger movements. J Neurophysiol 94: 3325–3341, 2005. doi: 10.1152/jn.00007.2005. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Rivlis G. Partial reconstruction of muscle activity from a pruned network of diverse motor cortex neurons. J Neurophysiol 97: 70–82, 2007. doi: 10.1152/jn.00544.2006. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schöner G, Latash ML. Identifying the control structure of multijoint coordination during pistol shooting. Exp Brain Res 135: 382–404, 2000. doi: 10.1007/s002210000540. [DOI] [PubMed] [Google Scholar]
- Scott SH. The role of primary motor cortex in goal-directed movements: insights from neurophysiological studies on non-human primates. Curr Opin Neurobiol 13: 671–677, 2003. doi: 10.1016/j.conb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Scott SH. Optimal feedback control and the neural basis of volitional motor control. Nat Rev Neurosci 5: 532–546, 2004. doi: 10.1038/nrn1427. [DOI] [PubMed] [Google Scholar]
- Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol 586: 1217–1224, 2008. doi: 10.1113/jphysiol.2007.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. The computational and neural basis of voluntary motor control and planning. Trends Cogn Sci 16: 541–549, 2012. doi: 10.1016/j.tics.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Scott SH. A functional taxonomy of bottom-up sensory feedback processing for motor actions. Trends Neurosci 39: 512–526, 2016. doi: 10.1016/j.tins.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Scott SH, Cluff T, Lowrey CR, Takei T. Feedback control during voluntary motor actions. Curr Opin Neurobiol 33: 85–94, 2015. doi: 10.1016/j.conb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol 77: 826–852, 1997. [DOI] [PubMed] [Google Scholar]
- Selen LP, Shadlen MN, Wolpert DM. Deliberation in the motor system: reflex gains track evolving evidence leading to a decision. J Neurosci 32: 2276–2286, 2012. doi: 10.1523/JNEUROSCI.5273-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Pâquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol 94: 2353–2378, 2005. doi: 10.1152/jn.00989.2004. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Kalaska JF. Changes in the temporal pattern of primary motor cortex activity in a directional isometric force versus limb movement task. J Neurophysiol 80: 1577–1583, 1998. [DOI] [PubMed] [Google Scholar]
- Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29: 13255–13263, 2009. doi: 10.1523/JNEUROSCI.0892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol 121: 1680–1689, 2010. doi: 10.1016/j.clinph.2010.02.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy KV, Kaufman MT, Sahani M, Churchland MM. A dynamical systems view of motor preparation: implications for neural prosthetic system design. Prog Brain Res 192: 33–58, 2011. doi: 10.1016/B978-0-444-53355-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy KV, Sahani M, Churchland MM. Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci 36: 337–359, 2013. doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14: 278–291, 2013. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett 23: 7–12, 1981. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 6: 309–331, 2014. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Miller L, Andersen T, Houk JC. Synaptic linkages between red nucleus cells and limb muscles during a multi-joint motor task. Exp Brain Res 102: 546–550, 1995. doi: 10.1007/BF00230659. [DOI] [PubMed] [Google Scholar]
- Smith AM, Hepp-Reymond MC, Wyss UR. Relation of activity in precentral cortical neurons to force and rate of force change during isometric contractions of finger muscles. Exp Brain Res 23: 315–332, 1975. doi: 10.1007/BF00239743. [DOI] [PubMed] [Google Scholar]
- Stark E, Drori R, Abeles M. Partial cross-correlation analysis resolves ambiguity in the encoding of multiple movement features. J Neurophysiol 95: 1966–1975, 2006. doi: 10.1152/jn.00981.2005. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Sakai ST, Qi H-X, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: potential substrate for parkinsonian tremor. J Comp Neurol 455: 378–395, 2003. doi: 10.1002/cne.10499. [DOI] [PubMed] [Google Scholar]
- Strick PL. The influence of motor preparation on the response of cerebellar neurons to limb displacements. J Neurosci 3: 2007–2020, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Kim CC. Input to primate motor cortex from posterior parietal cortex (area 5). I. Demonstration by retrograde transport. Brain Res 157: 325–330, 1978. doi: 10.1016/0006-8993(78)90035-5. [DOI] [PubMed] [Google Scholar]
- Suminski AJ, Mardoum P, Lillicrap TP, Hatsopoulos NG. Temporal evolution of both premotor and motor cortical tuning properties reflect changes in limb biomechanics. J Neurophysiol 113: 2812–2823, 2015. doi: 10.1152/jn.00486.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussillo D, Churchland MM, Kaufman MT, Shenoy KV. A neural network that finds a naturalistic solution for the production of muscle activity. Nat Neurosci 18: 1025–1033, 2015. doi: 10.1038/nn.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter BA, Migliore M, Shepherd GM. Intrinsic electrophysiology of mouse corticospinal neurons: a class-specific triad of spike-related properties. Cereb Cortex 23: 1965–1977, 2013. doi: 10.1093/cercor/bhs184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M, Boline J, Smyrnis N, Georgopoulos AP, Ashe J. On the relations between single cell activity in the motor cortex and the direction and magnitude of three-dimensional static isometric force. Exp Brain Res 109: 367–376, 1996. doi: 10.1007/BF00229620. [DOI] [PubMed] [Google Scholar]
- Tanji J, Wise SPS. Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 45: 467–481, 1981. [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. J Neurosci 27: 13241–13250, 2007. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci 3: 391–398, 2000. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci 5: 1226–1235, 2002. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]
- Van Acker GM 3rd, Amundsen SL, Messamore WG, Zhang HY, Luchies CW, Cheney PD. Equilibrium-based movement endpoints elicited from primary motor cortex using repetitive microstimulation. J Neurosci 34: 15722–15734, 2014. doi: 10.1523/JNEUROSCI.0214-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilis T, Hore J, Meyer-Lohmann J, Brooks VB. Dual nature of the precentral responses to limb perturbations revealed by cerebellar cooling. Brain Res 117: 336–340, 1976. doi: 10.1016/0006-8993(76)90743-5. [DOI] [PubMed] [Google Scholar]
- Viviani P, Cenzato M. Segmentation and coupling in complex movements. J Exp Psychol Hum Percept Perform 11: 828–845, 1985. doi: 10.1037/0096-1523.11.6.828. [DOI] [PubMed] [Google Scholar]
- Viviani P, McCollum G. The relation between linear extent and velocity in drawing movements. Neuroscience 10: 211–218, 1983. doi: 10.1016/0306-4522(83)90094-5. [DOI] [PubMed] [Google Scholar]
- Viviani P, Terzuolo C. Trajectory determines movement dynamics. Neuroscience 7: 431–437, 1982. doi: 10.1016/0306-4522(82)90277-9. [DOI] [PubMed] [Google Scholar]
- Weber DJ, He J. Adaptive behavior of cortical neurons during a perturbed arm-reaching movement in a nonhuman primate. Prog Brain Res 143: 477–490, 2004. doi: 10.1016/S0079-6123(03)43045-8. [DOI] [PubMed] [Google Scholar]
- Weiler J, Gribble PL, Pruszynski JA. Goal-dependent modulation of the long-latency stretch response at the shoulder, elbow, and wrist. J Neurophysiol 114: 3242–3254, 2015. doi: 10.1152/jn.00702.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel BL, Rustioni A, Dreyer DA, Loe PR, Allen EE, Metz CB. Thalamic projections to S-I in macaque monkey. J Comp Neurol 178: 385–409, 1978. doi: 10.1002/cne.901780302. [DOI] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, Datta SR. Mapping sub-second structure in mouse behavior. Neuron 88: 1121–1135, 2015. doi: 10.1016/j.neuron.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham CL, Fisher KM, Edgley SA, Baker SN. Corticospinal inputs to primate motoneurons innervating the forelimb from two divisions of primary motor cortex and area 3a. J Neurosci 36: 2605–2616, 2016. doi: 10.1523/JNEUROSCI.4055-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR. Amplitude of responses to perturbation in primate sensorimotor cortex as a function of task. J Neurophysiol 44: 1139–1147, 1980a. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Correlations between task-related activity and responses to perturbation in primate sensorimotor cortex. J Neurophysiol 44: 1122–1138, 1980b. [DOI] [PubMed] [Google Scholar]
- Wolpert D. The Real Reason for Brains. Filmed at TEDGlobal, Edinburgh, Scotland, July 2011 https://www.ted.com/talks/daniel_wolpert_the_real_reason_for_brains.
- Wolpert DM. Probabilistic models in human sensorimotor control. Hum Mov Sci 26: 511–524, 2007. doi: 10.1016/j.humov.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci 3, Suppl: 1212–1217, 2000. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science 269: 1880–1882, 1995. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Matsushita M. Spinocerebellar projections from the thoracic cord in the cat, as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol 258: 1–27, 1987. doi: 10.1002/cne.902580102. [DOI] [PubMed] [Google Scholar]
- Yang L, Michaels JA, Pruszynski JA, Scott SH. Rapid motor responses quickly integrate visuospatial task constraints. Exp Brain Res 211: 231–242, 2011. doi: 10.1007/s00221-011-2674-3. [DOI] [PubMed] [Google Scholar]
- Yttri EA, Dudman JT. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 533: 402–406; advance online publication, 2016. doi: 10.1038/nature17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzecki P, Strick PL, Asanuma H. Input to primate motor cortex from posterior parietal cortex (area 5). II. Identification by antidromic activation. Brain Res 157: 331–335, 1978. doi: 10.1016/0006-8993(78)90036-7. [DOI] [PubMed] [Google Scholar]