Motor expertise is associated with functional/structural brain plasticity. How such neuroplastic reorganization translates into altered motor learning processes remains elusive. We investigated endurance athletes (EA) and nonathletes (NA) in a multimodal balance task (MBT). EA showed superior static balance performance (SBT), whereas MBT-induced SBT improvements did not differ between groups. Functional near-infrared spectroscopy recordings revealed a differential MBT training-induced decrease of deoxygenated hemoglobin in left primary motor cortex and inferior parietal lobe between groups.
Keywords: functional near infrared spectroscopy, motor learning, endurance athletes, neuroplasticity, balance
Abstract
Studies suggested that motor expertise is associated with functional and structural brain alterations, which positively affect sensorimotor performance and learning capabilities. The purpose of the present study was to unravel differences in motor skill learning and associated functional neuroplasticity between endurance athletes (EA) and nonathletes (NA). For this purpose, participants had to perform a multimodal balance task (MBT) training on 2 sessions, which were separated by 1 wk. Before and after MBT training, a static balance task (SBT) had to be performed. MBT-induced functional neuroplasticity and neuromuscular alterations were assessed by means of functional near-infrared spectroscopy (fNIRS) and electromyography (EMG) during SBT performance. We hypothesized that EA would showed superior initial SBT performance and stronger MBT-induced improvements in SBT learning rates compared with NA. On a cortical level, we hypothesized that MBT training would lead to differential learning-dependent functional changes in motor-related brain regions [such as primary motor cortex (M1)] during SBT performance. In fact, EA showed superior initial SBT performance, whereas learning rates did not differ between groups. On a cortical level, fNIRS recordings (time × group interaction) revealed a stronger MBT-induced decrease in left M1 and inferior parietal lobe (IPL) for deoxygenated hemoglobin in EA. Even more interesting, learning rates were correlated with fNIRS changes in right M1/IPL. On the basis of these findings, we provide novel evidence for superior MBT training-induced functional neuroplasticity in highly trained athletes. Future studies should investigate these effects in different sports disciplines to strengthen previous work on experience-dependent neuroplasticity.
NEW & NOTEWORTHY Motor expertise is associated with functional/structural brain plasticity. How such neuroplastic reorganization translates into altered motor learning processes remains elusive. We investigated endurance athletes (EA) and nonathletes (NA) in a multimodal balance task (MBT). EA showed superior static balance performance (SBT), whereas MBT-induced SBT improvements did not differ between groups. Functional near-infrared spectroscopy recordings revealed a differential MBT training-induced decrease of deoxygenated hemoglobin in left primary motor cortex and inferior parietal lobe between groups.
recent animal and human studies provided compelling evidence that there is a strong link between motor skill learning and neuroplasticity on a functional and structural level (Costa et al. 2004; Dayan and Cohen 2011; Kleim et al. 1998; Li et al. 2001; Miyachi et al. 2002; Plautz et al. 2000; Taubert et al. 2012). For example, human studies could indicate that short-term motor skill learning is associated with distinct functional reorganization within the human motor system and generally improves the interaction between certain cortical and subcortical brain areas (Floyer-Lea and Matthews 2005; Lehéricy et al. 2005; Sakai et al. 1999). Using functional magnetic resonance imaging (fMRI), Karni et al. (1995) demonstrated that long-term motor skill learning of a simple finger-to-thumb opposition task, where participants had to move their fingers in a specific order, is associated with functional reorganization within the primary motor cortex (M1). Subsequent studies confirmed and extended these findings by showing that learning of sequential finger movements evoked a slowly evolving reorganization within M1 over the course of weeks and that this change in M1 follows more dynamic, rapid changes in the cerebellum, striatum, and other motor-related cortical areas over the course of days (Ungerleider et al. 2002). Although these studies indicated that M1 might be a key region for motor skill learning (Kantak et al. 2012), its influence and amount of functional reorganization is, however, strongly dependent on task complexity and other factors such as attentional resources (Hazeltine et al. 1997; Stefan et al. 2004).
Apart from learning-induced functional changes, it has been shown that skill acquisition is also associated with structural brain plasticity in gray matter (GM) and white matter (WM) (Draganski et al. 2004; Driemeyer et al. 2008; Gryga et al. 2012; Scholz et al. 2009; Taubert et al. 2010, 2012). One of the first studies that disclosed a brain-behavior interaction on a structural level was performed by Draganski et al. (2004). In that study, participants had to learn a three-ball juggling cascade over a period of 3 mo. Structural MRI (sMRI) measurements revealed reversible, training-induced structural alterations in GM of task-related brain regions such as visual motion areas. Similarly, complex balance task learning has been shown to induce dynamic neuroplasticity on both functional and structural levels (Mégrot and Bardy 2006; Taubert et al. 2010, 2011, 2012, 2016). Furthermore, it has been shown that motor skill learning can be facilitated by means of noninvasive brain stimulation such as transcranial direct current stimulation (tDCS) (Kaminski et al. 2016; Nitsche et al. 2003; Reis and Fritsch 2011; Saucedo Marquez et al. 2013; Stagg et al. 2011). These results provided compelling evidence that motor skill learning can be facilitated by modulating brain activity during the learning process.
However, the influence of prior physical activity during the process of motor skill learning as a performance enhancer has so far not been investigated in great detail. In analogy to animal studies (Zhang et al. 2005), there is preliminary evidence that endurance training is capable of improving motor performance and memory (Erickson et al. 2011; Statton et al. 2015). For example, it has been shown that one bout of intense exercise performed immediately before or after a motor task is practiced is sufficient to improve the long-term retention of a motor skill (Roig et al. 2012). Furthermore, Statton et al. (2015) indicated increased movement speed and accuracy after a bout of moderate aerobic exercise (running). Apart from alterations in motor performance, several studies also indicated that aerobic exercise is also capable of evoking improvements in cognitive performance (Erickson et al. 2011). Hence, endurance training, which is assumed to support neurogenesis, has also been used as an interventional strategy to counteract the age-related decline in cognitive and motor performance (Barnes et al. 2003; van Boxtel et al. 1997).
Additionally, there is preliminary evidence that (long term) physical activity leads to specific functional and structural brain alterations (Bullitt et al. 2009; Colcombe et al. 2006; Erickson et al. 2012; Voss et al. 2010). It has also been shown that such brain alterations are specific to the individual training regime (Jäncke et al. 2009; Park et al. 2009; Schlaffke et al. 2014). For example, GM volume was increased in hand areas of M1 of handball players, whereas GM volume was increased in foot areas of M1 of ballet dancers (Meier et al. 2016). These results suggest that the observed structural adaptations are sport specific and are manifested in brain regions associated with the neural processing of sport-specific skills.
Regarding underlying mechanisms, endurance training might stress the oxygen saturation in the brain and thus promote angiogenesis in movement-relevant brain regions. Furthermore, endurance training increases the distribution of neurotransmitters in the brain, which has a direct influence on the information processes taking place (Ploughman 2008). Last but not least, neurotrophin levels in the brain are highly regulated by endurance training, which promotes neuronal growth (Joundi et al. 2012; Ploughman 2008; Schiffer et al. 2009; Seifert et al. 2010; Zoladz et al. 2008). For example, brain-derived neurotrophic factor (BDNF) has been assumed to be a key regulator for activity-dependent synaptic plasticity and plays a central role in (motor) learning and memory processes (Cunha et al. 2010; Egan et al. 2003; Gómez-Pinilla et al. 2007; Hall et al. 2000; Ploughman et al. 2007; Ploughman 2008; Roig et al. 2012; Tang et al. 2008). Hence, the aim of the present study was to indirectly investigate this issue in endurance athletes (EA) and compare the effects on motor skill learning with those in a group of nonathletes (NA). In addition, we assessed learning-induced functional brain alterations and associated neuromuscular changes by means of functional near-infrared spectroscopy (fNIRS) and electromyography (EMG) measures.
fNIRS, a noninvasive method of functional neuroimaging, involves the quantification of chromophore concentrations resolved from the measurement of relative changes in oxygenated (oxyHb) and deoxygenated hemoglobin (deoxyHb). The use of fNIRS relies, in analogy to fMRI, on the principle of neurovascular coupling also known as the hemodynamic or blood oxygenation level-dependent (BOLD) response. In contrast to fMRI, fNIRS can be used to record brain processing during complex whole body movements, because it is less susceptible to motion artifacts. Alterations in both chromophores (oxyHb and deoxyHb) are assumed to be indicators for changes in neural processing (Obrig and Villringer 2003; Schroeter et al. 2002; Strangman et al. 2002).
The present study is based on previous work, which collectively assumes that the brain of endurance athletes differs in functional and structural plasticity compared with the brain of nonathletes due to an increased distribution of neurotrophic growth factor BDNF (Kumpulainen et al. 2015; Raichlen et al. 2016; Wood et al. 2016). These findings suggest that regular and long-term physical activity lead to an improvement in motor memory. Additionally, we assume, in line with the “neural efficiency” hypothesis proposed by Dunst et al. (2014), that EA show less brain activation during the performance of sport-related motor tasks (balance performance). Similar findings have been observed by Guo et al. (2017), who showed lower brain activation levels in expert table tennis players compared with NA, even in resting-state brain networks.
The primary aim of the present study was to investigate whether multimodal balance task (MBT) training, consisting of static and dynamic balance components, will enhance performance in a static balance task (SBT) and if short-term functional reorganizational changes can be observed after a single training session. In addition, we investigated the extent to which EA differ in their initial SBT performance compared with NA. Furthermore, we reasoned that EA will show better learning and retention rates after MBT training. This hypothesis is based on previous studies in pianists (also an established model for motor expertise), which have shown higher motor learning rates in experts compared with nonexperts (Hund-Georgiadis and von Cramon 1999; Pascual-Leone et al. 1995). It is further strengthened by a recent study showing enhanced implicit motor learning in athletes compared with nonathletes in a serial reaction-time task (Verburgh et al. 2016). To demonstrate that SBT performance gains are in fact related to MBT-training, we tested a control group (CG) of participants without MBT training that had to perform SBT before and after a 20-min rest period. On a cortical level, we hypothesized that MBT training would lead to differential learning-dependent alterations in brain activity within M1 leg area during SBT performance. This hypothesis is based on previous studies showing reorganizational changes within M1 during motor skill learning (Dayan and Cohen 2011; Kaminski et al. 2016; Kantak et al. 2012; Taubert et al. 2016). For example, Taubert et al. (2016) could demonstrate that short-term balance training is capable of evoking structural gray matter changes in M1 leg and not in other M1 regions, such as M1 hand. Furthermore, the functional relevance of M1 leg has also been proved by a recent tDCS study that showed tDCS-induced enhanced learning capabilities during dynamic balance task training (Kaminski et al. 2016). Even though the spatial resolution of fNIRS recordings is less accurate compared with fMRI, there have been studies showing clear differences in activation patterns for hand and foot movements (Batula et al. 2017; Koenraadt et al. 2012; Sukal-Moulton et al. 2014).
Regarding EMG assessments, we expected training-induced reductions in EMG activity after MBT training, which is in line with previous EMG studies showing a learning-related reduction in muscular activity (Engelhorn 1983; Lohse et al. 2010; Taubert et al. 2010; Zachry et al. 2005). On an exploratory level, we aimed at investigating potential learning-related differences in neuromuscular adaptations between EA and NA.
MATERIAL AND METHODS
Participants
A total of 43 healthy, right-handed participants (20 female) were enrolled in the present study after written informed consent was obtained. Study procedures were approved by the local ethics committee of the University of Leipzig. The study was performed in accordance with the Declaration of Helsinki. The group of EA consisted of 11 male and 7 female athletes (n = 18). EA were on average 25.06 ± 4.68 yr old (mean ± SD). EA trained on average 14.31 ± 4.02 h/wk. The EA group consisted of seven triathletes (competing in Olympic-, middle-, and long-distance triathlons), seven middle/long-distance runners (competing in running events from 800 to 10,000 m), and four cyclists (competing in road races). Their levels ranged from the ambitious broad-spectrum sportsman to the professional sportsman with national and international competitions. Within the NA group, a total of 7 male and 8 female participants (n = 15) with an age of 25.47 ± 4.23 yr and sports-related activities of 1.67 ± 1.25 h/wk were tested.
We used a quasi-experimental design (Fig. 1A) where each participant was tested in 2 sessions that were separated by ~1 wk (7 ± 2 days). Study procedures were identical for test days 1 and 2 (TD1 and TD2). Participants were asked to report their daily activities 48 h before TD1 and TD2, as well as their individual amount of sleep the night before the experimental sessions, to sufficiently control for this matter.
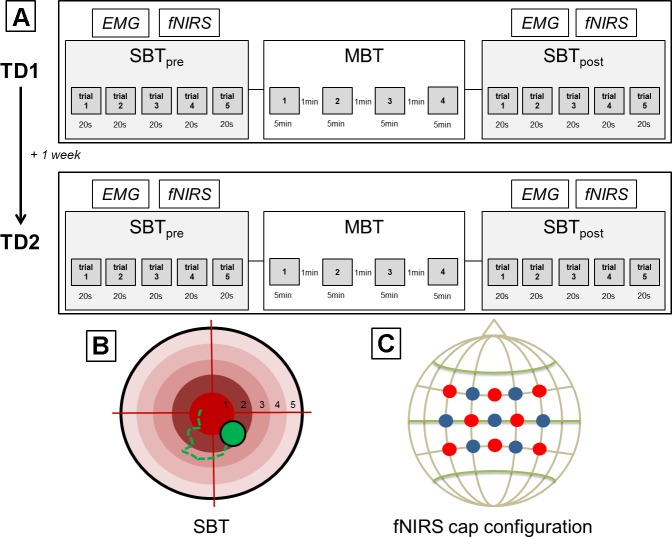
Fig. 1.
Study design. A: procedures for test day 1 (TD1) and test day 2 (TD2). Within each session, participants had to perform five 20-s trials of a static balance task (SBT) separated by 20-s rest periods (SBTpre). Subsequently, multimodal balance task (MBT) training was performed by endurance athletes (EA) and nonathletes (NA) for a total duration of 20 min (see text for details). Immediately after termination of MBT, SBT performance was reassessed (SBTpost). Control group (CG) participants only performed SBT twice, before and after a 20-min rest period (without MBT training). B: simplified illustration of SBT. The tracking ball (green) could be moved by subtle shifts in body sways. The target zone consisted of 5 circles where 1 indicated the best possible score and 5 the lowest. C: illustration of the fNIRS configuration used during SBT. Detectors are shown as blue dots and transmitters as red dots.
Within each session, participants had to perform five 20-s trials of an SBT (primary outcome measure; see below for details) separated by 20-s rest periods. Subsequently, MBT training was performed for a total duration of 20 min. MBT consisted of five static and eight dynamic components (see below). Static components lasted ~10 s, whereas dynamic components lasted ~20 s. Static and dynamic components were tested in pseudorandomized order. Immediately after termination of MBT, SBT performance was reassessed. During SBT (before and after MBT), cortical activity within the sensorimotor system was recorded from each participant using fNIRS (5 × 20 s vs. 5 × 20-s rest trials, block design). In addition, EMG measurements were performed during SBT over left and right tibialis anterior muscles (see below for details). The tibialis anterior muscles were chosen as target muscles because of their important role during static and dynamic balance conditions as suggested by previous studies (Day et al. 2013; Di Giulio et al. 2009; Laughton et al. 2003).
Furthermore, a control group (CG) was tested to ensure that potential behavioral changes in SBT are in fact MBT training related and not a mere test-retest improvement. A total of 5 male and 5 female participants (n = 10) in this group with an age of 25.40 ± 4.18 yr and sports-related activities of 1.40 ± 1.15 h/wk had to perform the SBT before and after a 20-min rest period (no MBT training).
Static and Multimodal Balance Task
To assess SBT performance, we used a balance board [MFT Challenge Disc; Trend Sport Trading (TST), Grosshöflein, Austria] that was mounted on a central ball joint and was freely rotatable with a maximum tilt angle of 12°. The balance board had a diameter of 53 cm and was connected to a personal computer via USB cable. Displacement of the balance board was detected by a 3-dimensional inclination sensor with a measuring range of 20° and an accuracy of 0.5°. Displacements of the balance board were recorded with a sampling rate of 100 Hz throughout the respective trials.
During SBT, participants were instructed to stand on the balance board with their feet in shoulder-width position, ~30 cm apart, and to move and keep a tracking ball in the center of a target zone (Fig. 1B) that was displayed on a computer screen. The tracking ball could be moved by subtle shifts in body sways. The target zone consisted of five circles. The measured value resulted from a point system between 1 (inner ring) and 5 (outer ring) where 1 indicates the best possible score and 5 the lowest. The longer the tracking ball was held in or near the center of the target zone, the lower (i.e., better) the score.
MBT training consisted of both static and dynamic components in different subtests. However, the static tasks were different from the primary outcome measure. Participants had to hold the balance board in one predefined target region for a total duration of 10 s. Dynamic tasks included tests of translatory, rotatory, and free movements of a target point that had to be tracked with the tracking ball. During translational tasks, the target point appeared for ~2 s in either the mediolateral or anteroposterior direction on screen. Additionally, there were conditions where the target point was moving in horizontal or vertical direction and had to be continuously tracked with corresponding movements of the balance board. During rotation tasks, the target point rotated in either clockwise or counterclockwise orientation. Furthermore, during free movements, the target point either appeared randomly on the screen for ~2 s or moved continuously in a randomized way and had to be tracked accordingly. These tasks were employed with the intention of creating a multimodal training regime consisting of static and dynamic balance components. The measured value was the score for each task, which corresponds to the relative time in which the tracking ball was at the target point [time in target (TIT), %]. Participants received feedback about their individual performance in the respective tasks after completion of MBT training. MBT had to be trained 4 times (trials 1–4) with a break of 1 min between each trial.
Data were analyzed using the software SPSS Statistics 22 (IBM, Armonk, NY). As a first step, normal distribution of data was evaluated by using the Shapiro-Wilk test. The test for normal distribution revealed that SBT and MBT data were normally distributed, which is why only parametric tests were used for subsequent statistical analyses. For SBT, five repetitions of the task were averaged for pre- and posttests on TD1 and TD2. Differences in SBT baseline values (pretest), learning rates, and retention rates (i.e., differences in performance between TD1post and TD2pre, %) between groups were assessed using an univariate analysis of variance (uANOVA) with the factor group (EA vs. NA). Performance gains within groups were assessed using a two-sample t-test. Because of initial performance differences in SBT between EA and NA (see results), data were normalized to the respective pretest values (in %) for subsequent analysis steps.
For MBT, data for respective subtests (static and dynamic components) were analyzed using a repeated-measures ANOVA (rmANOVA) with the factors time (trials 1–4) and group (EA vs. NA). Differences in baseline (pretests) and retention rates between groups were assessed using uANOVA with the factor group (EA vs. NA). Retention rates within groups were assessed using a two-sample t-test. As a significance level, α ≤ 0.05 was determined for all tests. If necessary, data were corrected for sphericity using Greenhouse-Geisser correction. Partial eta-squared () for ANOVAs are provided as measures of effect size and used to aid in the interpretation of inferential statistics. As a rule of thumb, introduced by Miles and Shevlin (2001), ≥ 0.01 is considered to be a small, ≥ 0.06 a medium, and ≥ 0.14 a large effect.
For the CG (without MBT training), we also computed the test-retest reliability using an intraclass correlation coefficient (ICC) to investigate whether the chosen SBT is a reliable measure of static balance performance.
Functional Near-Infrared Spectroscopy
fNIRS recordings during SBT were performed using the NIRSport measuring system (NIRx Medical Technologies, Glen Head, NY) for each participant. For this purpose, a cap was placed on the head of the participant, to which a defined number of optodes were attached, which were placed directly on the scalp. The center of the cap was placed according to the international 10–20 system over the vertex (Cz) of each participant. Cz was determined over the intersection of the courses nasion to inion and left preauricular point to right preauricular point according to Jurcak et al. (2007). The arrangement of the optodes allowed the measurement of brain activity within the sensorimotor system (Fig. 1C). An fNIRS configuration with a total number of 15 optodes (8 sources and 7 detectors) was chosen. The infrared light was emitted by sources with wavelengths of 760 and 850 nm (Pereira et al. 2007) and was measured with a recording frequency of 7.81 Hz. Spectroscopically, NIRSport operated via a so-called continuous wave method; i.e., sources emit light at constant frequency and intensity (Scholkmann et al. 2014).
The preprocessing and evaluation of fNIRS data was carried out with the software nirsLAB (v2016; NIRx Medical Technologies). First, the signal quality of the individual channels was checked by means of a coefficient of variation (CV). The exclusion value used was set at 15%. Subsequently, the data were subjected to baseline correction (10 s before onset) and then filtered (bandpass filter: low cutoff frequency = 0.01 Hz, high cutoff frequency = 0.2 Hz) to attenuate high-frequency noise and cardiovascular artifacts (Huppert et al. 2009). Finally, a time series analysis of the hemodynamic states was calculated according to Cope and Delpy (1988). Data were analyzed and evaluated using the NIRS-SPM (statistical parametric mapping) software (Ye et al. 2009). For this purpose, preprocessed data sets were transferred to the generalized linear model (GLM) assessment within the scope of the SPM level 1 analysis to obtain beta values. Evaluation on the basis of group level was subsequently carried out in the form of a two-sample t-test using SPM level 2, which allowed statistical statements on topographically different activity areas between groups. An intermediate step using the software MATLAB (The MathWorks, Natick, MA) was necessary for calculation of pre-post comparisons within groups on TD1 and TD2. Beta values of pre- and posttest data sets of all participants were subtracted and then statistically evaluated using SPM level 2. Visualization of hemodynamic data (maps of significant decreases/increases in both chromophores) was performed using the Brain Function Mapping Tool by Wang et al. (2016). The results of all topographical analyses were classified as statistically significant at a significance level of α ≤ 0.05. Furthermore, multiple correlations were performed between alterations in neurovascular coupling as assessed with fNIRS (oxy- and deoxyHb) and potential SBT behavioral gains and MBT training-induced EMG alterations. Because 22 correlations were performed (one for each fNIRS channel), the alpha (α) level was set to P = 0.0023. Hence, only P values <0.0023 were considered significant.
To transform topographic maps from the recorded hemodynamic responses, the fNIRS probe positions (x, y, z space) were registered to MNI (Montreal Neurological institute) space. This was done via the neuronavigation device Brainsight (version 2; Rogue Research, Montreal, QC, Canada). Subsequently, the respective MNI coordinates for each fNIRS probe were fed into a probabilistic atlas to allow the assignment of the hemodynamic response alterations during SBT to a specific brain region. For that purpose, the Juelich histological (cyto- and myeloarchitectonic) atlas was used within the program FSL (FMRIB Software Library v5.0, created by the Analysis Group, FMRIB, Oxford, UK; Eickhoff et al. 2005, 2006, 2007).
Electromyography
EMG recordings were carried out using the TELEmyo Desktop Direct Transmission System (DDTS; Noraxon, Scottsdale, AZ; input impedance >100 MΩ, common mode rejection ratio >100 dB, baseline noise <1 µV RMS, base gain 200). EMG recordings were performed using the software MyoResearch (MR 3.8; Noraxon). EMG was recorded at a sampling rate of 1,500 Hz, low-pass filtered at 500 Hz, and converted (analog to digital) with 16-bit accuracy in the signal range ±5 V. EMG electrodes (bipolar surface disk electrodes, Ag-AgCl, electrode area 95 mm2; Ambu, Copenhagen, Denmark) with electrode gel were attached to the left and right anterior tibialis muscles using adhesive tape. Interelectrode distance was 20 mm following the SENIAM recommendations (Hermens 1999).
EMG preprocessing was carried out with the software ProEMG (Motion Laboratory Systems, Baton Rouge, LAUSA). Task-related EMG bursts were subjected to an offset correction and to a second-order Butterworth high-pass filter (cutoff 15 Hz). Subsequently, root mean square (RMS) values were calculated for each muscle (left and right tibialis) and averaged to compute a compound RMS value. These RMS values were transferred to SPSS Statistics 22 for further statistical analyses. Statistical comparisons were performed in analogy to behavioral analysis (for details, see above). Furthermore, a correlational analysis was performed between alterations in neuromuscular activity and potential SBT behavioral gains.
RESULTS
Behavioral Data TD1
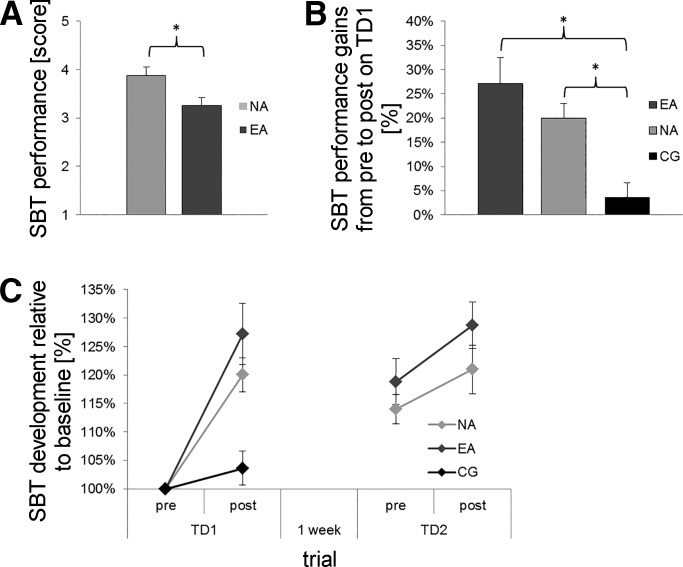
SBT performance (TD1pre) differed significantly between groups [EA: 3.26 ± 0.16 points vs. NA: 3.88 ± 0.17 points; uANOVA, main effect of group: F(1,31) = 6.857, P = 0.014, = 0.181] indicating superior SBT performance in EA as compared with NA (Fig. 2A).
Fig. 2.
SBT results. A: initial SBT performance on TD1pre. Values are means ± SE; dark gray bars represent endurance athletes (EA), and light gray bars represent nonathletes (NA). *P < 0.5 indicates significantly better initial SBT performance for EA compared with NA. B: SBT performance gains (%) from TD1pre to TD1post. Values are means ± SE; dark gray bars represent EA, light gray bars represent NA, and black bars represent the control group (CG). C: SBT development (relative to baseline, %) on TD1 and TD2. Symbol colors are defined in A. All values are normalized to baseline (TD1pre). *P < 0.5 indicates significant differences.
MBT training resulted in significant performance gains (Table 1) for static and dynamic components [static task (stabilization): F(2,62) = 15.963, P = 0.000, = 0.340; dynamic task (translation): F(2,62) = 10.168, P = 0.000, = 0.247], although there was no difference between groups [time × group interaction for stabilization: F(2,62) = 0.840, P = 0.436, = 0.026; translation: F(2,62) = 1.997, P = 0.144, = 0.061]. On the other hand, MBT training in dynamic tasks including rotation [time × group: F(2,62) = 0.441, P = 0.645, = 0.014] and free movements [time × group: F(2,62) = 0.667, P = 0.517, = 0.021] did not show significant performance improvements.
Table 1.
MBT performance gains on TD1 and TD2 for EA and NA
| Performance Gains TD1, % |
Performance Gains TD2, % |
|||
|---|---|---|---|---|
| EA | NA | EA | NA | |
| Stabilization | 22.29 ± 4.26 | 33.86 ± 11.11 | 6.31 ± 1.69 | 8.21 ± 4.17 |
| Translation | 38.89 ± 5.65 | 18.40 ± 7.24 | 14.46 ± 4.41 | 15.75 ± 5.15 |
| Rotation | 32.08 ± 10.10 | 18.24 ± 6.79 | 7.68 ± 4.35 | 10.61 ± 4.23 |
| Free movement | 1.23 ± 3.95 | 19.66 ± 11.62 | 13.71 ± 7.27 | 36.67 ± 16.07 |
| Total score | 23.68 ± 4.01 | 23.10 ± 5.87 | 8.38 ± 1.84 | 11.37 ± 2.04 |
Values are presented as means ± SE of percent improvement in performance gains from MBT trial 1 vs. trial 4 on test day 1 (TD1) and test day 2 (TD2) for endurance athletes (EA) and nonathletes (NA).
MBT-induced effects on SBT revealed a significant improvement (TD1pre vs. TD1post) in both groups (paired t-test, EA: 27.17 ± 5.38%, P = 0.001; NA: 20.02 ± 2.96%, P = 0.001), although there was no difference in behavioral gains between groups [uANOVA, main effect of group: F(1,31) = 1.213, P = 0.279, = 0.038].
To confirm that the observed behavioral effects in EA and NA were in fact related to MBT training and not a test-retest improvement, a control group (CG) had to perform SBT before and after a 20-min rest period. We found no statistically significant alterations in SBT performance (paired t-test, CG: 3.61 ± 3.00%, P = 0.260). This finding was confirmed by a good intrasession reliability (according to Larsson et al. 1999) for SBT [ICC (3,1) = 0.895]. Subsequent comparisons revealed CG improvement to be significantly lower compared with NA [uANOVA, main effect of group: F(1,23) = 13.622, P = 0.001, = 0.327] and EA [uANOVA, main effect of group: F(1,26) = 22.563, P = 0.000, = 0.465; Fig. 2B].
EMG and fNIRS Data TD1
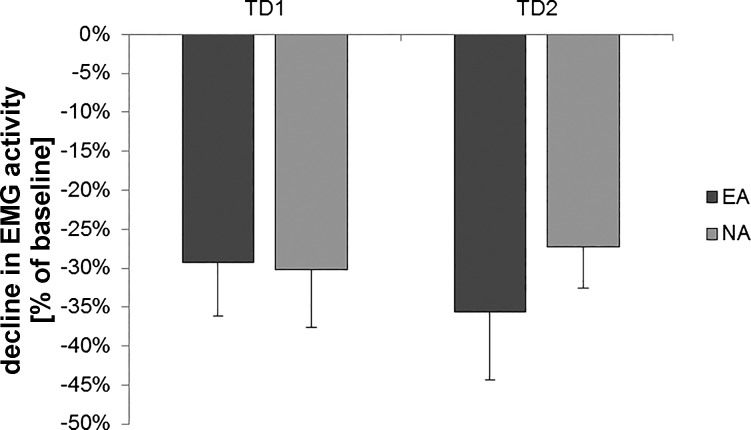
Mean EMG activity before and after MBT training during SBT (SBTpre vs. SBTpost) showed a significant decline in both groups (paired t-test, EA: −29.33 ± 6.80%, P = 0.001; NA: −30.15 ± 7.42%, P = 0.001), although there was no difference between groups [uANOVA, main effect of group: F(1,31) = 0.007, P = 0.935, = 0.000; Fig. 3]. A correlation analysis between the decline in EMG activity and SBT performance gains revealed no significant associations for either EA (r = −0.266, P = 0.285) and NA (r = 0.231, P = 0.407) or for all participants (r = −0.110, P = 0.541).
Fig. 3.
EMG results. Decline in tibialis anterior EMG activity during SBT performance on TD1 and TD2. Values are means ± SE (%baseline); dark gray bars represent endurance athletes (EA), and light gray bars represent nonathletes (NA).
Hemodynamic responses during SBT.
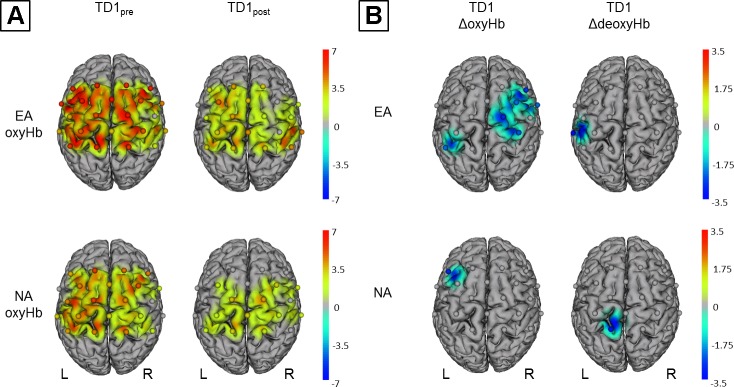
During SBT (TD1pre), we found significant activations in both EA and NA (Fig. 4A). EA revealed significantly higher oxyHb levels in every measured brain area, including premotor cortex (PMC), supplementary motor area (SMA), primary motor cortex (M1), somatosensory cortex (SSC), superior parietal lobe (SPL), and left and right inferior parietal lobe (IPL). For NA, oxyHb levels were significantly increased in PMC, SMA, M1, SSC, SPL, and left IPL. For oxyHb levels during TD1post (EA and NA), see Fig. 4A. No such activations were observed for deoxyHb in EA and NA for either TD1pre or TD1post.
Fig. 4.
Within-group comparisons for oxyHb and deoxyHb on TD1. Channels (centered between transmitters and detectors) are shown for each image (L, left hemisphere; R, right hemisphere). Decreases are illustrated in dark blue and increases in dark red; colors represent t-values. All images are thresholded at P < 0.05. A: topographic images show significant increased oxyHb levels during SBTpre and SBTpost on TD1. B: topographic images show significant decreases in both oxyHb and deoxyHb from TD1pre to TD1post (see text for details).
Baseline SBT comparison between EA and NA.
Comparing hemodynamic responses during SBTpre by means of fNIRS revealed significant lower deoxyHb in right M1 and PMC during task performance for EA compared with NA (Fig. 5A). On the other hand, no such changes could be observed for oxyHb.
Fig. 5.
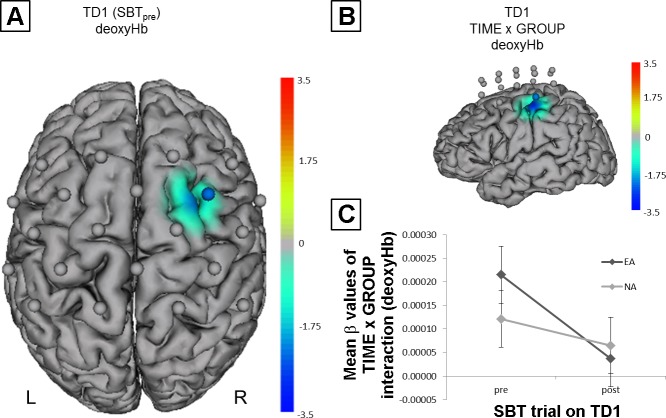
Initial fNIRS results (A) and time × group interaction (B and C). Channels (centered between transmitters and detectors) are shown for each image (L, left hemisphere; R, right hemisphere). Decreases are illustrated in dark blue and increases in dark red; colors represent t-values. All images are thresholded at P < 0.05. A: initial fNIRS results (SBTpre) on TD1 showing significant lower deoxyHb in right primary motor cortex (M1) and premotor cortex (PMC) during task performance for endurance athletes (EA) compared with nonathletes (NA). B: comparison of MBT-induced hemodynamic alterations during SBT performance (fNIRS SBTpre vs. SBTpost) revealed a significant time × group interaction for deoxyHb in left M1 and inferior parietal lobe (IPL). C: mean beta values (±SE) of time × group interaction (deoxyHb). The plot shows a significantly stronger decline of deoxyHb from TD1pre to TD1post in EA compared with NA.
MBT-training related changes in hemodynamic response.
Comparing MBT-induced hemodynamic alterations during SBT performance (fNIRS SBTpre vs. SBTpost) revealed a significant time × group interaction for deoxyHb in left M1 and IPL (Fig. 5, B and C), whereas no such changes were evident for oxyHb. Within-group comparisons revealed MBT-induced alterations for oxyHb and deoxyHb (Fig. 4B). For EA, we observed a decrease of oxyHb in right SPL, M1, and PMC as well as in left SPL. DeoxyHb decreased in left M1 and IPL. For NA, we observed a decrease in left PMC (oxyHb) and in left SPL (deoxyHb).
A correlation analysis between behavioral improvements in SBT and hemodynamic response alterations for all participants revealed a significant negative association within right M1/IPL for oxyHb (r = −0.550, P = 0.001, αcorr = 0.0023; Fig. 6, A and B) but not for deoxyHb (−0.158 ≤ r ≤ 0.267, P > 0.0023). Within-group correlations revealed no significant association for either oxyHb (EA: −0.582 ≤ r ≤ 0.261, P > 0.0023; NA: −0.555 ≤ r ≤ 0.089, P > 0.0023) or deoxyHb (EA: −0.116 ≤ r ≤ 0.410, P > 0.0023; NA: −0.560 ≤ r ≤ 0.224, P > 0.0023). Furthermore, correlations between EMG alterations and MBT training-induced changes in oxyHb/deoxyHb revealed no significant association.
Fig. 6.
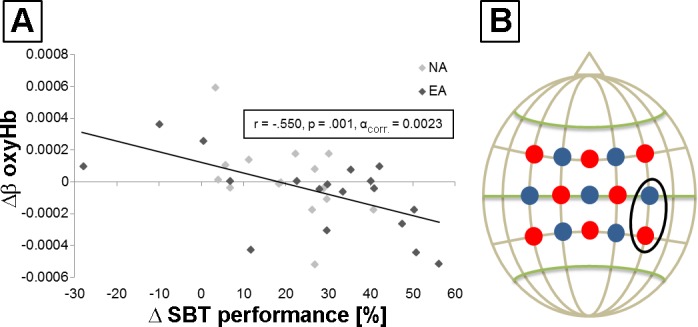
Correlation analysis between behavioral improvements in SBT and hemodynamic response alterations. A: scatter plot for all participants [dark gray diamonds, endurance athletes (EA); light gray diamonds, nonathletes (NA)] shows a significant negative association within right M1/IPL for oxyHb (r = −0.550, P = 0.001, αcorr = 0.0023). Δβ oxyHb and ΔSBT performance are the differences TD1post minus TD1pre. B: fNIRS configuration showing the channel of the significant negative association (black circle). Detectors are shown as blue dots and transmitters as red dots.
Behavioral Data TD2
Comparing SBT performance after 1 wk (TD1 SBTpost vs. TD2 SBTpre) revealed a significant decline in both groups (paired t-test, EA: −17.24 ± 7.18%, P = 0.028; NA: −8.65 ± 3.45%, P = 0.025), although there was no difference in retention rates between groups [uANOVA, main effect of group: F(1,31) = 1.023, P = 0.320, = 0.032; Fig. 2C]. On TD2, MBT training did not result in any significant performance gains for static and dynamic components [time × group interaction, stabilization: F(1.652,51.207) = 1.518, P = 0.230, = 0.047; translation: F(2,62) = 1.519, P = 0.227, = 0.047; rotation: F(2,62) = 0.172, P = 0.842, = 0.006; free movement: F(2,62) = 0.404, P = 0.670, = 0.013]. However, SBT performance before and after MBT training showed significant improvements within groups (paired t-test, EA: 10.38 ± 4.67%, P = 0.040; NA: 8.26 ± 3.74%, P = 0.045), although there was no difference in behavioral gains between groups [uANOVA, main effect of group: F(1,31) = 0.119, P = 0.733, = 0.004; Fig. 2C]. Furthermore, the comparison of TD1pre and TD2post revealed significant performance gains for EA (paired t-test, 28.73 ± 4.05%, P = 0.000) and NA (paired t-test 20.97 ± 4.25%, P = 0.0001), although there were no differences in SBT gains between groups [uANOVA, main effect of group: F(1,31) = 1.738, P = 0.197, = 0.053].
EMG and fNIRS Data TD2
Mean EMG activity before and after MBT training during SBT (SBTpre vs. SBTpost) showed a significant decline in both groups (EA: −35.66 ± 8.69%, P = 0.001; NA: −27.26 ± 5.26%, P = 0.000), although there was no difference between groups [uANOVA, main effect of group: F(1,31) = 0.620, P = 0.437, = 0.020; Fig. 3). Comparing MBT-induced hemodynamic alterations during SBT performance (fNIRS SBTpre vs. SBTpost) revealed no time × group interaction. The only significant change after MBT training was a decrease for oxyHb in left M1 and SMA within EA. For TD2, we also did not find any significant association between behavioral gains in SBT and hemodynamic response alterations.
DISCUSSION
The present study provides novel evidence that short-term multimodal balance task (MBT) training on 2 training days (TD1 and TD2) separated by 1 wk induced significant performance gains in a static balance task (SBT). Interestingly, endurance athletes (EA) showed initially superior performance in SBT compared with nonathletes (NA), although there was no difference in MBT training-induced behavioral gains between groups. The fact that EA showed superior performance in a static balance task is in line with a previous investigation by Raschner et al. (2008), who indicated that athletes show better postural control compared with NA. In that study, ski racers performed a variety of static and dynamic balance tasks and achieved significantly better scores then NA, which is clearly indicative of a training-induced manifestation of postural balance regulation. This assumption is supported by two investigations by Lephart et al. (1996) and Aydin et al. (2002). In these studies, it was shown that training-induced alterations in proprioceptive stability in knee and ankle joints of gymnasts could be responsible for improved postural control and balance regulation. Because EA usually integrate athletic and strength exercises into their training routine, it is reasonable to assume that this translates into superior performance in SBT compared with performance by NA. Further studies confirmed the relationship between strength training and postural stability (Anderson and Behm 2005; Bruhn et al. 2004). In general, athletes show superior balance performance compared with control participants (Hrysomallis 2011). In particular, it has been shown that endurance athletes such as swimmers show enhanced balance performance compared with NA (Davlin 2004). This may be due to the fact that athletes require strong balance skills to move efficiently/economically. Furthermore, some studies indicate that endurance training is indeed capable of evoking changes in postural control and balance capabilities (Buchner et al. 1997; Marques et al. 2017; Streckmann et al. 2014).
We further hypothesized that EA would show higher SBT performance gains, given that long-term expertise or training is assumed to facilitate learning skill (di Cagno et al. 2014; Faubert 2013; Verburgh et al. 2016). Even though the underlying neural mechanisms still remain elusive, several studies have indicated that experts show increased gray matter volume and enhanced functional brain processing (Alves et al. 2013; Hänggi et al. 2015; Vaquero et al. 2016; Verburgh et al. 2014; Wei et al. 2011; Wimshurst et al. 2016), which in turn might translate into superior learning capabilities.
Interestingly, we did not find differential training-induced SBT performance improvements between EA and NA. Although the underlying mechanisms cannot be explained with the present study design, it is tempting to speculate that EA might have reached a ceiling effect, because initial performance in SBT was already superior in EA compared with NA. However, this assumption is rather unlikely given that EA showed significant performance gains from TD1pre to TD2post. A possible reason for the lack of difference between EA and NA might be related to the outcome measure to capture performance gains, because SBT is a relatively unspecific task. As already pointed out, some studies have indicated that endurance training is capable of evoking changes in postural control and balance capabilities (Buchner et al. 1997; Marques et al. 2017; Streckmann et al. 2014). These studies showed improvements in dynamic components of balance (Marques et al. 2017) in both dynamic and static components. Additionally, static components of postural control might play only a subordinate role in individual training routines of EA. Hence, we cannot exclude the possibility that EA might show superior performance gains in other static and/or dynamic balance tasks.
MBT training resulted in differential hemodynamic response alterations in left M1 and IPL for EA compared with NA (decrease in deoxyHb, time × group interaction). These results indicate that EA showed a stronger MBT training-induced decrease in deoxyHb than NA. No such changes could be observed for oxyHb. Although we can only speculate about the lack of effects for oxyHb, the current concept for BOLD signal in fMRI suggests that decreased deoxyHb concentration is associated with higher BOLD signal (Buxton et al. 1998). Therefore, a stronger decrease in deoxyHb might be explained by reduced brain activity in EA compared with NA. Among other motor-related brain regions, IPL and M1 have recently been identified as brain regions that are important during observation and motor imagery of different balance tasks (Nachev et al. 2008; Slobounov et al. 2005; Taubert et al. 2010, 2011, 2016). Hence, those brain regions might also play an important role in balance regulation during SBT.
The observed MBT training-related decrease in neurovascular coupling is in line with previous fMRI studies that have shown a training-related decrease of motor-related brain regions even after a single training session (Dayan and Cohen 2011; Floyer-Lea and Matthews 2005; Karni et al. 1995; Sakai et al. 1999). Hence, it is reasonable to assume that a decrease in oxyHb and/or deoxyHb would point to more efficient neural processing as suggested by the “neural efficiency” hypothesis (Dunst et al. 2014).
It is also important to note that hemodynamic response alterations during SBT were observed for either oxyHb or deoxyHb. This fact is in line with the current discussion about reliability and validity of both chromophores with respect to the indirect measurement of cortical activity via fNIRS (Obrig and Villringer 2003; Schroeter et al. 2002; Strangman et al. 2002). For example, Plichta et al. (2006) concluded that deoxyHb is limited regarding reproducibility of the localization over time and over subjects, compared with oxyHb. In accordance, several fNIRS studies have described arbitrary behavior of deoxyHb (Maki et al. 1996; Miyai et al. 2001; Watanabe et al. 1996). Contrarily, other studies have drawn conclusions on active brain regions based on deoxyHb alterations (Durduran et al. 2004; Sato et al. 2007; Shibuya et al. 2008). Regarding the assessment of neural activity with fNIRS, there are studies that have used a simultaneous increase in oxyHb and decrease in deoxyHb as indicators of neural activity. Based on the aforementioned findings, the current literature is very inconsistent regarding the interpretation of alterations in oxyHb and deoxyHb.
Even though cortical processing during postural control and balance performance has mainly been investigated in prefrontal brain regions by means of fNIRS (Ferrari et al. 2014; Mahoney et al. 2016), a recent study pointed to the important role of motor-related brain regions such as SMA, M1, and S1 in balance control (Herold et al. 2017). Our results not only confirm these findings but also extend them by showing that MBT training is capable of inducing short-term reorganizational changes in brain areas such as SPL, M1, and PMC (decrease in oxyHb and/or deoxyHb).
Furthermore, we have demonstrated that EA show differential MBT training-induced changes in neurovascular coupling, compared with NA. More specifically, we observed a time × group interaction for deoxyHb in left M1 and IPL, indicating that EA show a stronger MBT training-induced reduction in deoxyHb. These results provide at least indirect evidence that athletes, compared with NA, seem to respond differently to a short MBT training intervention, although this in turn does not seem to translate into overt behavioral gains given that SBT performance changes did not differ between EA and NA.
Even though balance training-induced hemodynamic response alterations have also been described recently, there seem to be contradictory findings with respect to the directionality of the observed fNIRS alterations in oxyHb and/or deoxyHb. Whereas Hiyamizu et al. (2014) observed an increase in oxyHb within SMA after a single dynamic balance task training session, Ono et al. (2015) reported a training-related decrease of oxyHb in prefrontal brain areas such as the frontopolar cortex (FPC) after 20 h of dance video game training. Although the underlying mechanisms remain elusive, it is tempting to speculate that task specificity and difficulty, as well as the duration of balance training, might play a pivotal role in the directionality of the observed effects in neurovascular coupling.
Regarding EMG activity in tibialis anterior muscle during SBT, both groups showed significant declines from pre- to postmeasurement. These results suggest more efficient neuromuscular processing during the time course of motor skill learning. These findings are strengthened by previous EMG studies, which showed a decreased EMG activity during motor skill learning (Engelhorn 1983; Lohse et al. 2010; Taubert et al. 2010; Zachry et al. 2005). However, there are also studies indicating an increase in EMG activity (Darling and Cooke 1987; de Groot et al. 2003; Spencer and Thelen 1999) after skill learning.
The interpretation of the present MBT-induced cortical, neuromuscular, and behavioral alterations is limited for several reasons. Despite the fact that no power analysis was performed, the study provided effect size estimates for all statistical comparisons. Most of the significant findings show strong effect sizes even with such a small number of participants. Furthermore, because the intervention took place as MBT training between pre- and postmeasurement, this is not an intervention study in the classical sense. Within the intervention, the SBT task was not specifically trained, but rather MBT training with static (a modified SBT task) and dynamic components was used. Therefore, it was not a direct learning of a movement, but rather a direct motor transfer effect from the trained skill (MBT) to the skill required in the SBT task (primary outcome measure). Additionally, fNIRS findings must be interpreted with caution because, given our interoptode distance, the penetration depth of the infrared light is only ~3 cm. Hence, subcortical alterations obviously cannot be captured with this kind of imaging technique. Furthermore, fNIRS findings might be confounded by extracerebral alterations such as scalp blood flow (Tachtsidis and Scholkmann 2016). Finally, we assessed brain activity predominantly over motor-related brain regions and did not use a whole brain configuration. Hence, MBT-induced cortical alterations outside the human motor system might also contribute to the observed behavioral effects.
Conclusions
The present study provides novel evidence that EA show superior static balance performance (SBT) compared with NA. However, even though subsequent MBT-training resulted in significant SBT improvements, there was no difference in behavioral gains between groups. fNIRS recordings revealed a significantly stronger MBT training-induced functional decrease for deoxyHb in left primary motor cortex and inferior parietal lobe in EA compared with NA. Furthermore, we found a significant association between MBT training-induced hemodynamic response alterations for oxyHb and SBT improvements within right M1/IPL. In sum, our study indicates that endurance exercise may promote mechanisms of neuroplasticity in motor-related brain regions.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.S. and P.R. conceived and designed research; O.S. performed experiments; O.S., D.C., and R.K. analyzed data; O.S. and P.R. interpreted results of experiments; O.S. and D.C. prepared figures; O.S. and P.R. drafted manuscript; O.S., D.C., R.K., and P.R. edited and revised manuscript; O.S., D.C., R.K., and P.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ramona Menger for helping in the recruitment process.
REFERENCES
- Alves H, Voss MW, Boot WR, Deslandes A, Cossich V, Salles JI, Kramer AF. Perceptual-cognitive expertise in elite volleyball players. Front Psychol 4: 36, 2013. doi: 10.3389/fpsyg.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K, Behm DG. The impact of instability resistance training on balance and stability. Sports Med 35: 43–53, 2005. doi: 10.2165/00007256-200535010-00004. [DOI] [PubMed] [Google Scholar]
- Aydin T, Yildiz Y, Yildiz C, Atesalp S, Kalyon TA. Proprioception of the ankle: a comparison between female teenaged gymnasts and controls. Foot Ankle Int 23: 123–129, 2002. doi: 10.1177/107110070202300208. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 51: 459–465, 2003. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- Batula AM, Mark JA, Kim YE, Ayaz H. Comparison of Brain Activation during Motor Imagery and Motor Movement Using fNIRS. Comput Intell Neurosci 2017: 5491296, 2017. doi: 10.1155/2017/5491296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn S, Kullmann N, Gollhofer A. The effects of a sensorimotor training and a strength training on postural stabilisation, maximum isometric contraction and jump performance. Int J Sports Med 25: 56–60, 2004. doi: 10.1055/s-2003-45228. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Cress ME, de Lateur BJ, Esselman PC, Margherita AJ, Price R, Wagner EH. A comparison of the effects of three types of endurance training on balance and other fall risk factors in older adults. Aging (Milano) 9: 112–119, 1997. [DOI] [PubMed] [Google Scholar]
- Bullitt E, Rahman FN, Smith JK, Kim E, Zeng D, Katz LM, Marks BL. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. AJNR Am J Neuroradiol 30: 1857–1863, 2009. doi: 10.3174/ajnr.A1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med 39: 855–864, 1998. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61: 1166–1170, 2006. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Cope M, Delpy DT. System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput 26: 289–294, 1988. doi: 10.1007/BF02447083. [DOI] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol 14: 1124–1134, 2004. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci 3: 1, 2010. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling WG, Cooke WG. Movement related EMGs become more variable during learning of fast accurate movements. J Mot Behav 19: 311–331, 1987. doi: 10.1080/00222895.1987.10735415. [DOI] [PubMed] [Google Scholar]
- Davlin CD. Dynamic balance in high level athletes. Percept Mot Skills 98, Suppl 3: 1171–1176, 2004. doi: 10.2466/pms.98.3c.1171-1176. [DOI] [PubMed] [Google Scholar]
- Day JT, Lichtwark GA, Cresswell AG. Tibialis anterior muscle fascicle dynamics adequately represent postural sway during standing balance. J Appl Physiol (1985) 115: 1742–1750, 2013. doi: 10.1152/japplphysiol.00517.2013. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454, 2011. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot S, Veeger HE, Hollander AP, van der Woude LH. Short-term adaptations in co-ordination during the initial phase of learning manual wheelchair propulsion. J Electromyogr Kinesiol 13: 217–228, 2003. doi: 10.1016/S1050-6411(03)00018-X. [DOI] [PubMed] [Google Scholar]
- di Cagno A, Battaglia C, Fiorilli G, Piazza M, Giombini A, Fagnani F, Borrione P, Calcagno G, Pigozzi F. Motor learning as young gymnast’s talent indicator. J Sports Sci Med 13: 767–773, 2014. [PMC free article] [PubMed] [Google Scholar]
- Di Giulio I, Maganaris CN, Baltzopoulos V, Loram ID. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol 587: 2399–2416, 2009. doi: 10.1113/jphysiol.2009.168690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature 427: 311–312, 2004. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Driemeyer J, Boyke J, Gaser C, Büchel C, May A. Changes in gray matter induced by learning–revisited. PLoS One 3: e2669, 2008. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunst B, Benedek M, Jauk E, Bergner S, Koschutnig K, Sommer M, Ischebeck A, Spinath B, Arendasy M, Bühner M, Freudenthaler H, Neubauer AC. Neural efficiency as a function of task demands. Intelligence 42: 22–30, 2014. doi: 10.1016/j.intell.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durduran T, Yu G, Burnett MG, Detre JA, Greenberg JH, Wang J, Zhou C, Yodh AG. Diffuse optical measurement of blood flow, blood oxygenation, and metabolism in a human brain during sensorimotor cortex activation. Opt Lett 29: 1766–1768, 2004. doi: 10.1364/OL.29.001766. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269, 2003. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32: 570–582, 2006. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36: 511–521, 2007. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335, 2005. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Engelhorn R. Agonist and antagonist muscle EMG activity pattern changes with skill acquisition. Res Q Exerc Sport 54: 315–323, 1983. doi: 10.1080/02701367.1983.10605315. [DOI] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108: 3017–3022, 2011. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Weinstein AM, Sutton BP, Prakash RS, Voss MW, Chaddock L, Szabo AN, Mailey EL, White SM, Wojcicki TR, McAuley E, Kramer AF. Beyond vascularization: aerobic fitness is associated with N-acetylaspartate and working memory. Brain Behav 2: 32–41, 2012. doi: 10.1002/brb3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faubert J. Professional athletes have extraordinary skills for rapidly learning complex and neutral dynamic visual scenes. Sci Rep 3: 1154, 2013. doi: 10.1038/srep01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M, Bisconti S, Spezialetti M, Basso Moro S, Di Palo C, Placidi G, Quaresima V. Prefrontal cortex activated bilaterally by a tilt board balance task: a functional near-infrared spectroscopy study in a semi-immersive virtual reality environment. Brain Topogr 27: 353–365, 2014. doi: 10.1007/s10548-013-0320-z. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol 94: 512–518, 2005. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 148: 893–906, 2007. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryga M, Taubert M, Dukart J, Vollmann H, Conde V, Sehm B, Villringer A, Ragert P. Bidirectional gray matter changes after complex motor skill learning. Front Syst Neurosci 6: 37, 2012. doi: 10.3389/fnsys.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Li A, Yu L. “Neural efficiency” of athletes’ brain during visuo-spatial task: an fMRI study on table tennis players. Front Behav Neurosci 11: 72, 2017. doi: 10.3389/fnbeh.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci 3: 533–535, 2000. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hänggi J, Langer N, Lutz K, Birrer K, Mérillat S, Jäncke L. Structural brain correlates associated with professional handball playing. PLoS One 10: e0124222, 2015. doi: 10.1371/journal.pone.0124222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain 120: 123–140, 1997. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Hermens HJ. European Recommendations for Surface ElectroMyoGraphy. Results of the SENIAM Project. Enschede, The Netherlands: Roessingh Research and Development, 1999. [Google Scholar]
- Herold F, Orlowski K, Börmel S, Müller NG. Cortical activation during balancing on a balance board. Hum Mov Sci 51: 51–58, 2017. doi: 10.1016/j.humov.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Hiyamizu M, Maeoka H, Matsuo A, Morioka S. Effects of self-action observation on standing balance learning: a change of brain activity detected using functional near-infrared spectroscopy. NeuroRehabilitation 35: 579–585, 2014. doi: 10.3233/NRE-141153. [DOI] [PubMed] [Google Scholar]
- Hrysomallis C. Balance ability and athletic performance. Sports Med 41: 221–232, 2011. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, von Cramon DY. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res 125: 417–425, 1999. doi: 10.1007/s002210050698. [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Diamond SG, Franceschini MA, Boas DA. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl Opt 48: D280–D298, 2009. doi: 10.1364/AO.48.00D280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäncke L, Koeneke S, Hoppe A, Rominger C, Hänggi J. The architecture of the golfer’s brain. PLoS One 4: e4785, 2009. doi: 10.1371/journal.pone.0004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Lopez-Alonso V, Lago A, Brittain JS, Fernandez-Del-Olmo M, Gomez-Garre P, Mir P, Jenkinson N, Cheeran B, Brown P. The effect of BDNF val66met polymorphism on visuomotor adaptation. Exp Brain Res 223: 43–50, 2012. doi: 10.1007/s00221-012-3239-9. [DOI] [PubMed] [Google Scholar]
- Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage 34: 1600–1611, 2007. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kaminski E, Steele CJ, Hoff M, Gundlach C, Rjosk V, Sehm B, Villringer A, Ragert P. Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin Neurophysiol 127: 2455–2462, 2016. doi: 10.1016/j.clinph.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Kantak SS, Mummidisetty CK, Stinear JW. Primary motor and premotor cortex in implicit sequence learning–evidence for competition between implicit and explicit human motor memory systems. Eur J Neurosci 36: 2710–2715, 2012. doi: 10.1111/j.1460-9568.2012.08175.x. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158, 1995. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol 80: 3321–3325, 1998. [DOI] [PubMed] [Google Scholar]
- Koenraadt KL, Duysens J, Smeenk M, Keijsers NL. Multi-channel NIRS of the primary motor cortex to discriminate hand from foot activity. J Neural Eng 9: 046010, 2012. doi: 10.1088/1741-2560/9/4/046010. [DOI] [PubMed] [Google Scholar]
- Kumpulainen S, Avela J, Gruber M, Bergmann J, Voigt M, Linnamo V, Mrachacz-Kersting N. Differential modulation of motor cortex plasticity in skill- and endurance-trained athletes. Eur J Appl Physiol 115: 1107–1115, 2015. doi: 10.1007/s00421-014-3092-6. [DOI] [PubMed] [Google Scholar]
- Larsson B, Månsson B, Karlberg C, Syvertsson P, Elert J, Gerdle B. Reproducibility of surface EMG variables and peak torque during three sets of ten dynamic contractions. J Electromyogr Kinesiol 9: 351–357, 1999. doi: 10.1016/S1050-6411(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture 18: 101–108, 2003. doi: 10.1016/S0966-6362(02)00200-X. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele P-F, Pélégrini-Issac M, Waechter T, Ugurbil K, Doyon J. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 102: 12566–12571, 2005. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart SM, Giraldo JL, Borsa PA, Fu FH. Knee joint proprioception: a comparison between female intercollegiate gymnasts and controls. Knee Surg Sports Traumatol Arthrosc 4: 121–124, 1996. doi: 10.1007/BF01477265. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron 30: 593–607, 2001. doi: 10.1016/S0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Lohse KR, Sherwood DE, Healy AF. How changing the focus of attention affects performance, kinematics, and electromyography in dart throwing. Hum Mov Sci 29: 542–555, 2010. doi: 10.1016/j.humov.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Mahoney JR, Holtzer R, Izzetoglu M, Zemon V, Verghese J, Allali G. The role of prefrontal cortex during postural control in Parkinsonian syndromes a functional near-infrared spectroscopy study. Brain Res 1633: 126–138, 2016. doi: 10.1016/j.brainres.2015.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki A, Yamashita Y, Watanabe E, Koizumi H. Visualizing human motor activity by using non-invasive optical topography. Front Med Biol Eng 7: 285–297, 1996. [PubMed] [Google Scholar]
- Marques EA, Figueiredo P, Harris TB, Wanderley FA, Carvalho J. Are resistance and aerobic exercise training equally effective at improving knee muscle strength and balance in older women? Arch Gerontol Geriatr 68: 106–112, 2017. doi: 10.1016/j.archger.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégrot F, Bardy BG. Changes in phase space during learning an unstable balance. Neurosci Lett 402: 17–21, 2006. doi: 10.1016/j.neulet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Meier J, Topka MS, Hänggi J. Differences in cortical representation and structural connectivity of hands and feet between professional handball players and ballet dancers. Neural Plast 2016: 6817397, 2016. doi: 10.1155/2016/6817397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J, Shevlin M. Applying Regression & Correlation. A Guide for Students and Researchers. London: Sage, 2001. [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res 146: 122–126, 2002. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14: 1186–1192, 2001. doi: 10.1006/nimg.2001.0905. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856–869, 2008. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: 619–626, 2003. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Obrig H, Villringer A. Beyond the visible–imaging the human brain with light. J Cereb Blood Flow Metab 23: 1–18, 2003. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Ono Y, Noah JA, Zhang X, Nomoto Y, Suzuki T, Shimada S, Tachibana A, Bronner S, Hirsch J. Motor learning and modulation of prefrontal cortex: an fNIRS assessment. J Neural Eng 12: 066004, 2015. doi: 10.1088/1741-2560/12/6/066004. [DOI] [PubMed] [Google Scholar]
- Park IS, Lee KJ, Han JW, Lee NJ, Lee WT, Park KA, Rhyu IJ. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum 8: 334–339, 2009. doi: 10.1007/s12311-009-0100-1. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995. [DOI] [PubMed] [Google Scholar]
- Pereira VJ, Linden KG, Weinberg HS. Evaluation of UV irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Res 41: 4413–4423, 2007. doi: 10.1016/j.watres.2007.05.056. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem 74: 27–55, 2000. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Herrmann MJ, Ehlis AC, Baehne CG, Richter MM, Fallgatter AJ. Event-related visual versus blocked motor task: detection of specific cortical activation patterns with functional near-infrared spectroscopy. Neuropsychobiology 53: 77–82, 2006. doi: 10.1159/000091723. [DOI] [PubMed] [Google Scholar]
- Ploughman M. Exercise is brain food: the effects of physical activity on cognitive function. Dev Neurorehabil 11: 236–240, 2008. doi: 10.1080/17518420801997007. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Attwood Z, White N, Doré JJ, Corbett D. Endurance exercise facilitates relearning of forelimb motor skill after focal ischemia. Eur J Neurosci 25: 3453–3460, 2007. doi: 10.1111/j.1460-9568.2007.05591.x. [DOI] [PubMed] [Google Scholar]
- Raichlen DA, Bharadwaj PK, Fitzhugh MC, Haws KA, Torre GA, Trouard TP, Alexander GE. Differences in resting state functional connectivity between young adult endurance athletes and healthy controls. Front Hum Neurosci 10: 610, 2016. doi: 10.3389/fnhum.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschner C, Lembert S, Platzer HP, Patterson C, Hilden T, Lutz M. [S3-Check–evaluation and generation of normal values of a test for balance ability and postural stability]. Sportverletz Sportschaden 22: 100–105, 2008. doi: 10.1055/s-2008-1027239. [DOI] [PubMed] [Google Scholar]
- Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol 24: 590–596, 2011. doi: 10.1097/WCO.0b013e32834c3db0. [DOI] [PubMed] [Google Scholar]
- Roig M, Skriver K, Lundbye-Jensen J, Kiens B, Nielsen JB. A single bout of exercise improves motor memory. PLoS One 7: e44594, 2012. doi: 10.1371/journal.pone.0044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Pütz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci 19: RC1, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Ito M, Suto T, Kameyama M, Suda M, Yamagishi Y, Ohshima A, Uehara T, Fukuda M, Mikuni M. Time courses of brain activation and their implications for function: a multichannel near-infrared spectroscopy study during finger tapping. Neurosci Res 58: 297–304, 2007. doi: 10.1016/j.neures.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Saucedo Marquez CM, Zhang X, Swinnen SP, Meesen R, Wenderoth N. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci 7: 333, 2013. doi: 10.3389/fnhum.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer T, Schulte S, Hollmann W, Bloch W, Strüder HK. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res 41: 250–254, 2009. doi: 10.1055/s-0028-1093322. [DOI] [PubMed] [Google Scholar]
- Schlaffke L, Lissek S, Lenz M, Brüne M, Juckel G, Hinrichs T, Platen P, Tegenthoff M, Schmidt-Wilcke T. Sports and brain morphology–a voxel-based morphometry study with endurance athletes and martial artists. Neuroscience 259: 35–42, 2014. doi: 10.1016/j.neuroscience.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, Wolf M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85: 6–27, 2014. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci 12: 1370–1371, 2009. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Zysset S, Kupka T, Kruggel F, Yves von Cramon D. Near-infrared spectroscopy can detect brain activity during a color-word matching Stroop task in an event-related design. Hum Brain Mapp 17: 61–71, 2002. doi: 10.1002/hbm.10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol 298: R372–R377, 2010. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Sadamoto T, Sato K, Moriyama M, Iwadate M. Quantification of delayed oxygenation in ipsilateral primary motor cortex compared with contralateral side during a unimanual dominant-hand motor task using near-infrared spectroscopy. Brain Res 1210: 142–147, 2008. doi: 10.1016/j.brainres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Slobounov S, Hallett M, Stanhope S, Shibasaki H. Role of cerebral cortex in human postural control: an EEG study. Clin Neurophysiol 116: 315–323, 2005. doi: 10.1016/j.clinph.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Thelen E. A multimuscle state analysis of adult motor learning. Exp Brain Res 128: 505–516, 1999. doi: 10.1007/s002210050873. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 49: 800–804, 2011. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statton MA, Encarnacion M, Celnik P, Bastian AJ. A single bout of moderate aerobic exercise improves motor skill acquisition. PLoS One 10: e0141393, 2015. doi: 10.1371/journal.pone.0141393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol 92: 66–72, 2004. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17: 719–731, 2002. doi: 10.1006/nimg.2002.1227. [DOI] [PubMed] [Google Scholar]
- Streckmann F, Kneis S, Leifert JA, Baumann FT, Kleber M, Ihorst G, Herich L, Grussinger V, Gollhofer A, Bertz H. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol 25: 493–499, 2014. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- Sukal-Moulton T, de Campos AC, Stanley CJ, Damiano DL. Functional near infrared spectroscopy of the sensory and motor brain regions with simultaneous kinematic and EMG monitoring during motor tasks. J Vis Exp (94): e52391, 2014. doi: 10.3791/52391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachtsidis I, Scholkmann F. False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics 3: 031405, 2016. doi: 10.1117/1.NPh.3.3.031405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett 431: 62–65, 2008. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30: 11670–11677, 2010. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P. Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage 57: 1492–1498, 2011. doi: 10.1016/j.neuroimage.2011.05.078. [DOI] [PubMed] [Google Scholar]
- Taubert M, Mehnert J, Pleger B, Villringer A. Rapid and specific gray matter changes in M1 induced by balance training. Neuroimage 133: 399–407, 2016. doi: 10.1016/j.neuroimage.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Taubert M, Villringer A, Ragert P. Learning-related gray and white matter changes in humans: an update. Neuroscientist 18: 320–325, 2012. doi: 10.1177/1073858411419048. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78: 553–564, 2002. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- van Boxtel MP, Paas FG, Houx PJ, Adam JJ, Teeken JC, Jolles J. Aerobic capacity and cognitive performance in a cross-sectional aging study. Med Sci Sports Exerc 29: 1357–1365, 1997. doi: 10.1097/00005768-199710000-00013. [DOI] [PubMed] [Google Scholar]
- Vaquero L, Hartmann K, Ripollés P, Rojo N, Sierpowska J, François C, Càmara E, van Vugt FT, Mohammadi B, Samii A, Münte TF, Rodríguez-Fornells A, Altenmüller E. Structural neuroplasticity in expert pianists depends on the age of musical training onset. Neuroimage 126: 106–119, 2016. doi: 10.1016/j.neuroimage.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Verburgh L, Scherder EJA, van Lange PA, Oosterlaan J. Executive functioning in highly talented soccer players. PLoS One 9: e91254, 2014. doi: 10.1371/journal.pone.0091254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburgh L, Scherder EJA, van Lange PAM, Oosterlaan J. The key to success in elite athletes? Explicit and implicit motor learning in youth elite and non-elite soccer players. J Sports Sci 34: 1782–1790, 2016. doi: 10.1080/02640414.2015.1137344. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, Alves H, Heo S, Szabo AN, White SM, Wójcicki TR, Mailey EL, Gothe N, Olson EA, McAuley E, Kramer AF. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci 2: 32, 2010. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yan J, Wen J, Yu T, Li X. An intracranial electroencephalography (iEEG) brain function mapping tool with an application to epilepsy surgery evaluation. Front Neuroinform 10: 15, 2016. doi: 10.3389/fninf.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E, Yamashita Y, Maki A, Ito Y, Koizumi H. Non-invasive functional mapping with multi-channel near infra-red spectroscopic topography in humans. Neurosci Lett 205: 41–44, 1996. doi: 10.1016/0304-3940(96)12376-4. [DOI] [PubMed] [Google Scholar]
- Wei G, Zhang Y, Jiang T, Luo J. Increased cortical thickness in sports experts: a comparison of diving players with the controls. PLoS One 6: e17112, 2011. doi: 10.1371/journal.pone.0017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimshurst ZL, Sowden PT, Wright M. Expert-novice differences in brain function of field hockey players. Neuroscience 315: 31–44, 2016. doi: 10.1016/j.neuroscience.2015.11.064. [DOI] [PubMed] [Google Scholar]
- Wood KN, Nikolov R, Shoemaker JK. Impact of long-term endurance training vs. guideline-based physical activity on brain structure in healthy aging. Front Aging Neurosci 8: 155, 2016. doi: 10.3389/fnagi.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JC, Tak S, Jang KE, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage 44: 428–447, 2009. doi: 10.1016/j.neuroimage.2008.08.036. [DOI] [PubMed] [Google Scholar]
- Zachry T, Wulf G, Mercer J, Bezodis N. Increased movement accuracy and reduced EMG activity as the result of adopting an external focus of attention. Brain Res Bull 67: 304–309, 2005. doi: 10.1016/j.brainresbull.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dunlap M, Forman E, Grondin R, Gask DM. Treadmill exercise improves motor and working memory functions in aged rhesus monkeys. Program No. 199. Neuroscience 2005 Abstracts. Washington, DC: Society for Neuroscience, 2005. [Google Scholar]
- Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 59, Suppl 7: 119–132, 2008. [PubMed] [Google Scholar]