Abstract
Many central pattern generator (CPG)-mediated behaviors are episodic, meaning that they are not continuously ongoing; instead, there are pauses between bouts of activity. This raises an interesting possibility, that the neural networks that mediate these behaviors are not operating under “steady-state” conditions; i.e., there could be dynamic changes in motor activity as it stops and starts. Research in the feeding system of the mollusk Aplysia californica has demonstrated that this can be the case. After a pause, initial food grasping responses are relatively weak. With repetition, however, responses strengthen. In this review we describe experiments that have characterized cellular/molecular mechanisms that produce these changes in motor activity. In particular, we focus on cumulative effects of modulatory neuropeptides. Furthermore, we relate Aplysia research to work in other systems and species, and develop a hypothesis that postulates that changes in response magnitude are a reflection of an efficient feeding strategy.
Keywords: invertebrate, neuromodulation, neuropeptides, feeding microstructure
some central pattern generators (CPGs) are active all the time. This is particularly likely to be true when it is essential that the mediated behavior be continuously ongoing. For example, this is the case for the heartbeat in the leech (Calabrese et al. 2016; Kristan et al. 2005; Lamb and Calabrese 2011). Other CPG-mediated behaviors are episodic, meaning that bouts of rhythmic activity are separated by periods of quiescence. Most studies of the neural basis of episodic behavior have focused on the decision-making process that turns the CPG on or off (e.g., Getting and Dekin 1985; Kristan et al. 2005; Staras et al. 2003).
Because CPGs that mediate episodic behaviors are not active all the time, they do not necessarily operate under “steady-state” conditions. A less commonly investigated question is whether the process of starting and stopping has inherent consequences of its own. One experimentally advantageous system where this issue has been addressed is the feeding system of the mollusk Aplysia (Dacks et al. 2012; Friedman and Weiss 2010; Friedman et al. 2015; Proekt et al. 2004, 2007). In this review we describe this research and its relevance to work in other systems.
Feeding in Aplysia
To begin, we provide a brief overview of feeding behavior in Aplysia and the network that mediates it. These sections are not meant to be comprehensive because their primary purpose is simply to provide sufficient background information for subsequent sections of the text. The following is a list of more extensive reviews of the feeding network (Cropper et al. 2004; Elliott and Susswein 2002; Jing et al. 2017). Feeding in Aplysia is similar to behaviors in many species in that it can be divided into appetitive (anticipatory) and consummatory phases (Craig 1917, 1918; Kupfermann 1974a, 1974b). Appetitive behaviors include locomotion and head movements (Bablanian et al. 1987; Nagahama et al. 1993, 1994; Teyke et al. 1990). Consummatory behaviors include “bites,” “bite-swallows,” and “swallows” (Kupfermann 1974b). The research described in this review focuses on consummatory behaviors.
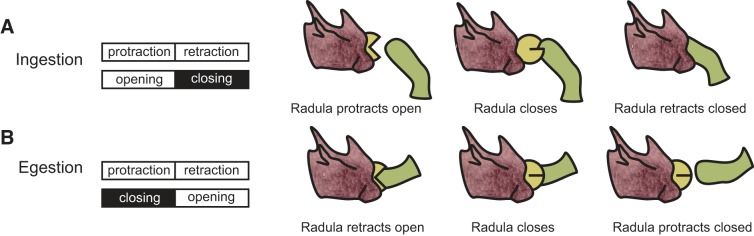
To ingest food, Aplysia (and a number of other mollusks) utilize a toothed chitinous structure, known as the radula (Elliott and Susswein 2002; Howells 1942; Wentzell et al. 2009). The radula can move forward or backward in the buccal cavity; i.e., it can protract or retract. Additionally, in Aplysia the radula has a longitudinal fold in its center that acts as a hinge and allows its two halves to open and close (Howells 1942; Kupfermann 1974b). During ingestive behaviors, the radula protracts open and retracts closed (Fig. 1A). This combination of movements will pull food into the buccal cavity (Church and Lloyd 1994; Morton and Chiel 1993a, 1993b).
Fig. 1.
Radula movements during ingestive (A) and egestive (B) feeding in Aplysia. In both types of behaviors radula protraction precedes radula retraction. Behaviors differ in the relative timing of radula opening and closing. Radula closing is the power phase of the behavior. During ingestion, the radula protracts open and retracts closed (A). In contrast, during egestion, the radula protracts closed and retracts open (B).
In addition to ingestion, Aplysia are also capable of egestion (Kupfermann 1974b) (Fig. 1B). Egestion can be triggered when an object cannot be swallowed (Kupfermann 1974b; Morton and Chiel 1993a, 1993b). During egestive behaviors, the radula protracts closed (instead of open) and retracts open (instead of closed). This combination of radula movements will push food (or an object) out of the buccal cavity.
Feeding in Aplysia is Mediated by a Multifunctional CPG
Organized oscillatory activity of the feeding circuitry in Aplysia can be triggered in the absence of the periphery, indicating the involvement of a CPG (Church and Lloyd 1994; Plummer and Kirk 1990; Rosen et al. 1991; Susswein and Byrne 1988). In this regard, Aplysia is similar to other species. Thus consummatory feeding movements are known to be CPG mediated in mammals (Doty et al. 1967; Lund 2011; Wiesenfeld et al. 1977) and in other mollusks and invertebrates (Benjamin 2012; Benjamin and Rose 1979; Brierley et al. 1997; Elliott and Benjamin 1985; Elliott and Susswein 2002; Kemenes et al. 2001; Marder and Calabrese 1996; Schoofs et al. 2010; Staras et al. 1998).
The Aplysia feeding CPG is like a number of other CPGs in that it is primarily, but not exclusively, composed of interneurons (Dembrow et al. 2003; Hurwitz et al. 1994, 1997; Hurwitz and Susswein 1996; Jing et al. 2004, 2011; Jing and Weiss 2001, 2002; Kabotyanski et al. 1998; Plummer and Kirk 1990; Sasaki et al. 2009, 2013, 2007; Susswein and Byrne 1988). Furthermore, it has a modular organization (Jing et al. 2004; Jing and Weiss 2001, 2002). Modules are often defined as groups of neurons that create distinct, coordinated body movements (Briggman and Kristan 2008). Importantly, in Aplysia, one set of neurons controls radula protraction/retraction movements, and a second set controls radula opening/closing movements (Jing and Weiss 2002).
The feeding network is multifunctional in that it can generate more than one type of motor program (e.g., ingestive and egestive; Cropper et al. 2004). Although multifunctionality has been most clearly demonstrated in studies of motor systems (e.g., Getting 1989; Kristan et al. 1988; Marder and Calabrese 1996), more recently it has become apparent that it is more universal. For example, reconfiguration of frontoparietal and frontotemporal networks has been observed during working memory tasks in humans (Braun et al. 2015).
Feeding in Aplysia is Episodic
Although CPGs that mediate certain ingestive behaviors can be continuously active (e.g., Selverston et al. 1976), most CPGs that control food intake have pauses between periods of activity. In some species the term “microstructure” has been used to describe the patterning of ingestive responses (Fig. 2) (Bowdan 1988; Davis and Smith 1992; Itskov et al. 2014; Ma et al. 2016; Mendez et al. 2016; Stellar and Shrager 1985). For example, when Drosophila feed, what are known as “sips” are organized into “bursts” (Itskov et al. 2014). There are multiple bursts per feeding session, and interburst intervals are significantly longer than intervals between sips. In Aplysia, investigators have not explicitly used the term microstructure but have described bouts of feeding responses (Susswein et al. 1983, 1984).
Fig. 2.
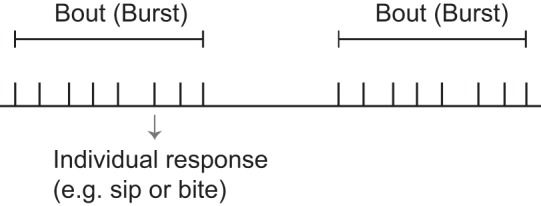
Schematic representation of the microstructure of an episodic behavior such as feeding. Each tick represents an individual response (e.g., in the context of feeding a bite or a sip). Responses are grouped into bouts. Interbout intervals are significantly longer than interresponse intervals.
Repetition Priming in Feeding Behavior
A number of factors can determine or influence feeding microstructure. Namely, there is often an effect of the motivational state of the animal, i.e., how hungry it is (Davis et al. 1999; Itskov et al. 2014; Yapici et al. 2016). Second, characteristics of the ingested food can be important (Davis and Smith 1992; Hurwitz and Susswein 1992; Susswein et al. 1986; Yapici et al. 2016). In a number of situations, changes in microstructure reflect a change in the total number of feeding responses, which produces an overall change in food intake. For example, the rate of food consumption generally decreases as animals satiate (Davis et al. 1999, 2001; Itskov et al. 2014; Kupfermann 1974b; Kupfermann and Carew 1974; Stellar and Shrager 1985; Susswein and Kupfermann 1975; Susswein et al. 1976). Microstructure is often characterized with the use of techniques that determine whether or not a response occurred. These data are used to compute response frequency (Davis and Smith 1992; Itskov et al. 2014; Wong et al. 2009).
In Aplysia, investigators have also quantified changes in response magnitude (Cullins et al. 2015; Susswein et al. 1976; Weiss et al. 1986; Ye et al. 2006). Experiments utilizing these techniques have addressed the question of whether or not the efficacy of behavior is impacted by how it is patterned. That this is an interesting issue is suggested by the fact that variables that have the same general effect on overall response number do not necessarily have identical effects on feeding microstructure. For example, sham feeding in rats can increase the number of licks by increasing the number of clusters (without increasing cluster size). In contrast, within a certain range, increases in sucrose concentration increase cluster size (Davis and Smith 1992). This suggests that particular patterns of activity may be appropriate under different conditions. An interesting question without an obvious answer is, why would this be the case?
Experiments in Aplysia have studied the effect of patterning on the magnitude of radula opening and closing (i.e., food grasping behaviors) (Susswein et al. 1976; Weiss et al. 1986; Zhurov et al. 2005). This work has demonstrated that when intact animals begin to feed, the initial bite is relatively weak and ineffective. With successive responses, however, there are progressive increases in bite strength. Similar changes have been noted when egestive responses have been monitored in semi-intact preparations (Zhurov et al. 2005). This indicates that there are changes in the efficacy of feeding in Aplysia as it stops and starts. Feeding responses are initially weak but are enhanced by repetition.
The progressive changes in feeding responses observed in Aplysia have been referred to as repetition priming (Cropper et al. 2014; Dacks et al. 2012; Friedman and Weiss 2010; Friedman et al. 2015; Proekt et al. 2004). Repetition priming is a tractable form of implicit memory that has been extensively documented in other species, including humans (Fowler et al. 1985; Kristjánsson and Campana 2010; Tresch et al. 1995; Yashar et al. 2013). In some of these situations the priming is not necessarily mediated by changes in motor or premotor pathways. In other cases, however, it clearly is (Tresch et al. 1995; Yashar et al. 2013). Thus repetition priming is ubiquitous and is becoming increasingly popular as a phenomenon that can be used to study implicit memory. Nevertheless, outside of the Aplysia research, there have been few efforts to characterize its underlying cellular/molecular mechanisms in a tractable model system.
How Is Repetition Priming in the Feeding Network Mediated at the Cellular/Molecular Level?
Episodic behavior is not unusual. In fact, it is probably more common than continuous activity. Consequently, mechanisms that induce dynamic changes in feeding in Aplysia potentially operate in a number of other systems. Experiments that have studied plasticity in feeding have taken advantage of the fact that motor activity can be induced in experimentally advantageous, “reduced” preparations. For example, feeding in molluscs can be triggered in preparations in which the periphery remains attached (e.g., in whole feeding head preparations) or in more reduced semi-intact preparations (Evans and Cropper 1998; Jing and Weiss 2005; Kabotyanski et al. 2000; McClellan 1982; McManus et al. 2012; Weiss et al. 1986; Willows 1980). Additionally, feeding motor programs can be triggered in the isolated nervous system (Church and Lloyd 1994; Morgan et al. 2002; Rosen et al. 1991; Sánchez and Kirk 2001). Thus motor activity can be triggered in preparations that can be used to characterize cellular/molecular mechanisms responsible for dynamic changes in motor output.
In Aplysia (and many other model systems), motor activity is initiated in reduced preparations by stimulating neurons that provide input to the behavior-mediating CPG. These neurons can be sensory (Beenhakker et al. 2004; Beenhakker and Nusbaum 2004; Blitz et al. 2004; Combes et al. 1999), command (Frost and Katz 1996; Kupfermann and Weiss 1978), or command-like neurons (Church and Lloyd 1994; Kemenes et al. 2001; Rosen et al. 1991; Sánchez and Kirk 2001). In Aplysia, ingestive activity is most commonly triggered by stimulating a command-like cell, cerebral buccal interneuron 2 (CBI-2; Fig. 3) (Church and Lloyd 1994; Morgan et al. 2002; Rosen et al. 1991; Sánchez and Kirk 2001). CBI-2 is activated by food contact, and with steady-state stimulation it triggers biting-like movements in semi-intact preparations (Jing and Weiss 2005; Rosen et al. 1991). Egestive activity is most commonly triggered by stimulating the esophageal nerve (EN; Fig. 3) (Dacks and Weiss 2013; Friedman et al. 2009, 2015; Proekt et al. 2004, 2007; Siniscalchi et al. 2016; Zhurov et al. 2005). The EN contains the processes of sensory neurons that innervate the esophagus (Kuslansky et al. 1987). In intact animals, rejection responses are triggered when an object that cannot be ingested makes esophageal contact (Kupfermann 1974b). Electrical stimulation of the EN in semi-intact preparations produces rejection movements (Chiel et al. 1986).
Fig. 3.
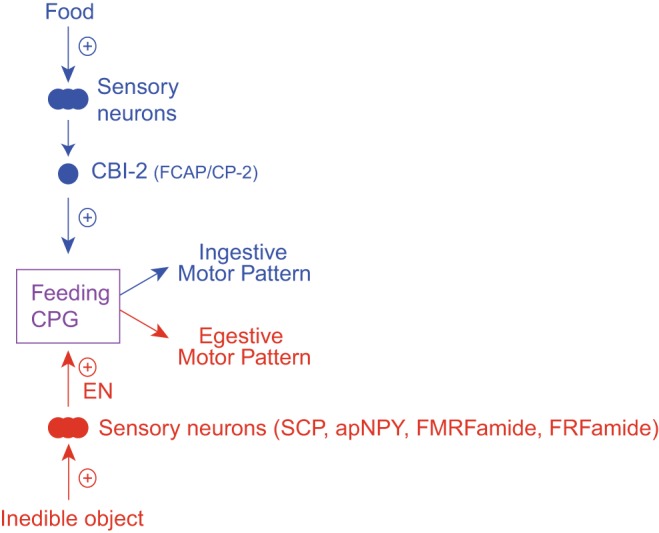
Organization of the feeding circuitry in Aplysia. Ingestive motor activity is triggered when food activates sensory neurons that excite cerebral buccal interneurons (CBIs) such as CBI-2. CBI-2 is a cholinergic neuron that contains feeding circuit-activating peptide (FCAP) and cerebral peptide-2 (CP-2) (Koh et al. 2003; Morgan et al. 2000; Sweedler et al. 2002). CBI-2 activates the feeding central pattern generator (CPG), and with repeated stimulation an ingestive motor program is induced. Egestive motor activity is triggered when afferents with processes in the esophageal nerve (EN) are activated, e.g., by the presence of an inedible object. EN afferents also activate the feeding CPG and contain the modulatory peptides SCP (small cardioactive peptides), apNPY (Aplysia neuropeptide Y), FMRFamide, and the RFamide peptides (Jing et al. 2007; Vilim et al. 2010; Wu et al. 2010). With repeated stimulation of the EN, egestive motor programs are triggered.
With repeated induction, the “articulation” of feeding motor programs improves.
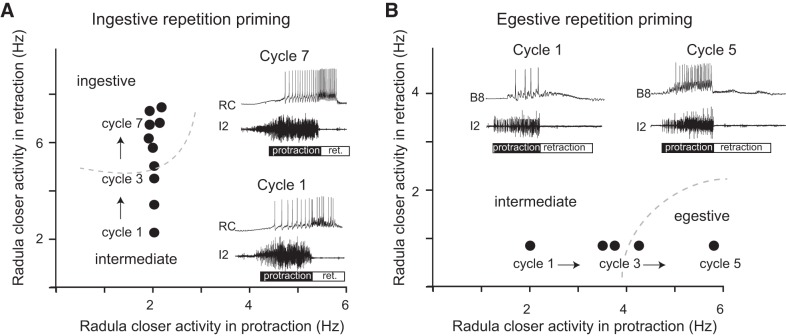
Because the efficacy of feeding behavior in Aplysia is influenced by its microstructure, it would be expected that episodic induction would impact feeding motor programs. A number of studies have demonstrated that this is the case. For example, when CBI-2 is stimulated, the first cycle of the evoked motor program is not well “articulated” (Fig. 4A) (Dacks et al. 2012; Friedman and Weiss 2010; Proekt et al. 2004, 2007). Activity in radula protraction motor neurons precedes activity in radula retraction motor neurons (as it should). However, radula closer motor neurons fire at a low frequency during both radula protraction and retraction. During protraction, radula opener motor neurons fire with the radula closer neurons at comparable (low) frequencies. Activity is referred to as being poorly articulated because it is not clearly patterned in what is likely to be a functional sense.
Fig. 4.
Repetition priming in the feeding circuit. Plotted is the firing frequency of a radula closer motor neuron during radula protraction (x-axis) and radula retraction (y-axis). Dashed lines mark clusters of ingestive and egestive activity. Cycles of activity were evoked with an intercycle interval of 30 s by using either CBI-2 to induce ingestive repetition priming (A) or the EN to induce egestive repetition priming (B). Insets are typical intracellular recordings from a radula closer motor neuron (RC; top trace) and extracellular recordings from the I2 nerve (bottom trace). Activity in the I2 nerve marks the protraction phase of the motor program. Note that in both A and B, the first cycle that was triggered was poorly articulated. The radula closer motor neuron fired at a relatively low frequency (cycle 1 in both A and B). With repeated input activation, however, program definition occurred. For example, with repeated stimulation of CBI-2, motor activity became clearly ingestive (e.g., the firing frequency of the radula closer motor neuron increased during retraction; A). With repeated stimulation of the EN, motor programs became egestive (e.g., the firing frequency of the radula closer motor neuron increased during protraction; B). [Data are replotted from Friedman et al. (2009) and Friedman and Weiss (2010).]
These data presumably provide at least a partial explanation for the observation that feeding responses triggered after a period of inactivity are relatively weak (Susswein et al. 1976; Weiss et al. 1986; Zhurov et al. 2005). In general, the amplitude of a muscle contraction in the feeding system is heavily influenced by motor neuron firing frequency (Cohen et al. 1978; Friedman et al. 2009). Consequently, low-frequency activity generally produces a weak contraction. Furthermore, in this context, the coactivation of antagonistic motor neurons is presumably counterproductive.
Motor programs change, however, if they are repeatedly induced and the intercycle interval is relatively short (i.e., tens of seconds instead of minutes). For example, the firing frequency of radula closer motor neurons progressively increases during radula retraction (Fig. 4A) (Dacks et al. 2012; Proekt et al. 2004, 2007), and the firing frequency of radula opener motor neurons progressively increases during protraction (Friedman and Weiss 2010; Friedman et al. 2009). Experiments conducted in semi-intact preparations have demonstrated that these increases in firing frequency are sufficient to alter radula movements. Thus frequencies observed after repetition priming are sufficient to significantly open and close the radula (Friedman et al. 2009). In contrast, frequencies observed during initial cycles of motor activity are not (Friedman et al. 2009).
With repeated stimulation of the “egestive” input to the CPG, the EN, “opposite” changes in motor activity are observed. Motor programs become egestive, in part due to progressive increases in the firing frequency of radula closing motor neurons during protraction (instead of retraction) (Fig. 4B) (Dacks et al. 2012; Proekt et al. 2004, 2007). Additionally, motor neurons that produce radula opening are recruited during radula retraction (instead of protraction) (Friedman et al. 2009, 2015).
To summarize, these data indicate that episodic induction impacts feeding motor programs. After a period of network inactivity, the initial motor program triggered is not well articulated. Antagonistic motor neurons that open and close the radula are coactive. Furthermore, these motor neurons fire at relatively low frequencies. With repeated induction, however, cycles of motor activity progressively change. Motor neurons fire at higher frequencies, and phase relationships of antagonistic motor neurons become more clearly defined.
Repetition priming is an example of a dynamic change in motor activity.
It has been suggested that it is important to view multifunctional networks as continuous dynamical systems (Briggman and Kristan 2008). Thus, not only are multifunctional networks capable of generating more than one discrete state, but they transition between states in a manner that has certain dynamics that can be described. Studies of repetition priming in the feeding network beautifully illustrate the importance of characterizing transition dynamics. For example, it has been demonstrated that with repetition priming, one type of motor program can slowly evolve into another; i.e., egestive activity is slowly converted to ingestive (Proekt et al. 2004). As is described in more detail below, this finding has informed mechanistic experiments that that have sought to determine how repetition priming is mediated at the cellular/molecular level.
Modulatory neuropeptides reconfigure motor activity: chemical coding in the feeding network.
A specific question that has been addressed is, why are feeding motor programs reconfigured when they are repeatedly induced with a relatively short intercycle interval? Interestingly, it has been noted that this does happen when motor activity is triggered using CBI-2 and the EN, but does not happen if motor activity is repeatedly triggered from within the CPG itself, e.g., by stimulating a protraction interneuron (Siniscalchi et al. 2016). Inputs to the feeding CPG, i.e., CBI-2 and the EN, contain modulatory neuropeptides (Fig. 3). For example, CBI-2, which is cholinergic (Hurwitz et al. 2003), also contains feeding circuit-activating peptide (FCAP) (Koh et al. 2003; Sweedler et al. 2002) and cerebral peptide 2 (CP-2) (Fig. 3) (Morgan et al. 2000). Occlusion (and other) experiments have indicated that both peptides play an important role in determining phase and timing relationships of motor neurons during ingestive motor programs (Friedman and Weiss 2010; Koh et al. 2003; Koh and Weiss 2005, 2007; Morgan et al. 2000).
Stimulation of the EN releases a number of modulatory neuropeptides, including small cardioactive peptide (SCP) (Wu et al. 2010) and Aplysia neuropeptide Y (apNPY) (Fig. 3) (Jing et al. 2007). In addition, neural processes in the EN contain FMRFamide and the FRFamide peptides (Vilim et al. 2010). FMRFamide and the FRFamide peptides act together to promote egestive activity (Friedman et al. 2015; Vilim et al. 2010).
In summary, data indicate that modulatory peptides released when CPG inputs are repeatedly activated play an essential role in configuring motor activity as repetition priming occurs. One set of peptides makes activity ingestive; a second set makes activity egestive. These results are consistent with the idea that a form of “chemical coding” is used in the selection of feeding motor programs.
A further question that has been addressed in the context of radula opening is, do ingestive and egestive peptides act via different second messenger systems? Radula opening motor neurons are behavior specific. Thus, when motor programs become ingestive, the motor neuron B48 is recruited during the protraction phase of the motor program (Friedman et al. 2009). In contrast, a second motor neuron, B44, is recruited during the retraction phase when programs become egestive (Friedman et al. 2009). In both cases, recruitment results from a peptide-mediated increase in motor neuron excitability. During ingestion, the excitability of B48 is increased by FCAP/CP2 released from CBI-2 (Friedman and Weiss 2010; Perkins et al. 2013; Perkins and Weiss 2012). During egestion, the excitability of B48 is decreased (Perkins et al. 2013; Perkins and Weiss 2012), and the excitability of B44 is increased by SCP released from afferents with processes in the EN (Friedman and Weiss 2010; Wu et al. 2010). Effects of FCAP/CP2 are cAMP mediated, whereas effects of SCP are PKC mediated (Friedman and Weiss 2010; Friedman et al. 2015; Perkins et al. 2013; 2015). These data therefore suggest that chemical coding in the feeding system is observed intracellularly (as well as intercellularly).
The “chemical coding” notion dates back to at least the 1960s (Grossman 1960; Heller et al. 1980; Kupfermann 1967, 1970; Truman 1978). In a number of characterized circumstances, the behavior (or motor program) is specified by a hormone or blood borne substance. Effects of these blood borne substances are often studied in experiments in which they are bath applied and network alterations are determined under steady-state conditions. Ingestive and egestive modulators in the Aplysia feeding system are obviously different. They are released as neurotransmitters from neurons that provide input to a network activated in an episodic manner. Consequently, motor activity changes progressively. Work in the feeding system therefore indicates that behavioral selection does not always occur in an all-or-none manner. Instead, it can occur in a progressive manner.
Multifunctionality in the feeding network.
Research in other systems has established that the ability of a network to be multifunctional results from the fact that its anatomical connectivity is not necessarily the same as its functional connectivity (Briggman and Kristan 2008; Getting 1989; Marder 2012). Functional connectivity depends on factors that can vary, i.e., synaptic strength and neuronal excitability. Both types of network parameters are altered when the feeding network is reconfigured to generate either ingestive or egestive activity (for specifics, see Dacks et al. 2012; Dacks and Weiss 2013; Friedman and Weiss 2010; Friedman et al. 2009, 2015; Proekt et al. 2004, 2007).
Neuromodulation as a general mechanism for repetition priming.
Why do changes in feeding motor programs occur gradually? Currently, there is no definitive answer to this question, but it has been suggested that it is a consequence of the persistence of neuromodulation (Cropper et al. 2014). Presumably, effects of modulators released during one cycle of a motor program last for tens of seconds, so are still present if the next cycle of activity is triggered with a relatively short delay. Consequently, modulatory effects summate and become cumulatively larger (Fig. 5) (Cropper et al. 2014). This type of model presumably explains why repetition priming is not observed in the feeding network when interresponse intervals are relatively long (i.e., minutes as opposed to seconds).
Fig. 5.
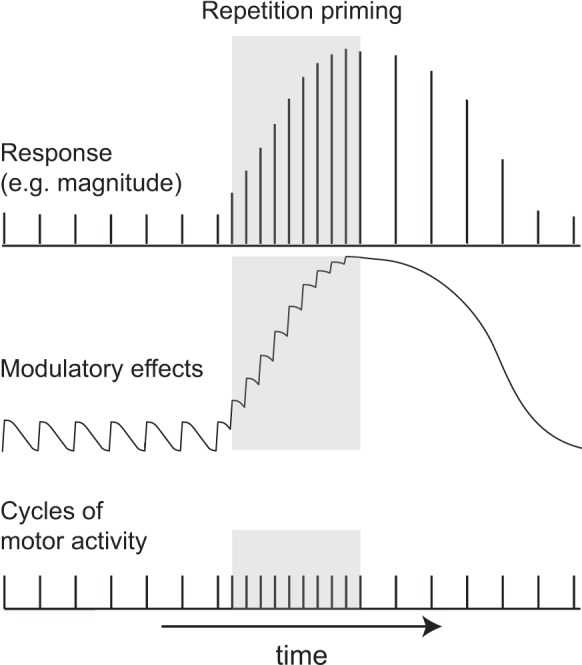
Schematic illustrating the hypothesized role of endogenous modulation in the induction of repetition priming. Repetition priming is observed when cycles of motor activity (bottom trace) are triggered with a short interstimulus interval (ISI; as is the case during the time indicated by gray shading). This results in progressive improvements in performance, which can be measured in a number of ways (e.g., as progressive increases in response magnitude; top trace). It has been suggested that priming results from cumulative effects of modulators (middle trace), which summate when there is a short ISI. Effects of repetition priming persist. This is apparent when the ISI is lengthened, e.g., returned to the control value (bottom trace to the right of the gray shading). Note that performance is still enhanced at this point (top trace to the right of the gray shading). This is likely to be a consequence of the fact that it takes time for cumulative effects of modulators to dissipate (middle trace to the right of the gray shading). [In the manner of Fig. 1 from Cropper et al. (2014).]
All nervous systems appear to be subject to neuromodulation (Marder 2012). This makes it possible that neuromodulation serves as a mechanism for repetition priming in other species. Furthermore, in the Aplysia feeding system, priming is observed because modulators are present within the feeding network itself. Consequently, they are classified as “intrinsic” (Cropper et al. 1987). This type of arrangement has been described in other systems. For example, it is observed in the circuitry that mediates escape swimming in Tritonia (Katz 1998; Katz and Frost 1995a, 1995b; Sakurai et al. 2006, 2007; Sakurai and Katz 2003, 2009). Here, serotonin is present in a CPG element, the dorsal swim interneuron (DSI). This cell exerts effects on a second interneuron, C2, which is also crucial for the behavior. Effects of 5-HT are in part mediated via interaction with metabotropic receptors (Clemens and Katz 2001, 2003) and persist long enough that they are likely to carry over from cycle to cycle (Katz and Frost 1997).
Repetition Priming Creates a “State” That Persists
As discussed above, data suggest that effects of modulators released during a single cycle of an Aplysia feeding motor program persist for seconds but not minutes (Friedman and Weiss 2010; Friedman et al. 2009, 2015; Proekt et al. 2004, 2007). However, effects of repetition priming clearly last longer (e.g., more than 10 min for ingestive priming; Friedman et al. 2009; Proekt et al. 2004). The difference is likely to be a reflection of the fact that summation of the modulatory signal occurs during repetition priming as a result of the repeated network activation (Fig. 5) (Cropper et al. 2014). Because the summated signal is considerably larger than the modulatory signal generated by a single cycle of a motor program, it takes longer to dissipate.
Possible Physiological Consequences of Repetition Priming
Repetition priming requires repeated activation of CPG inputs so that cycles of motor activity are triggered with a relatively short intercycle interval (Cropper et al. 2014; Dacks et al. 2012; Friedman and Weiss 2010; Friedman et al. 2015). At present there are no data that directly indicate when this occurs in the intact animal. It is, however, most likely that it happens in the maintained presence of a stimulus (such as food). In this situation, there is likely to be maintained afferent activation, which will trigger repeated motor activity either directly (in the case of EN afferents) or indirectly (in the case of the sensory neurons that activate CBI-2). Furthermore, when food is continuously present, there are likely to be changes within the feeding CPG itself. Thus food presentation results in a form of operant conditioning that is manifested as changes in membrane and synaptic properties of pattern-initiating neurons (Brembs et al. 2004; Lorenzetti et al. 2006; Mozzachiodi et al. 2008; Nargeot et al. 2007, 2009; Sieling et al. 2014). These changes tend to make feeding movements more frequent and regular (which will obviously tend to promote repetition priming).
Energy management.
That feeding responses get progressively stronger when they are repeated makes sense given the fact that what is altered is a component of behavior that is important for moving food in or out of the buccal cavity (i.e., radula opening and closing). For example, in the context of ingestive activity, Aplysia presumably generate bites with weak radula opening/closing movements when they are in the vicinity of food but are not able to maintain contact with it. In contrast, when food contact is maintained, radula opening and closing are enhanced. This arrangement is likely to be energetically favorable in that food-grasping movements are only potentiated when they are actually needed (i.e., food is present so can be ingested).
“Energy management” in the context of feeding behavior has been demonstrated in other species. For example, Lymnaea generate two different types of bites: appetitive and consummatory (Crossley et al. 2016). The two types of bites differ in that swallowing motor neurons fire at a lower frequency when bites are appetitive. Consequently, they require less energy. When Lymnaea search for food, bites are appetitive (relatively low energy). Consummatory bites (high energy) are reserved for the situation where food is detected. Food-related changes in feeding behavior are also observed in Caenorhabditis elegans. Namely, C. elegans feed on bacteria via rhythmic contractions and relaxations of the pharynx (i.e., pharyngeal pumping; Trojanowski et al. 2016). Pumping dynamics are influenced by the availability of food in the environment (Lee et al. 2017). Worms expend more energy (e.g., pump at a higher frequency) when the concentration of bacteria in the environment is high, and less energy when there is less food available (i.e., the concentration of bacteria is low).
Role in stabilizing behavior.
Other physiological consequences of repetition priming in Aplysia presumably stem from the fact that it is mediated by modulatory neurotransmitters that exert persistent, second messenger-mediated effects. Interestingly, this can have an impact on the ability of the network to task switch. In the Aplysia feeding network this has been demonstrated in the situation where egestive repetition priming is followed by an attempt to induce an ingestive cycle of a motor program (Fig. 6). In this situation a form of task set inertia is observed (Proekt et al. 2004). Thus, immediately after egestive repetition priming, stimulation of the ingestive input to the CPG (CBI-2) triggers a cycle of a motor program that is egestive. Activity only gradually becomes ingestive as CBI-2 stimulation is maintained.
Fig. 6.
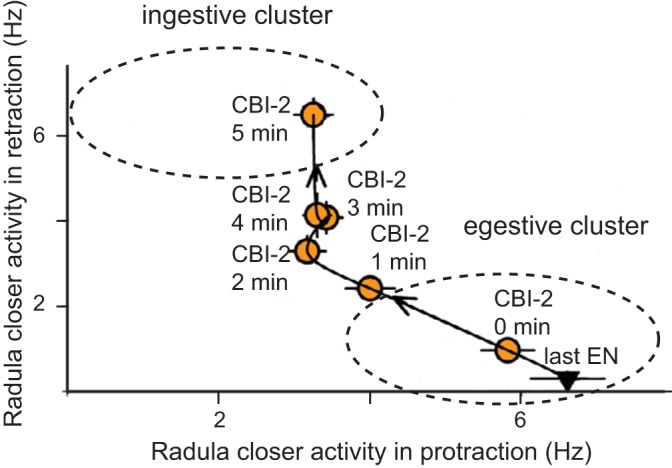
Task switch inertia in the Aplysia feeding circuitry. Motor programs were triggered by repeated stimulation of the EN with a short ISI (which induced egestive repetition priming; not shown). As would be expected, the last cycle of activity was egestive (inverted triangle, last EN). Motor programs were then triggered by stimulating CBI-2 with a relatively long ISI (i.e., once a minute). Immediately after egestive priming, the first CBI-2 induced cycle remained in the egestive cluster, which is marked by an ellipse (yellow circle, CBI-2 at 0 min). With repeated stimulation, CBI-2 programs returned to the ingestive cluster, also marked by an ellipse (yellow circles: CBI-2 at 1, 2, 3, 4, and 5 min). [Data are replotted from Proekt et al. (2004).]
It is possible that inertia in this context is beneficial because it serves as a “stabilizer.” Thus it is likely that it would make behavior relatively resistant to disruption due to a brief, and therefore inconsequential, alteration in the external environment. In the context of feeding, it may be important to “stabilize” the egestive state because this state may be a reflection of the maintained presence of a noxious or harmful stimulus. Task-switch inertia is not unique to Aplysia. It has been documented in a number of species (including humans) (Bratzke et al. 2009; Heuer et al. 2004; Orban de Xivry and Lefèvre 2016). However, in higher order animals, cellular/molecular changes that impair task switching have not been described.
Summary and Conclusion
In this review we describe dynamic changes in motor activity that are observed during an episodic behavior—feeding in the mollusk Aplysia. In this experimentally advantageous system, much progress has been made in experiments that have demonstrated that episodic induction results in dynamic reconfiguration of network activity. Activity is reconfigured by intrinsic neuromodulators that exert effects that persist and summate. This is likely to be responsible, at least in part, for the progressive increases in the magnitude of feeding responses that are observed when they are repeatedly generated.
Many human behaviors, like feeding in Aplysia, are episodic. Furthermore, they are mediated by neural networks that contain intrinsic neuromodulators. These modulators exert persistent second messenger-mediated effects that have the potential to summate. It is, therefore, likely that a number of episodic behaviors are dynamically altered as they stop and start via mechanisms that are similar to those that mediate repetition priming in the Aplysia feeding network. In future work it will be important to determine whether this is indeed the case.
GRANTS
This work was supported by National Institutes of Health Grants NS066587, NS070583, and MH051393.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.C.C., J.J., M.H.P., and K.R.W. conceived and designed research; E.C.C., J.J., M.H.P., and K.R.W. analyzed data; E.C.C., J.J., M.H.P., and K.R.W. interpreted results of experiments; E.C.C. prepared figures; E.C.C. drafted manuscript; E.C.C., J.J., and K.R.W. edited and revised manuscript; E.C.C., J.J., M.H.P., and K.R.W. approved final version of manuscript; J.J. and M.H.P. performed experiments.
REFERENCES
- Bablanian GM, Weiss KR, Kupfermann I. Motor control of the appetitive phase of feeding behavior in Aplysia. Behav Neural Biol 48: 394–407, 1987. doi: 10.1016/S0163-1047(87)90957-5. [DOI] [PubMed] [Google Scholar]
- Beenhakker MP, Blitz DM, Nusbaum MP. Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol 91: 78–91, 2004. doi: 10.1152/jn.00741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci 24: 6741–6750, 2004. doi: 10.1523/JNEUROSCI.1682-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin PR. Distributed network organization underlying feeding behavior in the mollusk Lymnaea. Neural Syst Circuits 2: 4, 2012. doi: 10.1186/2042-1001-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin PR, Rose RM. Central generation of bursting in the feeding system of the snail, Lymnaea stagnalis. J Exp Biol 80: 93–118, 1979. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Beenhakker MP, Nusbaum MP. Different sensory systems share projection neurons but elicit distinct motor patterns. J Neurosci 24: 11381–11390, 2004. doi: 10.1523/JNEUROSCI.3219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdan E. Microstructure of feeding by tobacco hornworm caterpillars, Manduca sexta. Entomol Exp Appl 47: 127–136, 1988. doi: 10.1111/j.1570-7458.1988.tb01127.x. [DOI] [Google Scholar]
- Bratzke D, Rolke B, Steinborn MB, Ulrich R. The effect of 40 h constant wakefulness on task-switching efficiency. J Sleep Res 18: 167–172, 2009. doi: 10.1111/j.1365-2869.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Walter H, Erk S, Romanczuk-Seiferth N, Haddad L, Schweiger JI, Grimm O, Heinz A, Tost H, Meyer-Lindenberg A, Bassett DS. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci USA 112: 11678–11683, 2015. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembs B, Baxter DA, Byrne JH. Extending in vitro conditioning in Aplysia to analyze operant and classical processes in the same preparation. Learn Mem 11: 412–420, 2004. doi: 10.1101/lm.74404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley MJ, Yeoman MS, Benjamin PR. Glutamatergic N2v cells are central pattern generator interneurons of the Lymnaea feeding system: new model for rhythm generation. J Neurophysiol 78: 3396–3407, 1997. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB Jr. Multifunctional pattern-generating circuits. Annu Rev Neurosci 31: 271–294, 2008. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- Calabrese RL, Norris BJ, Wenning A. The neural control of heartbeat in invertebrates. Curr Opin Neurobiol 41: 68–77, 2016. doi: 10.1016/j.conb.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiel HJ, Weiss KR, Kupfermann I. An identified histaminergic neuron modulates feeding motor circuitry in Aplysia. J Neurosci 6: 2427–2450, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church PJ, Lloyd PE. Activity of multiple identified motor neurons recorded intracellularly during evoked feedinglike motor programs in Aplysia. J Neurophysiol 72: 1794–1809, 1994. [DOI] [PubMed] [Google Scholar]
- Clemens S, Katz PS. Identified serotonergic neurons in the Tritonia swim CPG activate both ionotropic and metabotropic receptors. J Neurophysiol 85: 476–479, 2001. [DOI] [PubMed] [Google Scholar]
- Clemens S, Katz PS. G protein signaling in a neuronal network is necessary for rhythmic motor pattern production. J Neurophysiol 89: 762–772, 2003. doi: 10.1152/jn.00765.2002. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Weiss KR, Kupfermann I. Motor control of buccal muscles in Aplysia. J Neurophysiol 41: 157–180, 1978. [DOI] [PubMed] [Google Scholar]
- Combes D, Meyrand P, Simmers J. Dynamic restructuring of a rhythmic motor program by a single mechanoreceptor neuron in lobster. J Neurosci 19: 3620–3628, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Proc Natl Acad Sci USA 3: 685–688, 1917. doi: 10.1073/pnas.3.12.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. Appetites and aversions as constituents of instincts. Biol Bull 34: 91–107, 1918. doi: 10.2307/1536346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper EC, Evans CG, Hurwitz I, Jing J, Proekt A, Romero A, Rosen SC. Feeding neural networks in the mollusc Aplysia. Neurosignals 13: 70–86, 2004. doi: 10.1159/000076159. [DOI] [PubMed] [Google Scholar]
- Cropper EC, Friedman AK, Jing J, Perkins MH, Weiss KR. Neuromodulation as a mechanism for the induction of repetition priming. Curr Opin Neurobiol 29: 33–38, 2014. doi: 10.1016/j.conb.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper EC, Lloyd PE, Reed W, Tenenbaum R, Kupfermann I, Weiss KR. Multiple neuropeptides in cholinergic motor neurons of Aplysia: evidence for modulation intrinsic to the motor circuit. Proc Natl Acad Sci USA 84: 3486–3490, 1987. doi: 10.1073/pnas.84.10.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley M, Staras K, Kemenes G. A two-neuron system for adaptive goal-directed decision-making in Lymnaea. Nat Commun 7: 11793, 2016. doi: 10.1038/ncomms11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullins MJ, Shaw KM, Gill JP, Chiel HJ. Motor neuronal activity varies least among individuals when it matters most for behavior. J Neurophysiol 113: 981–1000, 2015. doi: 10.1152/jn.00729.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks AM, Siniscalchi MJ, Weiss KR. Removal of default state-associated inhibition during repetition priming improves response articulation. J Neurosci 32: 17740–17752, 2012. doi: 10.1523/JNEUROSCI.4137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks AM, Weiss KR. Latent modulation: a basis for non-disruptive promotion of two incompatible behaviors by a single network state. J Neurosci 33: 3786–3798, 2013. doi: 10.1523/JNEUROSCI.5371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106: 217–228, 1992. doi: 10.1037/0735-7044.106.1.217. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Singh B. A microstructural analysis of the control of water and isotonic saline ingestion by postingestional stimulation. Physiol Behav 66: 543–548, 1999. doi: 10.1016/S0031-9384(98)00325-4. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP, Singh B, McCann DL. The impact of sucrose-derived unconditioned and conditioned negative feedback on the microstructure of ingestive behavior. Physiol Behav 72: 393–402, 2001. doi: 10.1016/S0031-9384(00)00442-X. [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Jing J, Proekt A, Romero A, Vilim FS, Cropper EC, Weiss KR. A newly identified buccal interneuron initiates and modulates feeding motor programs in Aplysia. J Neurophysiol 90: 2190–2204, 2003. doi: 10.1152/jn.00173.2003. [DOI] [PubMed] [Google Scholar]
- Doty RW, Richmond WH, Storey AT. Effect of medullary lesions on coordination of deglutition. Exp Neurol 17: 91–106, 1967. doi: 10.1016/0014-4886(67)90125-2. [DOI] [PubMed] [Google Scholar]
- Elliott CJ, Benjamin PR. Interactions of pattern-generating interneurons controlling feeding in Lymnaea stagnalis. J Neurophysiol 54: 1396–1411, 1985. [DOI] [PubMed] [Google Scholar]
- Elliott CJ, Susswein AJ. Comparative neuroethology of feeding control in molluscs. J Exp Biol 205: 877–896, 2002. [DOI] [PubMed] [Google Scholar]
- Evans CG, Cropper EC. Proprioceptive input to feeding motor programs in Aplysia. J Neurosci 18: 8016–8031, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CA, Napps SE, Feldman L. Relations among regular and irregular morphologically related words in the lexicon as revealed by repetition priming. Mem Cognit 13: 241–255, 1985. doi: 10.3758/BF03197687. [DOI] [PubMed] [Google Scholar]
- Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci 30: 8906–8919, 2010. doi: 10.1523/JNEUROSCI.1287-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Weiss KR, Cropper EC. Specificity of repetition priming: the role of chemical coding. J Neurosci 35: 6326–6334, 2015. doi: 10.1523/JNEUROSCI.4562-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Zhurov Y, Ludwar BC, Weiss KR. Motor outputs in a multitasking network: relative contributions of inputs and experience-dependent network states. J Neurophysiol 102: 3711–3727, 2009. doi: 10.1152/jn.00844.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Katz PS. Single neuron control over a complex motor program. Proc Natl Acad Sci USA 93: 422–426, 1996. doi: 10.1073/pnas.93.1.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci 12: 185–204, 1989. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Getting PA, Dekin MS. Mechanisms of pattern generation underlying swimming in Tritonia. IV. Gating of central pattern generator. J Neurophysiol 53: 466–480, 1985. [DOI] [PubMed] [Google Scholar]
- Grossman SP. Eating or drinking elicited by direct adrenergic or cholinergic stimulation of hypothalamus. Science 132: 301–302, 1960. doi: 10.1126/science.132.3422.301. [DOI] [PubMed] [Google Scholar]
- Heller E, Kaczmarek LK, Hunkapiller MW, Hood LE, Strumwasser F. Purification and primary structure of two neuroactive peptides that cause bag cell afterdischarge and egg-laying in Aplysia. Proc Natl Acad Sci USA 77: 2328–2332, 1980. doi: 10.1073/pnas.77.4.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Kleinsorge T, Klein W, Kohlisch O. Total sleep deprivation increases the costs of shifting between simple cognitive tasks. Acta Psychol (Amst) 117: 29–64, 2004. doi: 10.1016/j.actpsy.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Howells HH. The structure and function of the alimentary canal of Aplysia punctata. Q J Microsc Sci 83: 357–397, 1942. [Google Scholar]
- Hurwitz I, Goldstein RS, Susswein AJ. Compartmentalization of pattern-initiation and motor functions in the B31 and B32 neurons of the buccal ganglia of Aplysia californica. J Neurophysiol 71: 1514–1527, 1994. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Kupfermann I, Susswein AJ. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J Neurophysiol 78: 1305–1319, 1997. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Kupfermann I, Weiss KR. Fast synaptic connections from CBIs to pattern-generating neurons in Aplysia: initiation and modification of motor programs. J Neurophysiol 89: 2120–2136, 2003. doi: 10.1152/jn.00497.2002. [DOI] [PubMed] [Google Scholar]
- Hurwitz I, Susswein AJ. Adaptation of feeding sequences in Aplysia oculifera to changes in the load and width of food. J Exp Biol 166: 215–235, 1992. [Google Scholar]
- Hurwitz I, Susswein AJ. B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol 75: 1327–1344, 1996. [DOI] [PubMed] [Google Scholar]
- Itskov PM, Moreira JM, Vinnik E, Lopes G, Safarik S, Dickinson MH, Ribeiro C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat Commun 5: 4560, 2014. doi: 10.1038/ncomms5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Cropper EC, Hurwitz I, Weiss KR. The construction of movement with behavior-specific and behavior-independent modules. J Neurosci 24: 6315–6325, 2004. doi: 10.1523/JNEUROSCI.0965-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Cropper EC, Weiss KR. Network functions of electrical coupling present in multiple and specific sites in behavior-generating circuits. In: Network Functions and Plasticity: Perspectives from Studying Neuronal Electrical Coupling in Microcircuits, edited by Jing J. London: Elsevier/Academic, 2017, p. 79–107. [Google Scholar]
- Jing J, Sasaki K, Perkins MH, Siniscalchi MJ, Ludwar BC, Cropper EC, Weiss KR. Coordination of distinct motor structures through remote axonal coupling of projection interneurons. J Neurosci 31: 15438–15449, 2011. doi: 10.1523/JNEUROSCI.3741-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci 27: 3490–3502, 2007. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci 21: 7349–7362, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci 22: 6228–6238, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Weiss KR. Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15: 1712–1721, 2005. doi: 10.1016/j.cub.2005.08.051. [DOI] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Byrne JH. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol 79: 605–621, 1998. [DOI] [PubMed] [Google Scholar]
- Kabotyanski EA, Baxter DA, Cushman SJ, Byrne JH. Modulation of fictive feeding by dopamine and serotonin in Aplysia. J Neurophysiol 83: 374–392, 2000. [DOI] [PubMed] [Google Scholar]
- Katz PS. Neuromodulation intrinsic to the central pattern generator for escape swimming in Tritonia. Ann N Y Acad Sci 860, 1 NEURONAL MECH: 181–188, 1998. doi: 10.1111/j.1749-6632.1998.tb09048.x. [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: serotonin mediates both neuromodulation and neurotransmission by the dorsal swim interneurons. J Neurophysiol 74: 2281–2294, 1995a. [DOI] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation in the Tritonia swim CPG: the serotonergic dorsal swim interneurons act presynaptically to enhance transmitter release from interneuron C2. J Neurosci 15: 6035–6045, 1995b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Frost WN. Removal of spike frequency adaptation via neuromodulation intrinsic to the Tritonia escape swim central pattern generator. J Neurosci 17: 7703–7713, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes G, Staras K, Benjamin PR. Multiple types of control by identified interneurons in a sensory-activated rhythmic motor pattern. J Neurosci 21: 2903–2911, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol 90: 2074–2079, 2003. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- Koh HY, Weiss KR. Peptidergic contribution to posttetanic potentiation at a central synapse of Aplysia. J Neurophysiol 94: 1281–1286, 2005. doi: 10.1152/jn.00073.2005. [DOI] [PubMed] [Google Scholar]
- Koh HY, Weiss KR. Activity-dependent peptidergic modulation of the plateau-generating neuron B64 in the feeding network of Aplysia. J Neurophysiol 97: 1862–1867, 2007. doi: 10.1152/jn.01230.2006. [DOI] [PubMed] [Google Scholar]
- Kristan WB Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol 76: 279–327, 2005. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kristan WB Jr, Wittenberg G, Nusbaum MP, Stern-Tomlinson W. Multifunctional interneurons in behavioral circuits of the medicinal leech. Experientia 44: 383–389, 1988. doi: 10.1007/BF01940531. [DOI] [PubMed] [Google Scholar]
- Kristjánsson A, Campana G. Where perception meets memory: a review of repetition priming in visual search tasks. Atten Percept Psychophys 72: 5–18, 2010. doi: 10.3758/APP.72.1.5. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying: possible neuroendocrine function of bag cells of abdominal ganglion of Aplysia californica. Nature 216: 814–815, 1967. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying by extracts of neuroendocrine cells (bag cells) of abdominal ganglion of Aplysia. J Neurophysiol 33: 877–881, 1970. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Dissociation of the appetitive and consummatory phases of feeding behavior in Aplysia: a lesion study. Behav Biol 10: 89–97, 1974a. doi: 10.1016/S0091-6773(74)91694-0. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol 10: 1–26, 1974b. doi: 10.1016/S0091-6773(74)91644-7. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Carew TJ. Behavior patterns of Aplysia californica in its natural environment. Behav Biol 12: 317–337, 1974. doi: 10.1016/S0091-6773(74)91503-X. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Weiss KR. Command neuron concept. Behav Brain Sci 1: 3–10, 1978. doi: 10.1017/S0140525X00059057. [DOI] [Google Scholar]
- Kuslansky B, Weiss KR, Kupfermann I. Mechanisms underlying satiation of feeding behavior of the mollusc Aplysia. Behav Neural Biol 48: 278–303, 1987. doi: 10.1016/S0163-1047(87)90836-3. [DOI] [PubMed] [Google Scholar]
- Lamb DG, Calabrese RL. Neural circuits controlling behavior and autonomic functions in medicinal leeches. Neural Syst Circuits 1: 13, 2011. doi: 10.1186/2042-1001-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Iwanir S, Kopito RB, Scholz M, Calarco JA, Biron D, Levine E. Serotonin-dependent kinetics of feeding bursts underlie a graded response to food availability in C. elegans. Nat Commun 8: 14221, 2017. doi: 10.1038/ncomms14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti FD, Mozzachiodi R, Baxter DA, Byrne JH. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nat Neurosci 9: 17–19, 2006. doi: 10.1038/nn1593. [DOI] [PubMed] [Google Scholar]
- Lund JP. Chapter 15–chew before you swallow. Prog Brain Res 188: 219–228, 2011. doi: 10.1016/B978-0-444-53825-3.00020-6. [DOI] [PubMed] [Google Scholar]
- Ma C, Kessler S, Simpson A, Wright G. A novel behavioral assay to investigate gustatory responses of individual, freely-moving bumble bees (Bombus terrestris). J Vis Exp (113): e54233, 2016. doi: 10.3791/54233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996. [DOI] [PubMed] [Google Scholar]
- McClellan AD. A differential displacement transducer for measuring relative movement in biological systems. J Neurosci Methods 5: 309–316, 1982. doi: 10.1016/0165-0270(82)90001-2. [DOI] [PubMed] [Google Scholar]
- McManus JM, Lu H, Chiel HJ. An in vitro preparation for eliciting and recording feeding motor programs with physiological movements in Aplysia californica. J Vis Exp (70): e4320, 2012. doi: 10.3791/4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Maidment NT, Murphy NP. Parsing the hedonic and motivational influences of nociceptin on feeding using licking microstructure analysis in mice. Behav Pharmacol 27: 516–527, 2016. doi: 10.1097/FBP.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol 87: 49–61, 2002. doi: 10.1152/jn.00438.2001. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol 84: 1186–1193, 2000. [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 172: 17–32, 1993a. doi: 10.1007/BF00214712. [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 173: 519–536, 1993b. doi: 10.1007/BF00197761. [DOI] [PubMed] [Google Scholar]
- Mozzachiodi R, Lorenzetti FD, Baxter DA, Byrne JH. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nat Neurosci 11: 1146–1148, 2008. doi: 10.1038/nn.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama T, Weiss KR, Kupfermann I. Effects of cerebral neuron C-PR on body postural muscles associated with a food-induced arousal state in Aplysia. J Neurophysiol 70: 1231–1243, 1993. [DOI] [PubMed] [Google Scholar]
- Nagahama T, Weiss KR, Kupfermann I. Body postural muscles active during food arousal in Aplysia are modulated by diverse neurons that receive monosynaptic excitation from the neuron C-PR. J Neurophysiol 72: 314–325, 1994. [DOI] [PubMed] [Google Scholar]
- Nargeot R, Le Bon-Jego M, Simmers J. Cellular and network mechanisms of operant learning-induced compulsive behavior in Aplysia. Curr Biol 19: 975–984, 2009. doi: 10.1016/j.cub.2009.05.030. [DOI] [PubMed] [Google Scholar]
- Nargeot R, Petrissans C, Simmers J. Behavioral and in vitro correlates of compulsive-like food seeking induced by operant conditioning in Aplysia. J Neurosci 27: 8059–8070, 2007. doi: 10.1523/JNEUROSCI.1950-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Lefèvre P. A switching cost for motor planning. J Neurophysiol 116: 2857–2868, 2016. doi: 10.1152/jn.00319.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MH, Cropper EC, Weiss KR. Modulatory masking: Activation of distinct currents in a single cell supports temporary reversals of response character in a multi-functional network. Program No. 559.05/ZZ9. 2013 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2013. [Google Scholar]
- Perkins MH, Cropper EC, Weiss KR. Persistent activation of a cyclic nucleotide gated sodium current contributes to the maintenance of a primed state in a multi-functional network. Program No. 420.28/P12. 2015 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2015. [Google Scholar]
- Perkins MH, Weiss KR. Repetition priming of two antagonistic Aplysia feeding behaviors is mnemonically independent. Program No. 86.10/OO1. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012. [Google Scholar]
- Plummer MR, Kirk MD. Premotor neurons B51 and B52 in the buccal ganglia of Aplysia californica: synaptic connections, effects on ongoing motor rhythms, and peptide modulation. J Neurophysiol 63: 539–558, 1990. [DOI] [PubMed] [Google Scholar]
- Proekt A, Brezina V, Weiss KR. Dynamical basis of intentions and expectations in a simple neuronal network. Proc Natl Acad Sci USA 101: 9447–9452, 2004. doi: 10.1073/pnas.0402002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proekt A, Jing J, Weiss KR. Multiple contributions of an input-representing neuron to the dynamics of the Aplysia feeding network. J Neurophysiol 97: 3046–3056, 2007. doi: 10.1152/jn.01301.2006. [DOI] [PubMed] [Google Scholar]
- Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci 11: 3630–3655, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Calin-Jageman RJ, Katz PS. Potentiation phase of spike timing-dependent neuromodulation by a serotonergic interneuron involves an increase in the fraction of transmitter release. J Neurophysiol 98: 1975–1987, 2007. doi: 10.1152/jn.00702.2007. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Darghouth NR, Butera RJ, Katz PS. Serotonergic enhancement of a 4-AP-sensitive current mediates the synaptic depression phase of spike timing-dependent neuromodulation. J Neurosci 26: 2010–2021, 2006. doi: 10.1523/JNEUROSCI.2599-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Katz PS. Spike timing-dependent serotonergic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. J Neurosci 23: 10745–10755, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Katz PS. State-, timing-, and pattern-dependent neuromodulation of synaptic strength by a serotonergic interneuron. J Neurosci 29: 268–279, 2009. doi: 10.1523/JNEUROSCI.4456-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez JA, Kirk MD. Cerebral-buccal pathways in Aplysia californica: synaptic connections, cooperative interneuronal effects and feedback during buccal motor programs. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 187: 801–815, 2001. doi: 10.1007/s00359-001-0251-0. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Brezina V, Weiss KR, Jing J. Distinct inhibitory neurons exert temporally specific control over activity of a motoneuron receiving concurrent excitation and inhibition. J Neurosci 29: 11732–11744, 2009. doi: 10.1523/JNEUROSCI.3051-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Cropper EC, Weiss KR, Jing J. Functional differentiation of a population of electrically coupled heterogeneous elements in a microcircuit. J Neurosci 33: 93–105, 2013. doi: 10.1523/JNEUROSCI.3841-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Due MR, Jing J, Weiss KR. Feeding CPG in Aplysia directly controls two distinct outputs of a compartmentalized interneuron that functions as a CPG element. J Neurophysiol 98: 3796–3801, 2007. doi: 10.1152/jn.00965.2007. [DOI] [PubMed] [Google Scholar]
- Schoofs A, Niederegger S, van Ooyen A, Heinzel HG, Spiess R. The brain can eat: establishing the existence of a central pattern generator for feeding in third instar larvae of Drosophila virilis and Drosophila melanogaster. J Insect Physiol 56: 695–705, 2010. doi: 10.1016/j.jinsphys.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Selverston AI, Russell DF, Miller JP, King DG. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7: 215–290, 1976. doi: 10.1016/0301-0082(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Sieling F, Bédécarrats A, Simmers J, Prinz AA, Nargeot R. Differential roles of nonsynaptic and synaptic plasticity in operant reward learning-induced compulsive behavior. Curr Biol 24: 941–950, 2014. doi: 10.1016/j.cub.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Siniscalchi MJ, Cropper EC, Jing J, Weiss KR. Repetition priming of motor activity mediated by a central pattern generator: the importance of extrinsic vs. intrinsic program initiators. J Neurophysiol 116: 1821–1830, 2016. doi: 10.1152/jn.00365.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras K, Kemenes G, Benjamin PR. Pattern-generating role for motoneurons in a rhythmically active neuronal network. J Neurosci 18: 3669–3688, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras K, Kemenes I, Benjamin PR, Kemenes G. Loss of self-inhibition is a cellular mechanism for episodic rhythmic behavior. Curr Biol 13: 116–124, 2003. doi: 10.1016/S0960-9822(02)01435-5. [DOI] [PubMed] [Google Scholar]
- Stellar E, Shrager EE. Chews and swallows and the microstructure of eating. Am J Clin Nutr 42, Suppl: 973–982, 1985. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Byrne JH. Identification and characterization of neurons initiating patterned neural activity in the buccal ganglia of Aplysia. J Neurosci 8: 2049–2061, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susswein AJ, Gev S, Achituv Y, Markovich S. Behavioral patterns of Aplysia fasciata along the Mediterranean coast of Israel. Behav Neural Biol 41: 7–22, 1984. doi: 10.1016/S0163-1047(84)90667-8. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Gev S, Feldman E, Markovich S. Activity patterns and time budgeting of Aplysia fasciata under field and laboratory conditions. Behav Neural Biol 39: 203–220, 1983. doi: 10.1016/S0163-1047(83)90859-2. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Kupfermann I. Bulk as a stimulus for satiation in Aplysia. Behav Biol 13: 203–209, 1975. doi: 10.1016/S0091-6773(75)91903-3. [DOI] [PubMed] [Google Scholar]
- Susswein AJ, Kupfermann I, Weiss KR. Stimulus control of biting in Aplysia. J Comp Physiol 108: 75–96, 1976. doi: 10.1007/BF00625442. [DOI] [Google Scholar]
- Susswein AJ, Schwarz M, Feldman E. Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J Neurosci 6: 1513–1527, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweedler JV, Li L, Rubakhin SS, Alexeeva V, Dembrow NC, Dowling O, Jing J, Weiss KR, Vilim FS. Identification and characterization of the feeding circuit-activating peptides, a novel neuropeptide family of Aplysia. J Neurosci 22: 7797–7808, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyke T, Weiss KR, Kupfermann I. Appetitive feeding behavior of Aplysia: behavioral and neural analysis of directed head turning. J Neurosci 10: 3922–3934, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Miller CL, Sinnamon HM. Priming of locomotor initiation by electrical stimulation in the hypothalamus and preoptic region in the anesthetized rat. Physiol Behav 57: 641–648, 1995. doi: 10.1016/0031-9384(94)00307-6. [DOI] [PubMed] [Google Scholar]
- Trojanowski NF, Raizen DM, Fang-Yen C. Pharyngeal pumping in Caenorhabditis elegans depends on tonic and phasic signaling from the nervous system. Sci Rep 6: 22940, 2016. doi: 10.1038/srep22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JW. Hormonal control of invertebrate behavior. Horm Behav 10: 214–234, 1978. doi: 10.1016/0018-506X(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Vilim FS, Sasaki K, Rybak J, Alexeeva V, Cropper EC, Jing J, Orekhova IV, Brezina V, Price D, Romanova EV, Rubakhin SS, Hatcher N, Sweedler JV, Weiss KR. Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors. J Neurosci 30: 131–147, 2010. doi: 10.1523/JNEUROSCI.3282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KR, Chiel HJ, Koch U, Kupfermann I. Activity of an identified histaminergic neuron, and its possible role in arousal of feeding behavior in semi-intact Aplysia. J Neurosci 6: 2403–2415, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzell MM, Martínez-Rubio C, Miller MW, Murphy AD. Comparative neurobiology of feeding in the opisthobranch sea slug, Aplysia, and the pulmonate snail, Helisoma: evolutionary considerations. Brain Behav Evol 74: 219–230, 2009. doi: 10.1159/000258668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld Z, Halpern BP, Tapper DN. Licking behavior: evidence of hypoglossal oscillator. Science 196: 1122–1124, 1977. doi: 10.1126/science.558653. [DOI] [PubMed] [Google Scholar]
- Willows AO. Physiological basis of feeding behavior in Tritonia diomedea. II. Neuronal mechanisms. J Neurophysiol 44: 849–861, 1980. [DOI] [PubMed] [Google Scholar]
- Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One 4: e6063, 2009. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Vilim FS, Hatcher NG, Due MR, Sweedler JV, Weiss KR, Jing J. Composite modulatory feedforward loop contributes to the establishment of a network state. J Neurophysiol 103: 2174–2184, 2010. doi: 10.1152/jn.01054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Cohn R, Schusterreiter C, Ruta V, Vosshall LB. A taste circuit that regulates ingestion by integrating food and hunger signals. Cell 165: 715–729, 2016. doi: 10.1016/j.cell.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashar A, Makovski T, Lamy D. The role of motor response in implicit encoding: evidence from intertrial priming in pop-out search. Vision Res 93: 80–87, 2013. doi: 10.1016/j.visres.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Ye H, Morton DW, Chiel HJ. Neuromechanics of multifunctionality during rejection in Aplysia californica. J Neurosci 26: 10743–10755, 2006. doi: 10.1523/JNEUROSCI.3143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov Y, Proekt A, Weiss KR, Brezina V. Changes of internal state are expressed in coherent shifts of neuromuscular activity in Aplysia feeding behavior. J Neurosci 25: 1268–1280, 2005. doi: 10.1523/JNEUROSCI.3361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]