Abstract
Clostridium difficile infections can be life-threatening but are increasingly being treated successfully with fecal microbiota transplantation (FMT). We report two patients with alopecia universalis who developed subsequent hair regrowth after FMT for treatment of recurrent C. difficile infections. Gut microbiota may have immunomodulatory effects in autoimmune conditions such as alopecia areata, and further study may elucidate disease mechanisms and lead to alternative treatment options for these patients for whom treatment options are currently limited.
Introduction
Alopecia areata (AA) is an autoimmune, inflammatory condition of the hair follicle. It is classified as patchy, totalis, and universalis, depending on the degree of hair loss.1 Fecal microbiota transplant (FMT) is increasingly being used to treat patients with Clostridium difficile infection (CDI). Gut microbiota have recently emerged as potential immunomodulators with the capacity to elicit physiologic or pathologic responses in the host.2
Case 1
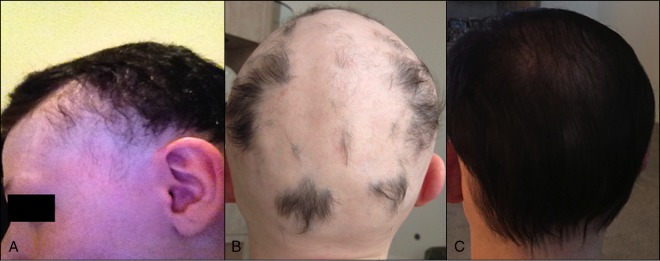
A 38-year-old man with alopecia universalis diagnosed 10 years previously presented with bloody diarrhea and abdominal pain. On physical examination, he had diffuse abdominal tenderness, and laboratory evaluation revealed leukocytosis. Abdominal computed tomography (CT) showed inflammation of the terminal ileum. Stool C. difficile polymerase chain reaction was positive, and the patient improved on metronidazole. He was treated with oral vancomycin for 2 further episodes with similar symptoms and C. difficile-positive stools. After FMT for recurrent CDI, he experienced sustained resolution of his CDI. At follow-up 8 weeks later, he reported new hair growth on his head, face, and arms (Figure 1). His alopecia had previously been refractory to steroid injections, and he was on no other therapies for alopecia at that time. Three years later, he continues to have patchy hair growth on his arms, scalp and face, which he shaves regularly.
Figure 1.
Hair regrowth on a 38-year-old patient’s (A) face and (B) scalp 8 weeks after FMT.
Case 2
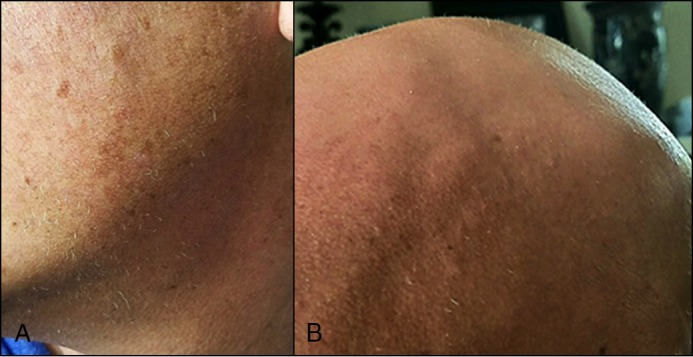
A 20-year-old man with severe ileocolic Crohn’s disease was treated with FMT after requiring 5 recurrent courses of antibiotics for CDI. He remained CDI-free after the procedure. He had been diagnosed with alopecia universalis approximately 2 years prior to FMT, which had been treated with intralesional corticosteroid injections, topical steroids, squaric acid, and laser treatments with no measurable improvement. After FMT, the patient had significant improvement in his hair loss. Only 2 additional intralesional corticosteroid injections with the same strength were done after FMT without any further treatment. Prior to FMT, he was persistently noted to have stage S4b alopecia (95–99% hair loss) despite intralesional injections, but he improved at least 2 grades to stage S2 (25–49% hair loss) after FMT (Figure 2). Notably, while the steroid injections had only been administered to his scalp, after FMT he has had regrowth of hair in other areas of his body as well.
Figure 2.
Hair regrowth on a 20-year-old patient’s scalp after FMT. (A) The patient’s scalp when he first started losing his hair at age 16. The patient’s scalp (B) a few months and (C) 1.5 years after FMT.
Discussion
This report highlights 2 patients with coexisting AA and recurrent CDI who experienced hair regrowth after FMT. This suggests not only an intestinal effect but a profound immunological response to FMT. Though still poorly understood, FMT is emerging as an effective therapy for recurrent CDI, and it is being investigated as a treatment option for other inflammatory conditions.
AA is one of the most common autoimmune disorders, affecting about 4.5 million people in the United States.1 Onset typically occurs in younger patients, with 66% presenting before age 30 and only 20% presenting after age 40. It is thought to be a T-cell-mediated autoimmune disease that attacks the hair follicle, and both the innate and adaptive immune systems have been implicated in the pathogenesis.1 Specifically, T-helper 17 (Th17) cells and interleukin-17 (IL-17) have been implicated in the development of AA.3,4
Current treatments for AA include topical and intralesional steroids for less severe cases and contact immunotherapy for extensive hair loss. A recent study by Avitabile et al showed decreased Th17 and IL-17 infiltrates in the skin of AA patients after successful contact immunotherapy with squaric acid dibutylester.5 Other treatments on the horizon for AA include Janus kinase (JAK) inhibitors and systemic immunomodulators. JAK inhibitors have been shown to be efficacious in inflammatory skin conditions like psoriasis and dermatitis, and they are being investigated in inflammatory bowel disease. Animal models show curative effects for AA with general or topical JAK inhibitors.6 Systemic immunomodulators such as ustekinumab (IL-12/IL-23p40 antibody), which has shown efficacy as induction and maintenance therapy for Crohn’s disease, and apremilast (phosphodiesterase-4 inhibitor) have been proven effective in other inflammatory conditions, such as psoriasis, and show promise for AA.6,7
Evidence linking gut microbiota and immunologic effects in the host is growing. A recent study by Maslowski and Mackay looked at microbiota as the basis for increased incidence of autoimmune conditions in developed countries.8 Short-chain fatty acids have been shown to have anti-inflammatory effects through regulation of T-cells and release of IL-10. They found that western diets, which tend to contain more fat and less fiber, were associated with different compositions of gut bacterial species and lower levels of short-chain fatty acids, potentially driving diseases such as asthma, diabetes, and allergies.8 Helicobacter pylori infection has been implicated in immune thrombocytopenia, illustrative of the potential for gut microbiota to trigger extraintestinal autoimmune disease.9
More recently, clinical trials have focused on the role of FMT in inflammatory bowel disease (IBD).10 Although the mechanism of IBD remains unclear, there is evidence suggesting that an inappropriate immune response to gut microbiota plays a role in the pathogenesis.11 Published case reports have suggested improvement in multiple sclerosis, Parkinson’s disease, and idiopathic thrombocytopenic purpura after FMT.12-14
We present 2 case reports where notable improvement in AA was observed after FMT was performed for recurrent CDI. Further study of gut microbiota in patients with autoimmune alopecia may elucidate disease mechanisms and provide evidence to support clinical trials of FMT in this population for whom treatment options are currently limited.
Disclosures
Author Contributions: All authors contributed equally to manuscript creation. CR Kelly is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Alkhalifah A, Alsantali A, Wang E, et al. Alopecia areata update: Part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177.. [DOI] [PubMed] [Google Scholar]
- 2.Geva-Zatorsky N, Sefik E, Kua L, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 2012;42:2215–20. [DOI] [PubMed] [Google Scholar]
- 4.Tojo G, Fujimura T, Kawano M, et al. Comparison of interleukin-17-producing cells in different clinical types of alopecia areata. Dermatology. 2013;227:78–82. [DOI] [PubMed] [Google Scholar]
- 5.Avitabile S, Sordi D, Garcovich S, L, et al. Effective squaric acid dibutylester immunotherapy is associated with a reduction of skin infiltrating T-helper (Th)1 and Th17 cells in alopecia areata patients. J Dermatol. 2015;42:98–9. [DOI] [PubMed] [Google Scholar]
- 6.Dainichi T, Kabashima K. Alopecia areata: What’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86(1):3–12. [DOI] [PubMed] [Google Scholar]
- 7.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 8.Maslowski KM, Mackay CR. Diet, gut microbiota, and immune responses. Nat Immunol. 2011;12:5–9. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki T, Matsushima M, Masui A, et al. Effect of Helicobacter pylori eradication in patients with chronic idiopathic thrombocytopenic purpura: A randomized controlled trial. Am J Gastroenterol. 2005;100(6):1265–70. [DOI] [PubMed] [Google Scholar]
- 10.Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: Indications, methods, evidence and future directions. G Curr Gastroenterol Rep. 2013;15:337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Round JL, Lee SM, Li J, et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332(6032):974–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) [abstract]. Am J Gastroenterol. 2011;106(S352). [Google Scholar]
- 13.Anathaswamy A. Fecal transplant eases symptoms of Parkinson’s. New Scientist Issue. 2011;2796:19. [Google Scholar]
- 14.Borody TJ, Nowak A, Torres M, et al. Bacteriotherapy in chronic fatigue syndrome: A retrospective sreview. Am J Gastroenterol. 2012;107(Suppl 1):S591–2. [Google Scholar]