Abstract Abstract
The family Doradidae (Siluriformes) is an important group of fishes endemic to freshwater ecosystems in South America. Some cytogenetic studies have been conducted focused on the group; however, there are no reports on the occurrence of B chromosomes for the family. In this paper the chromosomal characteristics of Platydoras armatulus (Valenciennes, 1840), Pterodoras granulosus (Valenciennes, 1821) and Ossancora punctata (Kner, 1855) were investigated through classical cytogenetics approaches. The conventional staining reveals 2n=58 in Platydoras armatulus and Pterodoras granulosus, however with distinct karyotypic formulae, possibly originated by pericentric inversions. In Ossancora punctata a derivate karyotype was described with 2n=66 and predominance of acrocentric chromosomes. The C banding pattern was resolutive in discriminating the three species, being considered an important cytotaxonomic marker. All species showed B chromosomes totally heterochromatic with non-Mendelian segregation during meiosis and low frequencies in mitotic cells. The probably origin of these additional elements was through fragmentations of chromosomes of the standard complement, which occurred recently and independently in these three species. The diploid number observed in Ossancora punctata is an evidence of centric fusions and up to the moment it is the highest diploid number reported for Doradidae.
Keywords: Centric fusion, chromosomal rearrangements, diploid number, neotropical fish, pericentric inversions, supernumerary chromosome
Introduction
Cytogenetic studies in Doradidae are scarce and restricted to nine species. Eight of these bear 58 chromosomes and single (NORs) in terminal positions (Eler et al. 2007, Milhomem et al. 2008). The exception to this pattern is Trachydoras paraguayensis (Eigenmann & Ward, 1907) with 56 chromosomes and single NORs in an interstitial position (Fenocchio et al. 1993, Baumgärtner et al. 2016). The members of Doradidae are popularly named thorny catfish and comprise 94 species and 33 genera (Froese and Pauly 2016) endemic to freshwater ecosystems in South America. The family is easily diagnosed among catfishes by the presence of a row of bony midlateral scutes, each usually bearing a single thorn (Birindelli 2014). Phylogenetic studies based on molecular (Moyer et al. 2004, Arce et al. 2013) and morphological (Birindelli 2014) data support the monophyly of Doradidae.
Supernumerary chromosomes have already been reported in several neotropical Siluriformes families, however up to now they have not been observed in Doradidae (Carvalho et al. 2008, Lui et al. 2009). These additional elements were described in different organisms and can originate in two ways: from chromosomal rearrangements in chromosomes from the A complement (the most common), or as a consequence of interspecific crosses. Regardless of their origin, the majority of B chromosomes due to not possess genes and follow an independent evolutionary path characterized by structural differentiation mechanisms, including the accumulation of different repetitive DNA sequences (Camacho et al. 2000).
In most organisms, the B chromosomes are dispensable elements, as their presence is not associated with phenotypic alterations. However, there are exceptions, as described in Nectria haematococca Samuels & Rossman, 1999 where the Bs possess resistance genes which grant a better pathogenicity (Coleman et al. 2009), and in Lithochromis rubripinnis Seehausen, 1998 in which B chromosomes have a functional effect in sex determination (Yoshida et al. 2011). According to Valente et al. (2016), the recent development of molecular biology associated with the advances in next-generation sequencing technologies have increased knowledge about the biological importance of B chromosomes, revealing that the presence of many genes and other transcriptionally active sequences can modulate the activity of autosomal genes.
In the present study, the karyotypic structure of Platydoras armatulus, Pterodoras granulosus and Ossancora punctata was investigated in mitotic and meiotic cells. This comparative analysis to provide a better understanding of the karyotype diversification in Doradidae, reporting for the first time the occurrence of B chromosomes and discussing the probably origins of this feature in this family.
Material and methods
In the present study, cytogenetic analyses were performed on 9 females and 8 males of Platydoras armatulus; 3 females and 6 males of Ossancora punctata, all collected in the Miranda river, in Corumbá, Mato Grosso do Sul, in the Brazilian Pantanal (19°31'25"S 57°02'26"W). Additionally, 5 females and 4 males of Pterodoras granulosus also collected in the Paraná river, in Pauliceia, São Paulo, Brazil (21°06'10.26"S 51°47'14.1"W), were analyzed. The collection of specimens was authorized by ICMBio. After processing and subsequent fixation of the material, all specimens were deposited in the Museu de Zoologia da Universidade Estadual de Londrina (data available via SpeciesLink).
Before euthanasia (48 hours), the specimens received an intraperitoneal injection of 2 ml of Broncho-vaxom (bacterial lysate) to trigger an inflammatory process and hence increase the number of kidney cells in mitotic division (Molina et al. 2010). After this, all specimens were anesthetized with clove oil (eugenol) and sacrificed to obtain the mitotic chromosomes from kidney cells (Bertollo et al. 1978) and meiotic chromosomes from testis cells (Kligerman and Bloom 1977). The metaphasic chromosomes were classified as metacentric, submetacentric, subtelocentric and acrocentric according to ratio of arms proposed by Levan et al. (1964). The heterochromatin pattern was determined using the C-banding technique (Sumner 1972) with a modification in staining phase (Lui et al. 2012).
Results
Platydoras armatulus
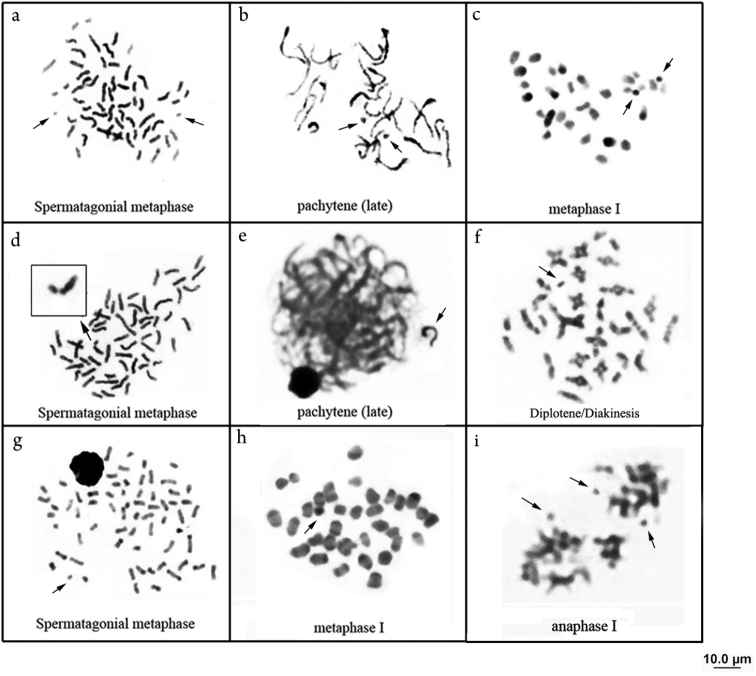
All specimens analyzed exhibited 58 chromosomes (22m + 14sm + 18st + 4a). Eleven samples showed cells carrying from 1-3 B chromosomes (Fig. 1a) with interindividual frequencies ranging from 5.25% to 61.90% (Table 1). C-banding evidenced heterochromatin blocks in the pericentromeric and terminal regions in the short arm of pairs 3, 5, 10, 12, 14, 15, 18, 19, 24, 26, 28 and in the long arm of pairs 3, 4, 12. Interstitial heterochromatin regions also occurred in pairs 2, 21 and 25. The B chromosomes are totally heterochromatic (Fig. 1b). C-banding applied to meiotic cells confirmed the results observed in mitosis in: spermatogonial metaphase with 58 chromosomes (Fig. 2a); late pachytene (more condensed) (Fig. 2b) and metaphase I, with 29 bivalents (Fig. 2c).
Figure 1.
Karyotypes after conventional staining and C-banding – Platydoras armatulus a Giemsa staining reveals 2n=58 (22m+14sm+18st+4a) and 1-3 B chromosomes b C-banding pattern characterized by the many heterochromatin blocks in different positions, including in B chromosomes. Pterodoras granulosus c Giemsa staining also reveals 2n=58 but with distinct karyotypic formulae: 16m+16sm+14st+12a and 1 B chromosome d a few heterochromatin blocks was evidenced after C banding, observe the B chromosome totally heterochromatic. Ossancora punctata e After Giemsa staining it was observed 2n=66 (12m+8sm+6st+40a), the high number of subtelocentric and acrocentric chromosomes is a remarkable feature of this specie f C-banding reveals heterochromatin regions in terminal position and in B chromosomes.
Table 1.
Frequencies of supernumerary chromosomes in Platydoras armatulus, Pterodoras granulosus and Ossancora punctata. ♀= female; ♂= male.
| Species/Samples | Sex | Number of B/cell | Total of cells | Cells with B | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Platydoras armatulus | |||||||
| 4156 | ♂ | 17 | 6 | 8 | 4 | 35 | 51.42% |
| 4157 | ♀ | 29 | 4 | 0 | 0 | 33 | 12.12% |
| 4158 | ♂ | 33 | 6 | 3 | 2 | 43 | 23.25% |
| 4159 | ♀ | 14 | 0 | 0 | 0 | 14 | 0% |
| 4160 | ♂ | 16 | 8 | 12 | 6 | 42 | 61.90% |
| 4161 | ♂ | 17 | 7 | 10 | 5 | 39 | 56.41% |
| 5032 | ♂ | 22 | 4 | 1 | 0 | 27 | 18.51% |
| 5320 | ♀ | 10 | 0 | 0 | 0 | 10 | 0% |
| 5322 | ♀ | 18 | 1 | 0 | 0 | 19 | 5.26% |
| 5325 | ♀ | 15 | 0 | 0 | 0 | 15 | 0% |
| 7 | ♂ | 22 | 3 | 2 | 1 | 28 | 21.42% |
| 8 | ♂ | 31 | 1 | 0 | 2 | 34 | 8.82% |
| 9 | ♀ | 25 | 0 | 0 | 0 | 25 | 0% |
| 80 | ♂ | 11 | 1 | 3 | 0 | 15 | 26.66% |
| 81 | ♀ | 19 | 0 | 0 | 0 | 19 | 0% |
| 82 | ♀ | 21 | 0 | 1 | 1 | 23 | 8.69% |
| 83 | ♀ | 28 | 0 | 0 | 0 | 28 | 0% |
| Pterodoras granulosus | |||||||
| 601 | ♀ | 16 | 2 | 0 | 0 | 18 | 12.5% |
| 602 | ♀ | 15 | 3 | 0 | 0 | 18 | 20% |
| 603 | ♂ | 54 | 9 | 0 | 0 | 63 | 16.6% |
| 604 | ♀ | 63 | 15 | 0 | 0 | 78 | 23.8% |
| 617 | ♂ | 32 | 4 | 0 | 0 | 36 | 12.5% |
| 618 | ♀ | 21 | 0 | 0 | 0 | 21 | 0% |
| 619 | ♀ | 23 | 0 | 0 | 0 | 23 | 0% |
| 628 | ♂ | 16 | 0 | 0 | 0 | 16 | 0% |
| 631 | ♂ | 35 | 6 | 0 | 0 | 41 | 17.1% |
| Ossancora punctata | |||||||
| 4561 | ♀ | 25 | 5 | 3 | 1 | 34 | 26.47% |
| 4566 | ♂ | 23 | 7 | 2 | 4 | 36 | 36.11% |
| 5119 | ♂ | 21 | 4 | 2 | 3 | 30 | 30% |
| 5120 | ♂ | 32 | 0 | 3 | 2 | 37 | 13.51% |
| 5692 | ♂ | 31 | 1 | 0 | 0 | 32 | 3.12% |
| 5694 | ♀ | 14 | 0 | 0 | 0 | 14 | 0% |
| 5695 | ♀ | 6 | 0 | 0 | 0 | 6 | 0% |
| 5696 | ♂ | 23 | 1 | 0 | 0 | 24 | 4.16% |
| 5332 | ♂ | 32 | 3 | 0 | 4 | 39 | 17.94% |
Figure 2.
Meiotic cells in different phases with B chromosomes evidenced after C-banding - Platydoras armatulus a spermatogonial metaphase with 58 chromosomes and 2 B chromosomes b late pachytene with bivalents in advanced condensation stage, note two B chromosomes forming univalents without homologies of standard complement c metaphase I with 27 bivalents and two B chromosomes. Pterodoras granulosus d spermatogonial metaphase composed by 58 chromosomes and one B chromosome e late pachytene, the isolated univalent probably correspond to B chromosome f diplotene/diakinesis with 27 bivalents and one B chromosome, note the high number of chiasms. Ossancora punctata g spermatogonial metaphase with 66 chromosomes and one B chromosome h metaphase I reveals heterochromatic B chromosome and 33 bivalents i anaphase I, observe the late segregation of B chromosomes.
Pterodoras granulosus
Conventional staining with Giemsa revealed 58 chromosomes, with a karyotype formula 16m + 16sm + 14st + 12a. In six specimens, one acrocentric supernumerary chromosome was observed (Fig. 1c) with interindividual frequencies ranging from 12.5% to 23.8% (Table 1). C-banding identified few blocks of heterochromatin restricted in some centromeres, short arm of pair 19 and in B chromosome (Fig. 1d). In meiotic analyses the B chromosomes were observed totally heterochromatic in: spermatogonial metaphase (Fig. 2d), late pachytene with the B chromosome isolated (Fig. 2e) and diplotene/diakinesis (Fig. 2f).
Ossancora punctata
The studied specimens presented 66 chromosomes and a karyotype formula of 12m + 8sm + 6st + 40a. Among all nine specimens analyzed, seven exhibited B chromosomes (Fig. 1e), and the frequencies were considered low, ranging from 3.12 % to 36.11 % of the specimen cells (Table 1). C-banding revealed pericentromeric heterochromatin in most chromosomes, as well as terminal blocks on the long arm of subtelo-acrocentric chromosomes and at the both ends of most metacentric chromosomes (Fig. 1f). The microchromosomes also presented themselves entirely heterochromatic in meiotic analyses in: spermatogonial metaphase (Fig. 2g), metaphase I with 33 bivalents (Fig. 2h) and anaphase (Fig. 2i).
Discussion
Phylogenetic analysis based on morphological and molecular data supports the monophyly of Doradidae that, together with Auchenipteridae, constitutes the superorder Doradoidea (Moyer et al. 2004, Arce et al. 2013, Birindelli 2014). According to some authors, the ancestor of Doradidae had a karyotype composed by 58 chromosomes (Eler et al. 2007, Milhomem et al. 2008, Baumgärtner et al. 2016). In fact, this diploid number is present in Wertheimeria maculata Steindachner, 1877 (Eler et al. 2007) the species considered, with Kalyptodoras bahiensis, the sister group of this family (Birindelli 2014).
Notwithstanding, not all doradid species have 2n = 58, as is the case of Trachydoras paraguayensis with 2n = 56 chromosomes (Fenocchio et al. 1993, Baumgärtner et al. 2016) and Ossancora punctata with 2n = 66 (present study). Baumgärtner et al. (2016) identify (ITS) in Trachydoras paraguayensis, demonstrating the emergence of the 2n = 56 from a karyotype with 58 chromosomes by centric fusion. Diversely, in Ossancora punctata the 2n = 66 is the largest diploid number ever reported for the family and probably originated due to centric fissions resulting in a karyotype with many subtelocentric and acrocentric chromosomes. These variations in diploid numbers show that the pericentric inversions are not the only chromosomal rearrangements that generate macro-structural variability (Eler et al. 2007, Milhomem et al. 2008).
The dispersion of heterochromatic regions is a high variable in Doradidae. Pterodoras granulosus exhibited few blocks, similarly described for Platydoras costatus Linnaeus, 1758 (Milhomem et al. 2008), but distinct from the pattern observed in Platydoras armatulus which exhibited many chromosomes bearing heterochromatin blocks in terminal and interstitial positions. This divergence observed in Platydoras can be an excellent cytogenetic marker, because these two species have 58 chromosomes and similar karyotypic formulae. The heterochromatin pattern of Ossancora punctata is similar to that described in Hassar wilderi Kindle, 1895 (Eler et al. 2007), Hassar orestis Steindachner, 1875, Hassar sp. and Tenellus ternetzi Eigenmann, 1925 (Milhomem et al. 2008) with many terminal blocks, some of these located in both chromosome arms. This C-band pattern reinforced the phylogenetic proximity between these three genera, which constitute one of the most derived clades of Doradidae (Birindelli 2014).
Cytogenetic studies in Neotropical Siluriformes revealed the occurrence of B chromosomes in more than 25 species, including representatives of the families Heptapteridae, Callichthyidae, Pimelodidae, Pseudopimelodidae, Auchenipteridae, Tricomycteridae and Loricariidae (Lui et al. 2009). The B chromosomes of Platydoras armatulus, Pterodoras granulosus and Ossancora punctata presented similar structural characteristics, even though the frequencies in mitotic cells were highly variable. This numerical variability is an evidence of the non-Mendelian segregation theory proposed by Jones and Rees (1982) and occurs because the B chromosomes possess a delayed migration during anaphase, as can be observed in some germ cells of Ossancora punctata (Fig. 2i). Another feature visualized in some spermatocytes was the presence of B chromosomes forming a univalent isolated of the standard complement. This meiotic behavior suggested a structural differentiation of B chromosomes in relation to the standard complement due to accumulation of different families from repetitive DNA (Camacho et al. 2000).
In Neotropical fish, the mechanisms responsible for the origin and evolution of B chromosomes remain unclear, as several theories were proposed (Lui et al. 2009, Blanco et al. 2012, and others). The B microchromosomes were described in distinct neotropical fishes, including Schizodon Agassiz, 1829, Astyanax Baird et Girard, 1854, Moenkhausia Eigenmann, 1903, Cyphocharax Fowler, 1906, Steindacnerina Fowler, 1906, Prochilodus Agassiz, 1829, Rhamdia, Bleeker, 1858 Iheringichthys Eigenmann et Norris, 1900, Callichthys Scopoli, 1777, Megalonema Eigenmann, 1912, Pimelodella, Eigenmann et Eigenmann, 1888 and Loricaria Linnaeus, 1758 (Carvalho et al. 2008, Lui et al. 2009). An interesting hypothesis to explain the origin of these additional genomic elements is the fragmentation in standard karyotype (Sampaio et al. 2015). Considering the morphological type, non-Mendelian segregation and low frequencies in mitotic cells, it seems likely that the B chromosomes observed in Platydoras armatulus, Pterodoras granulosus and Ossancora punctata have a recent origin from fragmentation in chromosomes from A complement.
This study contributed with relevant information to the better understanding of the karyotype variability in Doradidae. In this family, the 2n=58 is considered a primitive condition, such that the chromosomal diversification is based primarily on pericentric inversions and at lower frequency fissions and fusions. Additionally, the mitotic and meiotic analysis revealed at the first time in Doradidae the occurrence of B chromosomes, which originated recently from fragmentations in chromosomes of standard complement. Additional studies such as the isolation and molecular characterization of these chromosomes can be resolutive in confirming its origin and evolution.
Acknowledgements
The authors are grateful to the Universidade Estadual de Londrina (UEL), Centro de Ciências Biológicas (CCB), Departamento de Biologia Geral for providing the laboratory infrastructure to carry out this work; CAPES and Fundação Araucária (JLB,641/2014) for their financial support; and ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade), for permitting the collection of biological material.
Citation
Takagui FH, Dias AL, Birindelli JLO, Swarça AC, Rosa R, Lui RL, Fenocchio AS, Giuliano-Caetano L (2017) First report of B chromosomes in three neotropical thorny catfishes (Siluriformes, Doradidae). Comparative Cytogenetics 11(1): 55–64. https://doi.org/10.3897/CompCytogen.v11i1.10496
References
- Arce MH, Reis ER, Geneva AJ, Sabaj PHM. (2013) Molecular phylogeny of thorny catfishes (Siluriformes:Doradidae). Molecular Phylogenetics and Evolution 67: 560–577. https://doi.org/10.1016/j.ympev.2013.02.021 [DOI] [PubMed] [Google Scholar]
- Baumgärtner L, Paiz LM, Margarido VP, Portela-Castro ALB. (2016) Cytogenetics of the thorny catfish Trachydoras paraguayensis (Eigenmann & Ward, 1907), (Siluriformes, Doradidae): evidence of pericentric inversions and chromosomal fusion. Cytogenetic Genome and Research 149(3): 201–2016. https://doi.org/10.1159/000448126 [DOI] [PubMed] [Google Scholar]
- Bertollo LAC, Takahashi CS, Moreira-Filho O. (1978) Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae). Revista Brasileira de Genetica 1: 103–120. [Google Scholar]
- Birindelli JLO. (2014) Phylogenetic relationships of the South American Doradoidea (Ostariophysi: Siluriformes). Neotropical Ichthyology 12: 451–564. https://doi.org/10.1590/1982-0224-20120027 [Google Scholar]
- Blanco DR, Vicari MR, Artoni RF, Traldi JB, Moreira-Filho O. (2012) Chromosomal Characterization of Armored Catfish Harttia longipinna (Siluriformes, Loricariidae): First Report of B chromosomes in the genus. Zoological Science 29: 604–609. https://doi.org/10.2108/zsj.29.604 [DOI] [PubMed] [Google Scholar]
- Camacho JPM, Sharbel TF, Beukeboom LW. (2000) B-chromosome Evolution. Philosophical Transactions of the Royal Society Biological Sciences. 55: 163–178. https://doi.org/10.1098/rstb.2000.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RA, Martins-Santos IC, Dias AL. (2008) B chromosomes: an update about their occurrence in freshwater Neotropical fishes (Teleostei). Journal of Fish Biology 72: 1907–1932. https://doi.org/10.1111/j.1095-8649.2008.01835.x [Google Scholar]
- Coleman JJ, Rounsley SD, Rodriguez-Carrez M, Kuo A. (2009) The Genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genetics 5: e1000618 https://doi.org/10.1371/journal.pgen.1000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eler ES, Dergam JA, Vênere PC, Paiva LC, Miranda GA, Oliveira AA. (2007) The karyotypes of the thorny catfishes Wertheimeria maculata Steindachner, 1877 and Hassar wilderi Kindle, 1895 (Siluriformes, Doradidae) and their relevance in doradids chromosomal evolution. Genetica 130: 99–103. https://doi.org/10.1007/s10709-006-0023-4 [DOI] [PubMed] [Google Scholar]
- Fenocchio AS, Jorge LC, Venere PC, Bertollo LAC. (1993) Karyotypic characterization and nucleolus organizer regions in three species of Doradidae (Pisces, Siluriformes). Revista Brasileira de Genetica 4: 1097–1101. [Google Scholar]
- Froese R, Pauly D. (2016) FishBase: World Wide Web electronic publication. http://www.fishbase.org [accessed 12 June 2016]
- Jones RN, Rees H. (1982) B Chromosomes. London, Academic Press, 266 pp. [Google Scholar]
- Kligerman AD, Bloom SE. (1977) Rapid chromosome preparations from solid tissues of fishes. Journal of the Fisheries Research Board of Canadá 34: 266–269. https://doi.org/10.1139/f77-039 [Google Scholar]
- Levan A, Fredga K, Sandberg AA. (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52: 201–220. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x [Google Scholar]
- Lui RL, Blanco DR, Margarido VP, Moreira-Filho O. (2009) First description of B chromosomes in the family Auchenipteridae, Parauchenipterus galeatus (Siluriformes) of the São Francisco River basin (MG, Brazil). Micron 40: 552–559. https://doi.org/10.1016/j.micron.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Lui RL, Blanco DR, Moreira-Filho O, Margarido VP. (2012) Propidium iodide for making heterochromatin more evident in the C-banding technique. Biotechnic & Histochemistry 87(7): 433–438. https://doi.org/10.3109/10520295.2012.696700 [DOI] [PubMed] [Google Scholar]
- Milhomem SSR, Souza ACP, Nascimento AL, Carvalho JR, Jr, Feldberg E, Pieczarka JC, Nagamachi CY. (2008) Cytogenetic studies in fishes of the genera Hassar, Platydoras and Opsodoras (Doradidae, Siluriformes) from Jarí and Xingú rivers, Brazil. Genetics and Molecular Biology 31: 256–260. https://doi.org/10.1590/S1415-47572008000200017 [Google Scholar]
- Molina WF, Alves DEO, Araújo WC, Martinez PA, Silva MFM, Costa GWWF. (2010) Performance of human immunostimulating agents in the improvement of fish cytogenetic preparations. Genetics and Molecular Research 9: 1807–1814. https://doi.org/10.4238/vol9-3gmr840 [DOI] [PubMed] [Google Scholar]
- Moyer GR, Burr BM, Krajewski C. (2004) Phylogenetic relationship of thorny catfishes (Siluriformes, Doradidae) inferred from molecular and morphological data. Zoological Journal of Linnean Society 140: 551–575. https://doi.org/10.1111/j.1096-3642.2004.00114.x [Google Scholar]
- Sampaio TR, Gouveia JG, da Silva CRM, Dias AL, da Rosa R. (2015) Molecular analysis of the B microchromosomes in Steindacnerina insculpita (Characiformes: Curimatidae) by microdissection. Cytogenetic and Genome Research 146(1): 51–57. https://doi.org/10.1159/000381932. [DOI] [PubMed] [Google Scholar]
- Sumner AMT. (1972) A simple technique for demonstrating centromeric heterochromatin. Experimental Cell Research 75: 304–306. https://doi.org/10.1016/0014-4827(72)90558-7 [DOI] [PubMed] [Google Scholar]
- Valente GT, Nakajima RT, Fantinatti BEA, Marques DF, Almeida RO, Simões RP, Martins C. (2016) B chromosomes: from cytogenetics to systems biology. Chromosoma. https://doi.org/10.1007/s00412-016-0613-6 [DOI] [PubMed]
- Yoshida K, Terai Y, Mizoiri S, Aibara M, Nishihara H, Watanabe M. (2011) B chromosomes have a functional effect on female sex determination in Lake Victoria cichlid fishes. PLoS Genetics 7: e1002203 https://doi.org/10.1371/journal.pgen.1002203 [DOI] [PMC free article] [PubMed] [Google Scholar]