Abstract
Itraconazole (ITR) is a potent antifungal drug. However, poor aqueous solubility limits its permeation ability across the human nail plate. Therefore, in this project, ITR was converted to hydrochloride salt (ITR-HCl) to improve its solubility and to render it amenable to iontophoresis. ITR-HCl was characterized by spectroscopic methods and antifungal efficacy was evaluated in comparison to the base. In vitro and ex vivo transport studies (passive and iontophoresis) were carried out across the porcine hoof membrane and excised human cadaver toe using two different protocols; continuous delivery of drug for 24 h and pulsed delivery of drug for 3 days (8 h/day). The antifungal efficacy of ITR-HCL was comparable to ITR. Iontophoresis was found to be more effective than passive mode of delivery of ITR-HCL. In both iontophoresis as well as passive mode of delivery, the pulsed protocol resulted in more ungual and trans-ungual delivery of drug than continuous protocol. ITR-HCL could be delivered into and across the nail plate by iontophoresis. Human cadaver toe appears to be a good model to investigate the ungual delivery of drugs.
Keywords: Cadaver toe, iontophoresis, itraconazole, itraconazole hydrochloride, porcine hoof membrane
Introduction
Onychomycosis is fungal infection in the toe and finger nails that is mainly caused by Trichophyton rubrum and candida (dermatophytes)1. Approximately 2–13% of general population is affected by onychomycosis2. Onychomycosis could have significant negative impact on patient’s emotional, social and occupational life.
Currently, onychomycosis is treated by administration of drug via oral as well as topical route. Topical route is the most preferred owing to its advantages such as less severe side effects, non-invasiveness, drug-delivery at the site of infection, patient compliance and lower cost of treatment3. However, poor water-solubility and poor permeability of the drugs across the nail plate limit the topical route of application for the treatment of onychomycosis. Iontophoresis across the nail plate has already been demonstrated to be effective in delivering drugs to the nail apparatus4–13. Recently a clinical trial was performed on iontophoretic ungula patches intended for delivering terbinafine. Clinical trial for power paper iontophoresis patch device was conducted on 38 volunteers. After 8–12 weeks treatment, 84% of patients was reported mycologically cure14.
Itraconazole (ITR) is one of the most effective to treat nail fungus. However, long time oral use of ITR is known to cause severe gastric and systemic side effects such as nausea and vomiting15. According to the FDA, “patients who have signs of ventricular dysfunction such as congestive heart failure or a history of congestive heart failure should not take ITR orally for treatment of onychomycosis”. Topical delivery has not been successful as the ungula permeation was poor. Due to lack of charge on the ITR eliminates the applicability of iontophoresis technique. Therefore, ITR-HCl salt was prepared to render it amenable to iontophoresis.
Methods and materials
Materials
Itraconazole (ITR, molecular weight = 705.2) was purchased from VWR international (Atlanta, GA). Phosphate buffer saline at pH 7.4 (0.138 M of sodium chloride, 0.0027 M potassium chloride) and acetonitrile were purchased from Omnipur and Fisher chemicals (Hanover Park, IL). Excised human cadaver toes were purchased from Science Care (Phoenix, AZ). The Porcine hoof was obtained from Pontotoc slaughtering house (Pontotoc, MS).
Synthesis of ITR-HCl
ITR suspension (40 g/800 mL of acetone) was prepared in a round-bottom flask with three necks. Anhydrous hydrogen chloride gas was bubbled slowly into the suspension while heated under reflux. Hydrogen chloride gas was prepared using solid sodium chloride and concentrated sulfuric acid (H2SO4). After about 30 min, the suspension turned into solution and eventually in the next 5–10 min, ITR-HCl was precipitated. The hydrogen chloride gas was continued to pass for next 2 h. The slurry was allowed to stand for 24 h and then the precipitate was collected, washed with acetone and dried at 105 °C16.
Characterization of ITR-HCl
Differential scanning calorimetry
The Differential scanning calorimetry (DSC) (PerkinElmer, San Jose, CA) for ITR and ITR-HCl was recorded in the range of 3 to 250 °C at a heating rate 10 °C/min and nitrogen flow rate of 22 ml/min.
Mass spectroscopy
Mass spectroscopy of ITR and ITR-HCl was performed using matrix-assisted laser desorption/ionization technique (MALDI-SYNAPT MS/HDMS). Dimethyl sulfoxide was used to dissolve the samples. Samples were scanned from intensity of 200 m/z to 5000 m/z.
Solubility measurement
Solubility studies of ITR-HCl was carried out in water, isopropanol and mixtures of water & isopropanol (90:10, 80:20, 70:30, 60:40 and 50:50 v/v percentage) at pH 3 for preparing appropriate solution to perform iontophoresis. The pH of the solvent system was adjusted using 0.01 N HCl. Excess amount of ITR-HCl was added to the solvents and sonicated for 15 min at room temperature. Suspensions were shaken continuously on the rotary shaker for 2 days, and then filtered using disposable syringe filters (0.45 μm). Amount of dissolved ITR was estimated by HPLC.
Antifungal activity testing of ITR and ITR-HCl
Clinical and laboratory standards institute method was used for antifungal activity testing of ITR and ITR-HCl. Testing Organisms were obtained from the American Type Culture Collection (Manassas, VA) (Candida albicans, Candida krusei, Cryptococcus neoformans, Aspergillus fumigatus, Trichophyton Rubrum). The suspensions of testing organisms for Candida and Cryptococcus species were prepared from 3–5 colonies using sabouraud dextrose (SD) agar in sterile normal saline and incubated for 24–72 h. In the case of trichophyton species, potato dextrose medium was used to prepare the suspension and allowed to incubate for 1 to 2 weeks. Standard curves between optical density and CFU/ml of saline suspensions of test organisms were prepared to calculate the sample assay inocula at 630 nm.
Assay inocula were prepared by diluting organism suspension in the incubation broth (RPMI 1640 broth for Candida spp., SD broth for C. neoformans, and 5% Alamar blue–RPMI 1640 broth for Trichophyton spp) to reach the final inocula (1.5 × 103 CFU/ml for Candida spp. and Cryptococcus spp. or 2.7 × 104 CFU/ml for Trichophyton spp). Verification of inocula for each assay was carried out by platting on SD or PD agar for colony reckoning.
ITR and ITR-HCl samples were diluted by 20% dimethyl sulfoxide–saline to provide enough volume for all organisms and transferred in duplicate to 96-well flat-bottom microplates. ITR and ITR-HCl samples were assayed using two folds serial dilutions by preparing a total of 11 test concentrations. All the sample were diluted and 10 μL of the diluted samples were transferred in duplicate microplate as in template and made up the final volume up to 200 μl of all diluted samples using organism inocula.
Reading of Candida spp. and C. neoformans in the duplicate microplates were done using the Biotek Powerwave XS plate reader (Biotek, Winooski, VT) at 530 nm after and before incubation (Candida spp. at 35 °C for 48 h; C. neoformans at 35 °C for 72 h). Reading of A. fumigatus and Trichophyton spp were carried out using the Polarstar Galaxy plate reader (BMG Lab Technologies, Germany) at the excitation and emission wavelength of 544 nm and 590 nm prior to and after incubation (Trichophyton spp. at 35 °C for 5 days). IC50, MIC and MFC were calculated using the XLFit software (dose-response model 201; IDBS, Alameda, CA)17.
The Minimum inhibitory concentration (MIC) is defined as the lowest concentration that allows no detectable growth (or no more than 20% growth for the azoles and caspofungin). The Minimum Fungicidal Concentration (MFC) is defined as the lowest test concentration that allows no growth of the organism on agar. Half-maximal inhibitory concentration (IC50) is defined as a quantitative measurement of amount of drug that is needed to inhibit half of the biological process17.
In vitro drug transport studies across hoof membrane
In vitro drug transport studies were performed across the porcine hoof membrane as a model for human nail using Franz diffusion cells. Hoof membrane was excised, cleaned and prepared to have a thickness of 150–200 μm using scalpel. The hoof membrane was hydrated with phosphate buffer saline overnight prior to securing it between donor and receiver compartments. Active diffusion area (0.3 cm2) of the hoof membrane was used to perform transport studies. For iontophoresis, platinum and silver chloride electrodes were fixed at 2 mm distance from the hoof membrane in the donor and receiver compartments respectively. Constant DC current (0.5 mA/cm2) was applied using custom designed iontophoresis device (Active dose II iontophoresis delivery unit, Transport Pharmaceuticals, Boston, MA). The donor compartment was filled with 0.5 ml of ITR-HCl solution prepared by dissolving 1 mg of drug in 1 mL of water and isopropanol (1:1) mixture at pH 3. Receiver compartment was filled with 5 ml mixture of isopropanol and phosphate buffer saline (1:1) mixture at pH 3. Magnetic bars of 3 mm length were used to stir (600 rpm) the solution in receiver compartment for uniform distribution of the drug. Passive transport studies were performed simultaneously using same set up of Franz diffusion cells without application of current. Samples (200 μl) were collected at fixed time points from the receiver compartment and analyzed by HPLC6,7.
The passive and iontophoresis transport studies were performed following two different protocols. First protocol included continuous studies for 24 h and Pulsed protocol involved transport studies for 8 h/day for three days. In case of continuous protocol studies, the formulation in the donor was replaced every 8 hours with fresh formulation. In the pulsed mode, the hoof surface was washed after each 8-hour episode of transport studies and allowed to be in contact with the receiver compartment for remaining 16 hours.
Extraction of ITR from hoof membrane
After in vitro transport studies, the hoof membrane was detached from the adapter. Active diffusion area (0.3 cm2) of the membrane was marked and excised using metric punch apparatus before washing. Each nail plate was washed out by shaking (2 times) in the water and isopropanol mixture (1:1 at pH 3) and 95% ethanol with the help of forceps. This process was performed alternatively five times with both solvents. The nail plate was wiped each time using Kimwipe® (Kimberly Clark, Pleasant Praire, WI). Each plate was cut into small pieces and solubilized in 2 ml of 1 M sodium hydroxide solution by subjecting to ultrasonic treatment at 37 °C for 2 h. Six milliliters 6 ml of ethyl acetate was added to the final solution and ethyl acetate layer was separated out to another glass vial. Two milliliters of 1 M hydrochloric acid was added to the remaining sodium hydroxide layer for reverse extraction. To this solution, another 6 ml of ethyl acetate was added and ethyl acetate layer was separated out to another glass vial. Both ethyl acetate glass vials were mixed together and ethyl acetate was evaporated using nitrogen gas to get solid crystals of ITR-HCl. Finally, ITR-HCl was dissolved in water and isopropanol mixture (1:1) at pH 3 prior to analysis18.
Iontophoresis with excised human cadaver toe
Hydroxypropylmethyl cellulose gel (2% HPMC) incorporated with ITR-HCl was prepared using water and isopropanol (1:1) mixture adjusted to pH 3. Excised human cadaver toe model was used to perform the ex vivo drug transport studies. The cadaver toes were dipped in 0.5% gentamycin solution and dried one day prior to use in the study and stored at −20 °C, the toes were thawed at room temperature and then the studies were performed at room temperature conditions. Custom-designed patch was prepared to carry out the transport studies similar to that discussed earlier18. Adhesive backing membrane was used to fix electrode to the nail plate. Polyurethane foam pad was used to expose the drug and current on the fixed area. HPMC gel was filled up in the fixed area using spatula. Counter electrode filled with conductive gel (no drug) was adhered to the bottom of the toe. Anodal and cathodal electrodes were used as the active and counter electrodes respectively. A constant DC (0.5 mA/cm2) was applied for 24 h (again following two different protocols as described in section 2.6) using iontophoresis device. Passive transport studies were performed simultaneously with iontophoresis studies using same set up on the toe without current application. The amount of drug was estimated by HPLC after extraction of drug from the nail plate and nail bed8.
Extraction of ITR from nail plate and nail bed
Following transport studies, the nail plate was detached from the intact toe using blunt forceps and scalpel. Nail surface was washed (protocol discussed in section 2.7) to get rid of the adhering drug. Active diffusion area of nail plate was excised using a metric punch. Eventually, amount of ITR was extracted from the nail plate and measured.
Nail bed was separated carefully from the intact toe. Nail bed was homogenized and dissolved in the 1 M sodium hydroxide. The drug was extracted from sodium hydroxide solution using same procedure, detailed in section 2.718.
Analytical method
The amount of ITR was determined by high performance liquid chromatography system (HPLC, waters, 1525) consisting of an auto sampler (waters 717 plus), phenomenex C18 (2) 100 R analytical column (4.6 mm × 150 mm, luna, 5.0 μm), waters dual wavelength UV detector (2487). Mobile phase was prepared by combination of three solvents, Acetonitrile, nanopure water and diethylamine (70:30:0.05). Elution of drug was carried out isocratically at 32 °C using a flow rate of 1.0 ml/min and 30 μl injection volume. ITR was detected at 261 nm. Calibration curve was prepared using a range from 0.01–10 μg/ml (R2=0.99)3.
Statistical analysis
Statistical analysis of ITR-HCl permeation studies was performed by student t-test. The p value less than 0.05 was considered significant difference.
Results and discussion
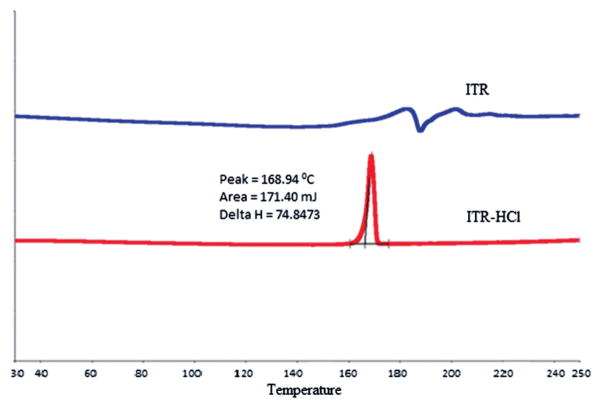
ITR-HCl was characterized by Differential scanning calorimetry (DSC) and MASS spectroscopy. According to the DSC thermogram, sharp melting endothermic peak was found in case of ITR at 171 °C. However, endothermic melting peak was not found in case of ITR-HCl indicating likely modification of the base into salt15 (Figure 1).
Figure 1.
DSC (PerkinElmer, San Jose, CA) thermogram of ITR and ITR–HCl.
Mass Spectroscopy of ITR and ITR-HCl was carried out by Matrix-assisted laser desorption/ionization technique (MALDI-SYNAPT MS/HDMS). According to mass spectra, peak of ITR was found at 705.64 m/z. ITR-HCl formation was confirmed by appearance of peak at ~741.02 m/z.
Solubility studies of ITR-HCl were performed in water, isopropanol, and mixture of water and isopropanol at pH 3 (Table 1). The maximum solubility of ITR-HCl found in water and isopropanol mixture (50:50 v/v) at pH 3 was 37.52 mg/ml which was ~181-folds more when compared with ITR (0.207 mg/ml).
Table 1.
The solubility of ITR and ITR-HCl (mg/ml) in different solvent systems.
| Solubility of ITR and ITR-HCl (mg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Water | Isopropanol | Water pH 3 | Water:Isopropanol ratio (pH 3) | |||||
|
| ||||||||
| 90:10 | 80:20 | 70:30 | 60:40 | 50:50 | ||||
| ITR | – | 0.288 | – | – | – | – | 0.112 | 0.207 |
| ITR-HCl | 0.01 | 15.15 | 0.04 | 0.06 | 0.09 | 0.13 | 0.41 | 37.52 |
Antifungal activity assays of ITR and ITR-HCl were performed on the fungal cultures such as T. rubrum, C. albicans, C. krusei, A. fumigatus and C. neoformans. According to the results, antifungal activity of ITR and ITR-HCl did not differ significantly in terms of IC50, MIC and MFC levels against all the fungal species tested in this work (Table 2).
Table 2.
Antifungal activity of ITR and ITR-HCl on different fungus species.
| Organisms | ITR (μg/ml) | ITR-HCl (μg/ml) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| IC50 | MIC | MFC | IC50 | MIC | MFC | |
| T. rubrum | 0.063 ± 0.02 | 0.26 ± 0.08 | >20.0 | 0.06 ± 0.01 | 0.31 ± 0.05 | >20.0 |
| C. albicans | 0.18 ± 0.07 | >20.0 | >20.0 | 0.11 ± 0.05 | >20.0 | >20.0 |
| C. krusei | 0.23 ± 0.03 | 0.87 ± 0.001 | 1.15 ± 0.25 | 0.27 ± 0.144 | 0.84 ± 0.005 | 1.04 ± 0.15 |
| A. fumigatus | 0.50 ± 0.17 | 1.04 ± 0.35 | 6.33 ± 1.35 | 0.29 ± 0.03 | 1.06 ± 0.25 | 6.67 ± 1.04 |
| C. neoformans | <0.02 | 0.027 ± 0.002 | 0.03 ± 0.001 | <0.02 | 0.02 ± 0.002 | 0.03 ± 0.001 |
All the values are an average of n =3.
Transport studies
Transport studies of ITR-HCl were performed using porcine hoof as well as excised human toe model18. The transport studies were performed by passive and iontophoresis modes using two different protocols. Continuous protocol involved continuous application of formulation for 24 hours. However, in case of continuous protocol also, the applied formulation was replaced every 8 hours for the sake of having sufficient chloride ions in the donor compartment. In non-continuous or pulsed protocol, the duration of application was at 8 h per day for three days (equivalent to 24 hours) across the porcine hoof membrane as well as in human toe model. These protocols were selected to compare the drug delivery efficiency in two different application conditions. The continuous protocol represents a condition where the subject would be applied with a device for prolonged duration. The pulsed protocol represents wearing the device only for a few hours a day for multiple days. In case of in vitro studies using Franz cell, pH 3 solvent system was used in the receiver compartment to maintain sink conditions. Previous studies have shown that formulation with pH 3 did not affect the constitution or permeability of the nail plate5,7.
Anodal iontophoresis was performed because of positive charge on the ITR. Platinum wire was used as an anodal electrode in the donor compartment because ITR was found to be precipitated due to pH change (pH 3–3.8) of drug solution in the donor compartment by interaction with Ag electrode. In case of platinum wire, pH of the donor compartment was dropped from 3.0 to 2.5 after the application of iontophoresis for 8 h. After every 8 h, drug solution in the donor compartment was replaced with fresh drug solution to avoid further drop in pH. AgCl electrode was used as cathode electrode in the receiver compartment. The receiver compartment pH was increased to 3.4 at the end of 24 hours. Replacement of fresh buffer solution after each sampling was found to keep the pH from going above 3.4.
Passive versus iontophoresis
In case of continuous protocol, the cumulative amount of ITR in the receiver compartment after application of iontophoresis across the hoof membrane was 0.91 ± 0.11 μg/cm2 which was ~30-folds (p<0.05) more than passive (0.03 ± 0.01 μg/cm2). The amount of drug retained in the hoof membrane by iontophoresis was 4.8 ± 1.2 μg/mg which was ~5-folds (p<0.05) more than passive (0.95 ± 0.54 μg/mg).
In case of pulsed protocol, the cumulative amount of drug transported in the receiver compartment by iontophoresis was 2.12 ± 0.30 μg/cm2 which was ~27-folds (p<0.05) more when compared to the passive (0.08 ± 0.01 μg/cm2). On the other hand, the amount of drug found in the hoof membrane by the application of iontophoresis was 4.95 ± 1.52 μg/mg which was ~4-folds (p<0.05) more than passive (1.3 ± 0.60 μg/mg; Table 3). These studies have clearly demonstrated the ability of iontophoresis to enhance the delivery of ionic drugs across the nail plate. Iontophoresis was also found to enhance the drug holding capacity of the nail plate (Table 4).
Table 3.
Amount of ITR was found in the receiver compartment (μg/cm2) and hoof membrane (μg/mg) after in vitro transport studies.
| Amount of ITR | ||||
|---|---|---|---|---|
|
| ||||
| Mode of drug loading | In vitro studies | |||
|
| ||||
| 24 h study | 3 days (8 h/day) study | |||
|
|
|
|||
| Receiver compartment (μg/cm2) | Hoof membrane (μg/mg) | Receiver compartment (μg/cm2) | Hoof membrane (μg/mg) | |
| Passive | 0.03 ± 0.01 | 0.95 ± 0.54 | 0.08 ± 0.01 | 1.3 ± 0.60 |
| Iontophoresis | 0.91 ± 0.11 | 4.8 ± 1.2 | 2.12 ± 0.30 | 4.95 ± 1.52 |
Table 4.
Amount of drug was found in the nail bed (μg/mg) and nail plate (μg/mg) after ex vivo transport studies.
| Amount of ITR | ||||
|---|---|---|---|---|
|
| ||||
| Mode of drug loading | Ex vivo studies | |||
|
| ||||
| 24 h study | 3 days (8 h/day) study | |||
|
|
|
|||
| Nail bed (μg/mg) | Nail plate (μg/mg) | Nail bed (μg/mg) | Nail plate (μg/mg) | |
| Passive | 0.003 ± 0.09 | 1.61 ± 0.73 | 0.11 ± 0.07 | 1.75 ± 0.98 |
| Iontophoresis | 1.17 ± 0.60 | 4.64 ± 1.14 | 2.65 ± 1.09 | 4.96 ± 1.64 |
Continuous versus pulsed protocol
In both passive as well as iontophoresis modes, the permeation of drug across the hoof membrane was significantly higher in case of pulse protocol as compared to continuous protocol. In the case of pulse protocol, although the duration of application of formulation is same as continuous protocol, there is pause time between the episodes, during which significant amount of drug could diffuse into the sub-ungual tissues (receiver compartment in case of Franz cell studies). This is likely to render the nail more receptive to drug uptake during the subsequent episode of application. Whereas, in the case of continuous protocol, the saturation of nail plate is likely to hamper the delivery of drug. However, regardless of the protocol, the amount of drug in the hoof membrane appears to saturate and did not differ significantly between continuous and pulsed protocols.
Human toe versus porcine hoof model
Porcine hoof has been suggested as a good model for human nail plate19. A good correlation between the permeability of drugs across the bovine hoof with that across the human nail plate has been reported by Mertin and Lippold20. To assess if there exists any correlation between the porcine hoof in Franz cell model with excised cadaver toe model, two correlation plots were created. The amount of drug permeated across the hoof membrane at a given mode and protocol of delivery was matched with the amount of drug permeated across the nail plate into the nail bed when same delivery mode and protocol was used. Similarly, the drug loaded in the hoof in Franz cell experiments was matched with the levels in the nail plate in toe model. The drug load in the porcine hoof membrane versus drug loaded in the nail plate showed an excellent correlation (R2=0.93; Figure 2). Whereas, the correlation between the amount of drug permeated across the hoof membrane into the receiver compartment and the amount of drug found in the nail bed was relatively modest (R2=0.56; Figure 3). The reason for this poor correlation is likely due to lack of clearance in the toe model. Although, the few number of data points are available for correlation, there appears to be a clear trend of positive correlation which is likely to strengthen with the inclusion of additional data in the future. The present studies have demonstrated that the excised human toe model could be an acceptable model to investigate the ungual drug delivery, despite its limitations.
Figure 2.
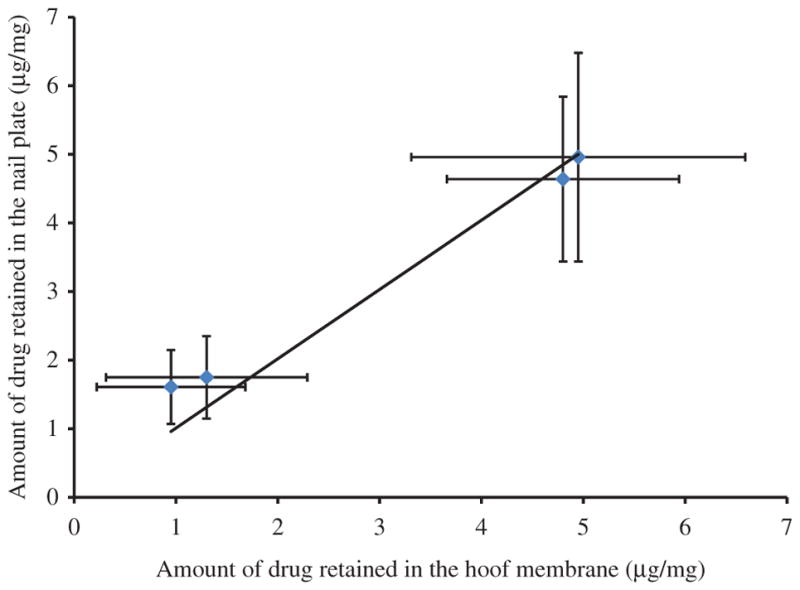
Correlation between amount of drug retained in the nail plate and amount of drug retained in the hoof membrane after transport studies.
Figure 3.
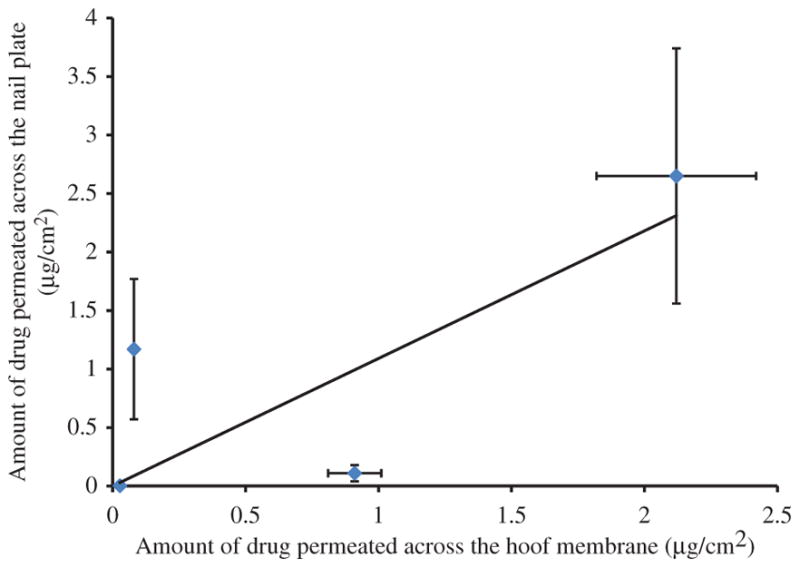
Correlation between amount of drug permeated across the nail plate and amount of drug permeated across the hoof membrane.
Conclusion
In vitro and ex vivo transport studies have demonstrated the feasibility of iontophoresis technique to enhance the trans-ungual delivery of ITR. Iontophoresis also enhanced the amount of drug loaded in the nail/hoof. Pulsed application protocol was found to be superior over the continuous application protocol in both passive as well as iontophoresis mode of trans-ungual drug delivery. The level of drug found in the nail bed/receiver compartment was estimated more than MIC level. This means in clinical practice, dividing the duration of application into multiple episodes would be more beneficial to the subject than continuous application of iontophoresis over long time.
Acknowledgments
The authors would like to thank Dr. Amala Dass and Vijay Reddy Jupally for ESI-MS measurements (Department of Chemistry, University of Mississippi).
Footnotes
Declaration of interest
This project was partially funded by a grant number AI 27094 from National Institute of Allergyand Infectious Diseases (NIAID). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415–29. doi: 10.1128/cmr.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hao J, Smith KA, Li SK. Iontophoretically enhanced ciclopirox delivery into and across human nail plate. J Pharm Sci. 2009;98:3608–16. doi: 10.1002/jps.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26. doi: 10.1016/s0378-5173(01)00989-9. [DOI] [PubMed] [Google Scholar]
- 4.Murthy SN, Waddell DC, Shivakumar HN, et al. Iontophoretic permselective property of human nail. J Dermatol Sci. 2007;46:150–2. doi: 10.1016/j.jdermsci.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Nair AB, Kim HD, Chakraborty B, et al. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis. J Pharm Sci. 2009;98:4130–40. doi: 10.1002/jps.21711. [DOI] [PubMed] [Google Scholar]
- 6.Nair AB, Vaka SR, Sammeta SM, et al. Trans-ungual iontophoretic delivery of terbinafine. J Pharm Sci. 2009;98:1788–96. doi: 10.1002/jps.21555. [DOI] [PubMed] [Google Scholar]
- 7.Nair AB, Kim HD, Davis SP, et al. An ex vivo toe model used to assess applicators for the iontophoretic ungual delivery of terbinafine. Pharm Res. 2009;26:2194–201. doi: 10.1007/s11095-009-9934-y. [DOI] [PubMed] [Google Scholar]
- 8.Nair AB, Sammeta SM, Kim HD, et al. Alteration of the diffusional barrier property of the nail leads to greater terbinafine drug loading and permeation. Int J Pharm. 2009;375:22–7. doi: 10.1016/j.ijpharm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Shivakumar HN, Juluri A, Desai BG, Murthy SN. Ungual and transungual drug delivery. Drug Dev Ind Pharm. 2012;38:901–11. doi: 10.3109/03639045.2011.637931. [DOI] [PubMed] [Google Scholar]
- 10.Nair AB, Chakraborty B, Murthy SN. Effect of polyethylene glycols on the trans-ungual delivery of terbinafine. Curr Drug Deliv. 2010;7:407–14. doi: 10.2174/156720110793566308. [DOI] [PubMed] [Google Scholar]
- 11.Nair AB, Vaka SR, Murthy SN. Transungual delivery of terbinafine by iontophoresis in onychomycotic nails. Drug Dev Ind Pharm. 2011;37:1253–8. doi: 10.3109/03639045.2011.568946. [DOI] [PubMed] [Google Scholar]
- 12.Murthy SN. Iontophoresis for treating nail diseases. Ther Deliv. 2013;4:647–50. doi: 10.4155/tde.13.42. [DOI] [PubMed] [Google Scholar]
- 13.Murthy SN, Maibach HI. Topical nail products and ungual drug delivery. Florida: CRC Press; 2013. pp. 165–87. [Google Scholar]
- 14.Gupta AK, Simpson F. Device-based therapies for onychomycosis treatment. Skin Therapy Lett. 2012;17:4–9. [PubMed] [Google Scholar]
- 15.Trey SM, Wicks DA, Mididoddi PK, Repka MA. Delivery of itraconazole from extruded HPC films. Drug Dev Ind Pharm. 2007;33:727–35. doi: 10.1080/03639040701199225. [DOI] [PubMed] [Google Scholar]
- 16.Tao T, Zhao Y, Wu J, Zhou B. Preparation and evaluation of itraconazole dihydrochloride for the solubility and dissolution rate enhancement. Int J Pharm. 2009;367:109–14. doi: 10.1016/j.ijpharm.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 17.Li XC, Jacob MR, Khan SI, et al. Potent in vitro antifungal activities of naturally occurring acetylenic acids. Antimicrob Agents Chemother. 2008;52:2442–8. doi: 10.1128/AAC.01297-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobue S, Sekiguchi K, Nabeshima T. Intracutaneous distributions of fluconazole, itraconazole, and griseofulvin in Guinea pigs and binding to human stratum corneum. Antimicrob Agents Chemother. 2004;48:216–23. doi: 10.1128/AAC.48.1.216-223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myoung Y, Choi HK. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Eur J Pharm Sci. 2003;20:319–25. doi: 10.1016/j.ejps.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Mertin D, Lippold BC. In-vitro permeability of the human nail and of a keratin membrane from bovine hooves: prediction of the penetration rate of antimycotics through the nail plate and their efficacy. J Pharm Pharmacol. 1997;49:866–72. doi: 10.1111/j.2042-7158.1997.tb06127.x. [DOI] [PubMed] [Google Scholar]