ABSTRACT
Chronic wasting disease (CWD) is a naturally occurring, fatal neurodegenerative disease of cervids. The potential for swine to serve as hosts for the agent of CWD is unknown. The purpose of this study was to investigate the susceptibility of swine to the CWD agent following experimental oral or intracranial inoculation. Crossbred piglets were assigned to three groups, intracranially inoculated (n = 20), orally inoculated (n = 19), and noninoculated (n = 9). At approximately the age at which commercial pigs reach market weight, half of the pigs in each group were culled (“market weight” groups). The remaining pigs (“aged” groups) were allowed to incubate for up to 73 months postinoculation (mpi). Tissues collected at necropsy were examined for disease-associated prion protein (PrPSc) by Western blotting (WB), antigen capture enzyme immunoassay (EIA), immunohistochemistry (IHC), and in vitro real-time quaking-induced conversion (RT-QuIC). Brain samples from selected pigs were also bioassayed in mice expressing porcine prion protein. Four intracranially inoculated aged pigs and one orally inoculated aged pig were positive by EIA, IHC, and/or WB. By RT-QuIC, PrPSc was detected in lymphoid and/or brain tissue from one or more pigs in each inoculated group. The bioassay was positive in four out of five pigs assayed. This study demonstrates that pigs can support low-level amplification of CWD prions, although the species barrier to CWD infection is relatively high. However, detection of infectivity in orally inoculated pigs with a mouse bioassay raises the possibility that naturally exposed pigs could act as a reservoir of CWD infectivity.
IMPORTANCE We challenged domestic swine with the chronic wasting disease agent by inoculation directly into the brain (intracranially) or by oral gavage (orally). Disease-associated prion protein (PrPSc) was detected in brain and lymphoid tissues from intracranially and orally inoculated pigs as early as 8 months of age (6 months postinoculation). Only one pig developed clinical neurologic signs suggestive of prion disease. The amount of PrPSc in the brains and lymphoid tissues of positive pigs was small, especially in orally inoculated pigs. Regardless, positive results obtained with orally inoculated pigs suggest that it may be possible for swine to serve as a reservoir for prion disease under natural conditions.
KEYWORDS: chronic wasting disease, prions, swine, transmissible spongiform encephalopathy
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) or prion diseases are fatal neurodegenerative diseases. Naturally occurring TSEs include chronic wasting disease (CWD) in cervids, scrapie in sheep, bovine spongiform encephalopathy (BSE) in cattle, and sporadic and familial prion diseases in humans.
The potential for swine to serve as hosts for the agent of CWD is unknown. A naturally occurring TSE has not been reported in swine (1, 2). Intracranial challenge of swine with brain tissue from patients with kuru, a human prion disease, was not successful (3), although at the time of those studies, molecular tests for disease-associated prion protein (PrPSc) were not available. Pigs have been shown to be susceptible to BSE following parenteral inoculation (simultaneously by the intraperitoneal, intravenous, and intracranial routes), to ovine BSE following intracranial inoculation (4), and to ovine scrapie following intracranial inoculation (5) but not to BSE after an oral challenge with a large amount of infected brain material (6–8).
The CWD agent has a wide host range among cervids and can be experimentally transmitted to several other species. Naturally occurring CWD has been reported in cervids, including mule deer (Odocoileus hemionus) (9–11), Rocky Mountain elk (Cervus elaphus nelson) (11, 12), white-tailed deer (Odocoileus virginianus) (10, 11), moose (Alces alces shirasi) (13, 14), and reindeer (Rangifer tarandus tarandus) (15). In addition, Eurasian red deer (Cervus elaphus) (16), Eurasian fallow deer (Dama dama) (17), Asian muntjac deer (Muntiacus reevesi) (18), and reindeer (19, 20) have been shown to be susceptible to CWD following experimental inoculation. CWD has been experimentally transmitted to noncervid species, including sheep (21), cattle (22–25), domestic cats (26, 27), ferrets (28, 29), nonhuman primates (30–32), and laboratory rodents (reviewed in reference 33).
Pigs could be exposed to CWD infectivity via two main routes, (i) exposure of farmed or pet swine (Sus scrofa domesticus) to contaminated feed and (ii) exposure of feral swine (S. scrofa) to CWD-infected carcasses or contaminated environments. In the United States, feeding of ruminant by-products to ruminants is prohibited but feeding of ruminant materials to swine, mink, and poultry still occurs. Therefore, it is possible that, if a CWD-affected cervid carcass entered the food chain through a commercial slaughterhouse, domesticated farmed and pet swine could be exposed to CWD infectivity in commercially prepared rations. As of 2015, feral pigs have been reported in 39 U.S. states (34), and in 12 of these states, CWD has been detected in free-ranging cervid populations (35). Environmental contamination with CWD infectivity in excreta or decomposing carcasses contributes to horizontal transmission of CWD in mule deer (10). Prion infectivity has been shown to persist on the surface of contaminated plant leaves and roots (36) and in soil (37–39). Therefore, feral pigs could be exposed to infectivity through scavenging of CWD-affected carcasses, by consumption of contaminated vegetation, and while rooting around in the soil during foraging. In this study, we demonstrate that swine are susceptible to the CWD agent following oral or intracranial experimental inoculation and accumulate PrPSc in both brain and lymphoid tissues. Detection of PrPSc in brain and lymphoid tissues from orally inoculated pigs at 6 months after inoculation raises the possibility that naturally exposed pigs could potentially be a reservoir for CWD prions.
RESULTS
Clinical presentation.
All pigs culled at 6 mpi (8 months of age; eight intracranially [i.c.] inoculated, nine orally inoculated) were clinically normal, with the exception of one pig (no. 35) that was noted to be limping on its left front and rear legs. Four i.c. inoculated pigs and one orally inoculated pig developed intercurrent lameness from approximately 30 mpi, usually beginning with the feet and legs and progressing to difficulty rising. At approximately 41 mpi, four clinically normal pigs (one noninoculated, three orally inoculated) were culled to reduce animal density in the containment space. Neurological signs were observed in one pig (no. 27; incubation period, 64 mpi) that included difficulty rising, and muscle fasciculations and tremors after rising. Pig 27 also had skin abrasions and/or ulceration over pressure points and polyarthritis. All other pigs were found dead or culled because of intercurrent disease, most commonly lameness that was not responsive to treatment.
Detection of PrPSc.
To determine if pigs inoculated with the agent of CWD accumulate misfolded prion protein in the central nervous system, we assayed the brain stem by Western blotting (WB), enzyme immunoassay (EIA), immunohistochemistry (IHC), and in vitro real-time quaking-induced conversion (RT-QuIC). Results of screening of brain stem material from all pigs by WB and EIA and results of additional testing of animals that were PrPSc positive by either screening test are shown in Table 1.
TABLE 1.
Detection and characterization of PrPSc from selected pigs
| Treatment group and animal no. | Incubation period (mpi) | Overall result |
Antigen capture EIA result | WB result | IHC result | PK sensitivity | RT-QuIC result | RT-QuIC AFR | |
|---|---|---|---|---|---|---|---|---|---|
| CNSa | LRSb | ||||||||
| Control market wt | |||||||||
| 1 | 0 | − | NTc | NAd | NA | NT | NT | NT | NT |
| 2 | 0 | − | NT | − | − | NT | NT | NT | NT |
| 3 | 0 | − | NT | − | − | NT | NT | NT | NT |
| 4 | 6 | − | NT | − | − | NT | NT | NT | NT |
| 5 | 6 | − | − | − | − | − | NA | − | 0 |
| Control aged | |||||||||
| 6 | 25 | − | NT | − | − | NT | NT | NT | NT |
| 7 | 41 | − | NT | − | − | NT | NT | NT | NT |
| 8 | 46 | − | NT | − | − | NT | NT | NT | NT |
| 9 | 73 | − | − | − | − | − | NA | − | 0 |
| i.c. inoculated market wt | |||||||||
| 10 | 0 | − | NT | − | − | NT | NT | NT | NT |
| 11 | 0 | − | NT | − | − | NT | NT | NT | NT |
| 12 | 6 | + | + | − | − | − | NA | + | 0.031 |
| 13 | 6 | − | NT | − | − | NT | NT | NT | NT |
| 14 | 6 | + | + | − | − | − | NA | + | 0.025 |
| 15 | 6 | + | + | + | − | − | sensitive | − | 0 |
| 16 | 6 | − | + | − | − | − | NA | − | 0 |
| 17 | 6 | − | NT | − | − | NT | NT | NT | NT |
| 18 | 6 | + | + | − | − | − | NA | + | 0.120 |
| 19 | 6 | − | − | − | − | − | NA | − | 0 |
| i.c. inoculated aged | |||||||||
| 20 | 30 | − | NT | − | − | NT | NT | NT | NT |
| 21 | 30 | − | NT | − | − | NT | NT | NT | NT |
| 22 | 30 | + | + | − | − | − | NA | + | 0.080 |
| 23 | 30 | − | NT | − | − | NT | NT | NT | NT |
| 24 | 42 | + | + | + | − | − | sensitive | + | 0.030 |
| 25 | 45 | + | + | + | − | + | resistant | + | 0.180 |
| 26 | 56 | + | + | + | − | + | resistant | + | 0.190 |
| 27 | 64 | + | − | + | + | − | resistant | + | 0.170 |
| 28 | 73 | + | + | + | + | + | resistant | + | 0.210 |
| 29 | 73 | + | + | − | − | − | NA | + | 0.050 |
| Orally inoculated market wt | |||||||||
| 32 | 6 | + | + | − | − | − | NA | + | 0.070 |
| 38 | 6 | + | + | − | − | − | NA | + | 0.010 |
| 30 | 6 | − | + | − | − | − | NA | − | 0 |
| 36 | 6 | − | + | − | − | − | NA | − | 0 |
| 37 | 6 | − | + | − | − | − | NA | − | 0 |
| 34 | 6 | − | − | − | − | − | NA | − | 0 |
| 31 | 6 | − | NT | − | − | NT | NT | NT | NT |
| 33 | 6 | − | NT | − | − | NT | NT | NT | NT |
| 35 | 6 | − | NT | − | − | NT | NT | NT | NT |
| Orally inoculated aged | |||||||||
| 39 | 19 | − | + | − | − | − | NA | − | 0 |
| 40 | 41 | − | NT | − | − | NT | NT | NT | NT |
| 41 | 41 | + | + | − | − | − | NA | + | 0.029 |
| 42 | 41 | − | NT | − | − | NT | NT | NT | NT |
| 43 | 45 | + | + | − | − | − | NA | + | 0.020 |
| 44 | 55 | + | + | − | − | − | NA | + | 0.030 |
| 45 | 64 | + | − | + | − | + | sensitive | + | 0.010 |
| 46 | 65 | − | NT | − | − | NT | NT | NT | NT |
| 47 | 65 | − | NT | − | − | NT | NT | NT | NT |
| 48 | 72 | + | − | − | − | − | NA | + | 0.010 |
CNS, central nervous system.
LRS, lymphoreticular system.
NT, sample not tested.
NA, result not applicable.
WB.
By WB, PrPSc was detected in brain tissue from two i.c. inoculated pigs (no. 27 and 28) necropsied at 64 and 73 mpi, respectively (Table 1).
The migration pattern of samples from pigs inoculated i.c. with the CWD agent was different from that of either the sample from a pig inoculated with classical BSE or the original CWD inoculum (Fig. 1). While the monoglycosylated (middle) band was most prominent in the sample from the pig inoculated with the BSE agent, the diglycosylated (top) band was most prominent in the sample from the pig inoculated with the CWD agent and the original CWD inoculum.
FIG 1.
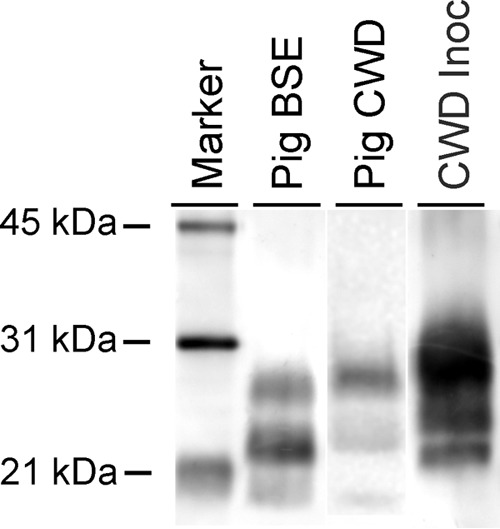
WB analysis demonstrating a unique PrPSc profile in brain samples from pigs with CWD. The positive brain sample from a pig inoculated with the CWD agent (pig CWD) has a slightly higher migration than the brain sample from a pig inoculated with the agent of classical BSE (pig BSE) and a much lower migration than the CWD inoculum (CWD Inoc). The diglycosylated band (topmost band in each lane) is more prominent in the pig CWD and CWD Inoc samples, while the monoglycosylated (middle) band is most prominent in the pig BSE sample. The blot was developed with monoclonal antibody L42. Note that because of the sparse PrPSc accumulation in the brains of inoculated pigs, the blot shown is a composite; see Materials and methods for details.
EIA.
By EIA, misfolded protein was detected in brain tissue from 1/10 i.c. inoculated market weight pigs, 5/10 i.c. inoculated aged pigs (42 to 73 mpi), 0/9 orally inoculated market weight pigs, and 1/10 orally inoculated aged pigs (Table 1).
RT-QuIC.
By RT-QuIC, PrPSc was detected in brain stem material from 3/6 i.c. inoculated market weight pigs, 7/7 i.c. inoculated aged pigs, 2/6 orally inoculated market weight pigs, and 5/6 orally inoculated aged pigs (Table 1; Fig. 2). For each positive sample, we quantified the seeding activity based on the amyloid formation rate (AFR), which is the reciprocal of the time (in hours) that it takes for a reaction to reach the threshold, defined as the mean baseline fluorescence plus 5 standard deviations. For i.c. inoculated pigs (n = 10), the mean AFR of each animal ranged from 0.025 to 0.210. For orally inoculated pigs (n = 7), the range of mean AFRs was 0.010 to 0.029 (Table 1; Fig. 2). Average RT-QuIC data, generated by calculating the mean of all replicates from all of the animals in each challenge group, are shown in Fig. 3.
FIG 2.
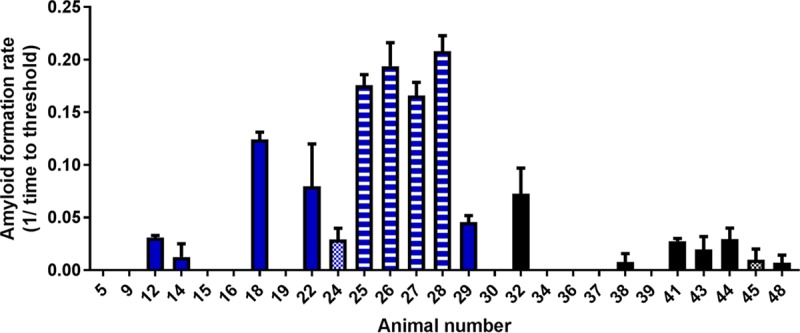
AFRs (RT-QuIC) and PK sensitivity (EIA) of PrPSc from pig brain samples. Treatment groups: animals 5 and 9, noninoculated controls; 12 to 19, i.c. inoculated market weight pigs; 22 to 29, i.c. inoculated aged pigs; 30 to 38, orally inoculated market weight pigs; 39 to 48, orally inoculated aged pigs. PK sensitivity: solid fill, PK sensitivity not determined (EIA negative); horizontal stripe fill, PK resistant; checked fill, PK sensitive.
FIG 3.
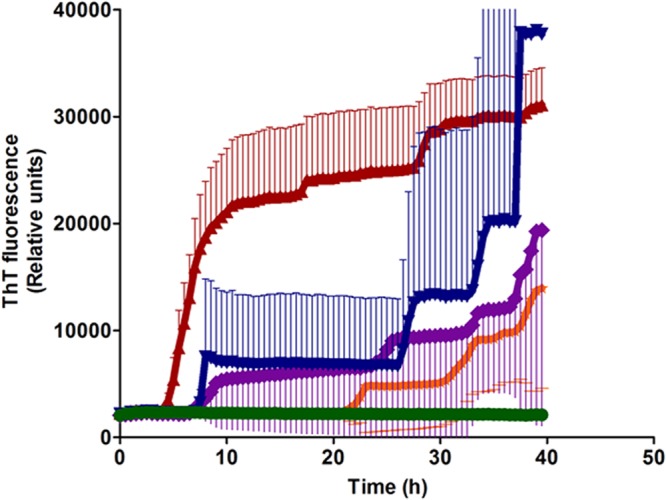
Results of RT-QuIC assays of brain homogenate from inoculated and negative-control pigs. Shown are the average percent thioflavin T (ThT) fluorescence readings (thick lines) with standard deviations (thin lines) determined from all replicates (four replicate reactions per animal) from all of the pigs in each challenge group. Red, i.c. inoculated aged pigs (n = 7); blue, i.c. inoculated market weight pigs (n = 6); purple, orally inoculated market weight pigs (n = 6); orange, orally inoculated aged pigs (n = 6); green, noninoculated control pigs (n = 2).
Differential PK sensitivity of brain stem samples.
To investigate possible biochemical properties of PrPSc that may have contributed to the variation in aggregation kinetics observed in the RT-QuIC assay, the EIA optical density of matched samples was measured with and without treatment with proteinase K (PK). The difference in optical density between non-PK-treated and PK-treated samples allows us to estimate the relative PK resistance of the PrPSc present in the brains of infected pigs (40).
PrPSc in EIA-positive brain tissue from one i.c. inoculated market weight pig (no. 15), one orally inoculated aged pig (no. 45), and one i.c. inoculated aged pig (no. 24) was PK sensitive. PrPSc from the remaining four pigs with samples positive by EIA, all from the i.c. inoculated aged pig group, was PK resistant (Table 1). PK titration of all EIA-positive samples was performed, and the results were consistent across PK concentrations of 0.4 to 50 μg/ml.
Six brain samples were EIA and RT-QuIC positive. Of these, the four samples that were PK resistant had higher AFRs (range, 0.17 to 0.21), while the two samples that were PK sensitive had lower AFRs (0.01 and 0.03) (Fig. 2).
Detection of PrPSc in lymphoid tissues.
To determine if pigs inoculated with the CWD agent accumulate misfolded prion protein in lymphoid tissues, EIA and RT-QuIC were applied to samples of the retropharyngeal lymph node (RPLN), palatine tonsil, and mesenteric lymph node (MLN). Full results for individual pigs are shown in Table 2.
TABLE 2.
Detection of PrPSc in lymphoid tissues by antigen capture EIA and RT-QuIC assay
| Treatment group and animal no. | Incubation period (mpi) | Overall result | RPLNb |
Tonsil |
MLNc |
|||
|---|---|---|---|---|---|---|---|---|
| EIA result | RT-QuIC result | EIA result | RT-QuIC result | EIA result | RT-QuIC result | |||
| Noninoculated controls | ||||||||
| 5 | 6 | − | − | − | − | − | − | − |
| 9 | 73 | − | NAa | NA | − | − | − | − |
| i.c. inoculated market wt | ||||||||
| 12 | 6 | + | − | − | − | − | − | + |
| 14 | 6 | + | − | + | − | + | − | − |
| 15 | 6 | + | − | + | − | + | − | + |
| 16 | 6 | + | − | + | − | + | − | + |
| 18 | 6 | + | − | − | − | − | − | + |
| 19 | 6 | − | − | − | − | − | − | − |
| i.c. inoculated aged | ||||||||
| 22 | 30 | + | − | − | − | − | − | + |
| 24 | 42 | + | NA | NA | − | + | NA | NA |
| 25 | 45 | + | − | + | − | − | − | − |
| 26 | 56 | + | − | + | − | + | NA | NA |
| 27 | 64 | − | NA | NA | − | − | NA | NA |
| 28 | 73 | + | NA | NA | − | + | − | + |
| 29 | 73 | + | − | + | − | − | − | − |
| Orally inoculated market wt | ||||||||
| 30 | 6 | + | − | − | − | − | − | + |
| 32 | 6 | + | − | − | − | + | − | + |
| 34 | 6 | − | − | − | − | − | − | − |
| 36 | 6 | + | − | − | − | + | − | + |
| 37 | 6 | + | − | + | − | − | + | + |
| 38 | 6 | + | − | − | − | + | − | + |
| Orally inoculated aged | ||||||||
| 39 | 19 | + | − | − | − | − | − | + |
| 41 | 41 | + | − | + | − | − | − | + |
| 43 | 45 | + | − | NA | − | + | NA | NA |
| 44 | 55 | + | NA | NA | − | − | − | + |
| 45 | 64 | − | − | NA | − | − | NA | NA |
| 48 | 72 | − | NA | NA | − | − | − | − |
NA, sample not available.
RPLN, retropharyngeal lymph node.
MLN, mesenteric lymph node.
All of the lymphoid tissues tested were PrPSc negative by EIA, with the exception of those of pig 37 (orally inoculated market weight pig), which had a positive MLN. By the RT-QuIC assay, PrPSc was detected in lymphoid tissues of the head (RPLN, palatine tonsil) in 3/6 i.c. inoculated market weight pigs, 5/7 i.c. inoculated aged pigs, 4/6 orally inoculated market weight pigs, and 2/6 orally inoculated aged pigs. The MLN was positive in 5/6 orally inoculated market weight pigs, 3/4 orally inoculated aged pigs (samples were not available from 2 pigs), 4/6 i.c. inoculated market weight pigs, and 2/4 i.c. inoculated aged pigs. Overall, the MLN was positive in 14/19 (74%) samples examined, the RPLN was positive in 8/18 (44%), and the tonsil was positive in 10/25 (40%).
Histopathology and IHC.
To determine if pigs inoculated with the CWD agent develop spongiform lesions or accumulate misfolded prion protein in the brain, coronal brain sections were examined by light microscopy after hematoxylin and eosin staining and by IHC.
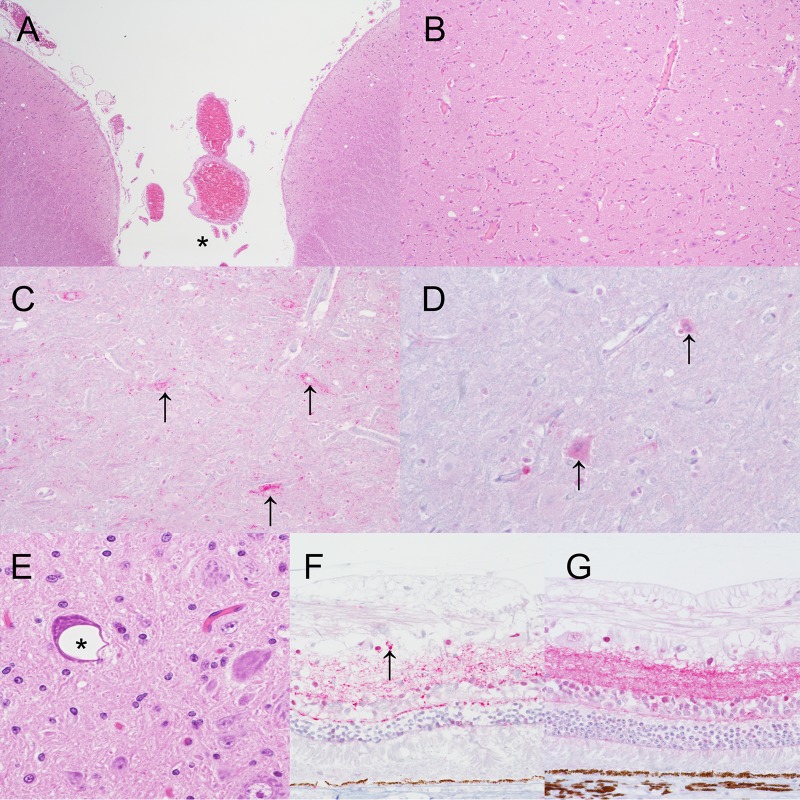
Occasional neuropil vacuolation and white matter vacuolation were present in different brain sections of control and inoculated pigs. Small to medium-sized gray matter vacuoles were seen in the colliculus of at least one pig from each treatment group, including control pigs (Fig. 4A, pig 7, and B, pig 25). Vacuolation and PrPSc deposition in the colliculus were present in two pigs (no. 25 and 26) from the i.c. inoculated aged pig group (Fig. 4C, pig 25). Intraneuronal vacuolation was observed in large neurons of the dorsal motor nucleus of the vagus nerve (DMNV) in the medulla at the level of the obex (Fig. 4E, pig 38). This type of vacuolation was present in pigs from all of the market weight treatment groups, including noninoculated control pigs, and in aged control pigs. PrPSc deposition in association with DMNV vacuolation was not observed in any pigs.
FIG 4.
Vacuolar change and PrPSc in the brain and eye. (A) Brain stem of pig 7 showing incidental, i.e., unrelated to prion disease, neuropil vacuolation in the colliculus. *, midline (hematoxylin and eosin staining; original magnification, ×4). (B) Higher-magnification view of panel A (original magnification, ×10). (C) Brain stem of pig 25 showing intraneuronal PrPSc immunoreactivity (arrows) in neurons in the colliculus (anti-PrP monoclonal antibody L42; original magnification, ×20). (D) Brain stem of noninoculated control pig 8 showing non-disease-specific intraneuronal immunolabeling (arrows) in neurons in the colliculus (anti-PrP monoclonal antibody L42; original magnification, ×40). (E) Brain stem of pig 38 showing incidental intraneuronal vacuolation (*) in the dorsal motor nucleus of the vagus nerve (hematoxylin and eosin staining; original magnification, ×40). (F) Retina of pig 26 showing granular to punctate PrPSc immunoreactivity in the inner and outer plexiform layers with occasional intraglial deposits (arrow) (anti-PrP monoclonal antibody L42; original magnification, ×40). (G) Retina of noninoculated control pig 4 showing non-disease-specific immunolabeling (anti-PrP monoclonal antibody L42; original magnification, ×40).
Positive PrPSc immunoreactivity was observed in samples from four pigs. In the brain, PrPSc immunoreactivity appeared as the intraneuronal type (coarse granular deposits of PrPSc in the neuronal perikarya surrounding the nucleus) in large neurons of the rostral medulla reticular formation (pig 26), midbrain colliculus (pigs 25 and 26), midline thalamic nuclei and hypothalamus (pigs 45 and 28), or septal nuclei (pig 28) (Fig. 4C).
PrPSc immunoreactivity was also seen in the retina of one pig, i.e., granular to punctate immunoreactivity in the inner and outer plexiform layers with occasional intraglial deposits (Fig. 4F, pig 26). Disease-specific PrPSc immunoreactivity was not seen in any other tissues, although nonspecific immunolabeling was common (Fig. 4D, brain stem, and G, retina).
Mouse bioassay.
To determine if pigs inoculated with the CWD agent accumulate infectious material, brain stem material from selected pigs was bioassayed in Tg002 mice that express porcine prion protein at normal levels (5).
Pigs from the i.c. inoculated market weight (pig 18) and i.c. inoculated aged (pigs 27 and 28) groups and the orally inoculated aged group (pig 48) produced positive bioassay results (Table 3). In mice inoculated with brain material from pig 18 (an i.c. inoculated market weight pig), the average incubation period was 244 days postinoculation (dpi) (2/28 mice). In mice inoculated with brain material from pig 27 (i.c. inoculated market aged pig group), the average incubation period was 167 (range, 140 to 220) dpi (3/29 mice). Two out of 27 mice were positive in the group inoculated with brain material from pig 28; 1 mouse was found dead at 314 dpi, and the other was euthanized at the end of the study at 701 dpi. The highest attack rate resulted from the orally inoculated aged pig (no. 48), with 14/28 mice positive and an average incubation period of 263 (range, 111 to 621) dpi.
TABLE 3.
Results of bioassays of brain material from selected pigs in Tg002 mice that express porcine prion protein
| Donor animal no. | Donor treatment group (donor incubation period [mpi]) | Tg002 mouse |
|
|---|---|---|---|
| Attack ratea | Mean incubation period (dpi) | ||
| 18 | i.c. inoculated market wt (6) | 2/29 | 244 |
| 27 | i.c. inoculated aged (64) | 3/29 | 167 |
| 28 | i.c. inoculated aged (73) | 2/27 | 314, 701b |
| 32 | Orally inoculated market wt (6) | 0/28 | >700 |
| 48 | Orally inoculated aged (72) | 14/28 | 263 |
PrPSc in the brains of mice was detected by an antigen capture EIA.
The survival times of these two mice are so disparate that calculation of a mean incubation period would not be meaningful.
All of the pigs that produced a positive bioassay result also had a positive RT-QuIC result. In addition, pigs 27 and 28 were positive by WB (both pigs), EIA (both pigs), and IHC (pig 28 only). Bioassay of brain tissue from pig 32 in the orally inoculated market weight group was unsuccessful (0/28 mice; the study ended at 702 dpi) (Table 3), although PrPSc was detected in the brain of this pig by RT-QuIC (Table 1).
DISCUSSION
We demonstrated that PrPSc can be detected as early as 6 months postinoculation (mpi) in brain and lymphoid tissues of pigs inoculated orally or i.c. with the CWD agent. We show that pigs inoculated with CWD rarely develop neurologic signs suggestive of prion disease, although PrPSc can be detected in brain samples. Furthermore, neuropathological changes are often equivocal and the amount of PrPSc present is generally low, so sensitive methods such as RT-QuIC and bioassay were used for PrPSc detection.
Prion infection was subclinical in most of the pigs in this study; PrPSc was detected in brain tissue from 18 pigs, but neurologic signs suggestive of prion disease were observed in only 1 pig. This pig developed clinical signs of difficulty in rising and signs of tremor. Both of these clinical signs have been reported previously in pigs challenged with the BSE agent (6) or the sheep-passaged BSE agent (4). A number of pigs developed persistent recumbency with difficulty in rising, but these clinical signs were attributed to musculoskeletal lameness rather than neurological disease.
Similar to pigs with BSE (8), PrPSc accumulation was sparse and did not necessarily correlate with the degree of spongiform change. In addition to having a restricted distribution, the range of morphological types of PrPSc was limited to just the intraneuronal type. Prominent intraneuronal immunolabeling is also a feature of scrapie in pigs (5). In contrast, a wider variety of PrPSc deposit types has been described in pigs challenged with the cow-passaged (6, 8) or sheep-passaged (4) BSE agent.
A mild spongiform change was observed in the brains of both inoculated and noninoculated pigs, suggesting that the presence of a spongiform change in the brain should not be used as the sole diagnostic test for CWD in pigs. Similar to results reported by others, microscopic changes in negative-control and inoculated pigs were limited to occasional scattered vacuoles in the neuropil or white matter throughout the brain (1), neuropil vacuolation of the superficial layers of the rostral colliculus (1, 8), and occasional neuronal vacuolation in the dorsal motor nucleus of the vagus nerve (1, 2, 8). Since the above microscopic changes can be observed in both noninoculated control and inoculated pigs, when present in inoculated pigs, they are considered equivocal, i.e., not related to prion disease. Colocalization of neuropil vacuolation and intraneuronal PrPSc deposits was present in the rostral colliculus of two pigs in our study, but vacuolation did not extend to deeper layers of the rostral colliculi or to other areas of the brain (8), so it was considered equivocal.
Limited microscopic and immunohistopathologic changes observed in the brains of pigs with CWD compared to pigs inoculated with cow- or sheep-adapted BSE suggests that the species barrier to CWD transmission to pigs is higher than that to BSE transmission to pigs. Despite this, pigs are able to accumulate misfolded prion protein and CWD infectivity.
By standard diagnostic tests (WB, EIA, and IHC), PrPSc was detected in brain or lymphoid tissues from eight pigs in this study. The number of positive animals and tissues, in particular lymphoid tissues, was much higher when the RT-QuIC assay was used. By RT-QuIC, PrPSc was detected in brain and lymphoid tissues that were PrPSc negative by all other tests. This is not surprising, considering that RT-QuIC is reported to be at least as sensitive as a bioassay (41) and 10,000 times as sensitive as EIA and WB for the detection of scrapie seeding activity in goat brain samples (42). With the exception of IHC, diagnostic tests were performed with brain stem samples since this brain region is the preferred site for statutory diagnostic testing. Testing of additional brain regions might have revealed PrPSc accumulation elsewhere in the brain, as was observed by IHC.
The RT-QuIC assay allows quantification of the seeding activity of prions in the samples on the basis of AFRs. The AFR is calculated as the reciprocal of the time it takes for a reaction to reach the threshold (i.e., 1/time to threshold in hours). A higher AFR reflects a shorter time taken to reach the threshold, which can also be termed a shorter “lag phase.” Lag phases have previously been shown to be inversely correlated with seed concentration in RT-QuIC reactions (41, 43, 44). Since the AFRs of samples from i.c. inoculated aged pigs tended to be higher than those of samples from orally inoculated aged pigs, it follows that the relative amount of PrPSc in the brain is larger in i.c. inoculated pigs. This seems logical, considering that PrPSc in the inoculum was delivered directly into the brain in i.c. inoculated pigs but delivered to peripheral tissues (oral cavity and gastrointestinal tract) in orally inoculated pigs.
We observed that the AFR of samples from positive animals that were determined to be PK sensitive was approximately 1 order of magnitude lower than the AFR of samples from positive animals that were PK resistant. Although the interpretation of these observations is limited by the small sample size and the fact that samples were not normalized for total protein content, it appears that there may be a relationship between AFR and PK sensitivity.
One hypothesis is that larger seed particles present more seeding surfaces than smaller particles and thus support faster RT-QuIC kinetics (45). In scrapie-infected hamsters, PK-sensitive PrPSc molecules from low-molecular-weight aggregates are made up of fewer PrP units (i.e., are smaller) than PK-resistant PrPSc aggregates (46, 47). Combining these observations with our own results, we hypothesize that the smaller average seed particle size of PK-sensitive PrPSc may result in slower RT-QuIC kinetics and lead to lower AFRs and longer lag times. However, as stated above, this hypothesis is based on a small number of samples.
The detection of PrPSc in lymphoid tissues from the heads and guts of CWD-infected pigs raises the possibility that pigs are able to shed prions in excreta, as has been shown for saliva (48–51) and feces (52–54) from CWD-affected cervids. Unfortunately, saliva and feces samples were not collected in the present study.
PrPSc was detected in brain and lymphoid tissues from orally inoculated pigs killed at approximately market weight. These results suggest that, if they were to be exposed to sufficient amounts of CWD infectivity, pigs in commercial swine production systems would have the potential to accumulate CWD prions by the time they reach market weight.
In the case of feral pigs, exposure to the agent of CWD through scavenging of CWD-affected cervid carcasses or through consumption of prion-contaminated plants or soil could allow feral pigs to serve as a reservoir of CWD infectivity. The range and numbers of feral pigs are predicted to continue to increase because of the ability of pigs to adapt to many climates, reproduce year-round, and survive on a varied diet (55). The range of CWD-affected cervids also continues to spread, increasing the likelihood of overlap of ranges of feral pigs and CWD-affected environments.
We demonstrate here that PrPSc accumulates in lymphoid tissues from pigs inoculated i.c. or orally with the CWD agent and can be detected as early as 6 months after inoculation. Clinical disease suggestive of prion disease developed only in a single pig after a long (64-month) incubation period. This raises the possibility that CWD-infected pigs can shed prions into their environment long before they develop clinical disease. However, the small amounts of PrPSc detected in our study pigs combined with the low attack rates in Tg002 mice suggest that there is a relatively strong species barrier to CWD prion transmission to pigs.
MATERIALS AND METHODS
Ethics statement.
All of our animal experiments were reviewed and approved by the National Animal Disease Center (NADC) Institutional Animal Care and Use Committee (IACUC; protocols 3510 [swine] and 2422 [mice]) and were carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC) and the Guide for the Care and Use of Agricultural Animals in Research and Teaching (Federation of Animal Science Societies, Champaign, IL). Pigs were observed daily for clinical signs of disease and euthanized and necropsied at approximately 6 mpi or when unequivocal signs of prion disease such as behavior changes, decreased feed intake, loss of body condition, ataxia, prolonged recumbency, or inability to rise were confirmed by a veterinarian or when euthanasia was necessary because of intercurrent illness or injury that could not be remediated by veterinary care. Euthanasia was performed by intravenous injection of sodium pentobarbital in accordance with the manufacturer's instructions.
Inoculum preparation.
The pooled CWD inoculum was prepared from three brains from white-tailed deer that were inoculated i.c. with brain material from CWD-affected elk, white-tailed deer, or mule deer (NADC IACUC protocol 3347) (56). All donor deer were homozygous for glycine (G/G) at PRNP codon 96 and serine (S/S) at codon 138. The brain tissue was ground in a mechanical grinder and mixed with phosphate-buffered saline (PBS) to produce a 10% (wt/vol) homogenate.
Animal procedures.
Crossbred piglets were inoculated at 8 weeks of age. Pigs inoculated i.c. (n = 20) received a single dose of 0.75 ml of 10% (wt/vol) CWD brain homogenate as described previously (57). Orally inoculated pigs (n = 19) received 15 ml of 10% (wt/vol) CWD brain homogenate by syringe with a soft feeding tube on 4 consecutive days (total dose, 45 ml). Pigs inoculated i.c. and orally were housed in separate pens. At 2 weeks postinoculation, noninoculated control pigs were introduced into the pens with the inoculated pigs.
At 6 to 7 months of age, approximately the time at which commercial pigs reach market weight, half of the pigs in each group were culled (“market weight” groups) as follows: eight i.c. inoculated pigs, nine orally inoculated pigs, and two control pigs. The remaining pigs (“aged” groups) were allowed to incubate for up to 73 mpi when the study ended. Swine were observed daily for the development of clinical signs.
Mouse bioassay.
Infectivity in brain tissue from selected pigs was assayed via intracranial inoculation of Tg002 mice that express porcine prion protein (GenBank porcine sequence accession no. GU595061) at approximately 1× the expression level of prion protein in FVB mice (5). Samples of brain stem at the level of the obex were prepared as 10% (wt/vol) homogenates in PBS. Mice were inoculated i.c. with 20 μl of 10% (wt/vol) brain homogenate as described previously (58). Mice were monitored daily and euthanized when they displayed unequivocal neurological signs (difficulty moving, poor coordination, inability to move, anorexia) or at the time of study termination (approximately 700 dpi). Brain samples from mice were prepared as 10% (wt/vol) brain homogenates in PBS as described previously (59). PrPSc was detected by EIA as described below.
Sample collection.
A full necropsy was performed on all pigs, including collection of two sets of tissue samples. To minimize potential cross-contamination, one pathologist collected tissues from the head and a second pathologist collected tissues from the rest of the body. Single-use instruments were not used. One set of tissues included representative sections of liver, kidney, spleen, skin, striated muscles (heart, tongue, diaphragm, masseter, triceps, biceps femoris, psoas major), lymphoid tissues of the head (pharyngeal tonsil, palatine tonsil, medial RPLN), other lymph nodes (mesenteric, hepatic, renal, popliteal, prescapular), nasal turbinates, lung, esophagus, small intestine, cecum, colon, rectal mucosa, stomach, adrenal gland, pituitary gland, reproductive tissues, peripheral nervous system (trigeminal ganglion, optic nerve, sciatic nerve, vagus nerve), brain (hemisections of cerebral cortex, hippocampus, cerebellum, superior colliculus, and brain stem, including obex), and eye (retina). Formalin-fixed tissues were fixed in 10% neutral buffered formalin, moved to 70% ethyl alcohol after 48 h, embedded in paraffin wax, sectioned, and stained with hematoxylin and eosin for light microscopy. The second set of tissues was frozen.
Selection of animals and tissues for PrPSc detection.
Frozen brain stem tissue from all pigs was screened for the presence of PrPSc by antigen capture EIA and WB. Fixed tissues from pigs that were positive by WB and/or EIA were examined by IHC. In addition, representative pigs from across the range of survival times in each group were also examined by IHC. Brain stem material from the pig with the longest incubation period in each treatment group was bioassayed in Tg002 mice. PrPSc detection by QuIC assay was applied to frozen brain stem and lymphoid tissues from all pigs that were positive by any other test (EIA, WB, IHC, bioassay), as well as additional animals, so that six or seven animals per group and across a range of survival times were tested.
IHC.
All paraffin-embedded tissues were immunostained with anti-PrP monoclonal antibody L42 by an automated immunohistochemical method for detection of PrPSc as described previously (60).
Antigen capture EIA.
Brain homogenates were homogenized in 1× PBS at a concentration of 20% (wt/vol) and assayed with a commercially available EIA kit (HerdChek BSE-Scrapie Ag Test; IDEXX Laboratories, Westbrook, ME) as previously described (61). Assays were performed in accordance with the manufacturer's instructions. The EIA kit instructions indicated three protocols (standard, short, and ultrashort). The short protocol was used to test tissue samples in the present study. Each tissue sample homogenate was assayed in a single well along with negative and positive controls supplied with the kit. Two conjugate concentrate products were included with the kit, a conjugate concentrate intended for use with brain samples obtained from small ruminants (SRB-CC) and a conjugate concentrate intended for use with brain samples obtained from cattle or lymph node or spleen samples obtained from small ruminants (CC). In this study, SRB-CC conjugate was used to test samples obtained from mice expressing pig prion protein. Absorbance at 450 nm was measured (SpectraMax 190; Molecular Devices, Sunnyvale, CA) by using a reference wavelength of 620 nm. Cutoff values were established for each run in accordance with the kit instructions, whereby 0.180 was added to the mean negative-control value. Samples were interpreted as positive if the absorbance at 450 nm minus the reference value at 620 nm was above the established cutoff value.
EIA-based PK sensitivity testing.
Sensitivity to PK was determined by the EIA protocol described above but with the addition of a pretesting PK treatment step (40). Briefly, for each animal, two 100-μl aliquots of 20% (wt/vol) brain homogenate were prepared; 5 μl of 1 mg/ml PK (USB Corporation, Cleveland, OH, USA) was added to one aliquot, and 5 μl of PBS was added to the second aliquot. Both aliquots were incubated for 1 h at 37°C with shaking at 1,000 rpm, followed by the addition of 1.0 μl of 100 mg/ml PK inhibitor (Pefabloc; Roche Diagnostics, Mannheim, Germany). The absorbance of each sample was determined by EIA as described above. Samples for which the non-PK-treated aliquot was EIA positive and the PK-treated aliquot was EIA negative were classified as PK sensitive. Samples for which the non-PK-treated aliquot was EIA positive and the PK-treated aliquot was EIA positive were classified as PK resistant.
WB.
Samples for WB were collected from the brain stem at the level of the obex and the midbrain between the optic and oculomotor nerves dorsal to the pituitary as previously reported (57). Tissues were homogenized and enriched as described previously (22), with the following modifications. After the pellets were resuspended in 100 μl of water, samples were digested with PK at a final enzyme concentration of 0.4 U/ml (8 μg/ml) at 37°C for 1 h. Digestion was stopped by the addition of a serine protease inhibitor (Pefabloc SC; Roche Diagnostics GmbH, Mannheim, Germany) to a final concentration of 1 mg/ml. Western blots were developed with mouse anti-PrP monoclonal antibody L42, which targets amino acids 145 to 163 of the ovine prion protein sequence (62), at a 1:500 dilution (0.1 μg/ml).
Because of the sparse PrPSc accumulation in the brains of inoculated pigs, the blot in Fig. 1 is a composite. The pig CWD sample was enriched and loaded at 100 mg/eq. The pig BSE positive-control tissue was provided by the APHA Biological Archive (Addlestone, United Kingdom).
Expression and purification of the recombinant PrP substrate.
The recombinant prion protein (rPrP) used in the RT-QuIC assay was expressed and purified by a previously reported standard protocol (41, 63). Briefly, rPrP composed of Syrian hamster PrP residues 90 to 231 in the pET vector was transformed into Escherichia coli Rosetta2(DE3) cells and purified from inclusion bodies by fast protein liquid chromatography as described previously (44, 64).
RT-QuIC assay for brain and lymphoid tissue samples.
We included brain and lymphoid tissue homogenates from clinical CWD-affected white-tailed deer, age group-matched noninoculated pigs, and a blank (buffer) as controls. Samples were collected by a strict aseptic technique to minimize the risk of cross-contamination. All of the samples were run by using a blinded study design (N.K., S.M.).
Prior to testing, brain and lymphoid tissue samples were homogenized in 1× PBS at a concentration of 20% (wt/vol) tissue and then further homogenized by repeated pipetting and sonication in a cup sonicator with two pulses of 30 s. The samples were then further diluted to a concentration of 0.02% in sample dilution buffer (0.025% SDS in 1× PBS).
The RT-QuIC assay was performed by previously published protocols (41, 65), with slight modifications as described previously (64). All samples were run in quadruplicate. The reaction mixtures consisted of 5 μg of protein from the brain and lymphoid tissue homogenates that were used as seed in a 100-μl total reaction volume. A sample was considered positive if the fluorescence intensity of at least half the replicate wells crossed the threshold, which was calculated as the mean fluorescence of the negative-control sample plus 10 standard deviations (66–68). For each positive sample, we quantified the seeding activity on the basis of the AFR, which is the reciprocal of the time (in hours) that it takes for a reaction to reach the threshold, defined as the mean baseline fluorescence plus 5 standard deviations (41, 65). The AFR was calculated by using all four replicates of each sample. Data analysis was performed with BioTek' s Gen5 software version 2.07.17 and BMG's MARS software version 5.2.R8.
ACKNOWLEDGMENTS
We thank Martha Church, Kevin Hassall, Robyn Kokemuller, Joe Lesan, Leisa Mandell, Dennis Orcutt, and Trudy Tatum for providing critical technical support to this project. We are grateful to the APHA Biological Archive for providing tissue from a pig with BSE for use as a positive control for IHC and WB analyses. We also acknowledge Byron Caughey for providing the rPrP construct used in this study.
The PoPrP-Tg002 mice were produced by Glenn Telling through specific cooperative agreement 58-3625-7-649 with the University of Kentucky.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal-opportunity employer.
REFERENCES
- 1.Jahns H, Callanan JJ, Sammin DJ, McElroy MC, Bassett HF. 2006. Survey for transmissible spongiform encephalopathies in Irish pigs fed meat and bone meal. Vet Rec 159:137–142. doi: 10.1136/vr.159.5.137. [DOI] [PubMed] [Google Scholar]
- 2.Köfler M, Seuberlich T, Maurer E, Heim D, Doherr M, Zurbriggen A, Botteron C. 2006. TSE surveillance in small ruminants and pigs: a pilot study. Schweiz Arch Tierheilkd 148:341–342, 344–348. (In German.) doi: 10.1024/0036-7281.148.7.341. [DOI] [PubMed] [Google Scholar]
- 3.Gajdusek DC, Gibbs CJ Jr, Alpers M. 1967. Transmission and passage of experimental “kuru” to chimpanzees. Science 155:212–214. http://life.umd.edu/classroom/HONR299J/Science%201967.pdf. [PubMed] [Google Scholar]
- 4.Hedman C, Bolea R, Marin B, Cobriere F, Filali H, Vazquez F, Pitarch JL, Vargas A, Acin C, Moreno B, Pumarola M, Andreoletti O, Badiola JJ. 2016. Transmission of sheep-bovine spongiform encephalopathy to pigs. Vet Res 47:14. doi: 10.1186/s13567-015-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenlee JJ, Kunkle RA, Smith JD, West Greenlee MH. 2016. Scrapie in swine: a diagnostic challenge. Food Safety 4:110–114. doi: 10.14252/foodsafetyfscj.2016019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells GA, Hawkins SA, Austin AR, Ryder SJ, Done SH, Green RB, Dexter I, Dawson M, Kimberlin RH. 2003. Studies of the transmissibility of the agent of bovine spongiform encephalopathy to pigs. J Gen Virol 84:1021–1031. doi: 10.1099/vir.0.18788-0. [DOI] [PubMed] [Google Scholar]
- 7.Dawson M, Wells GA, Parker BN, Scott AC. 1990. Primary parenteral transmission of bovine spongiform encephalopathy to the pig. Vet Rec 127:338. [PubMed] [Google Scholar]
- 8.Ryder SJ, Hawkins SA, Dawson M, Wells GA. 2000. The neuropathology of experimental bovine spongiform encephalopathy in the pig. J Comp Pathol 122:131–143. doi: 10.1053/jcpa.1999.0349. [DOI] [PubMed] [Google Scholar]
- 9.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Miller MW, Wild MA. 2004. Epidemiology of chronic wasting disease in captive white-tailed and mule deer. J Wildl Dis 40:320–327. doi: 10.7589/0090-3558-40.2.320. [DOI] [PubMed] [Google Scholar]
- 11.Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, Spowart RA, O'Rourke KI, Miller JM, Merz PA. 1997. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in north central Colorado. J Wildl Dis 33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Williams ES, Young S. 1982. Spongiform encephalopathy of Rocky Mountain elk. J Wildl Dis 18:465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]
- 13.Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW. 2007. A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 43:309–314. doi: 10.7589/0090-3558-43.2.309. [DOI] [PubMed] [Google Scholar]
- 14.Kreeger TJ, Montgomery DL, Jewell JE, Schultz W, Williams ES. 2006. Oral transmission of chronic wasting disease in captive Shira's moose. J Wildl Dis 42:640–645. doi: 10.7589/0090-3558-42.3.640. [DOI] [PubMed] [Google Scholar]
- 15.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikoren T. 2016. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res 47:88. doi: 10.1186/s13567-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balachandran A, Harrington NP, Algire J, Soutyrine A, Spraker TR, Jeffrey M, Gonzalez L, O'Rourke KI. 2010. Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late stage distribution of protease-resistant prion protein. Can Vet J 51:169–178. [PMC free article] [PubMed] [Google Scholar]
- 17.Hamir AN, Greenlee JJ, Nicholson EM, Kunkle RA, Richt JA, Miller JM, Hall M. 2011. Experimental transmission of chronic wasting disease (CWD) from elk and white-tailed deer to fallow deer by intracerebral route: final report. Can J Vet Res 75:152–156. [PMC free article] [PubMed] [Google Scholar]
- 18.Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes-Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA, Mathiason CK. 2013. Mother to offspring transmission of chronic wasting disease in Reeves' muntjac deer. PLoS One 8:e71844. doi: 10.1371/journal.pone.0071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore SJ, Kunkle R, Greenlee MH, Nicholson E, Richt J, Hamir A, Waters WR, Greenlee J. 2016. Horizontal transmission of chronic wasting disease in reindeer. Emerg Infect Dis 22:2142–2145. doi: 10.3201/eid2212.160635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell GB, Sigurdson CJ, O'Rourke KI, Algire J, Harrington NP, Walther I, Spraker TR, Balachandran A. 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One 7:e39055. doi: 10.1371/journal.pone.0039055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamir AN, Kunkle RA, Cutlip RC, Miller JM, Williams ES, Richt JA. 2006. Transmission of chronic wasting disease of mule deer to Suffolk sheep following intracerebral inoculation. J Vet Diagn Invest 18:558–565. doi: 10.1177/104063870601800606. [DOI] [PubMed] [Google Scholar]
- 22.Greenlee JJ, Nicholson EM, Smith JD, Kunkle RA, Hamir AN. 2012. Susceptibility of cattle to the agent of chronic wasting disease from elk after intracranial inoculation. J Vet Diagn Invest 24:1087–1093. doi: 10.1177/1040638712461249. [DOI] [PubMed] [Google Scholar]
- 23.Hamir AN, Cutlip RC, Miller JM, Williams ES, Stack MJ, Miller MW, O'Rourke KI, Chaplin MJ. 2001. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. J Vet Diagn Invest 13:91–96. doi: 10.1177/104063870101300121. [DOI] [PubMed] [Google Scholar]
- 24.Hamir AN, Kunkle RA, Cutlip RC, Miller JM, O'Rourke KI, Williams ES, Miller MW, Stack MJ, Chaplin MJ, Richt JA. 2005. Experimental transmission of chronic wasting disease agent from mule deer to cattle by the intracerebral route. J Vet Diagn Invest 17:276–281. doi: 10.1177/104063870501700313. [DOI] [PubMed] [Google Scholar]
- 25.Hamir AN, Kunkle RA, Miller JM, Greenlee JJ, Richt JA. 2006. Experimental second passage of chronic wasting disease (CWD(mule deer)) agent to cattle. J Comp Pathol 134:63–69. doi: 10.1016/j.jcpa.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes-Klug J, Hoover EA. 2013. Susceptibility of domestic cats to chronic wasting disease. J Virol 87:1947–1956. doi: 10.1128/JVI.02592-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seelig DM, Nalls AV, Flasik M, Frank V, Eaton S, Mathiason CK, Hoover EA. 2015. Lesion profiling and subcellular prion localization of cervid chronic wasting disease in domestic cats. Vet Pathol 52:107–119. doi: 10.1177/0300985814524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartz JC, Marsh RF, McKenzie DI, Aiken JM. 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology 251:297–301. doi: 10.1006/viro.1998.9427. [DOI] [PubMed] [Google Scholar]
- 29.Sigurdson CJ, Mathiason CK, Perrott MR, Eliason GA, Spraker TR, Glatzel M, Manco G, Bartz JC, Miller MW, Hoover EA. 2008. Experimental chronic wasting disease (CWD) in the ferret. J Comp Pathol 138:189–196. doi: 10.1016/j.jcpa.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Marsh RF, Kincaid AE, Bessen RA, Bartz JC. 2005. Interspecies transmission of chronic wasting disease prions to squirrel monkeys (Saimiri sciureus). J Virol 79:13794–13796. doi: 10.1128/JVI.79.21.13794-13796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Race B, Meade-White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, Parnell M, Striebel J, Priola SA, Ward A, Williams ES, Race R, Chesebro B. 2009. Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 15:1366–1376. doi: 10.3201/eid1509.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Race B, Meade-White KD, Phillips K, Striebel J, Race R, Chesebro B. 2014. Chronic wasting disease agents in nonhuman primates. Emerg Infect Dis 20:833–837. doi: 10.3201/eid2005.130778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurt TD, Sigurdson CJ. 2016. Cross-species transmission of CWD prions. Prion 10:83–91. doi: 10.1080/19336896.2015.1118603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Agriculture National Feral Swine Mapping System. 2017. United States feral swine distribution map. Southeastern Cooperative Wildlife Disease Study, College of Veterinary Medicine, The University of Georgia, Athens, GA: http://swine.vet.uga.edu/nfsms/index.jsp Accessed 13 February 2017. [Google Scholar]
- 35.U.S. Geological Survey National Wildlife Health Center. 2017. Chronic wasting disease (CWD). U.S. Geological Survey National Wildlife Health Center, Madison, WI: https://www.nwhc.usgs.gov/disease_information/chronic_wasting_disease/index.jsp. Accessed 13 February 2017. [Google Scholar]
- 36.Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C. 2015. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep 11:1168–1175. doi: 10.1016/j.celrep.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. 2006. Prions adhere to soil minerals and remain infectious. PLoS Pathog 2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 3:e93. doi: 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. 2007. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Martinez AB, Garrido JM, Zarranz JJ, Arteagoitia JM, de Pancorbo MM, Atares B, Bilbao MJ, Ferrer I, Juste RA. 2010. A novel form of human disease with a protease-sensitive prion protein and heterozygosity methionine/valine at codon 129: case report. BMC Neurol 10:99. doi: 10.1186/1471-2377-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilham JM, Orru CD, Bessen RA, Atarashi R, Sano K, Race B, Meade-White KD, Taubner LM, Timmes A, Caughey B. 2010. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dassanayake RP, Orru CD, Hughson AG, Caughey B, Graca T, Zhuang D, Madsen-Bouterse SA, Knowles DP, Schneider DA. 2016. Sensitive and specific detection of classical scrapie prions in the brains of goats by real-time quaking-induced conversion. J Gen Virol 97:803–812. doi: 10.1099/jgv.0.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peden AH, McGuire LI, Appleford NE, Mallinson G, Wilham JM, Orru CD, Caughey B, Ironside JW, Knight RS, Will RG, Green AJ, Head MW. 2012. Sensitive and specific detection of sporadic Creutzfeldt-Jakob disease brain prion protein using real-time quaking-induced conversion. J Gen Virol 93:438–449. doi: 10.1099/vir.0.033365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kondru N, Manne S, Greenlee J, West Greenlee H, Anantharam V, Halbur P, Kanthasamy A, Kanthasamy A. 2017. Integrated organotypic slice cultures and RT-QuIC (OSCAR) assay: implications for translational discovery in protein misfolding diseases. Sci Rep 7:43155. doi: 10.1038/srep43155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vascellari S, Orru CD, Hughson AG, King D, Barron R, Wilham JM, Baron GS, Race B, Pani A, Caughey B. 2012. Prion seeding activities of mouse scrapie strains with divergent PrPSc protease sensitivities and amyloid plaque content using RT-QuIC and eQuIC. PLoS One 7:e48969. doi: 10.1371/journal.pone.0048969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzaban S, Friedlander G, Schonberger O, Horonchik L, Yedidia Y, Shaked G, Gabizon R, Taraboulos A. 2002. Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41:12868–12875. doi: 10.1021/bi025958g. [DOI] [PubMed] [Google Scholar]
- 47.Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, Soto C, Requena JR. 2006. Isolation and characterization of a proteinase K-sensitive PrP(Sc) fraction. Biochemistry 45:15710–15717. doi: 10.1021/bi0615442. [DOI] [PubMed] [Google Scholar]
- 48.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 49.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hoover EA. 2009. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS One 4:e5916. doi: 10.1371/journal.pone.0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8:e74377. doi: 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. 2009. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 4:e7990. doi: 10.1371/journal.pone.0007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulford B, Spraker TR, Wyckoff AC, Meyerett C, Bender H, Ferguson A, Wyatt B, Lockwood K, Powers J, Telling GC, Wild MA, Zabel MD. 2012. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J Wildl Dis 48:425–434. doi: 10.7589/0090-3558-48.2.425. [DOI] [PubMed] [Google Scholar]
- 55.Wilcox J, Van Vuren D. 2009. Wild pigs as predators in oak woodlands of California. J Mammal 90:114–118. doi: 10.1644/08-MAMM-A-017.1. [DOI] [Google Scholar]
- 56.Hamir AN, Richt JA, Miller JM, Kunkle RA, Hall SM, Nicholson EM, O'Rourke KI, Greenlee JJ, Williams ES. 2008. Experimental transmission of chronic wasting disease (CWD) of elk (Cervus elaphus nelsoni), white-tailed deer (Odocoileus virginianus), and mule deer (Odocoileus hemionus hemionus) to white-tailed deer by intracerebral route. Vet Pathol 45:297–306. doi: 10.1354/vp.45-3-297. [DOI] [PubMed] [Google Scholar]
- 57.Greenlee JJ, Smith JD, Kunkle RA. 2011. White-tailed deer are susceptible to the agent of sheep scrapie by intracerebral inoculation. Vet Res 42:107. doi: 10.1186/1297-9716-42-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith JD, Nicholson EM, Greenlee JJ. 2013. Evaluation of a combinatorial approach to prion inactivation using an oxidizing agent, SDS, and proteinase K. BMC Vet Res 9:151. doi: 10.1186/1746-6148-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith JD, Nicholson EM, Foster GH, Greenlee JJ. 2013. Exposure of RML scrapie agent to a sodium percarbonate-based product and sodium dodecyl sulfate renders PrPSc protease sensitive but does not eliminate infectivity. BMC Vet Res 9:8. doi: 10.1186/1746-6148-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greenlee JJ, Smith JD, West Greenlee MH, Nicholson EM. 2012. Clinical and pathologic features of H-type bovine spongiform encephalopathy associated with E211K prion protein polymorphism. PLoS One 7:e38678. doi: 10.1371/journal.pone.0038678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith JD, Greenlee JJ. 2014. Detection of misfolded prion protein in retina samples of sheep and cattle by use of a commercially available enzyme immunoassay. Am J Vet Res 75:268–272. doi: 10.2460/ajvr.75.3.268. [DOI] [PubMed] [Google Scholar]
- 62.Hardt M, Baron T, Groschup MH. 2000. A comparative study of immunohistochemical methods for detecting abnormal prion protein with monoclonal and polyclonal antibodies. J Comp Pathol 122:43–53. doi: 10.1053/jcpa.1999.0343. [DOI] [PubMed] [Google Scholar]
- 63.Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, Onwubiko HA, Priola SA, Caughey B. 2008. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 5:211–212. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]
- 64.West Greenlee MH, Lind M, Kokemueller R, Mammadova N, Kondru N, Manne S, Smith J, Kanthasamy A, Greenlee J. 2016. Temporal resolution of misfolded prion protein transport, accumulation, glial activation, and neuronal death in the retinas of mice inoculated with scrapie. Am J Pathol 186:2302–2309. doi: 10.1016/j.ajpath.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orru CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. 2015. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio 6:e02451-14. doi: 10.1128/mBio.02451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Groveman BR, Orru CD, Hughson AG, Bongianni M, Fiorini M, Imperiale D, Ladogana A, Pocchiari M, Zanusso G, Caughey B. 2017. Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol 4:139–144. doi: 10.1002/acn3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. 2015. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol 96:210–219. doi: 10.1099/vir.0.069906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bongianni M, Orru C, Groveman BR, Sacchetto L, Fiorini M, Tonoli G, Triva G, Capaldi S, Testi S, Ferrari S, Cagnin A, Ladogana A, Poleggi A, Colaizzo E, Tiple D, Vaianella L, Castriciano S, Marchioni D, Hughson AG, Imperiale D, Cattaruzza T, Fabrizi GM, Pocchiari M, Monaco S, Caughey B, Zanusso G. 2017. Diagnosis of human prion disease using real-time quaking-induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol 74:155–162. doi: 10.1001/jamaneurol.2016.4614. [DOI] [PubMed] [Google Scholar]