ABSTRACT
Latency-associated nuclear antigen (LANA) is a multifunctional protein encoded by members of the Rhadinovirus genus of gammaherpesviruses. Studies using murine gammaherpesvirus 68 (MHV68) demonstrated that LANA is important for acute replication, latency establishment, and reactivation in vivo. Despite structural similarities in their DNA-binding domains (DBDs), LANA homologs from Kaposi sarcoma-associated herpesvirus (KSHV) and MHV68 exhibit considerable sequence divergence. We sought to determine if KSHV and MHV68 LANA homologs are functionally interchangeable. We generated an MHV68 virus that encodes KSHV LANA (kLANA) in place of MHV68 LANA (mLANA) and evaluated the virus's capacity to replicate, establish and maintain latency, and reactivate. kLANA knock-in (KLKI) MHV68 was replication competent in vitro and in vivo but exhibited slower growth kinetics and lower titers than wild-type (WT) MHV68. Following inoculation of mice, KLKI MHV68 established and maintained latency in splenocytes and peritoneal cells but did not reactivate efficiently ex vivo. kLANA repressed the MHV68 promoter for ORF50, the gene that encodes the major lytic transactivator protein RTA, while mLANA did not, suggesting a likely mechanism for the KLKI MHV68 phenotypes. Bypassing this repression by providing MHV68 RTA in trans rescued KLKI MHV68 replication in tissue culture and enabled detection of KLKI MHV68 reactivation ex vivo. These data demonstrate that kLANA and mLANA are functionally interchangeable for establishment and maintenance of latency and suggest that repression of lytic replication by kLANA, as previously shown with KSHV, is a kLANA-specific function that is transferable to MHV68.
IMPORTANCE Kaposi sarcoma-associated herpesvirus (KSHV) and murine gammaherpesvirus 68 (MHV68) are members of the Rhadinovirus genus of gammaherpesviruses. These viruses establish lifelong infections that place their respective human and murine hosts at risk for cancer. Latency-associated nuclear antigen (LANA) is a conserved Rhadinovirus protein that is necessary for long-term chronic infection by these viruses. To better understand the conserved functions performed by LANA homologs, we generated a recombinant MHV68 virus that encodes the KSHV LANA protein in place of the MHV68 LANA homolog. We determined that the KSHV LANA protein is capable of supporting MHV68 latency in a mouse model of chronic infection but also functions to repress viral replication. This work describes an in vivo model system for defining evolutionarily conserved and divergent functions of LANA homologs in Rhadinovirus infection and disease.
KEYWORDS: Kaposi sarcoma-associated herpesvirus, LANA, gammaherpesvirus, latency-associated nuclear antigen, latent infection, lytic infection, murid herpesvirus 4, murine gammaherpesvirus 68, small animal model
INTRODUCTION
Gammaherpesviruses (GHV) are a subfamily of herpesviruses that infect a large number of humans worldwide, with 95% of the adult population harboring at least one GHV infection (1). Like all herpesviruses, GHVs are large, enveloped viruses that contain a double-stranded DNA genome within a protein capsid (2). GHV are lymphotropic and include the human pathogens Epstein-Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus (KSHV). While GHV infections typically are not associated with severe disease, they can cause cancers and lymphoproliferative diseases, especially in immunocompromised patients such as organ transplant recipients or persons with AIDS (3). For instance, KSHV establishes latency in B cells and causes primary effusion lymphoma and multicentric Castleman disease, as well as the AIDS-defining endothelial malignancy Kaposi sarcoma (4). However, due to stringent species restriction, it is difficult to study pathogenesis and virus-host interactions using EBV or KSHV in small-animal models. Murine gammaherpesvirus 68 (MHV68), a natural rodent pathogen that is closely related to KSHV, provides a tractable model for defining mechanisms of GHV infection and disease in vivo (5).
During primary infection, GHVs undergo acute productive (lytic) replication. Dissemination of progeny virions following acute replication seeds the B cell compartment and other cell types where the virus establishes latency (6, 7). GHV latency is characterized by minimal viral gene expression while the viral genome is maintained as an episome in the nucleus of the host cell (8). For members of the Rhadinovirus genus such as KSHV and MHV68, the latency-associated nuclear antigen (LANA) plays an important role in establishing and maintaining viral latency (9–12). Encoded by the conserved gene ORF73, LANA homologs are expressed with immediate-early (IE) kinetics during lytic replication, and LANA is one of the few viral proteins expressed during latency. LANA homologs function as transcriptional regulators of viral and host genes and as inhibitors of host tumor suppressors, especially p53 (13–16). LANA is abundantly expressed in all known KSHV-related cancers, suggesting a role in cellular transformation (13). Indeed, transgenic expression of KSHV LANA in mice promotes the development of lymphoid hyperplasia and lymphoma (17).
The KSHV and MHV68 LANA (kLANA and mLANA, respectively) proteins have diverged considerably (13, 18). Amino acid sequence identity is less than 30% (18), and kLANA has an expanded internal repeat region that is not present in mLANA which contributes to the kLANA protein being nearly four times as long (13). Despite this divergence, both kLANA and mLANA have positionally conserved nuclear localization sequences and N-terminal proline and serine (P/S)-rich regions and exhibit remarkable structural homology in their DNA-binding domains (DBDs) (13, 19). Both kLANA and mLANA also engage a conserved sequence element in their cognate terminal repeats (TRs) (20, 21), and through this element, kLANA and mLANA mediate replication of TR-containing plasmids in vitro (11, 20, 22). These findings support the dogma that LANA homologs facilitate episome maintenance by tethering viral genomes to host chromosomes during cell division. Consistent with this notion, LANA-null MHV68 does not establish latency in mice following intranasal (i.n.) inoculation (10, 12). Taken together, these studies support the hypothesis that the capacity to maintain viral genomes during latency is conserved among LANA homologs despite the divergent evolution of these proteins within their respective hosts. However, whether KSHV and MHV68 LANA homologs are functionally interchangeable for establishment and maintenance of latency is not known. Moreover, due to the lack of a tractable small-animal model for KSHV infection, it is not known whether kLANA is necessary for latency establishment in a living host where immune surveillance actively combats infection.
There are also apparent functional differences between kLANA and mLANA. For MHV68, LANA facilitates efficient lytic replication by preventing infection-associated p53 responses that promote cell death and by recruiting heat shock cognate protein 70 (Hsc70) to the nuclei of infected cells, thereby promoting progression of replication (12, 14, 16, 23). In contrast, KSHV LANA is thought to actively suppress lytic replication by repressing transcription of ORF50 (24, 25), an essential gene that encodes the replication and transactivator protein RTA. Since RTA is both necessary and sufficient for entry into the lytic replication cycle (26), repression of RTA by kLANA is thought to promote viral latency. It is not known if mLANA represses the MHV68 ORF50 promoter (mORF50-pro) in a similar fashion. The impact that these differences have on chronic infection has not been tested.
In this study, we sought to evaluate kLANA contributions to viral infection and persistence in vivo. We generated an MHV68 recombinant virus in which the endogenous ORF73 gene that encodes mLANA was replaced by ORF73 from KSHV. With this reagent, we evaluated the capacity of kLANA to functionally replace mLANA in support of viral replication, latency, and reactivation in vivo. This work establishes a system for evaluating kLANA contributions to Rhadinovirus infection and disease and for delineating similarities and differences between two distantly related LANA homologs.
RESULTS
Generation of KSHV LANA knock-in (KLKI) MHV68.
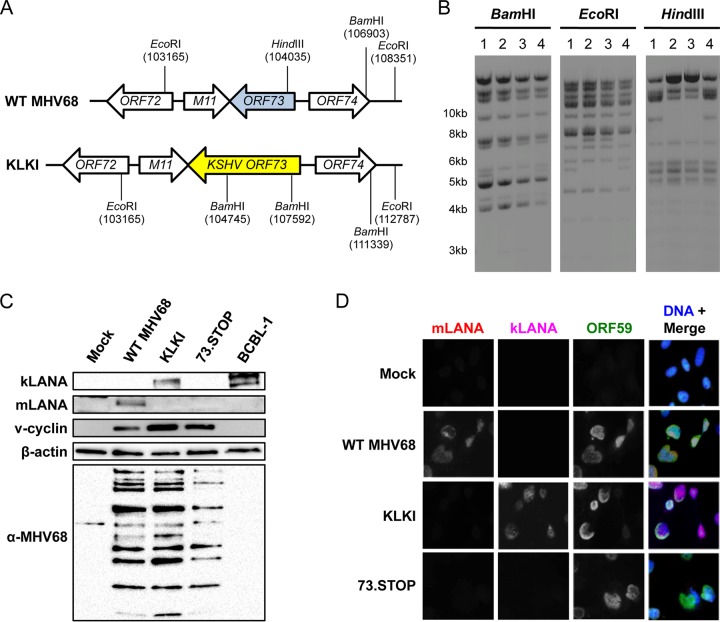
The KSHV and MHV68 LANA proteins exhibit structural homology in their DNA-binding domains and are thought to perform similar functions during the establishment and maintenance of latency (13, 19). However, kLANA and mLANA are not well conserved at the amino acid level (18), and it is not known whether LANA homologs are functionally interchangeable. Moreover, because KSHV does not readily infect mice, specific roles for kLANA in the pathogenesis of viral infection are not directly testable. In order to define similarities and differences in the functions of kLANA and mLANA, especially in a small-animal model, we generated an MHV68 recombinant virus that encodes kLANA instead of mLANA by replacing the MHV68 ORF73 gene with KSHV ORF73 (Fig. 1A and B). The resultant kLANA knock-in (KLKI) MHV68 was viable and expressed kLANA, but not mLANA, in immunoblot analyses performed as a nonquantitative steady-state evaluation of lysates from infected murine 3T12 fibroblasts (Fig. 1C). Lysates from wild-type (WT) MHV68 or mLANA-null MHV68 (73.STOP)-infected cells and KSHV-infected PEL cells were used as comparative controls. Importantly, KLKI MHV68 efficiently expressed v-cyclin, which is encoded by the adjacent ORF72 gene and cotranscribed with the product of ORF73, indicating that v-cyclin production was not adversely affected by replacement of MHV68 ORF73 with its KSHV counterpart. KLKI and WT MHV68 also comparably produced viral structural antigens detected with MHV68 antiserum. Similar results were observed in indirect immunofluorescence analyses, and kLANA, like mLANA, localized to nuclei of productively infected 3T12 fibroblasts (Fig. 1D). These data demonstrate that KLKI MHV68 expresses kLANA in place of mLANA.
FIG 1.
Derivation of an MHV68 recombinant that encodes KSHV LANA. (A) Schematic representation of the ORF73 locus and surrounding genes for WT MHV68 and KSHV LANA knock-in (KLKI) MHV68. Locations of recognition sites for specific restriction enzymes are indicated. (B) Restriction fragment length polymorphism analysis of WT MHV68 (lane 1), two independent clones of KLKI MHV68 (lanes 2 and 3), and a KLKI MHV68 WT revertant (KLKI.MR; lane 4). BACs were digested with the indicated restriction enzymes and resolved by agarose-gel electrophoresis. (C) 3T12 cells were mock infected or infected with WT, KLKI, or LANA-null (73.STOP) MHV68 at an MOI of 10 PFU/cell. Lysates were collected 18 h postinfection, and proteins were resolved by SDS-PAGE. Immunoblot analyses were performed using antibodies that recognize the indicated proteins. BCBL-1 lysates were used as a positive control for kLANA expression. (D) MEFs were infected with WT, KLKI, or 73.STOP MHV68 at an MOI of 20 PFU/cell. Cells were fixed 18 h postinfection and stained for indirect immunofluorescence analyses using antibodies that recognize the indicated proteins. DNA was stained with DAPI.
Effect of kLANA expression on MHV68 lytic replication.
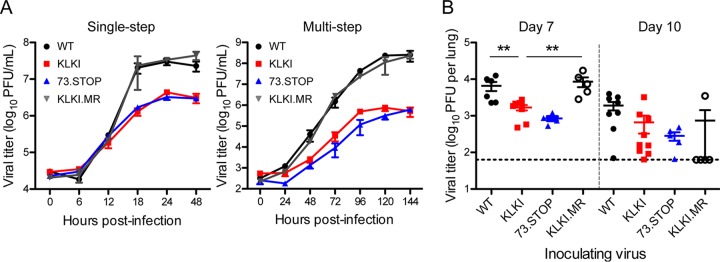
To determine whether the presence of kLANA impacts the MHV68 infectious cycle, we first evaluated KLKI MHV68 replication in single- and multistep growth assays. Although KLKI titers increased over time, compared to WT MHV68 and KLKI marker rescue (KLKI.MR) viruses, KLKI exhibited a replication defect that was comparable to that observed for mLANA-null 73.STOP, producing titers approximately 10-fold lower than the titers produced by WT viruses (Fig. 2). To define the effect of kLANA expression on MHV68 replication in vivo, we inoculated C57BL/6 mice i.n. with KLKI MHV68 and determined viral titers in the lungs on days 7 and 10 postinfection. Again, KLKI titers were more than 10-fold lower than the titers of WT virus (WT MHV68 and KLKI.MR) on days 7 and 10 postinfection but were slightly higher than mLANA-null 73.STOP virus titers. Together, these data indicate that KLKI MHV68 exhibits an attenuated lytic replication phenotype.
FIG 2.
KLKI replication is reduced in vitro and in vivo. (A) NIH 3T12 fibroblasts were infected with WT, KLKI, KLKI.MR, or 73.STOP MHV68 at an MOI of 5 PFU/cell (single-step; left panel) or 0.05 PFU/cell (multi-step; right panel). Viral titers were determined at the indicated times postinfection by plaque assay. (B) C57BL/6 mice were infected intranasally with 1,000 PFU of WT, KLKI, KLKI.MR, or 73.STOP MHV68. Mice were sacrificed at the indicated times postinfection, and viral titers in lung homogenates were determined by plaque assay. Each dot represents one mouse. Error bars represent standard errors of the means. **, P < 0.01 (determined using an unpaired, two-tailed Student's t test).
KLKI MHV68 establishes long-term latency in mice.
KSHV LANA homologs and MHV68 LANA homologs are both critical for multiple aspects of viral latency. mLANA is necessary for latency establishment and reactivation from latency following intranasal inoculation of mice with MHV68 (10, 12, 27). kLANA maintains viral episomes inside latently infected cells (9). Moreover, the LANA binding sites (LBS) in the MHV68 and KSHV TRs are remarkably well conserved (20, 21), and the DBDs of mLANA and kLANA are nearly identical in structure (19), suggesting that certain functions of LANA are conserved across species.
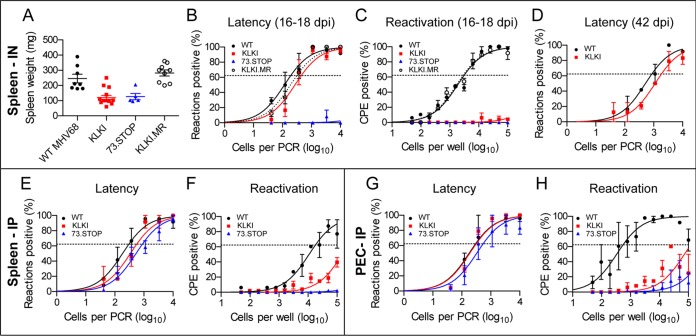
Given the dependence of MHV68 on mLANA for latency establishment in spleens of mice following i.n. inoculation, we sought to determine whether kLANA could functionally replace mLANA in the same assay. In contrast to mLANA-null 73.STOP virus, KLKI MHV68 established latency in spleens of infected mice at levels comparable to WT MHV68 and KLKI.MR following i.n. inoculation (Table 1) (Fig. 3B). Moreover, KLKI latency was maintained, as the frequencies of splenocytes latently infected with KLKI and WT MHV68 were similar on day 42 postinfection (Fig. 3D). However, neither KLKI MHV68 nor 73.STOP caused splenomegaly, which is characteristic of infection with WT MHV68 (Fig. 3A). Further, KLKI MHV68 failed to reactivate ex vivo (Table 2) (Fig. 3C). KLKI.MR behaved like WT virus during acute replication, latency establishment, and reactivation, confirming that offsite mutations were not the cause of the KLKI MHV68 phenotypes.
TABLE 1.
Frequencies of cells harboring MHV68 genomes in latently infected micea
| Virus | Harvest day pi | No. of cells harboring indicated MHV68 genome/total no. of cells |
||
|---|---|---|---|---|
| Spleen (i.n.) | Spleen (i.p.) | PEC (i.p.) | ||
| WT MHV68 | 16–18 | 1/200 | 1/300 | 1/300 |
| 42 | 1/790 | |||
| KLKI MHV68 | 16–18 | 1/480 | 1/570 | 1/330 |
| 42 | 1/1,600 | |||
| 73.STOP | 16–18 | N.D. | 1/900 | 1/570 |
| KLKI.MR | 16–18 | 1/330 | ||
pi, postinfection; N.D., not determined (the frequency was below the limit of detection for the assay).
FIG 3.
kLANA is sufficient to support MHV68 latency establishment and maintenance, but not reactivation. C57BL/6 mice were intranasally (IN) (A to D) or intraperitoneally (IP) (E to H) inoculated with 1,000 PFU of WT, KLKI, KLKI.MR, or 73.STOP MHV68. Spleens (A to F) or PECs (G and H) were harvested on days 16 to 18 postinfection (A to C and E to H) or on day 42 postinfection (D). (A) Harvested spleens were weighed. Each dot represents one mouse. Error bars represent standard errors of the means. (B, D, E, and G) Single-cell suspensions were serially diluted, and frequencies of cells harboring MHV68 genomes were determined using limiting-dilution PCR analysis. (C, F, and H) Reactivation frequencies were determined by ex vivo plating of serially diluted cells on an indicator monolayer. Cytopathic effect was scored 2 to 3 weeks postplating. Groups of 3 to 5 mice were pooled for each infection and analysis. Data represent means of results of two independent infections. Error bars represent standard errors of the means.
TABLE 2.
Frequencies of latently infected cells reactivating ex vivo on days 16 to 18 postinfection
| Virus | No. of latently infected cells reactivating ex vivo/total no. of cells |
||
|---|---|---|---|
| Spleen (i.n.) | Spleen (i.p.) | PEC (i.p.) | |
| WT MHV68 | 1/3,300 | 1/18,000 | 1/500 |
| KLKI MHV68 | N.D.a | N.D. | N.D. |
| 73.STOP | N.D. | N.D. | N.D. |
| KLKI.MR | 1/2,300 | ||
| KLKI MHV68 (on RTA-expressing cells) | 1/120,000 | ||
| KLKI.MR (on RTA-expressing cells) | 1/5,000 | ||
N.D., not determined (the frequency was below the limit of detection for the assay).
Although mLANA-null 73.STOP is incapable of establishing latency after i.n. inoculation, this mutant virus does chronically infect mice following intraperitoneal (i.p.) inoculation (27). In mice infected via the i.p. route, KLKI MHV68 established latency in both splenocytes and peritoneal exudate cells (PECs) at levels that were comparable to those seen with WT MHV68 (Fig. 3E and G). However, consistent with the phenotypes seen following i.n. inoculation, KLKI reactivation was substantially reduced following i.p. inoculation, resembling reactivation levels exhibited by mLANA-null 73.STOP (Fig. 3F and H). Together, these data indicate that the presence of kLANA is sufficient in the absence of mLANA to support the establishment and maintenance of MHV68 latency, thereby indicating that LANA homologs from divergent species encode conserved functions important for latency. These results also indicate that kLANA is not capable of replacing the function(s) of mLANA during MHV68 reactivation from latently infected explanted cells.
kLANA represses MHV68 ORF50 promoter activity and replication.
ORF50 encodes the lytic replication transactivator RTA in both KSHV and MHV68 (26). This IE gene is a master regulator of progression into the viral lytic gene expression cascade and is both necessary and sufficient for driving viral lytic replication and reactivation (28–30). In KSHV-infected tumor cells, kLANA represses the KSHV ORF50 promoter (24, 25). This kLANA function may be partially responsible for the “latent” phenotype of KSHV infection observed in most cell types, as repression of ORF50 by kLANA limits lytic viral gene expression in cells infected de novo (31, 32). In contrast, MHV68 undergoes efficient lytic replication in most cell types, and mLANA, which is also a transcription regulator, facilitates viral replication (14, 20, 23, 33).
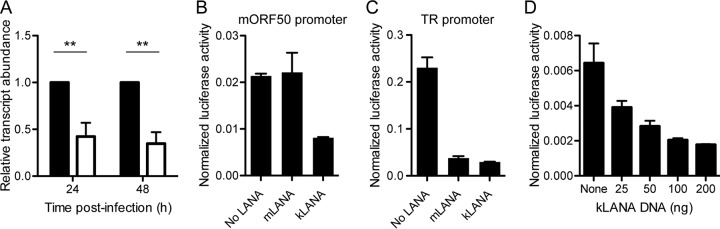
Given that KLKI MHV68 exhibited attenuated lytic replication and reactivation, we speculated that kLANA was able to repress the ORF50 promoter in MHV68. As an initial test of this hypothesis, we performed quantitative reverse transcription-PCR (qRT-PCR) analyses to compare orf50 transcription levels in KLKI MHV68-infected cells to those in WT virus-infected cells. Despite representing an IE gene product, orf50 transcription was reduced over 50% in KLKI-infected cells following infections performed at low multiplicities of infection (MOI) (Fig. 4A). We next tested the capacity of kLANA and mLANA to repress MHV68 ORF50 promoter (mORF50-pro) activity in luciferase reporter assays. While mLANA had no effect on mORF50-pro activity, cells transfected with kLANA exhibited a ca. 60% reduction in mORF50-pro activity (Fig. 4B). Moreover, kLANA-mediated repression of mORF50-pro was dose dependent (Fig. 4D). However, in agreement with previous work (20), kLANA and mLANA equally repressed a promoter contained in the MHV68 terminal repeats (Fig. 4C), confirming the functionality of both LANA constructs. Together, these data demonstrate that kLANA is capable of repressing the MHV68 ORF50 promoter.
FIG 4.
kLANA, but not mLANA, represses the MHV68 ORF50 promoter. (A) NIH 3T12 fibroblasts were infected with WT MHV68 (black bars) or KLKI MHV68 (white bars) at an MOI of 0.5 PFU/cell. RNA was isolated at the indicated times postinfection, and orf50 transcripts were quantified by qRT-PCR relative to gapdh using the ΔΔCT method. Data are normalized to MHV68 genomic DNA that was purified and quantified in parallel. Values for WT infection were set to 1. Data represent means of results of two independent infections that were analyzed in technical triplicate. Error bars represent standard errors of the means. **, P < 0.01 (determined using one-way analysis of variance [ANOVA]). (B to D) 293T cells were cotransfected with empty vector, mLANA- or kLANA-encoding plasmids, and 50 ng of plasmid DNA encoding luciferase under the control of the MHV68 proximal ORF50 promoter (B) or the TR promoter (C) and a simian virus 40 (SV40) renilla luciferase normalization control plasmid. (D) Cells were transfected as described for panel B using increasing amounts of kLANA-encoding plasmid. Dual luciferase assays were performed 24 h posttransfection. Data represent means of results from triplicate samples. Error bars indicate standard errors of the means.
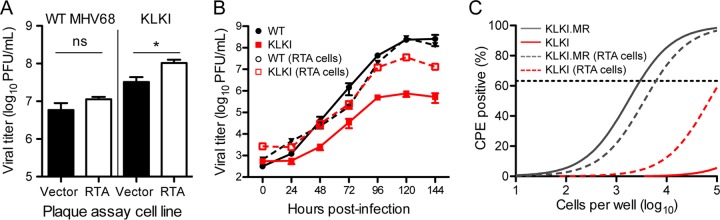
We reasoned that if repression of RTA in MHV68 by kLANA is the main cause of KLKI attenuation, providing RTA in trans might bypass the replication defect observed for KLKI MHV68, while minimally impacting WT MHV68. As a preliminary test of this hypothesis, we performed an efficiency-of-plating assay comparing WT MHV68 and KLKI plaque titers following titration on cells that stably express RTA in comparison to vector control cells (34). While WT MHV68 titer was minimally enhanced in RTA-expressing cells, KLKI MHV68 titers were ca. 3-fold higher in the presence of RTA (Fig. 5A). We also retested KLKI replication in low-MOI multistep replication assays, comparing viral replication on cells that expressed RTA to levels on control cells. Again, while the levels of WT MHV68 titers were not enhanced, KLKI MHV68 titers were approximately 50-fold higher at the 120-h time point in RTA-expressing cells (Fig. 5B). Finally, we reevaluated reactivation, comparing ex vivo reactivation from splenocytes on monolayers of indicator fibroblasts that stably express RTA to control fibroblast results. Again, while KLKI.MR control virus reactivation was not enhanced on RTA-expressing cells, the presence of RTA in the indicator monolayer enabled detection of KLKI MHV68 reactivation (Fig. 5C). Note that exogenous RTA was present only in the indicator monolayer and was not provided to the latently infected splenocytes. This indicates that KLKI MHV68 actually reactivated in this ex vivo assay, albeit much less efficiently than KLKI.MR, and that provision of RTA in trans simply enabled detection of low levels of reactivating virus that were not detectable in the standard assay. Together, these data indicate that repression of RTA by kLANA may contribute to the replication defect observed with KLKI MHV68.
FIG 5.
Exogenous RTA bypasses KLKI MHV68 replication and reactivation defects. (A) WT MHV68 and KLKI MHV68 were titrated by plaque assay on 3T12 fibroblasts that stably expressed RTA or contained the empty vector. Data represent means of results from triplicate samples. Error bars represent standard errors of the means. *, P < 0.05 (determined using an unpaired, two-tailed Student's t test); ns, not significant. (B) NIH 3T12 fibroblasts were infected with WT, KLKI, KLKI.MR, or 73.STOP MHV68 at an MOI of 0.05 PFU/cell. Viral titers were determined at the indicated times postinfection by plaque assay on 3T12 fibroblasts that stably expressed RTA or contained the empty vector. (C) C57BL/6 mice were intranasally infected with 1,000 PFU of KLKI or KLKI.MR MHV68. Spleens were harvested on days 16 to 18 postinfection. Reactivation frequencies were determined by ex vivo plating of serially diluted cells on indicator monolayers of 3T12 fibroblasts that stably expressed RTA or contained an empty vector. Cytopathic effect (CPE) was scored 2 to 3 weeks postplating. Groups of 3 to 5 mice were pooled for each infection and analysis. Data represent means of results of two independent infections. Results of nonlinear regression analyses are shown.
DISCUSSION
Herpesviruses are thought to have coevolved with their hosts (35). It is therefore likely that KSHV and MHV68, whose respective natural hosts are humans and rodents, diverged hundreds of millions of years ago. Since KSHV does not readily infect small animals, MHV68 infection of mice is often used as a surrogate system for understanding the importance in vivo of specific viral gene products for viral replication, chronic infection, and development of disease (8, 36). While KSHV and MHV68 clearly are related viruses with many conserved open reading frames (ORFs) and likely diverged from a common ancestor (18, 37), it is possible that the roles of even conserved viral gene products evolved to suit each virus's needs in its respective host. For instance, although the DBDs of kLANA and mLANA are structurally conserved (19), their amino acid sequence identity is only ca. 25% (18). Further, while mLANA is only 314 amino acids in length, kLANA is over 1,100 amino acids long. The difference in size is due in large part to the expansion of an internal repeat region in kLANA that separates the C-terminal DBD from a disordered proline and serine (P/S)-rich N-terminal region (21). The generation of knock-in viruses in which MHV68 genes are replaced with their KSHV homologs can provide insight into whether gene products have retained specific functions or evolved in manners that alter their impact on the pathogenesis of infection. By extension, these studies may enhance the value of the model of MHV68 infection of mice to reveal in general how members of the Rhadinovirus genus infect and colonize a host and promote disease. They may also provide new model systems for preclinical tests of drugs that target KSHV gene products to limit infection-related diseases, especially cancers associated with chronic viral infection.
kLANA is sufficient for MHV68 latency establishment and maintenance.
Whether viral proteins are interchangeable between KSHV and MHV68 is largely unexplored but has been addressed for KSHV and MHV68 homologs of v-cyclin and v-G-protein-coupled receptor (v-GPCR) (38, 39). In evaluations of lytic replication, virus-induced pneumonia, survival of infected endothelial cells, and reactivation from latently infected PECs, KSHV v-cyclin was capable of functionally replacing MHV68 v-cyclin (38). An MHV68 recombinant virus encoding the KSHV GPCR promoted enhanced angiogenesis and the formation of KS-like lesions in mice (39). Despite the differences in the two proteins, we found that MHV68 encoding kLANA in place of mLANA established and maintained latency following intranasal inoculation of mice. Since mLANA is a requirement for MHV68 latency in the spleen following infection by this route (10, 12) (Fig. 3), these data demonstrate that the presence of kLANA is sufficient to enable MHV68 latency establishment and maintenance in a mouse, indicating that latency functions of mLANA are conserved in kLANA. This finding also provides support for the hypothesis that the cis elements necessary for maintenance of the viral genome were preserved as MHV68 and KSHV diverged. Indeed, we and others previously noted the remarkable similarity of sequences within the MHV68 and KSHV TRs bound by the respective LANA proteins (20, 21).
In terms of the proteins themselves, the capacity of kLANA to replace mLANA for MHV68 latency indicates that the protein sequences or structural features necessary for maintaining the viral genome must be conserved between kLANA and mLANA. This would seem to rule out a role for the kLANA internal repeat region in this process, given the absence of such a sequence in other LANA homologs, including mLANA (13). It is possible that the nucleosome docking sequence present at the kLANA N terminus plays a role in MHV68 genome maintenance (40), but this seems less likely given that this stretch of amino acids is not well conserved in mLANA. Similarly, a region in the kLANA N-terminal domain that precedes the repeat region proposed to facilitate KSHV episome maintenance is absent in mLANA (41). On the other hand, the structural similarity in kLANA and mLANA DBDs, paired with the necessity of residues in the mLANA DBD for latency establishment, LBS binding, and TR plasmid replication (11, 19–21), supports the prediction that the DBD is the primary domain in kLANA required for KLKI latency establishment and maintenance in vivo. While it is clear from previous studies that both kLANA and mLANA DBDs bind a conserved cis element in the viral TR (20, 21, 42), it also is notable that histones coprecipitate with both mLANA and kLANA in protein-protein interaction studies (40, 43, 44). Whether similar sequences in kLANA and mLANA direct histone binding as a means to tether viral episomes to host chromosomes remains to be determined.
Repression of MHV68 lytic replication by kLANA.
Although kLANA is sufficient for MHV68 latency, KLKI MHV68 exhibited a lytic replication defect compared to WT MHV68. While kLANA is an activator of a promoter proximal to ORF73, thereby driving ORF73 transcription, it represses both the KSHV TR promoter and the KSHV ORF50 promoter (13). Repression of ORF50, the gene that encodes RTA, by kLANA is proposed as a mechanism by which kLANA promotes and enforces KSHV latency (24, 25). Indeed, LANA-null KSHV exhibits enhanced lytic gene expression relative to WT virus (31, 32). In contrast, mLANA facilitates efficient MHV68 replication both in vitro and in vivo, a phenotype partly dependent on mLANA's capacity to limit p53 induction during lytic replication and during reactivation from latency (12, 14, 16). Given these disparate phenotypes, we tested the hypothesis that kLANA represses the MHV68 ORF50 promoter and found that kLANA, but not mLANA, was capable of repressing MHV68 ORF50 promoter activity. Because provision of RTA in trans rescued KLKI replication and enabled detection of reactivating virus (see Fig. 4 and 5), it stands to reason that kLANA-mediated repression of ORF50 is responsible for the slower growth kinetics and reduced titers produced by KLKI MHV68. We cannot rule out the possibility that an mLANA-specific role in facilitating lytic replication is lacking in kLANA. These two hypotheses are not mutually exclusive, and the generation of knock-in viruses that encode kLANA/mLANA chimeras may allow experiments to further address this issue. Conversely, one might observe enhanced lytic gene expression were mLANA to be expressed in KSHV. Nonetheless, our data suggest that the capacity to suppress lytic replication is a kLANA-specific function that is transferrable to MHV68.
Unlike LBS sites in the TR, conserved sequences are not present in promoters for ORF50 in KSHV and MHV68. We therefore expect that recruitment of polycomb repressive complex (PRC) proteins by kLANA mediates the general repression of lytic gene expression for MHV68 in a manner similar to that which occurs during de novo KSHV infection (32). As we did not identify PRC proteins in complex with mLANA (23), PRC protein binding is likely a kLANA-specific adaptation. We therefore suspect that amino acid motifs or domains unique to kLANA, such as the internal repeat region, are necessary for this function.
The transferability of RTA repression by kLANA into MHV68 suggests that this is an intrinsic function of kLANA. The fact that this kLANA-specific function exists highlights the biological importance of kLANA-mediated repression of lytic replication, despite the fact that its impact on KSHV infection is not immediately obvious. It is possible that a more latent phenotype facilitates immune evasion or reduces infection-associated pathologies. Enforced latency by kLANA could therefore better enable long-term chronic infection or enhance spread to new hosts. MHV68, on the other hand, may benefit by reactivating more frequently to colonize a much-shorter-lived host population. With this in mind, it will be of interest to evaluate the functions of other LANA homologs for their capacities to both enable latency and suppress lytic replication as a further test of this hypothesis.
Altogether, the work described here highlights the potential utility of LANA knock-in MHV68 recombinants for defining functional similarities and differences in Rhadinovirus LANA homologs. Through such approaches, we may better understand the mechanisms by which LANA proteins dictate outcomes of infection, including the impact of suppressing lytic replication on the host antiviral response or cancer development. Moreover, given that kLANA is fully functional for MHV68 latency, KLKI infection should provide a unique small-animal model for screening compounds that target LANA function.
MATERIALS AND METHODS
Ethics statement.
Mouse experiments performed for this study were carried out in accordance with NIH, USDA, and University of Arkansas for Medical Sciences (UAMS) Division of Laboratory Animal Medicine and IACUC guidelines. The protocol supporting this study was approved by the UAMS Institutional Animal Care and Use Committee (animal use protocol 3587). Mice were anesthetized prior to inoculations and sacrificed humanely at the end of experiments.
Cells and viruses.
HEK 293T cells and NIH 3T12 and Swiss-albino 3T3 fibroblasts were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (cMEM). Cells were cultured at 37°C with 5% CO2 and ∼99% humidity. Murine embryonic fibroblasts (MEFs) were harvested from C57BL/6 mouse embryos and immortalized as previously described (45). Viruses used in this study included bacterial artificial chromosome (BAC)-derived wild-type MHV68 (46), mLANA-null MHV68 (73.STOP) (12), and kLANA knock-in MHV68 (KLKI) and KLKI marker rescue (KLKI.MR) characterized in this study. Viral stocks were produced by transfecting Cre-expressing Vero cells with BAC DNA to facilitate removal of the loxP-flanked BAC vector, followed by amplification in 3T12 fibroblasts.
Generation of recombinant viruses.
En passant mutagenesis was performed to generate KLKI and KLKI.MR viruses (47). The targeting construct was generated by inserting a kanamycin cassette into the KSHV ORF73 gene (from BAC16 [48]) using traditional restriction-enzyme-mediated cloning, and Gibson Assembly (New England BioLabs) was used to insert ∼450-bp flanking regions homologous to the MHV68 genome to facilitate double homologous recombination such that the KSHV LANA gene replaced the MHV68 LANA gene. The Gibson assembled construct was PCR amplified, purified with a Qiaquick purification kit (Qiagen), and treated with DpnI (NEB) to remove the methylated plasmid template. The PCR product was isolated on an agarose gel and purified with a QIAquick MinElute gel extraction kit (Qiagen). The purified amplicon was electroporated into freshly prepared electrocompetent Escherichia coli (strain GS1783.5) containing the wild-type MHV68 BAC. Kanamycin-resistant integrates were selected on plates containing 25 μg/ml kanamycin and 30 μg/ml chloramphenicol for 48 h. Kanamycin-resistant integrates were screened for proper recombination by PCR using primers that bind upstream and downstream of the inserted kanamycin gene. Positive cointegrates were resolved by the addition of 1% l-arabinose and a change in temperature from 32°C to 42°C to induce removal of the kanamycin resistance gene and of excess genetic material, resulting in a scarless mutation. Correct insertion was confirmed by PCR screening and sequencing. A restriction fragment length polymorphism (RFLP) analysis was performed to evaluate the integrity of newly derived BACs.
Viral replication assays.
Tissue culture replication assays were performed by incubating monolayers of NIH 3T12 fibroblasts, 3T12 fibroblasts transduced with empty murine stem cell virus (MSCV)-puro retrovirus, or RTA-stable 3T12 fibroblasts (34) at a specified MOI. Cells were lysed at the specified time points and subjected to freeze-thaw lysis. Viral titers were determined by plaque assay as previously described (49). For determining viral titers from infected lungs, minced tissue was homogenized by hypotonic disruption (0.3× incomplete DMEM) and mechanical disruption in a Mini-Beadbeater-16 cell disruptor (Biospec Products). Homogenates were subjected to freeze-thaw lysis in an ethanol/dry-ice bath. Viral titers were determined by plaque assay.
Mouse infections and tissue harvests.
Male and female C57BL/6 mice were purchased from Jackson Laboratories or were bred in-house. Eight-to-ten-week-old mice were anesthetized using isoflurane and inoculated with 1,000 PFU of virus diluted in incomplete DMEM (20 μl) for intranasal (i.n.) inoculations or injected with 1,000 PFU of virus diluted in incomplete DMEM (200 μl) for intraperitoneal (i.p.) inoculations. Splenocytes and peritoneal exudate cells (PECs) were harvested 16 to 18 days postinfection or 42 days postinfection, as described previously (45).
Limiting-dilution analyses.
Limiting-dilution (LD)-PCR analyses to quantify frequencies of latently infected splenocytes and peritoneal exudate cells (PECs) were performed as previously described (50). Briefly, 3-fold serial dilutions of latently infected cells were diluted in a background of uninfected 3T12 fibroblasts. After overnight digestion with proteinase K, cells were subjected to a nested PCR targeting the ORF50 region of the viral genome. Single-copy sensitivity and the absence of false-positive amplicons were confirmed using control standards. Amplicons were visualized for quantitation using ethidium bromide staining in 1.5% agarose gel electrophoresis. Ex vivo reactivation efficiency was determined as previously described (45). Briefly, 2-fold serial dilutions of latently infected splenocytes or PECs were plated on MEF monolayers. Death of the MEF monolayer due to the presence of reactivating virus provided a means for microscopic visualization of reactivation. The presence of preformed infectious virus was detected by plating mechanically disrupted cells on MEF indicator monolayers in parallel. Cytopathic effect was observed on days 14 and 21 postinfection.
Immunoblot analyses.
Immunoblot analyses were performed as previously described (49). Briefly, cells were lysed with radio immunoprecipitation (RIPA) buffer (150 mM NaCl, 20 mM Tris, 2 mM EDTA, 1% NP-40, 0.25% deoxycholate) supplemented with phosphatase and protease inhibitors. Samples were centrifuged at 16,000 × g to remove insoluble debris. The protein content for each sample was quantified using a Bio-Rad DC protein assay (Bio-Rad). Samples were diluted in 6× Laemmli sample buffer and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Resolved proteins were transferred to nitrocellulose membranes (Thermo Scientific), and blots were probed with the indicated primary antibodies. Antibody-bound proteins were recognized using horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch). Chemiluminescent signal was detected using a ChemiDoc MP imaging system (Bio-Rad) on blots treated with Clarity ECL reagent (Bio-Rad).
Immunofluorescence analyses.
3T3 fibroblasts were plated on glass coverslips and infected as described above. Infected cells were washed with cold phosphate-buffered saline (PBS) and fixed in 10% phosphate-buffered formalin. Cells were permeabilized for 15 min with 0.5% Triton X-100–Tris-buffered saline (TBS) at room temperature. Supernatants were aspirated, and cells were incubated in blocking buffer (5% bovine serum albumin [BSA], 1% normal donkey serum, 0.1% Triton X-100, TBS) for 30 min at room temperature. Cells were incubated with the indicated primary antibodies diluted in blocking buffer at 4°C overnight to probe for mLANA, kLANA, and MHV68 ORF59. Samples were washed three times with cold wash buffer (0.1% Triton X-100, TBS). Fluorophore-conjugated secondary antibodies specific to primary antibodies diluted in blocking buffer were added to the samples for 1 h in the dark at room temperature. Samples were washed three times with wash buffer, and glass coverslips were mounted on slides using ProLong Antifade Gold reagent with DAPI (4′,6-diamidino-2-phenylindole) (Sigma-Aldrich) to stain DNA. Cells were imaged by fluorescence microscopy using ×60 magnification on an Eclipse Ti-U fluorescence microscope (Nikon). Images were acquired with a D5-QilMc digital camera and analyzed using NIS-Elements software (Nikon).
Nucleic acid isolation and qPCR.
NIH 3T12 cells were infected with WT or KLKI MHV68 at an MOI of 0.5 PFU/cell. Total DNA and RNA were isolated using a DNeasy blood and tissue kit (Qiagen) and an RNeasy kit (Qiagen), respectively, according to the manufacturer's instructions. cDNA was synthesized from 200 ng of total RNA using Superscript IV (Thermo Fisher) according to the manufacturer's instructions. DNA or cDNA was diluted in RT2 SYBR green Fluor quantitative PCR (qPCR) Mastermix (Qiagen) and analyzed by quantitative real-time PCR in an Applied Biosystems StepOne Plus thermocycler. Cycling conditions were 10 min at 95°C followed by 40 cycles of 15 s at 95°C and 1 min at 60°C, using primers specific to the viral ORF50 gene or cellular GAPDH gene (12, 14). Biological duplicate samples were analyzed in technical triplicate using the ΔΔCT comparative threshold cycle method as previously described (14). gapdh was the cellular housekeeping transcript. Fold change in orf50 transcription for KLKI was calculated relative to WT MHV68 (arbitrarily set to 1.0) for each time point. Transcript levels were normalized to viral DNA present in each sample using the following formula: [2−ΔΔCT (cDNA)/2–ΔΔCT (gDNA)].
Reporter assays.
HEK293T cells were transiently transfected as indicated. Reporter constructs included the proximal MHV68 ORF50 promoter in firefly luciferase reporter vector pGL2 (Promega [51]) the and MHV68 terminal repeat promoter in firefly luciferase reporter vector pGL3 (Promega [20]). Cells were washed with PBS and lysed using passive lysis buffer 18 h postinfection. Reporter assays were performed using a dual-luciferase reporter assay system (Promega) following the manufacturer's instructions.
Antibodies.
Primary antibodies used in this study included rat anti-KSHV LNA-1 (13-210-100; Advanced Biotechnologies, Inc.), rabbit polyclonal v-cyclin antiserum (52), rabbit polyclonal mLANA antiserum (20), mouse polyclonal MHV68 antiserum (49), chicken anti-ORF59 IgY (53), and mouse monoclonal anti-β-actin (catalog no. A2228; Sigma-Aldrich). Fluorophore-conjugated secondary antibodies used in this study included Alexa Fluor goat anti-mouse 647, Alexa Fluor goat anti-rabbit 568, and Alexa Fluor goat anti-chicken 488 (Invitrogen Life Technologies).
Statistics.
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, San Diego, CA). Significance was determined by a two-tailed unpaired Student's t test with a 95% confidence interval.
ACKNOWLEDGMENTS
We thank present and past members of the Forrest laboratory for helpful discussions and suggestions. We thank Skip Virgin and Terry Dermody for their input on the project.
This work was supported by Public Health Service Award R01-CA167065 from the National Cancer Institute of the National Institutes of Health to J.C.F. Additional support was provided by grant P20-GM103625 from the National Institute of General Medical Sciences of the National Institutes of Health. The funders played no role in study design, data collection, or interpretation of data.
REFERENCES
- 1.Virgin HW, Wherry EJ, Ahmed R. 2009. Redefining chronic viral infection. Cell 138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed). 2013. Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett 305:163–174. doi: 10.1016/j.canlet.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesri EA, Cesarman E, Boshoff C. 2010. Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer 10:707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damania B, Pipas JM. 2009. DNA tumor viruses. Springer Science+Business Media, New York, NY. [Google Scholar]
- 6.Flaño E, Husain SM, Sample JT, Woodland DL, Blackman MA. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J Immunol 165:1074–1081. doi: 10.4049/jimmunol.165.2.1074. [DOI] [PubMed] [Google Scholar]
- 7.Moser JM, Farrell ML, Krug LT, Upton JW, Speck SH. 2006. A gammaherpesvirus 68 gene 50 null mutant establishes long-term latency in the lung but fails to vaccinate against a wild-type virus challenge. J Virol 80:1592–1598. doi: 10.1128/JVI.80.3.1592-1598.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speck SH, Ganem D. 2010. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8:100–115. doi: 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballestas ME, Chatis PA, Kaye KM. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 10.Fowler P, Marques S, Simas JP, Efstathiou S. 2003. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J Gen Virol 84:3405–3416. doi: 10.1099/vir.0.19594-0. [DOI] [PubMed] [Google Scholar]
- 11.Habison AC, Beauchemin C, Simas JP, Usherwood EJ, Kaye KM. 2012. Murine gammaherpesvirus 68 LANA acts on terminal repeat DNA to mediate episome persistence. J Virol 86:11863–11876. doi: 10.1128/JVI.01656-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorman NJ, Willer DO, Speck SH. 2003. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J Virol 77:10295–10303. doi: 10.1128/JVI.77.19.10295-10303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballestas ME, Kaye KM. 2011. The latency-associated nuclear antigen, a multifunctional protein central to Kaposi's sarcoma-associated herpesvirus latency. Future Microbiol 6:1399–1413. doi: 10.2217/fmb.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest JC, Paden CR, Allen RD III, Collins J, Speck SH. 2007. ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J Virol 81:11957–11971. doi: 10.1128/JVI.00111-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesri EA, Feitelson MA, Munger K. 2014. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 15:266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sifford JM, Stahl JA, Salinas E, Forrest JC. 16 December 2015. Murine gammaherpesvirus-68 LANA and SOX homologs counteract ATM-driven p53 activity during lytic viral replication. J Virol doi: 10.1128/JVI.02867-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. 2006. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest 116:735–742. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virgin HW IV, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol 71:5894–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellert J, Weidner-Glunde M, Krausze J, Richter U, Adler H, Fedorov R, Pietrek M, Ruckert J, Ritter C, Schulz TF, Luhrs T. 2013. A structural basis for BRD2/4-mediated host chromatin interaction and oligomer assembly of Kaposi sarcoma-associated herpesvirus and murine gammaherpesvirus LANA proteins. PLoS Pathog 9:e1003640. doi: 10.1371/journal.ppat.1003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paden CR, Forrest JC, Tibbetts SA, Speck SH. 2012. Unbiased mutagenesis of MHV68 LANA reveals a DNA-binding domain required for LANA function in vitro and in vivo. PLoS Pathog 8:e1002906. doi: 10.1371/journal.ppat.1002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponnusamy R, Petoukhov MV, Correia B, Custodio TF, Juillard F, Tan M, Pires de Miranda M, Carrondo MA, Simas JP, Kaye KM, Svergun DI, McVey CE. 2015. KSHV but not MHV-68 LANA induces a strong bend upon binding to terminal repeat viral DNA. Nucleic Acids Res 43:10039–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skalsky RL, Hu J, Renne R. 2007. Analysis of viral cis elements conferring Kaposi's sarcoma-associated herpesvirus episome partitioning and maintenance. J Virol 81:9825–9837. doi: 10.1128/JVI.00842-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salinas E, Byrum SD, Moreland LE, Mackintosh SG, Tackett AJ, Forrest JC. 2015. Identification of viral and host proteins that interact with murine gammaherpesvirus 68 latency-associated nuclear antigen during lytic replication: a role for Hsc70 in viral replication. J Virol 90:1397–1413. doi: 10.1128/JVI.02022-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan K, Kuppers DA, Verma SC, Robertson ES. 2004. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J Virol 78:6585–6594. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu F, Day L, Gao SJ, Lieberman PM. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J Virol 80:5273–5282. doi: 10.1128/JVI.02541-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staudt MR, Dittmer DP. 2007. The Rta/Orf50 transactivator proteins of the gamma-herpesviridae. Curr Top Microbiol Immunol 312:71–100. [DOI] [PubMed] [Google Scholar]
- 27.Paden CR, Forrest JC, Moorman NJ, Speck SH. 2010. Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J Virol 84:7214–7224. doi: 10.1128/JVI.00133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlova IV, Virgin HW IV, Speck SH. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J Virol 77:5731–5739. doi: 10.1128/JVI.77.10.5731-5739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu TT, Tong L, Rickabaugh T, Speck S, Sun R. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J Virol 75:9262–9273. doi: 10.1128/JVI.75.19.9262-9273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu TT, Usherwood EJ, Stewart JP, Nash AA, Sun R. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol 74:3659–3667. doi: 10.1128/JVI.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Zhou F, Ye F, Gao SJ. 2008. Genetic disruption of KSHV major latent nuclear antigen LANA enhances viral lytic transcriptional program. Virology 379:234–244. doi: 10.1016/j.virol.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toth Z, Papp B, Brulois K, Choi YJ, Gao SJ, Jung JU. 2016. LANA-mediated recruitment of host polycomb repressive complexes onto the KSHV genome during de novo infection. PLoS Pathog 12:e1005878. doi: 10.1371/journal.ppat.1005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottinger M, Pliquet D, Christalla T, Frank R, Stewart JP, Schulz TF. 2009. The interaction of the gammaherpesvirus 68 orf73 protein with cellular BET proteins affects the activation of cell cycle promoters. J Virol 83:4423–4434. doi: 10.1128/JVI.02274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Ward C, Yeasmin R, Skiena S, Krug LT, Forrest JC. 2017. A codon-shuffling method to prevent reversion during production of replication-defective herpesvirus stocks: implications for herpesvirus vaccines. Sci Rep 7:44404. doi: 10.1038/srep44404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharp PM. 2002. Origins of human virus diversity. Cell 108:305–312. doi: 10.1016/S0092-8674(02)00639-6. [DOI] [PubMed] [Google Scholar]
- 36.Barton E, Mandal P, Speck SH. 2011. Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 29:351–397. [DOI] [PubMed] [Google Scholar]
- 37.Gao L, Qi J. 2007. Whole genome molecular phylogeny of large dsDNA viruses using composition vector method. BMC Evol Biol 7:41. doi: 10.1186/1471-2148-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KS, Suarez AL, Claypool DJ, Armstrong TK, Buckingham EM, van Dyk LF. 2012. Viral cyclins mediate separate phases of infection by integrating functions of distinct mammalian cyclins. PLoS Pathog 8:e1002496. doi: 10.1371/journal.ppat.1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Zhu L, Lu X, Feldman ER, Keyes LR, Wang Y, Fan H, Feng H, Xia Z, Sun J, Jiang T, Gao SJ, Tibbetts SA, Feng P. 2015. Recombinant murine gamma herpesvirus 68 carrying KSHV G protein-coupled receptor induces angiogenic lesions in mice. PLoS Pathog 11:e1005001. doi: 10.1371/journal.ppat.1005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbera AJ, Chodaparambil JV, Kelley-Clarke B, Joukov V, Walter JC, Luger K, Kaye KM. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856–861. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- 41.De León Vázquez E, Juillard F, Rosner B, Kaye KM. 2014. A short sequence immediately upstream of the internal repeat elements is critical for KSHV LANA mediated DNA replication and impacts episome persistence. Virology 448:344–355. doi: 10.1016/j.virol.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu J, Renne R. 2005. Characterization of the minimal replicator of Kaposi's sarcoma-associated herpesvirus latent origin. J Virol 79:2637–2642. doi: 10.1128/JVI.79.4.2637-2642.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cotter MA II, Robertson ES. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254–264. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- 44.Kaul R, Verma SC, Robertson ES. 2007. Protein complexes associated with the Kaposi's sarcoma-associated herpesvirus-encoded LANA. Virology 364:317–329. doi: 10.1016/j.virol.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weck KE, Barkon ML, Yoo LI, Speck SH, Virgin HI. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J Virol 70:6775–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler H, Messerle M, Wagner M, Koszinowski UH. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J Virol 74:6964–6974. doi: 10.1128/JVI.74.15.6964-6974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 634:421–430. doi: 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- 48.Brulois KF, Chang H, Lee AS, Ensser A, Wong LY, Toth Z, Lee SH, Lee HR, Myoung J, Ganem D, Oh TK, Kim JF, Gao SJ, Jung JU. 2012. Construction and manipulation of a new Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome clone. J Virol 86:9708–9720. doi: 10.1128/JVI.01019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahl JA, Paden CR, Chavan SS, MacLeod V, Edmondson RD, Speck SH, Forrest JC. 2012. Amplification of JNK signaling is necessary to complete the murine gammaherpesvirus 68 lytic replication cycle. J Virol 86:13253–13262. doi: 10.1128/JVI.01432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weck KE, Kim SS, Virgin HI, Speck SH. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol 73:3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu S, Pavlova IV, Virgin HW IV, Speck SH. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J Virol 74:2029–2037. doi: 10.1128/JVI.74.4.2029-2037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dyk LF, Hess JL, Katz JD, Jacoby M, Speck SH, Virgin HI. 1999. The murine gammaherpesvirus 68 v-cyclin gene is an oncogene that promotes cell cycle progression in primary lymphocytes. J Virol 73:5110–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Upton JW, van Dyk LF, Speck SH. 2005. Characterization of murine gammaherpesvirus 68 v-cyclin interactions with cellular cdks. Virology 341:271–283. doi: 10.1016/j.virol.2005.07.014. [DOI] [PubMed] [Google Scholar]