ABSTRACT
Herpes simplex virus (HSV) infection is restricted to epithelial cells and neurons and is controlled by CD8 T cells. These cells both traffic to epithelial sites of recurrent lytic infection and to ganglia and persist at the dermal-epidermal junction for up to 12 weeks after lesion resolution. We previously showed that cutaneous lymphocyte-associated antigen (CLA), a functional E-selectin ligand (ESL), is selectively expressed on circulating HSV-2-specific CD8 T cells. CLA/ESL mediates adhesion of T cells to inflamed vascular endothelium. Later stages in T-cell homing involve chemokines (Ch) and lymphocyte chemokine receptors (ChR) for vascular wall arrest and diapedesis. Several candidate ChR have been implicated in skin homing. We measured cell surface ChR on HSV-specific human peripheral blood CD8 T cells and extended our studies to HSV-1. We observed preferential cell surface expression of CCR10 and CXCR3 by HSV-specific CD8 T cells compared to CD8 T cells specific for control viruses, Epstein-Barr virus (EBV) and cytomegalovirus (CMV), and compared to bulk memory CD8 T cells. CXCR3 ligand mRNA levels were selectively increased in skin biopsy specimens from persons with recurrent HSV-2, while the mRNA levels of the CCR10 ligand CCL27 were equivalent in lesion and control skin. Our data are consistent with a model in which CCL27 drives baseline recruitment of HSV-specific CD8 T cells expressing CCR10, while interferon-responsive CXCR3 ligands recruit additional cells in response to virus-driven inflammation.
IMPORTANCE HSV-2 causes very localized recurrent infections in the skin and genital mucosa. Virus-specific CD8 T cells home to the site of recurrent infection and participate in viral clearance. The exit of T cells from the blood involves the use of chemokine receptors on the T-cell surface and chemokines that are present in infected tissue. In this study, circulating HSV-2-specific CD8 T cells were identified using specific fluorescent tetramer reagents, and their expression of several candidate skin-homing-associated chemokine receptors was measured using flow cytometry. We found that two chemokine receptors, CXCR3 and CCR10, are upregulated on HSV-specific CD8 T cells in blood. The chemokines corresponding to these receptors are also expressed in infected tissues. Vaccine strategies to prime CD8 T cells to home to HSV lesions should elicit these chemokine receptors if possible to increase the homing of vaccine-primed cells to sites of infection.
KEYWORDS: herpes simplex virus, cell trafficking, chemokines, T cells, cytotoxic, CD8 T cell, chemokine receptors
INTRODUCTION
In immunocompetent persons, herpes simplex virus (HSV) infections are restricted to mucosal and keratinized epithelia and neuronal ganglia. Recruited, circulating HSV-specific CD8 T cells and tissue resident memory (TRM) T cells each participate in the control of recurrent infection (1). The appearance of cytotoxic HSV-2-specific T cells in human recurrent lesions correlates temporally and spatially with viral clearance and lesion resolution (2). Lymphocytes traffic into skin by slowing and then stopping on the inner wall of dermal venules, followed by extravasation into tissue (3, 4). Loose adhesion to the endothelium involves cutaneous lymphocyte-associated antigen (CLA), a carbohydrate modification of the extracellular region of transmembrane proteins. The CLA ligand, E-selectin (ESL), is upregulated on dermal venule endothelial cells in response to gamma interferon (IFN-γ). The subsequent cell arrest and diapedesis steps involve chemokines (Ch) and corresponding lymphocyte transmembrane chemokine receptors (ChR). Myriad human Ch and ChR mediate diverse tissue-specific leukocyte homing programs (5). Because human HSV-specific CD8 T cells circulate, traffic to infected tissues, and also remain in place as TRM cells, the programming of ChR expression may be complex, with patterns depending on the anatomical site of specimen collection. We previously showed that TRM cells that localize to the dermo-epidermal junction after HSV-2 lesion resolution are relatively deficient in ChR mRNA (6). In this study, we investigated ChR expressed by HSV-specific CD8 T cells in the circulation.
We found previously that HSV-2-specific CD8 T cells in blood overexpress CLA, specifically bind to soluble and cell-expressed E-selectin (7), and can be enriched from blood by gating for high CLA expression (8). HSV-2 lesions show CLA expression on infiltrating T cells and high venular E-selectin by immunostaining (7, 9). We used T-cell receptor beta chain (TRB) CDR3 methods to identify HSV-2-specific T-cell clonotypes and documented clonotypes shared between active lesions and blood HSV-2-specific T cells, consistent with ingress of cells from the circulation (6, 10–12). There are fewer human data available concerning the homing of HSV-1-specific T cells in humans, but we have defined HSV-1 type-specific epitopes and used tetramers to demonstrate TRM cells in situ in HSV-1-infected neural ganglia (13).
Ch-ChR pairings implicated in skin homing have been functionally classified as mediating either homeostatic or inflammatory immune cell trafficking (14). CCR4 and CCR10 are overexpressed on human circulating T cells that coexpress CLA/ESL (15–19) and have been implicated in pathological skin conditions (16, 20–24). In addition, lymphocytes from normal skin overexpress CCR6 (25). CCR8 expression on normal skin lymphocytes ranges from about 50% to nearly 100% depending on isolation techniques (14, 25). Among the ligands for these candidate skin-homing ChR, CCL27 and CCL28 are constitutively expressed by keratinocytes, implicating CCR10 in the possible homeostatic retention of cells in skin (16), although recent skin TRM cell studies have not detected remarkable CCR10 expression (26).
Murine HSV studies have identified inflammatory, functional roles for CXCR3 and its ligands, CXCL9 (MIG), CXCL10 (IP-10), and CXCL11 (ITAC), in models of HSV infection. Each CXCR3 ligand corresponds to an interferon-stimulated gene (27–29). In ocular HSV-1 models, local mRNA analyses show strong upregulation of CXCR3 ligands (30). HSV-1-specific CD8 T cells migrate toward CXCR3 gradients in murine models (31). Functional studies using HSV-2 mouse vaginal models indicate roles for the CXCR3 axis in disease resistance (32).
There are few human data on Ch/ChR and HSV infections. HSV vesicle fluid contains MIP1α (CCL3), MIP1β (CCL4), and RANTES (CCL5), Ch ligands for CCR1 and CCR5 (33). CD4+ T cells near sites of HSV-2 reactivation express CCR5 and persist for months after the lesion has healed (34). Human CD8 TRM cells in healed HSV lesions are generally low in ChR expression (6). In the present study, we used flow cytometry to measure expression patterns of the candidate skin-homing ChR CCR4, CCR6, CCR8, CCR10, and CXCR3 on circulating cells directly ex vivo. Control CD8 T cells specific for the herpesviruses Epstein-Barr virus (EBV) and cytomegalovirus (CMV), which do not typically infect skin or neurons, were also analyzed. Additionally, we used microarrays to investigate the Ch and ChR transcript levels in HSV-2-infected and matched normal skin. Our findings suggest roles both for the CXCR3 axis for homing of cells during inflammatory situations and for CCR10, possibly in the transit of more constitutive TRM-like cells.
RESULTS
Participants and virus-specific T cells.
Screening of peripheral blood mononuclear cells (PBMC) from HLA-appropriate donors identified 28 persons for detailed study (Table 1), including 12 men (43%) and 16 women (57%). Among the 28 participants, 9 contributed only HSV data, 7 were studied for both HSV- and control virus-specific T cells, and 12 samples were tested for control viruses only. Tetramer-positive and -negative CD8α+ T-cell populations were well separated (see representative dot plots in Fig. 1A). For HSV, we studied 19 different HSV-specific CD8 T-cell populations from 16 donors, as some participants were tetramer positive for more than one HSV epitope. Among these 16, 7 were HSV-1 and HSV-2 coinfected, while 9 were infected with only HSV-2. Ten tests used the HSV-2 type-specific tetramer B7-RPR, while four focused on HSV-1 type-specific T cells, the latter using two distinct HSV-1 type-specific tetramers in two donors each. Five donors were tested with the HSV type-common tetramer A2-FLW. Among these, two were HSV-2 monoinfected and three were HSV-1 and HSV-2 coinfected. Therefore, the overall HSV data set includes 12 tests of HSV-2-monoreactive cells elicited by HSV-2 infection, 4 tests of HSV-1-monoreactive cells elicited by HSV-1 infection, 2 tests of HSV-1/HSV-2-cross-reactive cells elicited by HSV-2 infection in persons seronegative for HSV-1, and 3 tests of tetramer A2-FLW conducted for HSV-1- and HSV-2-coinfected persons that are therefore ambiguous with regard to HSV type(s) provoking the immune response. Control CD8 T-cell populations were specific for EBV (n = 15 persons) or CMV (n = 12 persons). Not all control populations were tested for each homing-related marker (see Table 1, footnote e).
TABLE 1.
Participants and virus-specific CD8 T-cell populations studied
| Subject | Sexa | Serologyb |
HSV history and durationc |
HLA typed |
HSV tetramer(s) positive on PBMC ex vivo screening used for ChR studies |
Control tetramerse | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HSV-1 | HSV-2 | Oral | Genital | A | B | HSV-1 type specific | HSV type common | HSV-2 type specific | |||
| HSV T-cell studies only | |||||||||||
| 1 | F | − | + | None | Unk | 03 | 07 | B7-RPR | |||
| 2 | M | − | + | 34.5 | 24.5 | 03,31 | 07,60 | B7-RPR | |||
| 4 | F | + | + | None | 33.5 | 01,26 | 07,08 | B7-RPR | |||
| 6 | M | − | + | None | None | 02,03 | 07,44 | B7-RPR | |||
| 7 | F | − | + | None | 26.5 | 01,02 | 08,13 | A2-FLW | |||
| 9 | M | + | + | Unk | 39.5 | 02,01 | 07,44 | A2-FLW | B7-RPR | ||
| 17 | M | − | + | None | 8.6 | 02,03 | 07,62 | B7-RPR | |||
| 22 | M | + | + | 12.5 | 10.5 | 01,11 | 27 | A1-SAL, A1-FTD | |||
| 25 | F | + | + | 23.5 | 8.3 | 01,31 | 60 | A1-SAL, A1-FTD | |||
| HSV and control T-cell studies | |||||||||||
| 5 | F | + | + | None | 38.4 | 02,11 | 08,15 | A2-FLW | CMV, EBV | ||
| 10 | M | − | + | None | 21.5 | 02,34 | 15,35 | A2-FLW | CMV | ||
| 11 | M | + | + | None | None | 02 | 08,44 | A2-FLW | CMV, EBV | ||
| 14 | F | − | + | None | 9.3 | 02,03 | 07,13 | B7-RPR | EBV | ||
| 16 | M | − | + | None | 12.3 | 02,24 | 07,27 | B7-RPR | EBV | ||
| 26 | M | + | + | None | 8.4 | 02,03 | 07,44 | B7-RPR | EBV | ||
| 28 | F | − | + | None | 12.5 | 03,33 | 07,14 | B7-RPR | CMV, EBV | ||
| Control T-cell studies only | |||||||||||
| 3 | F | − | + | 23 | 25.0 | 01,02 | 08,57 | CMV | |||
| 8 | F | − | + | None | 12.7 | 02,26 | 07,27 | EBV | |||
| 12 | F | − | + | None | Unk | 02 | 27,37 | CMV | |||
| 13 | F | + | + | Unk | 11.5 | 02,26 | 14,27 | EBV | |||
| 15 | F | − | + | None | 20.5 | 02,03 | 14 | CMV, EBV | |||
| 18 | M | + | + | Unk | 17.9 | 02,24 | 35 | CMV | |||
| 19 | M | − | + | 11.5 | 11.5 | 02,31 | 44,64 | EBV | |||
| 20 | F | − | + | None | 38.5 | 02 | 44,62 | CMV, EBV | |||
| 21 | F | + | − | None | None | 01,02 | 07,62 | CMV, EBV | |||
| 23 | F | − | + | Unk | 19.5 | 02,68 | 44,49 | CMV, EBV | |||
| 24 | M | − | + | None | 25.5 | 01,02 | 08,44 | CMV, EBV | |||
| 27 | F | + | − | 51.1 | 6.9 | 02,03 | 07,62 | EBV | |||
F, female; M, male.
Serostatus by type-specific immunoblot at the closest time point available to the date of blood draw.
Years from symptom onset to blood draw. None, no symptoms; Unk, unknown (history not available).
HLA class I allele(s) to the two-digit level.
Among the control virus-specific populations, the numbers of subjects tested were 11 and 13 for CMV and EBV, respectively, for CLA, 11 and 14 for CCR4, CCR6, CCR7, and CXCR3, 10 and 11 for CCR8, and 10 and 14 for CCR10.
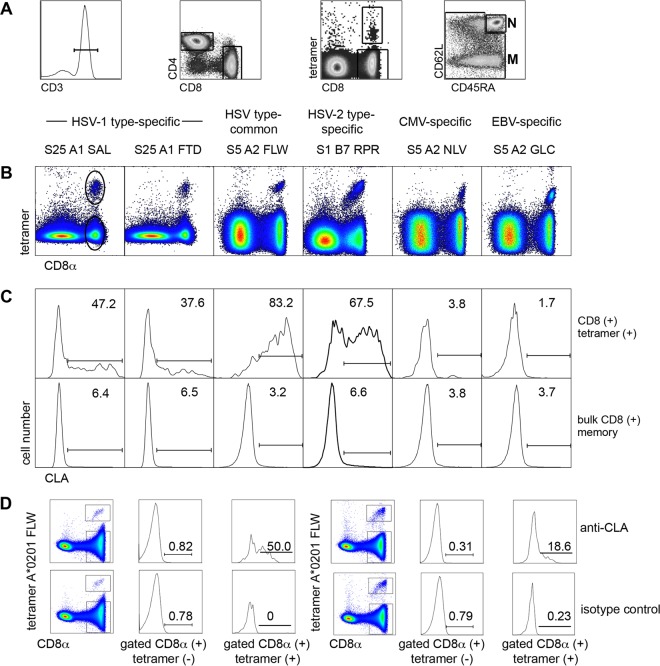
FIG 1.
Expression of CLA by circulating herpesvirus-specific CD8+ T cells. (A) Gating scheme after selection of single lymphocytes using forward/side scatter. CD3+ (first image) CD8+ CD4− (second image) cells are gated for CD8+ tetramer-positive cells (third image). CD8+ tetramer-negative cells are gated (fourth image) into bulk naive (N) (CD45RA+ CD62L+) and bulk memory (M) (all others). (B) Representative dot plots of gated live singlicate CD3+ CD4− T cells stained with anti-CD8α and APC-labeled tetramers specific for the indicated viruses, labeled with subject number (S), short HLA allele name, and first three viral peptide amino acids. Circles in the left dot plot are representative gating of CD8+ tetramer-positive or -negative cells. (C) Histograms of CLA expression by gated CD8+ tetramer-positive cells (upper) and CD8+ tetramer-negative cells (lower) from the dot plots above each histogram. Numbers are percentages of gated cells positive for CLA in the indicated region. (D) Isotype control staining of gated HSV-specific tetramer-positive and tetramer-negative CD8 T cells from two representative participants showing specific and positive staining with anti-CLA for tetramer-positive cells but minimal staining with isotype control rat IgM MAb.
CLA expression by virus-specific T cells.
We confirmed our previous (7) finding that HSV-2-specific CD8 T cells overexpress CLA in comparison to other CD8+ T cells. For each tetramer stain, we used bulk memory CD8+ T cells, comprising all CD62L− cells and CD62L+ CD45RA− central memory cells as an internal control (gating shown in Fig. 1A). We found that circulating HSV-1-specific and HSV-2-secific T cells overexpress CLA in comparison to bulk memory CD8+ cells (representative data, Fig. 1B and C; summary, Fig. 2; P = 0.006, 0.0007, and <0.0001 compared to EBV-specific, CMV-specific, and bulk memory CD8 T cells, respectively). The proportion of tetramer-positive cells expressing CLA was 4- to 16-fold higher for HSV-reactive than for CMV- or EBV-specific CD8 T cells or for bulk memory CD8 T cells (Fig. 1C, Fig. 2). We separately examined four HLA A*0201+, HSV-2-seropositive subjects with well-separated populations of CD8+ HSV A2-FLW tetramer-positive cells with anti-CLA and an isotype control monoclonal antibody (MAb). We again detected strong CLA expression on the gated CD8+ tetramer-positive but not the gated CD8+ tetramer-negative T cells, with essentially zero staining of either population with a rat IgM isotype control (results for two subjects are shown in Fig. 1D; same pattern in two other subjects [data not shown]).
FIG 2.
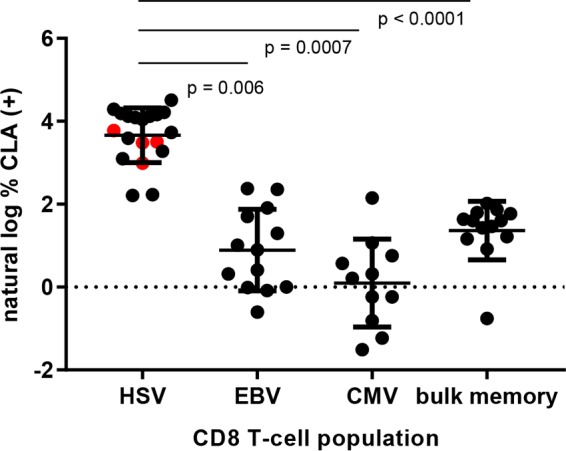
CLA expression by virus-specific CD8 T cells in direct ex vivo stains (left three groups) or bulk memory CD8 T cells (right). The x axis indicates origin of peptide in HLA class I tetramer used to identify virus-specific CD8 T cells. Red data points for HSV indicate data derived from HSV-1-specific tetramers. Data are graphed as the natural log of the percent CLA+ cells. Horizontal bars indicate means, and error bars indicate standard deviations. P values are indicated for selected comparisons.
Expression of chemokine receptors by CD8 T-cell populations.
The expression of ChR by circulating CD8+ T cells was evaluated using allophycocyanin (APC)-labeled tetramers and phycoerythrin (PE)-labeled anti-ChR and isotype control MAbs. As discussed in Materials and Methods, attention was paid to setting thresholds for evaluating positive ChR expression. Figure 3 shows representative data for participant S28 cells stained with HSV-2 type-specific tetramer B7-RPR. Including all participants (Fig. 4), for bulk memory CD8 T cells, we noted low expression of CCR8 and intermediate expression of CCR10. Expression of CCR6 was highly variable, while expression of CCR4 was generally high. We detected overall relatively high expression of CCR7 (Fig. 4), which was generally higher on naive (CD62L+ CDR45RA+) and central memory (CD62L+ CD45RA−) CD8 T cells than on effector memory/effector cells (CD62L−) (data not shown).
FIG 3.
Representative data for ChR expression by virus-specific CD8+ T cells for subject S28 and HSV-2 tetramer B7-RPR. (A) Dot plots showing expression of ChR (y axis, receptor names on top) by CD8+ bulk naive, bulk memory, or HSV tetramer-positive cells, with CLA expression on the x axis. Numbers are percentages of gated cells in each quadrant. (B) Expression levels for isotype control MAb species, fluorochrome and isotype matched to the anti-ChR MAb immediately above and naive cells. y axis thresholds are set in each case to 0.5% anti-isotype-positive events.
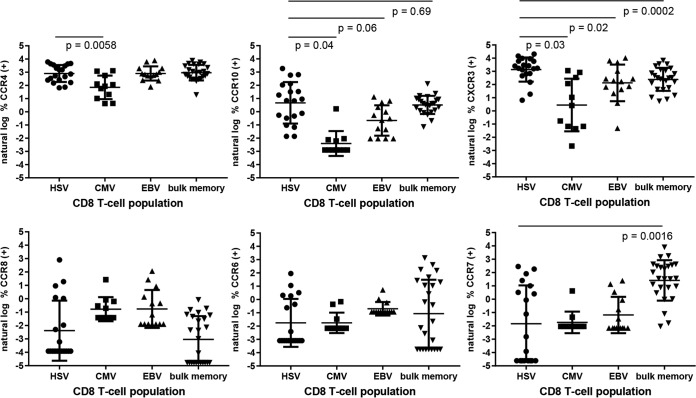
FIG 4.
ChR expression by circulating virus-specific CD8 T cells (left three groups in each graph) or bulk memory CD8 T cells (right). Data are shown as the natural log of the percent ChR+ cells. P values are shown for CCR10 and CXCR3; P values for other ChR are >0.05. Zero measurements were assigned a value of the natural log of 50% of the lowest measured value. Error bars indicate standard deviations.
Among circulating virus-specific cells, HSV-specific CD8 T cells had higher expression of CXCR3 than CMV- or EBV-specific CD8 T cells or bulk memory T cells. Specifically, the mean differences between the natural log percentage of HSV-specific cells and the natural log percentage of other cells expressing CXCR3 were 2.5, 1.0, and 0.7 for CMV-specific, EBV-specific, and bulk memory CD8+ T cells, respectively, with P values of 0.0321, 0.0242, and 0.002, respectively. Expression of CCR10 was also higher for HSV- than for CMV-specific lymphocytes, with a mean natural log percent difference value of 3 (P = 0.0427). CCR10 expression was almost statistically significantly higher for HSV-specific than for EBV-specific CD8 T cells (P = 0.0639). CCR4 was overexpressed by HSV-specific CD8 T cells compared to CMV-specific cells, with a mean log2 percentage difference of 1.5 (P = 0.0058). The expression of CCR6, CCR8, and CCR7 did not differ between HSV- and CMV- or EBV-specific CD8 T cells. A very wide range of CCR7 expression was detected; overall, CCR7 was underexpressed on HSV-specific T cells compared to bulk memory CD8 T cells (Fig. 4).
Relationship between CLA expression and chemokine receptor expression.
Having observed overexpression of CXCR3 and CCR10 by HSV-specific CD8 T cells, we determined if these ChR were preferentially coexpressed by CLA+ cells. Gating for HSV-specific CD8 T cells, dot plots showed a pattern of higher CCR10 expression by CLA+ than by CLA− cells (see representative data in Fig. 3A; dot plot is 3rd row, 5th column). Data from all the participants for all 19 HSV tetramer-positive populations showed that CLA+ cells had higher CCR10 expression than CLA− cells (Fig. 5A; P < 0.0001). Similar analyses for CXCR3 did not disclose any difference by CLA+ versus CLA− HSV tetramer-positive CD8 T-cell populations (Fig. 5B; P = 0.46).
FIG 5.
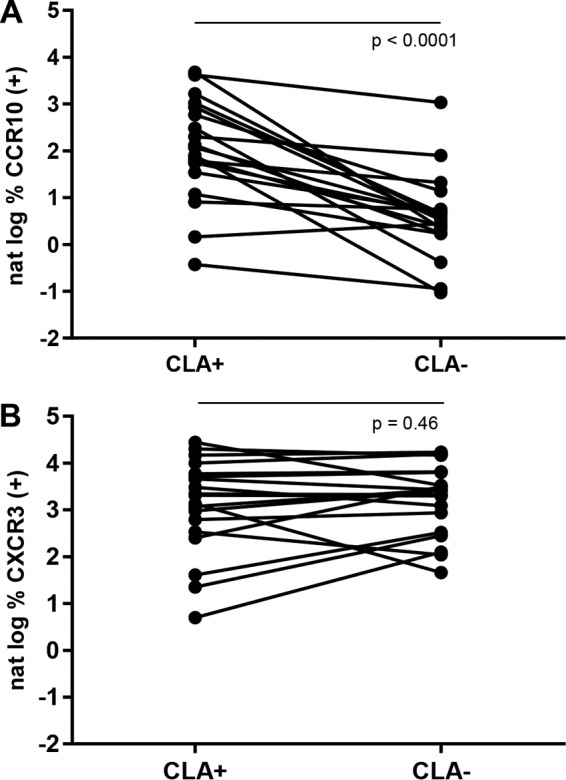
Circulating CLA+ HSV-specific CD8 T cells preferentially express CCR10. (A) Pairwise comparison of CCR10 expression by 19 gated tetramer-positive HSV-specific CD8 T-cell populations from 16 subjects differentiated by CLA expression. (B) Similar analysis for CXCR3 expression. P values are from two-tailed, Wilcoxon matched-pairs signed-rank tests.
Chemokine- and homing-related mRNA expression in HSV-2 and control biopsy specimens.
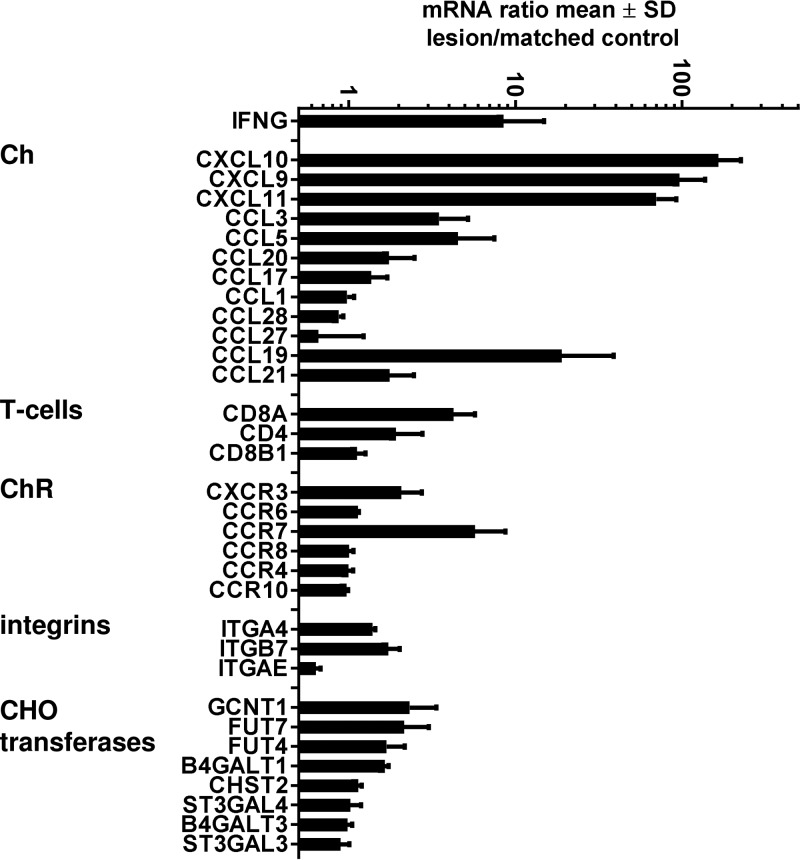
The ligands for CXCR3, CXCL9, CXCL10, and CXCL11 are highly responsive to interferon induction. We have previously reported high levels of IFN-γ in tissues of HSV-infected people (35); in the specimens analyzed in this study, IFN-γ mRNA levels were an average of 8-fold higher in HSV-2 lesions than in simultaneously obtained normal skin (Fig. 6). We observed higher levels of mRNA for all three known CXCR3 ligands, CXCL9, CXCL10, and CXCL11, in herpetic skin than in control tissue (Fig. 6). The levels of mRNA specific for CCL27 and CCL28, the ligands for CCR10, did not appear to differ between specimen types, and neither did mRNA levels for CCL20, CCL17, and CCL1, the ligands for CCR6, CCR4, and CCR8, respectively. The levels of CCL3 and CCR5 mRNA were moderately elevated, consistent with their protein detection in herpetic vesicle fluid and the attraction of a broad array of leukocytes to herpes lesions (33). The CD4+ and CD8α+ T-cell signature transcripts were increased. Among ChR, CXCR3 mRNA levels appeared to be increased in lesion biopsy specimens, consistent with an influx of CXCR3+ cells. Levels of most of the other mRNAs encoding the other ChR studied in PBMC were not increased in lesion biopsy specimens. The levels of CCR7 mRNA and of mRNA for the CCR7 ligand CCL19 (MIP1β) were also increased. The levels of GCNT1 and FUT7 mRNAs, encoding enzymes required for CLA synthesis, appeared to be mildly increased. A limited survey of integrin mRNAs related to leukocyte homing (ITGA4 and ITGAE, together encoding CD103, and ITGA4 and ITGA7, together encoding the α4β7 integrin, associated with gastrointestinal [GI] tract trafficking) did not reveal lesion enrichment. Statistical tests were not attempted with the small number of paired biopsy specimens studied.
FIG 6.
mRNA expression for selected immunologically relevant proteins in recurrent human HSV-2 genital skin lesion biopsy specimens (n = 3) and person-matched normal skin (n = 3). Genes are listed at the left. Bars show means of ratios of mRNA for HSV-2 skin compared to normal skin; error bars show standard deviations.
DISCUSSION
In this report, we have compared ChR expression by circulating human HSV-specific CD8 T cells with that of control memory CD8 T cells specific for other herpesvirus pathogens to gain insight into the homing potential of circulating memory T cells. The studies were undertaken because memory T cells are programmed for diverse patterns of gene expression and function (36). In the case of recurrent, localized infections due to HSV, we hypothesize that circulating memory cells include cells programming for trafficking and localization to sites of infection. Skin, mucosal surfaces, and the neural ganglia innervating these areas are rational target tissues. It is known that human HSV-specific effector cells are able to accumulate in overtly infected tissues to clear virus (2). While murine HSV studies of T-cell trafficking have been very informative, mice lack spontaneous HSV recurrences that could condition lymphocyte imprinting over time. Additionally, HSV has evolved immune escape functions for human, but not murine, CD8 T cells (37).
We found that HSV-specific circulating CD8 T cells overexpress CXCR3 compared to control EBV- and CMV-specific T cells and memory CD8 T cells. For CCR10, our results for higher expression by HSV-specific cells reached significance in comparison to that by CMV-specific T cells. Moreover, when we investigated coexpression of CLA and ChR, we observed that CCR10 expression was significantly higher among CLA+ than among CLA− HSV-specific CD8 T cells, suggesting that functional specialization for skin homing among CLA+ cells extends to ChR expression as well. In contrast, CLA+ HSV-specific circulating CD8+ T cells did not preferentially overexpress CXCR3. Our data can be interpreted in a model in which circulating CLA+ CXCR3+ HSV-specific CD8 T cells can home to sites of recurrent HSV infection, attracted by the high levels of CXCR3 ligands that we document at the transcriptional level in herpetic lesions. The cellular infiltrate in active HSV-2 lesions is known to contain CD8 and CD4 T cells (38), which could be the origin of the CXCR3 mRNA detected in lesional skin in our limited microarray studies. The origin and fate of CLA+ CCR10+ cells are less clear, but given the lack of clear induction of CCR10 ligands in herpetic lesions, we hypothesize that these cells, present at widely varied levels in individual donors, may represent lineages capable of recruitment or constitutive residence in skin mediated by CCR10 ligands.
Using new HSV-1 reagents, we were also able to extend previous observations (7) centered on HSV-2 to detect high expression of CLA by circulating HSV-1-specific CD8 T cells. CLA-high and CLA-negative subpopulations were observed for each virus-specific T-cell population. Most circulating T cells are permissive for CLA expression after entering the cell cycle in specific cytokine milieus (39). However, something other than T-cell receptor (TCR) stimulation likely maintained constitutive CLA expression on the circulating cells studied in this investigation because HSV recurrences are localized and intermittent. Many of our participants, for example, were persistently asymptomatic for herpetic lesions, and all were without symptoms at the time of blood collection. While asymptomatic, localized epithelial HSV reactivation occurs in most infected persons (40), we consider it unlikely that this is frequent or strong enough to cause TCR-driven CLA expression in the blood compartment. There is no gene encoding CLA per se; rather, CLA is a fucose-containing short branched carbohydrate that may decorate several cell surface proteins. We studied the mRNA levels for FUT7 and GCNT1, two genes in the CLA synthetic pathway (41). We hypothesize that epigenetic regulation of enzymes, such as FUT7, required for CLA synthesis is programmed into HSV-specific CD8 T cells, as shown for cytosine methylation in the FUT7 promoter region in other leukocytes (42), and we plan to address this experimentally. It is also not known if the CLA+ and CLA− cells evolve from clonally distinct precursors at the time or priming, develop more slowly over time into fixed lineages, or show plasticity for CLA expression, a question that can be addressed by TCR CDR3 sequencing of circulating tetramer-positive cells differing for CLA expression. We are collecting serial samples from a cohort with primary genital HSV-1 infection (43) to address both the kinetics of CLA acquisition and its clonal distribution.
Several studies have examined the relationship between the specificity, number or phenotype of HSV tetramer-positive cells, and HSV severity (44–47), but homing potential has not been studied. We do not yet have enough data to correlate homing potential with HSV infection phenotype. It is possible that circulating cells are second responders after failure of local cell-intrinsic, leukocyte-innate, or antigen-specific TRM cells. If this is true, we would predict that circulating cells capable of homing to inflamed lesions, for example, CXCR3+ cells, might correlate with more rapid lesion and shedding resolution, i.e., shorter recurrences and shedding episodes, but not necessarily with asymptomatic shedding, which could be controlled by rapid local responses in the absence of overt inflammation. It is interesting that some markers on circulating HSV-2-specific CD8 T cells, such as CCR7, displayed a very wide level of intersubject variability and may be particularly suitable for disease correlation studies. While CCR7 traditionally marks central memory cells able to home to lymph nodes, we also detected high levels of mRNA for CCR7 and for the CCR7 ligand CCL19 in the limited number of lesion biopsy specimens studied. The cellular origins of these signals are not yet known. Dually CXCR3- and CCR7-positive virus-specific CD8 T cells have been described for human blood (48), challenging the traditional central memory versus effector memory dichotomy. CCL19 and CCR7 can be expressed by dendritic cells (DC), and we and others have previously described enrichment of DC in HSV-2 lesions (34, 49).
ChR expression by circulating cells with the potential to migrate to perform effector functions and possibly become TRM cells, and ChR expression by TRM cells after their irreversible localization, has been studied in several systems. Many investigations have studied ChR patterns for circulating T cells specific for tissue-restricted infectious pathogens or allergens. However, ChR patterns can segregate with homing, cytokine, and effector profiles that overlap in complex ways. Overlapping the homing-associated expression of ChR, CD4 T-cell ChR expression can also vary with Th1, Th2, Th17, and TFH cytokine and effector potential, with these cells tending to express CXCR3, CCR4, CCR6, and CXCR5, respectively (50). Thus, CCR4 expression by circulating T cells specific for allergens (51) can be interpreted as programming for homing, Th2 cytokine expression, or both. In the context of HSV, specific CD4 T cells generally secrete the Th1 cytokines IFN-γ and interleukin 2 (IL-2) (52). HSV-specific CD8 T cells have strong IFN-γ and IL-2 secretion and cytolytic potential (53), but it remains unclear how master transcription factor, effector functions, and programming for homing interact for this T-cell subset. We recently demonstrated that HSV-specific TRM cells in human cervical biopsy specimens overexpress CD103 (αEβ7 integrin) at the protein level (12). Our limited studies of active HSV-2 lesions did not disclose overexpression of CD103-encoding mRNAs, perhaps reflecting the difference between leukocytes present between HSV-2 recurrences and those responding to acute viral replication.
Murine adoptive-transfer experiments show that circulating naive T cells can seed HSV-1-specific skin TRM cells (54) and that these precursors express CXCR3 (55). These precursors are also KLRG1−, a finding amenable to exploration in humans. Similar findings have been made in the murine vaginal HSV-2 system in which circulating cells can be attracted to the genital tract by CXCR3 ligands, where they are retained as TRM cells (56). We find that HSV-specific CD8 T cells overexpress CXCR3 in humans and have noted TRB CDR3 sequence overlap between circulating HSV-specific cells and TRM cells (6), consistent with a model in which circulating cells are the precursors of TRM cells. In murine Yersinia infection, CXCR3 is also involved in the migration of antigen-specific CD8 T cells to infected intestinal tissues that later become TRM cells (57). Human skin TRM cells generally lack most ChR (6) and murine brain CD8 TRM cells are generally low in ChR protein expression (58), consistent with loss of TRM cell migratory capacity. Recently, transcription factors have been identified in TRM cells that may enforce downregulation of some ChR and other mobility-associated receptors (59), but confirmatory findings in humans have not yet been reported.
The present report focuses on the natural human host for HSV in the setting of reactivation disease. Murine models, mostly emphasizing resistance to primary infection, highlight functional roles of other arms of innate and adaptive immunity. NK cell depletion can render mice susceptible to primary HSV infection (60). CD4 T cells, including local tissue-resident virus-specific cells, are involved in the resistance of mice vaccinated with live attenuated HSV-2 to vaginal challenge with a virulent strain (61). HSV-specific antibody can also protect mice from HSV challenge in the absence of acquired cellular immunity (62). Virus-specific CD8 T cells can protect mice from primary HSV-2 infection (63), but there are relatively few positive data emphasizing strong protection from CD8 T cells alone. Local virus-specific CD8 TRM can protect against exogenous reinfection, as a surrogate for reactivation, in an anatomically restricted fashion (64). Depletion of immune mice or ganglia with anti-CD8 reagents can lead to in vivo or in vitro evidence of HSV reactivation (65, 66), although mice do not reactivate in the manner that humans do, and recent experiments (67) have added complexity to the interpretation of these CD8 cell findings. Overall, the circulating virus-specific CD8 T cells emphasized in this report work in an extremely complex and dynamic milieu to contribute to the outcome of viral replication in the periphery during the chronic phase of HSV infections.
Our study had several strengths and weaknesses. Adhesion molecule and ChR expression are labile after restimulation in vitro and depend on the cytokine milieu such that ex vivo measures such as ours are preferred. Each HSV tetramer used has empirical proof that CD8 T cells specific for the peptide-HLA combination recognize bona fide HSV-infected antigen-presenting cells (13, 68, 69). In addition to the HSV specificities in this report, we showed that circulating HSV-2-reactive CD8 T cells with other, diverse fine specificities also overexpress CLA (8). It is likely, albeit not yet proven, that ChR programming is also shared across fine specificities. We were not able to determine if low CCR10 observed for CMV-specific CD8 T cells was associated with the exhausted, CD27− CD28− CD57+ and expanded phenotype noted for CMV-specific T cells (70). We recently showed that some T-cell clonotypes recognizing A2-FLW, used in this study, cross-react with a peptide homolog in varicella-zoster virus (VZV) (53). A portion of the cells studied in this investigation using A2-FLW could therefore derive from VZV infection, but we consider this unlikely, as VZV recurs rarely and VZV-monoreactive CD8 T cells are mostly below the limit for direct ex vivo tetramer detection to date (71). ChR were studied singly rather than in combination, such that informative subsets could be measured with more complex panels. The single cell studies mentioned above incorporated index sorting with a targeted panel of ChR antibodies and ChR mRNA detection to attempt to discern such complexity.
Interventions to prevent or treat HSV infections could benefit from insights into the homing of HSV-specific T cells. Preclinical models using systemic immunization and vaginal administration of CXCR3 ligands to attract HSV-specific T cells to the female genital tract have shown that these cells can become locally protective TRM cells (56). HSV reactivation severity has been modeled to be sensitive to small variations in T-cell density and timing, such that either TRM cells or rapid, efficient homing of effector cells may be beneficial (72). An understanding of the control mechanisms that program the expression of tissue homing to skin could eventually benefit vaccine design for HSV and other epithelial infections.
MATERIALS AND METHODS
Participants and specimens.
Participants were 18 years old or older, HIV-1 seronegative, and HSV-1 and/or HSV-2 seropositive (73), and they were not taking anti-HSV therapy or experiencing herpetic symptoms at the time of blood draw. HLA typing was performed at Bloodworks Northwest (Seattle, WA) (74). PBMC were separated from heparin-anticoagulated blood by centrifugation and used after cryopreservation for screening for tetramer positivity, or they were used fresh for ChR studies to preserve CD62L for memory gating (75). Protocols were approved by the UW Institutional Review Board, and all participants gave written informed consent.
HLA-peptide tetramers.
Allophycocyanin (APC)- and phycoerythrin (PE)-labeled tetramers from the Fred Hutchinson Immunology Core incorporated HLA A*0101 (A1), A*0201 (A2), or B*0702 (B7). PE or APC tetramers were used to screen PBMC for positive participants, and APC reagents were titrated and used for ChR studies. The HSV-2 type-specific tetramer was B7-RPR (HSV-2 UL49 gene product VP22 amino acids [aa] 49 to 57). HSV-1 type-specific tetramers were A1-FTD (HSV-1 UL48 product aa 479 to 488) and A1-SAL (HSV-1 UL48 product aa 90 to 99). The A2-FLW (HSV-2 UL25 product aa 372 to 380) tetramer contains a type-common epitope identical in HSV-1 and HSV-2. Controls were A2-NLV (CMV pp65 aa 595 to 603) and A2-GLC (EBV BMLF-1 aa 280 to 288) (7, 68, 76, 77).
Flow cytometry.
Screening assays used tetramers and anti-CD8α (68). For ChR studies, PBMC were plated at 3 × 106 in 96-well round-bottom plates or conical-bottom tubes. Unless otherwise indicated, all incubations were at room temperature. Following centrifugation at 2,000 rpm for 5 min and supernatant removal, 50 μl of viability dye (LIVE/DEAD fixable violet; Invitrogen, Carlsbad, CA) was added for 20 min, followed by phosphate-buffered saline (PBS) washes. Cells were incubated with FcR blocking reagent (Miltenyi Biotec, Auburn, CA) per the manufacturer in 100 μl for 10 min, followed by addition of titrated tetramer. After 30 min, 50 μl of MAb cocktail was added and cells were shifted to 4°C. MAbs were murine unless otherwise indicated. The cocktail contained anti-CD3-energy-coupled dye (clone UCHT1; Beckman Coulter, Miami, FL), anti-CD4-APC-H7 (clone RPA-T4; BD, San Jose, CA), anti-CD8α-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone SK1; BD), anti-CD45RA-Alexa Fluor 700 (clone HI100; BioLegend, San Diego, CA), anti-CD62L-PE-Cy7 (clone DREG-56; BioLegend), and rat anti-CLA-fluorescein isothiocyanate (FITC) (clone HECA-452; BD) combined with one of the following: anti-CCR4-PE (clone 205410; R&D Systems, Minneapolis, MN), anti-CCR6-PE (clone R6H1; eBioscience, San Diego, CA), rat anti-human CCR7-PE (clone 3D12; eBioscience), rat anti-human CCR8-PE (clone 191704; R&D), rat anti-human CCR10-PE (clone 314305; R&D), and anti-CXCR3-PE (clone 49801; R&D). PE-labeled, species-matched isotype control MAbs (R&D) were used at concentrations equivalent to those of the corresponding anti-ChR MAbs. FITC-labeled isotype control rat IgM (BioLegend; 400805) was used with separate specimens to rule out possible nonspecific staining with anti-CLA. Cells were stained sequentially with LIVE/DEAD, tetramer A2-FLW, and then a cocktail of anti-CD3, anti-CD8α, and either anti-CLA or isotype MAb. After 30 min, cells were washed and fixed in PBS–1% formaldehyde. Analysis used a BD LSR II and FACSDiva v6.1.1. At least 100 tetramer-positive cells were collected per specimen. Fluorescence minus one controls were run during panel development to ensure proper gating. Cutoffs for ChR antibodies were generally set at 0.5% isotype-PE-positive events in a cocktail containing the rest of the MAb panel. The CLA cutoff was set at the inflection point between the majority CLA-negative population and the shoulder of CLA-expressing cells, established for each participant. For other markers, bimodal distributions were present to establish cutoffs. Cells were defined as central memory (CD62L+ CD45RA−), effector memory (CD62L− CD45RA−), and effector (CD62L− CD45RA+) (78). For determination of CLA and ChR receptor expression by pooled memory cells, data were analyzed once per participant by gating for tetramer-negative CD8 T cells. Analysis used FlowJo v8.6.6 (Treestar, Inc., Ashland, OR). Initial gating used the lymphocyte forward/side scatter area, viability, and CD3 to define live T cells and then single positive gating for CD8α.
Biopsy microarray analysis.
Human participant and specimen information for HSV-2 lesion skin and matched normal skin biopsy samples has been provided previously (35). Briefly, 3-mm punch biopsy specimens were obtained from active symptomatic genital herpes recurrences at person-specific typical sites from persons with known genital HSV-2 infection and simultaneously from clinically normal epithelialized genital skin from the contralateral side. RNA extraction, amplification, hybridization, and analyses were performed as described previously (35). Genes from the Illumina Ref8-v2 bead arrays that were annotated to chemokine activity and chemokine receptor activity by the GoMiner program were selected, and their expression patterns were analyzed by SpotFire 9.1.2 (TIBCO Software, Inc., Palo Alto, CA).
Statistical analyses.
Linear mixed-effect models were used to compare the log-transformed percentage of CLA+ and ChR+ cells across the populations of HSV-specific, CMV-specific, EBV-specific, and bulk memory CD8 T cells. The mixed model accounts for the correlation between measurements from the same participant. The natural log-transformed percentage of T cells positive for CLA or ChR was used, replacing the log of zero with the log of half of the minimum nonzero measurement for that ChR and cell type. Calculations used SAS for Windows (version 9.3; SAS Institute Inc., Cary, NC). The natural logs of the percentages of CLA+ and CLA− cells expressing the ChR were compared using Wilcoxon matched-pairs signed-rank tests in Prism version 7 (GraphPad, La Jolla, CA).
Accession number(s).
The data sets in this report have been deposited in the NCI Gene Expression Omnibus (GEO) database under accession number GSE18527.
ACKNOWLEDGMENTS
Support by NIH grants P01AI30731 (D.M.K., A.W., and A.S.M.), R01AI094019 (D.M.K.), T32AI007509 (M.T.H.), T32AI007140 (M.T.H.), and UM1AI068618 (S.C.D.R.) is acknowledged. The study was funded in part by the University of Washington Center for AIDS Research (CFAR), an NIH program under award AI027757.
We acknowledge the staff of the Virology Research Clinic, the volunteers, and technical assistance from Tiana Chong and Christopher L. McClurkan.
REFERENCES
- 1.Iwasaki A. 2016. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol 34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 2.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest 101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher EC, Picker LJ. 1996. Lymphocyte homing and homeostasis. Science 272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 4.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 5.Schulz O, Hammerschmidt SI, Moschovakis GL, Forster R. 2016. Chemokines and chemokine receptors in lymphoid tissue dynamics. Annu Rev Immunol 34:203–242. doi: 10.1146/annurev-immunol-041015-055649. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, Jin L, Diem K, Koelle DM, Wald A, Robins H, Corey L. 2013. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature 497:494–497. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. 2002. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J Clin Invest 110:537–548. doi: 10.1172/JCI0215537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. 2003. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A 100:12899–12904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelle DM, Gonzalez JC, Johnson AS. 2005. Homing in on the cellular immune response to HSV-2 in humans. Am J Reprod Immunol 53:172–181. doi: 10.1111/j.1600-0897.2005.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barcy S, Huang ML, Corey L, Koelle DM. 2005. Longitudinal analysis of herpes simplex virus-specific CD4+ cell clonotypes in infected tissues and blood. J Infect Dis 191:2012–2021. doi: 10.1086/430389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posavad CM, Barcy S, Huang ML, Koelle DM, Polyak SJ, Corey L. 2000. Long-term persistence of herpes simplex virus-specific CD8+ CTL clones derived from genital lesions. J Immunol 165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 12.Posavad CM, Zhao L, Dong L, Jin L, Stevens CE, Magaret AS, Johnston C, Wald A, Zhu J, Corey L, Koelle DM. Enrichment of herpes simplex virus type 2 reactive mucosal T cells in the female genital tract. Mucosal Immunol, in press. doi: 10.1038/mi.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Velzen M, Jing L, Osterhaus AD, Sette A, Koelle DM, Verjans GM. 2013. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog 9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaerli P, Moser B. 2005. Chemokines: control of primary and memory T-cell traffic. Immunol Res 31:57–74. doi: 10.1385/IR:31:1:57. [DOI] [PubMed] [Google Scholar]
- 15.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, Dieu-Nosjean M-C, Orozco R, Ruzicka T, Lehmann P, Oldham E, Zlotnick A. 2000. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol 164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 16.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto H, Yen D, Marxhausen H, To W, Sedgwick T, Lehmann P, Zlotnick A. 2002. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med 8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. 1999. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 18.Colantonio L, Iellem A, Sinigaglia F, D'Ambrosio D. 2002. Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1/I-309. Eur J Immunol 32:3506–3514. [DOI] [PubMed] [Google Scholar]
- 19.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. 2006. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol 177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 20.Sebastiani S, Albanesi C, Nasorri F, Girolomoni G, Cavani A. 2002. Nickel-specific CD4(+) and CD8(+) T cells display distinct migratory responses to chemokines produced during allergic contact dermatitis. J Invest Dermatol 118:1052–1058. doi: 10.1046/j.1523-1747.2002.01771.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishida T, Utsunomiya A, Iida S, Inagaki H, Takatsuka Y, Kusumoto S, Takeuchi G, Shimizu S, Ito M, Komatsu H, Wakita A, Eimoto T, Matsushima K, Ueda R. 2003. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 9:3625–3634. [PubMed] [Google Scholar]
- 22.Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, Abuzahra F, Bowman E, Roszkiewicz J, Bieber T, Tuting T. 2005. Circulating clonal CLA(+) and CD4(+) T cells in Sezary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol 152:258–264. doi: 10.1111/j.1365-2133.2004.06325.x. [DOI] [PubMed] [Google Scholar]
- 23.Moed H, Boorsma DM, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ, Gibbs S, Rustemeyer T. 2004. Nickel-responding T cells are CD4+ CLA+ CD45RO+ and express chemokine receptors CXCR3, CCR4 and CCR10. Br J Dermatol 151:32–41. doi: 10.1111/j.1365-2133.2004.05975.x. [DOI] [PubMed] [Google Scholar]
- 24.Kakinuma T, Saeki H, Tsunemi Y, Fujita H, Asano N, Mitsui H, Tada Y, Wakugawa M, Watanabe T, Torii H, Komine M, Asahina A, Nakamura K, Tamaki K. 2003. Increased serum cutaneous T cell-attracting chemokine (CCL27) levels in patients with atopic dermatitis and psoriasis vulgaris. J Allergy Clin Immunol 111:592–597. doi: 10.1067/mai.2003.114. [DOI] [PubMed] [Google Scholar]
- 25.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. 2006. The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Olshansky M, Carbone FR, Ma JZ. 2016. Transcriptional analysis of T cells resident in human skin. PLoS One 11:e0148351. doi: 10.1371/journal.pone.0148351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. 2005. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med 202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch M, Moser B, Mackay CR. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molesworth-Kenyon SJ, Oakes JE, Lausch RN. 2005. A novel role for neutrophils as a source of T cell-recruiting chemokines IP-10 and Mig during the DTH response to HSV-1 antigen. J Leukoc Biol 77:552–559. doi: 10.1189/jlb.0904485. [DOI] [PubMed] [Google Scholar]
- 30.Araki-Sasaki K, Tanaka T, Ebisuno Y, Kanda H, Umemoto E, Hayashi K, Miyasaka M. 2006. Dynamic expression of chemokines and the infiltration of inflammatory cells in the HSV-infected cornea and its associated tissues. Ocul Immunol Inflamm 14:257–266. doi: 10.1080/09273940600943581. [DOI] [PubMed] [Google Scholar]
- 31.Ariotti S, Beltman JB, Borsje R, Hoekstra ME, Halford WP, Haanen JB, de Boer RJ, Schumacher TN. 2015. Subtle CXCR3-dependent chemotaxis of CTLs within infected tissue allows efficient target localization. J Immunol 195:5285–5295. doi: 10.4049/jimmunol.1500853. [DOI] [PubMed] [Google Scholar]
- 32.Thapa M, Carr DJ. 2009. CXCR3 deficiency increases susceptibility to genital herpes simplex virus type 2 infection: uncoupling of CD8+ T-cell effector function but not migration. J Virol 83:9486–9501. doi: 10.1128/JVI.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikloska Z, Danis VA, Adams S, Lloyd AR, Adrian DL, Cunningham AL. 1998. In vivo production of cytokines and beta (C-C) chemokines in human recurrent herpes simplex lesions—do herpes simplex virus-infected keratinocytes contribute to their production? J Infect Dis 177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng T, Zhu J, Klock A, Phasouk K, Huang ML, Koelle DM, Wald A, Corey L. 2009. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J Virol 83:12559–12568. doi: 10.1128/JVI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller SN, Gebhardt T, Carbone FR, Heath WR. 2013. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol 31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 37.Tomazin R, van Schoot NE, Goldsmith K, Jugovic P, Sempe P, Fruh K, Johnson DC. 1998. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J Virol 72:2560–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cunningham AL, Turner RR, Miller AC, Para MF, Merigan TC. 1985. Evolution of recurrent herpes simplex lesions: an immunohistologic study. J Clin Invest 75:226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LWMM. 1993. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tisue-selective homing receptor for skin-homing T cells. J Immunol 150:1122–1136. [PubMed] [Google Scholar]
- 40.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizukawa Y, Shitara K, Yamazaki Y, Teraki Y, Takahashi R, Narimatsu H, Shiohara T. 2001. Development and characterization of a monoclonal antibody specific for fucosyltransferase VII (fuc-TVII): discordant expression of CLA and Fuc-TVII in peripheral CD4+ and CD8+ T cells. J Invest Dermatol 117:743–747. doi: 10.1046/j.1523-1747.2001.t01-1-01447.x. [DOI] [PubMed] [Google Scholar]
- 42.Pink M, Ratsch BA, Mardahl M, Durek P, Polansky JK, Karl M, Baumgrass R, Wallner S, Cadenas C, Gianmoena K, Floess S, Chen W, Nordstroem K, Tierling S, Olek S, Walter J, Hamann A, Syrbe U. 2016. Imprinting of skin/inflammation homing in CD4+ T cells is controlled by DNA methylation within the fucosyltransferase 7 gene. J Immunol 197:3406–3414. doi: 10.4049/jimmunol.1502434. [DOI] [PubMed] [Google Scholar]
- 43.Gunby S, Jing L, Ott M, Wald A, Koelle D, Johnston C. 2017. T cell response to herpes simplex virus type 1 primary genital infection. 2017 Western Medical Research Conference, Carmel, CA. [Google Scholar]
- 44.Srivastava R, Khan AA, Garg S, Syed SA, Furness JN, Vahed H, Pham T, Yu HT, Nesburn AB, BenMohamed L. 9 November 2016. Human asymptomatic epitopes identified from the herpes simplex virus tegument protein VP13/14 (UL47) preferentially recall polyfunctional effector memory CD44highCD62LlowCD8+ TEM cells and protect “humanized” HLA-A*02:01 transgenic mice against ocular herpes. J Virol doi: 10.1128/JVI.01793-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. 2013. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 191:5124–5138. doi: 10.4049/jimmunol.1301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava R, Khan AA, Spencer D, Vahed H, Lopes PP, Thai NT, Wang C, Pham TT, Huang J, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J Immunol 194:2232–2248. doi: 10.4049/jimmunol.1402606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan AA, Srivastava R, Spencer D, Garg S, Fremgen D, Vahed H, Lopes PP, Pham TT, Hewett C, Kuang J, Ong N, Huang L, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes. J Virol 89:3776–3792. doi: 10.1128/JVI.03419-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. 2011. A human memory T cell subset with stem cell-like properties. Nat Med 17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donaghy H, Bosnjak L, Harman AN, Marsden V, Tyring SK, Meng TC, Cunningham AL. 2009. Role for plasmacytoid dendritic cells in the immune control of recurrent human herpes simplex virus infection. J Virol 83:1952–1961. doi: 10.1128/JVI.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sallusto F. 2016. Heterogeneity of human CD4(+) T cells against microbes. Annu Rev Immunol 34:317–334. doi: 10.1146/annurev-immunol-032414-112056. [DOI] [PubMed] [Google Scholar]
- 51.Wambre E, James EA, Kwok WW. 2012. Characterization of CD4+ T cell subsets in allergy. Curr Opin Immunol 24:700–706. doi: 10.1016/j.coi.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moss NJ, Magaret A, Laing KJ, Shaulov Kask A, Wang M, Mark KE, Schiffer JT, Wald A, Koelle DM. 3 July 2012. Peripheral blood CD4 T-cell and pDC reactivity to HSV-2 and pDC number do not correlate with the clinical or virologic severity of recurrent genital herpes. J Virol doi: 10.1128/JVI.00829-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jing L, Laing KJ, Dong L, Russell RM, Barlow RS, Haas JG, Ramchandani MS, Johnston C, Buus S, Redwood AJ, White KD, Mallal SA, Phillips EJ, Posavad CM, Wald A, Koelle DM. 2016. Extensive CD4 and CD8 T cell cross-reactivity between alphaherpesviruses. J Immunol 196:2205–2218. doi: 10.4049/jimmunol.1502366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. 2011. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 55.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. 2013. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 56.Shin H, Iwasaki A. 2012. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergsbaken T, Bevan MJ. 2015. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat Immunol 16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, Jellison ER, McCullough LD. 2016. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J Immunol 196:3318–3330. doi: 10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, Braun A, Wynne-Jones E, Behr FM, Stark R, Pellicci DG, Godfrey DI, Belz GT, Pellegrini M, Gebhardt T, Busslinger M, Shi W, Carbone FR, van Lier RA, Kallies A, van Gisbergen KP. 2016. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 60.Nandakumar S, Woolard SN, Yuan D, Rouse BT, Kumaraguru U. 2008. Natural killer cells as novel helpers in anti-herpes simplex virus immune response. J Virol 82:10820–10831. doi: 10.1128/JVI.00365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, Sengupta M, Herold BC, Jacobs WR. 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 4:e06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaney JE, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu TM, Kawoaka Y, Tevethia SS. 1998. Immunization with a single major histocompatibility class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol 72:9567–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. 2009. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 65.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. 2007. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J Immunol 179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. 2003. Herpes simplex virus-specific memory CD8(+) T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mott KR, Gate D, Matundan HH, Ghiasi YN, Town T, Ghiasi H. 2016. CD8+ T cells play a bystander role in mice latently infected with herpes simplex virus 1. J Virol 90:5059–5067. doi: 10.1128/JVI.00255-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. 2001. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J Immunol 166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 69.Jing L, Haas J, Chong TM, Bruckner JJ, Dann GC, Dong L, Marshak JO, McClurkan CL, Yamamoto TN, Bailer SM, Laing KJ, Wald A, Verjans GMGM, Koelle DM. 2012. Herpes simplex virus type 1 T-cells antigens in humans revealed by cross-presentation and genome-wide screening. J Clin Invest 122:654–673. doi: 10.1172/JCI60556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mekker A, Tchang VS, Haeberli L, Oxenius A, Trkola A, Karrer U. 2012. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog 8:e1002850. doi: 10.1371/journal.ppat.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sei JJ, Cox KS, Dubey SA, Antonello JM, Krah DL, Casimiro DR, Vora KA. 2015. Effector and central memory poly-functional CD4(+) and CD8(+) T cells are boosted upon ZOSTAVAX((R)) vaccination. Front Immunol 6:553. doi: 10.3389/fimmu.2015.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Koelle DM, Wald A, Corey L. 2010. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A 107:18973–18978. doi: 10.1073/pnas.1006614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. 1988. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 26:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koelle DM, Chen HB, McClurkan CM, Petersdorf EW. 2002. Herpes simplex virus type 2-specific CD8 cytotoxic T lymphocyte cross-reactivity against prevalent HLA class I alleles. Blood 99:3844–3847. doi: 10.1182/blood.V99.10.3844. [DOI] [PubMed] [Google Scholar]
- 75.Reimann KA, Chernoff M, Wilkening CL, Nickerson CE, Landay AL, The ACTG Immunology Advanced Technology Laboratories. 2000. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter trials. Clin Diagn Lab Immunol 7:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong L, Li P, Oenema T, McClurkan CL, Koelle DM. 2010. Public TCR use by herpes simplex virus-2-specific human CD8 CTLs. J Immunol 184:3063–3071. doi: 10.4049/jimmunol.0903622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laing KJ, Magaret AS, Mueller DE, Zhao L, Johnston C, De Rosa SC, Koelle DM, Wald A, Corey L. 2010. Diversity in CD8(+) T cell function and epitope breadth among persons with genital herpes. J Clin Immunol 30:703–722. doi: 10.1007/s10875-010-9441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]