ABSTRACT
Several lines of evidence indicate that cutaneous human papillomavirus (HPV) types belonging to the beta genus of the HPV phylogenetic tree synergize with UV radiation in the development of skin cancer. Accordingly, the E6 and E7 oncoproteins from some beta HPV types are able to deregulate pathways related to immune response and cellular transformation. Toll-like receptor 9 (TLR9), in addition to playing a role in innate immunity, has been shown to be involved in the cellular stress response. Using primary human keratinocytes as experimental models, we have shown that UV irradiation (and other cellular stresses) activates TLR9 expression. This event is closely linked to p53 activation. Silencing the expression of p53 or deleting its encoding gene affected the activation of TLR9 expression after UV irradiation. Using various strategies, we have also shown that the transcription factors p53 and c-Jun are recruited onto a specific region of the TLR9 promoter after UV irradiation. Importantly, the E6 and E7 oncoproteins from beta HPV38, by inducing the accumulation of the p53 antagonist ΔNp73α, prevent the UV-mediated recruitment of these transcription factors onto the TLR9 promoter, with subsequent impairment of TLR9 gene expression. This study provides new insight into the mechanism that mediates TLR9 upregulation in response to cellular stresses. In addition, we show that HPV38 E6 and E7 are able to interfere with this mechanism, providing another explanation for the possible cooperation of beta HPV types with UV radiation in skin carcinogenesis.
IMPORTANCE Beta HPV types have been suggested to act as cofactors in UV-induced skin carcinogenesis by altering several cellular mechanisms activated by UV radiation. We show that the expression of TLR9, a sensor of damage-associated molecular patterns produced during cellular stress, is activated by UV radiation in primary human keratinocytes (PHKs). Two transcription factors known to be activated by UV radiation, p53 and c-Jun, play key roles in UV-activated TLR9 expression. The E6 and E7 oncoproteins from beta HPV38 strongly inhibit UV-activated TLR9 expression by preventing the recruitment of p53 and c-Jun to the TLR9 promoter. Our findings provide additional support for the role that beta HPV types play in skin carcinogenesis by preventing activation of specific pathways upon exposure of PHKs to UV radiation.
KEYWORDS: UV radiation, Toll-like receptor 9, HPV38, primary keratinocytes, p53
INTRODUCTION
Toll-like receptor 9 (TLR9) is a transmembrane receptor that plays a fundamental role in innate immunity. It is expressed in endolysosomes by various human immune cells, including dendritic cells and B cells, and on nonimmune cells such as epithelial cells. TLR9 senses highly conserved unmethylated CpG motifs on viral and bacterial DNA, and its activation results in the production of proinflammatory cytokines and IFN-1.
Previous studies have shown that the basal levels of TLR9 expression are downregulated by various viruses, such as the alpha human papillomavirus type 16 (HPV16), the beta HPV38, Epstein-Barr virus, and Merkel cell polyomavirus (1–5). The blocking of TLR9 function by several oncogenic viruses is a crucial event related to host immune evasion and guarantees the persistence of the infection. However, the downregulation of TLR9 expression requires viral gene expression and occurs after virus entry and activation of the innate immune response. Therefore, it is plausible that inhibition of TLR9 signaling may also be necessary to deregulate additional events and to generate the ideal conditions for completion of the viral life cycle. In a previous study, we showed that overexpression of TLR9 in HPV38-immortalized keratinocytes results in accumulation of the cell cycle inhibitor p21WAF1/Cip1 and in decreased cellular proliferation (3). The inhibitory effect of TLR9 on cell cycle progression has also been observed in head and neck cancer cell lines, in which TLR9 expression resulted in a reduction of cellular proliferation and in inhibition of the cells' ability to grow in an anchorage-independent manner (6). It has also been shown that TLR9 signaling can be activated by the damage-associated molecular pattern molecules released by cells after exposure to several types of stresses (7). Because the DNA replication of HPV and other oncogenic viruses is entirely dependent on the proliferative status of the infected cells, inhibition of TLR9 expression may be needed to counteract the receptor's inhibitory effect on cellular proliferation in order to enable efficient viral replication. Beta HPV types are suspected, together with UV radiation, to promote the development of nonmelanoma skin cancer (8). Interestingly, it has been shown that the exposure of various cancer-derived cells to genotoxic stresses, including UV irradiation, results in an upregulation of TLR9 expression that is partially mediated by activation of the transcription factor p53 (9). However, the possible interplay between beta HPVs, UV radiation, and TLR9 signaling in human keratinocytes has not yet been elucidated. Here, we show that UV radiation induces activation of TLR9 expression in primary human keratinocytes (PHKs), the natural host cells of HPV. This event is mediated by the recruitment of p53 and c-Jun to the TLR9 promoter. Importantly, the beta HPV38 E6 and E7 oncoproteins prevent UV-mediated activation of TLR9.
RESULTS
TLR9 transcription is induced by several cellular stresses in primary human keratinocytes.
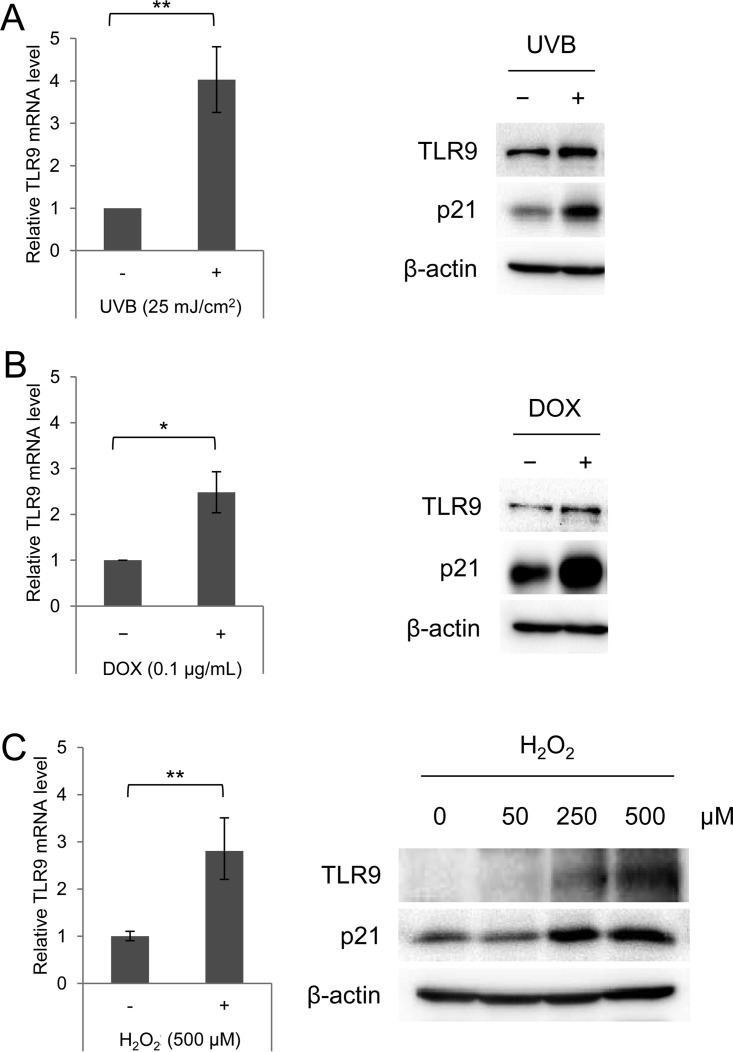
We previously showed that beta HPV38 E6 and E7 repress the basal TLR9 transcriptional levels in PHKs, which are naturally infected by the virus (3). Because it has also been shown that UV irradiation and other stresses activate TLR9 expression in several cell lines (8), we were interested in determining whether the viral proteins could also interfere with this UV-mediated transcriptional activation. As a first step, we evaluated the effect of UV radiation on TLR9 expression in PHKs. Cells were UVB irradiated at a dose of 25 mJ/cm2, cultured for 8 h, and then harvested and processed for extraction of total RNA and total proteins. Quantitative reverse transcription-PCR (RT-qPCR) showed that UV irradiation significantly increased the expression of TLR9 (Fig. 1A, left panel). Concordantly, TLR9 protein levels were increased in the UV-irradiated cells compared to in mock-irradiated cells (Fig. 1A, right panel). As expected, p21WAF1, a marker for activation of the cellular response to stress, was accumulated after UV irradiation (Fig. 1A, right panel).
FIG 1.
TLR9 transcription is induced by several cellular stresses in PHKs. (A) Total RNA and total proteins were extracted from UVB-irradiated (+) and mock-irradiated (−) PHKs; TLR9 expression levels were measured by RT-qPCR and normalized to GAPDH levels (left panel), and protein extracts were analyzed by immunoblotting for the indicated antibodies (right panel). (B and C) Total RNA and total proteins were extracted from PHKs treated (+) and mock treated (−) with 0.1 μg/ml doxorubicin (DOX) for 8 h (B) or with H2O2 (at the indicated concentrations) for 1 h (C). TLR9 expression levels were measured by RT-qPCR (left panels), and protein extracts were analyzed by immunoblotting for the indicated antibodies (right panels). The RT-qPCR data shown are the means from three independent experiments. The immunoblots shown are from one representative experiment of the three performed. *, P < 0.05; **, P < 0.01.
We also assessed whether other cellular stresses led to TLR9 transcriptional activation in PHKs. Similarly to UV radiation, doxorubicin (0.1 μg/ml) and H2O2 (500 μM) also induced an increase in TLR9 mRNA and protein levels (Fig. 1B and C).
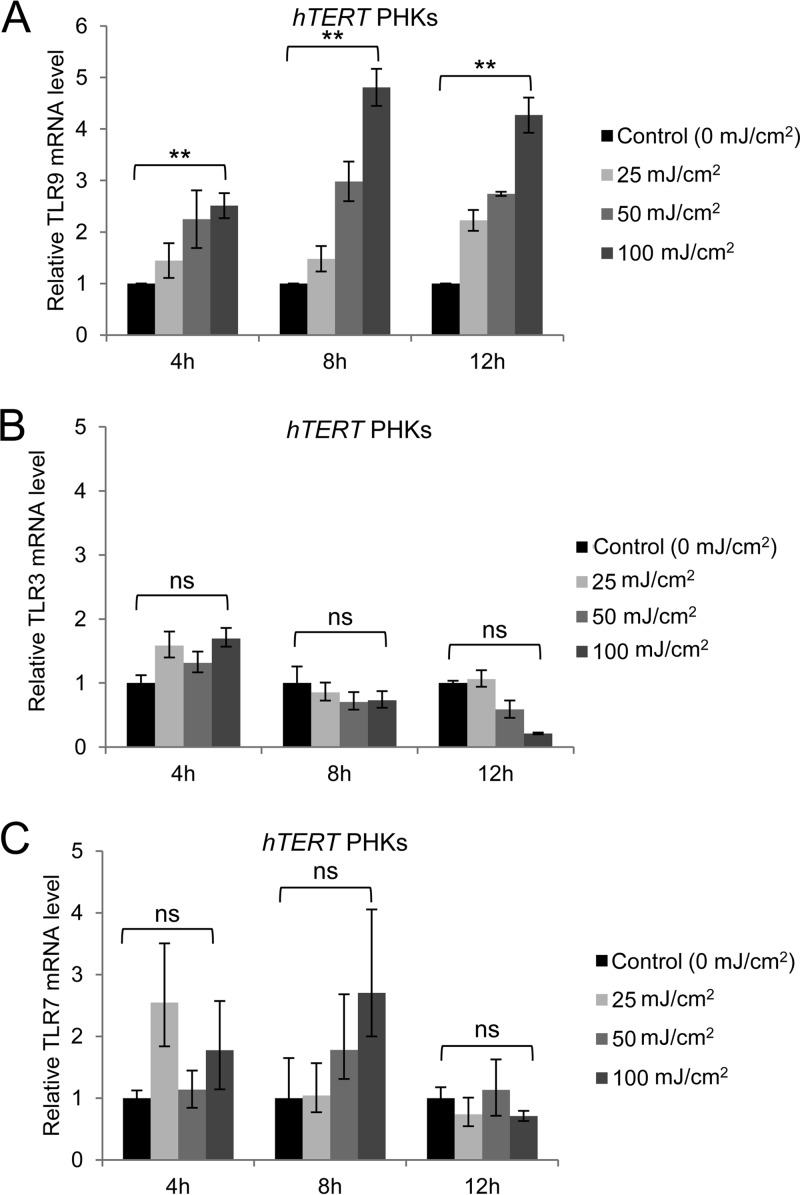
Activation of TLR9 transcription was observed following various doses of UV radiation and at various time points after irradiation in PHKs expressing the human telomerase reverse transcriptase gene (hTERT), which extends the life span of primary cells (Fig. 2A). To determine whether UV irradiation of hTERT PHKs could also lead to transcriptional activation of other TLRs, we evaluated the expression of two additional endosomal TLRs, TLR3 and TLR7, before and after UV irradiation. TLR3 mRNA was not increased by any dose of UV radiation (Fig. 2B). A small (not statistically significant) increase of expression was seen for TLR7, but only at 8 h postirradiation.
FIG 2.
Expression of TLR9, but not of TLR3 or TLR7, is significantly increased in hTERT PHKs exposed to various doses of UV radiation. hTERT PHKs were exposed to the doses of UV radiation indicated in the figure. After 4, 8, and 12 h, total RNA was propagated, and the expression levels of TLR9 (A), TLR3 (B), and TLR7 (C) were determined by RT-qPCR and normalized to GAPDH levels. **, P < 0.01; ns, not significant.
p53 plays a key role in UV-mediated TLR9 transcriptional activation.
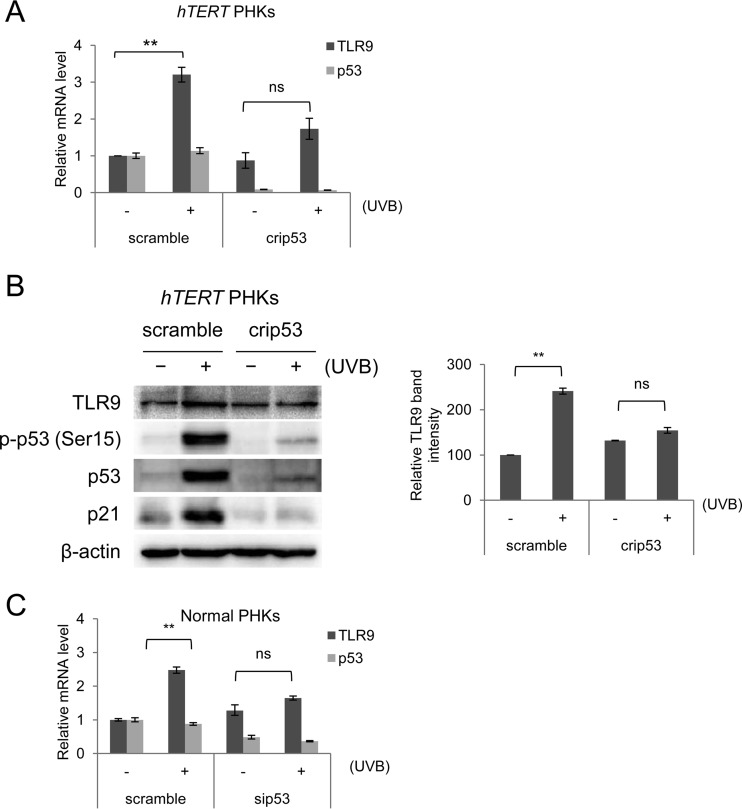
We next determined whether the activation of TLR9 by UV irradiation and other cellular stresses is mediated by p53 in PHKs as it has been observed to be in transformed human cancer cells (8). As an initial strategy, we generated p53 knockout PHKs using CRISPR/Cas9 technology. To increase the yield of p53 knockout cells, we also used hTERT PHKs. In the knockout cells, the expression of p53 was silenced by the deletion of its encoding gene, unlike in the mock-transfected cells, which were transfected with a CRISPR/Cas9 vector containing a scrambled sequence and retained p53 expression (Fig. 3A). In three independent experiments, the p53−/− PHKs showed no significant activation of TLR9 expression after UV irradiation versus nonirradiated cells, in contrast to the mock-transfected cells (Fig. 3A). Consistent with lower RNA expression levels, TLR9 protein was not accumulated in the UV-irradiated p53−/− PHKs (Fig. 3B). To eliminate the possibility that hTERT could interfere with TLR9 expression under any of the various conditions, we performed additional experiments with PHKs in which p53 expression was silenced by small interfering RNA (siRNA) rather than by CRISPR/Cas9-mediated p53 deletion. The key role of p53 in UV-mediated TLR9 transcriptional activation was apparent in those experiments as well (Fig. 3C).
FIG 3.
p53 plays a key role in UV-mediated TLR9 transcriptional activation. (A and B) hTERT PHKs either expressing wild-type p53 (scramble) or with CRISPR/Cas9-mediated p53 deletion (crip53) were UVB irradiated (+) or mock irradiated (−). (A) Total RNA was extracted, and TLR9 and p53 mRNA levels were measured by RT-qPCR. (B) Total protein extracts were analyzed by immunoblotting for the indicated antibodies (left panel). TLR9 band intensities were quantified and normalized to β-actin levels (right panel). (C) Normal PHKs were transfected with either a control siRNA (scramble) or an siRNA specific for p53 (sip53). After 40 h, the cells were UVB irradiated (+) or mock irradiated (−) as described in Materials and Methods. Total RNA was then extracted, and p53 and TLR9 mRNA levels were measured by RT-qPCR. The RT-qPCR data shown are the means from three independent experiments. The error bars represent the standard deviations of the three biological replicates. The immunoblots shown are from one representative experiment of the three performed. **, P < 0.01; ns, not significant.
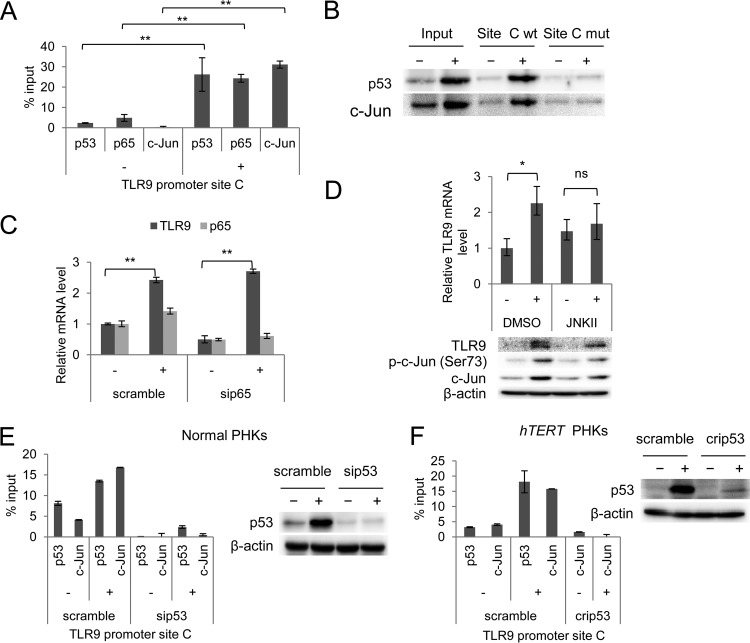
UV-induced TLR9 transcriptional activation is mediated by p53 and c-Jun recruitment at a specific region of the TLR9 promoter.
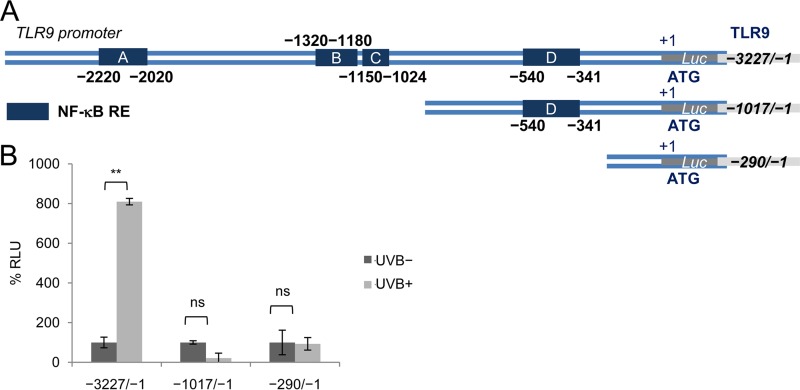
Subsequently, we performed transient-transfection experiments in a spontaneously immortalized human keratinocyte cell line (NIKS) using constructs containing various regions of the TLR9 promoter cloned in front of a luciferase reporter gene (Fig. 4A). Consistent with our observations in PHKs, UV radiation activated the TLR9 promoter (Fig. 4B). The UV-mediated activation is dependent on a promoter region of approximately 2,200 nucleotides; the deletion of this region prevented the increase of luciferase activity upon UV irradiation (Fig. 4B). The region contains three NF-κB-responsive elements (REs): A, B, and C (2, 4). We previously determined that RE C is involved in the basal transcriptional repression mediated by beta HPV38 E6 and E7 via the accumulation of ΔNp73α (3). Chromatin immunoprecipitation (ChIP) experiments showed that, upon UV irradiation, p53 is mainly recruited near NF-κB RE C (Fig. 5A). Interestingly, we observed that two additional transcription factors, p65 and c-Jun, were recruited in the same region of the TLR9 promoter after UV irradiation (Fig. 5A). We targeted these transcription factors because c-Jun is well known to be activated by UV radiation (10), and p65 has been shown to play both positive and negative regulatory roles in TLR9 expression (1, 11, 12). The ChIP results were confirmed by oligonucleotide pulldown assay using biotinylated DNA fragments of the TLR9 promoter (−1150/−1024) that encompassed the wild-type or mutated NF-κB RE C (Fig. 5B). Both the wild-type and the mutated biotinylated DNA probes were incubated with cellular extracts of UV-irradiated and nonirradiated hTERT PHKs. Only the wild-type DNA probe coprecipitated p53 and c-Jun (Fig. 5B). In the same assay, we also observed that p53 and c-Jun binding to the TLR9 promoter region increased upon UV irradiation.
FIG 4.
A TLR9 promoter region containing the three NF-B-responsive elements A, B, and C is required for UV-induced TLR9 transcriptional activation. (A) Schematic diagram of the three TLR9 promoter luciferase (Luc) constructs: the full-length (−3227/−1) construct containing four NF-κB REs (A, B, C, and D) and the two deleted (−1017/−1 and −290/-1) constructs. (B) NIKS cells were transfected with these TLR9 promoter constructs cloned in front of a luciferase reporter gene. After 40 h, the cells were either UVB irradiated at a dose of 25 mJ/cm2 (UVB+) or mock irradiated (UVB−), and then cultured in a humidified chamber for 8 h at 37°C. The cells were then harvested; their luciferase activities were measured and are expressed as percent relative luminescence units (% RLU). The data shown are the means from three independent experiments performed in triplicate. **, P < 0.01; ns, not significant.
FIG 5.
UV-induced TLR9 transcriptional activation is mediated by p53 and c-Jun recruitment at a specific region of the TLR9 promoter. (A) ChIP was performed in nonirradiated (−) and UVB-irradiated (+) normal PHKs using p53, p65, and c-Jun antibodies. Simultaneously, part of the total chromatin fraction (1/10) was used as the input. qPCR was performed using specific primers flanking NF-κB RE C within the TLR9 promoter. The histogram shows the relative amount of the promoter bound by the antibodies after subtracting the background of nonspecific IgG control, expressed as a percentage of the input. The data shown are representative of three independent experiments. (B) The UVB-irradiated (+) and nonirradiated (−) hTERT PHKs were processed for the oligonucleotide pulldown assay. Cell lysate was incubated with biotinylated probes containing NF-κB RE C of the TLR9 promoter, either wild-type (site C wt) or mutated (site C mut). DNA-associated proteins were recovered by precipitation with streptavidin beads and analyzed by immunoblotting. (C) Normal PHKs were transfected with a control siRNA (scramble) or an siRNA specific for p65 (sip65). After 40 h, the cells were UVB irradiated (+) or mock irradiated (−) as described in Materials and Methods. The total RNA was then extracted, and p65 and TLR9 mRNA levels were measured by RT-qPCR. The error bars represent the standard deviations of two biological replicates. (D) Normal PHKs were treated with either JNK inhibitor II (JNKII) or dimethyl sulfoxide (DMSO) and then UVB irradiated (+) or mock irradiated (−) as described in Materials and Methods. After 8 h, the total RNA was extracted, and the TLR9 mRNA levels were measured by RT-qPCR (upper panel). Simultaneously, total proteins were extracted and analyzed by immunoblotting for the indicated antibodies (lower panel). The error bars represent the standard deviations of two biological replicates. (E) Normal PHKs were transfected with a control siRNA (scramble) or an siRNA specific for p53 (sip53). After 40 h, the cells were UVB irradiated (+) or mock irradiated (−) as described in Materials and Methods; total cellular extracts were processed for ChIP with the indicated antibodies. (F) hTERT PHKs either expressing wild-type p53 (scramble) or with CRISPR/Cas9-mediated p53 deletion (crip53) were UVB irradiated (+) or mock irradiated (−) and then processed for ChIP with the indicated antibodies. *, P < 0.05; **, P < 0.01; ns, not significant.
Silencing the expression of p65 with a specific siRNA revealed that p65 was not essential for UV-induced transcriptional activation (Fig. 5C). In contrast, inhibition of c-Jun activation by a chemical inhibitor eliminated UV-induced TLR9 expression at both the mRNA and the protein levels (Fig. 5D).
Downregulation of p53 by a specific siRNA resulted in a significant decrease in the amount of c-Jun recruited to the TLR9 promoter in UV-irradiated normal PHKs (Fig. 5E), indicating that c-Jun and p53 may directly interact at the TLR9 promoter. The same result was observed in hTERT p53−/− PHKs (Fig. 5F).
HPV38 E6 and E7 prevent the UV-mediated activation of TLR9 expression.
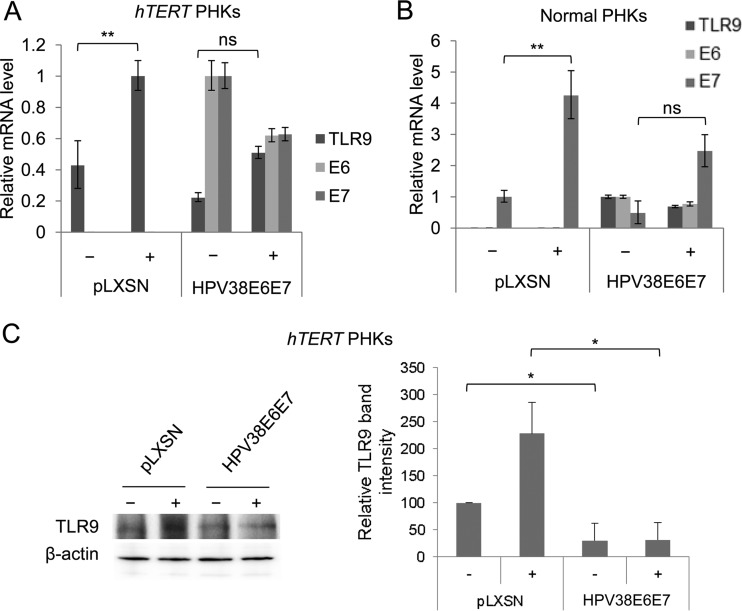
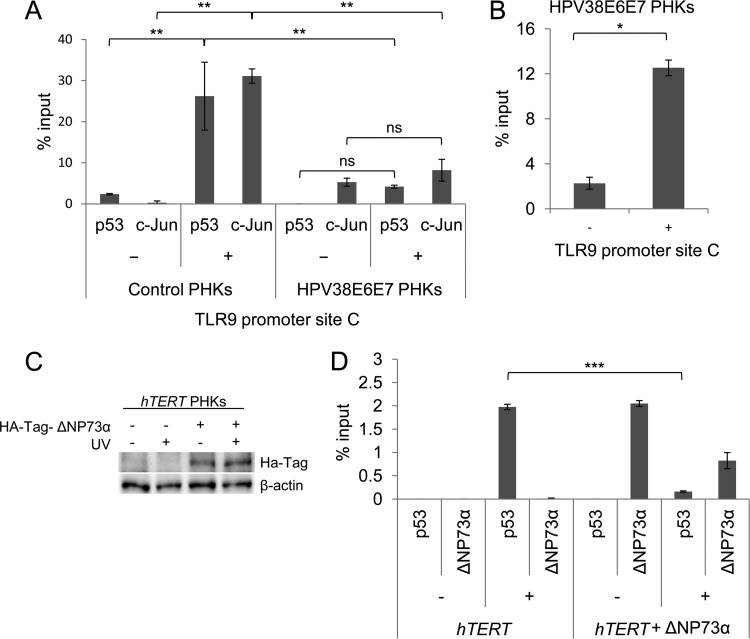
Our previous data showed that the beta HPV38 E6 and E7 oncoproteins inhibit the basal expression of TLR9 mRNA. Therefore, we evaluated whether the viral proteins could also interfere with UV-induced activation of TLR9 transcription. hTERT PHKs were retrotransduced either with a recombinant pLXSN retroviral vector encoding the HPV38 E6 and E7 oncoproteins or with an empty control vector. After total RNA preparation, TLR9 mRNA levels were measured by RT-qPCR. After UV irradiation, TLR9 expression levels were lower in the HPV38 E6 and E7 hTERT PHKs than in the control cells (Fig. 6A). Similar results were observed in normal PHKs (Fig. 6B), indicating that the results are independent of hTERT expression. Similarly, following UV irradiation, TLR9 protein levels were also lower in the hTERT PHKs expressing the two viral oncoproteins than in the mock-transduced cells (Fig. 6C). ChIP experiments revealed that p53 and c-Jun recruitment induced by UV radiation was severely affected by the viral oncoproteins (Fig. 7A). Moreover, recruitment of ΔNp73α on the same RE of the TLR9 promoter was significantly increased in UV-irradiated HPV38 E6 and E7 PHKs compared to nonirradiated cells (Fig. 7B), suggesting that p53 and ΔNp73α compete for binding to the TLR9 promoter, leading to an inverse regulation of its activity.
FIG 6.
HPV38 E6 and E7 prevent the UV-mediated activation of TLR9 expression. (A) hTERT PHKs were transduced with an empty control vector (pLXSN) or with pLXSN encoding HPV38 E6 and E7 (HPV38E6E7) and then UVB irradiated (+) or mock irradiated (−) as described in Materials and Methods. The efficiency of transduction was verified and TLR9 mRNA levels, as well as E6 and E7 mRNA levels, were measured by RT-qPCR. (B) Normal PHKs were transduced with the empty pLXSN vector (pLXSN) or with pLXSN encoding HPV38 E6 and E7 (HPV38E6E7) and then UVB irradiated (+) or mock irradiated (−) as described in Materials and Methods. After 8 h, total RNA was extracted, and the TLR9 and viral mRNA levels were analyzed by RT-qPCR. (C) Total protein extracts from hTERT PHKs that were transduced with the empty pLXSN vector (pLXSN) or with pLXSN encoding HPV38 E6 and E7 (HPV38E6E7) and then UVB irradiated (+) or mock irradiated (−) were analyzed by immunoblotting (left panel). The quantification of protein bands in three independent experiments is shown (right panel). The error bars represent the standard deviation of three biological replicates. *, P < 0.05; **, P < 0.01; ns, not significant.
FIG 7.
ΔNp73α prevents recruitment of p53 to the TLR9 promoter. (A) Normal PHKs (control PHKs) and PHKs expressing HPV38 E6 and E7 (HPV38E6E7 PHKs) were UVB irradiated (+) or not irradiated (−). Total cellular extracts were processed for ChIP with the indicated antibodies. The error bars represent the standard deviations of three biological replicates. (B) PHKs expressing HPV38 E6 and E7 (HPV38E6E7 PHKs) were UVB irradiated (+) or not irradiated (−). Total cellular extracts were processed for ChIP with anti-p73 antibody (OP108; Calbiochem). The error bars represent the standard deviations of two biological replicates. (C) hTERT PHKs were transiently transfected with pCDNA HA-ΔNp73α and UV irradiated (+) or mock irradiated (−). After 8 h, total proteins were extracted, and ΔNp73α protein levels were determined by immunoblotting using HA-Tag antibody. (D) Cells treated as described in panel C were processed for ChIP experiments using p53 and HA-Tag antibodies. The error bars represent the standard deviations of three biological replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.0001; ns, not significant.
To further corroborate this hypothesis about the competition between p53 and ΔNp73α at binding site C of the TLR9 promoter, we overexpressed ΔNp73α fused at the N terminus with the human influenza hemagglutinin tag (HA-Tag) in hTERT PHKs. Immunoblotting revealed that HA-Tag–ΔNp73α was synthesized in hTERT PHKs (Fig. 7C). ChIP experiments showed that HA-Tag–ΔNp73α was efficiently recruited at TLR9 promoter site C, which in turn decreased the binding of p53 induced by UV irradiation (Fig. 7D).
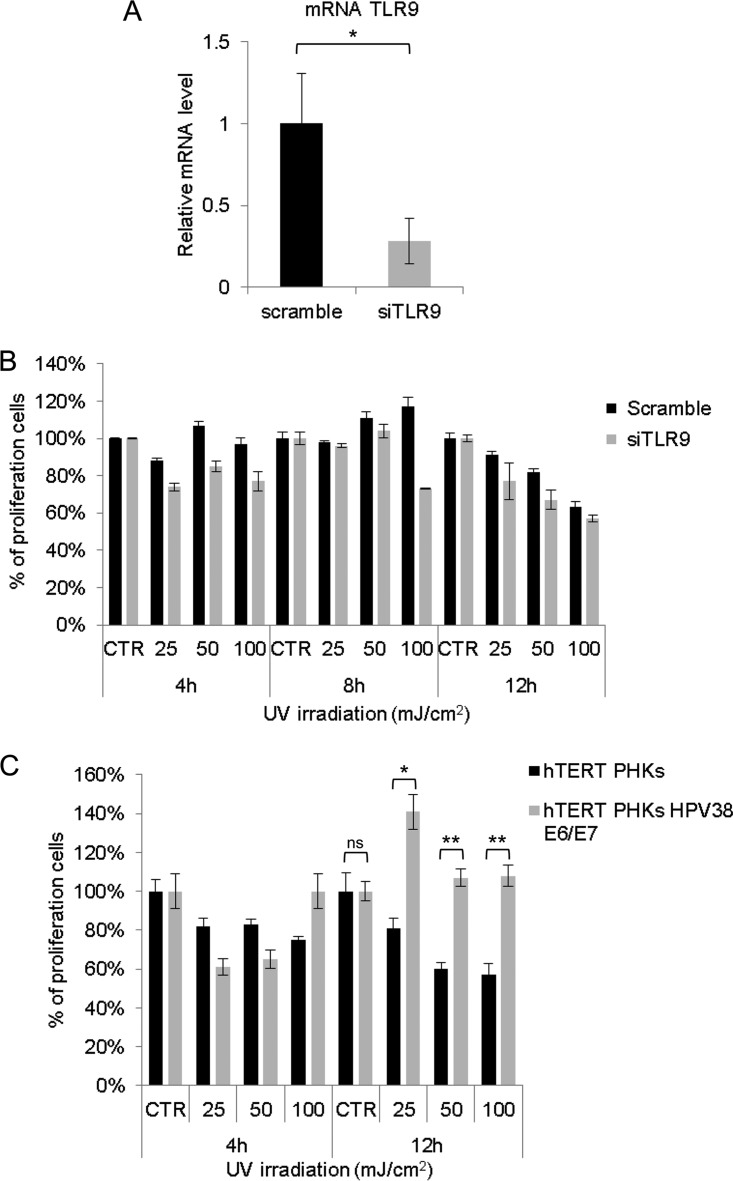
TLR9 gene silencing does not alter the cell viability of hTERT PHKs.
To gain insight into the role of TLR9 in the cellular stress response, we first evaluated whether the silencing of TLR9 gene expression may influence the cell viability of hTERT PHKs. No significant changes in cell viability were observed in hTERT PHKs transfected with scramble or TLR9 siRNA (Fig. 8A and B). In contrast, HPV38 E6/E7 hTERT PHKs showed a higher viability than did the mock-transfected cells after 12 h of UV irradiation (Fig. 8C), indicating that the virus is able to alter the cellular response to UV irradiation.
FIG 8.
Role of TLR9 in cell viability of hTERT PHKs exposed to various doses of UV radiation. Cells were transfected with scramble or siTLR9. After 48 h, cells were collected for total RNA preparation to determine TLR9 mRNA levels (A) or cultured and exposed to the doses of UV radiation indicated (per cm2) (B). (A) TLR9 expression was determined by quantitative RT-PCR. (B) Cell viability after 4, 8, and 12 h of UV irradiation was determined using the CellTiter 96 AQueous One Solution cell proliferation assay (MTS). (C) Cell viability of hTERT PHKs and HPV38 E6/E7 hTERT PHKs after exposure to UV radiation. Cells were exposed to various doses of UV radiation (per cm2) as indicated in the figure. After 4 and 12 h, cell viability was determined as described in panel B. CTR, control (0 mJ/cm2).
DISCUSSION
Beta HPV types are suspected to play a role, together with UV radiation, in the development of nonmelanoma skin cancer, the most common cancer among Caucasians (9, 13, 14). In vitro and in vivo mouse models have revealed that E6 and E7 from certain beta HPV types have the ability, like those from the high-risk alpha HPV types, to deregulate molecular pathways related to cell survival, proliferation, and apoptosis (9, 15). Transgenic mice expressing beta HPV oncoproteins under the control of the keratin 14 promoter showed higher susceptibility to UV-induced carcinogenesis than did wild-type animals (16). Although the keratin 14 HPV38 E6/E7 transgenic mice did not spontaneously develop any skin lesions during their life span, exposure to low doses of UV radiation for many weeks resulted in the development of premalignant skin lesions and subsequently squamous cell carcinoma (16). These findings indicate that beta HPV types have developed mechanisms to alter the response of normal cells to UV radiation, favoring skin carcinogenesis. In this study, we provide evidence implicating TLR9 in the PHK responses to UV irradiation and other stresses. Exposure of PHKs to UV radiation increases TLR9 expression, which is mediated by p53 and c-Jun. The product of the c-jun proto-oncogene was initially discovered as a component of the AP-1 transcription complex, which is activated by several extracellular stimuli, such as mitogenic factors and various forms of cellular stress (17), including UV irradiation (10). Many findings underscore the complexity of the c-Jun network, which is involved in the control of cell cycle progression and apoptosis (18, 19). It has been shown that c-Jun, via activation of the expression of the cyclin D1 gene, promotes the G1/S cell cycle transition (19). c-Jun is also able to prevent apoptosis induced by UV radiation (19). The use of c-jun+/+ and c-jun−/− fibroblasts allowed a more detailed characterization of c-Jun's role in the UV-induced cellular response (18). That study showed that c-Jun negatively regulates p53 recruitment to the p21WAF1 promoter. Accordingly, the loss of c-Jun was found to induce a prolonged cell cycle arrest in UV-irradiated fibroblasts, as well as p21WAF1 accumulation, with no signs of apoptotic events. Constitutive expression of c-Jun in c-jun−/− fibroblasts counteracts cell cycle arrest and promotes apoptosis by an unknown mechanism (18). On the basis of these findings, it has been proposed that c-Jun may facilitate cell recovery after the stress induced by UV irradiation. It would be interesting to evaluate the role of TLR9 in the context of the c-Jun network and stress induced by UV radiation; it is likely that TLR9 accumulation induced by UV radiation is required in order to coordinate the cell cycle arrest or apoptosis, according to the extent of damage. Regardless of TLR9's precise role, our data demonstrate that HPV38 E6 and E7 prevent the activation of UV-induced TLR9 expression. They also show that the activation of TLR9 expression induced by UV radiation is mediated by p53. It is reasonable to hypothesize that UV irradiation and other types of stress can lead to the accumulation of endogenous TLR9 ligands, activating specific signaling pathways. The physiological ligands that may induce TLR9 activation remain poorly characterized. We speculate that the inhibitory effect of TLR9 on cellular proliferation may be induced by damage-associated molecular pattern molecules, which are normally released by damaged or stressed tissues to alert the immune system of tissue injury. A recent study in a model of hepatocellular carcinoma showed that hypoxia-induced HMGB1 and the release of mitochondrial DNA (mtDNA) lead to the activation of TLR9-mediated tumor growth (20). Nucleic acids have been shown to be a potent trigger of innate inflammatory responses (21). Similar to CpG oligodeoxynucleotides, mtDNA is rich in CpG motifs; therefore, it is highly possible that TLR9 senses mtDNA in a similar way as it senses CpG oligodeoxynucleotides and other nucleic acids. We found that silencing TLR9 gene expression did not increase the resistance of hTERT PHKs to UV irradiation; therefore, it is possible that the activation of TLR9 transcription does not have an impact on intracellular events such as cell viability, but via TLR9 signaling and the production of cytokines, it could influence the recruitment of specific populations of immune cells in the skin upon UV irradiation.
In a previous study, we found evidence that, like mucosal high-risk HPV E6 and E7, HPV38 oncoproteins also inhibit the basal transcription of TLR9. We speculate that in the context of HPV infection, TLR9 expression is activated in response to the oncogenic stress. Therefore, the virus must inhibit this event in order to guarantee the survival and proliferation of the infected cells. Consistent with this hypothesis, TLR9 reexpression in HPV38 E6 and E7-immortalized keratinocytes resulted in a significant decrease in cellular proliferation and an increase in protein levels of the cell cycle inhibitor p21WAF1 (2). Concordantly, other studies have shown that loss of TLR9 expression may also lead to deregulation of the cell cycle and other events that may control transformation (6). In addition, clinical studies have shown that TLR9 expression is reduced in head and neck cancers (6).
Interestingly, another pathway involved in the antiviral response, the cyclic GMP–AMP synthase (cGAS)/STING pathway, is hampered by oncogenic viruses (22). cGAS detects intracellular DNA and subsequently signals via the adapter protein STING. The E7 oncoprotein from mucosal high-risk HPV16 is able to directly interact with STING, inhibiting its normal functions. Thus, the DNA sensors TLR9 and STING appear to be key targets for oncogenic viruses. However, there is no evidence indicating whether STING is also influenced by other HPV types, such as beta HPV38.
In conclusion, our data, in agreement with the findings of other studies, support a role of TLR9 in the response induced by various cellular stresses. In particular, our study provides possible explanations for the cooperation of UV radiation and beta HPV types in cellular transformation.
MATERIALS AND METHODS
Cell culture and treatment.
The experiments were carried out in PHKs isolated from neonatal foreskin and in a spontaneously immortalized human keratinocyte cell line, NIKS. The culture of cells, selection of antibiotics, and generation of high-titer retroviral supernatants were carried out using a previously described method (23). Transient-transfection experiments were performed using Lipofectamine 2000 transfection reagent (Invitrogen) or TransIT-Keratinocyte transfection reagent (Mirus) according to manufacturer protocols.
Cells covered with a thin layer of phosphate-buffered saline were exposed to a 25-mJ/cm2 dose of UVB radiation using the BIO-SUN UV irradiation system (Vilber Lourmat). After irradiation, the cells were cultured in a humidified chamber for 8 h at 37°C and then harvested. The cells were incubated for 1 h in media containing H2O2 at various concentrations (Sigma); others were incubated for 8 h in media containing 0.1 μg/ml doxorubicin (Sigma).
For evaluation of c-Jun inhibition, cells were incubated for 1 h in media containing JNK inhibitor II (CAS 129-56-6; Merck Millipore) at a final concentration of 30 μM and then UVB irradiated at a dose of 25 mJ/cm2 and cultured for 8 h in media containing 5 μM JNK inhibitor II. Cell viability was determined using the CellTiter 96 AQueous One Solution cell proliferation assay (MTS; Promega).
Gene silencing.
Gene silencing of p53 and p65 was achieved using synthetic siRNA (Table 1). siRNA or scrambled RNA at a concentration of 40 nM was transfected using Lipofectamine 2000 transfection reagent (Invitrogen) according to the standard protocol.
TABLE 1.
Sequences of siRNA and CRISPR/Cas9 vectors used for gene silencing
| Target | Orientationa | siRNA sequence (5′–3′) or description (source) |
|---|---|---|
| Scrambled RNA (negative control) | GGUGGAAGAGGUGGUGAGC | |
| p65 | siGenome SMART pool M-003533-02-0005, human RELA, NM_021975 (Thermo Scientific) | |
| p53 | CAAUGGUUCACUGAAGACCUU | |
| p53 vector 1 | F | TCCATTGCTTGGGACGGCAAGTTTT |
| R | TTGCCGTCCCAAGCAATGGACGGTG | |
| p53 vector 2 | F | CCATTGTTCAATATCGTCCGGTTTT |
| R | CGGACGATATTGAACAATGGCGGTG | |
| p53 vector 3 | F | CTCGGATAAGATGCTGAGGAGTTTT |
| R | TCCTCAGCATCTTATCCGAGCGGTG | |
| p53 vector 4 | F | CACTTTTCGACATAGTGTGGGTTTT |
| R | CCACACTATGTCGAAAAGTGCGGTG | |
| Scrambled vector | F | GGATGGACGGTAGAGGTGGGTTTT |
| R | CCACCTCTACCGTCCATCCCGGTG |
Abbreviations: forward (F), reverse (R).
The plasmids for CRISPR/Cas9 were obtained from the Addgene plasmid repository. All single-guide RNAs were designed by Thermo Fisher Scientific. The target sequence information is shown in Table 1. The CRISPR/Cas9 vectors were generated according to manufacturer protocols and then transiently transfected into keratinocytes. Purification of the cells carrying the CRISPR/Cas9 vectors was performed 48 h after transfection according to the manufacturer's protocol (GeneArt CRISPR Nuclease Vector Kit; Life Technologies).
Luciferase assay.
Transient transfections were conducted using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's protocol. Naturally immortalized keratinocytes (NIKS cells) were transfected with firefly luciferase TLR9 promoter vectors (0.5 μg). A pRL-TK Renilla reporter vector (15 ng) was used as an internal control. After 40 h, cells were UVB irradiated as previously described, then lysed, and luciferase activity was measured using a dual-luciferase reporter assay system (Promega). The expression of firefly luciferase relative to Renilla luciferase was expressed in percentage of relative luminescence units.
Reverse transcription and quantitative PCR.
Total RNA was extracted using the Absolutely RNA miniprep kit (Stratagene). The RNA obtained was reverse transcribed to cDNA using a RevertAid H Minus M-MuLV reverse transcriptase kit (Fermentas) according to the manufacturer's protocols. Real-time quantitative PCR (qPCR) was performed using the MESA Green qPCR MasterMix Plus for SYBR Assay (Eurogentec) with the primers listed in Table 2.
TABLE 2.
Sequences of primers used for RT-qPCR analyses, ChIP assays, and oligonucleotide pulldown assays
| Promoter or gene | Orientationa | Primer sequence (5′–3′) |
|---|---|---|
| TLR9 promoter NF-κB site C | F | GAGAGCACTCAGGGGAACAG |
| R | GGTCACATTCAGCCCCTAGA | |
| GAPDH | F | AAGGTGGTGAAGCAGGCGT |
| R | GAGGAGTGGGTGTCGCTGTT | |
| TLR9 | F | CGTCTTGAAGGCCTGGTGTTGA |
| R | CTGGAAGGCCTTGGTTTTAGTGA | |
| TLR3 | F | AACGACTGATGCTCCGAAG |
| R | TCCAGAGCCGTGCTAAGTTG | |
| TLR7 | F | TTGGCACCTCTCATGCTGTG |
| R | ACCATCTAGCCCCAAGGAGT | |
| p53 | F | GATGAAGCTCCCAGAATGCC |
| R | CAAGAAGCCCAGACGGAAAC | |
| p65 | F | GTCACCGGATTGAGGAGAAA |
| R | GCTCAGGGATGACGTAAAGG | |
| E6 | F | TCTGGACTCAAGAGGATTTTG |
| R | CACTTTAAACAATACTGACACC | |
| E7 | F | CAAGCTACTCTTCGTGATATAGTT |
| R | CAGGTGGGACACAGAAGCCTTAC | |
| Probes for oligonucleotide pulldown assays | F | Btn-GAGAGCACTCAGGGGAACAG |
| R | GGTCACATTCAGCCCCTAGA |
Abbreviations: forward (F), reverse (R).
Immunoblotting.
Total protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotting were performed as described previously (24). Antibodies against the following proteins were used: β-actin (clone C4; MP Biomedicals), TLR9 (2254; Cell Signaling Technology), p21WAF1 (2946; Cell Signaling Technology), p53 (DO-1) (sc-126; Santa Cruz Biotechnology), phospho-p53 (Ser15) (9284; Cell Signaling Technology), NF-κB p65 (3034; Cell Signaling Technology), c-Jun (H-79; Santa Cruz Biotechnology), phospho-c-Jun (Ser73) (9164; Cell Signaling Technology), and HA-Tag (3F10; Roche). Images were produced using the ChemiDoc XRS imaging system (Bio-Rad).
Chromatin immunoprecipitation.
ChIP was performed using the Shearing ChIP and OneDay ChIP kits (Diagenode) according to the manufacturer's instructions. Briefly, cells were sonicated to obtain DNA fragments of 200 to 500 bp. Sheared chromatin was immunoprecipitated with isotype control IgG or the indicated antibodies: against p53 (DO-1) (sc-126; Santa Cruz Biotechnology), NF-κB p65 (3034; Cell Signaling Technology), c-Jun (H-79; Santa Cruz Biotechnology), and HA-Tag (3F10; Roche). The eluted DNA was used as a template for qPCR. The primers used for qPCR are listed in Table 2.
Oligonucleotide pulldown assay.
Cells were lysed and sonicated as described previously (3). The TLR9 promoter (wild type or mutated) was used as a template to amplify the NF-κB RE region near site C. PCR amplification was performed using a biotinylated forward primer and a nonbiotinylated reverse primer (listed in Table 2). Amplicons were extracted from agarose gel using the MinElute gel extraction kit (Qiagen) and quantified. Then, 1 mg of prepared protein extract was incubated with 2 μg of biotin-TLR9 promoter probes and 10 μg of poly(dI-dC)–poly(dI-dC) for 16 h at 4°C. DNA-bound proteins were collected with streptavidin-agarose beads for 1 h and washed five times with HKMG buffer. DNA-bound proteins were then analyzed by immunoblotting.
Statistical analysis.
Statistical significance was determined using the Student t test. The levels of statistical significance for each experiment (P < 0.05, P < 0.01, P < 0.0001, or not significant) are indicated in the corresponding figures. The error bars in the graphs represent the standard deviations.
ACKNOWLEDGMENTS
We are grateful to all members of IARC's Infections and Cancer Biology Group for their support, to Nicole Suty for her help with preparation, and to Eleonora Feletto and Jessica Cox for editing the manuscript.
This work was partially supported by a grant awarded to M.T. by Fondation ARC (PJA 20151203192).
REFERENCES
- 1.Fathallah I, Parroche P, Gruffat H, Zannetti C, Johansson H, Yue J, Manet E, Tommasino M, Sylla BS, Hasan UA. 2010. EBV latent membrane protein 1 is a negative regulator of TLR9. J Immunol 185:6439–6447. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 2.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M. 2007. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol 178:3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 3.Pacini L, Savini C, Ghittoni R, Saidj D, Lamartine J, Hasan UA, Accardi R, Tommasino M. 2015. Downregulation of Toll-like receptor 9 expression by beta human papillomavirus 38 and implications for cell cycle control. J Virol 89:11396–11405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahzad N, Shuda M, Gheit T, Kwun HJ, Cornet I, Saidj D, Zannetti C, Hasan U, Chang Y, Moore PS, Accardi R, Tommasino M. 2013. The T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expression. J Virol 87:13009–13019. doi: 10.1128/JVI.01786-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent IE, Zannetti C, Lucifora J, Norder H, Protzer U, Hainaut P, Zoulim F, Tommasino M, Trépo C, Hasan U, Chemin I. 2011. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS One 6:e26315. doi: 10.1371/journal.pone.0026315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parroche P, Roblot G, Le Calvez-Kelm F, Tout I, Marotel M, Malfroy M, Durand G, McKay J, Ainouze M, Carreira C, Allatif O, Traverse-Glehen A, Mendiola M, Pozo-Kreilinger JJ, Caux C, Tommasino M, Goutagny N, Hasan UA. 2016. TLR9 reexpression in cancer cells extends the S-phase and stabilizes p16INK4a protein expression. Oncogenesis 5:e244. doi: 10.1038/oncsis.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shatz M, Menendez D, Resnick MA. 2012. The human TLR innate immune gene family is differentially influenced by DNA stress and p53 status in cancer cells. Cancer Res 72:3948–3957. doi: 10.1158/0008-5472.CAN-11-4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tommasino M. 2014. The human papillomavirus family and its role in carcinogenesis. Semin Cancer Biol 26:13–21. doi: 10.1016/j.semcancer.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Devary Y, Gottlieb RA, Lau LF, Karin M. 1991. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol 11:2804–2811. doi: 10.1128/MCB.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan UA, Zannetti C, Parroche P, Goutagny N, Malfroy M, Roblot G, Carreira C, Hussain I, Müller M, Taylor-Papadimitriou J, Picard D, Sylla BS, Trinchieri G, Medzhitov R, Tommasino M. 2013. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J Exp Med 210:1369–1387. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeshita F, Suzuki K, Sasaki S, Ishii N, Klinman DM, Ishii KJ. 2004. Transcriptional regulation of the human TLR9 gene. J Immunol 173:2552–2561. doi: 10.4049/jimmunol.173.4.2552. [DOI] [PubMed] [Google Scholar]
- 13.Lomas A, Leonardi-Bee J, Bath-Hextall F. 2012. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 14.Tuttleton Arron S, Jennings L, Nindl I, Rosl F, Bouwes Bavinck JN, Seçkin D, Trakatelli M, Murphy GM, Viral Working Group of the International Transplant Skin Cancer Collaborative (ITSCC) and Skin Care in Organ Transplant Patients Europe (SCOPE). 2011. Viral oncogenesis and its role in nonmelanoma skin cancer. Br J Dermatol 164:1201–1213. doi: 10.1111/j.1365-2133.2011.10322.x. [DOI] [PubMed] [Google Scholar]
- 15.Howley PM, Pfister HJ. 2015. Beta genus papillomaviruses and skin cancer. Virology 479-480:290–296. doi: 10.1016/j.virol.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viarisio D, Mueller-Decker K, Kloz U, Aengeneyndt B, Kopp-Schneider A, Gröne H-J, Gheit T, Flechtenmacher C, Gissmann L, Tommasino M. 2011. E6 and E7 from beta HPV38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 7:e1002125. doi: 10.1371/journal.ppat.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaulian E, Karin M. 2002. AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 18.Shaulian E, Schreiber M, Piu F, Beeche M, Wagner EF, Karin M. 2000. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell 103:897–908. doi: 10.1016/S0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 19.Wisdom R, Johnson RS, Moore C. 1999. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J 18:188–197. doi: 10.1093/emboj/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, Lotze M, Tang D, Tsung A. 2015. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J Hepatol 63:114–121. doi: 10.1016/j.jhep.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. 2006. TLR signaling. Cell Death Differ 13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 22.Lau L, Gray EE, Brunette RL, Stetson DB. 2015. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 350:568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 23.Caldeira S, Zehbe I, Accardi R, Malanchi I, Dong W, Giarrè M, de Villiers EM, Filotico R, Boukamp P, Tommasino M. 2003. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol 77:2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Accardi R, Dong W, Smet A, Cui R, Hautefeuille A, Gabet A-S, Sylla BS, Gissmann L, Hainaut P, Tommasino M. 2006. Skin human papillomavirus type 38 alters p53 functions by accumulation of ΔNp73. EMBO Rep 7:334–340. doi: 10.1038/sj.embor.7400615. [DOI] [PMC free article] [PubMed] [Google Scholar]