ABSTRACT
Human respiratory syncytial virus (RSV) is the leading cause of pediatric bronchiolitis and hospitalizations. RSV can also cause severe complications in elderly and immunocompromised individuals. There is no licensed vaccine. We previously generated a parainfluenza virus 5 (PIV5)-vectored vaccine candidate expressing the RSV fusion protein (F) that was immunogenic and protective in mice. In this work, our goal was to improve the original vaccine candidate by modifying the PIV5 vector or by modifying the RSV F antigen. We previously demonstrated that insertion of a foreign gene at the PIV5 small hydrophobic (SH)–hemagglutinin-neuraminidase (HN) junction or deletion of PIV5 SH increased vaccine efficacy. Additionally, other groups have demonstrated that antibodies against the prefusion conformation of RSV F have more potent neutralizing activity than antibodies against the postfusion conformation. Therefore, to improve on our previously developed vaccine candidate, we inserted RSV F at the PIV5 SH-HN gene junction or used RSV F to replace PIV5 SH. We also engineered PIV5 to express a prefusion-stabilized F mutant. The candidates were tested in BALB/c mice via the intranasal route and induced both humoral and cell-mediated immunity. They also protected against RSV infection in the mouse lung. When they were administered intranasally or subcutaneously in cotton rats, the candidates were highly immunogenic and reduced RSV loads in both the upper and lower respiratory tracts. PIV5-RSV F was equally protective when administered intranasally or subcutaneously. In all cases, the prefusion F mutant did not induce higher neutralizing antibody titers than wild-type F. These results show that antibodies against both pre- and postfusion F are important for neutralizing RSV and should be considered when designing a vectored RSV vaccine. The findings also that indicate PIV5-RSV F may be administered subcutaneously, which is the preferred route for vaccinating infants, who may develop nasal congestion as a result of intranasal vaccination.
IMPORTANCE Despite decades of research, human respiratory syncytial virus (RSV) is still a major health concern for which there is no vaccine. A parainfluenza virus 5-vectored vaccine expressing the native RSV fusion protein (F) has previously been shown to confer robust immunity against RSV infection in mice, cotton rats, and nonhuman primates. To improve our previous vaccine candidate, we developed four new candidates that incorporate modifications to the PIV5 backbone, replace native RSV F with a prefusion-stabilized RSV F mutant, or combine both RSV F and PIV5 backbone modifications. In this work, we characterized the new vaccine candidates and tested their efficacies in both murine and cotton rat models of RSV infection. Most importantly, we found that PIV5-based RSV vaccine candidates were efficacious in preventing lower respiratory tract infection as well as in reducing the nasal viral load when administered via the subcutaneous route.
KEYWORDS: PIV5, RSV, vaccine, subcutaneous
INTRODUCTION
Human respiratory syncytial virus (RSV) is a leading cause of pediatric bronchiolitis and pneumonia, resulting in over 3 million hospitalizations and 200,000 deaths globally each year in children under 5 years old (1). The disease burden is also significant among elderly and immunocompromised populations. An estimated 3 to 7% of healthy adults over 65 years of age and 4 to 10% of high-risk adults develop RSV infections annually, with the rate of mortality reaching 8% among hospitalized individuals (2).
RSV vaccine development has been ongoing since the 1960s, when the virus was first discovered. Numerous candidates have been developed and tested, but a vaccine has yet to be licensed. A formalin-inactivated RSV (FI-RSV) vaccine tested during the 1960s failed to protect against infection. Furthermore, it caused enhanced disease in vaccinees upon natural exposure during the following RSV season. The concern over enhanced disease has been a major hurdle for vaccine development (3–6). Vaccines developed by many approaches have been used, including live attenuated vaccines, vectored vaccines, subunit vaccines, and virus-like particles (7–10). Promising live attenuated and vectored vaccine candidates that are not known to potentiate disease upon natural RSV infection have been developed (11).
Parainfluenza virus 5 (PIV5) is a single-stranded, negative-sense RNA virus of the genus Rubulavirus in the family Paramyxoviridae (12). It has several distinguishing characteristics that make it an attractive vector for vaccine development. First, PIV5 can infect a variety of animals but causes no known disease. Its previous association with canine kennel cough led to its inclusion in the kennel cough vaccine, which has been used for over 3 decades without safety concerns for dogs or humans (13–16). Studies in the intervening years have confirmed that PIV5 does not cause kennel cough (17). Second, PIV5 can infect many cell types, including Vero cells, which are approved for use for vaccine production by FDA and WHO. Its ability to grow to titers as high as 108 PFU/ml in laboratory settings also makes it suitable for vaccine production (12). Lastly, PIV5 has already been used to develop vaccine candidates efficacious against different viral and bacterial pathogens, such as influenza virus, rabies virus, and Mycobacterium tuberculosis (18–25). For these reasons, PIV5 is a promising vector for the development of vaccines.
A PIV5-vectored RSV vaccine has recently been developed in which the coding sequence of the RSV fusion protein (F) is inserted between the hemagglutinin-neuraminidase (HN) and RNA-dependent RNA polymerase (L) genes of PIV5 [PIV5-RSV-F (HN-L)]. The vaccine candidate was immunogenic and highly efficacious at protecting mice, cotton rats, and nonhuman primates against RSV lower respiratory tract infection (26, 27). It has been hypothesized that the vaccine candidate can be improved by modifying the vector or by increasing the antigen expression level to better protect the upper respiratory tract from RSV infection. The small hydrophobic (SH) protein of PIV5 plays an important role in blocking tumor necrosis factor alpha-mediated apoptosis. Deletion of this gene from PIV5 induces apoptosis in infected cells through an intrinsic pathway, possibly making it a better vector for presenting foreign antigens (28). Previous work has shown that deletion of the SH gene from PIV5 improves the efficacy of a PIV5-based vaccine expressing H5 of the H5N1 influenza virus strain (the PIV5ΔSH-H5 vaccine). When tested in mice, the PIV5ΔSH-H5 vaccine increased antibody and cell-mediated immune responses to influenza virus and provided better protection against H5N1 infection than its SH-containing counterpart (21).
The PIV5-RSV-F vaccine candidate can also be improved by modifying the RSV F antigen. Previous work has suggested that RSV F in its prefusion conformation induces the production of antibodies with in vitro neutralizing activity more potent than that of antibodies induced by RSV F in its postfusion conformation (29, 30). A stabilized prefusion RSV F mutant called DS-Cav1 was recently crystallized and has been shown to induce higher neutralizing antibody titers than the postfusion conformation of F (31, 32). Introduction of the DS-Cav1 construct into a chimeric bovine/human PIV3 vector generated a vaccine candidate that was more immunogenic and protective than the same vector expressing postfusion RSV F (33).
In this work, we modified the previous PIV5-RSV-F (HN-L) candidate by deleting the SH gene of PIV5 or by replacing wild-type RSV F with the DS-Cav1 prefusion mutant as well as by combining both modifications into a vaccine. We tested their immunogenicity and protective efficacy against RSV infection in mice and cotton rats.
RESULTS
Generation and analysis of PIV5 expressing wild-type RSV F and a prefusion-stabilized RSV F mutant.
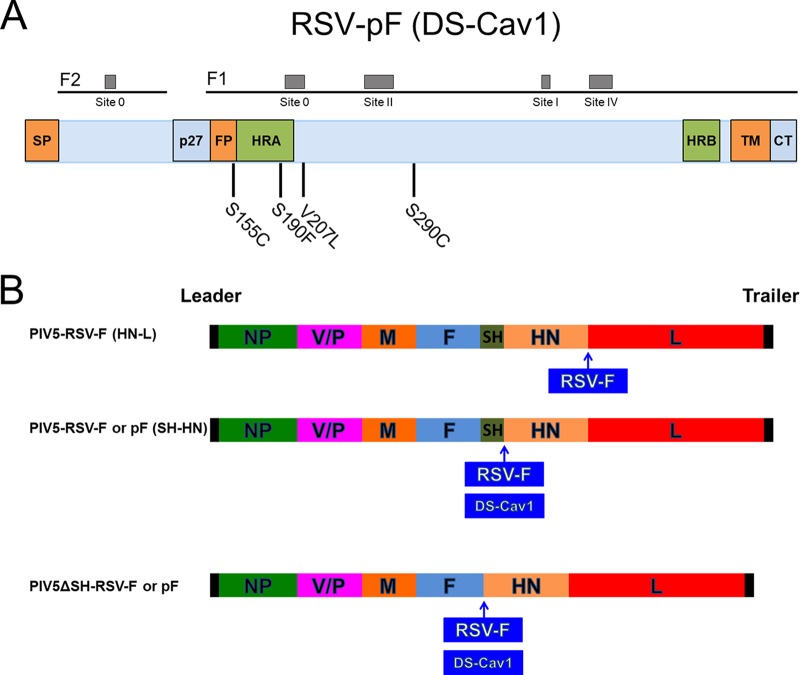
Previously, we reported that the SH-HN junction of PIV5 was the optimal site for inserting the hemagglutinin of influenza virus to give the best protection against infection (25). We also reported that deletion of the SH gene resulted in a more efficacious vector (21). To improve on the existing PIV5-RSV-F (HN-L) vaccine candidate, we inserted RSV F at the SH-HN junction to generate PIV5-RSV-F (SH-HN) or replaced PIV5 SH with RSV F to produce PIV5ΔSH-RSV-F. We also sought to improve the RSV F antigen. It has been previously reported that antibodies with the highest RSV-neutralizing activity are directed against the prefusion conformation of RSV F (29, 30). McLellan et al. previously obtained the crystal structure of a prefusion-stabilized RSV F mutant called DS-Cav1 (32). This mutant contains substitutions that introduce disulfide bond-forming cysteine pairs and fill cavities in the head of the prefusion structure (Fig. 1A). Therefore, we inserted the prefusion-stabilized RSV F mutant DS-Cav1 into PIV5 to generate PIV5-RSV-pF (SH-HN) and PIV5ΔSH-RSV-F. The new vaccine candidates were engineered using previously described methods and confirmed by sequencing (Fig. 1B) (26, 34).
FIG 1.
Schematics of PIV5-based vaccine candidates. (A) Schematic of substitutions in prefusion-stabilized RSV F (DS-Cav1). (B) Schematic of PIV5-RSV-F (SH-HN), PIV5ΔSH-RSV-F, PIV5-RSV-pF, PIV5ΔSH-RSV-pF. NP, nucleoprotein; V, V protein; P, phosphoprotein; M, matrix protein; F, fusion protein; SH, small hydrophobic protein; HN, hemagglutinin-neuraminidase protein; L, RNA-dependent RNA polymerase; RSV F, respiratory syncytial virus fusion protein; DS-Cav1, stabilized prefusion RSV F.
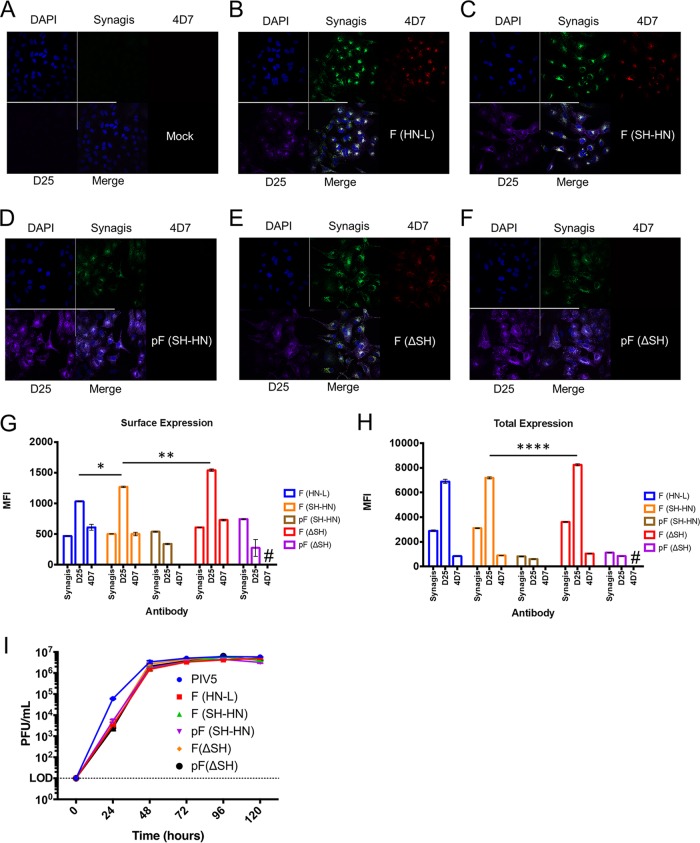
Expression of pre- and postfusion RSV F by the vaccine candidates was examined by fluorescence microscopy and flow cytometry using antibodies specific to prefusion F (antibody D25), postfusion F (antibody 4D7), and total F (palivizumab). Cells infected with candidates encoding wild-type F expressed mixtures of both prefusion and postfusion F (Fig. 2B, C, E, G, and H). Candidates encoding the DS-Cav1 construct expressed mostly prefusion F, as expected, but the level of total F expression by candidates encoding DS-Cav1 was lower than that by candidates encoding wild-type F. There appeared to be little to no postfusion F expression when it was examined by microscopy or flow cytometry (Fig. 2D and F to H). The level of F expression was similar in viruses with the wild-type PIV5 backbone and the backbone with the SH deletion (ΔSH) (Fig. 2B, C, E, G, and H). Furthermore, the F constructs localized both inside the cell and on the cell surface (Fig. 2G and H). Thus, wild-type F expressed from PIV5-based vectors consisted of a mixture or pre- and postfusion conformations, while the majority of F in DS-Cav1 was expressed in the prefusion conformation.
FIG 2.
Generation and characterization of recombinant PIV5-based vaccine candidates. (A to F) Detection of total RSV F, postfusion RSV F, and prefusion RSV F in infected A549 cells by immunofluorescence assay. Total RSV F was detected using palivizumab (Synagis) labeled with Alexa Fluor 488 (green). Postfusion RSV F was detected with the 4D7 antibody labeled with Cy3 (red). Prefusion RSV F was detected using the D25 antibody labeled with APC (purple). DAPI (4′,6-diamidino-2-phenylindole; blue) was used as a nuclear stain. Images are representative of those from three independent experiments. (G, H) Flow cytometry was used to examine surface (G) and total (H) RSV F, postfusion RSV F, and prefusion RSV F expression in A549 cells infected with the vaccine candidates. Antibodies were labeled as described in the legend to panels A to F, except that 4D7 was labeled with PE. The graphs present the mean fluorescence intensity (MFI) of cells expressing postfusion or prefusion F. Error bars represent the standard errors of the means. #, too few positive cells were available to analyze the mean fluorescence intensity. *, P ≤ 0.05; **, P ≤ 0.01; ****, P ≤ 0.0001. (I) Multicycle growth curves of PIV5, PIV5-RSV-F (HN-L) [F (HN-L)], PIV5-RSV-F (SH-HN) [F (SH-HN)], PIV5-RSV-pF (SH-HN) [pF (SH-HN)], PIV5ΔSH-RSV-F [F(ΔSH)], and PIV5ΔSH-RSV-pF [pF(ΔSH)] in Vero cells. Vero cells were infected at an MOI of 0.01 PFU per cell. Aliquots of the cell culture supernatant were collected every 24 h for 120 h. Plaque assays were performed in BHK21 cells to determine the virus titers at each time point. Growth curves were performed in triplicate. Error bars represent the standard errors of the means.
The new vaccine candidates grew to titers similar to those of the original PIV5-RSV-F (HN-L) candidate (Fig. 2I). In vitro stability studies of the PIV5-RSV-F (HN-L), PIV5-RSV-F (SH-HN), and PIV5ΔSH-RSV-F candidates showed that the insertions were retained through multiple passages in cell culture (35). These results indicate that incorporation of the RSV F and PIV5 backbone modifications did not significantly impact the titers of new candidates. Furthermore, modifications to the PIV5 backbone did not significantly impact the genetic stability of the candidates.
Immunogenicity and protective efficacy of vaccine candidates in mice.
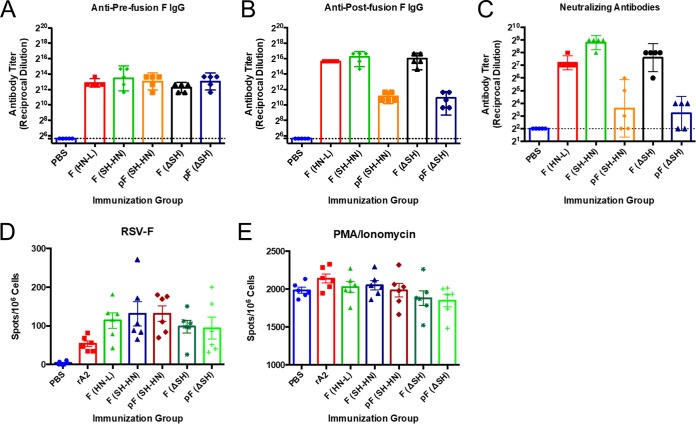
To examine the immunogenicity of the vaccine candidates, 6- to 8-week-old BALB/c mice were immunized once with 106 PFU of the different candidates, and serum was collected at 21 days postvaccination. The levels of antibodies against prefusion and postfusion F were measured by enzyme-linked immunosorbent assay (ELISA). The levels of serum neutralizing antibodies were measured by 50% plaque reduction assay.
All of the vaccine candidates induced similar levels of antibodies that bound to prefusion F (Fig. 3A). The constructs expressing wild-type F induced approximately 32-fold higher levels of postfusion F-specific antibodies than their counterparts expressing DS-Cav1 (Fig. 3B). All of the candidates induced serum neutralizing antibodies, but candidates expressing wild-type F induced titers approximately 8- to 32-fold higher than those induced by the DS-Cav1-expressing candidates. Animals immunized with PIV5-RSV-F (SH-HN) had an average neutralizing antibody titer that was 3-fold higher than that of animals immunized with PIV5-RSV-F (HN-L), but the difference was not statistically significant (Fig. 3C). The backbone consisting of PIV5 with the SH deletion (PIV5ΔSH) did not increase the immunogenicity of any of the constructs (Fig. 3A and B). Despite expressing mostly prefusion F, candidates encoding the DS-Cav1 construct did not increase the levels of prefusion F antibodies or neutralizing antibodies in mice.
FIG 3.
Humoral and cell-mediated immune responses in mice immunized with PIV5 or PIV5ΔSH expressing wild-type or prefusion RSV F. Six- to 8-week-old BALB/c mice were immunized intranasally with 106 PFU of PIV5-RSV-F (HN-L), PIV5-RSV-F (SH-HN), PIV5-RSV-pF (DS-Cav1), PIV5ΔSH-RSV-F, or PIV5ΔSH-RSV-pF (DS-Cav1). Serum samples were collected at 21 days postimmunization. (A, B) Serum IgG antibodies that bound prefusion or postfusion RSV F were measured by indirect ELISA. Purified prefusion-stabilized (A) or postfusion (B) RSV F was used as the coating antigen. Twofold serial dilutions of immune sera were incubated with the immobilized antigens, followed by incubation with HRP-conjugated goat anti-mouse IgG. The plates were developed, and the optical density at 450 nm was measured. Titers were defined as the reciprocal of the highest dilution at which the absorbance was 2 standard deviations above the average absorbance for sera from PBS-treated mice. (C) Serum neutralizing antibody titers were measured by plaque reduction assay. Twofold serial dilutions of heat-inactivated immune sera were incubated with 100 to 150 PFU of RSV/A/A2. Plaques were enumerated 7 days later, and the neutralizing antibody titer was defined as the reciprocal of the highest dilution at which there was a 50% reduction in input virus. The graphs present the geometric mean antibody titers for four to five mice per group, and error bars represent the 95% confidence intervals. (D, E) Six- to 8 week old BALB/c mice from a separate cohort were immunized as described in the legends to panels A to C. Splenocytes were collected at 27 days postimmunization, and cell-mediated immune responses were measured by ELISpot assay. Cells were stimulated with 200 ng/well of RSV F peptide (D) or PMA-ionomycin (E) for 48 h. The number of IFN-γ-secreting cells per 2 × 105 cells was enumerated. The graphs present the average number of spots per 106 cells for each group of six mice.
To examine the cell-mediated immune responses induced by the vaccine candidates, an enzyme-linked immunosorbent spot (ELISpot) assay was performed on splenocytes harvested from a separate cohort of immunized mice (Fig. 3D and E). All of the immunization groups had similar numbers of gamma interferon (IFN-γ)-secreting cells in response to RSV F peptide stimulation (Fig. 3D), with little to no stimulation by the green fluorescent protein (GFP) peptide being found (data not shown). Thus, all of the vaccine candidates elicited significant cell-mediated immune responses, regardless of their prefusion and postfusion F expression and regardless of the presence of SH in the vector backbone.
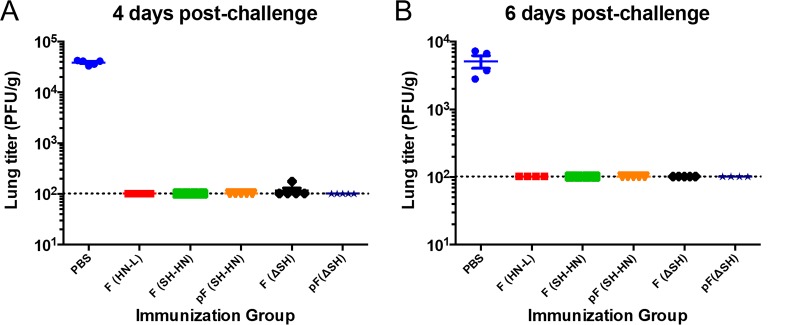
The mice were challenged at 28 days postimmunization with RSV type A strain A2 (RSV/A/A2) to determine the protective efficacies of the different vaccine candidates. Challenge virus was recovered from only one out of five mice in the PIV5ΔSH-RSV-F-immunized group at 4 days postchallenge. None of the mice in the other vaccination groups had detectable challenge virus in the lungs at 4 and 6 days postchallenge, showing that all of the candidates induced potent lower respiratory tract protection against RSV infection (Fig. 4A and B).
FIG 4.
Protection in mice immunized with PIV5 or PIV5ΔSH expressing wild-type or prefusion RSV F. Six- to 8-week-old BALB/c mice were immunized intranasally with 106 PFU of PIV5-RSV-F (HN-L), PIV5-RSV-F (SH-HN), PIV5-RSV-pF (DS-Cav1), PIV5ΔSH-RSV-F, or PIV5ΔSH-RSV-pF (DS-Cav1). The mice were challenged 28 days postimmunization with 106 PFU of RSV/A/A2. Four (A) and 6 (B) days later, the lungs were harvested to assess the viral load by plaque assay in Vero cells. The graphs present the average lung titer for four to five mice per group. Error bars represent the standard errors of the means.
Immunogenicity and protective efficacies of vaccine candidates in cotton rats.
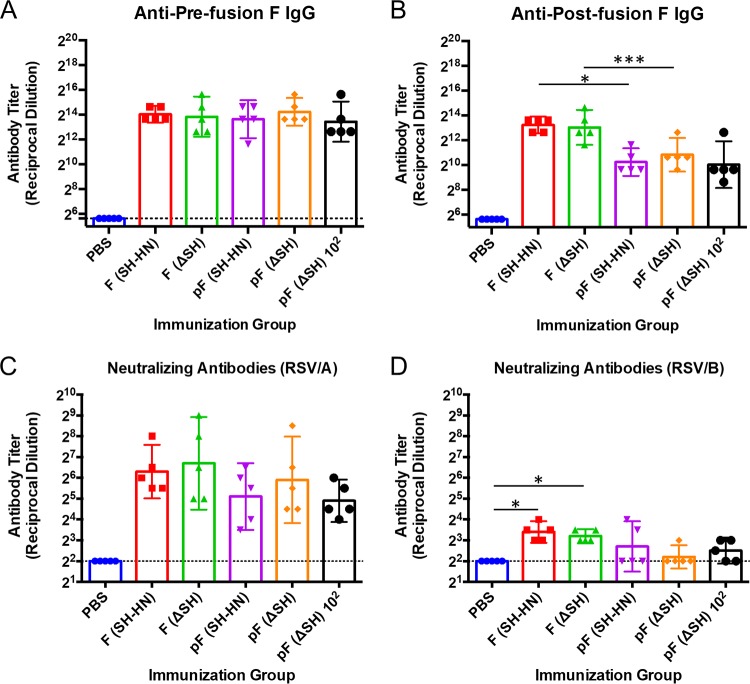
The vaccine candidates were further evaluated in cotton rats, a model more permissive for RSV infection (36). Previous findings indicated that PIV5-RSV-F (HN-L) induced potent protection against RSV in cotton rats infected with RSV at 105 PFU (27). Therefore, for this study, we intranasally immunized cotton rats with low doses (103 PFU) of PIV5-RSV-F (SH-HN), PIV5-RSV-pF (SH-HN), or the ΔSH counterparts to determine if differences between the vaccines could be observed at low doses. An additional group was immunized with 102 PFU of PIV5ΔSH-RSV-pF to determine whether the candidate was efficacious at an even lower dose. Similar to the findings in mice, all candidates elicited similar levels of anti-prefusion F IgG (Fig. 5A), while the DS-Cav1-expressing candidates induced 8-fold lower anti-postfusion F IgG titers than the other candidates (Fig. 5B). The candidates also induced similar levels of neutralizing antibody titers against RSV/A (Fig. 5C). The neutralizing antibody titers against RSV type B (RSV/B) were significantly lower than the titers against RSV/A (Fig. 5D). Only the animals immunized with PIV5-RSV-F (SH-HN) or PIV5ΔSH-RSV-F had significant levels of neutralizing antibodies against RSV/B (Fig. 5D).
FIG 5.
Humoral responses in cotton rats immunized with PIV5 or PIV5ΔSH expressing wild-type or prefusion RSV F. Cotton rats were intranasally immunized with PIV5-RSV-F (SH-HN), PIV5-RSV-pF (DS-Cav1), PIV5ΔSH-RSV-F, or PIV5ΔSH-RSV-pF (DS-Cav1). The groups received 102 or 103 PFU of the indicated vaccine virus. (A, B) Using serum collected at 28 days postinfection, the levels of IgG antibodies against prefusion (A) or postfusion (B) RSV F were measured by ELISA using chicken anti-cotton rat IgG conjugated to HRP. Serum neutralizing antibody titers against RSV/A/Tracy were measured in serum samples collected at 28 days postinfection. (C, D) The neutralizing activity of the serum samples against RSV/A/Tracy (C) and RSV/B/18537 (D) was measured. The neutralizing antibody titer was defined as the reciprocal of the serum dilution at which there was a 50% reduction in the viral CPE. The graphs present the geometric mean antibody titer for five cotton rats per group. Error bars represent 95% confidence intervals.
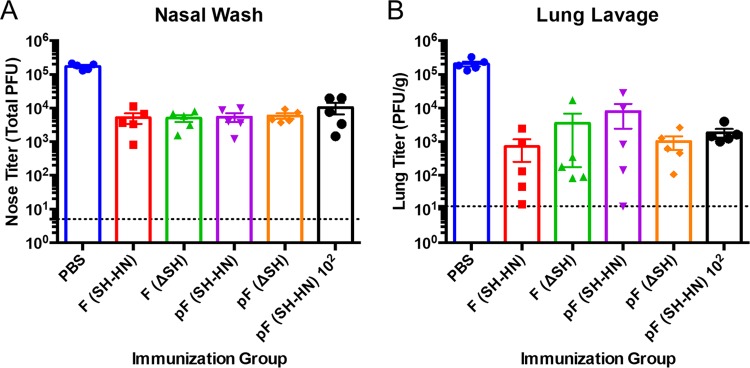
Cotton rats were challenged at 28 days postimmunization with 1.21 × 105 PFU of RSV/A/Tracy, which is 97% identical to RSV/A/A2. Immunization with the candidates reduced the viral loads in the nasal wash fluid by 1.40 to 1.66 log10 PFU (Fig. 6A). The viral loads in the lung lavage fluid were reduced by 2 to 3 log10 (Fig. 6B). There were no statistically significant differences in the postchallenge viral loads between the groups. Thus, all of the candidates were highly effective at inducing humoral immune responses and protecting against RSV challenge. Furthermore, immunization with 102 PFU of PIV5ΔSH-RSV-pF was as effective as immunization with 103 PFU.
FIG 6.
Protection in cotton rats immunized with PIV5 or PIV5ΔSH expressing wild-type or prefusion RSV F. Cotton rats were intranasally immunized with PIV5-RSV-F (SH-HN), PIV5-RSV-pF (DS-Cav1), PIV5ΔSH-RSV-F, or PIV5ΔSH-RSV-pF (DS-Cav1). Groups received 102 or 103 PFU of the indicated vaccine virus. Animals were challenged with 1.21 × 105 PFU of RSV/A/Tracy at 28 days postimmunization. Four days later, the nose (A) and lungs (B) were lavaged to determine the virus titer by plaque assay in HEp-2 cells. The graphs present the average lung titer for five rats per group. Error bars represent the standard errors of the means.
Evaluating the efficacies of wild-type F-expressing candidates using different routes of administration.
Since the DS-Cav1-expressing candidates were no more effective than the wild-type F-expressing candidates at inducing RSV-specific immunity and protection, we sought to evaluate the efficacies of PIV5-RSV-F (SH-HN) and PIV5ΔSH-RSV-F in cotton rats using alternate routes of administration. Cotton rats were intranasally or subcutaneously immunized with 105 PFU of the vaccine candidates. The candidates were also administered subcutaneously at 106 PFU. A group intranasally immunized with 105 RSV/A/A2 was used as a positive control for protection and safety. Mice sham vaccinated with phosphate-buffered saline (PBS) intranasally or intramuscularly were used as negative controls for protection. An additional group vaccinated with FI-RSV was included for comparison in safety studies. This group was intramuscularly vaccinated two times, with an interval of 28 days between the prime and boost vaccinations being used.
Serum samples were collected at 28 and 49 days postimmunization for serology studies. High levels of RSV-specific IgG antibodies were detected in all groups immunized with the vaccine candidates, with the titers being comparable to those in the RSV/A/A2-immunized group. The FI-RSV-immunized group had lower F-specific IgG titers overall, with antibody levels increasing between 28 and 49 days postimmunization due to boosting. The antibody titers of the groups immunized with the vaccine candidates were slightly higher at 28 days postimmunization than 49 days postimmunization (Fig. 7A). The highest average neutralizing antibody titers were observed in the group immunized with RSV/A/A2. Both PIV5-RSV-F (SH-HN) and PIV5ΔSH-RSV-F elicited significant neutralizing antibodies when they were administered by both the intranasal and subcutaneous routes. PIV5-RSV-F (SH-HN) administered intranasally or subcutaneously at 105 PFU induced similar levels of neutralizing antibodies. The subcutaneous 106-PFU dose of PIV5-RSV-F (SH-HN) did not improve the neutralizing antibody response. For the PIV5ΔSH-RSV-F candidate, 105 PFU induced slightly higher neutralizing antibody titers when it was administered intranasally than when it was given via the subcutaneous route. Increasing the subcutaneous dose of PIV5ΔSH-RSV-F to 106 PFU induced slightly higher neutralizing antibody titers. Similar to the serum IgG titers, the neutralizing antibody titers were higher at 28 days postimmunization than at 49 days postimmunization (Fig. 7B). Overall, 105 or 106 PFU of PIV5-RSV-F (SH-HN) given subcutaneously induced similar antibody titers as 105 PFU administered intranasally. Deletion of SH from the PIV5 backbone did not improve the humoral response to RSV in cotton rats.
FIG 7.
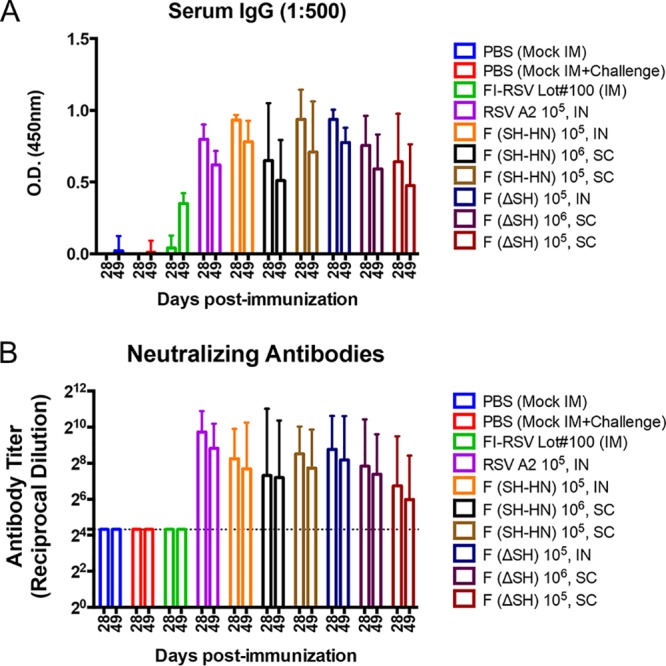
Humoral responses in cotton rats immunized with PIV5 or PIV5ΔSH expressing wild-type RSV F using different routes of administration. Cotton rats were immunized with RSV/A/A2, PIV5-RSV-F (SH-HN), or PIV5ΔSH-RSV-F. Animals received 105 PFU intranasally (IN) or 105 to 106 PFU subcutaneously (SC), as indicated. Animals vaccinated with FI-RSV were immunized intramuscularly (IM). (A) Serum samples from day 28 postimmunization were diluted 1:500, and the anti-postfusion RSV F IgG level was measured by ELISA. IgG levels are shown as the absorbance at 450 nm. The graphs present the average IgG measurement for five animals per group. Error bars represent the standard errors of the 95% confidence intervals. (B) The titers of serum neutralizing antibody against RSV/A/A2 were measured. The neutralizing antibody titer was defined as the reciprocal of the highest dilution at which there was a 60% reduction in the viral CPE. The graphs present the geometric mean neutralizing antibody titer for five cotton rats per group. Error bars represent the 95% confidence intervals.
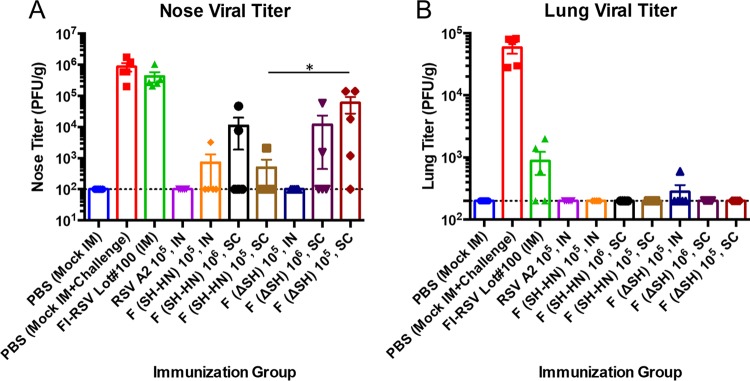
The cotton rats were intranasally challenged at 49 days postimmunization with 105 PFU of RSV/A/A2. Nasal and lung tissues were collected at 5 days postchallenge to determine the viral loads. No challenge virus was detected in the nasal tissues of animals vaccinated with RSV/A/A2. Immunization with 105 PIV5-RSV-F (SH-HN) intranasally or subcutaneously reduced the nasal viral loads by 3.5 log10, and impressively, four out of five cotton rats had no detectable virus in the nose. Increasing the subcutaneous dose to 106 PFU did not improve protection. Remarkably, immunization with PIV5ΔSH-RSV-F intranasally resulted in no detectable nasal viral load. Subcutaneous administration of the vaccine candidate PIV5ΔSH-RSV-F was less efficacious, reducing the nasal viral loads by 1 to 2 log10. As expected, sham-vaccinated animals or FI-RSV-vaccinated animals had high viral loads in the nose (Fig. 8A). Animals immunized with PIV5-RSV-F (SH-HN) by either the intranasal or subcutaneous route had no detectable virus in the lung, comparable to the findings for animals immunized with RSV/A/A2. Animals immunized subcutaneously with PIV5ΔSH-RSV-F also had no detectable virus in the lung. Only one out of five animals immunized intranasally with PIV5ΔSH-RSV-F had any virus detected in the lung (2.5 log10 PFU/g). FI-RSV vaccination conferred modest protection against lower respiratory tract infection (Fig. 8B). All of the PIV5-based vaccine candidates provided robust upper and lower respiratory tract protection when they given by both the intranasal and subcutaneous routes. The PIV5-RSV-F (SH-HN) candidate appeared to be slightly more efficacious than the PIV5ΔSH-RSV-F candidate when it was administered via the subcutaneous route, with the postchallenge nasal titers for the 105-PFU dose being significantly lower.
FIG 8.
Protection in cotton rats immunized with PIV5 or PIV5ΔSH expressing wild-type RSV F using different routes of administration. Cotton rats were immunized with RSV/A/A2, PIV5-RSV-F (SH-HN), or PIV5ΔSH-RSV-F. Animals received 105 PFU intranasally or 105 to 106 PFU subcutaneously, as indicated. Animals vaccinated with FI-RSV were immunized intramuscularly. Animals were challenged with 105 PFU of RSV/A/A2 at 49 days postimmunization. Five days later, nose (A) and lung (B) tissues were harvested to determine viral loads by plaque assay in HEp-2 cells. The graphs present the average nose and lung titers for five rats per group. Error bars represent the standard errors of the means.
Evaluating the safety of wild-type F-expressing candidates using different routes of administration.
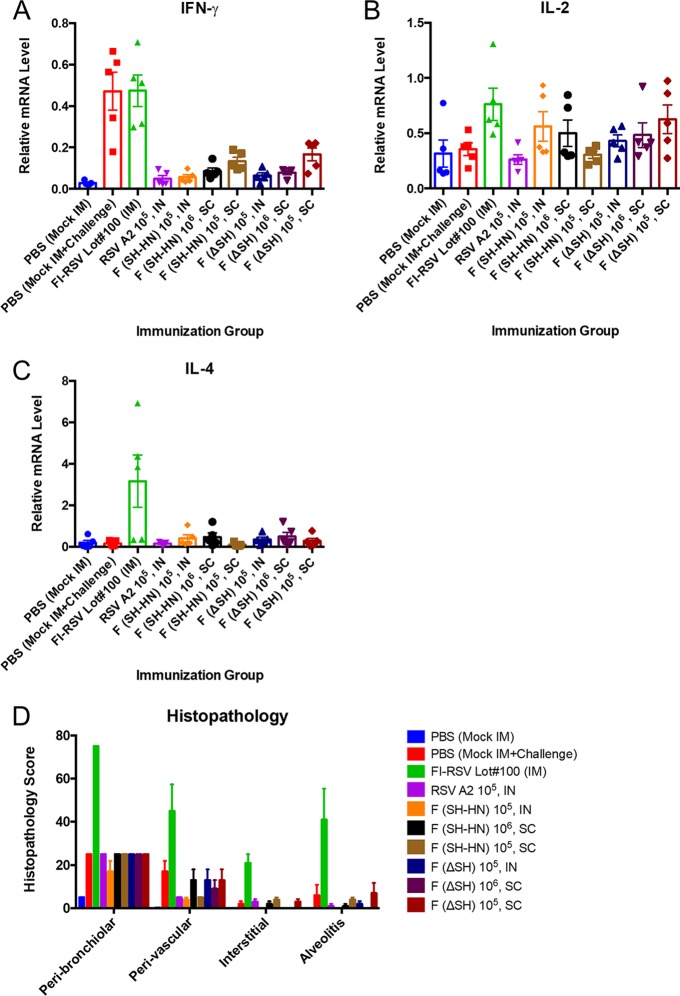
In addition to testing the efficacies of the vaccine candidates, we examined their safety. Since the FI-RSV vaccine causes enhanced disease postchallenge that can be recapitulated in cotton rats, we sought to determine whether there was any evidence of enhanced disease caused by our vaccine candidates (36). Since enhanced disease is associated with a Th2-biased immune response, cytokine levels in lung tissues at 5 days postchallenge were measured by quantitative real-time PCR (qPCR) (37). Specifically, the levels of IFN-γ, interleukin-2 (IL-2), and IL-4 mRNA were measured. IFN-γ mRNA levels were the highest in the sham-immunized and FI-RSV-immunized groups. IFN-γ mRNA levels were similarly low between groups immunized with the PIV5-based candidates and the RSV-immunized group (Fig. 9A). IL-2 mRNA levels were similar in all groups, but the average IL-2 level in the FI-RSV-immunized group was significantly higher than that in the other groups (Fig. 9B). The IL-4 mRNA level was significantly elevated only in the FI-RSV-vaccinated group, consistent with the histopathology results and the enhanced disease phenotype (Fig. 9C and D).
FIG 9.
Postchallenge pulmonary cytokine levels and lung histology of cotton rats immunized with PIV5 or PIV5ΔSH expressing wild-type RSV F. Cotton rats were immunized with RSV/A/A2, PIV5-RSV-F (SH-HN), or PIV5ΔSH-RSV-F. Animals received 105 PFU intranasally or 105 to 106 PFU subcutaneously, as indicated. Animals vaccinated with FI-RSV were immunized intramuscularly. Animals were challenged with 105 PFU of RSV/A/A2 at 49 days postimmunization. (A to C) At 5 days postchallenge, lung tissue was collected for RNA isolation and qPCR. The levels of IFN-γ (A), IL-2 (B), and IL-4 (C) mRNA were normalized to the β-actin mRNA levels in each sample. The graphs present the average relative mRNA levels for five rats per group. (D) At 5 days postchallenge, the lungs were harvested and fixed in 10% neutral buffered formalin. Lung sections were stained with hematoxylin and eosin. The sections were scored on a scale ranging from 0 to 4 for peribronchiolitis, perivasculitis, interstitial pneumonia, and alveolitis. The scores were then converted to a 0 to 100% histopathology scale. The graphs present the average scores for five rats per group. Error bars represent the standard errors of the means.
Hematoxylin and eosin (H&E)-stained lung sections from the different groups were examined for four hallmarks of pulmonary inflammation: peribronchiolitis, perivasculitis, interstitial pneumonia, and alveolitis. Histopathology was scored on a severity scale ranging from 0 to 4 and converted to a 0 to 100% scale. The largest lesions were observed in the FI-RSV-immunized, RSV-challenged group. Pulmonary changes were moderate in the groups immunized with PIV5-RSV-F (SH-HN) or PIV5ΔSH-RSV-F (intranasally or subcutaneously), with the levels not exceeding those observed in the RSV-immunized, RSV-challenged group or the sham-immunized, RSV-challenged group (Fig. 9D). On the basis of the cytokine profile and the histopathology results, the PIV5-based vaccine candidates did not appear to cause enhanced disease upon challenge with RSV.
DISCUSSION
In this study, we characterized and evaluated recombinant PIV5-based RSV vaccine candidates expressing wild-type or prefusion-stabilized RSV F in a PIV5 or PIV5ΔSH backbone. The candidates were evaluated in mice and cotton rats for immunogenicity, protection against RSV infection, and safety.
Candidates with wild-type F expressed F as a mixture of the prefusion and postfusion conformations, with a bias toward the postfusion conformation. Candidates with DS-Cav1 expressed mostly prefusion RSV F. The deletion of SH slightly increased the level of prefusion RSV F expression but did not appear to alter the ratio of prefusion and postfusion RSV F expression. We consistently detected lower levels of DS-Cav1 than wild-type F expression even when their insertion sites were the same. It is possible that this lower level of DS-Cav1 expression resulted from the lower translation efficiency of DS-Cav1 or a shorter half-life of DS-Cav1. Alternatively, the lower levels of DS-Cav1 expression could have been due to the antibodies used. To determine the levels of RSV F and DS-Cav1 expression (surface and total) in cells, we had to rely on monoclonal antibodies. It is possible that these antibodies did not recognize DS-Cav1 in the same way that they recognized wild-type F.
Recent studies have shown that antibodies against the prefusion conformation of RSV F are responsible for the majority of the neutralizing activity (29). Therefore, we initially hypothesized that candidates expressing the prefusion-stabilized F construct DS-Cav1 would induce the best neutralizing antibody response. However, PIV5-RSV-F (SH-HN), which expressed wild-type F, elicited the highest neutralizing antibody titers in the mouse model. This virus induced similar levels of anti-prefusion F IgG but larger amounts of anti-postfusion F IgG than the DS-Cav1-expressing counterpart. While antibodies against prefusion F account for the majority of neutralizing activity, antibodies against postfusion F are also important. Palivizumab and motavizumab, two highly neutralizing antibodies against RSV F, recognize epitopes that are exposed in both the prefusion and postfusion conformations (38, 39). The higher neutralizing antibody titers in PIV5-RSV-F (SH-HN)-vaccinated mice may have been due to the increased levels of anti-postfusion F antibodies, indicating that postfusion-specific antibodies are protective. Regardless of the in vitro neutralizing antibody titers, all of the PIV5-vectored vaccine candidates induced potent lower respiratory tract protection against RSV challenge in mice.
The vaccine candidates were also evaluated in cotton rats, which are more permissive to RSV infection and allowed examination of upper and lower respiratory tract protection against challenge. All PIV5 or PIV5ΔSH candidates expressing prefusion or postfusion RSV F were highly immunogenic and protective when administered via the intranasal route. The greatest reductions in viral load were observed in the lower respiratory tract, but the candidates also reduced the upper respiratory tract viral load. However, modification did not appear to improve the reduction of the viral load in the upper respiratory tract. Similar to the results of the mouse study, the prefusion F-expressing candidates induced lower IgG titers against postfusion F than the other candidates. Overall, there was no evidence of prefusion RSV F being more immunogenic or protective than wild-type RSV F in our PIV5-based vaccine candidates in the mouse or cotton rat studies.
Liang et al. (33) recently published the results of studies examining the immunogenicity and efficacy of live attenuated chimeric bovine/human PIV3-vectored vaccines expressing wild-type, prefusion, or postfusion RSV F. When evaluated in the hamster model, vaccine candidates expressing prefusion F were no more immunogenic or efficacious than candidates expressing wild-type F. The candidates expressing prefusion F only induced higher in vitro neutralizing antibody titers in the absence of guinea pig complement (GPC), leading the authors to conclude that these antibodies were more potent (33). In our studies, antibodies from mice immunized with candidates expressing prefusion F were only modestly neutralizing in the absence of guinea pig complement, with an average neutralizing titer of 1:4 (data not shown). While these titers were higher than those induced by wild-type F-expressing candidates under these conditions, the biological relevance of these results is unclear since complement-mediated host defense is active in the pulmonary environment. It is unlikely that vaccine-induced antibodies will be expected to function in a complement-free system (40). In the study of Liang et al., the prefusion F-expressing candidates outperformed only candidates expressing postfusion F (33). For subunit vaccines that generally express only postfusion F, there is a rationale to develop a prefusion-stabilized F mutant. However, for vectored vaccines that express wild-type F in both the prefusion and postfusion conformations, there is little evidence that stabilized prefusion F is a better antigen in our system. It is worth noting that our results may differ from those of Liang et al. (33) due to the use of different animal models: we employed the mouse and cotton rat models, whereas Liang et al. used the hamster model.
We also examined whether the PIV5ΔSH construct would induce better immunity over wild-type PIV5 when used as a vaccine vector. Previous research has shown that the deletion of SH from PIV5 improves the immunogenicity and efficacy of an intranasal PIV5-based H5N1 influenza virus vaccine (21). In the current studies with RSV, the two vectors performed similarly when administered via the intranasal route. When administered via the subcutaneous route, the wild-type PIV5 vector appeared to confer better protection against RSV challenge. The discrepancies observed between the PIV5- and PIV5ΔSH-vectored RSV and influenza virus vaccines may be due to both the route of administration and the antigen that is being expressed. In the case of RSV, PIV5 expressing wild-type RSV F appears to be the best candidate for administration via the subcutaneous route. Subcutaneous immunization with PIV5-RSV-F (SH-HN) was just as effective as intranasal immunization. The subcutaneous route is particularly advantageous for immunizing newborns because the problems associated with intranasal vaccination and nasal congestion can be avoided. There was no evidence of enhanced disease postchallenge in any of the cotton rats that received PIV5- or PIV5ΔSH-vectored vaccines intranasally or subcutaneously. Therefore, our vaccines are safe and effective candidates that can be adapted for various immunization routes.
All of the PIV5-based RSV vaccine candidates evaluated in this study were highly immunogenic and safe and provided protection against RSV challenge. Expression of a prefusion-stabilized RSV F antigen did not improve vaccine-induced immune responses or protection, suggesting that the expression of wild-type F in live viral vectors may be the best approach. Furthermore, the nature of the antigen and the route of administration should be considered when making modifications to the PIV5 vector. The findings from this study suggest that PIV5-RSV-F (SH-HN) and PIV5ΔSH-RSV-F are promising vaccine candidates that can be administered intranasally or subcutaneously.
MATERIALS AND METHODS
Cells.
Vero and HEp-2 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) plus 100 IU/ml penicillin and 100 μg/ml streptomycin (1% P/S; Mediatech Inc., Manassas, VA, USA). MDBK cells were grown in DMEM containing 5% FBS and 1% P/S. Cells were prepared 1 day prior to infection and achieved approximately 90% confluence by the following day.
Plasmids and virus rescue.
To obtain the DS-Cav1-coding sequence, the mutations described by McLellan et al. were introduced into the wild-type F sequence from RSV/A/A2 by splicing by overlap extension (31). The cDNA clones of PIV5 expressing RSV F or the DS-Cav1 mutant were constructed using previously described methods (21, 26, 34). Primer sequences are available upon request. Infectious viruses were rescued in BHK21 cells as previously described (26).
Viruses.
Recombinant PIV5 or PIV5ΔSH isolates expressing RSV F or DS-Cav1 were propagated in MDBK cells as previously described (26, 34). Briefly, MDBK cells were infected at a multiplicity of infection (MOI) of 0.01 PFU per cell in DMEM and 1% bovine serum albumin (BSA). After adsorption for 1 to 2 h, the inoculum was replaced with DMEM containing 2% FBS and 1% P/S. After 4 to 5 days of incubation at 37°C in 5% CO2, the cell medium was collected and centrifuged to remove the debris. The supernatant was mixed with 0.1 volume of 10× sucrose-phosphate-glutamate (SPG) buffer, flash-frozen in liquid nitrogen, and stored at −80°C.
The RSV/A/A2 strain used in the mouse studies was grown in Vero cells as previously described (26, 41). The RSV/A/Tracy and RSV/A/A2 strains used in the cotton rat studies were propagated in HEp-2 cells. FI-RSV (lot number 100) was produced in the mid-1960s by Pfizer Inc. and was stored and used on-site at Sigmovir, Inc.
IFAs.
Direct and indirect immunofluorescence assays (IFAs) were performed to detect total RSV F, prefusion RSV F, and postfusion RSV F in cells infected with the vaccine candidates. A549 cells seeded onto coverslips were infected with the vaccine candidates. Forty-eight hours later, the cells were fixed with 2% formaldehyde in phosphate-buffered saline (PBS) and immunostained with antibodies that bound total RSV F (palivizumab, site II specific), postfusion F (antibody 4D7, site I specific), and prefusion F (antibody D25, site φ specific). Palivizumab and the D25 antibody were directly labeled with Zenon Alexa Fluor 488 and Zenon allophycocyanin (APC) complexes, respectively, according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA). The 4D7 antibody was indirectly labeled with goat anti-mouse IgG conjugated to Cy3. Samples were permeabilized by adding 0.1% saponin to the immunostaining buffers. The coverslips were mounted with ProLong Gold antifade reagent and imaged using a Nikon A1R confocal microscope (Nikon Instruments Inc., Melville, NY).
Flow cytometry.
A549 cells were infected with the vaccine candidates. Forty-eight hours later, the cells were harvested with 10 mM EDTA and immunostained with palivizumab, D25, and 4D7 antibodies conjugated to Zenon Alexa Fluor 488, Zenon phycoerythrin (PE), or Zenon APC. For surface staining, the cells were immunostained and then fixed with 1% formaldehyde. For total staining, the cells were first fixed and permeabilized with BD Cytofix/Cytoperm buffer followed by immunostaining. Since a single cell can present both pre- and postfusion F, we determined the levels of protein expression based on the percentage of RSV F-positive cells that were positive for prefusion RSV F, postfusion RSV F, or both. Flow cytometry was performed using a BD LDR II flow cytometer (Franklin Lakes, NJ).
Recombinant PIV5 growth curves.
Six-well plates of Vero cells were infected with PIV5 or the vaccine candidates at an MOI of 0.01 PFU per cell. After adsorption for 1 to 2 h at 37°C in 5% CO2, the inocula were replaced with 2 ml of DMEM supplemented with 2% FBS and 1% P/S. One-hundred-microliter samples of supernatant were collected at 0, 24, 48, 72, 96, and 120 h postinfection. Each sample was mixed with 0.1 volume of 10× SPG buffer, flash-frozen in liquid nitrogen, and stored at −80°C. The virus titers in the samples were quantified by plaque assay. There were three replicates for each virus.
Plaque assays.
PIV5 plaque assays were performed as previously described (23). Briefly, 10-fold serial dilutions were prepared in DMEM with 1% BSA. One hundred microliters of each dilution was transferred to 6-well plates of BHK21 cells in a total infection volume of 1 ml. After adsorption for 1 to 2 h at 37°C in 5% CO2, the inocula were aspirated and cell monolayers were overlaid with DMEM containing 10% tryptose phosphate broth (TPB), 2% FBS, 1% P/S, and 1% low-melting-point agarose. After 5 days, the cells were fixed with 2% formaldehyde, the overlays were removed, and the cells were stained with crystal violet to visualize the plaques.
For mouse studies, RSV plaque assays were performed in Vero cells and plaques were visualized by immunostaining with RSV F-specific antibody as previously described (26, 42). For cotton rat studies, plaque assays were performed in HEp-2 cells and plaques were visualized by staining with crystal violet.
Mouse studies.
All mouse studies were performed according to the protocols approved by the Institutional Animal Care and Use Committee at the University of Georgia. Six- to 8-week-old female BALB/c mice were anesthetized by intraperitoneal injection of 200 μl of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin). The mice were intranasally immunized with 106 PFU of the vaccine candidates or RSV/A/A2 in 100-μl volumes. Mice treated intranasally with 100 μl of PBS served as negative controls. At 21 days postimmunization, serum samples were collected via cheek bleed. At 28 days postimmunization, the mice were challenged with 106 PFU of RSV/A/A2 in a 100-μl volume. At 4 and 6 days after challenge, mice were humanely sacrificed and lungs were collected to determine the viral burden as previously described (26, 42).
For ELISpot experiments, mice were sacrificed at 28 days postimmunization and spleens were harvested for splenocyte isolation.
Cotton rat study 1: comparing PIV5-RSV-pF/F (SH-HN) and PIV5ΔSH-RSV-pF/F.
All experiments were performed in accordance with guidelines set forth by the National Institutes of Health and the United States Department of Agriculture, using protocols approved by the Baylor College of Medicine Investigational Animal Care and Use Committee. Thirty cotton rats (Sigmodon hispidus) were divided into six groups consisting of 5 animals each. The animals were lightly anesthetized with isoflurane and intranasally immunized with 103 PFU of PIV5-RSV-F, PIV5-RSV-pF, PIV5ΔSH-RSV-F, or PIV5ΔSH-RSV-pF in 100-μl volumes. An additional group was intranasally immunized with 102 PFU of PIV5ΔSH-RSV-pF. Cotton rats treated with 100 μl of PBS served as negative controls. Serum samples were collected at 21 and 28 days after immunization and 4 days after challenge for serological studies. Animals were challenged on day 28 with 1.21 × 105 PFU of RSV/A/Tracy in 100 μl. Four days later, the animals were euthanized by CO2 asphyxiation and the lungs and noses were lavaged to determine the viral burden.
Cotton rat study 2: comparing PIV5-RSV-F (SH-HN) and PIV5ΔSH-RSV-F safety and efficacy using different routes of administration.
Cotton rats were maintained and handled according to the National Institutes of Health guidelines and protocols approved by the Sigmovir Institutional Animal Care and Use Committee.
Fifty 6- to 8-week-old inbred female cotton rats were divided into 10 groups containing 5 animals each. The animals were anesthetized and then immunized intranasally, subcutaneously, or intramuscularly, as indicated in Fig. 7 to 9. Sham-vaccinated groups and the FI-RSV-vaccinated group were boosted 28 days after the primary immunization. All other groups received only a single dose on day 0. Serum samples were collected 28 and 49 days after the primary immunization. Animals were also challenged on day 49 with 105 PFU of RSV/A/A2. At 5 days after challenge, the cotton rats were euthanized by CO2 asphyxiation. Nasal tissue and the left lung section were harvested, homogenized in Hanks' balanced salt solution (HBSS) and 10% SPG buffer, and used for virus titration. The right lung section was inflated with 10% formalin for histopathology. The lingular lung segments were flash frozen for RNA isolation and qPCR.
Anti-RSV-F IgG ELISA.
Antibodies against prefusion and postfusion conformations of RSV F were measured by indirect ELISA. Immulon 2HB 96-well microtiter plates were coated with 50 μl of purified prefusion or postfusion RSV F at 2 μg/ml in PBS and incubated overnight at 4°C. The proteins were expressed and purified as described previously (43). After the plates were blocked in blocking buffer for 1 h (3% nonfat dry milk dissolved in PBS containing 0.05% Tween 20 [PBST]), 2-fold serial dilutions of serum were made in blocking buffer. One hundred microliters of each dilution was transferred to the plates and the plates were incubated for 2 h at room temperature. The plates were washed with PBST, and 50 μl of diluted secondary antibody (horseradish peroxidase [HRP]-conjugated goat anti-mouse IgG or HRP-conjugated chicken anti-cotton rat IgG; 1:2,500) was added to each well, followed by incubation for 1 h at room temperature. The plates were washed and developed with 100 μl/well of SureBlue Reserve tetramethylbenzidine (TMB) substrate at room temperature. The reaction was stopped with 100 μl/well of 1 N HCl after 5 to 7 min. The optical density (OD) at 450 nm was read using a BioTek Epoch microplate reader. The endpoint antibody titer was defined as the highest serum dilution at which the OD was greater than 2 standard deviations above the mean OD for the naive serum.
For cotton rat study 2, ELISA plates were coated with purified RSV F protein extracted from RSV/A/A2-infected HEp-2 cells. The plates were blocked, washed, and then incubated with 4-fold serial dilutions of serum for 1 h at room temperature. The plates were then incubated with rabbit anti-cotton rat IgG (1:4,000) for 1 h at room temperature, followed by incubation with HRP-conjugated goat anti-rabbit IgG (1:4,000) for 1 h at room temperature. The plates were developed with TMB substrate for 15 min, and the reaction was stopped with TMB-Stop solution. The optical density at 450 nm was read.
Neutralization assays. (i) Mouse study: plaque reduction assay.
Mouse neutralizing antibody titers were determined by plaque reduction assay. Serum samples were heat inactivated at 56°C for 30 min. Twofold serial dilutions of samples were prepared in Opti-MEM I medium supplemented with 2% FBS, 1% P/S, and 5% guinea pig complement (GPC). The samples were incubated with 150 PFU/25 μl of RSV/A/A2 for 1 h at 37°C in 5% CO2, transferred to Vero cell monolayers, adsorbed for 1 h, and overlaid with 0.8% methylcellulose medium. After incubation for 7 days, the cells were fixed in 60% acetone–40% methanol and immunostained to visualize the plaques. The neutralizing antibody titers were defined as the highest serum dilution at which 50% neutralization of the input virus control was achieved.
(ii) Cotton rat study 1: microneutralization assay.
Cotton rat neutralizing antibodies from study 1 were determined by microneutralization assay. Twofold serial dilutions of heat-inactivated serum were incubated with RSV/A/Tracy or RSV/B/18537, transferred to HEp-2 cell monolayers, and incubated at 37°C in 5% CO2. The cells were fixed with 10% neutral buffered formalin and stained with crystal violet to visualize the cytopathic effect. The neutralizing antibody titer was defined as the highest serum dilution at which a 50% reduction in the cytopathic effect (CPE) was observed.
(iii) Cotton rat study 2: plaque reduction assay.
Cotton rat neutralizing antibody titers from study 2 were measured by plaque reduction assay. Fourfold dilutions of heat-inactivated serum were incubated with 25 to 50 PFU of RSV/A/A2 for 1 h at room temperature. Serum-virus mixtures were transferred to HEp-2 cell monolayers, adsorbed for 1 h, and overlaid with 0.75% methylcellulose medium. After 4 days at 37°C in 5% CO2, the cells were fixed and stained with crystal violet to visualize the plaques. The neutralizing antibody titer was determined at the 60% reduction endpoint of the virus control using the statistical program plqard.manual.entry.
ELISpot assay.
At 28 days postimmunization, spleens were harvested from the mice and pressed through 70-μm-mesh-size strainers into HBSS to isolate splenocytes. The cells were collected by centrifugation, treated with red blood cell lysis buffer (0.15 M NH4Cl, 1 M KHCO3, 0.1 mM Na2EDTA [ACK buffer]), washed with HBSS, and resuspended in complete tumor medium (CTM).
The BD mouse IFN-γ ELISpot assay set was used to measure IFN-γ-secreting splenocytes from vaccinated mice. One day prior to performing the assay, ELISpot assay plates were activated with 70% ethanol, washed with water, and coated with capture antibody overnight at 4°C. The next day, the plates were washed and blocked with CTM for at least 2 h at room temperature. The blocking solution was discarded, and peptides were added to the wells (200 ng of peptide/50 μl of CTM/well). The peptides used were RSV F (amino acids 85 to 93) and GFP (amino acids 200 to 208). Phorbol myristate acetate (PMA)-ionomycin was used to nonspecifically stimulate the cells. Cells (2.5 × 105 cells/50 μl) were added to each well, and the plates were incubated for 48 h at 37°C in 5% CO2. The plates were then immunostained according to the manufacturer's instructions, and the spots were enumerated using an ImmunoSpot analyzer.
Quantitative real-time PCR (qPCR).
Total RNA was extracted from lung tissue using an RNeasy kit (Qiagen). To prepare cDNA, 1 μg of total RNA was combined with SuperScript II reverse transcriptase (Invitrogen) and oligo(dT) primer. Real-time PCR mixtures were prepared using Bio-Rad iQ SYBR green supermix and 0.5 μM primer. The reactions were run on a Bio-Rad iCycler instrument, and baseline cycle and cycle threshold (CT) values were calculated using the PCR baseline-subtracted curve fit mode of iQ5 software. Quantitation of the relative amount of DNA was applied to all samples. Standard curves were generated using serially diluted cDNA that had the highest levels of the transcript of interest. CT values were plotted against the log10 cDNA dilution factor. The standard curves were used to convert the CT values from the experimental samples to relative expression units. The relative expression units were then normalized to the level of β-actin mRNA in the corresponding sample.
Histology.
Lungs were dissected, inflated with 10% neutral buffered formalin, and then immersed in the same fixative. The lungs were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Peribronchiolitis, perivasculitis, interstitial pneumonia, and alveolitis were scored in a blind fashion on a severity scale ranging from 0 to 4. The scores were then converted to a 0 to 100% histopathology scale.
Statistics.
Statistical analysis was performed using GraphPad Prism software (version 6 for Macintosh; GraphPad Software, La Jolla, CA). Analysis of variance (ANOVA) followed by Tukey multiple-comparison tests were used to analyze the level of RSV F expression, antibody titers, and challenge viral loads.
ACKNOWLEDGMENTS
We thank all members of Biao He's laboratory for their helpful discussions and technical assistance. Additionally, we thank Patricia Jorquera from Ralph Tripp's laboratory for the RSV and GFP peptides.
We also thank the teams at the Baylor College of Medicine and Sigmovir Biosystems, Inc., for carrying out the cotton rat studies under RDB/DMID/NIAID/NIH contracts HHSN272201000004I and HHSN272201000006I, respectively.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/JVI.00559-17.
REFERENCES
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 4.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 89:435–448. [DOI] [PubMed] [Google Scholar]
- 5.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 6.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 7.Karron RA, Buchholz UJ, Collins PL. 2013. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. 2009. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J 28:655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 9.Power UF, Plotnicky-Gilquin H, Huss T, Robert A, Trudel M, Ståhl S, Uhlén M, Nguyen TN, Binz H. 1997. Induction of protective immunity in rodents by vaccination with a prokaryotically expressed recombinant fusion protein containing a respiratory syncytial virus G protein fragment. Virology 230:155–166. doi: 10.1006/viro.1997.8465. [DOI] [PubMed] [Google Scholar]
- 10.Quan F-S, Kim Y, Lee S, Yi H, Kang S-M, Bozja J, Moore ML, Compans RW. 2011. Viruslike particle vaccine induces protection against respiratory syncytial virus infection in mice. J Infect Dis 204:987–995. doi: 10.1093/infdis/jir474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. 2013. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 31(Suppl 2):B209–B215. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamb R, Kolakofsky D. 2001. Paramyxoviridae: the viruses and their replication. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 13.Binn LN, Eddy GA, Lazar EC, Helms J, Murnane T. 1967. Viruses recovered from laboratory dogs with respiratory disease. Proc Soc Exp Biol Med 126:140–145. doi: 10.3181/00379727-126-32386. [DOI] [PubMed] [Google Scholar]
- 14.Azetaka M, Konishi S. 1988. Kennel cough complex: confirmation and analysis of the outbreak in Japan. Nippon Juigaku Zasshi 50:851–858. doi: 10.1292/jvms1939.50.851. [DOI] [PubMed] [Google Scholar]
- 15.Cornwell HJ, McCandlish IA, Thompson H, Laird HM, Wright NG. 1976. Isolation of parainfluenza virus SV5 from dogs with respiratory disease. Vet Rec 98:301–302. doi: 10.1136/vr.98.15.301. [DOI] [PubMed] [Google Scholar]
- 16.McCandlish IA, Thompson H, Cornwell HJ, Wright NG. 1978. A study of dogs with kennel cough. Vet Rec 102:293–301. doi: 10.1136/vr.102.14.293. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg FJ, Lief FS, Todd JD, Reif JS. 1971. Studies of canine respiratory viruses. I. Experimental infection of dogs with an SV5-like canine parainfluenza agent. Am J Epidemiol 94:147–165. [DOI] [PubMed] [Google Scholar]
- 18.Tompkins SM, Lin Y, Leser GP, Kramer KA, Haas DL, Howerth EW, Xu J, Kennett MJ, Durbin RK, Durbin JE, Tripp R, Lamb RA, He B. 2007. Recombinant parainfluenza virus 5 (PIV5) expressing the influenza A virus hemagglutinin provides immunity in mice to influenza A virus challenge. Virology 362:139–150. doi: 10.1016/j.virol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Mooney A, Gabbard JD, Gao X, Xu P, Place RJ, Hogan RJ, Tompkins SM, He B. 2013. Recombinant parainfluenza virus 5 expressing HA of influenza A virus H5N1 protected mice against lethal high pathogenic avian influenza H5N1 challenge. J Virol 87:354–362. doi: 10.1128/JVI.02321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Gabbard JD, Mooney A, Gao X, Chen Z, Place RJ, Tompkins SM, He B. 2013. Single dose vaccination of a recombinant parainfluenza virus 5 expressing NP from H5N1 provides broad immunity against influenza A viruses. J Virol 87:5985–5993. doi: 10.1128/JVI.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Gabbard JD, Mooney A, Chen Z, Tompkins SM, He B. 2013. Efficacy of parainfluenza virus 5 mutants expressing hemagglutinin from H5N1 influenza A virus in mice. J Virol 87:9604–9609. doi: 10.1128/JVI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Gabbard J, Johnson S, Dlugolenski D, Phan S, Tompkins M, He B. 2015. Efficacy of a parainfluenza virus 5 (PIV5)-based H7N9 vaccine in mice and guinea pigs: antibody titer towards HA was not a good indicator for protection. PLoS One 10:e0120355. doi: 10.1371/journal.pone.0120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Zhou M, Gao X, Zhang G, Ren G, Gnanadurai CW, Fu ZF, He B. 2013. A novel rabies vaccine based on a recombinant parainfluenza virus 5 expressing rabies virus glycoprotein. J Virol 87:2986–2993. doi: 10.1128/JVI.02886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Gupta T, Xu P, Phan S, Pickar A, Yau W, Karls RK, Quinn FD, Sakamoto K, He B. 2015. Efficacy of parainfluenza virus 5 (PIV5)-based tuberculosis vaccines in mice. Vaccine 33:7217–7224. doi: 10.1016/j.vaccine.2015.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mooney AJ, Li Z, Gabbard JD, He B, Tompkins SM. 2013. Recombinant parainfluenza virus 5 vaccine encoding the influenza virus hemagglutinin protects against H5N1 highly pathogenic avian influenza virus infection following intranasal or intramuscular vaccination of BALB/c mice. J Virol 87:363–371. doi: 10.1128/JVI.02330-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phan SI, Chen Z, Xu P, Li Z, Gao X, Foster SL, Teng MN, Tripp RA, Sakamoto K, He B. 2014. A respiratory syncytial virus (RSV) vaccine based on parainfluenza virus 5 (PIV5). Vaccine 32:3050–3057. doi: 10.1016/j.vaccine.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Phan S, DiStefano DJ, Citron MP, Callahan CL, Indrawati L, Dubey SA, Heidecker GJ, Govindarajan D, Liang X, He B, Espeseth AS. 2017. A single-dose recombinant parainfluenza virus 5-expressing respiratory syncytial virus (RSV) F or G protein protected cotton rats and African green monkeys from RSV challenge. J Virol 91:e00066-17. doi: 10.1128/JVI.00066-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Bright AC, Rothermel TA, He B. 2003. Induction of apoptosis by paramyxovirus simian virus 5 lacking a small hydrophobic gene. J Virol 77:3371–3383. doi: 10.1128/JVI.77.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magro M, Mas V, Chappell K, Vázquez M, Cano O, Luque D, Terrón MC, Melero JA, Palomo C. 2012. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A 109:3089–3094. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Yassine HM, Moin SM, Killikelly AM, Chuang G-Y, Druz A, Georgiev IS, Rundlet EJ, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Nason MC, Capella C, Peeples ME, Ledgerwood JE, McLellan JS, Kwong PD, Graham BS. 2015. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 7:309ra162. doi: 10.1126/scitranslmed.aac4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, Kumar A, Modjarrad K, Zheng Z, Zhao M, Xia N, Kwong PD, Graham BS. 2013. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 340:1113–1117. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GBE, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang G-Y, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko S-Y, Todd J-P, Rao S, Graham BS, Kwong PD. 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang B, Surman S, Amaro-Carambot E, Kabatova B, Mackow N, Lingemann M, Yang L, McLellan JS, Graham BS, Kwong PD, Schaap-Nutt A, Collins PL, Munir S. 2015. Enhanced neutralizing antibody response induced by respiratory syncytial virus prefusion F protein expressed by a vaccine candidate. J Virol 89:9499–9510. doi: 10.1128/JVI.01373-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He B, Paterson RG, Ward CD, Lamb RA. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237:249–260. doi: 10.1006/viro.1997.8801. [DOI] [PubMed] [Google Scholar]
- 35.Phan SI, Adam CM, Chen Z, Citron MP, Liang X, Espeseth AS, Wang D, He B. 2017. Genetic stability of parainfluenza virus 5-vectored human respiratory syncytial virus vaccine candidates after in vitro and in vivo passage. J Virol 91:e00559-17. doi: 10.1128/JVI.00559-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boukhvalova MS, Prince GA, Blanco JCG. 2009. The cotton rat model of respiratory viral infections. Biologicals 37:152–159. doi: 10.1016/j.biologicals.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol 151:2032–2040. [PubMed] [Google Scholar]
- 38.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci U S A 108:9619–9624. doi: 10.1073/pnas.1106536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McLellan JS, Yang Y, Graham BS, Kwong PD. 2011. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol 85:7788–7796. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watford WT, Ghio AJ, Wright JR. 2000. Complement-mediated host defense in the lung. Am J Physiol Lung Cell Mol Physiol 279:L790–L798. [DOI] [PubMed] [Google Scholar]
- 41.Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine. Proc Natl Acad Sci U S A 92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol 72:2871–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn JA, Durr E, Swoyer R, Cejas PJ, Horton MS, Galli JD, Cosmi SA, Espeseth AS, Bett AJ, Zhang L. 2016. Stability characterization of a vaccine antigen based on the respiratory syncytial virus fusion glycoprotein. PLoS One 11:e0164789. doi: 10.1371/journal.pone.0164789. [DOI] [PMC free article] [PubMed] [Google Scholar]