ABSTRACT
We previously demonstrated that the combination of synthetic small-molecule Toll-like receptor 4 (TLR4) and TLR7 ligands is a potent adjuvant for recombinant influenza virus hemagglutinin, inducing rapid and sustained immunity that is protective against influenza viruses in homologous, heterologous, and heterosubtypic murine challenge models. Combining the TLR4 and TLR7 ligands balances Th1 and Th2-type immune responses for long-lived cellular and neutralizing humoral immunity against the viral hemagglutinin. Here, we demonstrate that the protective response induced in mice by this combined adjuvant is dependent upon TLR4 and TLR7 signaling via myeloid differentiation primary response gene 88 (MyD88), indicating that the adjuvants function in vivo via their known receptors, with negligible off-target effects, to induce protective immunity. The combined adjuvant acts via MyD88 in both bone marrow-derived and non-bone marrow-derived radioresistant cells to induce hemagglutinin-specific antibodies and protect mice against influenza virus challenge. The protective efficacy generated by immunization with this adjuvant and recombinant hemagglutinin antigen is transferable with serum from immunized mice to recipient mice in a homologous, but not a heterologous, H1N1 viral challenge model. Depletion of CD4+ cells after an established humoral response in immunized mice does not impair protection from a homologous challenge; however, it does significantly impair recovery from a heterologous challenge virus, highlighting an important role for vaccine-induced CD4+ cells in cross-protective vaccine efficacy. The combination of the two TLR agonists allows for significant dose reductions of each component to achieve a level of protection equivalent to that afforded by either single agent at its full dose.
IMPORTANCE Development of novel adjuvants is needed to enhance immunogenicity to provide better protection from seasonal influenza virus infection and improve pandemic preparedness. We show here that several dose combinations of synthetic TLR4 and TLR7 ligands are potent adjuvants for recombinant influenza virus hemagglutinin antigen induction of humoral and cellular immunity against viral challenges. The components of the combined adjuvant work additively to enable both antigen and adjuvant dose sparing while retaining efficacy. Understanding an adjuvant's mechanism of action is a critical component for preclinical safety evaluation, and we demonstrate here that a combined TLR4 and TLR7 adjuvant signals via the appropriate receptors and the MyD88 adaptor protein. This novel adjuvant combination contributes to a more broadly protective vaccine while demonstrating an attractive safety profile.
KEYWORDS: adjuvant, influenza virus, TLR4, TLR7, Toll-like receptor, vaccine
INTRODUCTION
Influenza remains a major global health issue, and the efficacy of currently available vaccines is limited (1, 2). Influenza A subtype H1 and H3 viruses and influenza B viruses were the etiologic agents in recent seasonal outbreaks (3). Influenza A viruses were also responsible for several global pandemics in the last century, including the 1918 Spanish influenza (H1N1), 1957 Asian influenza (H2N2), 1968 Hong Kong influenza (H3N2), and the 2009 swine origin influenza (H1N1) pandemics (4). Current influenza vaccines induce humoral protection that is correlated with a hemagglutination-inhibiting (HAI) response to the immunodominant globular head (5). While the majority of neutralizing antibodies target epitopes in the globular head domain of the viral hemagglutinin (HA), its antigenic regions are highly variable and continually undergo antigenic drift, resulting in evasion of neutralization induced by natural infection and immunization (3, 5). One major focus of improved influenza vaccine development has been rational antigen composition (6–13). However, developing an appropriate adjuvant may be equally important in optimal vaccine design (14–18).
Current seasonal influenza virus vaccines are based on predictions of which influenza virus strains will circulate in the following season. Trivalent or quadrivalent vaccines containing H1N1 and H3N2 influenza A virus antigens plus one or two influenza B virus components, respectively, are the most widely distributed (6). The majority of influenza virus-associated mortality occurs in the elderly population. In 2015, the U.S. Food and Drug Administration (FDA) approved marketing for a vaccine targeted toward the elderly population; this vaccine, Fluad, was the first seasonal influenza virus vaccine containing an adjuvant (19, 20). Fluad, a trivalent vaccine produced from three influenza virus strains (two subtype A and one type B), is formulated with the adjuvant MF59 (Novartis), an oil-in-water emulsion of squalene oil. The addition of an adjuvant affords a higher level of protective antibodies in at-risk populations, such as elderly people (21).
We previously reported on a novel viral vaccine adjuvant that is comprised of two synthetic ligands for Toll-like receptor 4 (TLR4) and TLR7 (18). 1Z105 is a substituted pyrimido[5,4-b]indole compound specific for the TLR4-MD2 complex (22), and 1V270 is a phospholipid-conjugated TLR7 agonist (23, 24). The combined adjuvant was added to recombinant chimeric HA (rHA) antigens containing the highly conserved HA stalk domain, and immunized mice were protected against a heterosubtypic challenge virus (18). Heterosubtypic protection is associated with broadly reactive antibodies to HA stalk epitopes (9, 13, 17, 18, 25–29). Critically, histological examination and cytokine profiling revealed that intramuscular (i.m.) administration of 1Z105 or 1V270 resulted in a less reactogenic response than did a squalene-based adjuvant, AddaVax, which is similar to MF59 (18). Intramuscular immunization with the new vaccines did not induce substantial local or systemic inflammation; however, further preclinical testing is warranted to better understand the adjuvant's in vivo mechanism of action. Here, we evaluated the combined adjuvant for off-target effects in a series of mice with mutations, determined the correlates of protective immunity induced, and assessed different dose combinations of 1Z105 and 1V270 for additive effects to reduce toxicity by minimizing the adjuvant concentrations needed to provide complete efficacy.
RESULTS
The combined TLR4/TLR7 adjuvant promoted a reduction in lung viral titers and decreased morbidity following homologous influenza virus challenge.
Because weight loss and lung viral titers are indications of influenza severity in mice (30), we closely examined the relationship between lung viral titer, morbidity, and the adjuvants in a homologous challenge model. Briefly, wild-type (WT; BALB/c) mice were immunized with the recombinant hemagglutinin (HA) from influenza A/Puerto Rico/8/1934 (rPR/8) in combination with the same dose of synthetic adjuvant, 1V270, 1Z105, or 1V270 and 1Z105 combined, as previously reported (18). An MF59-like squalene adjuvant, AddaVax, and vehicle (rPR/8 without adjuvant) were used as comparators. Three weeks after immunization, the mice were challenged intranasally (i.n.) with homologous virus (PR/8) and monitored for morbidity, which was measured by weight loss. Six days postinfection, the mice were sacrificed and lung viral titers were assessed in a plaque assay. Mice that received 1Z105, 1V270, or the 1V270/1Z105 combination had less weight loss on days 3 to 6 than did mice that received no adjuvant (P < 0.05) (Fig. 1A) or on days 4 to 6 compared to mice that received AddaVax. Interestingly, the viral titer was significantly reduced in mice that received the 1V270/1Z105 combination (P < 0.01) or 1V270 alone (P < 0.05), but there was only a statistical trend in the mice that received 1Z105, which also trended toward more weight loss on day 6 (Fig. 1B). As expected, the reduction in morbidity correlated with viral lung titers on day 6 in individual mice (Pearson correlation coefficient r = 0.86; P < 0.0001) (Fig. 1C). These results support that the previously reported increases in antiviral antibody titers elicited with the combined adjuvant (18) are associated with the reduction in morbidity from homologous viral challenge.
FIG 1.
1V270/1Z105-induced reduction in lung viral titers correlated with decreased morbidity from influenza virus challenge. WT (BALB/c) mice (4 to 5 animals/group) were immunized with rPR/8 HA adjuvanted with 1V270/1Z105, 1V270, 1Z105, AddaVax, or no adjuvant and subsequently challenged with PR/8 virus 3 weeks after immunization. (A) Virus-challenged mice were monitored for morbidity as measured by weight loss until day 6 postinfection (DPI), when they were sacrificed. Statistical significance of weight loss was assessed by multiple t tests compared to the no-adjuvant group. (B) Lung viral titers from mice sacrificed on day 6 postinfection were quantified by plaque assays. The limit of detection for the plaque assay was 10 PFU/ml. Statistical significance was assessed by an ANOVA for comparison to the no-adjuvant group, and also with the Kruskal-Wallis test. (C) The percentage of body weight lost on day 6 postinfection was plotted against the lung viral titer for each mouse, and a linear regression curve was generated. The Pearson correlation coefficients (r) are shown. *, P < 0.05; **, P < 0.01.
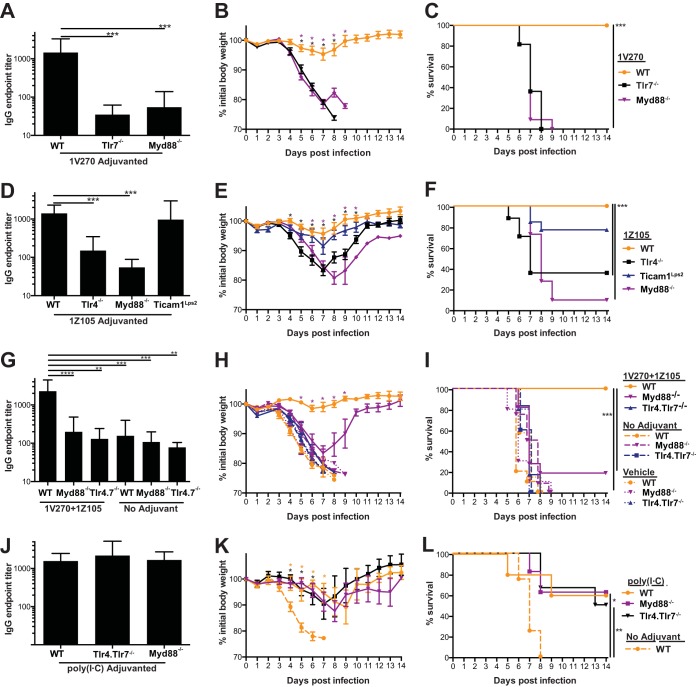
Induction of protective antiviral immunity to the combined 1V270/1Z105 adjuvant is TLR4, TLR7, and MyD88 dependent in vivo.
Although 1V270 and 1Z105 were developed as synthetic TLR7 and TLR4 agonists, respectively, the in vivo adjuvant effect may be due to off-target effects. To test if the adjuvant effect is restricted to the known receptors for these compounds, mice deficient in TLR4 and its signaling adaptors, MyD88 and TRIF, and TLR7 (which only utilizes MyD88) were immunized and assayed for antigen-specific IgG titers and monitored for morbidity and mortality to a homologous viral challenge. Hence, 1V270 (the TLR7 agonist) was tested for efficacy in WT (C57BL/6), Tlr7−/−, and Myd88−/− mice that were immunized with rPR/8 HA mixed with adjuvant or vehicle alone. After 3 to 4 weeks, the mice were bled and challenged with PR/8 virus. The WT mice generated an antibody titer significantly greater than that in Tlr7−/− or Myd88−/− mice (P < 0.001) (Fig. 2A) and were significantly protected from PR/8 virus-induced weight loss (P < 0.05) (Fig. 2B) and mortality (P < 0.001) (Fig. 2C). All of the Tlr7−/− and Myd88−/− mice immunized with 1V270 succumbed to viral challenge, indicating that TLR7 and its signaling adaptor protein MyD88 are required for 1V270 activity.
FIG 2.
The protective efficacy of the 1V270/1Z105 combined adjuvant is dependent upon TLR4, TLR7, and MyD88. WT (C57BL/6) and Tlr4−/−, Tlr7−/−, Myd88−/−, or Ticam1Lps2 mice were immunized with rPR/8 HA with adjuvant or vehicle alone 3 to 4 weeks before being bled and challenged with PR/8 virus. (A to C) WT (C57BL/6), Tlr7−/−, and Myd88−/− mice (11 to 12 animals/group) were immunized with rPR/8 HA plus 1V270. (A) Total serum IgG was quantified in an ELISA with PR/8 virus substrate, and the mice were subsequently challenged and followed for morbidity (B), based on weight loss, and mortality (C). The statistical significance of weight loss was assessed for each group compared to the WT, as indicated by the colors of the asterisks, by multiple t tests. (D to F) WT (C57BL/6), TLR4−/−, Myd88−/−, and Ticam1Lps2 mice (11 to 17 animals/group) were immunized with rPR/8 HA plus 1Z105. (D) Total serum IgG was quantified by ELISA with PR/8 virus substrate, and the mice were subsequently challenged and followed for morbidity (E), assayed by weight loss, and mortality (F). Statistical significance of weight loss was assessed for each group compared to the WT, as indicated by the colors of the symbols, by multiple t tests. (G to I) WT (C57BL/6), Myd88−/−, and Tlr4.Tlr7−/− mice (6 to 11 animals/group) were immunized with rPR/8 plus 1V270/1Z105, rPR/8 in PBS, or vehicle alone. (G) Total serum IgG was quantified by ELISA with PR/8 virus substrate, and the mice were subsequently challenged and followed for morbidity (H), assayed by weight loss, and mortality (I). Statistical significance of weight loss was assessed for each group compared to the WT by multiple t tests, and only the statistically significant differences between the WT animals immunized with 1V270/1Z105 and the Myd88−/− animals immunized with 1V270/1Z105 are indicated (purple asterisks). (J to L) WT (C57BL/6), Myd88−/−, and Tlr4.Tlr7−/− mice (5 to 6 animals/group) were immunized with rPR/8 HA plus poly(I·C). A second group of WT (C57BL/6) mice (4 animals) was immunized with rPR/8 in PBS. (J) Total serum IgG was quantified by ELISA with PR/8 virus substrate, and the mice were subsequently challenged and followed for morbidity (K), assayed by weight loss, and mortality (L). Statistical significance for IgG endpoint titers, compared to those in WT mice receiving an adjuvanted immunization, was assessed by using the Kruskal-Wallis test. Statistical significance of survival was assessed by the Mantel-Cox test to compare treated and WT mice receiving adjuvanted immunizations as indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
The TLR4 agonist (1Z105) was similarly tested in WT (C57BL/6), Tlr4−/−, TicamLps2, and Myd88−/− mice for its adjuvant effect. The WT and Ticam1Lps2 mice developed similar antibody titers (Fig. 2D); however, Tlr4 −/− and Myd88−/− mice had significantly impaired antibody responses, indicating that 1Z105 is dependent upon TLR4 signaling through MyD88, but not TRIF (P < 0.001) (Fig. 2D). Subsequent challenge with PR/8 virus resulted in significant morbidity, based on observed weight losses in the Tlr4−/− and Myd88−/− groups compared to the WT mice (P < 0.05) (Fig. 2E). Survival of Tlr4−/− and Myd88−/− mice immunized with 1Z105 was significantly impaired compared to WT mice (P < 0.001) (Fig. 2F). The Ticam1Lps2 mice maintained weight and survived, similar to WT mice, indicating that this signaling adaptor is dispensable for the response to 1Z105 (Fig. 2E and F); however, TLR4 and MyD88 are necessary for the induction of protective antiviral immunity.
The combined 1V270/1Z105 adjuvant was further examined in double-deficient Tlr4.Tlr7−/− mice and Myd88−/− mice (see Materials and Methods for further details on the mutant mouse strains used in this study). With the combined adjuvant, the antibody response and protective effect against viral challenge were dependent on the presence of TLR4, TLR7, and MyD88 (Fig. 2G to I). The abilities of the Tlr4.Tlr7−/− and Myd88−/− mice to respond to adjuvanted rPR/8 HA were confirmed by using poly(I·C), an agonist for TLR3 and melanoma differentiation-associated protein-5 (MDA5), an intracellular retinoic acid-inducible gene I (RIG-I)-like receptor. Tlr4.Tlr7−/− and Myd88−/− mice immunized with rPR/8 adjuvanted with poly(I·C) generated an antibody response comparable to that in WT mice (Fig. 2J). These immunized Tlr4.Tlr7−/− and Myd88−/− mice were protected from weight loss and survived PR/8 viral challenge, similar to WT mice (Fig. 2K and L). Hence, the Tlr4.Tlr7−/− and Myd88−/− mice are capable of mounting protective antiviral immune responses with an alternative adjuvant, and the in vivo efficacy of 1V270/1Z105 is dependent upon intact TLR4 and TLR7 signaling via the MyD88 adaptor.
Bone marrow-derived and radioresistant cells contribute to combined 1V270/1Z105 induction of antigen-specific humoral immunity and protective vaccine efficacy.
To ascertain whether the protective immune responses induced by 1V270/1Z105 required MyD88 signaling in connective tissue cells at the i.m. injection site or in bone marrow (BM)-derived immune cells, we generated chimeric mice. The chimeric mice were generated with BM from WT (C57BL/6) and Myd88−/− mice and lethally irradiated recipients. Irradiated Myd88−/− mice reconstituted with WT BM were designated Myd88WT BM, irradiated WT mice that received Myd88−/− BM were labeled WTMyd88 BM, and recipients reconstituted with homologous bone marrow, namely, WTWT BM and Myd88Myd88 BM, served as controls. These mice were immunized i.m. with 1V270/1Z105-adjuvanted rPR/8 HA and assayed for HA-specific immunoglobulin production in an enzyme-linked immunosorbent assay (ELISA). The Myd88Myd88 BM mice developed significantly lower antiviral IgG titers than did WTWT BM mice (Fig. 3A) (P < 0.0001). WTMyd88 BM mice (P < 0.0001) and Myd88WT BM mice (P < 0.05) developed significantly lower IgG titers than WTWT BM mice. Myd88WT BM mice developed a significantly higher mean titer than Myd88Myd88 BM mice (P < 0.01). IgG titers in WTMyd88 BM mice trended higher than those in Myd88Myd88 BM mice, and they were not significantly different from IgG titers in Myd88WT BM mice. These results indicate that MyD88 signaling in both radioresistant and radiosensitive compartments contribute to the adjuvant-induced IgG response.
FIG 3.
Induction of IgG antibodies and protective efficacy are dependent upon MyD88 expression in bone marrow-derived and radioresistant cells. (A) BM chimeric mice, including Myd88WT BM (n = 23), WTMyd88 BM (n = 21), WTWT BM (n = 24), and Myd88Myd88 BM (n = 10) mice, were immunized with 1V270/1Z105-adjuvanted rPR/8 HA protein at days 0 and 14 and bled on day 35; sera were assayed for total IgG in an ELISA with PR/8 virus substrate. The data shown are pooled from two independent experiments. Bone marrow chimeric mice were challenged with 10 mLD50 of PR/8 virus on day 36 after the first immunization and assayed for morbidity (B), measured by body weight loss, and mortality (C) for each of the following BM chimeric groups: Myd88WT BM (n = 9), WTMyd88 BM (n = 8), WTWT BM (n = 15), and Myd88Myd88 BM (n = 5). Significant differences in body weights compared to the WTWT BM mice are indicated by the asterisk colors. (D) The IgG endpoint titer was plotted against the minimum percentage of initial body weight lost for each animal, and the Pearson correlation coefficient (r) and linear regression line are included. Statistical significance for IgG endpoint titers was assessed by the Kruskal-Wallis test. Statistical significance of weight loss was assessed by t tests adjusted for multiple comparisons. Statistical significance of survival was assessed by the Mantel-Cox test to compare WT mice receiving adjuvanted immunizations, as indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To determine the contribution of these compartments to protective vaccine efficacy, these mice were challenged with a lethal dose of PR/8 virus and followed for morbidity, assessed by weight loss (Fig. 3B), and mortality (Fig. 3C). Mice deficient in MyD88 in either radioresistant WTMyd88 BM or radiosensitive WTMyd88 BM cell pools had significantly greater weight loss (P < 0.05) and mortality (P < 0.01) than WTWT BM mice; however, they survived significantly longer than Myd88Myd88 BM mice (WTMyd88 BM, P < 0.01; Myd88WT BM, P < 0.001). The maximum percentage of body weight lost by each animal was plotted against the IgG endpoint titer (EPT; Pearson correlation coefficient r = 0.76, P < 0.001) (Fig. 3D). Again, these data support a role for MyD88 signaling in 1V270/1Z105-adjuvanted vaccines in both radioresistant and BM-derived cell populations.
IgG and CD4+ cells induced by 1V270/1Z105-adjuvanted rHA contribute to protection from viral challenge.
To formally assess the role of IgG in the protection induced by the combined adjuvant, WT (BALB/c) mice were immunized at 0 and 3 weeks with either rHA from PR/8 or influenza A/California/04/2009 (pandemic H1N1) (Cal/09) plus 1V270/1Z105 or with unadjuvanted antigen. The mice were bled 10 weeks after the first immunization, and sera were heat inactivated and passively transferred by intraperitoneal (i.p.) injection into recipient WT (BALB/c) mice. Mice were challenged with homologous PR/8 virus and followed for morbidity, assayed by weight loss (P < 0.05) (Fig. 4A) and mortality (P < 0.01) (Fig. 4B) or challenged with mouse-adapted heterologous NL/09 and followed for morbidity (P < 0.05) (Fig. 4C) and mortality (P < 0.01) (Fig. 4D). A control group of mice immunized with rCal/09 and challenged with influenza A/Netherlands/602/2009 (NL/09) was included to demonstrate homologous protection in the pandemic H1N1 model. The passive transfer of antibodies from mice immunized with 1V270/1Z105 attenuated weight loss and conferred protection from lethal infectious homologous challenge, but not from a heterologous challenge.
FIG 4.
Passive serum transfer and CD4+ cells induced by 1V270/1Z105-adjuvanted rHA contribute to protection from lethal influenza virus challenge. Immune sera were generated from mice by immunization with rPR/8 or rCal/09 HA adjuvanted with 1V270/1Z105 or no adjuvant at 0 and 3 weeks and administered to recipient mice. Mice immunized with rPR/8 were challenged with homologous PR/8 virus and followed for morbidity (A), as assayed by weight loss, and mortality (B). Mice receiving immune sera from either rPR/8- or rCal/09-vaccinated animals were challenged with NL/09 virus, such that heterologous protection was assayed using homologous protection as the control group, and these animals were followed for morbidity (C), based on body weight loss, and mortality (D). Statistical significance of weight loss was assessed by multiple t tests, comparing the 1V270/1Z015 versus no adjuvant groups (A) and the Cal/09 homologous control (C), as indicated by the colors of the asterisks. (E and F) Mice were immunized with rPR/8 HA plus 1V270/1Z105, and 3 to 4 weeks later they were treated with the MAb GK 1.5 to deplete CD4+ cells or the MAb LTF-2 (isotype) as a control. Two days after administration of the MAbs, mice were challenged with homologous PR/8 virus and assayed for morbidity, based on weight loss, and mortality (animal survival data are shown in parentheses) (E) or with heterologous NL/09 virus and assayed for morbidity by weight loss and mortality (animal survival data are shown in parentheses) (F). For the heterologous challenge, two independent experiments with 10 mice/group and 19 to 20 mice/group were conducted and combined. Statistical significance of weight loss was assessed based on multiple t tests. Statistical significance of survival was assessed with the Mantel-Cox test. *, P < 0.05; **, P < 0.01.
The previously reported protective efficacy to heterologous challenge in the 1V270/1Z105-immunized mice could not be entirely attributed to an in vivo neutralizing effect of the antibody response (18). CD4+ cells are associated with high-affinity antibody induction and isotype switching, but they also can have other effector functions. To examine if they also play a role in the observed protective efficacy, WT (BALB/c) mice were immunized with rPR/8 HA and 1V270/1Z105. Three weeks later, at the time when a protective antibody response is established, the mice were then treated with a monoclonal antibody (MAb) to deplete CD4+ cells or isotype controls. The depletion was confirmed by flow cytometry (>99% depletion) of peripheral blood cells from a separate cohort of unchallenged animals. The mice were treated with antibody at days −2 and +1 relative to challenge with homologous PR/8 virus (Fig. 4E) or heterologous NL/09 virus (Fig. 4F). Neither group of mice immunized with rPR/8 HA plus 1V270/1Z105 lost substantial weight following a lethal homologous viral challenge (Fig. 4E). Similarly, both antibody-treated groups of mice lost significant weight with the heterologous viral challenge, but the CD4+ cell-depleted mice recovered more slowly from the NL/09 infection than the isotype control-treated mice and demonstrated significantly lower body weights on days 10 and 11 postinfection (P < 0.05) (Fig. 4F). Depletion of CD4+ cells did not impair protection from a lethal homologous challenge after an antibody response had been established. However, CD4+ depletion delayed the recovery from a heterologous challenge, indicating a role for CD4+ cells when cross-reactive antibodies alone are insufficient for protection from heterologous viral exposure.
1V270 and 1Z105 work additively to induce antigen-specific IgG and protect mice from influenza virus challenge.
The above data indicated that TLR4 and TLR7 signaling via MyD88 are indispensable for the induction of influenza virus-specific antibodies and survival after vaccination with the combined adjuvant. To further study whether the combination of two agonists can provide adjuvant dose-sparing effects, WT (BALB/c) mice were immunized with rPR/8 HA and 1V270, 1Z105, or the 1V270/1Z105 combined adjuvant in graded doses. After 3 weeks, mice were bled and subsequently challenged with PR/8 virus and monitored for weight loss. Increasing doses of 1V270 or 1Z105 as individual adjuvants resulted in increased anti-HA antibodies, determined by ELISA. The log EPT was a linear function of the log dose of 1V270 (Fig. 5A). On a log scale, 1Z105 had an additive effect on 1V270 [F(3, 64), P < 0.0001]; however, 1Z105 did not change the general 1V270 dose-response relationship, apart from this additive effect [test for overall interaction, F(9, 64), P = 0.11]. A test of individual interaction terms showed that at the 2 highest dose levels of 1V270, the additive effect of 1V270 was attenuated at its highest dose (P < 0.02 for each term), consistent with a threshold effect at the highest doses. The percent body weight maintained following lethal virus challenge correlated with the endpoint titers (Fig. 5B) (Pearson correlation coefficient r = 0.91, P < 0.0001). Near-maximal endpoint titers and protection against the morbidity of viral challenge were seen in mice that received 1V270 (0.2 nmol) and 1Z105 (40 nmol), suggesting that the potential for toxicity could be reduced by minimizing the adjuvant doses in the formulation necessary to induce a fully efficacious response.
FIG 5.
1V270 and 1Z105 work additively to induce antigen-specific IgG and protect mice from morbidity after viral challenge. (A) WT BALB/c mice (4 to 5 animals/group) were immunized with rPR/8 HA with or without adjuvant at the indicated doses or with antigen only in the vehicle control group. Animals were bled 3 weeks after immunization and subsequently challenged with PR/8 virus. Antigen-specific antibody endpoint titers were determined by ELISA with the PR/8 virus substrate, and the endpoint IgG titer for all groups demonstrated a dose response to both single agents 1V270 and 1Z105. The log endpoint titer is a linear function of the log dose of 1V270 (P < 0.05), and 1Z105 has an additive effect on 1V270 (P < 0.0001). However, 1Z105 does not change the 1V270 dose-response relationship, apart from this additive effect (for the overall interaction, P = 0.11), except for the very highest doses (see the text). (B) Body weight data for each group receiving the indicated doses of adjuvant or control were plotted as a function of the endpoint titer. The percent initial body weight change on day 6 following a lethal viral challenge correlated with the endpoint titers (Pearson correlation coefficient r = 0.91, P < 0.0001).
DISCUSSION
The few FDA-approved adjuvants on the market have compelled the discovery, synthesis, and characterization of new compounds as vaccine adjuvants (16). TLR agonists have been examined in preclinical models as target-directed adjuvants (16, 31, 32). The AS04 adjuvant licensed for use in GlaxoSmithKline's Cervarix vaccine suggests that TLR4-based ligands, namely, monophosphoryl lipid A (MPLA), are safe and effective adjuvants for a recombinant viral vaccine (33). Other combinations of TLR4 and TLR7/8 adjuvants are in preclinical trials in nonhuman primates (34–36). Specific to influenza virus vaccines, we and others have reported that combinations of TLR4 and TLR7 agonists as adjuvants successfully augment protective immune responses and can also broaden the cross-protection to HA globular head antigens as well as HA stalk antigens, as evidenced in heterologous and heterosubtypic murine challenge models (18, 35). The induction of broad immunity to influenza viruses may depend upon induction of a robust humoral and cellular immune response. Here, we have demonstrated that 1V270/1Z105 induces antigen-specific humoral and cellular immunity, both of which contribute to in vivo efficacy.
In addition to the role of HAI antibodies (18) in passively transferred immune sera, we have demonstrated a role for cross-protective CD4+ cells in a rHA subunit vaccine adjuvanted with a combination of synthetic TLR4 and TLR7 ligands. CD4+ cells are known to play a role in human immunity to influenza viruses (37, 38). However, the majority of studies have reported that upon development of CD4+ immunity to internal viral proteins generated by natural infection, namely, nucleoprotein (NP) or matrix protein, inactivated vaccines or subunit vaccines target internal viral proteins rather than adjuvanted rHA. Several recent clinical trials of influenza virus vaccines coupled with adjuvants utilizing diverse mechanisms of action demonstrated that these vaccines induce CD4+ cells (39–41), and a correlation between CD4+ cell induction and the development of HAI antibodies has been reported for influenza virus vaccines adjuvanted with MF59 and Matrix-M, a saponin-based adjuvant (42, 43). In a murine model, rHA adjuvanted with glucopyranosyl lipid adjuvant, a synthetic TLR4 ligand similar to MPLA, and a stable emulsion of oil in water was dependent upon the presence of CD4+ cells at the time of immunization to induce protective immunity (14). To our knowledge, however, few mouse studies have formally evaluated the protective role of HA-specific CD4+ cells in a murine model of influenza virus immunization and challenge with CD4+ cell depletion occurring after the development of a protective immune response. Interestingly, a recent study that evaluated a trivalent virosomal vaccine adjuvanted with Matrix-M demonstrated a cross-protective role for CD4+ cells that were primarily directed against HA and the viral neuraminidase, with a smaller population of CD4+ cells specific for the viral NP (44). Here, we definitively showed that a TLR4/TLR7-adjuvanted rHA influenza virus vaccine induces CD4+ cellular responses that play an active role in protecting mice during heterologous influenza virus challenge.
Although adjuvanted vaccines may enhance immunity, regulatory agencies are appropriately concerned with vaccine safety (16, 45, 46). Here, we examined the combined adjuvant for off-target effects and systematically evaluated a reduction in dose to minimize any potential toxicities. We previously reported that the combined 1V270/1Z105 adjuvant did not induce a systemic inflammatory response, and it compared well to the squalene-based emulsion AddaVax in this regard (18); however, determining the in vivo mechanism of action is a critical step in the preclinical evaluation of novel adjuvants. With knockout mouse models, we demonstrated that 1Z105 and 1V270 are strictly dependent upon TLR4 and TLR7, respectively, signaling through MyD88 without any discernible off-target effects. The combination of small molecules targeting TLR4 and TLR7 results in the ability to substantially reduce the adjuvant dose.
Adjuvants with multiple molecular and cellular targets are desirable if efficacy results from preclinical models are to be translated into use in humans with genetically diverse backgrounds. 1V270/1Z105 works via both TLR4 and TLR7 and exerts its effects on both peripheral radioresistant cells as well as bone marrow-derived cells. Activating multiple receptors on different populations of adaptive and innate immune cells will help to improve vaccine responses in community-based populations. In particular, this strategy has great potential to overcome immune senescence in older patients, who are at the highest risk for complications from influenza virus infections. As we previously described, the TLR4:TLR7 ratio affects the balance of Th1:Th2-type immunity (18). Moving forward, optimization of the TLR4:TLR7 ratio should provide an opportunity to optimize both safety and efficacy as the compounds are formulated for clinical development, and this is an important area of research for developing next-generation adjuvanted vaccines.
MATERIALS AND METHODS
Animals.
Animal experiments performed at the University of California San Diego (UCSD), La Jolla, CA, USA, were approved by the UCSD Institutional Animal Care and Use Committee (IACUC; S00028 and S09331), and animal experiments performed at the Icahn School of Medicine at Mount Sinai (ISMMS) were approved by the ISMMS IACUC (LA13-00084). Seven- to 9-week-old C57BL/6 (WT) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Ticam1Lps2 mice were kindly provided by Bruce Beutler (University of Texas Southwestern Medical Center, Dallas, TX, USA). Ticam1 is the gene that encodes the TIR domain-containing adapter-inducing interferon-β (TRIF) protein. Tlr4−/−, Tlr7−/−, and Myd88−/− mice were gifts from Shizuo Akira (Osaka University, Osaka, Japan). These strains were backcrossed for 10 generations onto the C57BL/6 background at UCSD, and the Tlr4−/− and Tlr7−/− strains were intercrossed to generate the double-deficient mice (Tlr4.Tlr7−/−). For immunization with influenza virus antigens, 6- to 8 week-old WT female BALB/c and WT female C57BL/6 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Ticam1Lps2, Tlr4−/−, Tlr7−/−, and Myd88−/− animals for immunization with influenza virus antigens at ISMMS were purchased from the Jackson Laboratories (Bar Harbor, ME), bred in-house, and immunized at 6 to 10 weeks of age. The animals were anesthetized with ketamine-xylazine before intranasal viral infection.
Preparation of MyD88 and WT bone marrow chimeric mice.
Bone marrow chimeric mice were generated by injecting 107 bone marrow cells intravenously into whole-body-irradiated (950 cGy) recipient mice (47). The chimeric mice were immunized with rHA plus adjuvant 6 weeks after the bone marrow transfer. To evaluate chimerism, CD45.1 and CD45.2 congenic markers were used in WT and Myd88−/− mice, respectively. Five and 11 weeks after the bone marrow transfer, peripheral blood mononuclear cells from the recipient mice were stained for CD45 markers to analyze the percentage of donor cells by flow cytometry. The average chimerism percentages at 5 and 11 weeks after bone marrow transfer were determined to be 91.3% ± 3.1% and 96.4% ± 2.0% (means ± standard deviations), respectively.
Reagents.
The compounds 1Z105 and 1V270 were synthesized in the Carson laboratory at UCSD as previously described (22–24), dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) as 20 to 100 mM stock solutions, and stored at −20°C. The endotoxin levels of these compounds were determined by using Endosafe (Charles River Laboratory, Wilmington, MA, USA) and were confirmed to be less than 10 endotoxin units (EU)/μmol. AddaVax and VacciGrade high-molecular-weight (HMW) poly(I·C) were purchased from Invivogen (San Diego, CA).
Recombinant HA was prepared as previously described (48, 49). Briefly, rHA antigens derived from influenza virus A/Puerto Rico/8/1934 (H1N1) (PR/8) and A/California/04/2009 (pandemic H1N1) (Cal/09) were expressed from baculovirus vectors in High Five cells (BTI-TN-5B1-4 cells; subclone from the Vienna Institute of Biotechnology) in the laboratory as soluble trimers, by utilizing the T4 phage fibritin natural trimerization domain and a C-terminal hexahistidine tag for purification as previously described (48, 49). Protein was purified with Ni-nitrilotriacetic acid (NTA)–agarose beads (Qiagen, Hilden, Germany).
In vivo immunization studies.
BALB/c WT, C57BL/6 WT, Tlr4−/−, Tlr7−/−, Tlr4.Tlr7−/− (double knockout), Myd88−/−, and TicamLps2 mice were immunized i.m. with 1V270 (10.8 μg/injection, equivalent to 10 nmol/animal), 1Z105 (89.4 μg/injection, equivalent to 200 nmol/animal), or a combination of 1V270 (10 nmol/animal) and 1Z105 (200 nmol/animal), or at doses otherwise stated in the figure legends for the dose-response evaluation, in a total volume of 50 μl of 10% DMSO in saline (vehicle). Controls included no adjuvant (antigen in 10% DMSO in saline), vehicle, poly(I·C) [10 μg of poly(I·C) plus antigen in vehicle], and AddaVax (1:1 ratio with antigen in saline). An adjuvant-only control (10 nmol of 1V270 plus 200 nmol of 1Z105 in vehicle) was previously evaluated and found to have no effect on challenged mice relative to vehicle alone (18). All mice were immunized i.m. with rHA (2 μg rPR/8 HA per mouse, or 5 μg rPR/8 HA per mouse in the adjuvant dose-response experiment). All immunizations were delivered i.m. into the gastrocnemius muscle.
Murine influenza virus challenge.
Mouse-adapted (via serial passage through murine lungs in work performed before the Gain-of-Function Moratorium) A/Netherlands/602/2009 (H1N1) (NL/09) and A/Puerto Rico/8/1934 (H1N1) (PR/8) were grown in 8- to 10-day-old embryonated chicken eggs. The murine 50% lethal doses (mLD50s) were 10 PFU and 30 PFU, respectively, as determined in 8- to 10-week-old female BALB/c mice. Mice were anesthetized with ketamine-xylazine and infected i.n. in a volume of 50 μl of phosphate-buffered saline (PBS) with 100 PFU of NL/09 or 300 PFU of PR/8. All challenged animals were monitored daily for body weight and were sacrificed when they reached IACUC-approved endpoints.
Passive serum transfer study.
For the passive serum transfer study, 6- to 8-week-old WT BALB/c female mice were immunized at 0 and 3 weeks with rPR/8 HA plus 1V270/1Z105 or rPR/8 HA in vehicle. Mice were bled 10 weeks after the first immunization, and sera were purified via microcentrifugation. Sera were heat inactivated at 56°C, and 200-μl aliquots of sera were transferred via i.p. injection to naive 6- to 8-week-old BALB/c female mice. The mice were challenged with 10 mLD50 of PR/8 virus 3 h after passive serum transfer and monitored daily for body weight changes.
CD4+ cell depletion study.
CD4+ cells were depleted after vaccination. Briefly, BALB/c mice were immunized with rPR/8 HA plus 1V270/1Z105, and 3 to 4 weeks later the monoclonal antibody (MAb) GK 1.5 (200 μg/injection; BioXCell, West Lebanon, NH) was administered i.p. on days −2 and +1 relative to the virus challenge (day 0) to deplete CD4+ cells. The MAb LTF-2 (BioXCell) was used as an isotype control. Mice were challenged with homologous PR/8 virus or heterologous NL/09 virus and assayed for morbidity, based on weight loss, and mortality.
Measurement of antigen-specific antibodies.
Anti-influenza virus antibodies were measured in an ELISA using as the substrate PR/8 virus purified through a 30% sucrose cushion via ultracentrifugation by standard methods (18). EPTs were defined as the highest dilution of serum that resulted in a signal three times above the background level. Total IgG was detected as previously described (18).
Influenza virus titers.
Influenza virus titers were determined in MDCK cells via a standard plaque assay, described previously (50).
Statistical analysis.
For continuous outcomes, the data are presented as mean results and standard errors of the means (SEM). One-way analysis of variance (ANOVA) with Dunnett's post hoc test was used to compare multiple groups to a control group. Pearson's correlation tests were used to assess the linear association between two continuous variables. For the influenza virus ELISA endpoint titer data, the Kruskal-Wallis test with Dunn's correction for multiple comparisons was used to assess significance of differences compared to responses in WT animals that received immunizations with the indicated adjuvant. For weight loss curves, t tests correcting for multiple comparisons by the Holm-Sidak method were used to compare each day's weight to the indicated control group, and no adjustment was made for performing comparisons on multiple days for the same endpoints. For survival, Kaplan-Meier curves were plotted, and the log rank test was performed to assess if there was a significant difference in a survival outcome between groups. A two-way ANOVA model was applied to assess the dose-response relationship between an endpoint and the compounds; additive effects were assessed by testing the significance of the main effects, and a synergistic effect was assessed by testing the interaction effect of the two compounds. Prism 6 statistical software (GraphPad Software) and R (version 3.1.0; http://www.r-project.org) were used to obtain P values for comparisons between groups (a P value of <0.05 was considered significant).
ACKNOWLEDGMENTS
We acknowledge the NIH Adjuvant Discovery Program for funding (HHSN272200900034C and HHSN272201400051C [Carson] and HHSN272200900032C [Palese]). We are also grateful to the NIH/NIAID CEIRS CRIP program (HHSN272201400008C [Palese and Krammer]) and the Mount Sinai Medical Scientist Training Program Training Grant (T32 GM007280-41 [Margaret H. Baron]) for additional funding.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We thank Christa Caneda, Alast Ahmadi, and Shannon Shueyin Zhang for technical assistance.
REFERENCES
- 1.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. 2013. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 56:1363–1369. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Comanor L, Shay DK. 2006. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis 194:S82–S91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- 3.Hannoun C. 2013. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines 12:1085–1094. doi: 10.1586/14760584.2013.824709. [DOI] [PubMed] [Google Scholar]
- 4.Smith GJ, Bahl J, Vijaykrishna D, Zhang J, Poon LL, Chen H, Webster RG, Peiris JS, Guan Y. 2009. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A 106:11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox RJ. 2013. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother 9:405–408. doi: 10.4161/hv.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, Gust ID, Hampson AW, Hay AJ, Hurt AC, de Jong JC, Kelso A, Klimov AI, Kageyama T, Komadina N, Lapedes AS, Lin YP, Mosterin A, Obuchi M, Odagiri T, Osterhaus ADME, Rimmelzwaan GF, Shaw MW, Skepner E, Stohr K, Tashiro M, Fouchier RAM, Smith DJ. 2008. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 26:D31–D34. doi: 10.1016/j.vaccine.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 7.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1:e00018-10. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia-Sastre A, Moran TM, Palese P. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A 107:18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. 2014. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol 88:2340–2343. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krammer F, Palese P. 2014. Universal influenza virus vaccines: need for clinical trials. Nat Immunol 15:3–5. doi: 10.1038/nri3797. [DOI] [PubMed] [Google Scholar]
- 11.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F, Palese P, Steel J. 2015. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol 386:301–321. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- 13.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg CH, Roque R, Hoeven NV, Perrone L, Baldwin SL, Rininger JA, Bowen RA, Reed SG. 2012. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A 109:17585–17590. doi: 10.1073/pnas.1207308109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Gil L, Goff PH, Hai R, Garcia-Sastre A, Shaw ML, Palese P. 2013. A Sendai virus-derived RNA agonist of RIG-I as a virus vaccine adjuvant. J Virol 87:1290–1300. doi: 10.1128/JVI.02338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed SG, Orr MT, Fox CB. 2013. Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 17.Goff PH, Eggink D, Seibert CW, Hai R, Martinez-Gil L, Krammer F, Palese P. 2013. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One 8:e79194. doi: 10.1371/journal.pone.0079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goff PH, Hayashi T, Martinez-Gil L, Corr M, Crain B, Yao S, Cottam HB, Chan M, Ramos I, Eggink D, Heshmati M, Krammer F, Messer K, Pu M, Fernandez-Sesma A, Palese P, Carson DA. 2015. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J Virol 89:3221–3235. doi: 10.1128/JVI.03337-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. 2011. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines 10:447–462. doi: 10.1586/erv.11.23. [DOI] [PubMed] [Google Scholar]
- 20.Camilloni B, Basileo M, Valente S, Nunzi E, Iorio AM. 2015. Immunogenicity of intramuscular MF59-adjuvanted and intradermal administered influenza enhanced vaccines in subjects aged over 60: a literature review. Hum Vaccin Immunother 11:553–563. doi: 10.1080/21645515.2015.1011562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Buynder PG, Konrad S, Van Buynder JL, Brodkin E, Krajden M, Ramler G, Bigham M. 2013. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine 31:6122–6128. doi: 10.1016/j.vaccine.2013.07.059. [DOI] [PubMed] [Google Scholar]
- 22.Chan M, Hayashi T, Mathewson RD, Nour A, Hayashi Y, Yao S, Tawatao RI, Crain B, Tsigelny IF, Kouznetsova VL, Messer K, Pu M, Corr M, Carson DA, Cottam HB. 2013. Identification of substituted pyrimido[5,4-b]indoles as selective Toll-like receptor 4 ligands. J Med Chem 56:4206–4223. doi: 10.1021/jm301694x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan M, Hayashi T, Kuy CS, Gray CS, Wu CC, Corr M, Wrasidlo W, Cottam HB, Carson DA. 2009. Synthesis and immunological characterization of toll-like receptor 7 agonistic conjugates. Bioconjug Chem 20:1194–1200. doi: 10.1021/bc900054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan M, Hayashi T, Mathewson RD, Yao S, Gray C, Tawatao RI, Kalenian K, Zhang Y, Hayashi Y, Lao FS, Cottam HB, Carson DA. 2011. Synthesis and characterization of PEGylated toll like receptor 7 ligands. Bioconjug Chem 22:445–454. doi: 10.1021/bc1004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krammer F, Pica N, Hai R, Tan GS, Palese P. 2012. Hemagglutinin stalk-reactive antibodies are boosted following sequential infection with seasonal and pandemic H1N1 influenza virus in mice. J Virol 86:10302–10307. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, Garcia-Sastre A, Palese P, Treanor JJ, Krammer F. 2013. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol 87:4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, Garcia-Sastre A, Palese P. 2013. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 87:10435–10446. doi: 10.1128/JVI.01715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reference deleted.
- 29.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, Garcia-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin ME, Dieter JA, Luo Z, Baumgarth N, Solnick JV. 2012. Predicting the outcome of infectious diseases: variability among inbred mice as a new and powerful tool for biomarker discovery. mBio 3:e00199-12. doi: 10.1128/mBio.00199-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. 2011. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol 1:226–232. doi: 10.1016/j.coviro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Akira S. 2011. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci 366:2748–2755. doi: 10.1098/rstb.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, Salmerón J, McNeil S, Stapleton JT, Bouchard C, Martens MG, Money DM, Quek SC, Romanowski B, Vallejos CS, ter Harmsel B, Prilepskaya V, Fong KL, Kitchener H, Minkina G, Lim YKT, Stoney T, Chakhtoura N, Cruickshank ME, Savicheva A, da Silva DP, Ferguson M, Molijn AC, Quint WGV, Hardt K, Descamps D, Suryakiran PV, Karkada N, Geeraerts B, Dubin G, Struyf F. 2016. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis 16:1154–1168. doi: 10.1016/S1473-3099(16)30120-7. [DOI] [PubMed] [Google Scholar]
- 34.Kasturi SP, Kozlowski PA, Nakaya HI, Burger MC, Russo P, Pham M, Kovalenkov Y, Silveira EL, Havenar-Daughton C, Burton SL, Kilgore KM, Johnson MJ, Nabi R, Legere T, Sher ZJ, Chen X, Amara RR, Hunter E, Bosinger SE, Spearman P, Crotty S, Villinger F, Derdeyn CA, Wrammert J, Pulendran B. 2017. Adjuvanting a simian immunodeficiency virus vaccine with Toll-like receptor ligands encapsulated in nanoparticles induces persistent antibody responses and enhanced protection in TRIM5α restrictive macaques. J Virol 91:e01844-16. doi: 10.1128/JVI.01844-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer SS, Gangadhara S, Victor B, Shen X, Chen X, Nabi R, Kasturi SP, Sabula MJ, Labranche CC, Reddy PB, Tomaras GD, Montefiori DC, Moss B, Spearman P, Pulendran B, Kozlowski PA, Amara RR. 2016. Virus-like particles displaying trimeric simian immunodeficiency virus (SIV) envelope gp160 enhance the breadth of DNA/modified vaccinia virus Ankara SIV vaccine-induced antibody responses in rhesus macaques. J Virol 90:8842–8854. doi: 10.1128/JVI.01163-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zens KD, Farber DL. 2015. Memory CD4 T cells in influenza. Curr Top Microbiol Immunol 386:399–421. doi: 10.1007/82_2014_401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, Staples KJ, Dong T, Douek DC, McMichael AJ, Xu XN. 2012. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med 18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 39.Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE, Patel NB, Zak DE, Aderem A, Dong T, Del Giudice G, Rappuoli R, Cerundolo V, Pollard AJ, Pulendran B, Siegrist CA. 2016. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci U S A 113:1853–1858. doi: 10.1073/pnas.1519690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Sicilia J, Aristegui J, Omenaca F, Carmona A, Tejedor JC, Merino JM, Garcia-Corbeira P, Walravens K, Bambure V, Moris P, Caplanusi A, Gillard P, Dieussaert I. 2015. Safety and persistence of the humoral and cellular immune responses induced by 2 doses of an AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine administered to infants, children and adolescents: two open, uncontrolled studies. Hum Vaccin Immunother 11:2359–2369. doi: 10.1080/21645515.2015.1063754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen GK, Sjursen H, Nostbakken JK, Jul-Larsen A, Hoschler K, Cox RJ. 2014. Matrix M(TM) adjuvanted virosomal H5N1 vaccine induces balanced Th1/Th2 CD4+ T cell responses in man. Hum Vaccin Immunother 10:2408–2416. doi: 10.4161/hv.29583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spensieri F, Siena E, Borgogni E, Zedda L, Cantisani R, Chiappini N, Schiavetti F, Rosa D, Castellino F, Montomoli E, Bodinham CL, Lewis DJ, Medini D, Bertholet S, Del Giudice G. 2016. Early rise of blood T follicular helper cell subsets and baseline immunity as predictors of persisting late functional antibody responses to vaccination in humans. PLoS One 11:e0157066. doi: 10.1371/journal.pone.0157066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pedersen GK, Madhun AS, Breakwell L, Hoschler K, Sjursen H, Pathirana RD, Goudsmit J, Cox RJ. 2012. T-helper 1 cells elicited by H5N1 vaccination predict seroprotection. J Infect Dis 206:158–166. doi: 10.1093/infdis/jis330. [DOI] [PubMed] [Google Scholar]
- 44.Cox F, Baart M, Huizingh J, Tolboom J, Dekking L, Goudsmit J, Saeland E, Radošević K. 2015. Protection against H5N1 influenza virus induced by matrix-M adjuvanted seasonal virosomal vaccine in mice requires both antibodies and T cells. PLoS One 10:e0145243. doi: 10.1371/journal.pone.0145243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmed SS, Schur PH, MacDonald NE, Steinman L. 2014. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun 50:1–11. doi: 10.1016/j.jaut.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 46.Fox CB, Haensler J. 2013. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines 12:747–758. doi: 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi T, Gray CS, Chan M, Tawatao RI, Ronacher L, McGargill MA, Datta SK, Carson DA, Corr M. 2009. Prevention of autoimmune disease by induction of tolerance to Toll-like receptor 7. Proc Natl Acad Sci U S A 106:2764–2769. doi: 10.1073/pnas.0813037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krammer F, Grabherr R. 2010. Alternative influenza vaccines made by insect cells. Trends Mol Med 16:313–320. doi: 10.1016/j.molmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hai R, Martinez-Sobrido L, Fraser KA, Ayllon J, Garcia-Sastre A, Palese P. 2008. Influenza B virus NS1-truncated mutants: live-attenuated vaccine approach. J Virol 82:10580–10590. doi: 10.1128/JVI.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]