ABSTRACT
Primary infection of a plant with a pathogen that causes high accumulation of salicylic acid in the plant typically via a hypersensitive response confers enhanced resistance against secondary infection with a broad spectrum of pathogens, including viruses. This phenomenon is called systemic acquired resistance (SAR), which is a plant priming for adaption to repeated biotic stress. However, the molecular mechanisms of SAR-mediated enhanced inhibition, especially of virus infection, remain unclear. Here, we show that SAR against cucumber mosaic virus (CMV) in tobacco plants (Nicotiana tabacum) involves a calmodulin-like protein, rgs-CaM. We previously reported the antiviral function of rgs-CaM, which binds to and directs degradation of viral RNA silencing suppressors (RSSs), including CMV 2b, via autophagy. We found that rgs-CaM-mediated immunity is ineffective against CMV infection in normally growing tobacco plants but is activated as a result of SAR induction via salicylic acid signaling. We then analyzed the effect of overexpression of rgs-CaM on salicylic acid signaling. Overexpressed and ectopically expressed rgs-CaM induced defense reactions, including cell death, generation of reactive oxygen species, and salicylic acid signaling. Further analysis using a combination of the salicylic acid analogue benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) and the Ca2+ ionophore A23187 revealed that rgs-CaM functions as an immune receptor that induces salicylic acid signaling by simultaneously perceiving both viral RSS and Ca2+ influx as infection cues, implying its autoactivation. Thus, secondary infection of SAR-induced tobacco plants with CMV seems to be effectively inhibited through 2b recognition and degradation by rgs-CaM, leading to reinforcement of antiviral RNA silencing and other salicylic acid-mediated antiviral responses.
IMPORTANCE Even without an acquired immune system like that in vertebrates, plants show enhanced whole-plant resistance against secondary infection with pathogens; this so-called systemic acquired resistance (SAR) has been known for more than half a century and continues to be extensively studied. SAR-induced plants strongly and rapidly express a number of antibiotics and pathogenesis-related proteins targeted against secondary infection, which can account for enhanced resistance against bacterial and fungal pathogens but are not thought to control viral infection. This study showed that enhanced resistance against cucumber mosaic virus is caused by a tobacco calmodulin-like protein, rgs-CaM, which detects and counteracts the major viral virulence factor (RNA silencing suppressor) after SAR induction. rgs-CaM-mediated SAR illustrates the growth versus defense trade-off in plants, as it targets the major virulence factor only under specific biotic stress conditions, thus avoiding the cost of constitutive activation while reducing the damage from virus infection.
KEYWORDS: systemic acquired resistance, calmodulin-like protein, RNA silencing suppressor, cucumber mosaic virus, priming, RNA interference, innate immunity, plant viruses, salicylic acid signaling
INTRODUCTION
Being sessile, plants are exposed to pathogen attacks and diverse environmental stresses and are unable to evade exposure to subsequent attacks. Instead, plants retain the “memory” of experiences with pathogens and environmental stresses, enabling them to mount defense reactions to subsequent challenges more effectively. A number of antibiotics and pathogenesis-related proteins targeted against secondary infection are expressed more strongly and rapidly. This general phenomenon is called priming (1); priming induced by and against pathogens is called systemic acquired resistance (SAR) (2). SAR was discovered decades ago (3, 4) and has the potential to confer on crops enhanced resistance against diverse pathogens; for this reason, induction of SAR using chemical and biological agents has been explored. Studies in recent decades have dramatically unveiled the molecular mechanisms of SAR (2). SAR-induced plants systemically accumulate salicylic acid (5), an important phytohormone for mediating immune responses to pathogens (6, 7), including viruses (8). In Arabidopsis thaliana, the primed state of SAR is partly attributed to the action of the genes encoding the nonexpressor of pathogenesis-related proteins NPR1, NPR3, and NPR4, which have been shown to be salicylic acid receptors and mediators (9–12). In addition, epigenetic modifications in SAR-induced plants have been suggested to be involved in the primed state (13). The existence of transgenerational SAR (14) supports the involvement of epigenetic modifications because such modifications can be inherited in plants (15). Thus, the requirement of NPR1 for transgenerational SAR (14) implies that salicylic acid is also involved in the epigenetic modifications. Although systemic salicylic acid biosynthesis (i.e., including plant parts distant from the site of infection) is required for induction of SAR (6), salicylic acid derivatives and other chemical molecules recently have been identified as the systemic signaling molecules (5).
In contrast to our understanding of the mechanisms of how SAR is induced and maintained, even across generations, the exact mechanisms underlying the enhanced resistance against pathogens, especially viruses, at secondary infection sites in SAR-induced plants remain to be examined. One such mechanism may be RNA silencing, a major plant defense against diverse viruses, which is induced by double-stranded RNA (dsRNA) and targets its cognate RNAs for degradation (16, 17). RNA silencing and salicylic acid-mediated immunity cooperatively inhibit systemic infection by the plum pox virus (18). RNA-dependent RNA polymerase 1 (RdRp1), which is involved in antiviral immunity through its role in RNA silencing (19–23), is induced by salicylic acid (22, 23). The RNA silencing components dsRNA binding protein 4, Argonaute 2 (AGO2), and AGO4 are involved in salicylic acid-mediated and nucleotide binding site (NB)–leucine-rich repeat (LRR)-mediated immunity (24–26). On the other hand, resistance against cucumber mosaic virus (CMV) and tobacco mosaic virus was enhanced by applying exogenous salicylic acid to an A. thaliana triple mutant of the Dicer-like genes that was considered to completely lack antiviral RNA silencing, implying that SAR is independent of RNA silencing (27).
In this study, we revealed that a tobacco calmodulin-like molecule (a regulator of gene silencing calmodulin-like protein, thus designated rgs-CaM) is involved in SAR against CMV. rgs-CaM was initially isolated in a screen of tobacco proteins that interact with the helper component-proteinase (HC-Pro) of the tobacco etch virus (28). HC-Pro is a multifunctional protein found in viruses that are members of the genus Potyvirus and functions as an effector molecule that suppresses antiviral RNA silencing (RNA silencing suppressor [RSS]) (29–31). In a previous study, rgs-CaM was shown to be an endogenous RSS that suppresses virus-induced gene silencing (VIGS) by the potato virus X (PVX) vector, which was developed from a member of the genus Potexvirus (28). We and other groups confirmed that rgs-CaM has RSS activity (32–34) and facilitates infection by viruses in the genus Begomovirus via its RSS activity (34, 35). However, we also observed an antiviral function of rgs-CaM: it binds to and directs degradation of two viral RSSs, HC-Pro and CMV 2b, via autophagy, resulting in reinforcement of antiviral RNA silencing in virus-infected cells (32). The present study reconciled these antagonistic functions of rgs-CaM by revealing a phase change in the rgs-CaM function: the antiviral function is dormant in normally growing plants and activated after SAR is induced. Moreover, we found that rgs-CaM also functions as an immune receptor. Previously, necrotic symptoms and hypersensitive responses accompanied by programmed cell death were thought to be required for SAR induction (36). More recently, however, immune receptors, receptor-like kinases (RLK), and NB-LRR proteins, which mainly perceive pathogen invasion and mount defense responses in plants, have been shown to induce SAR via defense signaling regardless of whether cell death occurs (37, 38). In this study, we showed that rgs-CaM induces salicylic acid signaling via the simultaneous perception of both viral RSS and calcium ion (Ca2+) influx as virus infection cues, implying autoactivation of the antiviral function of rgs-CaM in SAR. This study shows that two conditional reactions of tobacco plants (Nicotiana tabacum) against CMV—recognition of CMV infection, which induces salicylic acid signaling, and inhibition of CMV infection after SAR induction—are mediated by a single host protein.
RESULTS
Overexpressed and ectopically expressed rgs-CaM induces cell death and defense reactions.
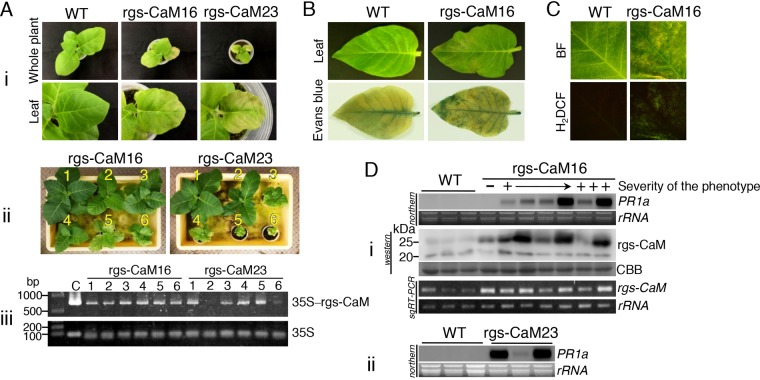
We became aware of the association between rgs-CaM and defense reactions other than RNA silencing by observing transgenic tobacco plants that constitutively overexpressed the rgs-CaM gene under the control of the cauliflower mosaic virus (CaMV) 35S promoter. Among a dozen transgenic lines, two showed dwarfing, deformation, and partial necrosis on their leaves (Fig. 1Ai, B, and C). These phenotypes were similar to those of lesion mimic mutants that involve hypersensitive response-like programmed cell death, which are accompanied by induction of reactive oxygen species (ROS) and immune signaling components, including salicylic acid (39, 40). In the transgenic plants showing these phenotypes, cell death was observed (Fig. 1B), ROS were generated (Fig. 1C), and mRNA of the gene for pathogenesis-related protein 1a (PR1a), an indicator of activation of salicylic acid signaling (41), was induced in the leaves (Fig. 1Di and ii). The severity of the lesion mimic phenotype (Fig. 1Aii) and PR1a levels (Fig. 1Di and ii) varied both among and within rgs-CaM-overexpressing lines. These results combined with our previous inoculation test that showed enhanced resistance against CMV in line rgs-CaM16 (32) indicate the possibility that the overexpressed rgs-CaM can induce cell death and immune responses and signaling, though it does not always do so. We confirmed this possibility by two additional experiments.
FIG 1.
Overexpressed and ectopically expressed rgs-CaM elicits immune responses in tobacco, implying a link between rgs-CaM and salicylic acid signaling. (Ai) Transgenic tobacco plants overexpressing rgs-CaM showed phenotypic characteristics indicating activation of immune responses, such as necrosis and dwarfing, at 7 weeks after sowing of transgenic lines 16 (rgs-CaM16) and 23 (rgs-CaM23). (Aii) Within each of these two transgenic lines, the severity of the lesion mimic phenotype was variable. Individual plants from each line are shown in order from mild (1) to severe (6) phenotypes. (Aiii) These individuals were confirmed to have the rgs-CaM transgene by detecting the 35S and rgs-CaM nucleotide sequences by PCR. PCR products amplified from the binary vector pBE2113-rgs-CaM, with which tobacco plants were transformed, with the same primer pairs were loaded as a control (lane C). (B and C) Cell death (B) and generation of reactive oxygen species (ROS) (C) in leaves were compared between transgenic tobacco overexpressing rgs-CaM and the wild type (WT) by Evans blue and 2′,7′-dichlorofluorescein diacetate (H2DCF) staining, respectively. BF, bright-field images. (Di) Expression of PR1a, an indicator of salicylic acid signaling, was investigated by Northern blotting. Samples from seven plants of transgenic line 16 were ordered from left to right by increasing severity of the phenotype. The PR1a mRNA level was investigated by Northern blotting. Overexpression of rgs-CaM in these plants was confirmed by Western blotting for its protein and by semiquantitative RT-PCR (sqRT-PCR) for its mRNA. Wild-type (WT) tobacco was used as a control. (Dii) Transgenic line 23, which overexpressed rgs-CaM and showed a phenotype similar to that of line 16, was also shown by Northern blotting to induce PR1a expression; as in the case of line 16, expression varied within the line. Coomassie brilliant blue-stained (CBB) and ethidium bromide-stained (rRNA) gels are shown as loading controls.
First, rgs-CaM was overexpressed in wild-type tobacco plants by infection with a PVX vector expressing rgs-CaM. Infection with this vector caused necrotic spots, whereas infection with the empty PVX vector or the vector expressing the rgs-CaM gene that lacks the initiation codon to express its encoded protein [PVX-rgs-CaM(−ATG)] did not (Fig. 2A). PR1a was induced significantly in leaves inoculated with the PVX vector expressing rgs-CaM but not in leaves inoculated with either the empty PVX vector or PVX-rgs-CaM(−ATG). Second, rgs-CaM was transiently expressed in protoplasts prepared from wild-type tobacco leaves. Protoplast transfection with an expression cassette containing rgs-CaM under the control of the CaMV 35S promoter resulted in cell death and ROS generation (Fig. 2B and C). In contrast, protoplast transfection with the negative-control expression cassette [rgs-CaM(−ATG)] did not significantly increase cell death or ROS generation. Taken together, these data suggest that overexpressed and ectopically expressed rgs-CaM induces immune responses and salicylic acid signaling.
FIG 2.
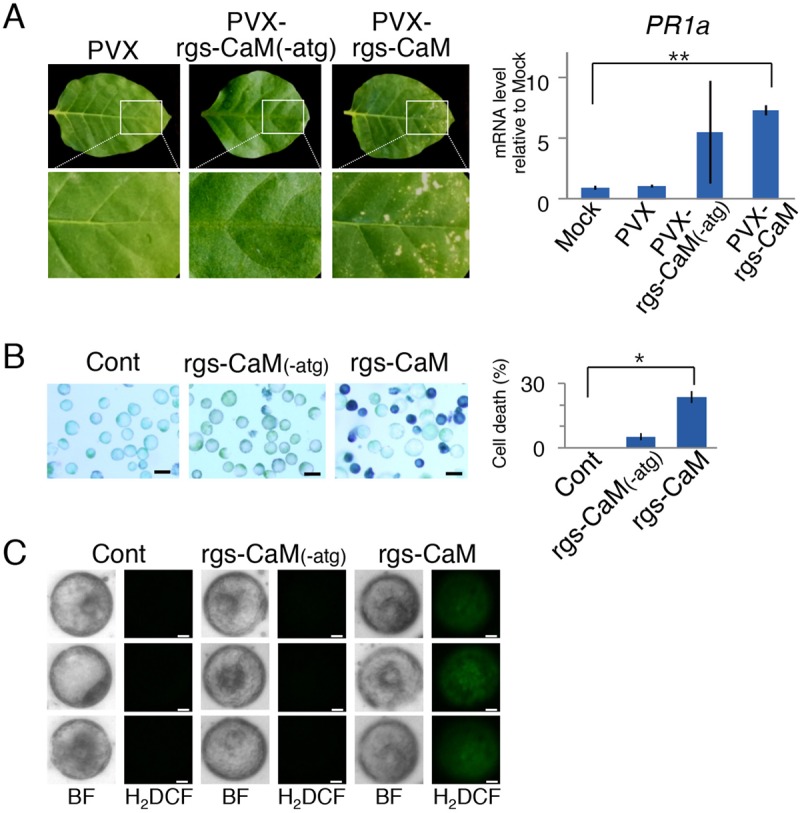
Defense responses and salicylic acid signaling were induced by transient expression of rgs-CaM. (A) A PVX vector expressing rgs-CaM (PVX-rgs-CaM), a PVX vector expressing the subgenomic RNA containing the rgs-CaM open reading frame without its initiation codon [PVX-rgs-CaM(−ATG)], and an empty vector (PVX) were inoculated into wild-type tobacco (cv. Xanthi) plants. Inoculated leaves at 7 days postinoculation are shown. Their PR1a expression was investigated by real-time PCR. The mRNA levels relative to that of mock-inoculated plants are shown in the bar graph (n = 4). Error bars indicate standard errors (SE). Student's t test was applied to the data; **, P < 0.01. (B) Protoplasts prepared from wild-type tobacco plants were transfected with expression cassettes with the rgs-CaM cDNA and the modified cDNA without the initiation codon [rgs-CaM(−ATG)] and stained with Evans blue. Black bars, 50 μm. The cell death rate (Evans blue-stained cells/total cells) is shown in the bar graph (n = 5). Error bars indicate SE. Student's t test was applied to the data; *, P < 0.05, relative to protoplasts without transfection (Cont). (C) When the protoplasts described in panel B were stained with H2DCF, protoplasts generating ROS were detected among those transfected with the rgs-CaM expression cassette. Among protoplasts transfected with rgs-CaM(−ATG) or not transfected (Cont), no H2DCF signal was detected. BF, bright-field images. White bars, 10 μm.
rgs-CaM is involved in salicylic acid signaling in response to CMV Y strain (CMV-Y) infection.
Because overexpressed and ectopically expressed rgs-CaM induced immune responses and salicylic acid signaling in transgenic plants (Fig. 1 and 2), we assume that endogenous rgs-CaM is also involved in the induction of these responses, including salicylic acid signaling. Viral infection induces various immune responses and signals that are mediated via phytohormones, including salicylic acid, and thus rgs-CaM may be involved in these responses. We tested this possibility using PVX and CMV.
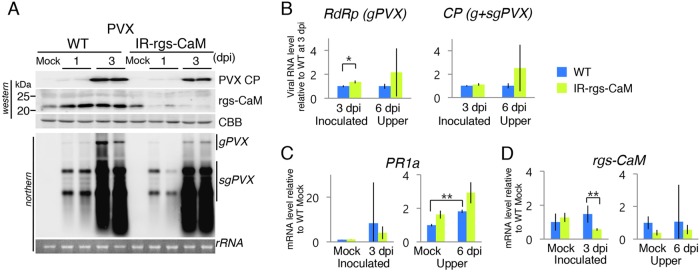
When rgs-CaM knockdown tobacco plants, in which rgs-CaM was suppressed by an inverted repeat (IR) transgene (32), were inoculated with PVX, the levels of PVX coat protein (CP) and genomic and subgenomic RNAs (gPVX and sgPVX) were observed by Western and Northern blotting, respectively. The sgPVX level was similar to that in inoculated wild-type tobacco plants, but CP and gPVX accumulated to a lesser extent (Fig. 3A). We reexamined whether rgs-CaM facilitates or inhibits PVX infection by using real-time PCR with more individual plants for each genotype (n = 8). Two primer pairs to amplify cDNAs of PVX RNAs were used (Fig. 3B). One was designed to amplify the cDNA from PVX genomic RNA (RdRp) and another to amplify the cDNA from both genomic and subgenomic RNAs of PVX (CP). PVX RNAs accumulated slightly more in inoculated leaves of the rgs-CaM knockdown plants, but a statistically significant difference was detected only for RdRp cDNA, indicative of PVX genomic RNA (Fig. 3B). In noninoculated upper leaves, PVX RNAs appeared to accumulate more in the rgs-CaM knockdown plants than in wild-type plants, but the difference was not statistically significant. We then examined whether salicylic acid signaling was induced in these plants by examining the mRNA level of PR1a. The PR1a mRNA level increased slightly but significantly in noninoculated upper leaves of wild-type tobacco plants (Fig. 3C). Similar results were obtained in the rgs-CaM knockdown plants, but the differences with the wild-type plants were not significant. Our results suggest that even if rgs-CaM is involved in the defense and induction of salicylic acid signaling against PVX infection, its contribution is minimal. Reduced rgs-CaM mRNA levels were not observed in mock-inoculated leaves of the rgs-CaM knockdown plants in comparison to those of wild-type plants, although the levels were reduced in rgs-CaM knockdown plants in the other cases (Fig. 3D). In a previous study, we obtained several lines of rgs-CaM knockdown plants (32) but could not propagate them because of their infertility. In the rgs-CaM knockdown tobacco plants used in the present study, we speculate that rgs-CaM expression was not as severely suppressed and thus this line was fertile.
FIG 3.
Susceptibility of rgs-CaM knockdown tobacco plants to PVX and salicylic acid signaling in response to PVX infection. (A) PVX was inoculated into rgs-CaM knockdown (IR-rgs-CaM) and wild-type (WT) tobacco plants. Accumulation of PVX CP and rgs-CaM and of PVX genomic and subgenomic RNAs (gPVX and sgPVX, respectively) was investigated in the inoculated leaves by Western and Northern blotting, respectively, at 1 and 3 days postinoculation (dpi). (B) The same type of inoculation as described for panel A was done with more individual plants (n = 8). Accumulation of PVX genomic RNA was measured by real-time PCR using a pair of primers for amplification of a partial cDNA sequence of viral RNA-dependent RNA polymerase (RdRp). Similarly, accumulation of PVX RNAs, including both genomic and subgenomic RNAs, was measured with a pair of primers for amplification of a partial cDNA of viral coat protein (CP). (C and D) The levels of PR1a (C) and rgs-CaM (D) mRNA were investigated by real-time PCR (n = 5). mRNA levels relative to those of mock-inoculated plants are shown. Bars indicate SE. Student's t test was applied to the data; *, P < 0.05. Coomassie brilliant blue-stained (CBB) and ethidium bromide-stained (rRNA) gels are shown as loading controls of Western and Northern blotting, respectively.
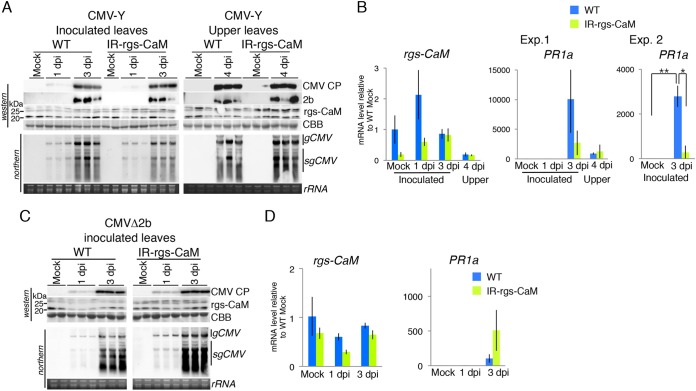
In contrast to the situation with PVX, we obtained quite different results with CMV-Y. CMV RNAs and CP accumulated to similar levels in both wild-type and rgs-CaM knockdown tobacco plants (Fig. 4A). PR1a expression was strongly induced in CMV-inoculated leaves of wild-type tobacco plants but to a lesser extent in the rgs-CaM knockdown plants (Fig. 4B). Although there was no statistically significant difference in PR1a levels in inoculated leaves between wild-type and rgs-CaM knockdown plants in the experiment shown in Fig. 4B, experiment 1 (n = 3), we repeated the experiment with more samples (n = 9) and detected a significantly higher PR1a level in the wild-type plants than in the knockdown plants (Fig. 4B, experiment 2). Moreover, reduced PR1a expression in CMV-Y-inoculated leaves of the rgs-CaM knockdown plants, compared with that in wild-type tobacco plants, was also detected previously (32). However, PR1a mRNA levels in the upper leaves of plants infected with CMV-Y (Fig. 4B) and in leaves inoculated with CMV that lacked the 2b RSS (CMVΔ2b) (Fig. 4D) were not lower in the rgs-CaM knockdown plants than in the wild-type plants. This was even though CMV RNAs and CP accumulated similarly in both wild-type and rgs-CaM knockdown plants (Fig. 4C). Considering that rgs-CaM physically interacts with the dsRNA binding site of 2b (32) and is a calmodulin-like protein with EF-hand motifs that bind to Ca2+ and probably transduce the Ca2+ signal (42), these results led us to hypothesize that rgs-CaM is an immune receptor. According to our model, in CMV-Y-infected epidermal cells in an inoculated leaf, 2b is expressed by CMV-Y, Ca2+ influx is derived from wounding caused by mechanical inoculation with carborundum (Fig. 5A), and salicylic acid signaling is reduced by knocking down of rgs-CaM (Fig. 4B, experiment 2). However, a noninoculated upper leaf (Fig. 5B) and a leaf inoculated with CMVΔ2b (Fig. 5C) lack either 2b expression or Ca2+ influx, and salicylic acid signaling (PR1a expression) is not reduced by knocking down of rgs-CaM (Fig. 4B and D). Therefore, we hypothesize that rgs-CaM induces salicylic acid signaling through perception of both 2b and Ca2+ influx as cues of the initial infection with CMV-Y in inoculated leaves.
FIG 4.
Implication of rgs-CaM involvement in salicylic acid signaling in response to infection by CMV. CMV-Y (A and B) and CMV lacking 2b (CMVΔ2b) (C and D) were inoculated into wild-type (WT) and rgs-CaM knockdown (IR-rgs-CaM) tobacco plants, and accumulation of CMV CP, 2b, and rgs-CaM proteins and CMV genomic and subgenomic RNAs (gCMV and sgCMV, respectively) (A and C) and the PR1a (experiment [Exp.] 1) and rgs-CaM mRNAs (B and D) were investigated (n = 3) as described for Fig. 3. (B, Exp. 2) The same type of inoculation as used for experiment 1 was done with more individual plants (n = 9), and the PR1a mRNA level was investigated. Error bars indicate SE. Student's t test was applied to the data; * and **, P < 0.05 and P < 0.01, respectively. Coomassie brilliant blue-stained (CBB) and ethidium bromide-stained (rRNA) gels are shown as loading controls.
FIG 5.
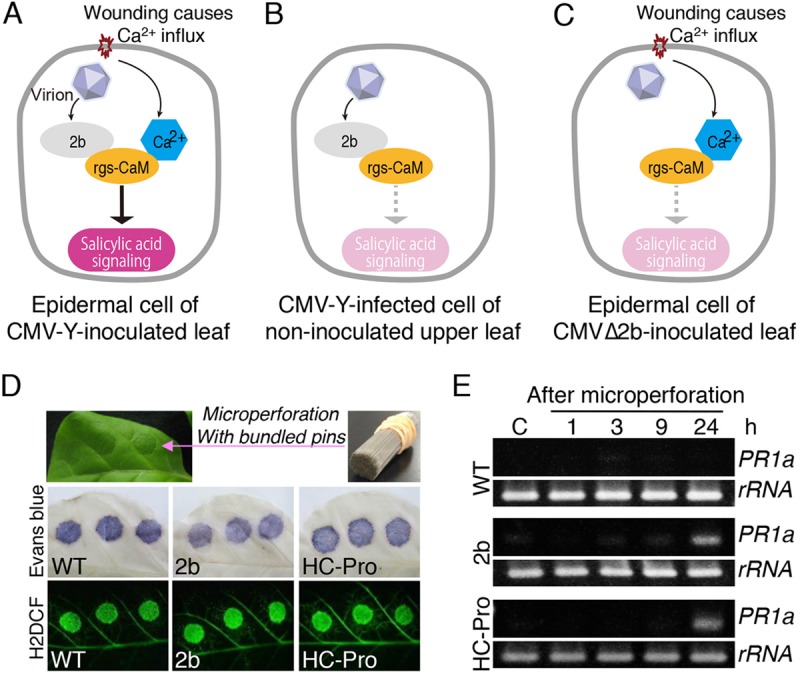
Model of salicylic acid signaling in response to CMV infection in tobacco plants (A to C) and salicylic acid signaling in response to wounding stress (D and E). (A to C) In this model, rgs-CaM functions as an immune receptor that perceives viral RSS and Ca2+. Tobacco plants induce salicylic acid signaling when rgs-CaM perceives both 2b and Ca2+ as CMV infection cues in an inoculated leaf (A) but not when rgs-CaM perceives either 2b or Ca2+ alone, e.g., in a noninoculated upper leaf (B) or in a leaf inoculated with CMV lacking 2b (CMVΔ2b) (C). (D) Transgenic tobacco plants expressing CMV 2b and ClYVV HC-Pro were microperforated by bundled pins. Immediately after microperforation, cell death (middle panels) and ROS generation (lower panels) were visualized by staining leaves with Evans blue or H2DCF, respectively. (E) Expression of PR1a was investigated by RT-PCR at different time points after microperforation of tobacco leaves.
rgs-CaM induces salicylic acid signaling via perception of both Ca2+ and viral RSS.
To examine this hypothesis, we used transgenic tobacco plants that constitutively express a viral RSS, i.e., either CMV 2b or HC-Pro of clover yellow vein virus (ClYVV); the latter was chosen because HC-Pro is known to interact with rgs-CaM (28, 32). We previously showed that the PR1a mRNA level did not increase in these transgenic tobacco plants, compared with that in wild-type tobacco plants, although the rgs-CaM mRNA level somewhat increased in transgenic plants (32). PR1a expression was monitored at different times in the transgenic tobacco plants after wounding stress caused by the opening of microperforations in leaves with a bundle of about 400 pins (Fig. 5D). PR1a expression was induced at a level detectable by reverse transcription PCR (RT-PCR) in the transgenic plants expressing 2b and HC-Pro 24 h after wounding but not in wild-type plants (Fig. 5E).
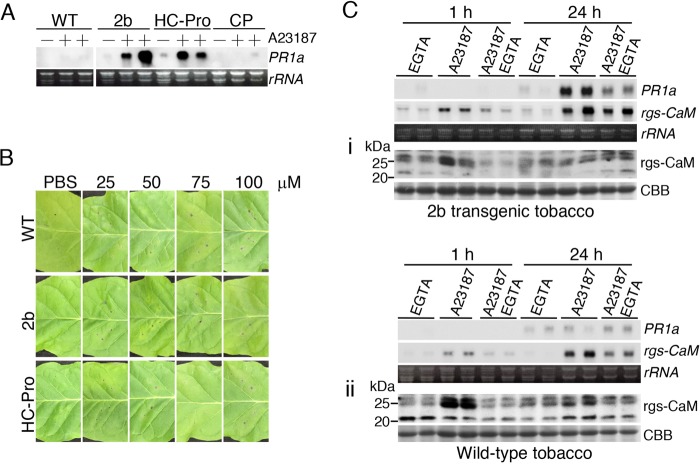
Wounding causes various changes and reactions associated with morphological damage in injured cells and surrounding cells, including Ca2+ influx and generation of ROS. In fact, ROS were generated at the wounding sites in leaves of both wild-type plants and transgenic tobacco plants expressing viral RSSs (Fig. 5D). To examine whether PR1a expression is caused by the Ca2+ influx that accompanies wounding, in addition to viral RSS, we infiltrated the leaves of transgenic tobacco plants expressing 2b or HC-Pro with a Ca2+ ionophore, A23187, which causes external Ca2+ influx and thus elevates intracellular Ca2+ levels by increasing its ability to cross biological membranes.
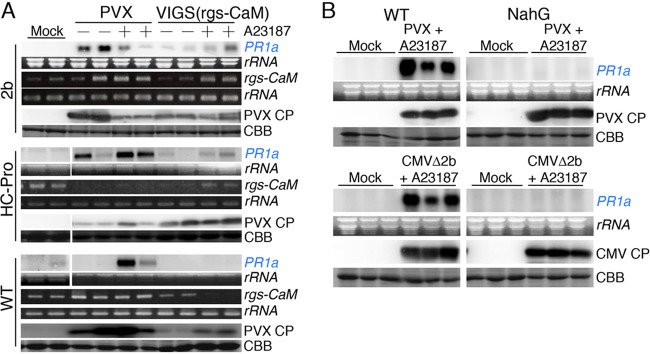
At 24 h after infiltration with A23187, PR1a was induced in transgenic tobacco plants expressing 2b or HC-Pro but not in transgenic tobacco plants expressing CMV CP or in wild-type tobacco plants (Fig. 6A). We confirmed that the PR1a expression was not due to a side effect of A23187: infiltration of A23187 did not cause cell death or other obvious morphological changes in these plant leaves (Fig. 6B), and concurrent treatment with EGTA, which chelates Ca2+, and A23187 antagonized PR1a expression (Fig. 6Ci). We note that PR1a was slightly induced in wild-type plants with A23187 infiltration (Fig. 6Cii). However, this slight PR1a induction seems to be qualitatively different from that induced by viral RSSs and Ca2+ influx, because the PR1a mRNA levels that were increased by Ca2+ in 2b-expressing plants were reduced in the presence of EGTA, whereas the PR1a levels induced by Ca2+ in wild-type plants treated with A23187 did not change in the presence of EGTA. We conclude that the expression of an RSS together with Ca2+ influx induces salicylic acid signaling but that neither RSS expression nor Ca2+ influx alone is sufficient. Ca2+ influx induced rgs-CaM expression (Fig. 6Ci and ii), consistent with our hypothesis that PR1a is induced via rgs-CaM. To test this further, we used a PVX vector that expresses the rgs-CaM mRNA sequence without its initiation codon to knock down the expression of endogenous rgs-CaM by VIGS [VIGS(rgs-CaM)]. When RSS-expressing tobacco plants were inoculated with the PVX empty vector, PR1a expression was induced even without A23187 treatment (Fig. 7A). We also found induction of PR1a in the empty-vector-infected wild-type tobacco plants treated with A23187. PR1a induction by infection of RSS-expressed plants with PVX without A23187 or by infiltration of PVX-infected tobacco leaves with A23187 is apparently discrepant to our hypothesis shown in Fig. 5A and discussed later in Discussion. Including these apparently discrepant cases, the PR1a inductions were reduced by infection with the VIGS(rgs-CaM) vector (Fig. 7A), suggesting that PR1a induction depends on rgs-CaM.
FIG 6.
Induction of salicylic acid signaling in viral RNA silencing suppressor (RSS)-expressing tobacco plants with Ca2+ influx. (A) A Ca2+ ionophore, A23187 (75 μM), was infiltrated into leaves of wild-type (WT) and transgenic tobacco plants expressing 2b, HC-Pro, or CMV CP. At 24 h after infiltration, the mRNA levels of PR1a were investigated by Northern blotting. + and −, infiltration of phosphate buffer (PBS) with and without A23187, respectively. (B) Tobacco leaves were infiltrated with A23187. A23187 was dissolved in PBS at the indicated concentrations and used to infiltrate wild-type (WT) and transgenic tobacco expressing RNA silencing suppressors CMV 2b and ClYVV HC-Pro. Photographs were taken 24 h after infiltration with A23187. (Ci and ii) To test whether PR1a induction was dependent on Ca2+ influx, EGTA (10 mM) was infiltrated along with A23187. PR1a and rgs-CaM mRNA levels and rgs-CaM protein levels were investigated by Northern and Western blotting, respectively, 1 and 24 h after infiltration. Coomassie brilliant blue-stained (CBB) and ethidium bromide-stained (rRNA) gels are shown as loading controls.
FIG 7.
PR1a induction depends on rgs-CaM. (A) Wild-type (WT) and transgenic tobacco expressing RNA silencing suppressors CMV 2b and ClYVV HC-Pro were inoculated with a PVX empty vector (PVX) and a PVX vector expressing the rgs-CaM ORF sequence lacking the initiation codon as a means of inducing VIGS of rgs-CaM [VIGS(rgs-CaM)]. These inoculated leaves were infiltrated with A23187 (+) or buffer alone (−) 3 days after inoculation with PVX. The levels of PR1a mRNA, PVX CP, and rgs-CaM mRNA were investigated by Northern blotting, Western blotting, and semiquantitative RT-PCR, respectively, 24 h after infiltration with A23187. Samples were also prepared from plants that were inoculated with buffer but not infiltrated (Mock). (B) WT and transgenic tobacco plants expressing salicylate hydroxylase (NahG), which antagonizes salicylic acid signaling, were inoculated with PVX and CMVΔ2b and infiltrated with A23187 at 3 days postinoculation. The levels of PR1a mRNA and viral CPs were investigated by Northern and Western blotting, respectively, 24 h after infiltration with A23187. Samples were also prepared from buffer-inoculated plants without infiltration (Mock). Coomassie brilliant blue-stained (CBB) and ethidium bromide-stained (rRNA) gels are shown as loading controls.
PR1a induction was suppressed when salicylate hydroxylase (nahG)-expressing tobacco plants, in which salicylic acid is converted to catechol and thus salicylic acid signaling is antagonized, were inoculated with the PVX empty vector or CMVΔ2b and then treated with A23187. These results indicate that salicylic acid signaling was induced in wild-type tobacco plants infected with either the empty PVX vector or CMVΔ2b when Ca2+ influx was artificially induced with A23187 (Fig. 7B).
rgs-CaM is necessary for enhanced resistance against CMV in SAR-induced tobacco plants.
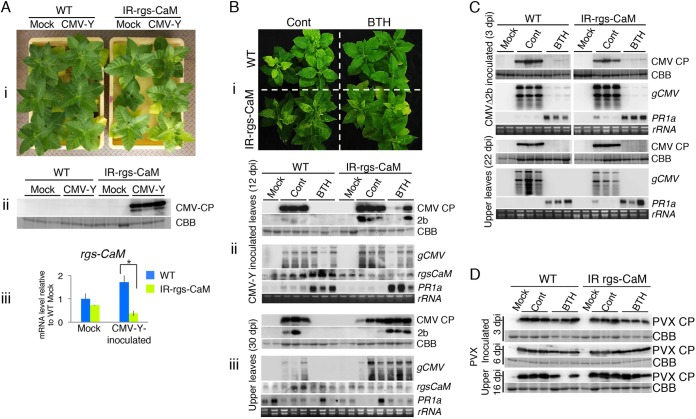
In addition to being an inducer of salicylic acid signaling, we found that rgs-CaM is involved in salicylic acid-mediated antiviral defense. The inoculation test results in Fig. 4 showed comparable accumulation levels of CMV CP and genomic RNAs in inoculated and upper leaves between wild-type and rgs-CaM knockdown plants, indicating that rgs-CaM does not interfere with CMV infection. However, when CMV was inoculated into relatively old tobacco plants (for example, 7 weeks after sowing [Fig. 8Ai]), the rgs-CaM knockdown plants developed systemic yellowing of leaves earlier than did the inoculated wild-type plants. At 16 days postinoculation (dpi), CMV could be detected by Western blotting only in noninoculated upper leaves of inoculated rgs-CaM knockdown plants (Fig. 8Aii). The tobacco plants described in Fig. 4 were inoculated at 4 weeks after sowing, suggesting that the antiviral function of rgs-CaM has two phases: it is dormant in normally growing young tobacco plants around 4 weeks after sowing but becomes activated by 7 weeks after sowing.
FIG 8.
Enhanced resistance against CMV-Y in SAR-induced tobacco plants depends on rgs-CaM. (Ai) Comparison of symptoms (yellowing) on noninoculated upper leaves of tobacco plants inoculated with CMV-Y. CMV-Y was inoculated into wild-type (WT) and rgs-CaM knockdown (IR-rgs-CaM) tobacco plants 7 weeks after sowing. The photograph was taken at 16 days postinoculation (dpi) with CMV-Y. All of the rgs-CaM knockdown tobacco plants that were inoculated with CMV-Y developed systemic symptoms on their leaves, but wild-type tobacco plants did not express symptoms. (Aii) The difference in susceptibility between wild-type and rgs-CaM knockdown plants was confirmed by detecting CMV CP in noninoculated upper leaves of these plants by Western blotting. (Aiii) The mRNA level of rgs-CaM relative to that of mock-inoculated wild-type plants was investigated by real-time PCR and is shown in the bar graph (n = 3). Error bars indicate SE. Student's t test was applied to the data; *, P < 0.05. (Bi) Five days after SAR induction by treatment with benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH), WT and IR-rgs-CaM tobacco plants were inoculated with CMV-Y. Control plants (Cont) were treated with a solution containing 1.4% (vol/vol) acetone and 0.2% Tween 20 (the solution used to dissolve BTH). Symptoms on upper leaves were photographed 30 dpi. (Bii and iii) CMV CP and 2b proteins were detected by Western blotting. CMV genomic and subgenomic RNAs (gCMV and sgCMV, respectively), rgs-CaM, and PR1a mRNA were detected by Northern blotting. Coomassie brilliant blue-stained (CBB) and ethidium bromide-stained gels are shown as loading controls. (C) Experiments similar to those shown in panel B were done with CMVΔ2b. (D) PVX CP accumulation in plants inoculated with PVX 5 days after BTH treatment. Accumulation of PVX CP was detected in inoculated and noninoculated upper leaves by Western blotting. CBB-stained gels are shown as loading controls. Control samples were prepared from buffer-inoculated plants (Mock).
What, then, is different between tobacco plants at 4 and 7 weeks after sowing that brings about the phase change of the antiviral function of rgs-CaM? A previous study showed that tobacco plants gradually accumulate salicylic acid during the 7 to 10 weeks after sowing and develop enhanced resistance against tobacco mosaic virus, probably because of the accumulated salicylic acid (43). Similar age- and salicylic acid-related resistance against CMV has been reported previously (44, 45). These studies prompted us to examine whether salicylic acid signaling affects rgs-CaM function by using a salicylic acid analog, benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH), which is a strong inducer of SAR via systemic induction of salicylic acid signaling (46, 47). Systemic symptom expression in leaves was delayed (Fig. 8Bi) and CMV accumulation was drastically reduced in BTH-treated wild-type tobacco plants relative to the untreated control (Fig. 8Bii and iii), confirming the enhancement of antiviral resistance by induction of SAR with BTH, as reported previously (48, 49). These effects were weakened in the rgs-CaM knockdown plants, indicating that the enhanced resistance to CMV induced by BTH depends on rgs-CaM (Fig. 8Bii and iii). Judging by the symptoms observed (Fig. 8Bi) and the results of Western blotting with samples of inoculated leaves (Fig. 8Bii), some resistance was still induced in BTH-treated rgs-CaM knockdown plants. This resistance might have been caused by the residual rgs-CaM in the knockdown plants or by a salicylic acid-mediated defense system that operates independently but in parallel to the rgs-CaM-mediated defense mechanism. To examine whether tobacco plants have a salicylic acid-mediated defense system(s) which is not linked to the rgs-CaM-mediated defense mechanism, we conducted similar experiments using CMVΔ2b and PVX because these viruses were considered to lack an RSS that interacts with rgs-CaM. When CMVΔ2b was inoculated into wild-type tobacco plants, CMVΔ2b accumulation was drastically reduced by BTH treatment even in rgs-CaM knockdown plants (Fig. 8C), indicating the existence of an independent salicylic acid-mediated defense system(s) that effectively inhibits CMV infection. When PVX was inoculated into wild-type tobacco plants in which SAR was induced by pretreatment with BTH, PVX CP accumulated in inoculated and upper leaves, although to a slightly lesser extent than in noninduced leaves (Fig. 8D). Similar results were obtained using the rgs-CaM knockdown tobacco plants. Thus, the SAR induced by BTH was relatively ineffective against PVX, compared with that against CMV-Y and CMVΔ2b, and we could not conclude whether rgs-CaM contributes to the low level of SAR against PVX.
Reduced accumulation of viral RSSs in SAR-induced transgenic tobacco cells and plants.
We previously demonstrated that rgs-CaM binds to and directs degradation of two viral RSSs, CMV 2b and ClYVV HC-Pro, via autophagy (32). The prerequisite of rgs-CaM for enhanced resistance against CMV but not against CMVΔ2b in SAR-induced plants implies that the rgs-CaM-mediated degradation of viral RSSs might be activated in the SAR-induced plants. Using cultured transgenic tobacco BY2 cells that constitutively express CMV 2b, we examined whether the degradation of 2b is activated by SAR induction. The 2b protein was detected in nuclei in untreated cells by immunofluorescent staining, but the fluorescent signal disappeared 1 h after BTH treatment (Fig. 9). The fluorescent signal was, however, retained in cells treated with both BTH and an autophagy inhibitor (either E64d or concanamycin A), suggesting that the degradation of 2b, probably via autophagy, was activated by SAR induction, which leads to resistance against CMV-Y infection.
FIG 9.
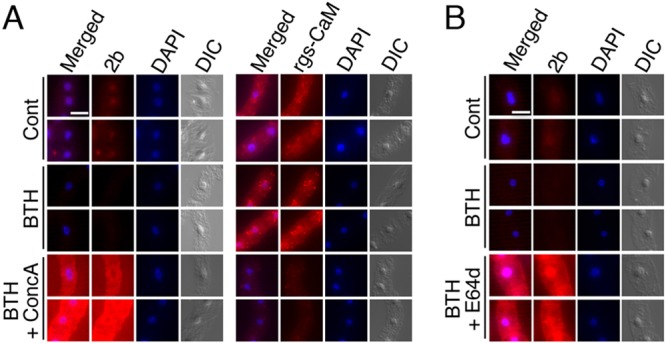
Degradation of CMV 2b is enhanced by benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH) in transgenic BY2 cultured tobacco cells expressing 2b. Transgenic BY2 cultured cells expressing 2b were treated with BTH by adding it to the medium at a final concentration of 10 μM with or without an inhibitor, concanamycin A (concA) at 0.1 μM (A) or E64d at 10 μM (B). The CMV 2b and rgs-CaM proteins were detected by immune staining using specific fluorescent secondary antibodies 1 h after treatment with BTH with or without an inhibitor. Nuclei were visualized by DAPI staining. Differential interference contrast (DIC) images are also shown. White bars, 25 μm.
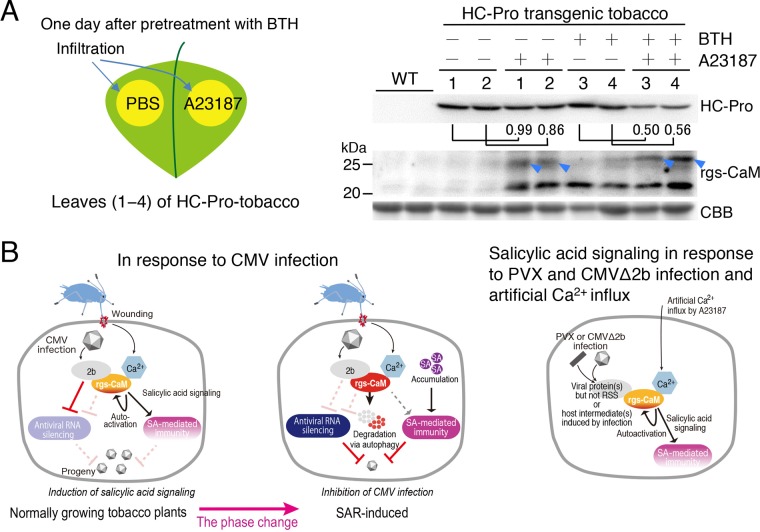
We then examined the effect of Ca2+ influx on accumulation of the HC-Pro protein in SAR-induced HC-Pro transgenic tobacco plants because Ca2+ influx is expected as a result of wounding during virus infection, as illustrated in Fig. 5A to C. A23187 treatment reduced the accumulation of the HC-Pro protein in SAR-induced HC-Pro tobacco plants (Fig. 10A). However, A23187 treatment had little effect on the accumulation of the HC-Pro protein in HC-Pro tobacco plants in which SAR was not induced, suggesting that HC-Pro expression is specifically inhibited in the initial virus-infected cells of SAR-induced tobacco plants. The upper band (around 25 kDa) of the rgs-CaM protein extracted from A23187-infiltrated leaf tissue of SAR-induced plants migrated a little more slowly in SDS-PAGE than that extracted from A23187-infiltrated leaf tissue of noninduced plants (Fig. 10A, right panel, blue arrowheads), implying a change in the rgs-CaM protein state as a result of SAR induction.
FIG 10.
Reduction of ClYVV HC-Pro accumulation in transgenic tobacco plants expressing HC-Pro (A) and schematic models of detection and counteraction of viral RSSs by rgs-CaM (B). (A, left) Four leaves (numbered 1 to 4) of individual transgenic plants expressing HC-Pro were treated with benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester (BTH). A23187 in PBS was infiltrated into one half of a leaf 1 day after BTH treatment; the other half was infiltrated with buffer (PBS). (A, right) The HC-Pro and rgs-CaM proteins were detected by Western blotting. Values under the HC-Pro panel are band intensities of samples from the leaf part infiltrated with A23187 relative to that without A23187 in the same leaf (leaves 1 to 4). (B, left) In normally growing tobacco plants, the rgs-CaM-mediated defense system does not inhibit CMV infection but induces salicylic acid (SA) signaling via perception of CMV 2b and Ca2+ as CMV infection cues. Blue arrowheads indicate bands detected by anti-rgs-CaM antibody. (B, center) When the phase of rgs-CaM is changed by SAR induction, subsequent CMV infection is inhibited by rgs-CaM-mediated anti-RSS defense reactions. rgs-CaM directs degradation of RSS (CMV 2b) via autophagy, resulting in reinforcement of antiviral RNA silencing in addition to SA-mediated antiviral immunity. (B, right) When plants are infected with PVX or CMVΔ2b and Ca2+ influx is artificially induced with A23187, SA signaling is induced, probably via perception by rgs-CaM of Ca2+ and viral proteins other than RSS or host intermediate proteins that are induced by virus infection.
DISCUSSION
This study revealed that a novel class of protein, calmodulin-like protein rgs-CaM, functions as an immune receptor for CMV infection and induces salicylic acid signaling, which is characteristic of immune responses against biotrophic pathogens, including viruses (8), and is required for SAR induction (2, 6). As mentioned in the introduction, the known immune receptors for pathogens in plants are mostly RLKs and NB-LRRs. RLKs perceive molecules that are conserved among pathogenic microorganisms but are not found in host plants (pathogen- or microorganism-associated molecular patterns [PAMPs or MAMPs]) and induce pattern-triggered immunity (PTI). Host-adapted pathogens develop effector molecules that suppress PTI and enable their colonization of plants. Another class of receptors, NB-LRRs, counteractively recognize pathogen effector proteins and induce strong defense reactions, called hypersensitive responses; this mechanism is termed effector-triggered immunity (ETI) (50, 51). Several NB-LRRs that perceive virus invasion and induce ETI have been identified (52), and recent studies of Arabidopsis RLKs (53, 54) suggest the existence of an immune receptor that perceives dsRNAs or other viral factors as viral PAMPs and induces PTI. In animals, Toll-interleukin 1-like receptors (TLRs), which are structurally similar to plant RLKs and NB-LRRs, perceive viral RNA and DNA in endosomes and on cell membranes (55). In addition, RIG-I and MDA5 for viral RNA and IFI16 and cGAS for viral DNA have been identified as receptors that perceive PAMPs in the cytoplasm and nucleus (56). A NOD-like receptor and other host factors have been implicated in recognition of viral infection (56). However, no CaM or CaM-like protein (CML) has previously been identified to be an immune receptor.
Plant CaMs and CMLs are Ca2+ sensors that play important roles in development and stress responses (57, 58). An increase in the Ca2+ concentration in the cytoplasm is one of the earliest events following exposure to environmental stresses, and Ca2+ is a crucial secondary messenger in the perception of these stresses. In plants, CaMs and CMLs constitute a relatively large family of Ca2+ sensor genes along with two other classes of proteins, calcineurin B-like proteins and Ca2+-dependent protein kinases (59). CaMs and CMLs bind a number of endogenous factors and have no obvious functional domains except for one to seven EF-hand motifs for binding Ca2+ and thus are considered to transduce Ca2+ signals by modifying the activity or conformation of their binding endogenous proteins (58). rgs-CaM, one of the tobacco CMLs, uniquely binds to exogenous proteins, diverse viral RSSs (including potyvirus HC-Pro, CMV, [the related] tomato aspermy virus 2b, and human immunodeficiency virus TAT), presumably via affinity to their positively charged dsRNA binding sites (28, 32), though there is no conserved amino acid sequence motif among these dsRNA binding domains. CaMs and CMLs are hub proteins, which bind to various substrate proteins through their relatively disordered binding sites (60). Homology modeling (32, 42) implies that rgs-CaM has a negatively charged disordered binding site for substrates, which is probably where rgs-CaM binds diverse viral RSSs. Since viral RSSs are considered to be effectors that suppress an antiviral PTI-like basal defense (RNA silencing), rgs-CaM is another class of receptor for viral effectors in addition to NB-LRRs. rgs-CaM perceives not only viral RSSs but also Ca2+ cues that induce salicylic acid signaling (Fig. 4 to 7). A recent structural and thermodynamic study by Makiyama et al. (42) revealed that rgs-CaM binds Ca2+ at three EF-hand motifs and suggested that Ca2+ binding at the two EF hands that show higher affinity to Ca2+ alters the conformation of rgs-CaM such that the negatively charged binding sites are more exposed. This supports our model that salicylic acid signaling is induced by the dual perception of viral RSS and Ca2+ by rgs-CaM (Fig. 5A to C and 10B). We assume that the dual perception of viral RSS and Ca2+ by rgs-CaM avoids nonspecific induction of salicylic acid signaling. Consistently, overexpression and ectopic expression of rgs-CaM did not always induce defense responses and salicylic acid signaling (Fig. 1). Because plant cells are surrounded by a cell wall, virus invasion seems to require mechanical wounding, which would cause Ca2+ influx in the virus-invaded cells. The normal mechanism of CMV infection in the field is via aphid feeding, and aphid feeding has been reported to cause Ca2+ influx in tobacco plants (61, 62). In general, defense responses against various abiotic and biotic stress responses involve Ca2+ fluxes (63), and virus infection is known to lead to an increase of the cytoplasmic Ca2+ concentration (64). We assume that this is why PR1a was induced in PVX-infected transgenic tobacco plants expressing viral RSSs without artificial Ca2+ influx induced by A23187 (Fig. 7A). Therefore, the dual perception of a viral component and Ca2+ seems suitable as a viral infection cue to specifically induce immune responses. One drawback to recognition of a viral RSS as an infection cue is that it is incapable of immediate induction of immune responses because most viral RSSs, including 2b and HC-Pro, are not included in the invading virion but are expressed during establishment of viral infection and viral multiplication. As described below, the rgs-CaM-induced immune responses do not appear to prevent primary virus infection; rather, salicylic acid signaling among them may contribute to prevent subsequent infection by viruses possessing RSSs that interact with rgs-CaM via its autoactivation in SAR-induced plants. Therefore, the rgs-CaM-induced immune responses do not necessarily need to be induced immediately. In the present study, the induction of rgs-CaM-mediated salicylic acid signaling after wounding of transgenic plants expressing viral RSSs took 24 h (Fig. 5E), which is slower than that seen with ETI (hypersensitive response) (65).
rgs-CaM may have the ability to induce salicylic acid signaling in response to viral or host proteins other than viral RSSs. Under natural conditions, rgs-CaM does not seem to be involved in the induction of salicylic acid signaling in response to PVX and CMVΔ2b infection (Fig. 3 and 4). However, when Ca2+ influx was artificially induced with A23187 in wild-type plants, salicylic acid signaling was induced by infection with either PVX or CMVΔ2b (Fig. 7), and salicylic acid signaling induced by PVX in the presence of Ca2+ was dependent on rgs-CaM (Fig. 7A). The triple gene block protein 1 (TGBp1) of PVX is an RSS. The suppression mechanism of RNA silencing by TGBp1 is not through binding to dsRNA; instead, TGBp1 has been reported to bind to AGO1 to AGO4 and leads to degradation of AGO1 via the 26S proteasome (66). Considering that rgs-CaM probably binds to the dsRNA binding sites of viral RSSs, rgs-CaM may not bind TGBp1. More strikingly, tobacco plants must be able to recognize CMV proteins other than its RSS (2b) for there to be induction of salicylic acid signaling by CMVΔ2b (Fig. 7B). At first glance, the results in Fig. 7 seem to contradict our conclusion that rgs-CaM perceives viral RSSs and Ca2+ as virus infection cues to induce salicylic acid signaling. One possible explanation is that rgs-CaM may have weak affinity to PVX and a CMV protein(s) other than 2b and can bind to them when Ca2+ influx is stimulated by A23187 infiltration (Fig. 10B, right panel). The substrate (RSS) binding domain of rgs-CaM was predicted to be more exposed when Ca2+ binds to rgs-CaM at its EF hands (42). Therefore, under specific conditions, such as when wild-type tobacco leaves that were infected with PVX or CMVΔ2b were subsequently infiltrated with A23187, rgs-CaM may perceive other PVX and CMV protein(s) to induce salicylic acid signaling. Another possibility is simply that rgs-CaM binds to a host intermediate(s) that is induced by virus infection for salicylic acid signaling.
RNA silencing and salicylic acid-mediated immunity are two major antiviral systems in plants, and their linkage has been suggested (18–26). The present study also revealed a link between RNA silencing and salicylic acid-mediated immunity via a single host factor, rgs-CaM, which suppresses antiviral RNA silencing as an endogenous RSS but induces salicylic acid signaling by perceiving viral RSS as an immune receptor (e.g., in the case of CMV). Pruss et al. (67) reported that transgenic tobacco plants expressing HC-Pro show enhanced resistance to both heterologous viruses that have different RSSs and fungal pathogens; depending on the pathogen, resistance could be either salicylic acid dependent or independent. The mechanism underlying this viral RSS-induced enhanced resistance against multiple pathogens remains unclear. In those transgenic plants (68), rgs-CaM could induce salicylic acid signaling in response to Ca2+ influx caused by infection with pathogens and thus partly contribute to the enhanced resistance in a salicylic acid-dependent manner.
Another significant observation of this study is uncovering a part of the molecular mechanism underlying the enhanced resistance against a virus in SAR-induced plants. We previously reported the antiviral function of rgs-CaM (32). The present study revealed that this antiviral function is not constitutively active but exhibits a phase change from dormant to activated after SAR induction via salicylic acid signaling (Fig. 4 and 8 to 10). We previously showed that, without artificial induction of SAR, the rgs-CaM-overexpressing transgenic tobacco plants (rgs-CaM16) inhibit CMV infection (32). However, this is not contradictory to the present study because overexpression of rgs-CaM induces salicylic acid signaling systemically in this transgenic line (Fig. 1) and thus induces SAR. Since CMV infection has been reported to induce salicylic acid signaling in this study (Fig. 4) and previously (44, 68, 69), one may expect that rgs-CaM autoactivates its antiviral function for SAR during CMV infection via its perception of CMV 2b. However, rgs-CaM did not effectively inhibit CMV infection in relatively young plants (Fig. 4) although it did in older plants (Fig. 8A). CMV 2b has been reported to interfere with salicylic acid and jasmonic acid signaling (44, 68, 69). Ca2+ influx induced by A23187 caused rgs-CaM protein accumulation in both wild-type and 2b-expressing transgenic plants (Fig. 6C). However, its accumulation level was lower in 2b-expressing plants, in which PR1a was induced, than in wild-type tobacco plants. Our previous study (32) suggested that both rgs-CaM and viral RSS proteins are posttranslationally regulated via the 26S proteasome and autophagy and that rgs-CaM directs degradation of these RSS proteins. The rgs-CaM-mediated degradation of viral RSS proteins was enhanced by salicylic acid signaling (Fig. 8 to 10). Overexpression of rgs-CaM did not always result in increased accumulation of rgs-CaM protein, induction of salicylic acid signaling, and other defense responses (Fig. 1), suggesting complex interactions (counteraction or neutralization) among rgs-CaM, 2b, salicylic acid signaling, and protein degradation pathways.
It is generally assumed that plants and animals inhibit infection by any pathogens to reduce the threat of disease. However, this and previous studies have shown biased reactions of tobacco plants against pathogenic viruses via the antagonistic functions of rgs-CaM. rgs-CaM was initially shown to be an endogenous RSS by using transgenic Nicotiana benthamiana in which the tobacco rgs-CaM gene was overexpressed by the CaMV 35S promoter (28). In that study, the overexpressed tobacco rgs-CaM gene interfered with VIGS of green fluorescent protein (GFP) by a PVX vector, resulting in increased fluorescence and accumulation of the GFP transgene and the PVX genomic RNA itself (28). Li et al. (34) reported that infection by tomato yellow leaf curl China virus, a member of the genus Begomovirus, was facilitated or inhibited in transgenic N. benthamiana plants in which rgs-CaM was overexpressed or silenced, respectively. They also confirmed the RSS activity of rgs-CaM (34, 70). Additionally, infection by tomato golden mosaic virus, another member of the genus Begomovirus, was shown to be facilitated in transgenic Arabidopsis plants in which Arabidopsis CML39, one of the proteins most similar to rgs-CaM among 50 Arabidopsis CMLs, was overexpressed (35). Taken together with data in this study, in normally growing plants, rgs-CaM facilitates infection by members of the genus Begomovirus, but not CMV (Cucumovirus) and PVX (Potexvirus), probably by its RSS activity but inhibits CMV infection by its phase-changed antiviral activity that directs degradation of CMV 2b via autophagy after SAR induction.
Constitutive activation of plant immune systems results in inhibition of plant growth (71), as also shown here by overexpression of rgs-CaM (Fig. 1). This trade-off between immunity and growth in plants has driven the evolution of immune receptors for recognition of pathogen invasion that effectively induce defense mechanisms only when needed. The receptor and conditional effector functions of rgs-CaM (that is, its phase change via SAR induction) suggest that tobacco changes its reaction to viral infection according to environmental conditions via rgs-CaM. rgs-CaM strongly inhibits infection by viruses that express RSSs that directly interact with it, such as CMV, only under environmental conditions with a high frequency of infection by pathogens, which leads to SAR induction (Fig. 10B, left and center panels). In general, viral RSSs function as virulence factors not only by enhancing virus multiplication that leads to increased expression of other viral virulence factors via suppressing antiviral RNA silencing but also by disrupting host gene expression controlled by the small-RNA pathways in infected cells. This biased and conditional antiviral defense system has presumably developed as a means of counteracting RSS-expressing virulent viruses to avoid the cost of constitutive defense activation while reducing the damage from the virus infection.
MATERIALS AND METHODS
PVX vectors carrying rgs-CaM cDNA and expression cassettes.
The rgs-CaM open reading frame (ORF) and the ORF lacking its initial codon were cloned between the ClaI and SalI sites of the PVX vector pPC2S (72) to generate PVX-rgs-CaM and PVX-rgs-CaM(−ATG) [VIGS(rgs-CaM)], respectively. After linearization of these plasmids by digestion with SpeI, infectious RNAs were transcribed by T7 RNA polymerase with the 7-methylguanosine-5′-phosphate cap analog (Thermo Fisher Scientific, Inc., Waltham, MA, USA) from the linearized plasmids and used as inocula for mechanical inoculation. The rgs-CaM ORFs with or without the initiation codon were also cloned between the XbaI and SacI sites of pE2113 (73), and the cloned plasmids, pE2113-rgs-CaM and pE2113-rgs-CaM(−ATG), were used for transfection of tobacco protoplasts to express rgs-CaM under the control of the CaMV 35S promoter.
Transgenic tobacco plants and virus inoculation.
Transgenic tobacco plants (Nicotiana tabacum cv. Xanthi), in which rgs-CaM was either overexpressed or knocked down, were made previously (32). Transgenic tobacco plants (N. tabacum cv. BY4) expressing viral RSSs were also made previously (32). Transgenic tobacco plants expressing CMV CP and NahG were made similarly to those expressing viral RSSs (32). T2 or later generations of transgenic tobacco plants, all of which were shown to be kanamycin resistant, were grown under a 16-h light/8-h dark photoperiod at 25°C for virus inoculation and other experiments. N. benthamiana leaves infected with CMV-Y, CMVΔ2b, which lacked 2b and was designated CMV-H1 in a previous study (74), and the PVX vectors were used as inocula for mechanical inoculation with carborundum and stored in a deep freezer at −80°C until needed.
BTH and Ca2+ ionophore treatment.
A salicylic acid analog, BTH, was spread on tobacco leaves with cotton tufts that were dipped in 1 mM BTH, 1.4% (vol/vol) acetone as a solvent, and 0.2% Tween 20. Phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4·12H2O, and 2 mM KH2PO4, pH 7.4) containing 75 μM Ca2+ ionophore A23187 (MilliporeSigma, St. Louis, MO, USA) was prepared by diluting A23187 stock solution (5 mg/ml of A23187 dissolved in dimethyl sulfoxide [DMSO]) with PBS, and the diluted A23187 solution with or without 10 mM EGTA was infiltrated into leaves with a syringe.
Preparation, transfection, and assays of tobacco protoplasts.
Tobacco mesophyll protoplasts were prepared from wild-type tobacco plants (N. tabacum cv. Xanthi) and transfected with pE2113 vectors as described previously (75). Assays following transfection were also carried out according to the method from the previous study (75). H2O2 signals, indicative of ROS generation, were visualized with 500 nM 2′,7′-dichlorofluorescein diacetate (H2DCF) (MilliporeSigma) 5 h after transfection. The images were observed with a fluorescence microscope (model DMI 6000B; Leica, Tokyo), and H2DCF signals were visualized with excitation at 488 nm (emission, 498 to 532 nm). Eleven hours after transfection, protoplasts were exposed to 0.04% Evans blue dye (an indicator of cell death) for 5 min and then observed with light microscopy (model BX51; Olympus, Tokyo).
RT-PCR, semiquantitative RT-PCR, real-time RT-PCR, and Northern blotting.
After tobacco leaves were ground in liquid nitrogen, total RNA was extracted using the TRIzol reagent according to the manufacturer's manual (Thermo Fisher Scientific). Each RNA sample was treated with RNase-free DNase I (Roche Diagnostics, Basel, Switzerland). First-strand cDNAs were synthesized from 1 μg of RNA extracts by a modified Moloney murine leukemia virus (MMLV) reverse transcriptase, ReverTra Ace (Toyobo, Osaka, Japan). Accumulation of viral genomic RNAs and endogenous mRNAs was detected by PCR in a mixture (25 μl) containing cDNAs corresponding to 0.05 μg RNA, 0.4 μM each of the specific primer pairs listed in Table 1, 0.2 mM deoxynucleoside triphosphate (dNTP), and 0.625 U Ex Taq DNA polymerase (TaKaRa, Otsu, Japan). PCR mixtures for PR1a were incubated for 2 min at 94°C, followed by 28 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 40 s, and PCR products were fractionated with 2% agarose gel electrophoresis. Semiquantitative RT-PCR was done for rgs-CaM by using 24 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 30 s and for 18S rRNA by using 15 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Real-time PCR was performed by using the DNA Engine Opticon 2 system (Bio-Rad Laboratories, Hercules, CA, USA) according to the method described in a previous study (76). The reaction mixture (25 μl) contained 0.625 U of Ex Taq (TaKaRa), Ex Taq buffer, 0.2 mM dNTP, 0.2 μM (each) forward and reverse primers listed in Table 1, SYBR green (30,000× dilution) (Thermo Fisher Scientific), and cDNA corresponding to 12.5 ng of total RNA. Samples were incubated for 2 min at 95°C, followed by 39 cycles of 95°C for 10 s, 58°C for rgs-CaM or 59°C for PR1a for 20 s, and 72°C for 20 s. Northern blotting was performed as described previously (77) using digoxigenin (DIG)-labeled cRNA probes (Roche Diagnostics). These probes were made from the target mRNA sequences, PVX genomic RNA sequence, and the conserved nucleotide sequence at the 3′-terminal regions of CMV genome segments by using the primers listed in Table 1. RNA samples (2 to 5 μg) were fractionated by denaturing agarose gel electrophoresis and transferred onto a nylon membrane (Hybond-N; GE Healthcare, Chicago, IL, USA). Chemiluminescence signals were quantitatively detected by a LAS-4000 mini PR Lumino-image analyzer (GE Healthcare).
TABLE 1.
Primers used for detection of viral genomic RNAs and endogenous gene expression
| Gene (accession no.) | Primer sequence (5′–3′)a |
|---|---|
| 18S rRNA | F CCGTAGTCCCTCTAAGAAGCTG |
| R GGTCCAGACATAGTAAGGATTG | |
| rgs-CaM (AF329729) | F TGATAGGAGCATTTGGAATGTATG |
| R ACTCATCAAAGTTGAGAACTCCATC | |
| F ACTATTACTACTGATTATCTTTCGA (semiQ PCR) | |
| R CCCAAGGCCAAAGAATTATGTACA (semiQ PCR) | |
| *F ACTATTACTACTGATTATCTTTCGA | |
| *R GGGATCCTAATACGACTCACTATAGGGGCAAATGCTCCTATCAATTCACT | |
| CaMV 35S promoter | F CCACTGACGTAAGGGATGACGC |
| R GTGTTCTCTCCAAATGAAATGA | |
| PR1a (X06361, Y00707) | F GAAGTGGCGATTTCATGACGGCTG |
| R CGAACCGAGTTACGCCAAACCACC | |
| *F ATGGGATTTGTTCTCTTTTCACAATTGCC | |
| *R AATTCTAATACGACTCACTATAGGGGAAGGTTCTTGATATCAAGCAG | |
| PVX genomic RNA | *F ATGTCAGCACCAGCTAGCACAACA |
| *R AATTCTAATACGACTCACTATAGGGACATTATGGTGGTAGCGTGAC | |
| F ACCAATCTTTTACAGACTCCACCAC (for RdRp) | |
| R CTCTAGATCATTAGCCGCTTCAACC (for RdRp) | |
| F AGGGTCAACTACCTCAACTACCAC (for CP) | |
| R TCCTTCCAAATAGCCTCAATCTTGC (for CP) | |
| CMV genomic RNA | *F GGCGGGAGCTGAGTTGGCAGTTCTGC |
| *R AATTCTAATACGACTCACTATAGGGGGTCTCCTTTTGGAGGCCCCCACGA |
Asterisks indicate primers used for making DIG-cRNA probes for Northern blotting. F, sense primer; R, antisense primer; semiQ, semiquantitative.
Western blotting.
Western blotting was carried out as described previously (32). Tobacco leaf tissues were homogenized in liquid nitrogen and then dissolved in 12-fold (vol/mass) urea-denaturing buffer containing 4.5 M urea, 1% (vol/vol) Triton X-100, 0.5% dithiothreitol (DTT), 0.0625 M Tris-HCl (pH 6.8), 2% (wt/vol) SDS, 5% mercaptoethanol, 5% sucrose, and 0.002% bromophenol blue. The extracts were centrifuged to collect the supernatants. Equal amounts of samples were separated by 10% SDS-PAGE. Fractionated proteins were then transferred to Immobilon polyvinylidene difluoride (PVDF) membranes (MilliporeSigma), and the blots were probed with anti-PVX CP, anti-CMV CP, anti-2b, and anti-rgs-CaM rabbit polyclonal antibodies. Proteins were visualized using anti-rabbit secondary antibodies conjugated to alkaline phosphatase, followed by treatment with CDP-Star solutions (Roche Diagnostics, Basel, Switzerland) for chemiluminescence detection. Chemiluminescent signals were quantitatively detected by a LAS-4000 mini PR Lumino-image analyzer (GE Healthcare).
Immunohistochemical studies with tobacco BY2 cultured cells.
Tobacco BY2 cultured cells were transformed with the CMV 2b gene under the control of the CaMV 35S promoter as described in a previous study (78), in which the transformed BY2 was called cell line Y2b-BY2. Transgenic BY2 cells expressing 2b were pretreated with 10 μM BTH with or without autophagy inhibitors E64d (10 μM) and concanamycin A (0.1 μM) for 1 h and then assayed for endogenous rgs-CaM and CMV 2b as described previously (32). The fixed cells were immunofluorescently stained with their specific primary and CF594 goat anti-rabbit IgG secondary antibodies (Biotium, Fremont, CA, USA). These cells were also fluorescently stained with 4′,6-diamino-2-phenylindole (DAPI) to detect nuclei. Photomicrographs were taken using a Leica DMI6000 B microscope (Leica Microsystems). Image colors were then reassigned using AF6000 v.1.5 software.
ACKNOWLEDGMENTS
We thank Peter Palukaitis for critically reading the manuscript.
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI grant numbers 25450055 and 16H04879 (to K.S.N.), the Novartis Foundation (to K.S.N.), and the Asahi Glass Foundation (to K.S.N.).
We declare no competing financial interests.
REFERENCES
- 1.Savvides A, Ali S, Tester M, Fotopoulos V. 2016. Chemical priming of plants against multiple abiotic stresses: mission possible? Trends Plant Sci 21:329–340. doi: 10.1016/j.tplants.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Fu ZQ, Dong X. 2013. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64:839–863. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- 3.Gilpatrick JD, Weintraub M. 1952. An unusual type of protection with the carnation mosaic virus. Science 115:701–702. doi: 10.1126/science.115.3000.701. [DOI] [PubMed] [Google Scholar]
- 4.Chester KS. 1933. The problem of acquired physiological immunity in plants. Q Rev Biol 8:275–324. doi: 10.1086/394440. [DOI] [Google Scholar]
- 5.Gao QM, Zhu S, Kachroo P, Kachroo A. 2015. Signal regulators of systemic acquired resistance. Front Plant Sci 6:228. doi: 10.3389/fpls.2015.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. 1994. A central role of salicylic acid in plant disease resistance. Science 266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 7.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 8.Palukaitis P, Carr JP. 2008. Plant resistance responses to viruses. J Plant Pathol 90:153–171. [Google Scholar]
- 9.Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. 1997. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88:57–63. doi: 10.1016/S0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- 10.Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong X. 2012. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attaran E, He SY. 2012. The long-sought-after salicylic acid receptors. Mol Plant 5:971–973. doi: 10.1093/mp/sss086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, Despres C. 2012. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1:639–647. doi: 10.1016/j.celrep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Conrath U, Beckers GJ, Langenbach CJ, Jaskiewicz MR. 2015. Priming for enhanced defense. Annu Rev Phytopathol 53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- 14.Luna E, Bruce TJ, Roberts MR, Flors V, Ton J. 2012. Next-generation systemic acquired resistance. Plant Physiol 158:844–853. doi: 10.1104/pp.111.187468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser MT, Aufsatz W, Jonak C, Luschnig C. 2011. Transgenerational epigenetic inheritance in plants. Biochim Biophys Acta 1809:459–468. doi: 10.1016/j.bbagrm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pumplin N, Voinnet O. 2013. RNA silencing suppression by plant pathogens: defence, counter-defence and counter-counter-defence. Nat Rev Microbiol 11:745–760. doi: 10.1038/nrmicro3120. [DOI] [PubMed] [Google Scholar]
- 17.Incarbone M, Dunoyer P. 2013. RNA silencing and its suppression: novel insights from in planta analyses. Trends Plant Sci 18:382–392. doi: 10.1016/j.tplants.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Alamillo JM, Saenz P, Garcia JA. 2006. Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco. Plant J 48:217–227. doi: 10.1111/j.1365-313X.2006.02861.x. [DOI] [PubMed] [Google Scholar]
- 19.Cao M, Du P, Wang X, Yu YQ, Qiu YH, Li W, Gal-On A, Zhou C, Li Y, Ding SW. 2014. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in Arabidopsis. Proc Natl Acad Sci U S A 111:14613–14618. doi: 10.1073/pnas.1407131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Ruiz H, Takeda A, Chapman EJ, Sullivan CM, Fahlgren N, Brempelis KJ, Carrington JC. 2010. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip mosaic virus infection. Plant Cell 22:481–496. doi: 10.1105/tpc.109.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang SJ, Carter SA, Cole AB, Cheng NH, Nelson RS. 2004. A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc Natl Acad Sci U S A 101:6297–6302. doi: 10.1073/pnas.0304346101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu D, Fan B, MacFarlane SA, Chen Z. 2003. Analysis of the involvement of an inducible Arabidopsis RNA-dependent RNA polymerase in antiviral defense. Mol Plant Microbe Interact 16:206–216. doi: 10.1094/MPMI.2003.16.3.206. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Fan B, Chen C, Chen Z. 2001. An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc Natl Acad Sci U S A 98:6516–6521. doi: 10.1073/pnas.111440998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu S, Jeong RD, Lim GH, Yu K, Wang C, Chandra-Shekara AC, Navarre D, Klessig DF, Kachroo A, Kachroo P. 2013. Double-stranded RNA-binding protein 4 is required for resistance signaling against viral and bacterial pathogens. Cell Rep 4:1168–1184. doi: 10.1016/j.celrep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Zhao H, Gao S, Wang WC, Katiyar-Agarwal S, Huang HD, Raikhel N, Jin H. 2011. Arabidopsis Argonaute 2 regulates innate immunity via miRNA393*-mediated silencing of a Golgi-localized SNARE gene, MEMB12. Mol Cell 42:356–366. doi: 10.1016/j.molcel.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharjee S, Zamora A, Azhar MT, Sacco MA, Lambert LH, Moffett P. 2009. Virus resistance induced by NB-LRR proteins involves Argonaute4-dependent translational control. Plant J 58:940–951. doi: 10.1111/j.1365-313X.2009.03832.x. [DOI] [PubMed] [Google Scholar]
- 27.Lewsey MG, Carr JP. 2009. Effects of DICER-like proteins 2, 3 and 4 on cucumber mosaic virus and tobacco mosaic virus infections in salicylic acid-treated plants. J Gen Virol 90:3010–3014. doi: 10.1099/vir.0.014555-0. [DOI] [PubMed] [Google Scholar]
- 28.Anandalakshmi R, Marathe R, Ge X, Herr JM Jr, Mau C, Mallory A, Pruss G, Bowman L, Vance VB. 2000. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290:142–144. doi: 10.1126/science.290.5489.142. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov KI, Eskelin K, Basic M, De S, Lohmus A, Varjosalo M, Makinen K. 2016. Molecular insights into the function of the viral RNA silencing suppressor HCPro. Plant J 85:30–45. doi: 10.1111/tpj.13088. [DOI] [PubMed] [Google Scholar]
- 30.Pruss G, Ge X, Shi XM, Carrington JC, Vance VB. 1997. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9:859–868. doi: 10.1105/tpc.9.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB. 1998. A viral suppressor of gene silencing in plants. Proc Natl Acad Sci U S A 95:13079–13084. doi: 10.1073/pnas.95.22.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakahara KS, Masuta C, Yamada S, Shimura H, Kashihara Y, Wada TS, Meguro A, Goto K, Tadamura K, Sueda K, Sekiguchi T, Shao J, Itchoda N, Matsumura T, Igarashi M, Ito K, Carthew RW, Uyeda I. 2012. Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus RNA silencing suppressors. Proc Natl Acad Sci U S A 109:10113–10118. doi: 10.1073/pnas.1201628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura H, Shin MR, Fukagawa T, Arita M, Mikami T, Kodama H. 2014. A tobacco calmodulin-related protein suppresses sense transgene-induced RNA silencing but not inverted repeat-induced RNA silencing. Plant Cell Tissue Organ Cult 116:47–53. doi: 10.1007/s11240-013-0381-4. [DOI] [Google Scholar]
- 34.Li F, Huang C, Li Z, Zhou X. 2014. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog 10:e1003921. doi: 10.1371/journal.ppat.1003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yong Chung H, Lacatus G, Sunter G. 2014. Geminivirus AL2 protein induces expression of, and interacts with, a calmodulin-like gene, an endogenous regulator of gene silencing. Virology 460-461:108–118. doi: 10.1016/j.virol.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 36.Durrant WE, Dong X. 2004. Systemic acquired resistance. Annu Rev Phytopathol 42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 37.Liu PP, Bhattacharjee S, Klessig DF, Moffett P. 2010. Systemic acquired resistance is induced by R gene-mediated responses independent of cell death. Mol Plant Pathol 11:155–160. doi: 10.1111/j.1364-3703.2009.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishina TE, Zeier J. 2007. Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J 50:500–513. doi: 10.1111/j.1365-313X.2007.03067.x. [DOI] [PubMed] [Google Scholar]
- 39.Lorrain S, Vailleau F, Balague C, Roby D. 2003. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci 8:263–271. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 40.Tang X, Xie M, Kim YJ, Zhou J, Klessig DF, Martin GB. 1999. Overexpression of Pto activates defense responses and confers broad resistance. Plant Cell 11:15–29. doi: 10.1105/tpc.11.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohshima M, Itoh H, Matsuoka M, Murakami T, Ohashi Y. 1990. Analysis of stress-induced or salicylic acid-induced expression of the pathogenesis-related 1a protein gene in transgenic tobacco. Plant Cell 2:95–106. doi: 10.1105/tpc.2.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makiyama RK, Fernandes CA, Dreyer TR, Moda BS, Matioli FF, Fontes MR, Maia IG. 2016. Structural and thermodynamic studies of the tobacco calmodulin-like rgs-CaM protein. Int J Biol Macromol 92:1288–1297. doi: 10.1016/j.ijbiomac.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Yalpani N, Shulaev V, Raskin I. 1993. Endogenous salicylic-acid levels correlate with accumulation of pathogenesis-related proteins and virus-resistance in tobacco. Phytopathology 83:702–708. doi: 10.1094/Phyto-83-702. [DOI] [Google Scholar]
- 44.Ji LH, Ding SW. 2001. The suppressor of transgene RNA silencing encoded by Cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol Plant Microbe Interact 14:715–724. doi: 10.1094/MPMI.2001.14.6.715. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Ruiz H, Murphy JF. 2001. Age-related resistance in bell pepper to Cucumber mosaic virus. Ann Appl Biol 139:307–317. doi: 10.1111/j.1744-7348.2001.tb00144.x. [DOI] [Google Scholar]
- 46.Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Rella MG, Meier B, Dincher S, Staub T, Uknes S, Metraux JP, Kessmann H, Ryals J. 1996. A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J 10:61–70. doi: 10.1046/j.1365-313X.1996.10010061.x. [DOI] [Google Scholar]
- 47.Gorlach J, Volrath S, Knauf-Beiter G, Hengy G, Beckhove U, Kogel KH, Oostendorp M, Staub T, Ward E, Kessmann H, Ryals J. 1996. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8:629–643. doi: 10.1105/tpc.8.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawton KA, Friedrich L, Hunt M, Weymann K, Delaney T, Kessmann H, Staub T, Ryals J. 1996. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J 10:71–82. doi: 10.1046/j.1365-313X.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- 49.Anfoka GH. 2000. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester induces systemic resistance in tomato (Lycopersicon esculentum. Mill cv. Vollendung) to Cucumber mosaic virus. Crop Prot 19:401–405. [Google Scholar]
- 50.Jones JD, Dangl JL. 2006. The plant immune system. Nature 444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 51.Miyashita Y, Atsumi G, Nakahara KS. 2016. Trade-offs for viruses in overcoming innate immunities in plants. Mol Plant Microbe Interact 29:595–598. doi: 10.1094/MPMI-05-16-0103-CR. [DOI] [PubMed] [Google Scholar]
- 52.Moffett P. 2009. Mechanisms of recognition in dominant R gene mediated resistance. Adv Virus Res 75:1–33. doi: 10.1016/S0065-3527(09)07501-0. [DOI] [PubMed] [Google Scholar]
- 53.Zorzatto C, Machado JP, Lopes KV, Nascimento KJ, Pereira WA, Brustolini OJ, Reis PA, Calil IP, Deguchi M, Sachetto-Martins G, Gouveia BC, Loriato VA, Silva MA, Silva FF, Santos AA, Chory J, Fontes EP. 2015. NIK1-mediated translation suppression functions as a plant antiviral immunity mechanism. Nature 520:679–682. doi: 10.1038/nature14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niehl A, Wyrsch I, Boller T, Heinlein M. 2016. Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. New Phytol 211:1008–1019. doi: 10.1111/nph.13944. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Sparrer KM, Gack MU. 2015. Intracellular detection of viral Nucleic acids. Curr Opin Microbiol 26:1–9. doi: 10.1016/j.mib.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheval C, Aldon D, Galaud JP, Ranty B. 2013. Calcium/calmodulin-mediated regulation of plant immunity. Biochim Biophys Acta 1833:1766–1771. doi: 10.1016/j.bbamcr.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 58.Bender KW, Snedden WA. 2013. Calmodulin-related proteins step out from the shadow of their namesake. Plant Physiol 163:486–495. doi: 10.1104/pp.113.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu X, Dunand C, Snedden W, Galaud JP. 2015. CaM and CML emergence in the green lineage. Trends Plant Sci 20:483–489. doi: 10.1016/j.tplants.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Patil A, Nakamura H. 2006. Disordered domains and high surface charge confer hubs with the ability to interact with multiple proteins in interaction networks. FEBS Lett 580:2041–2045. doi: 10.1016/j.febslet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Ren G, Wang X, Chen D, Wang X, Liu X. 2014. Effects of aphids Myzus persicae on the changes of Ca2+ and H2O2 flux and enzyme activities in tobacco. J Plant Interact 9:883–888. doi: 10.1080/17429145.2014.982221. [DOI] [Google Scholar]
- 62.Will T, van Bel AJ. 2006. Physical and chemical interactions between aphids and plants. J Exp Bot 57:729–737. doi: 10.1093/jxb/erj089. [DOI] [PubMed] [Google Scholar]
- 63.Lecourieux D, Ranjeva R, Pugin A. 2006. Calcium in plant defence-signalling pathways. New Phytol 171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, Frey TK, Yang JJ. 2009. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 46:1–17. doi: 10.1016/j.ceca.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsuda K, Mine A, Bethke G, Igarashi D, Botanga CJ, Tsuda Y, Glazebrook J, Sato M, Katagiri F. 2013. Dual regulation of gene expression mediated by extended MAPK activation and salicylic acid contributes to robust innate immunity in Arabidopsis thaliana. PLoS Genet 9:e1004015. doi: 10.1371/journal.pgen.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiu MH, Chen IH, Baulcombe DC, Tsai CH. 2010. The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol Plant Pathol 11:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pruss GJ, Lawrence CB, Bass T, Li QQ, Bowman LH, Vance V. 2004. The potyviral suppressor of RNA silencing confers enhanced resistance to multiple pathogens. Virology 320:107–120. doi: 10.1016/j.virol.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 68.Lewsey MG, Murphy AM, Maclean D, Dalchau N, Westwood JH, Macaulay K, Bennett MH, Moulin M, Hanke DE, Powell G, Smith AG, Carr JP. 2010. Disruption of two defensive signaling pathways by a viral RNA silencing suppressor. Mol Plant Microbe Interact 23:835–845. doi: 10.1094/MPMI-23-7-0835. [DOI] [PubMed] [Google Scholar]
- 69.Zhou T, Murphy AM, Lewsey MG, Westwood JH, Zhang HM, Gonzalez I, Canto T, Carr JP. 2014. Domains of the cucumber mosaic virus 2b silencing suppressor protein affecting inhibition of salicylic acid-induced resistance and priming of salicylic acid accumulation during infection. J Gen Virol 95:1408–1413. doi: 10.1099/vir.0.063461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Zhao N, Li Z, Xu X, Wang Y, Yang X, Liu SS, Wang A, Zhou X. 2017. A calmodulin-like protein suppresses RNA silencing and promotes geminivirus infection by degrading SGS3 via the autophagy pathway in Nicotiana benthamiana. PLoS Pathog 13:e1006213. doi: 10.1371/journal.ppat.1006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huot B, Yao J, Montgomery BL, He SY. 2014. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7:1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baulcombe DC, Chapman S, Santa Cruz S. 1995. Jellyfish green fluorescent protein as a reporter for virus infections. Plant J 7:1045–1053. doi: 10.1046/j.1365-313X.1995.07061045.x. [DOI] [PubMed] [Google Scholar]
- 73.Mitsuhara I, Ugaki M, Hirochika H, Ohshima M, Murakami T, Gotoh Y, Katayose Y, Nakamura S, Honkura R, Nishimiya S, Ueno K, Mochizuki A, Tanimoto H, Tsugawa H, Otsuki Y, Ohashi Y. 1996. Efficient promoter cassettes for enhanced expression of foreign genes in dicotyledonous and monocotyledonous plants. Plant Cell Physiol 37:49–59. doi: 10.1093/oxfordjournals.pcp.a028913. [DOI] [PubMed] [Google Scholar]
- 74.Matsuo K, Hong JS, Tabayashi N, Ito A, Masuta C, Matsumura T. 2007. Development of Cucumber mosaic virus as a vector modifiable for different host species to produce therapeutic proteins. Planta 225:277–286. doi: 10.1007/s00425-006-0346-5. [DOI] [PubMed] [Google Scholar]
- 75.Kim BM, Suehiro N, Natsuaki T, Inukai T, Masuta C. 2010. The P3 protein of Turnip mosaic virus can alone induce hypersensitive response-like cell death in Arabidopsis thaliana carrying TuNI. Mol Plant Microbe Interact 23:144–152. doi: 10.1094/MPMI-23-2-0144. [DOI] [PubMed] [Google Scholar]
- 76.Atsumi G, Kagaya U, Kitazawa H, Nakahara KS, Uyeda I. 2009. Activation of the salicylic acid signaling pathway enhances Clover yellow vein virus virulence in susceptible pea cultivars. Mol Plant Microbe Interact 22:166–175. doi: 10.1094/MPMI-22-2-0166. [DOI] [PubMed] [Google Scholar]
- 77.Yambao MLM, Yagihashi H, Sekiguchi H, Sekiguchi T, Sasaki T, Sato M, Atsumi G, Tacahashi Y, Nakahara KS, Uyeda I. 2008. Point mutations in helper component protease of clover yellow vein virus are associated with the attenuation of RNA-silencing suppression activity and symptom expression in broad bean. Arch Virol 153:105–115. doi: 10.1007/s00705-007-1073-3. [DOI] [PubMed] [Google Scholar]
- 78.Kanazawa A, Inaba J, Shimura H, Otagaki S, Tsukahara S, Matsuzawa A, Kim BM, Goto K, Masuta C. 2011. Virus-mediated efficient induction of epigenetic modifications of endogenous genes with phenotypic changes in plants. Plant J 65:156–168. doi: 10.1111/j.1365-313X.2010.04401.x. [DOI] [PubMed] [Google Scholar]