ABSTRACT
The castaneous (CAST) mouse, a wild-derived inbred strain, is highly susceptible to orthopoxvirus infection by intranasal and systemic routes. The 50% lethal intraperitoneal dose of vaccinia virus (VACV) was 3 PFU for CAST mice, whereas BALB/c mice survived 106 PFU. At all times and in all organs analyzed, virus titers were higher in CAST than in BALB/c mice. In individual CAST mice, luciferase-expressing VACV was seen to replicate rapidly leading to death, whereas virus levels increased for a few days and then declined in BALB/c mice. Increases in gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) were delayed and low in CAST mice compared to BALB/c mice following VACV infection or poly(I-C) inoculation, consistent with differences in innate immune responses. In addition, naive CAST mice had considerably lower numbers of NK and T cells than BALB/c mice. The percentage of IFN-γ-producing CD4+ and CD8+ T cells increased following infection of CAST mice only after considerable virus spread, and the absolute cell numbers remained low. Administration of exogenous IFN-γ or -α to CAST mice before or during the first days of infection suppressed virus replication and prolonged survival, allowing the mice to make adaptive CD4+ and CD8+ T cell responses that were necessary to clear the virus after cessation of interferon treatment. Thus, insufficient innate cytokine and cellular immune responses contribute to the unique susceptibility of CAST mice to VACV, whereas the adaptive immune response can be protective only if virus replication is suppressed during the first several days of infection.
IMPORTANCE Most inbred mouse strains are relatively resistant to orthopoxviruses. The castaneous (CAST) mouse is a notable exception, exhibiting extreme vulnerability to monkeypox virus, cowpox virus, and vaccinia virus and thus providing a unique model for studying pathogenicity, immunity, vaccines, and antiviral drugs. To fully utilize the CAST mouse for such purposes, it is necessary to understand the basis for virus susceptibility. We showed that naive CAST mice make low IFN-γ and TNF-α responses and have low levels of NK cells and CD4+ and CD8+ T cells compared to a resistant classical inbred mouse strain. Attenuating virus replication with one or more doses of exogenous IFN-α or -γ before or during the first few days of infection enabled the development of adaptive cellular immunity and clearance of virus. Further genetic studies may reveal the basis for the low innate immunity.
KEYWORDS: NK cells, T cells, adaptive immunity, immune deficiency, innate immunity, interferons, poxvirus, vaccinia virus
INTRODUCTION
During extensive mouse screening, we previously discovered that CAST/EiJ and CASA/RkJ, two independently inbred derivatives of Mus musculus castaneus (the Southeastern Asian house mouse), are highly susceptible to intranasal (i.n.) infections with monkeypox virus (MPXV), whereas 32 classical inbred strains, including BALB/c and C57BL/6 mice, are resistant (1, 2). Castaneous (CAST) mice are genetically distant from classical mouse strains (3, 4), and cross-breeding with resistant mice suggests that their susceptibility to MPXV depends on multiple loci (2). CAST mice are very susceptible to infections with additional orthopoxviruses such as vaccinia virus (VACV) and cowpox virus and also to influenza virus (5) but not to herpes simplex virus (6) or flaviviruses under the infection protocols tested (7).
Following i.n. infection, robust replication of MPXV occurs in the lungs of both BALB/c and CAST mice. Spread of MPXV to other internal organs is rapid in CAST mice, whereas the virus is largely restricted to the lungs in BALB/c mice. The spread to internal organs is likely the cause of mortality in CAST mice as the 50% lethal dose (LD50) is more than a log lower when MPXV is administered intraperitoneally (i.p.) compared to i.n. (14 PFU versus 680 PFU) (1). Clearance of MPXV from BALB/c mice correlated with a strong induction of gamma interferon (IFN-γ) and CCL5 in the lungs, which did not occur in CAST mice (8). Daily administration of IFN-γ by the i.n. route during the first 5 days of infection protected CAST mice against lethal i.n. infection with MPXV. Despite their susceptibility to primary infection, CAST mice are able to mount effective humoral and T cell responses when immunized with an attenuated strain of VACV and are protected against a subsequent MPXV challenge (1). The data point to a deficit in innate immunity; however, further investigations are needed, and neither NK nor T cell responses following primary infection of CAST mice with pathogenic orthopoxviruses had been analyzed.
The goal of the present study was to evaluate both the innate and adaptive immune responses of CAST mice in order to better understand their unique susceptibility to orthopoxvirus infection. CAST mice are susceptible to both MPXV and VACV strain WR with similar disease courses following i.n. administration (1, 6, 8). However, MPXV is a select agent requiring use of secure containment and special precautions to bring infected materials into common areas for special analyses such as flow cytometry. For this reason, we chose to use VACV strain WR in the present study. We discovered that CAST mice, compared to BALB/c mice, are exquisitely sensitive to i.p. infection with VACV. The induction of IFN-γ and tumor necrosis factor alpha (TNF-α) was reduced and delayed in CAST mice compared to BALB/c mice, consistent with an insufficient innate immune response. Naive CAST mice had very low levels of splenic NK cells and CD4+ and CD8+ T cells. Although the percentage of IFN-γ-producing T cells increased after infection, the absolute numbers remained low, and extensive virus spread had already occurred by this time. Exogenous IFN-γ, IFN-α, or poly(I-C) administered before or during the first few days of infection was sufficient to suppress virus replication long enough for adequate adaptive CD4+ and CD8+ T cell responses that were necessary to clear the virus. Thus, delayed and insufficient innate cytokine and cellular immune responses contribute to the sensitivity of CAST mice to orthopoxvirus infection.
RESULTS
Susceptibilities of CAST and BALB/c mice to systemic VACV infection.
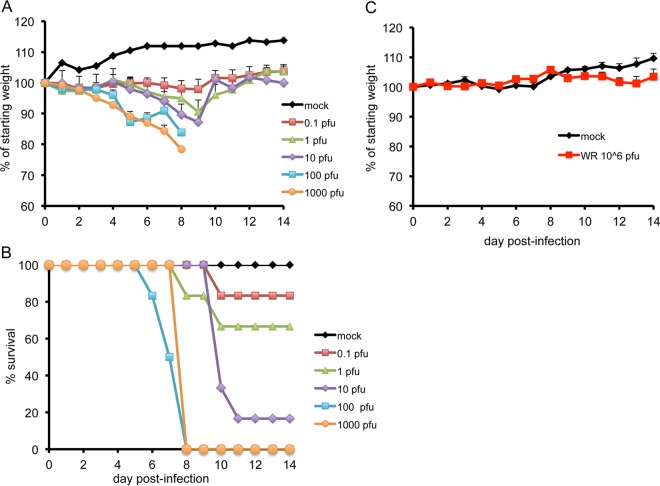
CAST mice are more susceptible to i.n. infection with VACV strain WR than are BALB/c mice, with LD50 values of approximately 102 and 2.2 × 104 PFU, respectively (6). To determine the susceptibility of CAST mice to i.p. inoculation of VACV, groups of mice were infected with 0.1 to 1,000 PFU of virus. BALB/c mice are resistant to VACV by this route and therefore were infected with a single high dose of 106 PFU. CAST mice infected with 100 and 1,000 PFU lost up to 22% of their starting weight, and all succumbed to death by day 8 postinfection (Fig. 1A and B). With 1 or 10 PFU, weight loss was delayed in comparison to the higher-dose groups and some deaths occurred between days 8 and 11 postinfection. No weight loss was observed in mice that received 0.1 PFU, although there was one death in this group. In contrast, BALB/c mice showed only minor signs of illness, no weight loss, and 100% survival at the 106-PFU dose (Fig. 1C). The LD50 values were 3 and >106 PFU for CAST and BALB/c mice, respectively. The greater susceptibility of CAST mice to inoculation of VACV by the i.p. route compared to i.n. was similar to results obtained with MPXV (1). Further experiments were carried out using the i.p. route because of the extreme sensitivity of CAST mice and striking difference from BALB/c mice as well as previous data suggesting that spread to internal organs is the cause of death even when the virus is administered i.n.
FIG 1.
Weight loss and survival. CAST mice (7 to 8 weeks old) were mock infected (n = 3) or infected with 0.1, 1, 10, 100 (n = 6 each group) or 1,000 (n = 3) PFU of VACV strain WR by the i.p. route. BALB/c mice (8 weeks old) were mock infected (n = 3) or infected with 106 PFU (n = 5). Animals were weighed daily. Standard deviations are plotted as error bars in panels A and C.
Virus replication in organs.
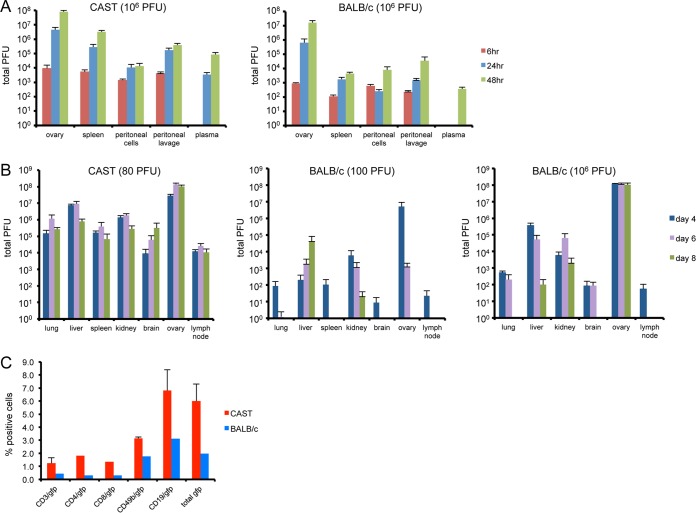
Groups of BALB/c and CAST mice were infected i.p. with 106 PFU of VACV and sacrificed at various times postinfection, and virus titers were determined by plaque assay. Virus was detected in the ovaries, spleen, and peritoneal cells and fluid at 6 h and was increased at 48 h postinfection in both mouse strains (Fig. 2A). Virus was not detected in the plasma until 24 h in CAST mice and 48 h in BALB/c mice. At all times and in all organs and compartments analyzed, virus titers were higher in CAST than in BALB/c mice.
FIG 2.
Replication of VACV in CAST and BALB/c mice. (A) CAST and BALB/c mice were injected i.p. with 106 PFU of VACV (n = 5) and sacrificed at 6, 24, and 48 h. Organs were homogenized, and virus titers were determined by plaque assay. (B) CAST mice were infected i.p. with 80 PFU and BALB/c mice with 102 or 106 PFU (n = 3 to 8), and organ titers were determined as in panel A. Error bars indicate standard deviations. (C) In vitro infection of spleen cells. Splenocytes were prepared from naive CAST and BALB/c mice and infected overnight with 10 PFU/cell of VV.NP-S-EGFP. Cells were stained for T cell, B cell, and NK cell subsets and enumerated by flow cytometry. The data are from three separate infections. Standard deviations are shown by error bars.
In order to follow replication for a longer period in the CAST mouse, we infected both strains with approximately 102 PFU and also repeated the infection of BALB/c mice with 106 PFU for comparison. Mice were euthanized on days 4, 6, and 8 postinfection, and virus yields were determined. At the low dose, there was a striking difference in the amount of virus in organs from CAST and BALB/c mice; e.g., the amount in CAST mice was 2 to 5 logs higher than that in BALB/c mice, except for the ovary, in which the difference was little more than 1 log (Fig. 2B). In addition, virus was no longer detected at day 8 in many organs, including lung, spleen, brain, ovary, and lymph node of BALB/c mice infected with the low dose, while the virus titers in organs of CAST mice remained uniformly high. Even at the high dose of 106 PFU, the virus yields in all organs except ovaries were lower in BALB/c mice than those in CAST mice infected with 80 PFU.
More virus was detected in the organs of CAST mice than in those of BALB/c mice, even at 6 h, which is before there is extensive virus replication. In vitro experiments were carried out to investigate whether there is a difference in the susceptibilities of cells from CAST and BALB/c mice to virus infection. Spleen cells from CAST and BALB/c mice were infected overnight with 10 PFU/cell of a VACV strain that expressed green fluorescent protein (GFP) under an early/late promoter. Analysis by flow cytometry showed that a relatively small percentage of cells were infected, in the following order: B cells > NK cells > T cells (Fig. 2C). The percentage of infected cells of each type was severalfold higher from CAST mice compared to BALB/c mice. This could represent an intrinsic difference in the susceptibilities to infection of cells from the two mouse strains. However, we will show in a subsequent section that BALB/c mice produce larger amounts of IFN-γ than CAST mice, and we considered that this might contribute to the lower numbers of spleen cells infected in vitro. Although this may be correct, the levels of IFN-γ and IFN-α in the supernatant were below our level of detection (data not shown).
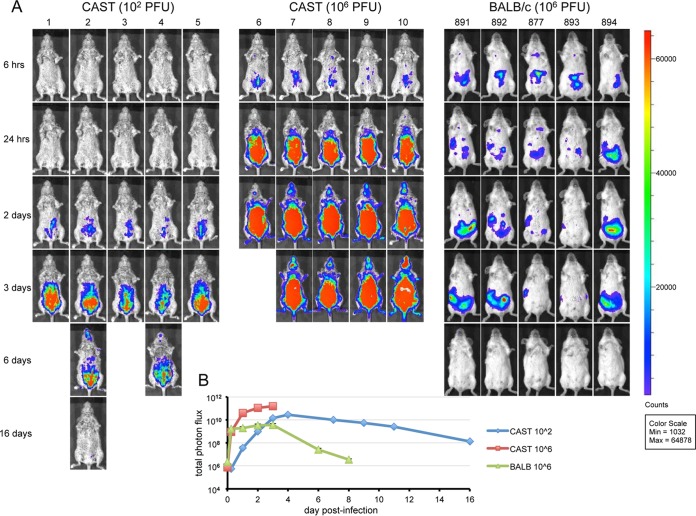
Bioluminescence imaging.
Bioluminescence imaging has the advantage of allowing tracking of virus spread in individual animals over the course of the disease. We infected mice with WRvFire, a recombinant VACV with no genes disrupted that expresses firefly luciferase under the control of an early/late promoter (9). Previous studies showed that luciferase expression does not affect virulence of VACV or MPXV (10, 11). Groups of CAST mice were infected i.p. with 102 or 106 PFU of WRvFire, whereas BALB/c mice were infected only with the higher dose. The animals were imaged repeatedly, starting at 6 h postinfection and continuing until death or clearance of virus. The images may be directly compared as the same exposure time and color scale were used. The luminescence in CAST and BALB/c mice was mainly localized to the abdominal area, although there were large differences in intensity and timing depending on the size of the inoculum and mouse strain (Fig. 3A). Living Image software was used to calculate the total photon flux (photons per second per square centimeter per steradian) of the entire animals, which was averaged for each group in Fig. 3B. At 6 h postinfection, the intensities of luminescence appeared similar in BALB/c and CAST mice infected with 106 PFU. However, luciferase levels in the BALB/c mice increased only slightly thereafter and began to diminish within a few days. In contrast, replication was rapid and robust in CAST mice infected with 106 PFU, with an increase in luminescence of more than 2 logs before the mice succumbed by day 4. At the 102-PFU dose in CAST mice, luminescence increased more slowly (Fig. 3B); although the photon flux appeared to be decreasing, there were only two survivors by day 6 and one by day 16 in the 102-PFU group. The maximum photon fluxes were 1.5 × 1011 and 2.7 × 1010 photons/s/cm2 in CAST mice infected at 106 and 102 PFU, respectively, compared to 3.5 × 109 photons/s/cm2 in BALB/c mice. Taken together, the data indicated that the greater susceptibility of CAST mice than BALB/c mice correlated with the failure to limit virus replication during the first few days and subsequently clear the virus.
FIG 3.
Bioluminescence imaging. CAST mice were infected i.p. with 102 or 106 PFU and BALB/c mice with 106 PFU of the recombinant VACV WRvFire expressing firefly luciferase. Animals were injected i.p. with luciferin on the indicated days and imaged as described in Materials and Methods. (A) Ventral views of surviving mice are shown on indicated days. (B) Total photon flux (photons per second per square centimeter per steradian) of the entire animals averaged for each group. The time 0 value represents background luminescence. Mouse identification numbers are shown at the top of the figure.
Cytokine responses.
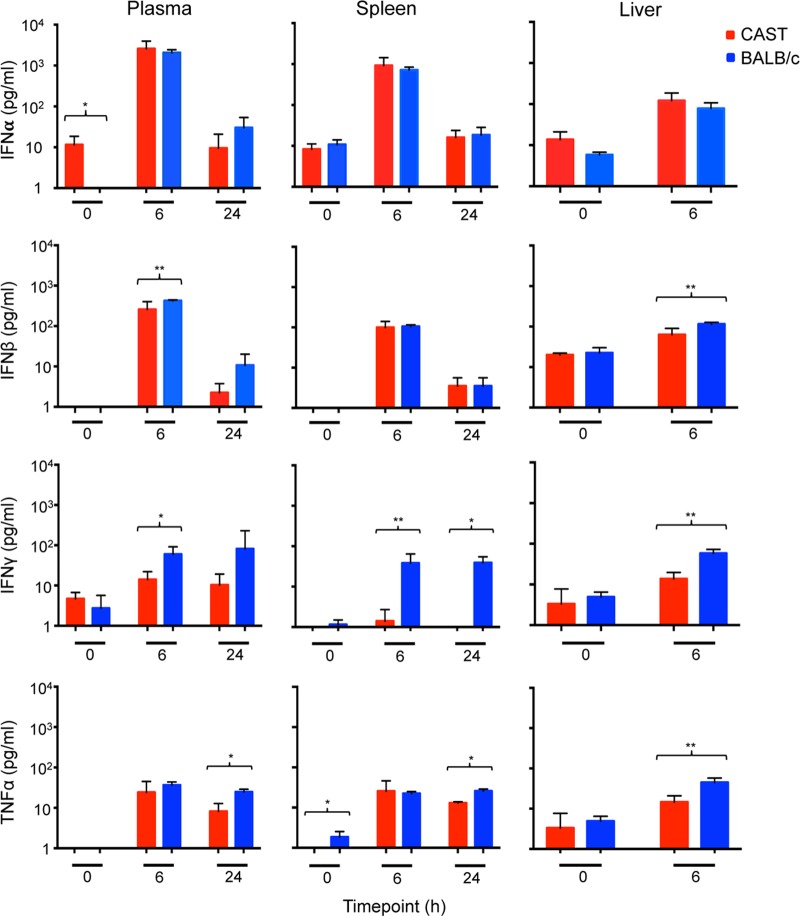
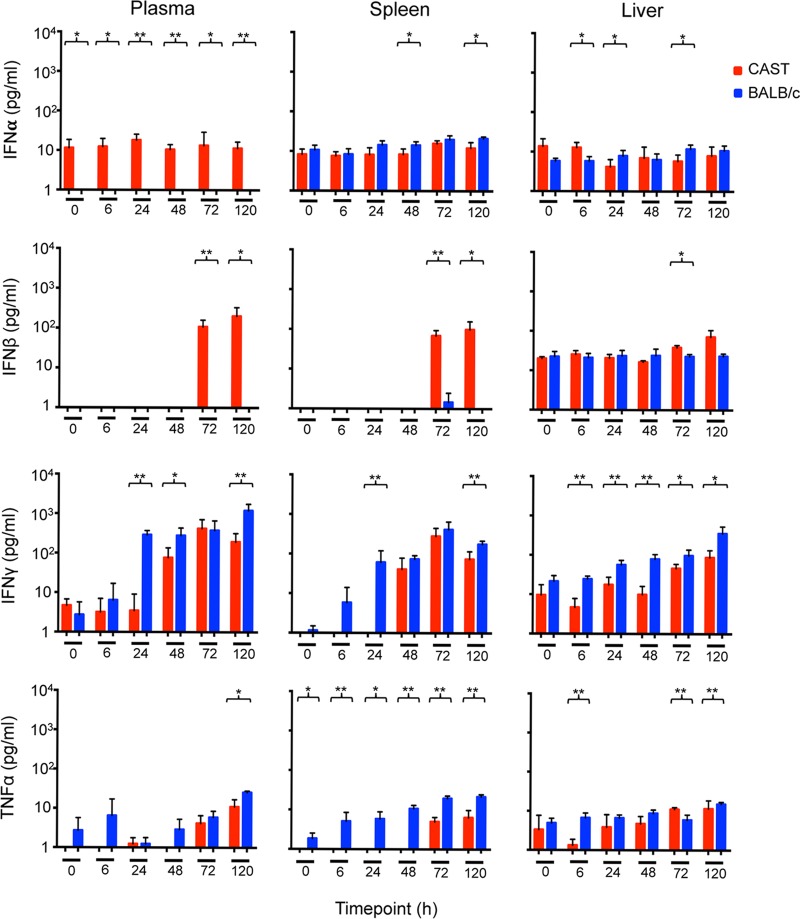
To investigate whether differences in the innate immune responses of CAST and BALB/c mice could contribute to their distinct sensitivities to VACV infection, we measured IFN-α, IFN-β, IFN-γ, and TNF-α levels in the plasma, spleen, and liver. The main producers of IFN-α, IFN-β, IFN-γ, and TNF-α are leukocytes, fibroblasts, NK cells plus T cells, and activated macrophages, respectively.
First, we wanted to determine the ability of CAST mice to make an innate immune response in the absence of virus infection. For this analysis, CAST and BALB/c mice received an i.p. injection of poly(I-C), a mimic of double-stranded RNA and a Toll-like receptor 3 (TLR3) ligand. IFN-α and IFN-β increased by about 2 logs in the plasma and spleens and somewhat less in the livers of both mouse strains, although IFN-β was slightly higher in the BALB/c mice (Fig. 4). There was also a slightly higher level of TNF-α in BALB/c mice compared to CAST mice. The most consistent difference was in IFN-γ, which was higher at each time point in all three organs of BALB/c mice than in CAST mice (Fig. 4).
FIG 4.
Induction of cytokines following inoculation of CAST and BALB/c mice with poly(I-C). Groups (n = 5) of CAST and BALB/c mice were inoculated i.p. with 200 μg of poly(I-C) and sacrificed at 6 and 24 h postinoculation. The 0-h group received no poly(I-C). Plasma and spleen and liver homogenates were assayed for IFN-α, IFN-β, IFN-γ, and TNF-α, and the mean values in picograms per milliliter were plotted at the indicated times. Parentheses and asterisks represent significant differences at the P = 0.05 (*) or P = 0.01 (**) level. Error bars indicate standard deviations.
Next, CAST mice were infected i.p. with a lethal dose of 200 PFU and BALB/c mice with a sublethal dose of 106 PFU of VACV. Despite the difference in the amounts of virus inoculated, we confirmed in this experiment that larger amounts of virus were present in the spleens of CAST mice compared to BALB/c mice from 24 h on and in the livers from 48 h on (not shown). In contrast to the cytokine results with poly(I-C), there was no increase in IFN-α following i.p. infection of CAST or BALB/c mice, and IFN-β only increased in CAST mice at 72 h, after extensive virus spread and likely tissue destruction occurred (Fig. 5). As with poly(I-C), the IFN-γ response was lower in CAST mice than in BALB/c mice at all times and tissues. TNF-α was also lower in CAST mice than in BALB/c mice (Fig. 5). Thus, CAST mice made lower IFN-γ and TNF-α responses than BALB/c mice in response to poly(I-C) and VACV.
FIG 5.
Induction of cytokines following infection of CAST and BALB/c mice with VACV. Groups (n = 5) of CAST and BALB/c mice were inoculated i.p. with 200 or 106 PFU of VACV, respectively. Infected mice were sacrificed at 6, 24, 48, 72, and 120 h postinoculation, and plasma and organs were harvested. The 0-h group received no virus. Plasma, and spleen and liver homogenates were assayed for IFN-α, IFN-β, IFN-γ, and TNF-α, and the mean values in picograms per milliliter were plotted at the indicated times. Parentheses and asterisks represent significant differences at the P = 0.05 (*) or P = 0.01 (**) level. Error bars indicate standard deviations.
Analysis of splenic NK cells.
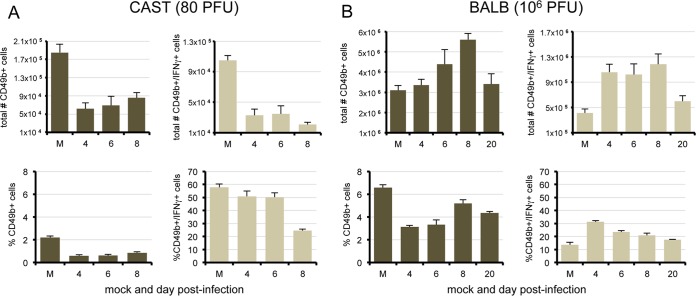
NK cells were of particular interest since they produce IFN-γ and TNF-α, are among the first lymphocytes to become activated following virus infection (12, 13), and are important in limiting the replication of VACV in mice (14–16). It was reported previously that i.p. infection of C57BL/6 mice with VACV results in decreased numbers of NK cells in the spleen during the first 2 days, followed by an increase between days 3 and 5 due to active proliferation (17). In the present study, BALB/c mice were infected i.p. with a sublethal dose of 106 PFU of VACV and sacrificed on days 4, 6, 8, and 20. Splenic NK cells were enumerated by flow cytometry using antibodies that recognize mouse CD3 and CD49b (18). Total BALB/c CD3− CD49b+ cell numbers increased and peaked on day 8, whereas the subset of IFN-γ-producing NK cells, determined after phorbol 12-myristate 13-acetate (PMA)-ionomycin stimulation, increased significantly (P = 0.0016) by day 4 and maintained high levels through day 8 (Fig. 6B).
FIG 6.
Splenic NK cells in CAST and BALB/c mice infected with VACV WR. CAST (A) and BALB/c (B) mice (n = 5) were mock infected (M) or infected i.p. with 80 or 106 PFU of VACV, respectively. NK cells were harvested from spleens, and the population of CD3− CD49b+ cells was enumerated by flow cytometry.
CAST mice were infected i.p. with a lethal dose of 80 PFU of VACV and sacrificed on days 4, 6, and 8. The total numbers of splenic NK cells in naive CAST mice were more than a log lower than in naive BALB/c mice, and they decreased further by day 4, with little recovery on subsequent days (Fig. 6A). Although a high percentage of CAST NK cells expressed IFN-γ, the absolute numbers were very low.
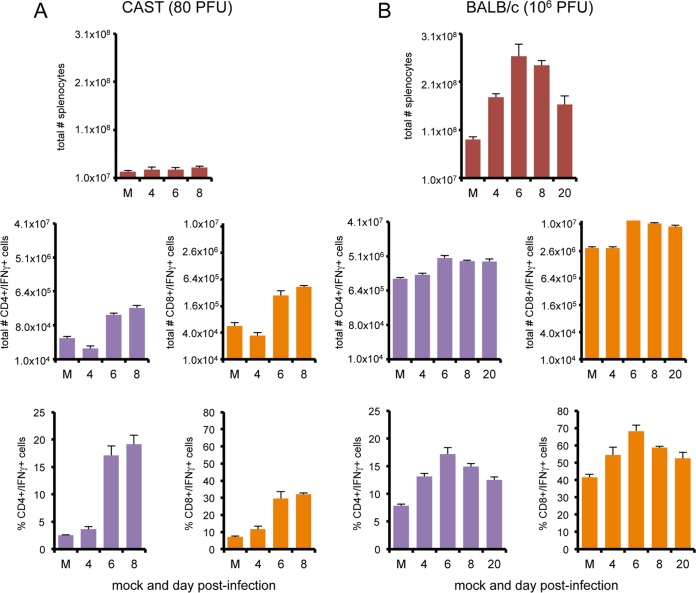
IFN-γ-producing CD4+ and CD8+ T cells.
CD4+ and CD8+ T cells provide additional sources of IFN-γ, although later after infection than NK cells. Indeed, previous studies showed that expansion of IFN-γ-producing CD4+ and CD8+ T cells occurs in the spleen, peaking on day 7 following i.p. infection of BALB/c mice with VACV WR (19). In the present study, infected CAST and BALB/c mice were euthanized on days 4, 6, and 8 postinfection and the total numbers of splenic cells and IFN-γ-producing CD4+ and CD8+ T cells were determined after PMA-ionomycin activation. BALB/c mice infected with 106 PFU of VACV exhibited nearly a 3-fold increase in the total number of spleen cells by day 6 postinfection and 3- and 4-fold increases in IFN-γ-producing CD4 and CD8 cells (Fig. 7B). The increases corresponded in time with clearance of virus.
FIG 7.
Splenic T cell populations in CAST and BALB/c mice infected with VACV WR. CAST (n = 5) (A) and BALB/c (n = 5) (B) mice were mock infected (M) or infected with 80 or 106 PFU of VACV WR, respectively. Spleen cells were activated with PMA-ionomycin. Total splenocytes and CD4+ and CD8+ subsets expressing IFN-γ were enumerated by flow cytometry on the days indicated. Error bars indicate standard deviations.
In naive CAST mice, there was a remarkably low number of total spleen cells (Fig. 7A), with the majority being B cells (data not shown). IFN-γ-producing CD4 and CD8 T cells increased 4- and 5-fold by day 6, but the absolute numbers remained low. Moreover, the increase occurred after extensive virus replication.
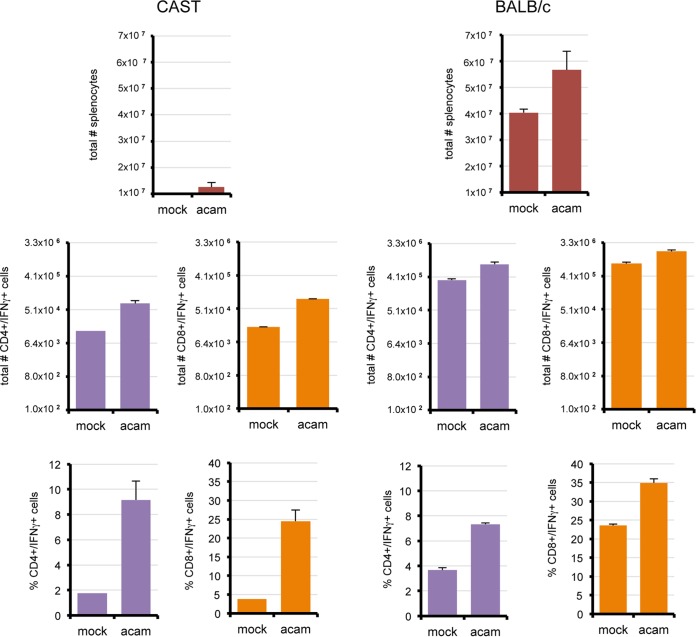
We were only able to measure cellular immune responses of CAST mice after infection with low doses of the virulent WR strain of VACV because of the rapid death at high doses. We considered the possibility that prolonged survival and better immune responses might occur with higher doses of an attenuated VACV strain. Preliminary studies with the ACAM2000 strain of VACV established that it was nonlethal for CAST and BALB/c mice, enabling use of equivalent high virus doses in both mouse strains. Groups of CAST and BALB/c mice were infected i.p. with 107 PFU and euthanized on day 8 postinfection. In BALB/c mice, the numbers of CD4+ IFN-γ+ and CD8+ IFN-γ+ spleen cells increased more than 2-fold (Fig. 8), which was slightly less than occurred after infection with VACV WR. CAST mice had nearly 6-fold increases in CD4+ IFN-γ+ and CD8+ IFN-γ+ cells (Fig. 8), also similar to what occurred upon infection with VACV WR. Despite the increases in the percentage of IFN-γ cells, the absolute numbers were still much lower in CAST mice than in BALB/c mice. NK IFN-γ+ cell numbers were not significantly changed upon infection of either mouse strain with ACAM2000 (not shown).
FIG 8.
Splenic T cell populations in CAST and BALB/c mice infected with VACV ACAM2000. CAST (n = 5) and BALB/c (n = 5) mice were infected i.p. with 107 PFU of VACV ACAM2000. Spleen cells were activated with PMA-ionomycin. Total splenocytes and CD4+ and CD8+ subsets were enumerated by flow cytometry on the days indicated. Error bars indicate standard deviations.
Protection of CAST mice by treatment with exogenous IFN-γ, IFN-α, or poly(I-C).
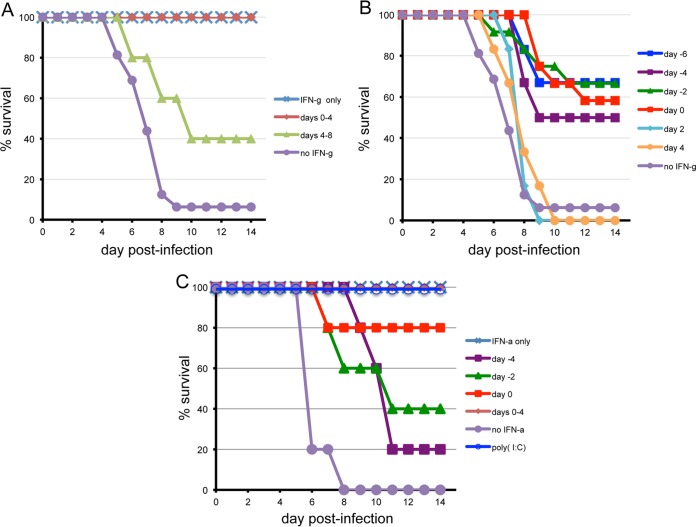
Because of the rapid replication of VACV WR leading to death in CAST mice, we were still uncertain whether they were capable of developing an effective adaptive immune response. To investigate this, we enhanced the weak innate immunity of CAST mice by providing exogenous cytokines to attenuate virus replication and allow a window of time for development of adaptive immunity. Delivery of IFN-γ on 5 consecutive days starting on the day of challenge fully protected mice from subsequent death, whereas a similar treatment regimen initiated 4 days after challenge was only partially protective (Fig. 9A). A single dose of IFN-γ, given on the day of challenge or up to 6 days prior to challenge, still protected 50% or more of the animals, whereas a single dose at 2 or 4 days after infection had no effect (Fig. 9B). Poly(I-C) or IFN-α also protected CAST mice. Survival was 100% when poly(I-C) was given on day 0 or when IFN-α was provided for the first 4 days (Fig. 9C). A single injection of IFN-α on day 0 protected 4 of 5 animals; there was less protection when IFN-α was given on day −2 or −4 (Fig. 9C). The ability of both type I and type II IFNs to protect indicated that the effect was not specific for the latter. The ability of CAST mice to survive even after cytokine administration was stopped suggested the development of an effective adaptive immune response.
FIG 9.
Exogenous IFN-γ, IFN-α, and poly(I-C) protected CAST mice against lethal infection with VACV WR. Doses of 5 × 103 units of IFN-γ (A and B) or IFN-α (C) or of 200 μg poly(I-C) were administered i.p. to CAST mice (n = 3 to 6) on multiple days or a single day as indicated. Survival was determined after i.p. infection with approximately 150 PFU of VACV WR. In panel B, data were combined from 2 separate experiments.
Role of T cells in clearing VACV following IFN-γ treatment.
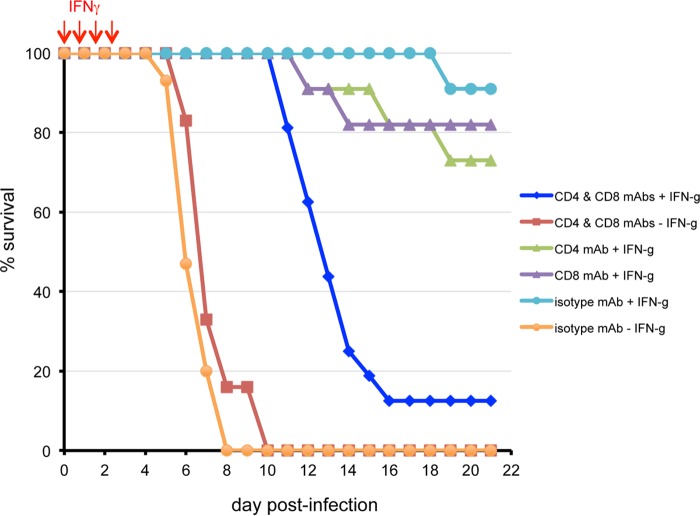
We assessed the importance of T cells during and after the period of IFN-γ treatment, by depleting CD4+ and/or CD8+ cells prior to infection. The extent of depletion was determined by flow cytometry and shown to be >98% for CD4+ and CD8+ T cells on days 5, 13, and 29 after administration. Mice were untreated or treated with exogenous IFN-γ on 4 consecutive days, beginning on the day of challenge. As expected, mice that did not receive IFN-γ mostly succumbed within 8 days, regardless of whether T cells were depleted or not (Fig. 10). Mice that received IFN-γ nearly all survived when T cells were not depleted (e.g., received control isotype monoclonal antibody [MAb]). When both CD4+ and CD8+ T cells were depleted, the mice survived for 5 or more days after the cessation of IFN-γ treatment, but most then succumbed (Fig. 10). When only one T cell subset was depleted, the survival rate was 70 to 80% (Fig. 10). Thus, both cell types contributed to protection.
FIG 10.
Effect of T cell depletion on protection afforded by exogenous IFN-γ. CAST mice were inoculated i.p. with antibodies to CD4 or CD8 cells alone or CD4 plus CD8 cells or with isotype-specific antibodies on days −3 and −4 and infected with 212 to 560 PFU of VACV on day 0. IFN-γ was administered daily on days 0 to 3. Survival data were combined from 3 separate experiments. Two experiments were performed with VACV WR and the third with VACV WRvFire. Bioluminescence data obtained with WRvFire were used for the experiment shown in Fig. 11.
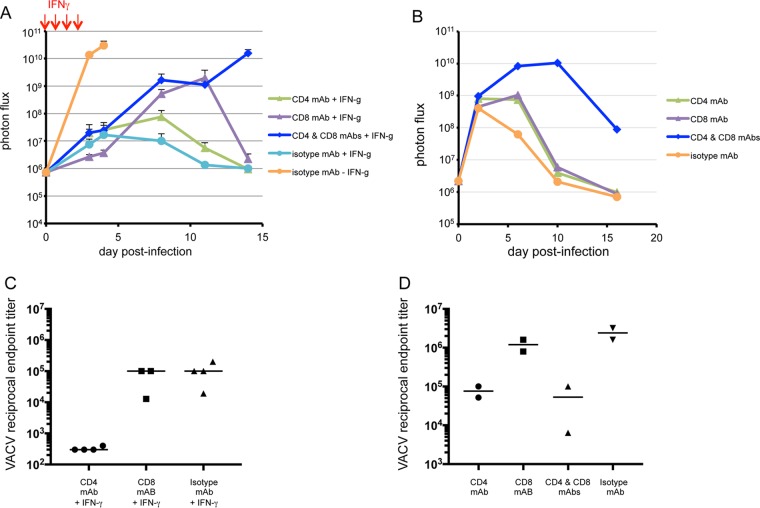
Insight into how T cell depletion and IFN-γ treatment affected virus replication was attained by imaging CAST mice at intervals after infection with a lethal dose (560 PFU) of WRvFire. In the absence of IFN-γ, virus replication was rapid and robust, with high levels of luminescence on day 4 in CAST mice (Fig. 11A). IFN-γ given on days 0 to 4 dramatically reduced virus replication regardless of whether T cells were depleted, as determined by luciferase activity on the final day of treatment. Clearing of virus occurred on subsequent days in mice receiving control isotype MAb (Fig. 11A). Mice depleted of either CD4+ or CD8+ T cells showed a rise in luminescence, which was higher in the CD8-depleted group but in each case was followed by clearing in survivors. However, mice that were depleted of both CD4+ and CD8+ T cells exhibited a progressive increase in luminescence that by day 14 reached a level equivalent to that in nontreated mice, and all succumbed. Thus, T cells were not required to restrict virus replication during the 4 days of IFN-γ treatment but were necessary to prevent extensive replication thereafter.
FIG 11.
Bioluminescence imaging and antibody production in infected CAST and BALB/c mice depleted of T cells. CAST (A) and BALB/c (B) mice were injected with MAbs to CD4 or CD8 cells alone or CD4 plus CD8 cells on days −3 and −4 and on day 0 infected with 560 or 106 PFU of VACV WRvFire, respectively. CAST mice were treated with IFN-γ on days 0 to 3 (indicated by red arrows). Bioluminescence imaging was performed on the days indicated. The survival of IFN-γ-treated CAST mice injected with isotype-, CD8-, CD4-, and CD4-plus-CD8-specific MAbs were 80, 60, 80, and 0%, respectively. No CAST mice injected with isotype-specific MAb survived in the absence of IFN-γ. On day 29, blood was obtained from 3 or 4 surviving CAST mice per group (C) and 2 BALB/c mice per group (D) and analyzed by ELISA for antibodies to VACV. Reciprocal endpoint titers are shown.
Virus replication was also followed by luminescence in T-cell-depleted BALB/c mice that were not treated with IFN-γ. Mice were infected with 106 PFU of WRvFire and imaged until day 16. Two days after infection, similar levels of virus were detected regardless of whether T cells were depleted or not (Fig. 11B). Virus was rapidly cleared in normal mice but was sustained for several days in mice that were either CD4+ or CD8+ T cell depleted and for a longer time when both subtypes were depleted. Nevertheless, the BALB/c mice all survived.
Antibody titers in the surviving mice from the luminescence experiments were determined on day 29. The titers of CD8-depleted mice were similar to those of the isotype controls, whereas the titers were lower in the surviving CD4-depleted CAST mice (Fig. 11C). Although, BALB/c mice lacking CD4+ T cells had lower antibody titers than the isotype control and CD8+ T-cell-depleted groups, the titers were still substantial and might have contributed to clearance. These results were consistent with the known role of CD4+ T cells in enhancing antibody responses.
Since depletion of CD4+ T cells led to a reduced VACV antibody response, we investigated the effect of depleting B cells with mouse anti-mouse CD20 MAb. Previous studies have shown that depletion is dependent on Fc receptor mechanisms, occurs mainly in the liver by Kupfer cells, and is dependent on circulatory dynamics (20–22). Although we could achieve 96 to 99% depletion in BALB/c mice, depletion occurred more slowly and was lower in CAST mice. In one experiment in which 200 μg of MAb was given i.p., the B cell numbers in the blood of CAST mice were reduced 70 to 73% by day 7, after which time a 4-day course of IFN-γ was started and the mice were infected. All mice survived regardless of whether B cells were depleted. In a second experiment, the mice were injected intravenously with 100 μg of MAb, resulting in 82 to 87% depletion of B cells by day 13, after which IFN-γ treatment was started and the mice were infected. Again, all mice survived. At the end of experiment 1, the anti-VACV enzyme-linked immunosorbent assay (ELISA) reciprocal endpoint titers varied from 1,600 to 300,000 (median, 19,200) in nondepleted mice and from <100 to 19,200 (median, 3,200) in B-cell-depleted mice. At the end of experiment 2, the titers varied from 19,200 to 200,000 (median, 52,000) in nondepleted mice and from <100 to 38,800 (median, 12,000) in B-cell-depleted mice. The antibody titers reflected the incomplete depletion of B cells. However, even individual animals with very low antibody titers survived, suggesting that T cells were most important.
DISCUSSION
Commonly used laboratory mouse strains are relatively resistant to infection by most orthopoxviruses, so that high doses are required to elicit disease and death. An exception is ectromelia virus, for which there are highly susceptible mouse strains. Experimental and genetic studies indicate type 1 cytokines, particularly IFN-γ, and the potency of the cell-mediated immune response account for differences between ectromelia-resistant C57BL/6 mice and susceptible BALB/c, A/J, and DBA/2 mice (23–25). Nevertheless, those ectromelia-susceptible as well as resistant strains are all resistant to MPXV (1, 26) and relatively resistant to other orthopoxviruses. However, SCID mice lacking functional B and T cells are susceptible to MPXV given i.p. or i.n. (27, 28), and STAT 1-deficient mice defective in type 1 and 2 IFN signaling are vulnerable to i.n. infection (28). Thus, MPXV has an intrinsic ability to replicate and spread in the mouse but is inhibited by immune mechanisms. It was therefore of interest to determine why CAST mice are more susceptible to orthopoxviruses, including MPXV, cowpox virus, and VACV, than more widely used strains such as BALB/c.
BALB/c mice are more susceptible to administration of VACV by the i.n. route than the i.p. route, whereas the reverse was true for CAST mice. Virus titrations demonstrated higher titers of virus in all abdominal organs of CAST mice compared to BALB/c mice starting at 6 h after i.p. infection. Live imaging of individual mice infected with a recombinant VACV expressing luciferase confirmed virus spread throughout the abdominal organs of CAST mice, leading to death in 4 to 8 days, depending on the dose. Although virus spread also occurred during the first few days in BALB/c mice, clearing subsequently occurred. The above scenario suggested to us a relative deficiency in the immediate innate immune response of CAST mice compared to BALB/c mice, which was confirmed by determining cytokine levels in the plasma, spleen, and liver. We found that BALB/c made more rapid and higher IFN-γ and TNF-α responses than CAST mice following infection with VACV or inoculation of poly(I-C). Rapid induction of IFN-α and -β occurred after injection of poly(I-C) but not VACV in both mouse strains.
The low level of IFN-γ in CAST mice may be related to a relative deficiency in NK cells. Of 32 inbred mouse strains examined for the number of NK cells in the blood (Mouse Phenome Database), CAST was number 29 and MOLF was 32. MOLF, like CAST, is a wild-derived inbred strain that is highly susceptible to MPXV (1) but was not further studied because of availability issues. In the present study, we found that the number of NK cells in spleens of naive CAST mice were more than a log lower than in BALB/c mice. CAST mice also had lower numbers of CD4+ and CD8+ T cells than BALB/c mice. Even though the percentages of cells capable of expressing IFN-γ increased in the spleens of CAST mice, the total numbers were still low, and by this time the virus had replicated extensively. A recent study showed that CAST mice are susceptible to influenza virus, which was also attributed to low numbers of NK cells and CD4+ and CD8+ T cells (5).
We considered that if the susceptibility of CAST mice is mainly due to an insufficient innate immune response, then administration of cytokines might alleviate the deficiency and provide a protected window during which an adaptive immune response could be established. On the other hand, the protection afforded by cytokines might be short-lived if CAST mice were also incapable of making a sufficient adaptive immune response. Our previous study had shown that providing exogenous IFN-γ i.n. during the first 5 days after i.n. infection of CAST mice with MPXV allowed the animals to survive, but the basis for this was not explored (8). In the present study, we demonstrated that either IFN-γ, IFN-α, or poly(I-C) administered before or immediately after infection prevented lethality following i.p. infection. Bioluminescent imaging revealed that IFN-γ reduced the virus load substantially and that the virus was cleared after halting treatment.
The above results led to the question of whether CD4+ and CD8+ cells contributed either to the initial reduction in virus load in IFN-γ-treated mice or the subsequent clearing of virus. Specific antibodies were used to deplete either CD4+ or CD8+ T cells alone or CD4+ and CD8+ cells together prior to infection and administration of IFN-γ. The depletion of both T cell subsets did not prevent the suppression of virus replication while IFN-γ was being provided. However, after IFN-γ administration was stopped, extensive virus replication occurred, leading to death of the T-cell-depleted animals. Some animals survived after depletion of only CD4+ or CD8+ T cells but not after depletion of both. Previous studies had shown that one T cell subset alone was sufficient to control infection in C57BL/6 mice infected i.p. with VACV strain WR (29). Thus, the IFN-γ (and presumably IFN-α) provided CAST mice with a window of time to develop a CD4+- and CD8+-dependent adaptive immune response. Since a reduction in anti-VACV antibodies occurred when CD4+ cells were depleted, we depleted B cells in additional experiments. Although depletion was variable and incomplete, all animals survived, including those that made low or insignificant anti-VACV antibody responses. Thus, the ability of CAST mice to clear virus after cessation of IFN-γ was dependent mainly on T cells and not B cells.
In conclusion, we propose that deficient innate immunity marked by very low numbers of NK cells in naive animals and a reduced IFN-γ response upon infection contribute to the susceptibility of CAST mice to VACV and presumably other orthopoxviruses. CAST mice can be protected by administration of exogenous IFN-γ but also by IFN-α. The key appears to be suppression of virus replication until the development of an adaptive immune response by CD4+ and CD8+ T cells. The basis for the susceptibility of CAST mice is likely to be complex based on initial genetic studies (2). The CAST mouse is one of the eight collaborative cross founder strains (http://csbio.unc.edu/CCstatus/index.py), which may facilitate further investigations.
MATERIALS AND METHODS
Cells and viruses.
BS-C-1 cells were maintained at 37°C and 5% CO2 in modified Eagle's minimal essential medium (Quality Biologicals, Inc., Gaithersburg, MD) supplemented with 8% heat-inactivated fetal bovine serum, 10 U of penicillin/ml, 10 mg streptomycin/ml, and 2 mM l-glutamine. The WR strain of VACV was propagated and purified as previously described (30).
Mice.
Female CAST/EiJ and BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, ME) and Taconic Biotechnology (Germantown, NY), respectively, and maintained in small ventilated microisolator cages.
Infection of animals.
On the day of infection, VACV was thawed, sonicated, and diluted in phosphate-buffered saline (PBS) containing 0.05% bovine serum albumin. Infections were performed by delivery of 100 to 200 μl of virus into the peritoneal cavity. Mock-infected animals were inoculated with a similar volume of diluent. The titer of each dose was verified by plaque assay on BS-C-1 cells. Animals were weighed and observed 5 to 7 days per week for up to 22 days postinfection. Animals that became moribund were euthanized in accordance with NIAID animal care and use guidelines. All experiments were performed in an animal biosafety level 2 (ABSL-2) or ABSL-3 facility with approval of the NIAID Animal Care and Use Committee.
Bioluminescence imaging.
An IVIS 200 system (PerkinElmer, Waltham, MA) was used for live imaging. d-Luciferin (PerkinElmer) was injected i.p. (150 μg/g body weight) 10 min prior to imaging. Animals were maintained under isoflurane anesthesia for the duration of the procedure. Luminescent images were collected for 1 to 60 s with small or medium binning factors. A single region of interest was drawn around the entire body, and light emission was measured in photons per second per square candela per steradian (photon flux). Acquisition and analysis were performed with Living Image software (PerkinElmer).
Titration of virus from infected animals.
Major organs (lung, liver, spleen, brain, kidney, ovary, and inguinal lymph nodes) were harvested at specified times postinfection or at time of death, placed in 2 to 3 ml of balanced salt solution containing 0.1% bovine serum albumin, and frozen at −80°C. Organs were thawed, homogenized with a GLH-1 mechanical grinder equipped with a hard-tissue probe (Omni International, Kennesaw, GA) and sonicated for three 45-s intervals in ice water. Tissue homogenates were clarified by centrifugation for 20 s at 400 × g in a 4515 microcentrifuge (Eppendorf, Hauppauge, NY). Peritoneal cavities of infected animals were washed with 2.5 to 5 ml of ice-cold RPMI medium (Quality Biologicals, Inc.), and cells were harvested by centrifugation at 2,500 rpm for 10 min at 4°C. Supernatants were saved, and cell pellets were suspended in RPMI–2% fetal bovine serum (FBS) and subjected to three freeze-thaw cycles prior to virus titration. Both tissue homogenate and peritoneal wash samples were aliquoted, frozen, and stored at −80°C. Virus titers were determined by serial dilution and plaque assay on BS-C-1 cells.
Stimulation and intracellular cytokine staining of lymphocytes.
Splenocytes were prepared from individual mice at specified times postinfection. For stimulation, 1.5 × 106 cells were incubated with 25 mg/ml of phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml of ionomycin (Sigma-Aldrich, St. Louis, MO). After 1 h at 37°C, brefeldin A (Sigma-Aldrich) was added to 10 μg/ml. Samples were incubated at 37°C for another 5 h and then at 4°C overnight. Cells were incubated with anti-CD16/32, clone 2.4G2 (a gift of Jack Bennink, NIAID), for 10 min then stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3e, phycoerythrin (PE)-conjugated anti-CD4, and peridinin chlorophyll a protein (PerCP-Cy5.5)-conjugated anti-CD8 for 1 h on ice. Alternatively, cells were stained with FITC-conjugated anti-CD3 and PE-conjugated anti-CD49b. After fixation and permeabilization, cells were stained with allophycocyanin (APC)-conjugated anti-IFN-γ for 1 h on ice and washed and suspended in 2% paraformaldehyde. All conjugated anti-mouse antibodies were purchased from BD Pharmingen (San Jose, CA). Approximately 150,000 cells were acquired on a FACSCaliber cytometer using Cell Quest software (BD Biosciences, San Jose, CA) and analyzed using Flowjo software (TreeStar, Cupertino, CA).
Poly(I-C) inoculation.
Endotoxin-free water was added to 1 mg of lyophilized poly(I-C) (Invivogen, San Diego, CA) to prepare a 1-mg/ml stock solution. Groups of BALB/c and CAST/EiJ mice were inoculated i.p. with 200 μl (200 μg) of reconstituted poly(I-C), and organ samples were collected 6 and 24 h later. To evaluate protection, CAST mice were challenged at 6 h after poly(I-C) administration.
Cytokine assays.
Cytokine measurements were performed on plasma, spleen, and liver samples from poly(I-C)-treated and VACV-infected CAST and BALB/c mice. Whole blood obtained from the submandibular vein was collected in EDTA-treated tubes at specified times, and red blood cells were removed by centrifugation at 2,000 × g for 8 min at 4°C. The plasma was stored at −80°C. Spleen and liver homogenates were treated with protease inhibitor, centrifuged at 2,000 × g for 30 s to pellet gross debris, and stored in aliquots at −80°C. IFN-α and IFN-β levels in plasma, spleen, and liver samples were determined in duplicate using the Verikine mouse IFN-α ELISA kit and mouse IFN-β high-sensitivity ELISA kit (PBL Assay Science, Piscataway, NJ) according to the manufacturers' instructions. IFN-γ and TNF-α levels in samples were analyzed by Milliplex xMAP cytokine bead array (EMD Millipore, Billerica, MA) using BioPlex 200 Luminex (Bio-Rad, Hercules, CA).
In vitro infection of spleen cells.
Splenocytes were prepared by Dounce homogenization of spleens followed by lysis of red blood cells and washing. Suspensions of cells at 107 per ml were infected with 10 PFU/cell of VV.NP-S-EGFP (expressing enhanced green fluorescent protein [EGFP]) (31, 32). After 2 h, the volume was increased 5-fold, and the infection was continued for 16 to 20 h at 37°C. Cells were washed, incubated with anti-CD16/32 clone 2.4G2 (gift of Jack Bennink, NIAID) for 10 min, and then stained with APC-conjugated anti-CD3e, PE-conjugated anti-CD4, and peridinin chlorophyll a protein (PerCP-Cy5.5)-conjugated anti-CD8 for 1 h on ice. Alternatively, cells were stained with APC-conjugated anti-CD3, PE-conjugated anti-CD49b, and PerCP-Cy5.5-conjugated CD19. Cells were washed and suspended in 2% paraformaldehyde. Approximately 200,000 cells were acquired on a FACSCaliber cytometer using CellQuest software (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (TreeStar, Cupertino, CA).
IFN-γ and IFN-α administration.
A dose of 5 × 103 units of recombinant mouse IFN-γ (Thermo Fisher Scientific, Waltham, MA) or IFN-α (EMD Millipore, Billerica, MA) in 100 to 200 μl of phosphate-buffered saline (PBS) was injected into the peritoneum of mice either once or on several consecutive days.
T cell depletion.
MAbs against CD4 (clone GK1.5), CD8 (clone YTS 169.4), or KLH (isotype control, clone LTF-2) (Bio X Cell, Lebanon, NH) were utilized for T cell depletion. Mice were injected i.p. with 0.2 mg of MAb in 200 μl of PBS on days 3 and 4 before virus challenge. The efficacy of depletion was tested by flow cytometry of cells harvested from whole blood on days 5 and 13 and from spleen when terminated on day 29.
B cell depletion.
Mouse anti-mouse CD20 (5D2) was generously donated by Genentech. For B cell depletion, mice were injected i.p. or intravenously with 100 to 200 μg of anti-CD20 MAb, or an isotype control was administered either i.p. or intravenously. The efficacy of depletion was tested by flow cytometry of cells from whole blood and measuring the VACV antibody response by ELISA.
VACV ELISA.
Endpoint serum IgG titers to VACV were determined as previously described (6).
ACKNOWLEDGMENTS
We thank Catherine Cotter for cells and viruses and the NIAID Comparative Medicine Branch for care of animals. Anti-CD16/32, clone 2.4G2, was a gift of Jack Bennink, and anti-CD20 MAb was generously donated by Genentech.
Research support was provided by the Division of Intramural Research, NIAID, NIH.
REFERENCES
- 1.Americo JL, Moss B, Earl PL. 2010. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. J Virol 84:8172–8180. doi: 10.1128/JVI.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Earl PL, Americo JL, Moss B. 2015. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology 481:161–165. doi: 10.1016/j.virol.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ideraabdullah FY, de la Casa-Esperon E, Bell TA, Detwiler DA, Magnuson T, Sapienza C, de Villena FP. 2004. Genetic and haplotype diversity among wild-derived mouse inbred strains. Genome Res 14:1880–1887. doi: 10.1101/gr.2519704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. 2011. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leist SR, Pilzner C, van den Brand JM, Dengler L, Geffers R, Kuiken T, Balling R, Kollmus H, Schughart K. 2016. Influenza H3N2 infection of the collaborative cross founder strains reveals highly divergent host responses and identifies a unique phenotype in CAST/EiJ mice. BMC Genomics 17:143. doi: 10.1186/s12864-016-2483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Americo JL, Sood CL, Cotter CA, Vogel JL, Kristie TM, Moss B, Earl PL. 2014. Susceptibility of the wild-derived inbred CAST/Ei mouse to infection by orthopoxviruses analyzed by live bioluminescence imaging. Virology 449:120–132. doi: 10.1016/j.virol.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangster MY, Heliams DB, MacKenzie JS, Shellam GR. 1993. Genetic studies of flavivirus resistance in inbred strains derived from wild mice: evidence for a new resistance allele at the flavivirus resistance locus (Flv). J Virol 67:340–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl PL, Americo JL, Moss B. 2012. Lethal monkeypox virus infection of CAST/EiJ mice is associated with a deficient interferon-gamma response. J Virol 86:9105–9112. doi: 10.1128/JVI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsley AC, Weisberg AS, Wagenaar TR, Moss B. 2006. Vaccinia virus entry into cells via a low pH-dependent-endosomal pathway. J Virol 80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luker KE, Hutchens M, Schultz T, Pekosz A, Luker GD. 2005. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology 341:284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Earl PL, Americo JL, Cotter CA, Moss B. 2015. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology 475:150–158. doi: 10.1016/j.virol.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama WM, Kim S, French AR. 2004. The dynamic life of natural killer cells. Annu Rev Immunol 22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 14.Burshtyn DN. 2013. NK cells and poxvirus infection. Front Immunol 4:7. doi: 10.3389/fimmu.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. 1983. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol 131:1531–1538. [PubMed] [Google Scholar]
- 16.Abboud G, Tahiliani V, Desai P, Varkoly K, Driver J, Hutchinson TE, Salek-Ardakani S. 2015. Natural killer cells and innate interferon gamma participate in the host defense against respiratory vaccinia virus infection. J Virol 90:129–141. doi: 10.1128/JVI.01894-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prlic M, Gibbs J, Jameson SC. 2005. Characteristics of NK cell migration early after vaccinia infection. J Immunol 175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 18.Arase H, Saito T, Phillips JH, Lanier LL. 2001. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (alpha 2 integrin, very late antigen-2). J Immunol 167:1141–1141. doi: 10.4049/jimmunol.167.3.1141. [DOI] [PubMed] [Google Scholar]
- 19.Harrington LE, van der Most R, Whitton JL, Ahmed R. 2002. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol 76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida J, Hamaguchi Y, Oliver JA, Ravetch JV, Poe JC, Haas KM, Tedder TF. 2004. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, Wu Y, Meng YG, Gribling P, Lin Z, Nguyen K, Tran T, Zhang Y, Rosen H, Martin F, Chan AC. 2005. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174:817–826. doi: 10.4049/jimmunol.174.2.817. [DOI] [PubMed] [Google Scholar]
- 22.Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, Bousso P. 2013. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest 123:5098–5103. doi: 10.1172/JCI70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delano ML, Brownstein DG. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J Virol 69:5875–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhri G, Panchanathan V, Buller RM, van den Eertwegh AJ, Claassen E, Zhou J, de Chaza R, Laman JD, Karupiah G. 2004. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc Natl Acad Sci U S A 101:9057–9062. doi: 10.1073/pnas.0402949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteban DJ, Buller RML. 2005. Ectromelia virus: the causative agent of mousepox. J Gen Virol 86:2645–2659. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- 26.Parker S, Buller RM. 2013. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol 8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osorio JE, Iams KP, Meteyer CU, Rocke TE. 2009. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One 4:e6592. doi: 10.1371/journal.pone.0006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stabenow J, Buller RM, Schriewer J, West C, Sagartz JE, Parker S. 2010. A mouse model of lethal infection for evaluating prophylactics and therapeutics against monkeypox virus. J Virol 84:3909–3920. doi: 10.1128/JVI.02012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu R, Johnson AJ, Liggitt D, Bevan MJ. 2004. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol 172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 30.Cotter CA, Earl PL, Wyatt LS, Moss B. 2015. Preparation of cell cultures and vaccinia virus stocks. Curr Protoc Microbiol 39:14A.3.1–14A.3.18. doi: 10.1002/9780471729259.mc14a03s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anton LC, Schubert U, Bacik I, Princiotta MF, Wearsch PA, Gibbs J, Day PM, Realini C, Rechsteiner MC, Bennink JR, Yewdell JW. 1999. Intracellular localization of proteasomal degradation of a viral antigen. J Cell Biol 146:113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. 2002. Visualizing priming of virus-specific CD8(+) T cells by infected dendritic cells in vivo. Nat Immunol 3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]