ABSTRACT
We previously reported that the T-cell receptor (TCR) repertoire of human T-cell lymphotropic virus type 1 (HTLV-1) Tax301-309-specific CD8+ cytotoxic T cells (Tax301-309-CTLs) was highly restricted and a particular amino acid sequence motif, the PDR motif, was conserved among HLA-A*24:02-positive (HLA-A*24:02+) adult T-cell leukemia/lymphoma (ATL) patients who had undergone allogeneic hematopoietic cell transplantation (allo-HSCT). Furthermore, we found that donor-derived PDR+ CTLs selectively expanded in ATL long-term HSCT survivors with strong CTL activity against HTLV-1. On the other hand, the TCR repertoires in Tax301-309-CTLs of asymptomatic HTLV-1 carriers (ACs) remain unclear. In this study, we directly identified the DNA sequence of complementarity-determining region 3 (CDR3) of the TCR-β chain of Tax301-309-CTLs at the single-cell level and compared not only the TCR repertoires but also the frequencies and phenotypes of Tax301-309-CTLs between ACs and ATL patients. We did not observe any essential difference in the frequencies of Tax301-309-CTLs between ACs and ATL patients. In the single-cell TCR repertoire analysis of Tax301-309-CTLs, 1,458 Tax301-309-CTLs and 140 clones were identified in this cohort. Tax301-309-CTLs showed highly restricted TCR repertoires with a strongly biased usage of BV7, and PDR, the unique motif in TCR-β CDR3, was exclusively observed in all ACs and ATL patients. However, there was no correlation between PDR+ CTL frequencies and HTLV-1 proviral load (PVL). In conclusion, we have identified, for the first time, a unique amino acid sequence, PDR, as a public TCR-CDR3 motif against Tax in HLA-A*24:02+ HTLV-1-infected individuals. Further investigations are warranted to elucidate the role of the PDR+ CTL response in the progression from carrier state to ATL.
IMPORTANCE ATL is an aggressive T-cell malignancy caused by HTLV-1 infection. The HTLV-1 regulatory protein Tax aggressively promotes the proliferation of HTLV-1-infected lymphocytes and is also a major target antigen for CD8+ CTLs. In our previous evaluation of Tax301-309-CTLs, we found that a unique amino acid sequence motif, PDR, in CDR3 of the TCR-β chain of Tax301-309-CTLs was conserved among ATL patients after allo-HSCT. Furthermore, the PDR+ Tax301-309-CTL clones selectively expanded and showed strong cytotoxic activities against HTLV-1. On the other hand, it remains unclear how Tax301-309-CTL repertoire exists in ACs. In this study, we comprehensively compared Tax-specific TCR repertoires at the single-cell level between ACs and ATL patients. Tax301-309-CTLs showed highly restricted TCR repertoires with a strongly biased usage of BV7, and PDR, the unique motif in TCR-β CDR3, was conserved in all ACs and ATL patients, regardless of clinical subtype in HTLV-1 infection.
KEYWORDS: HTLV-1 Tax, cytotoxic T cells, T-cell receptor, single-cell repertoire analysis
INTRODUCTION
Adult T-cell leukemia (ATL) is an aggressive T-cell malignancy that is caused by infection with human T-cell lymphotropic virus type 1 (HTLV-1) (1–4). Although most HTLV-1-infected individuals remain asymptomatic carriers (ACs) throughout their lifetime, approximately 5% develop ATL after a long latency period, 50 to 60 years, and these patients, especially with the acute type and lymphoma type in Shimoyama's classification (5), show a markedly poor prognosis (6, 7).
Some previous studies of T-cell immune responses in HTLV-1 infection have suggested that HTLV-1 Tax-specific CD8+ cytotoxic T cells (CTLs) help to control virus replication, leading to a reduction in the risk of disease onset for ACs or to the prevention of relapse in ATL patients who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT) (8–14). We previously observed an increase in Tax301-309-specific CTLs (Tax301-309-CTLs) in ATL patients who achieved complete remission after allo-HSCT (14). However, recent studies on the response of human CTLs to viral (15–17) or tumor (18) antigens have suggested that it is important to evaluate the quality rather than the quantity (frequency) of CTLs to determine the in vivo activity of CTLs.
In our previous study, we investigated the T-cell receptor (TCR) repertoire of HLA-A*24:02-restricted Tax301-309 (SFHSSLHLLF)-specific CTLs in ATL patients because A*24:02 is the most common HLA-A allele in Japan. In this qualitative evaluation of Tax301-309-CTLs at the single-cell level in four HLA-A*24:02-positive (HLA-A*24:02+) ATL patients who had undergone allo-HSCT, we found that TCR repertoires in Tax301-309-CTL of ATL patients were highly restricted, and a particular amino acid sequence motif, PDR, in complementarity-determining region 3 (CDR3) of the TCR-β chain was commonly used by several predominant Tax301-309-CTL clones in these ATL patients before and after allo-HSCT (19). Furthermore, we reported that only a few dominant Tax301-309-CTL clones, including the PDR+ Tax-CTL clone, persisted in ATL patients who had achieved complete remission for more than several years after allo-HSCT, and during this period the PDR+ Tax-CTL clone as a central clone selectively expanded, with strong CTL activities against HTLV-1 (14). These Tax301-309-CTLs, including PDR+ Tax-CTLs, were derived from an HTLV-1-negative donor and were assumed to be activated by the small amount of Tax protein on residual HTLV-1-infected cells in the recipients after allo-HSCT. These findings implied that the presence of the PDR+ Tax-CTL clone might contribute to the long-term survival of ATL patients who have undergone allo-HSCT, and the diversity of TCR repertoires in Tax301-309-CTLs might impact the disease status of ATL patients. Therefore, we were interested in whether there is a difference in TCR repertoires in Tax301-309-CTLs among HTLV-1-infected individuals before and after the onset of ATL (ACs and ATL patients) and, if such a difference does exist, the extent to which the difference in TCR repertoires is associated with the disease status in HTLV-1 infection.
In the present study, we comprehensively compared not only TCR repertoires but also the frequencies and phenotypes of Tax301-309-CTLs at the single-cell level between HLA-A*24:02+ ACs and ATL patients. AC subjects were further divided into stable ACs (sACs) and high-risk ACs (hrACs) according to the HTLV-1 proviral load (PVL) and the profile of cell adhesion molecule 1 (CADM1) versus CD7 in CD4+ cells in flow cytometry (20, 21).
RESULTS
Profile of CADM1 versus CD7 in CD4+ cells in flow cytometry accurately reflected the disease status in HTLV-1 infection.
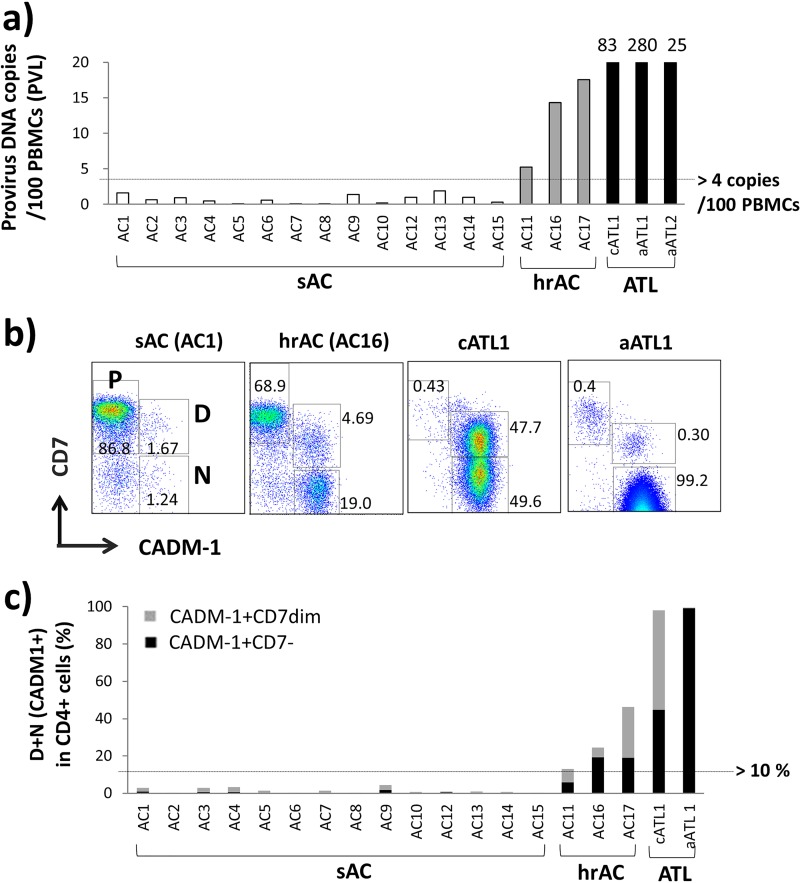
First, 17 ACs were separated into stable ACs (sACs) and high-risk ACs (hrACs) based on PVL, because a high PVL (more than 4 copies/100 peripheral blood mononuclear cells [PBMCs]) has been recognized as a major risk factor for the development of ATL in ACs (22). According to this definition, 3 (AC11, AC16, and AC17) of the 17 ACs were defined as hrACs and the other 14 were sACs (Fig. 1a). Furthermore, our group recently reported that the difference in three subpopulations divided according to the plot of CADM1 versus CD7 in CD4+ cells in HTLV-1-infected individuals, P (CADM1− CD7+), D (CADM1+ CD7dim), and N (CADM1+ CD7−), accurately reflected the disease status regarding HTLV-1 infection (HTLV-1 analysis system [HAS] flow profile) (20, 21). HTLV-1-infected cells and clones are efficiently enriched in CADM1+ subpopulations (D and N), and disease progression was reflected in an increase in the percentage of D plus N. In the aggressive ATL phase, loss of CD7 usually occurs, resulting in dominance of the N subpopulation in CD4+ cells.
FIG 1.
Proviral load (PVL) and profiles of CADM1 versus CD7 in CD4+ cells from ACs and ATL patients. (a) PVL of ACs (n = 17) and ATL patients (n = 3). ACs were divided into stable ACs (sACs) and high-risk ACs (hrACs) on the basis of a high PVL (more than four copies/100 PBMCs) (22). According to this definition, three ACs (AC11, AC16, and AC17) were defined as hrACs. (b) Representative profiles of CADM1 versus CD7 in CD4+ cells (HAS flow profiles) in each case of sAC, hrAC, cATL, and aATL. P (CADM1− CD7+), D (CADM1+ CD7dim), and N (CADM1+ CD7−) subpopulations were gated according to our previous report (20). (c) The proportion of CADM1+ (D + N gates) in CD4+ cells in the samples from sACs, hrACs, and ATL patients are summarized. sACs with PVLs of <4 copies/100 PBMCs had CADM1+ (D + N) values of less than 10%. hrACs (AC11, -16, and -17) with high PVLs (>4 copies/100 PBMCs) had CADM1+ values of more than 10%. Both chronic- and acute-type ATL patients had remarkably high frequencies of CADM1+ subpopulations.
We performed the HAS flow analysis for samples from ACs and ATL patients. Representative data are shown in Fig. 1b, and the profiles are summarized in Table 1. The HAS flow profiles of most ACs showed a low percentage of D plus N, indicating sACs. Three ACs (AC11, AC16, and AC17), who were defined as hrACs according to their high PVLs, showed increases in the CADM1+ (D plus N) gate (13.1% to 46.2%), indicating growth of HTLV-1-infected clones. Moreover, CD4+ cells in ATL patients fully expressed CADM1 with loss of CD7. These results are consistent with our previous reports (20, 21), and the ACs and ATL patients could be reasonably separated into three groups (sACs, hrACs, and ATL patients) according to their PVL and HAS flow profiles.
TABLE 1.
Clinical characteristics and flow cytometric profiles of HLA-A*24:02+ ACs and ATL patients examined in this studya
| Patient ID | Age (yr) | Sex | Clinical status | No. of WBCs (/μl) | Lymphocytes (%) | Atypical lymphocytes (%) | No. of provirus DNA copies (PVL)/100 PBMCs | sIL-2R (U/ml) | HAS flow prolife |
Tax-Tet (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CADM1−, P (%) | CADM1+ |
||||||||||||
| D (%) | N (%) | D + N (%) | |||||||||||
| AC1 | 48 | Female | sAC | 3,780 | 32.0 | 0.5 | 1.61 | 416 | 91.8 | 1.8 | 1.0 | 2.8 | 20.10 |
| AC2 | 46 | Female | sAC | 7,280 | 28.0 | 0 | 0.65 | 239 | 80.8 | 0.2 | 0.1 | 0.3 | 1.05 |
| AC3 | 63 | Male | sAC | 4,570 | 39.0 | 0 | 0.94 | 311 | 92.2 | 2.2 | 0.7 | 2.9 | 2.76 |
| AC4 | 56 | Female | sAC | 5,020 | 42.0 | 0 | 0.50 | 305 | 90.2 | 2.6 | 0.8 | 3.4 | 2.07 |
| AC5 | 58 | Male | sAC | 3,850 | 36.5 | 0.5 | 0.05 | 364 | 94.8 | 1.1 | 0.2 | 1.3 | 1.76 |
| AC6 | 61 | Female | sAC | 4,220 | 32.5 | 0 | 0.57 | 327 | 85.0 | 0.2 | 0.1 | 0.3 | 0.66 |
| AC7 | 53 | Female | sAC | 6,940 | 33.0 | 0.5 | 0.06 | 220 | 86.6 | 1.1 | 0.3 | 1.4 | 1.75 |
| AC8 | 49 | Female | sAC | 3,560 | 23.0 | 0.5 | 0.04 | 207 | 86.2 | 0.1 | 0.1 | 0.2 | 0.14 |
| AC9 | 71 | Male | sAC | 5,200 | 30.5 | 0.5 | 1.37 | 272 | 79.9 | 2.5 | 1.9 | 4.4 | 0.05 |
| AC10 | 43 | Male | sAC | 5,710 | 43.5 | 0 | 0.22 | 277 | 95.9 | 0.7 | 0.1 | 0.9 | 3.37 |
| AC12 | 56 | Female | sAC | 10,300 | 21.5 | 0.5 | 0.97 | 462 | 77.0 | 0.5 | 0.5 | 1.0 | 1.59 |
| AC13 | 70 | Male | sAC | 3,810 | 37.0 | 0 | 1.93 | 435 | 81.7 | 0.7 | 0.3 | 1.0 | 0.83 |
| AC14 | 67 | Female | sAC | 5,540 | 23.5 | 1.0 | 1.00 | 657 | 82.5 | 0.4 | 0.3 | 0.8 | 6.60 |
| AC15 | 52 | Female | sAC | 5,400 | 28.0 | 1.5 | 0.29 | 264 | 87.2 | 0.2 | 0.1 | 0.3 | 0.44 |
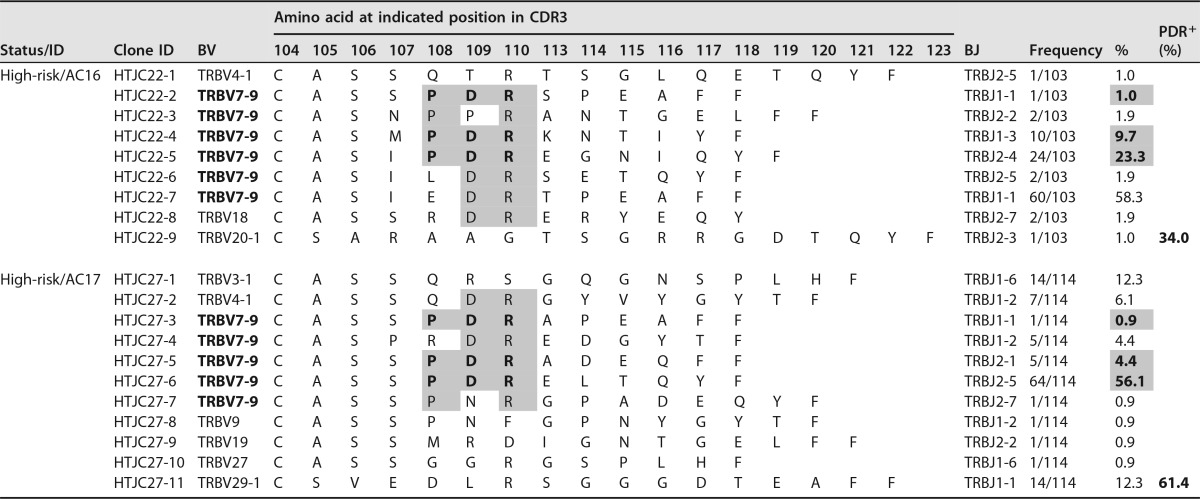
| AC11 | 63 | Male | hrAC | 6,070 | 25.0 | 1.0 | 5.26 | 378 | 71.1 | 7.1 | 6.0 | 13.1 | 3.12 |
| AC16 | 66 | Female | hrAC | 5,590 | 45.0 | 5.3 | 14.31 | 381 | 71.6 | 5.2 | 19.3 | 24.5 | 0.71 |
| AC17 | 56 | Male | hrAC | 8,780 | 32.0 | 1.0 | 17.53 | 700 | 50.0 | 27.2 | 19.0 | 46.2 | 2.53 |
| cATL1 | 60 | Male | ATL (chronic type) | 9,850 | 12.5 | 57.5 | 83.17 | 4,560 | 1.3 | 53.5 | 44.7 | 98.2 | 3.47 |
| aATL1 | 58 | Female | ATL (acute type) | 51,200 | 1.5 | 96.0 | 280.25 | 29,600 | 0.3 | 0.3 | 99.2 | 99.5 | UD |
| aATL2* | 57 | Male | ATL (acute type) | 1,430 | 18.0 | 0 | 25.2 | 900 | NA | NA | NA | NA | UD |
| aATL3* | 54 | Male | ATL (lymphoma type) | 1,270 | 54 | 5 | NA | 982 | NA | NA | NA | NA | 1.97 |
| aATL4 | 55 | Male | ATL(lymphoma type) | 1,310 | 25 | 1 | NA | 817 | NA | NA | NA | NA | 0.08 |
ID, identifier; WBCs, white blood cells; sIL-2R, soluble interleukin 2 receptor; AC, asymptomatic HTLV-1 carrier; sAC, asymptomatic HTLV-1 carrier without high risk of ATL development defined by the PVL (fewer than 4 copies of proviruses per 100 PBMCs); hrAC, high-risk asymptomatic HTLV-1 carrier who had more than 4 copies of proviruses per 100 PBMCs; cATL, chronic-type ATL; aATL, acute-type ATL; HAS flow profiles, distribution to the three subpopulations P (CADM1− CD7+), D (CADM1+ CD7dim), and N (CADM1+ CD7−) by plot of CADM1 versus CD7 for CD4+ cells in PB samples by flow cytometry; Tax-Tet (%), the proportion of Tax301-309/HLA tetramer-reacting cells to total CD8T cells in PB; NA, not analyzed; UD, under the detection limits. The clinical data for WBCs, lymphocytes, atypical lymphocytes, PVL, and sIL-2R were measured using samples that were obtained at a time close to the point of collecting samples for the other experiments. Furthermore, the clinical data and Tax-Tet (%) of these two patients were used as samples before allo-HSCT in a preceding study (19).
The frequencies and phenotypes of Tax-CTLs did not respond sensitively to the disease status in HTLV-1 infection.
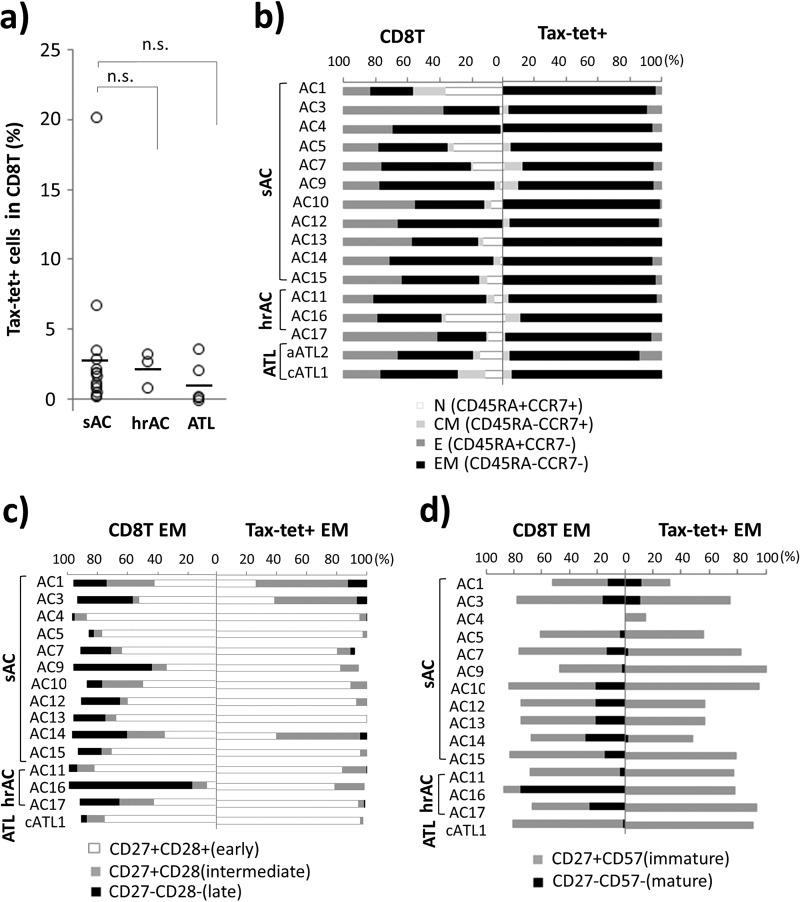
As shown in Fig. 2a and Table 1, Tax301-309-specific CTLs (Tax tetramer-positive cells) were effectively detected in PBMCs of sACs and hrACs before the onset of ATL (average frequencies in CD8+ T cells: 3.08% and 2.12%, respectively) with no apparent difference between them, even though a couple of sACs (AC1 and AC14) showed very high frequencies of Tax tetramer-positive cells (20.1% and 6.6%, respectively). After the onset of ATL, Tax tetramer-positive cells in three aATL patients (aATL1, -2, and -4) were inefficiently detected in PBMCs (less than 0.08% in CD8 T cells), except for two patients (cATL1 and aATL3) who showed relatively high frequencies (3.47% and 1.97%, respectively). As a result, we did not observe any essential difference in the frequencies of Tax tetramer-positive cells between sACs, hrACs, and ATL patients in this investigation.
FIG 2.
Frequencies and effector memory phenotypes of Tax301-309-CTLs in ACs and ATL patients. (a) Frequencies of Tax tetramer-positive CD8 T cells among sACs (n = 14), hrACs (n = 3), and ATL patients. Horizontal bars indicate the averages of Tax tetramer-positive cells for all groups. n.s., not significant. (b) Differentiation of subsets based on CD45RA CCR7 expression in Tax tetramer-positive cells. Tax tetramer-positive cells in the CD45RA− CCR7− effector memory subset (TEM-Tax-CTL) were further evaluated according to the expression of CD27 and CD28 (24) (c) and CD27 and CD57 (25, 26) (d) and compared to that of all of the CD8 T cells in the effector memory subset.
Furthermore, we assessed the phenotypes associated with T-cell differentiation for Tax tetramer-positive cells in the three groups. T cells can be phenotypically divided into the following four differentiation subsets based on CD45RA and CCR7 expression: CD45RA+ CCR7+ (naive [TN]), CD45RA− CCR7+ (central memory [TCM]), CCR7− CD45RA− (effector memory [TEM]), and CCR7− CD45RA+ (effector [TE]) (23). As shown in Fig. 2b, almost all Tax tetramer-positive cells in all of the cases examined for sACs, hrACs, and ATL patients exhibited striking similarities, with a predominance of the TEM phenotype (means, 92.8%, 90.9%, and 88.0%, respectively), with no essential difference among the groups. In contrast, all of the CD8+ T cells in all individuals in each group exhibited a heterogeneous distribution of naive, memory, and effector subsets. We further assessed the coordinate expression of T-cell differentiation markers, CD27 CD28 and CD27 CD57, on Tax tetramer-positive cells belonging to the TEM subset (TEM-Tax-CTLs), because it has been shown that both CD27 CD28 and CD27 CD57 profiles are useful for defining the following differentiation stages of memory T cells: CD27+ CD28+ (early), CD27+ CD28− (intermediate), CD27− CD28− (late) (24), CD27+ CD57− (immature), and CD27− CD57+ (mature) (25, 26). In fact, TEM-Tax-CTLs in most individuals showed CD27 expressed on lymphocytes with relatively young differentiation phenotypes, i.e., early, intermediate (Fig. 2c), and immature (CD27+ CD57−) (Fig. 2d), but not the senescent phenotypes late and mature. In contrast, all of the CD8+ T cells in the TEM subset showed relatively heterogeneous distributions, with an increase in the fraction of CD27 downregulation late or CD57-expressing mature phenotypes. Thus, Tax-CTLs of ACs and ATL patients commonly were CD27+ CD28+/−, which are relatively young effector memory phenotypes, irrespective of the disease status in HTLV-1 infection.
Highly restricted TCR repertoires of Tax-CTLs in ACs and ATL patients according to a strongly biased usage of the TCR-BV7 gene family.
We performed a single-cell TCR repertoire analysis for sorted individual Tax301-309-CTL (Tax tetramer-positive cells) and analyzed a total of 1,107 Tax tetramer-positive cells from 10 ACs (7 sACs [AC1, -3, -4, -5, -7, -9, and -10] and 3 hrACs [AC11, -16, and -17]) and 351 cells from 4 ATL patients (cATL1 and aATL2, -3, and -4).
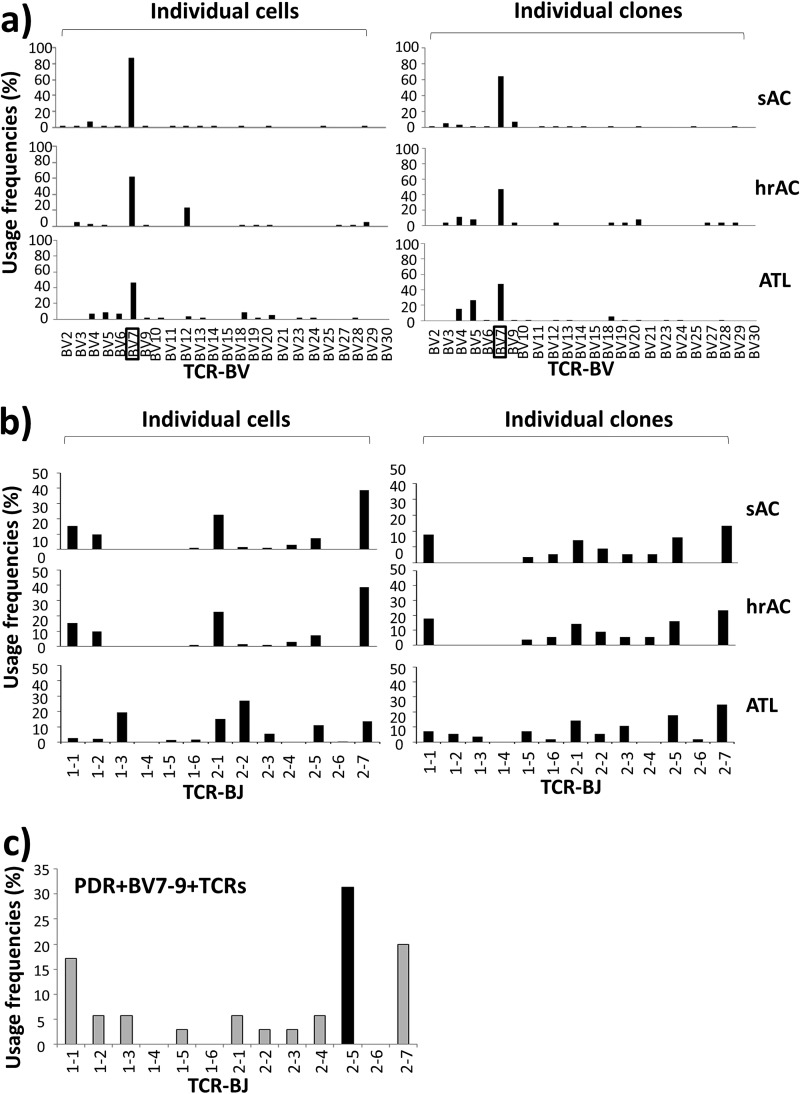
To estimate the differences in TCR repertoires of Tax301-309-CTLs between ACs and ATL patients, the TCR-BV gene family and TCR-BJ gene usages were first investigated for single-cell sorted Tax tetramer-positive cells in sACs, hrACs, and ATL patients at both the cellular and clonal levels. The usage frequencies for a given TCR-BV gene family or TCR-BJ genes were defined as the ratios of the number of Tax tetramer-positive cells or clones that used the genes to the total number of Tax tetramer-positive cells or clones detected in sACs (n = 7,782 cells and 56 clones), hrACs (n = 3,325 cells and 28 clones), and ATL patients (n = 4,351 cells and 56 clones). As shown in Fig. 3a, Tax301-309-CTLs of sACs, hrACs, and ATL patients commonly showed highly restricted TCR repertoires with a strongly biased usage of the BV7 gene family at both the cellular and clonal levels. This preference for BV7 of Tax-CTLs tended to decrease in the order sAC→hrAC→ATL at the cellular level (87.5%, 62.5%, and 47.9%, respectively) (Fig. 3a, left graph), whereas this trend was not observed at the clonal level (right graphs): sACs, hrACs, and ATL patients showed values of 64.3%, 46.4%, and 46.4%, respectively. This difference in the trend of the reduction in BV7 usage of Tax301-309-CTLs from ACs to ATL patients between the cellular and clonal levels might result from a decrease in the number of BV7+ cells that efficiently underwent clonal expansion in ATL patients compared to that in ACs in a cellular investigation, because it has been suggested that a high PVL may lead to the functional inactivation of CD8 T cells (27). In contrast, Tax301-309-CTLs of sACs, hrACs, and ATL patients commonly showed a wide variety of TCR-BJ gene usages at both the cellular and clonal levels (Fig. 3b).
FIG 3.
TCR repertoire bias for Tax301-309-CTLs in ACs and ATL patients analyzed at the single-cell level. Individual Tax-tetramer-positive cells or clones detected in sACs (n = 7,782 cells and 56 clones), hrACs (n = 3,325 cells and 28 clones), and ATL patients (n = 4,351 cells and 56 clones) were analyzed with respect to the usage frequencies of TCR-β genes. The usage frequency for a given BV gene family or BJ genes is described in Results. (a and b) TCR-BV repertoires (a) and TCR-BJ repertoires (b) in Tax301-309-CTLs at the cellular and clonal levels. (c) TCR-BJ repertoires of 35 PDR motif-expressing BV7-9+Tax301-309-CTL clones from ACs and ATL patients. Black and gray bars represent TCR-BJ genes with usage frequencies above and below the mean values + 2 SDs, respectively.
The PDR amino acid sequence motif was conserved in CDR3β of Tax-CTLs regardless of clinical subtype in HTLV-1 infection.
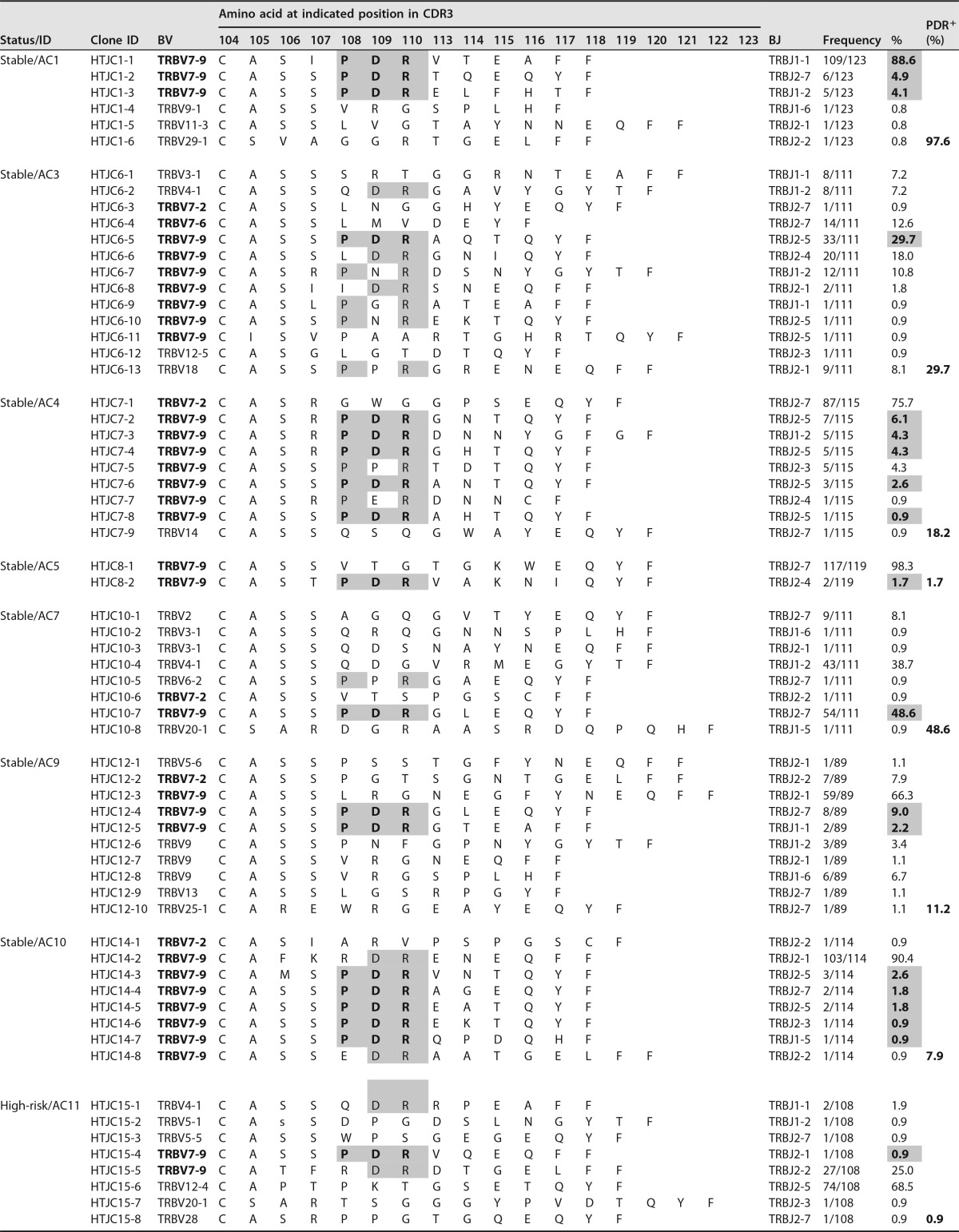
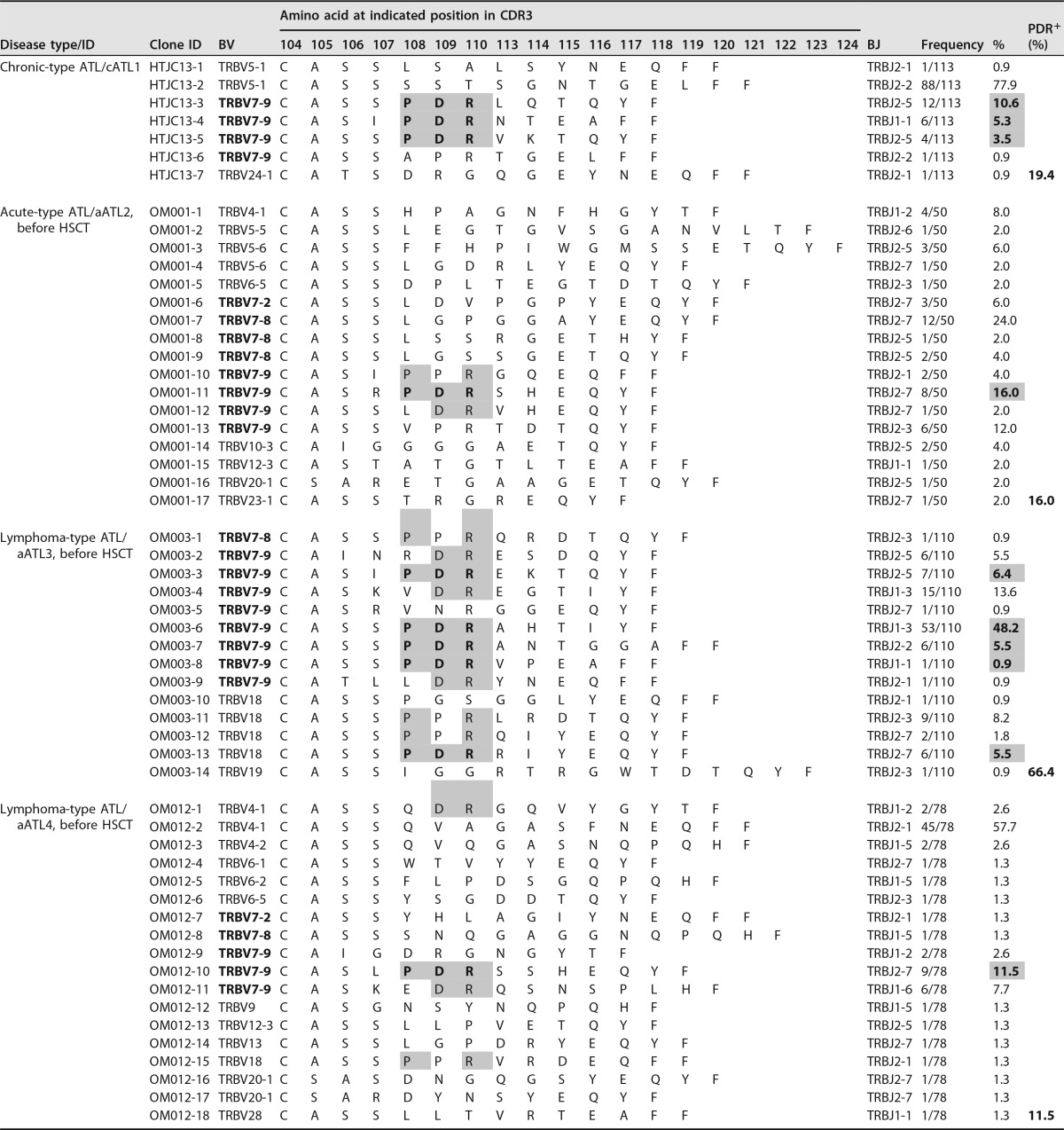
Tables 2 and 3 summarize the TCR-β CDR3 amino acid sequence information of Tax301-309-CTL clones detected in the analyzed Tax tetramer-positive cells from ACs (sACs and hrACs) and ATL patients, respectively. The amino acid sequences of Tax-CTL clones detected in two aATL patients (aATL2 and aATL3) before HSCT have already been reported as Pt-1 and Pt-3, respectively, previously (19).
TABLE 2.
TCR-β CDR3 amino acid sequences and frequencies of Tax301-309 tetramer-positive CTL clones among ACsa
TCR-β CDR3 amino acid sequences of individual HLA-A*2402-Tax301-309 tetramer-positive CTL clones (Tax-CTL clones) of 10 AC samples from seven sACs and three hrACs. Entries that are in bold and shaded indicate CDR3 sequences of the PDR motif at positions 108 to 110 and their frequencies in analyzed cells. Furthermore, the sums of the frequencies of the PDR+ repertoire in each sample are also represented as PDR+ (%). Entries that are shaded represent the conserved CDR3 amino acid sequence, which is P-R or -DR at positions 108 to 110 in CDR3 of each Tax-CTL clone.
TABLE 3.
TCR-β CDR3 amino acid sequences and frequencies of Tax301-309 tetramer-positive CTL clones among ATL patientsa
TCR-β CDR3 amino acid sequences of individual Tax301-309 tetramer-positive CTL clones (Tax-CTL clones) of four HLA-A*2402+ ATL patients, including one with chronic-type ATL (cATL), one with acute-type (aATL), and two with lymphoma-type ATL. Unfortunately, the TCR repertoire status of aATL1 was not examined, because Tax tetramer-positive cells were not detected in the sample. The totals for cells and clones included are 351 cells and 56 clones, respectively. For explanations of bold and shading, see footnote a to Table 2.
Interestingly, the CDR3β amino acid sequences of Tax301-309-CTL clones from all 10 ACs showed a remarkable degree of conservation of a particular motif, the PDR motif, at positions 108 to 110 in CDR3β of BV7-9+ TCRs, with a wide range of frequencies (0.9% to 97.6% in analyzed cells) (Table 2). Furthermore, conservation of the PDR motif in CDR3β of BV7-9+ Tax301-309-CTL clones was also found in both chronic (19.4%) and acute (11.5% to 66.4%) ATL patients (Table 3). Accordingly, we gathered 35 PDR motif-expressing BV7-9+ Tax301-309-CTL clones detected in the AC and ATL groups and investigated TCR-BJ gene usage. We defined usage as biased when the usage frequency of a given BJ gene was more than the mean value + 2 standard deviations (SDs) (7.7% + 18.9%) for the usage of 13 BJ genes. As a result, only BJ2-5 (31.4%) was used in a biased manner by PDR motif-expressing BV7-9+ Tax301-309-CTL clones (Fig. 3c). Furthermore, when we compared all of the CDR3β amino acid sequences detected in ACs and ATL patients, similar sequences with a P-R or -DR motif (hyphens indicate other amino acids at these positions) at positions 108 to 110 in CDR3β of Tax301-309-CTL clones expressing BV4-1, BV7-9, or BV18 were also frequently observed in four of seven sACs (AC3, -4, -7, and -10), three of three hrACs (AC11, -16, and -17), and three of four ATL patients (aATL2, -3, and -4). Thus, many of the CDR3β amino acid sequences of Tax301-309-CTL clones detected in ACs and ATL patients were similar, and PDR motif-expressing BV7-9+ Tax-specific TCRs were conserved in all samples from not only ACs but also ATL patients. The TCR bias for PDR+ Tax-CTL clones was likely to be in BV7-9/BJ2-5.
Relationship between the presence of PDR+ Tax-CTL and disease status in HTLV-1 infection.
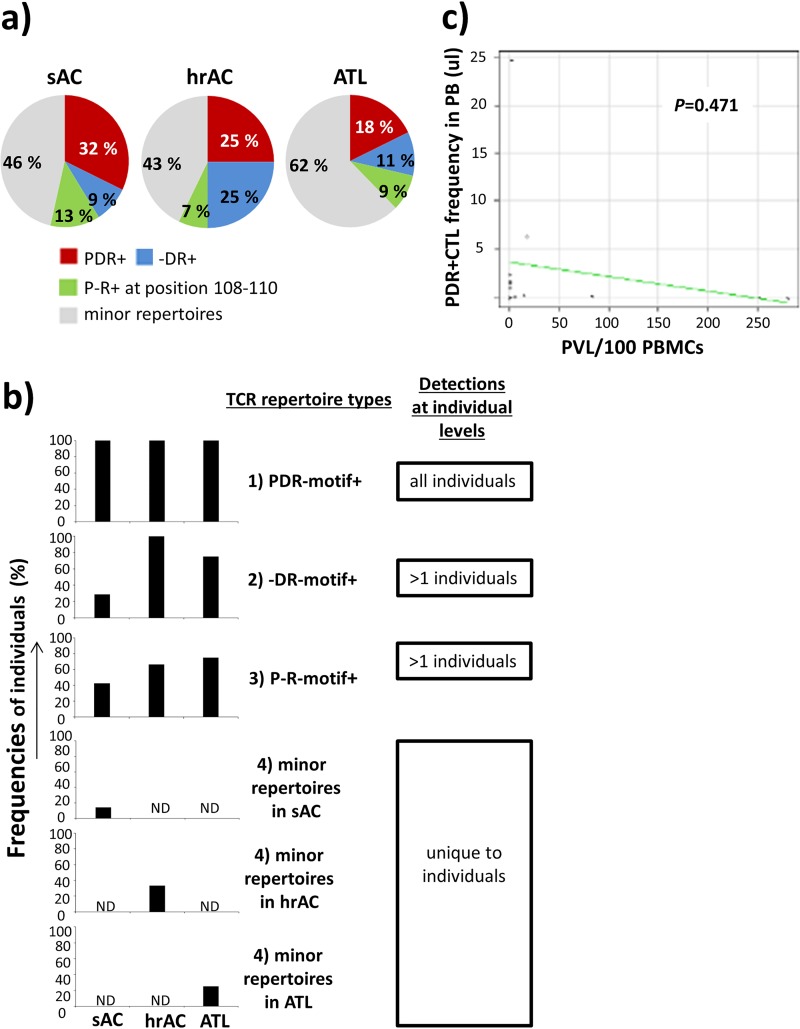
We classified all of the Tax301-309-CTL clonotypes detected in this investigation into four types based on their CDR3β amino acid sequences at positions 108 to 110: (i) PDR, (ii) -DR, and (iii) P-R as the three most frequent sequence motifs and (iv) minor repertoires that had no sequence motif in common with others. The ratios of the four clonotypes to the total number of clonotypes detected in the sACs, hrACs, and ATL patients are shown in Fig. 4a. The fraction of clonotypes expressing PDR, -DR, and P-R in ACs (sACs and hrACs) (approximately 55%) was greater than that in ATL patients (38%). Notably, the proportion of the PDR+ clonotype gradually decreased, in the order sACs, hrACs, and ATL (32%, 25%, and 18%, respectively). However, the decreasing trend of PDR+ clonotype was not statistically significant (P = 0.157).
FIG 4.
Consistent observation of PDR+ clonotypes in both ACs and ATL patients and its relation to PVL. Tax-specific TCR repertoires detected in sACs, hrACs, and ATL patients were classified into four types based on the CDR3β amino acid sequences at positions 108 to 110: (i) PDR+, (ii) -DR+, (iii) P-R+, and (iv) minor repertoires. (a) Ratios of the each repertoire type to the total number of detected TCR repertoires in sACs, hrACs, and ATL patients. (b) Summary of the detection frequencies of the four repertoire types among sACs, hrACs, and ATL patients at the individual level. (c) Relationships between the absolute frequencies of PDR motif+ Tax-CTL (PDR+CTL) in PB and PVL in 13 individuals for whom a single-cell TCR repertoire analysis was performed.
Furthermore, we determined the detection frequencies of these four Tax301-309-CTL clonotypes in sACs, hrACs, and ATL patients at the individual level. The results are shown in Fig. 4b. Interestingly, only the PDR+ clonotype was detected in all individuals among the sACs, hrACs, and ATL patients, whereas the -DR+ and P-R+ clonotypes were not detected in all individuals but were detected in two or more individuals (28.6% to 100%) in each group. Minor-TCR clonotypes detected in sACs, hrACs, and ATL groups, respectively, were not observed in the other groups. We thought that these clonotypes might be unique to individuals and may have been detected simply due to the sensitivity of our single-cell TCR repertoire analysis. Thus, PDR+ Tax-specific clonotypes were consistently found in individuals regardless of clinical subtype in HTLV-1 infection. Finally, we examined the correlation of the absolute frequencies of PDR+ Tax-CTL and PVL in all PB samples that had undergone a single-cell TCR repertoire analysis. No correlation between them was apparent (Fig. 4c).
DISCUSSION
In the present study, we showed that in contrast to the changes in the phenotypes (CADM1 versus CD7, HAS flow profile) of HTLV-1-infected CD4+ cells with ATL progression, neither the frequencies nor the differentiation phenotypes of Tax301-309-CTLs were responsive to the disease status of HTLV-1 infection, suggesting that simply measuring the frequency and phenotypes of Tax301-309-CTLs would not help to estimate the contribution of Tax301-309-CTLs to controlling the disease status. In fact, Tax301-309-CTLs showed a wide variety of frequencies not only among ACs who could effectively control HTLV-1 replication but also among ATL patients who could not. Furthermore, Tax301-309-CTLs of both ACs and ATL patients were consistently present as less differentiated, or relatively young, CD45RA− CCR7− effector memory CTLs based on the predominant phenotypes of CD27+, CD28+/−, and CD57− without major changes toward terminally differentiated or mature phenotypes (CD27−, CD28−, and CD57+), despite in vivo chronic stimulation by HTLV-1 in ATL progression. In contrast to this T-cell differentiation status, Tax-CTLs in patients with an inflammatory disease, HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), have shown a predominance of late differentiation (CD27− CD28−) (28). However, previous studies have revealed that CD27 and CD28 molecules are required for the generation and long-term maintenance of T-cell immunity in virus infection, and especially that the expression of CD27 can promote the survival of activated virus-specific T cells without affecting cell division activity by preventing the onset of TCR/CD3 stimulation (29, 30). Thus, the expression of CD27 and CD28 molecules on Tax-CTLs might be necessary for their survival under chronic stimulation by HTLV-1, and this may be affected by the status of HTLV-1-associated diseases (ATL or HAM/TSP) rather than PVL among HTLV-1-infected individuals.
Of the HTLV-1 antigens, such as env, gag, pol, and pX gene products, it has been shown that Tax is the most dominant target antigen of HTLV-1-specific CTLs (31). Especially, it has been considered that Tax301-309 is one of the major epitopes for HLA-A*24:02, which is one of the most common HLA-A alleles in Japan (9). Therefore, in the present study, to further understand the nature of protective CTL responses against Tax, we have investigated the differences in Tax301-309-specific TCR repertoires of HLA-A*24:02+ HTLV-1-infected individuals between individuals who controlled HTLV-1 (ACs) and individuals who developed ATL. In our initial studies on Tax301-309-specific TCR repertoires of HLA-A*24:02+ ATL patients who had undergone allo-HSCT, we found a common amino acid sequence motif, PDR, in CDR3β of Tax301–309-specific TCRs, not only in unrelated ATL patients but also in individual patients before and after allo-HSCT (19). This prompted us to investigate if selection of the PDR motif in CDR3β of Tax301–309-specific TCRs is independent of the disease status or if it is conserved in all HTLV-1-infected individuals. Therefore, in the present study, we compared 140 Tax301-309-specificTCR repertoires (clonotypes) composed of 1,458 Tax tetramer-positive cells between HLA-A*24:02+ ACs and ATL patients at the single-cell level.
Our results showed that TCR repertoires in Tax301-309-CTL of both ACs and ATL patients were highly restricted, with strongly biased usage of the BV7 gene family, and similar amino acid sequence motifs were frequently encountered: PDR, -DR, and P-R at positions 108 to 110 in CDR3β of Tax301-309-specific TCRs. The most important observation was that only PDR+ Tax-specific clonotypes were observed in all samples from both ACs and ATL patients in this study (Tables 2 and 3 and Fig. 4b). Furthermore, an investigation of the TCR repertoire for PDR+ Tax-specific clonotypes revealed that they were likely to show a TCR-β bias in BV7-9/BJ2-5 (Fig. 3c) and the composition of PDR+ Tax-specific clonotypes in Tax-CTLs might gradually decrease during the course of ATL development (Fig. 4a), although this phenomenon has not yet been clarified. Thus, Tax301-309-specific CTLs showed a TCR bias for the usage of BV7 and the consistent selection of the PDR motif in CDR3 under selective pressures that act on V-D-J gene recombination of the Tax301–309-specific TCR-β chain in the thymus (32–34) in HLA-A*24:02+ HTLV-1-infected individuals.
Since the 1990s, many studies on virus-specific memory T-cell responses to the same antigen epitope have described the presence of a TCR repertoire bias by shared sequences in their TCR-α and/or -β chains in multiple unrelated individuals, and such a TCR bias associated with the sharing of full TCR amino acid sequences or certain motifs in CDR3 has generally been referred to as ”public” (35, 36). To date, a TCR bias associated with shared sequences (public) of TCR-α/β for targeting antigen epitopes of, for example, Epstein-Barr virus (37, 38), influenza A virus (39), cytomegalovirus (40, 41), and human immunodeficiency virus (HIV) (42–45), has been identified among HLA-matched unrelated individuals. With regard to HTLV-1, a TCR repertoire bias with Tax11-19-specific CTLs bearing a shared CDR3β amino acid motif (PG-G) and expressing BV6-5/BJ2-7 has also been identified among HLA-A2+ patients with HAM/TSP (46), which is similar to our finding of a PDR motif in BV7-9-expressing Tax-CTLs among HLA-A*24:02+ ACs and ATL patients. Furthermore, there have been some reports on the effects of these biased TCR features on CTL efficiency and the clinical outcome in virus infections. For example, for HIV, one study demonstrated that a public clonotype of CTLs exhibited high levels of antigen sensitivity and TCR avidity, which provide functional advantages and enable the effective suppression of HIV replication, and a different study on long-term nonprogressors revealed that a type of public TCR motif-expressing HIV nef-specific CTL demonstrated effective cross-recognition of naturally occurring FL8 epitope variants and may be associated with a better clinical outcome (42). In the case of HTLV-1, the shared (public) TCR motif in HTLV-1 Tax11-19-specific CTLs mentioned above has been shown to be critical for maintenance of the tertiary conformation of the CDR3β loop (46).
In this study, we also identified a public TCR motif for the HLA-A*24:02+-restricted HTLV-1 Tax301-309 antigen epitope. However, we failed to show any obvious relationship between the frequency of PDR+ Tax-CTLs and disease status in HTLV-1 infection. This indicated that the presence of PDR+ Tax-CTLs was less likely to directly predict the efficiency of Tax301-309-CTLs at controlling HTLV-1 replication. However, if we consider our previous results in a functional assay for PDR+ Tax-CTLs in ATL patients after allo-HSCT, PDR+ Tax-CTLs might have strong activity against HTLV-1 with high affinity for Tax301-309 peptide, which may be associated with the efficient production of gamma interferon (IFN-γ) and cytotoxicity (19, 47). On the other hand, even if ATL patients have sufficient numbers of PDR-carrying Tax-CTLs, they seem to be as dysfunctional as non-PDR-carrying Tax-CTLs, because a high PVL might be involved in the systemic functional inactivation of CD8 T cells (27). The limitation of our study is the small number of patients evaluated, especially the number of hrACs and ATL patients, which did not allow us to form any definitive conclusions regarding the association between the frequency of PDR+ Tax-CTLs and disease status in HTLV-1 infection. A larger-scale and sequential study is needed before we can reach a definitive conclusion regarding the strength of the biological impact of PDR+ Tax-CTLs on the disease onset of ATL.
In conclusion, we showed that the frequencies and memory phenotypes of Tax301-309-CTLs were not useful indicators for estimating in vivo Tax301-309-CTL efficiency in ATL progression. However, according to the results obtained with our single-cell-based approach to TCR repertoires in Tax301-309-CTLs of HLA-A*24:02+ ACs and ATL patients, HLA-A*24:02+ HTLV-1-infected individuals might exhibit conservation of the PDR motif in CDR3β of BV7-9+ Tax301-309-specific TCRs, in accordance with a public TCR motif for the Tax301-309 epitope. The findings described here may help us to understand not only the comprehensive rules for T-cell regulation in HTLV-1 infection but also the performance of public TCR motif-carrying T cells in persistent virus infection.
MATERIALS AND METHODS
Cells.
Peripheral blood (PB) samples of 17 unrelated ACs (AC1 to -17) and 2 ATL patients (one each with acute-type ATL and chronic-type ATL) (aATL1 and cATL1) were obtained from the Institute of Medical Science, The University of Tokyo Hospital. PB samples from three cases of aATL (aATL2, -3, and -4) before allo-HSCT were obtained from Saitama Medical Center, Jichi Medical University. All patients with ATL were categorized into clinical subtypes according to Shimoyama's criteria (5), and the clinical characteristics of the tested individuals are summarized in Table 1. This study was approved by the institutional review boards of the University of Tokyo and Jichi Medical University. Written informed consent was obtained from all patients. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep (Axis-Shield PoC AS, Oslo, Norway) and cryopreserved in liquid nitrogen until use.
Quantification of HTLV-1 PVL.
The HTLV-1 proviral load (PVL) per 100 PBMCs was measured by a quantitative real-time PCR with HTLV-1 Tax-specific primers using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA), as described previously (22, 48).
CADM1 versus CD7 plot in CD4+ cells in flow cytometry.
PBMCs were stained using a combination of monoclonal antibodies (MAbs) against CADM1, CD7, CD3, CD4, and CD14 molecules. PE-conjugated CADM1 antibody (clone 3E1) was purchased from MBL (Tokyo, Japan). Allophycocyanin (APC)-conjugated CD7 antibody (clone CD7-6B7), CD4-APC-Cy7, and CD14-fluorescein isothiocyanate (FITC) were obtained from BioLegend (San Diego, CA). CD3-phycoerythrin (PE)-Cy7 was obtained from BD Biosciences (San Jose, CA). After cells reacted with these MAbs, they were washed twice in 2% fetal bovine serum (FBS)–phosphate-buffered saline (PBS) buffer, and 7-aminoactinomycin D (7-AAD; BD Biosciences) was added to the samples to stain dead cells immediately before acquisition using FACS Verse (BD Biosciences). Data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Phenotypic analysis and single-cell sorting of individual Tax tetramer-positive cells.
For the phenotypic analysis of Tax301-309-CTLs, PE-conjugated HTLV-1 Tax301–309 (SFHSLHLLF)-/HLA-A*24:02 tetramer reagents (MBL) and the following fluorescence-conjugated MAbs were used. CD3-FITC, CD8-APC-Cy7, CD27-PerCP-Cy5.5, CD28-FITC, and CD57-FITC were obtained from BioLegend, and CCR7-PE-Cy7 and CD45RA-APC were obtained from BD Biosciences. After cells were reacted with Tax tetramer reagent and antigen-specific MAbs, they were washed twice and subsequently analyzed by FACSAriaII (BD Biosciences). Individual CD3+ CD8+ Tax tetramer-positive cells were also single-cell sorted for further single-cell TCR repertoire analysis using the same instrument, as described below. The data were analyzed using FlowJo software.
Single-cell TCR repertoire analysis of Tax tetramer-positive cells.
The single-cell TCR repertoire analysis of Tax tetramer-positive cells was performed as described previously (19, 49). Amino acid sequences of CDR3 of the TCR-β chain (CDR3β) were analyzed from a total of 1,107 Tax tetramer-positive cells from 10 ACs (AC1, -3, -4, -5, -7, -9, -10, -11, -16, and -17) and from a total of 351 Tax tetramer-positive cells from 4 ATL patients (aATL2, -3, and -4 and cATL1). TCR repertoires in Tax tetramer-positive cells of aATL2 and aATL3 have been reported previously (19). A TCR repertoire analysis of aATL2 was performed using a bone marrow sample instead of a PB sample, because Tax tetramer-positive cells were undetectable in this patient's PB sample. CDR3 sequence data for each cell were analyzed by comparison with the IMGT human TCR gene database (http://www.imgt.org/).
Statistics.
The nonparametric Mann-Whitney two-tailed U test was used to evaluate the statistical significance of differences in the frequencies of Tax tetramer-positive cell between sACs and either hrACs or ATL patients. A two-group comparison of the absolute numbers of PDR motif-expressing Tax-specific CTLs (PDR+ Tax-CTLs) and PVL in PB that was subjected to a single-cell TCR repertoire analysis was performed with the nonparametric Wilcoxon rank sum test.
ACKNOWLEDGMENTS
This study was partially supported by The Research Award to Jichi Medical University Graduate Student (Yuko Ishihara); Hideki Nakasone was a recipient of AMED J-PRIDE (17fm0208015h0001). We thank Keisuke Takahashi, Sanae Suzuki, and Kiyomi Kubo at the Institute of Medical Science, The University of Tokyo, for their assistance with collecting samples and clinical data.
Yuko Ishihara and Yukie Tanaka designed the study, performed experiments, analyzed data, and wrote the manuscript. Seiichiro Kobayashi and Kaoru Uchimaru conducted the study, collected the data, and analyzed the data. Hideki Nakasone helped design experimental procedures. Koji Kawamura, Ayumi Gomyo, Jin Hayakawa, Masaharu Tamaki, Yu Akahoshi, Naonori Harada, Machiko Kusuda, Kazuaki Kameda, Tomotaka Ugai, Hidenori Wada, Kana Sakamoto, Miki Sato, Kiriko Terasako-Saito, Misato Kikuchi, Shun-ichi Kimura, Aki Tanihara, and Shinichi Kako collected clinical data and samples and interpreted results of experiments. Yoshinobu Kanda designed the project, analyzed data, and wrote the article.
We have no conflicting financial interests.
REFERENCES
- 1.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481–492. [PubMed] [Google Scholar]
- 2.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A 77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, Shirakawa S, Miyoshi I. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A 78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida M, Miyoshi I, Hinuma Y. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A 79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimoyama M. 1991. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol 79:428–437. [DOI] [PubMed] [Google Scholar]
- 6.Ishitsuka K, Tamura K. 2014. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol 15:e517–e526. doi: 10.1016/S1470-2045(14)70202-5. [DOI] [PubMed] [Google Scholar]
- 7.Tajima K. 1990. The 4th nation-wide study of adult T-cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. The T- and B-cell Malignancy Study Group. Int J Cancer 45:237–243. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu Y, Takamori A, Utsunomiya A, Kurimura M, Yamano Y, Hishizawa M, Hasegawa A, Kondo F, Kurihara K, Harashima N, Watanabe T, Okamura J, Masuda T, Kannagi M. 2009. Impaired Tax-specific T-cell responses with insufficient control of HTLV-1 in a subgroup of individuals at asymptomatic and smoldering stages. Cancer Sci 100:481–489. doi: 10.1111/j.1349-7006.2008.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harashima N, Kurihara K, Utsunomiya A, Tanosaki R, Hanabuchi S, Masuda M, Ohashi T, Fukui F, Hasegawa A, Masuda T, Takaue Y, Okamura J, Kannagi M. 2004. Graft-versus-Tax response in adult T-cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res 64:391–399. doi: 10.1158/0008-5472.CAN-03-1452. [DOI] [PubMed] [Google Scholar]
- 10.Okamura J, Utsunomiya A, Tanosaki R, Uike N, Sonoda S, Kannagi M, Tomonaga M, Harada M, Kimura N, Masuda M, Kawano F, Yufu Y, Hattori H, Kikuchi H, Saburi Y. 2005. Allogeneic stem-cell transplantation with reduced conditioning intensity as a novel immunotherapy and antiviral therapy for adult T-cell leukemia/lymphoma. Blood 105:4143–4145. doi: 10.1182/blood-2004-11-4193. [DOI] [PubMed] [Google Scholar]
- 11.Shiratori S, Yasumoto A, Tanaka J, Shigematsu A, Yamamoto S, Nishio M, Hashino S, Morita R, Takahata M, Onozawa M, Kahata K, Kondo T, Ota S, Wakasa K, Sugita J, Koike T, Asaka M, Kasai M, Imamura M. 2008. A retrospective analysis of allogeneic hematopoietic stem cell transplantation for adult T cell leukemia/lymphoma (ATL): clinical impact of graft-versus-leukemia/lymphoma effect. Biol Blood Marrow Transplant 14:817–823. doi: 10.1016/j.bbmt.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Yonekura K, Utsunomiya A, Takatsuka Y, Takeuchi S, Tashiro Y, Kanzaki T, Kanekura T. 2008. Graft-versus-adult T-cell leukemia/lymphoma effect following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 41:1029–1035. doi: 10.1038/bmt.2008.39. [DOI] [PubMed] [Google Scholar]
- 13.Kanda J, Hishizawa M, Utsunomiya A, Taniguchi S, Eto T, Moriuchi Y, Tanosaki R, Kawano F, Miyazaki Y, Masuda M, Nagafuji K, Hara M, Takanashi M, Kai S, Atsuta Y, Suzuki R, Kawase T, Matsuo K, Nagamura-Inoue T, Kato S, Sakamaki H, Morishima Y, Okamura J, Ichinohe T, Uchiyama T. 2012. Impact of graft-versus-host disease on outcomes after allogeneic hematopoietic cell transplantation for adult T-cell leukemia: a retrospective cohort study. Blood 119:2141–2148. doi: 10.1182/blood-2011-07-368233. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, Nakasone H, Yamazaki R, Wada H, Ishihara Y, Kawamura K, Sakamoto K, Ashizawa M, Machishima T, Sato M, Terasako K, Kimura S, Kikuchi M, Okuda S, Kako S, Kanda J, Tanihara A, Nishida J, Kanda Y. 2012. Long-term persistence of limited HTLV-I Tax-specific cytotoxic T cell clones in a patient with adult T cell leukemia/lymphoma after allogeneic stem cell transplantation. J Clin Immunol 32:1340–1352. doi: 10.1007/s10875-012-9729-5. [DOI] [PubMed] [Google Scholar]
- 15.Bangham CR. 2009. CTL quality and the control of human retroviral infections. Eur J Immunol 39:1700–1712. doi: 10.1002/eji.200939451. [DOI] [PubMed] [Google Scholar]
- 16.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, Feeney ME, Rodriguez WR, Basgoz N, Draenert R, Stone DR, Brander C, Goulder PJ, Rosenberg ES, Altfeld M, Walker BD. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol 77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asquith B, Mosley AJ, Barfield A, Marshall SE, Heaps A, Goon P, Hanon E, Tanaka Y, Taylor GP, Bangham CR. 2005. A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J Gen Virol 86:1515–1523. doi: 10.1099/vir.0.80766-0. [DOI] [PubMed] [Google Scholar]
- 18.Coulie PG, Connerotte T. 2005. Human tumor-specific T lymphocytes: does function matter more than number? Curr Opin Immunol 17:320–325. doi: 10.1016/j.coi.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Nakasone H, Yamazaki R, Sato K, Sato M, Terasako K, Kimura S, Okuda S, Kako S, Oshima K, Tanihara A, Nishida J, Yoshikawa T, Nakatsura T, Sugiyama H, Kanda Y. 2010. Single-cell analysis of T-cell receptor repertoire of HTLV-1 Tax-specific cytotoxic T cells in allogeneic transplant recipients with adult T-cell leukemia/lymphoma. Cancer Res 70:6181–6192. doi: 10.1158/0008-5472.CAN-10-0678. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Nakano K, Watanabe E, Ishigaki T, Ohno N, Yuji K, Oyaizu N, Asanuma S, Yamagishi M, Yamochi T, Watanabe N, Tojo A, Watanabe T, Uchimaru K. 2014. CADM1 expression and stepwise downregulation of CD7 are closely associated with clonal expansion of HTLV-I-infected cells in adult T-cell leukemia/lymphoma. Clin Cancer Res 20:2851–2861. doi: 10.1158/1078-0432.CCR-13-3169. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi S, Watanabe E, Ishigaki T, Ohno N, Yuji K, Nakano K, Yamochi T, Watanabe N, Tojo A, Watanabe T, Uchimaru K. 2015. Advanced human T-cell leukemia virus type 1 carriers and early-stage indolent adult T-cell leukemia-lymphoma are indistinguishable based on CADM1 positivity in flow cytometry. Cancer Sci 106:598–603. doi: 10.1111/cas.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, Ogata M, Kikuchi H, Sagara Y, Uozumi K, Mochizuki M, Tsukasaki K, Saburi Y, Yamamura M, Tanaka J, Moriuchi Y, Hino S, Kamihira S, Yamaguchi K. 2010. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood 116:1211–1219. doi: 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 24.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 25.Focosi D, Bestagno M, Burrone O, Petrini M. 2010. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol 87:107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 26.Hoji A, Connolly NC, Buchanan WG, Rinaldo CR Jr. 2007. CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1-specific memory CD8+ T cells. Clin Vaccine Immunol 14:74–80. doi: 10.1128/CVI.00250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oxenius A, Sewell AK, Dawson SJ, Gunthard HF, Fischer M, Gillespie GM, Rowland-Jones SL, Fagard C, Hirschel B, Phillips RE, Price DA. 2002. Functional discrepancies in HIV-specific CD8+ T-lymphocyte populations are related to plasma virus load. J Clin Immunol 22:363–374. doi: 10.1023/A:1020656300027. [DOI] [PubMed] [Google Scholar]
- 28.Sabouri AH, Usuku K, Hayashi D, Izumo S, Ohara Y, Osame M, Saito M. 2008. Impaired function of human T-lymphotropic virus type 1 (HTLV-1)-specific CD8+ T cells in HTLV-1-associated neurologic disease. Blood 112:2411–2420. doi: 10.1182/blood-2008-02-140335. [DOI] [PubMed] [Google Scholar]
- 29.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol 1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 30.Hendriks J, Xiao Y, Borst J. 2003. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med 198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kannagi M, Harada S, Maruyama I, Inoko H, Igarashi H, Kuwashima G, Sato S, Morita M, Kidokoro M, Sugimoto M, et al. . 1991. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int Immunol 3:761–767. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- 32.Pannetier C, Even J, Kourilsky P. 1995. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today 16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg WM, Moss PA, Bell JI. 1992. Variation in human T cell receptor V beta and J beta repertoire: analysis using anchor polymerase chain reaction. Eur J Immunol 22:541–549. doi: 10.1002/eji.1830220237. [DOI] [PubMed] [Google Scholar]
- 34.Spits H. 2002. Development of alphabeta T cells in the human thymus. Nat Rev Immunol 2:760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 35.Turner SJ, Doherty PC, McCluskey J, Rossjohn J. 2006. Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol 6:883–894. doi: 10.1038/nri1977. [DOI] [PubMed] [Google Scholar]
- 36.Venturi V, Price DA, Douek DC, Davenport MP. 2008. The molecular basis for public T-cell responses? Nat Rev Immunol 8:231–238. doi: 10.1038/nri2260. [DOI] [PubMed] [Google Scholar]
- 37.Argaet VP, Schmidt CW, Burrows SR, Silins SL, Kurilla MG, Doolan DL, Suhrbier A, Moss DJ, Kieff E, Sculley TB, Misko IS. 1994. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J Exp Med 180:2335–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tynan FE, Borg NA, Miles JJ, Beddoe T, El-Hassen D, Silins SL, van Zuylen WJ, Purcell AW, Kjer-Nielsen L, McCluskey J, Burrows SR, Rossjohn J. 2005. High resolution structures of highly bulged viral epitopes bound to major histocompatibility complex class I. Implications for T-cell receptor engagement and T-cell immunodominance. J Biol Chem 280:23900–23909. [DOI] [PubMed] [Google Scholar]
- 39.Moss PA, Moots RJ, Rosenberg WM, Rowland-Jones SJ, Bodmer HC, McMichael AJ, Bell JI. 1991. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci U S A 88:8987–8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price DA, Brenchley JM, Ruff LE, Betts MR, Hill BJ, Roederer M, Koup RA, Migueles SA, Gostick E, Wooldridge L, Sewell AK, Connors M, Douek DC. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med 202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trautmann L, Rimbert M, Echasserieau K, Saulquin X, Neveu B, Dechanet J, Cerundolo V, Bonneville M. 2005. Selection of T cell clones expressing high-affinity public TCRs within human cytomegalovirus-specific CD8 T cell responses. J Immunol 175:6123–6132. doi: 10.4049/jimmunol.175.9.6123. [DOI] [PubMed] [Google Scholar]
- 42.Gillespie GM, Stewart-Jones G, Rengasamy J, Beattie T, Bwayo JJ, Plummer FA, Kaul R, McMichael AJ, Easterbrook P, Dong T, Jones EY, Rowland-Jones SL. 2006. Strong TCR conservation and altered T cell cross-reactivity characterize a B*57-restricted immune response in HIV-1 infection. J Immunol 177:3893–3902. doi: 10.4049/jimmunol.177.6.3893. [DOI] [PubMed] [Google Scholar]
- 43.Dong T, Stewart-Jones G, Chen N, Easterbrook P, Xu X, Papagno L, Appay V, Weekes M, Conlon C, Spina C, Little S, Screaton G, van der Merwe A, Richman DD, McMichael AJ, Jones EY, Rowland-Jones SL. 2004. HIV-specific cytotoxic T cells from long-term survivors select a unique T cell receptor. J Exp Med 200:1547–1557. doi: 10.1084/jem.20032044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu XG, Lichterfeld M, Chetty S, Williams KL, Mui SK, Miura T, Frahm N, Feeney ME, Tang Y, Pereyra F, Labute MX, Pfafferott K, Leslie A, Crawford H, Allgaier R, Hildebrand W, Kaslow R, Brander C, Allen TM, Rosenberg ES, Kiepiela P, Vajpayee M, Goepfert PA, Altfeld M, Goulder PJ, Walker BD. 2007. Mutually exclusive T-cell receptor induction and differential susceptibility to human immunodeficiency virus type 1 mutational escape associated with a two-amino-acid difference between HLA class I subtypes. J Virol 81:1619–1631. doi: 10.1128/JVI.01580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iglesias MC, Almeida JR, Fastenackels S, van Bockel DJ, Hashimoto M, Venturi V, Gostick E, Urrutia A, Wooldridge L, Clement M, Gras S, Wilmann PG, Autran B, Moris A, Rossjohn J, Davenport MP, Takiguchi M, Brander C, Douek DC, Kelleher AD, Price DA, Appay V. 2011. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood 118:2138–2149. doi: 10.1182/blood-2011-01-328781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourcier KD, Lim DG, Ding YH, Smith KJ, Wucherpfennig K, Hafler DA. 2001. Conserved CDR3 regions in T-cell receptor (TCR) CD8(+) T cells that recognize the Tax11-19/HLA-A*0201 complex in a subject infected with human T-cell leukemia virus type 1: relationship of T-cell fine specificity and major histocompatibility complex/peptide/TCR crystal structure. J Virol 75:9836–9843. doi: 10.1128/JVI.75.20.9836-9843.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka Y, Yamazaki R, Terasako-Saito K, Nakasone H, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, Ishihara Y, Kawamura K, Sakamoto K, Ashizawa M, Sato M, Kimura S, Kikuchi M, Kako S, Kanda J, Tanihara A, Nishida J, Kanda Y. 2014. Universal cytotoxic activity of a HTLV-1 Tax-specific T cell clone from an HLA-A*24:02(+) patient with adult T-cell leukemia against a variety of HTLV-I-infected T-cells. Immunol Lett 158:120–125. doi: 10.1016/j.imlet.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y, Kobayashi S, Ohno N, Isobe M, Tsuda M, Zaike Y, Watanabe N, Tani K, Tojo A, Uchimaru K. 2011. Leukemic T cells are specifically enriched in a unique CD3(dim) CD7(low) subpopulation of CD4(+) T cells in acute-type adult T-cell leukemia. Cancer Sci 102:569–577. doi: 10.1111/j.1349-7006.2010.01833.x. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka-Harada Y, Kawakami M, Oka Y, Tsuboi A, Katagiri T, Elisseeva OA, Nishida S, Shirakata T, Hosen N, Fujiki F, Murao A, Nakajima H, Oji Y, Kanda Y, Kawase I, Sugiyama H. 2010. Biased usage of BV gene families of T-cell receptors of WT1 (Wilms' tumor gene)-specific CD8+ T cells in patients with myeloid malignancies. Cancer Sci 101:594–600. doi: 10.1111/j.1349-7006.2009.01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]