ABSTRACT
The RV144 HIV vaccine trial included a recombinant HIV glycoprotein 120 (gp120) construct fused to a small portion of herpes simplex virus 1 (HSV-1) glycoprotein D (gD) so that the first 40 amino acids of gp120 were replaced by the signal sequence and the first 27 amino acids of the mature form of gD. This region of gD contains most of the binding site for HVEM, an HSV receptor important for virus infection of epithelial cells and lymphocytes. RV144 induced antibodies to HIV that were partially protective against infection, as well as antibodies to HSV. We derived monoclonal antibodies (MAbs) from peripheral blood B cells of recipients of the RV144 HIV vaccine and showed that these antibodies neutralized HSV-1 infection in cells expressing HVEM, but not the other major virus receptor, nectin-1. The MAbs mediated antibody-dependent cellular cytotoxicity (ADCC), and mice that received the MAbs and were then challenged by corneal inoculation with HSV-1 had reduced eye disease, shedding, and latent infection. To our knowledge, this is the first description of MAbs derived from human recipients of a vaccine that specifically target the HVEM binding site of gD. In summary, we found that monoclonal antibodies derived from humans vaccinated with the HVEM binding domain of HSV-1 gD (i) neutralized HSV-1 infection in a cell receptor-specific manner, (ii) mediated ADCC, and (iii) reduced ocular disease in virus-infected mice.
IMPORTANCE Herpes simplex virus 1 (HSV-1) causes cold sores and neonatal herpes and is a leading cause of blindness. Despite many trials, no HSV vaccine has been approved. Nectin-1 and HVEM are the two major cellular receptors for HSV. These receptors are expressed at different levels in various tissues, and the role of each receptor in HSV pathogenesis is not well understood. We derived human monoclonal antibodies from persons who received the HIV RV144 vaccine that contained the HVEM binding domain of HSV-1 gD fused to HIV gp120. These antibodies were able to specifically neutralize HSV-1 infection in vitro via HVEM. Furthermore, we showed for the first time that HVEM-specific HSV-1 neutralizing antibodies protect mice from HSV-1 eye disease, indicating the critical role of HVEM in HSV-1 ocular infection.
KEYWORDS: herpes simplex virus, monoclonal antibody, HVEM, ADCC, ocular infection, HIV vaccine, glycoprotein D
INTRODUCTION
The RV144 HIV vaccine trial was a randomized, double-blind, placebo-controlled study with four injections of a recombinant canarypox vector vaccine (ALVAC-HIV; vCP1521) expressing HIV glycoprotein 120 (gp120) linked to the transmembrane-anchoring portion of gp41 and HIV gag and protease. A bivalent recombinant gp120 subunit vaccine (AIDSVAX B/E) (1) was given concurrently with the last two injections of ALVAC-HIV. RV144 had 31% efficacy to prevent HIV-1 infection in vaccine recipients. The booster component of the vaccine (AIDSVAX B/E) was a modified form of HIV gp120 with its first 40 amino acids (including the 29-amino-acid signal sequence and the N-terminal 11 amino acids of the mature form of gp120) replaced by the signal sequence and the first 27 amino acids of the mature form of herpes simplex virus 1 (HSV-1) glycoprotein D (gD) (Fig. 1A). The amino terminus of HSV-1 gD was fused to HIV gp120 to facilitate purification and increase expression of gp120 in CHO cells (2–4). While the deletion of the first 11 amino acids of the mature form of gp120 improved the ability of the AIDSVAX B/E vaccine to induce specific types of HIV antibodies compared with wild-type gp120, insertion of the HSV gD sequences did not impair HIV antibody production in nonhuman primates (5). Vaccination with RV144 induced antibodies to HIV, as well as to the HSV gD peptide in the vaccine, in the human clinical trial (6, 7).
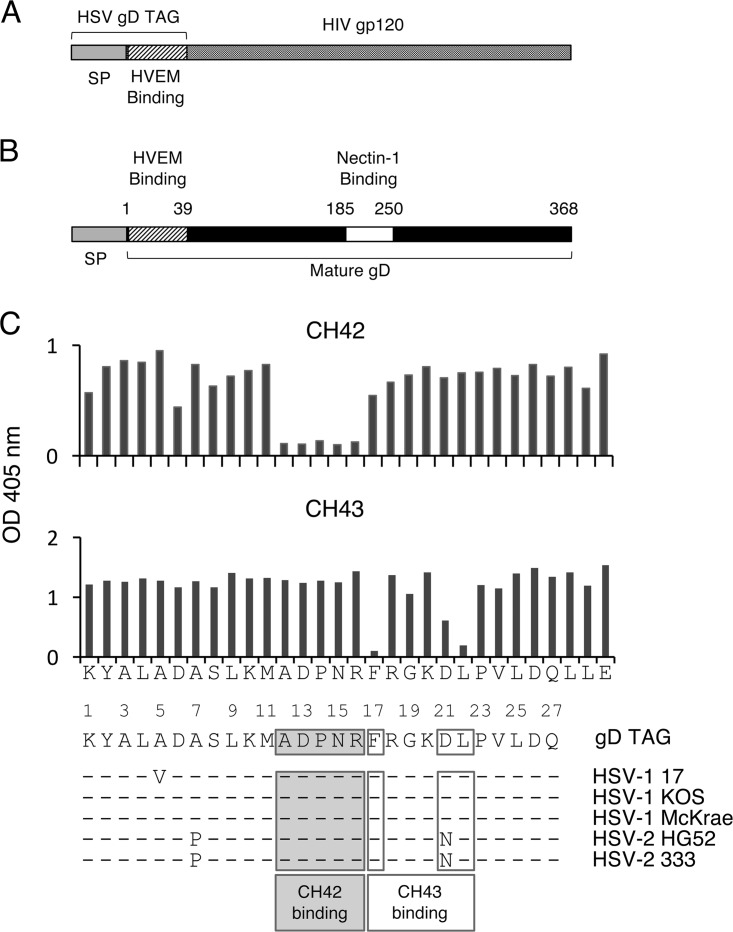
FIG 1.
Structures of AIDSVAX B/E, HSV-1 gD, and amino acids of HSV-1 gD critical for binding of monoclonal antibodies CH42 and CH43. (A) AIDSVAX B/E has the first 27 amino acids of the mature form of HSV-1 gD fused to gp120 without the first 40 amino acids of gp120. (B) HSV-1 gD has a signal sequence and HVEM and nectin-1 binding domains. (C) HSV-1 gD amino acids recognized by MAb CH42 and CH43 were determined by binding the MAbs to peptides containing alanine substitution mutations in HSV gD (the amino acid sequence of HSV-1 gD TAG peptide is shown on the x axis), followed by ELISA. The optical density (OD) at 405 nm for binding of MAbs to gD TAG is shown on the y axes. Amino acid sequence alignment of HSV-1 gD TAG to HSV-1 and HSV-2 gD in five commonly used virus strains is shown below; the dashes represent amino acids identical to those in HSV gD TAG. The numbers indicate amino acid positions in the mature form of gD.
HSV uses two principal receptors to enter cells, herpesvirus entry mediator (HVEM) and nectin-1 (8, 9). HVEM is a member of the tumor necrosis factor receptor family and is important for HSV entry into lymphocytes, fibroblasts, and epithelial cells. HVEM interacts with LIGHT and lymphotoxin-α (10), as well as BTLA (11) and CD160 (12). Nectin-1 is a member of the immunoglobulin superfamily, functions as an adhesion molecule, and interacts with afadin (13). Nectin-1 is important for entry of HSV into epithelial cells, fibroblasts, and especially neurons. The first 32 amino acids of the mature form of HSV-1 gD bind to HVEM (14, 15), while side chains of exposed amino acids in several regions of gD, especially amino acids 38, 132, 215, 220, 222, and 223, interact with nectin-1 (16) (Fig. 1B). Since AIDSVAX B/E contains the first 27 amino acids of the mature form of HSV-1 gD, persons receiving this vaccine might make antibody to HSV-1 that could neutralize HSV infection in vitro and reduce disease in an animal model of HSV infection.
The roles of HVEM and nectin-1 in HSV infection have been studied in mice with the two virus receptors knocked out (17–20). HVEM is critical for HSV-1 corneal infection but is not required for HSV-2 corneal, intravaginal, or intracranial infection or for HSV-1 intravaginal infection. In contrast, nectin-1 is critical for HSV-2 disease after genital and intracranial inoculation, as well as HSV-1 corneal infection. These studies imply that an antibody that specifically blocks the interaction of HSV with HVEM is likely to inhibit corneal infection with HSV-1. Here, we report that monoclonal antibodies (MAbs) derived from B cells of RV144 recipients that specifically target the HVEM binding site of HSV-1 gD neutralize HSV-1 infection of cells expressing HVEM, mediate HSV-1-specific antibody-dependent cellular cytotoxicity (ADCC), and reduce HSV-1 corneal disease and shedding in mice.
RESULTS
Isolation of MAbs to HSV-1 gD from B cells in the blood of RV144 vaccine recipients.
RNA isolated from memory B cells that bound to gD tetramer was used to derive HSV-1 gD-specific V(D)J sequences and was cloned into a mammalian expression vector encoding a human IgG1 backbone (21, 22). The MAbs were expressed in 293T cells transfected with IgH and IgL gene constructs and purified from culture supernatants. Of nine heavy chains isolated, seven were specific for the HSV-1 gD peptide. Two MAbs, CH42 and CH43, that showed strong binding to gD peptide were selected for further characterization. CH42 was derived from a B cell producing IgA2, and CH43 was derived from a B cell producing IgG1.
HSV gD MAbs CH42 and CH43 recognize amino acids at the N terminus of HSV-1 gD important for interacting with HVEM.
The gD binding epitopes for MAbs CH42 and CH43 were mapped using alanine scanning mutagenesis. MAb CH42 recognizes amino acids 12 to 16 (ADPNR), while CH43 recognizes amino acids 17, 21, and 22 (FXXXDL) (Fig. 1C). Amino acids 12 to 16 of HSV-1 gD are critical for the interaction of the viral glycoprotein with its cell receptor, HVEM, based on the crystal structure of gD bound to HVEM, binding assays, and amino acid mutagenesis of gD (14, 15). While HSV-1 gD amino acids 21 and 22 are not in direct contact with HVEM, they are located at the site where gD undergoes a conformational change upon binding to HVEM (15). Thus, MAbs CH42 and CH43 might block HSV-1 gD binding to HVEM or sterically inhibit the ability of gD to change its conformation during HVEM binding, respectively. Alignment of the HSV-1 gD sequence from AIDSVAX B/E rgp120 with sequences of gD from different strains of HSV-1 and HSV-2 showed nearly 100% amino acid identity, with the exception of gD amino acids 5, 7, and 21 (Fig. 1C). Most of the amino acid differences were due to changes in the sequence of HSV-2 gD. The MAb CH42 binding epitope (ADPNR) was identical to the sequences of HSV-1 and HSV-2 gD. The MAb CH43 binding epitope was identical to the sequence of HSV-1 gD but differed from HSV-2 gD, with an asparagine in the sequence of HSV-2 gD at amino acid 21 in place of an aspartic acid in HSV-1.
HSV gD MAbs CH42 and CH43 neutralize HSV-1 infection in cells expressing human HVEM, but not nectin-1.
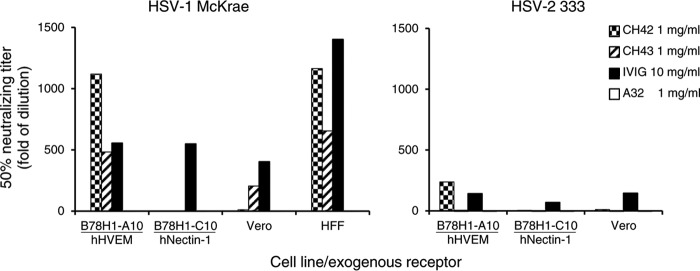
Since the HSV-1 gD sequence in AIDSVAX B/E contains the majority of the HSV-1 gD domain that interacts with HVEM (14, 15), but not with its other receptor, nectin-1 (16), we postulated that MAbs CH42 and CH43 might neutralize HSV infection by blocking entry via HVEM, but not nectin-1. Therefore, we performed neutralization assays in B78H1-A10 mouse cells that stably express human HVEM (but not nectin-1) or B78H1-C10 mouse cells that stably express human nectin-1 (but not HVEM). Both MAbs CH42 and CH43 neutralized HSV-1 infection in B78H1-A10 cells by 50% at 1:1,118 and 1:483 dilutions (equivalent to 0.89 μg/ml and 2.07 μg/ml), respectively. In contrast, neither MAb CH42 nor MAb CH43 was able to neutralize HSV-1 infection in B78H1-C10 cells. Human intravenous immunoglobulin (IVIG) neutralized HSV-1 infection by 50% at a 1:556 dilution in B78H1-A10 cells and at a 1:549 dilution in B78H1-C10 cells (equivalent to 18.0 μg/ml and 18.2 μg/ml), while the human IgG1 isotype control antibody A32 did not neutralize the viruses (Fig. 2).
FIG 2.
Neutralizing activity of MAbs CH42 and CH43 in B78H1-A10 mouse cells that express human HVEM (hHVEM) (but not nectin-1), B78H1-C10 mouse cells that express human nectin-1 (but not HVEM), Vero cells that express endogenous simian HVEM and nectin-1, and primary HFF. Stock concentrations of MAbs and human IVIG were 1 mg/ml and 10 mg/ml, respectively. Fifty percent HSV-neutralizing titers plotted as the reciprocal of the dilution are shown for HSV-1 McKrae and HSV-2 333 in the presence of MAbs CH42, CH43, and A32 (human IgG1 isotype control) or IVIG, a positive control. Similar results were obtained in two experiments, and a representative result is shown.
Neither MAb CH42 nor MAb CH43 was able to neutralize HSV-2 infection in B78H1-C10 cells (Fig. 2). However, MAb CH42 had neutralizing activity for HSV-2 infection in B78H1-A10 cells (the 50% neutralizing titer was at a 1:238 dilution, equivalent to 4.2 μg/ml); MAb CH43 had no neutralizing activity for HSV-2 infection in these cells. As noted above, the sequence of the CH43 binding site in HSV gD matches that in HSV-1 gD but differs by 1 amino acid in HSV-2 gD, which may explain the inability of MAb CH43 to neutralize HSV-2 infection. Human IVIG was able to neutralize HSV-2 infection of both B78H1-A10 and B78H1-C10 cells; 50% neutralizing titers were observed at 1:141 and 1:71 dilutions (equivalent to 71 μg/ml and 143 μg/ml), respectively.
Previously, we showed that HVEM from Vero cells (a simian cell line) was less effective than human HVEM in supporting HSV-2 infection (23). Therefore, we determined if MAbs CH42 and CH43 could neutralize virus infectivity in Vero cells. CH43 neutralized HSV-1, but not HSV-2, infection in Vero cells; the 50% HSV-1 neutralizing titer for CH43 in Vero cells was observed at a 1:205 dilution, lower than the 1:483 dilution observed in B78H1-A10 cells (equivalent to 4.9 μg/ml and 2.1 μg/ml, respectively), presumably due to simian HVEM in Vero cells versus human HVEM in B78H-A10 cells (Fig. 2). CH42, which had a titer higher than that of CH43 to neutralize HSV-1 in B78H1-A10 cells expressing human HVEM, had very low neutralizing activity in Vero cells against both HSV-1 and HSV-2; 50% neutralizing titers were observed at 1:11 and 1:10 dilutions, equivalent to 89 μg/ml and 100 μg/ml, respectively.
To determine if the MAbs could neutralize HSV-1 infection in primary human cells, we measured their neutralizing activities in human foreskin fibroblasts (HFF). CH42 and CH43 neutralized 50% of HSV-1 infection of HFF at 1:1,164 and 1:655 dilutions (equivalent to 0.86 μg/ml and 1.53 μg/ml), respectively (Fig. 2). However, residual HSV-1 infectivity remained even when high doses (100 μg/ml) of either antibody were used. Taken together, these findings indicate that both MAbs CH42 and CH43 can neutralize HSV-1 entry mediated by human HVEM.
HSV gD MAbs CH42 and CH43 mediate ADCC.
ADCC has been shown to be important for protection against HSV infection (24, 25). ADCC is mediated by effector cells in response to virus-specific antibodies bound to viral antigens expressed on the surfaces of infected cells. Classical ADCC is mediated by NK cells, but other cell types, including macrophages and neutrophils, can also mediate ADCC. We developed a nonradioactive method to detect HSV-specific ADCC NK cell activation by measuring induction of NK cell surface CD107a, a surrogate marker for degranulation of NK cells (26). Human NK-92-CD16 cells, which express the CD16 Fc receptor for IgG, were used as effector cells in the assay. Incubation of HSV-1-infected SK-N-SH cells in the presence of 5 μg/ml of CH42 or CH43 resulted in expression of CD107a on the surfaces of 49% or 29% of NK-92-CD16 cells, respectively (Fig. 3A). As a control, NK-92-CD16 cells were incubated with uninfected SK-N-SH cells in the presence of MAb or NK-92-CD16 cells were incubated with HSV-1-infected cells in the absence of MAb; in each of these control experiments, <5% of NK-92-CD16 cells expressed CD107a on their surfaces. The percentages of activated NK-92-CD16 cells induced by isotype (human IgG1) control antibody A32, which recognizes HIV gp120, were comparable in the presence or absence of HSV-1 infection (13% and 12%, respectively), indicating that the relatively high background with the isotype control MAb was not HSV-1 specific (Fig. 3A). A dose-response curve using various concentrations of CH42 and CH43 MAbs showed that CH42 induced maximum NK-92-CD16 cell-activating activity at lower concentrations of antibody than CH43 (Fig. 3B); 43.9% and 33.9% of NK-92-CD16 cells expressed CD107a on their surfaces after incubation with HSV-1-infected cells in the presence of 5 μg/ml of CH42 or 50 μg/ml of CH43, respectively. The isotype control antibody A32 induced NK-92-CD16 cell activation in >5% of cells only when the amount of antibody was >50 μg/ml, and its activity was not HSV-1 infection specific (Fig. 3B).
FIG 3.
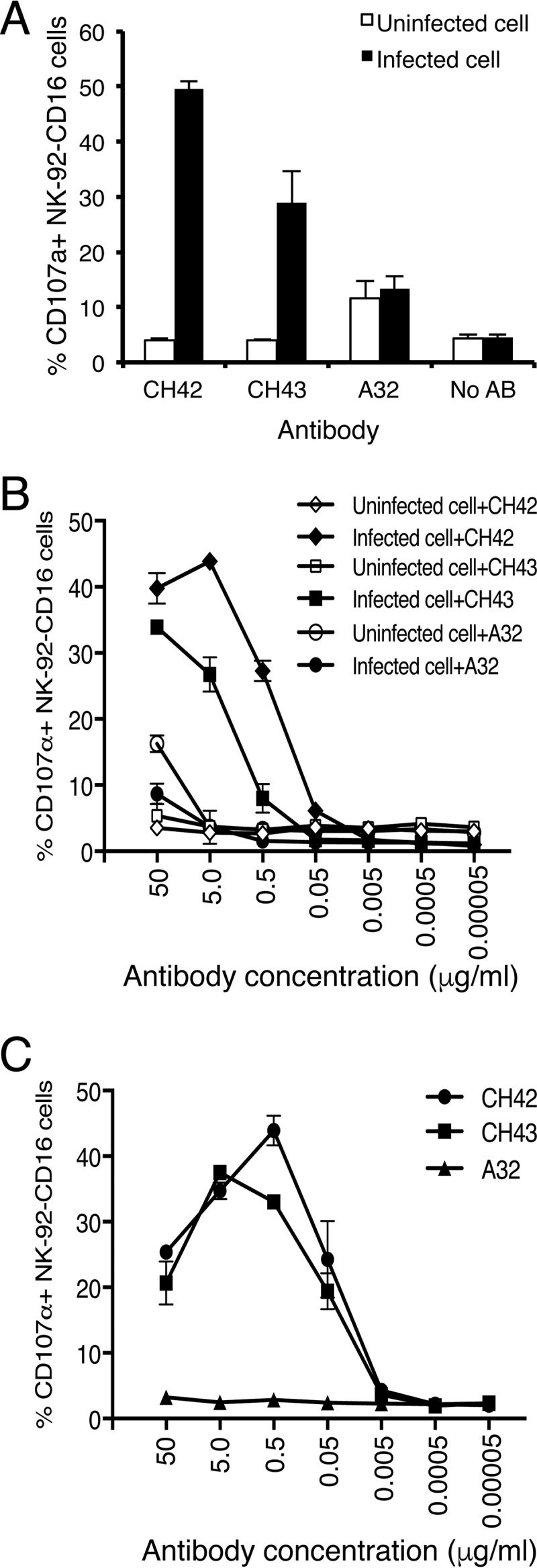
ADCC NK cell activation mediated by MAbs CH42 and CH43 incubated with NK-92-CD16 cells (which stably express GFP) and either HSV-1-infected SK-N-SH cells or HSV-1 gD-coated 96-well plates. (A) NK-92-CD16 cells (1 × 105) were incubated with HSV-1-infected or uninfected SK-N-SH cells in the presence or absence of MAbs at 5 μg/ml (CH42, CH43, and A32 [a human IgG1 isotype control] or no antibody [No AB]) for 5 h. Cell surface CD107a staining was then performed as a marker of NK cell activation. The percentages of NK-92-CD16 cells (GFP positive) that expressed CD107a on their surfaces are shown on the y axis. (B) Dose-response curve of ADCC using serial dilutions of MAb CH42, CH43, or isotype control A32 with HSV-1-infected or uninfected SK-N-SH cells. (C) Dose-response curve of NK-92-CD16 cell activation using serial dilutions of MAb CH42, CH43, or isotype control A32 in HSV-1 gD-coated 96-well plates. The diluted antibodies were added to the gD-coated wells and incubated for 15 min, and 5 × 105 NK-92-CD16 cells/well were added and incubated for 5 h. After washing with PBS, the cells were stained with 4 μg/ml APC-Cy7-conjugated anti-CD107a antibody and fixed with 10% paraformaldehyde. Activated degranulating NK cells (GFP+ CD107a+) were detected by flow cytometry. The percentages of CD107a+ NK-92-CD16 cells are shown. The error bars indicate standard deviations.
To show that the ADCC assay specifically measured antibody to HSV-1 gD, 96-well plates were coated with recombinant HSV-1 gD and then incubated with MAbs and NK-92-CD16 cells. MAbs CH42 and CH43 activated NK cells when used at a concentration of 0.05 μg/ml, while the isotype control MAb had no activity (Fig. 3C). MAbs CH42 and CH43 showed maximum NK cell activation (43.9% and 37.5%) at 0.5 μg/ml and 5 μg/ml, respectively.
HSV-1 gD MAbs CH42 and CH43 protect mice from lethal HSV-1 infection after corneal inoculation.
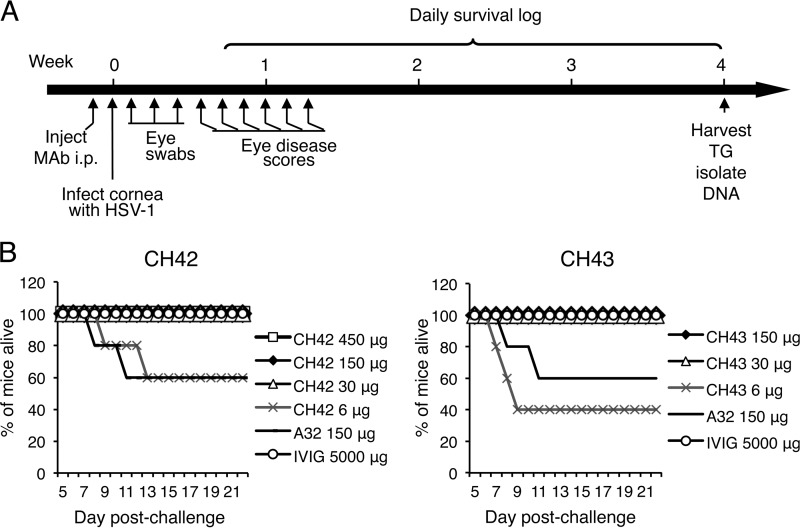
To determine if CH42 or CH43 has activity in vivo, we tested the antibodies in a mouse ocular model of HSV-1 infection. Mice were injected intraperitoneally (i.p.) with MAb, and 2 days later, the animals were infected by corneal scarification of both eyes with 2 × 105 PFU/eye of HSV-1 McKrae (Fig. 4A). Animals receiving 30 μg or more of CH42 or CH43 or 5 mg of human IVIG had 100% survival after HSV-1 inoculation (Fig. 4B).
FIG 4.
Survival of mice treated with MAb CH42, CH43, or isotype control (A32) or human IVIG and infected with HSV-1 by corneal scarification. (A) Eight-week-old BALB/c mice (5 animals/group) were injected i.p. with MAb CH42, CH43, or A32 or IVIG, and 2 days later, both eyes were infected with HSV-1 McKrae after corneal scarification. Eye swabs were obtained during the first 3 days after infection, disease scores were measured 4 to 9 days after infection, and trigeminal ganglia (TG) were harvested 4 weeks after infection. (B) Kaplan-Meier survival curves for days 5 to 21 after infection for mice treated with CH42 (left) or CH43 (right).
HSV-1 gD MAbs CH42 and CH43 reduce the severity of HSV-1 ocular disease in a dose-dependent manner.
Mice that received MAbs and were infected with HSV-1 were monitored for eye disease from days 4 to 9 after HSV-1 infection by corneal scarification. Both CH42 and CH43 significantly reduced ocular disease compared with isotype control antibody A32 (P = 0.0006 for CH42 versus A32; P = 0.0018 for CH43 versus A32; t test) (Fig. 5A). The reduction in ocular disease was dose dependent for both HSV MAbs. The correlation of ocular disease with the dose of each antibody was statistically significant (P = 0.0003 for CH42 and P = 0.011 for CH43, based on linear regression) (Fig. 5B).
FIG 5.
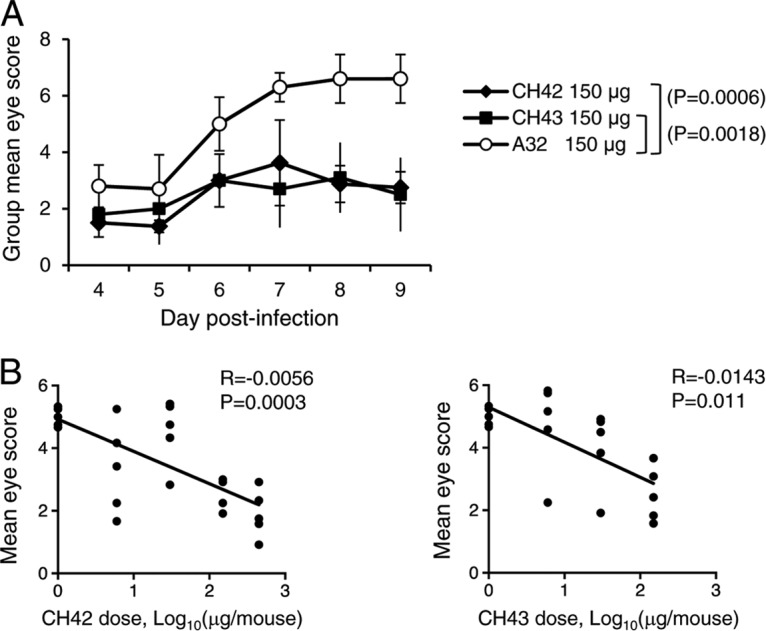
Eye scores of mice treated with MAb CH42, CH43, or isotype control (A32) and infected with HSV-1 by corneal scarification. The mice were treated and infected as described in the legend to Fig. 4. (A) Daily group mean eye disease scores in mice (5 animals/group) that received 150 μg of MAbs. The error bars indicate standard deviations. (B) Correlation of the dose of MAb (0, 6, 30, 150, and 450 μg/mouse for CH42 and 0, 6, 30, and 150 μg/mouse for CH43) with mean eye disease scores during the 6 days after infection. Each point represents the results for one mouse.
HSV-1 gD MAbs CH42 and CH43 reduce shedding of HSV-1 from the eye after ocular challenge in a dose-dependent manner.
Virus shedding from eyes was measured with corneal swabs on days 1 to 3 after corneal scarification with HSV-1. Both CH42 and CH43 reduced ocular shedding of HSV-1 compared with isotype control antibody A32 (P = 0.0543 for CH42 versus A32; P = 0.0337 for CH43 versus A32; t test) (Fig. 6A). While the reduction of virus shedding was modest, the reduction in ocular shedding was dose dependent for both HSV MAbs and was statistically significant (P = 0.0091 for CH42 and P = 0.028 for CH43 based on linear regression) (Fig. 6B).
FIG 6.
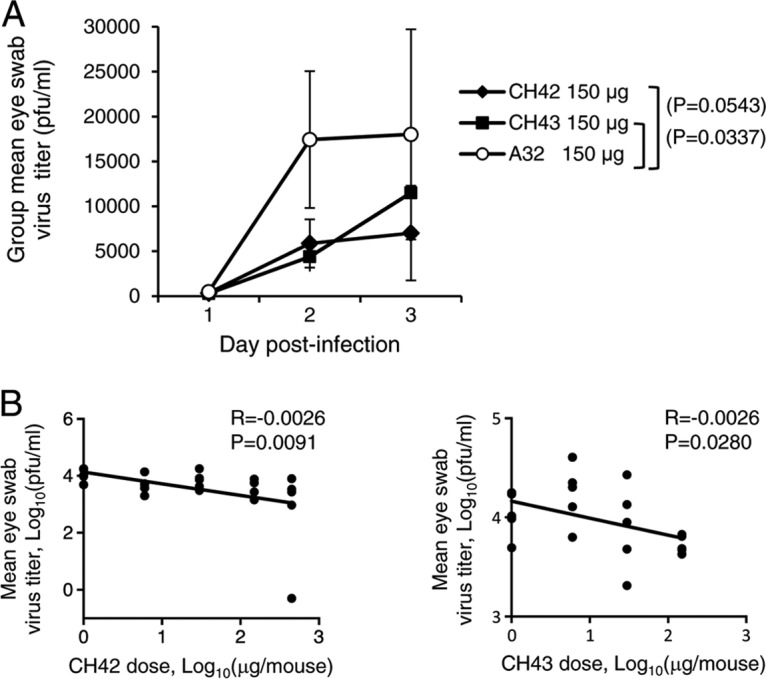
HSV-1 shedding from eyes of mice treated with MAb CH42, CH43, or isotype control (A32) and infected with HSV-1 by corneal scarification. The mice were treated and infected as described in the legend to Fig. 4. (A) Eye swabs were obtained for the first 3 days after infection, HSV-1 titers were measured in Vero cells, and daily group mean titers were determined. The error bars indicate standard deviations. (B) Correlation of the dose of MAb (0, 6, 30, 150, and 450 μg/mouse for CH42 and 0, 6, 30, and 150 μg/mouse for CH43) with the mean HSV-1 titer in eye swabs from mice during the first 3 days after infection. Each point represents the results for one mouse.
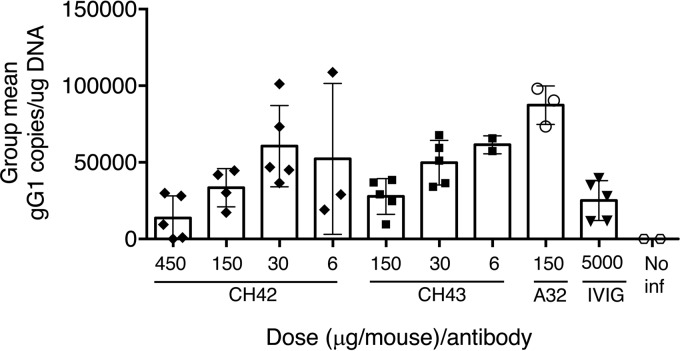
HSV-1 gD MAbs CH42 and CH43 reduce HSV-1 latency in trigeminal ganglia after ocular challenge.
Mice that received MAbs and were infected with HSV-1 were euthanized 4 weeks after HSV-1 infection, and trigeminal ganglia, the site of latent HSV-1 after ocular infection, were examined for HSV-1 DNA. Mice that received 150 μg of CH42 or 150 μg of CH43 had lower levels of HSV-1 DNA (means, 36,140 and 27,781 HSV-1 genomes/μg DNA, respectively) in latently infected trigeminal ganglia than those receiving isotype control antibody A32 (mean, 90,430 HSV-1 genomes/μg DNA) (Fig. 7). Taken together, these findings indicate that the HSV-1 MAbs protected mice from lethal infection, reduced ocular disease, and modestly reduced virus shedding and latency of virus in trigeminal ganglia compared with isotype control antibody.
FIG 7.
HSV-1 latent DNA loads in trigeminal ganglia of mice treated with MAb CH42, CH43, or isotype control (A32) or human IVIG and infected with HSV-1 by corneal scarification. The mice were treated and infected as described in the legend to Fig. 4; mice not infected with HSV-1 (No inf) served as a negative control. Four weeks after infection, the mice were euthanized, trigeminal ganglia were harvested, DNA was isolated, and HSV-1 DNA copies per microgram of DNA were determined by real-time PCR using primers and a probe specific for the HSV-1 gG gene. The error bars indicate standard deviations.
DISCUSSION
We report that two human MAbs derived from B cells of volunteers who received the RV144 HIV vaccine, in which the HSV-1 gD HVEM binding domain was fused to gp120, bind to amino acids in gD that are critical for the interaction of gD with HVEM and specifically block HSV-1 infection mediated by HVEM. In addition, these MAbs mediated ADCC and protected mice from lethal infection, reduced eye disease, and modestly reduced virus shedding and latency after corneal inoculation with HSV-1.
Multiple cellular receptors, including HVEM and nectin-1, can mediate HSV entry into cells. These receptors are differentially expressed on various cell types and are the principal determinant of susceptibility of cells to HSV infection. Lymphocytes express abundant HVEM, while neurons express nectin-1; both HVEM and nectin-1 are present on epithelial cells (9, 27–29). Neutralizing antibodies can prevent HSV infectivity by blocking virus binding to a specific receptor or to more than one receptor; therefore, antibodies may protect only some cell types from HSV infection. For example, antibody that blocks HSV binding to nectin-1 may neutralize HSV infection of neurons but not lymphocytes. In addition, antibodies also mediate other antiviral activities, including complement-dependent cytotoxicity, antibody-dependent phagocytosis, and ADCC (30). Certain antibody isotypes, such as IgG1 in humans and IgG2a in mice, are more potent for inducing ADCC. The combination of antibodies that both neutralize virus infection and mediate ADCC of infected cells may result in better protection from infection.
We found that two MAbs derived from B cells of recipients of the RV144 HIV vaccine bound to amino acids in HSV-1 gD critical for its interaction with HVEM and had high levels of HSV-1-neutralizing activity in mouse cells expressing human HVEM but had no neutralizing activity against virus infection of mouse cells expressing human nectin-1. These antibodies had low HSV-1-neutralizing activity in Vero cells that express simian HVEM and simian nectin-1 (31). We previously showed that an HSV-2 gD mutant (HSV2-gD27) that is unable to use nectin-1 as a receptor but still uses HVEM was unable to infect Vero cells unless the cells were engineered to express human HVEM (23). These data indicate that HSV infection of Vero cells is dependent on nectin-1 as a receptor; therefore, we postulated that CH42 and CH43 would have low neutralizing activity in Vero cells. While MAb CH42 had very low neutralizing activity against HSV-1 infection of Vero cells, MAb CH43 was able to efficiently neutralize HSV-1 infection in these cells. This disparity is likely due to differences where MAbs CH42 and CH43 bind to gD. Binding of the first 32 amino acids of gD to HVEM results in formation of a hairpin-like domain at the amino terminus of gD with amino acid 21 at the site where the hairpin folds (14). A large portion of the surface of HSV-1 gD that interacts with nectin-1 is predicted to be covered by this hairpin-like domain (16). Thus, while HVEM and nectin-1 bind to different sites on gD, the binding of gD to HVEM is predicted to reduce or eliminate the ability of gD to bind to nectin-1 (16). MAbs CH42 and CH43 bind to different sites at the amino terminus of gD; CH42 binds to amino acids 12 to 16, while CH43 binds to amino acids 17, 21, and 22. Binding of MAb CH43 to gD might mimic binding of HVEM to gD, resulting in formation of a hairpin-like domain (with a fold at amino acid 21 of gD) that could partially block the surface of gD, important for interacting with nectin-1, and reduce HSV-1 infection of Vero cells mediated by nectin-1. While MAbs CH42 and CH43 had potent neutralizing activity against HSV-1 infection of HFF, they were unable to completely block HSV-1 infection of the cells. This could be due to HSV-1 receptors other than HVEM on the surfaces of the cells.
In addition to neutralizing activity, antibodies may also induce ADCC. MAbs CH42 and CH43 were constructed with a human IgG1 backbone and therefore have the potential to elicit ADCC. Both MAbs induced NK cell activation after incubation with HSV-1-infected cells or purified HSV-1 gD on plates. Activated NK cells can kill virus-infected cells and also produce a number of antiviral cytokines, including interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (32). HSV-infected cells are susceptible to lysis by mononuclear-cell-mediated ADCC within 2 to 3 h after infection in vitro (33). Passive transfer of MAbs that did not have HSV-neutralizing activity in vitro protected ≥50% of mice from lethal HSV-2 challenge; protection of mice was correlated with the ability of the MAbs to induce ADCC (24). Passive transfer of serum from mice immunized with an HSV-2 mutant that had little or no HSV-neutralizing antibody but significant levels of ADCC antibody protected wild-type mice, but not Fcγ receptor knockout mice, from challenge with HSV-2 (25). High levels of maternal or neonatal anti-HSV ADCC antibody in humans or high levels of anti-HSV neutralizing antibody in neonates correlated with protection of babies from disseminated HSV infection (34). Therefore, MAbs that have both HSV-1-neutralizing activity to block entry of virus into cells and ADCC activity to kill virus-infected cells may be more effective at preventing HSV infection than MAbs with only virus-neutralizing activity.
MAbs to the receptor binding sites of other viruses, including Ebola virus (35), Epstein-Barr virus (EBV) (36), Middle East respiratory syndrome (MERS) virus (37), severe acute respiratory syndrome (SARS) coronavirus (38), influenza virus (39), poliovirus (40), and HIV (41), can potently neutralize virus infection. MAbs DL11 and E317 were previously reported to block both the nectin-1 and HVEM receptor binding sites (42–44). Thus, a vaccine that induces an antibody that blocks HSV binding to HVEM and nectin-1 might be more effective at protecting epithelial cells from infection than a vaccine that targets the HVEM binding domain of gD alone.
Both of the major receptors for HSV, HVEM and nectin-1, are present on murine corneal epithelial cells (45, 46), as well as on human corneal epithelial cells, conjunctival epithelial cells, and retinal pigment epithelial cells (27–29). Studies of HVEM and nectin-1 knockout mice indicate that HVEM is important for HSV-1 infection of the mouse vaginal tract (20), brain (19), and eyes (17, 18). Further studies showed that HVEM may induce corneal disease after HSV infection independently of its role in virus entry (47). HSV-1 infection of corneal epithelial cells from wild-type mice results in induction of multiple cytokines and chemokines, including interleukin 6 (IL-6) and CXCL10, which are induced at significantly lower levels in corneal epithelial cells from HVEM knockout mice (48). IL-6 and CXCL10 are important for ocular disease associated with HSV-1 infection (49) and may recruit inflammatory cells into the eye, resulting in corneal damage. Importantly, induction of IL-6 and CXCL10 in corneal epithelial cells by HVEM does not require entry of HSV-1 through HVEM, since an HSV HVEM entry-null mutant (lacking amino acids 7 to 15 of gD) also results in cytokine induction (48). Increased numbers of HVEM-positive monocytes and neutrophils are present in the cornea during acute and chronic ocular infection, respectively, of mice with HSV-1 (50), and these cells may also contribute to the pathogenesis of virus-associated eye disease.
While several studies have shown that passive systemic transfer of MAbs can prevent or reduce disease due to HSV-1 keratitis (51–59), the ability of MAbs that specifically block HSV-1 infection mediated by HVEM to prevent HSV-1 disease in mice has not been reported. Since MAbs CH42 and CH43 block HSV-1 infection mediated specifically by HVEM, our observation that these MAbs reduce HSV-1 infection of the mouse cornea supports the hypothesis that HVEM plays a critical role in HSV-1 ocular infection as an HSV-1 entry receptor, in addition to its role in inducing ocular inflammation.
MATERIALS AND METHODS
Human blood samples.
Peripheral blood mononuclear cells (PBMCs) were obtained from vaccine recipients who enrolled in the RV144 vaccine trial (NCT00223080), which was approved, as previously reported (1), by the Ethics Committees of the Walter Reed Army Institute of Research, Mahidol University, the Royal Thai Army, and the Thai Ministry of Public Health.
Isolation of MAbs.
PBMCs from HSV-seronegative RV144 vaccine recipients who subsequently developed HSV gD antibody after vaccination were stained with fluorescently labeled antibodies to identify memory B cells (live CD3− CD14− CD16− CD235a− CD19+ surface IgD−) that reacted with a gD tetramer (biotin-KKKKYALADASLKMADPNRFRGKDLPVLDQLLE). Memory B cells that bound the gD tetramer (∼1% of memory B cells) were sorted as individual cells into 96-well plates. Human MAbs were constructed from the cells using recombinant-DNA techniques as described previously (22, 60, 61). Briefly, the B cells were sorted into 96-well plates containing an RNA stabilization reagent and were stored at −80°C prior to further manipulation. RNA in the wells was used for reverse transcription (RT)-PCR to amplify the V(D)J regions of both heavy and light chains; the resulting PCR product was used for overlapping PCR to directly construct a linear cassette (22) based on the human IgG1 backbone. MAbs were expressed in 293T cells transfected with the constructs; culture supernatant was used in enzyme-linked immunosorbent assay (ELISA) for screening. For large-scale production of antibodies, genes were synthesized (GenScript) and used to produce linear cassettes that were transfected into 293T cells; antibodies from the culture supernatants were isolated using protein A/G columns. The ability of the recombinant MAbs to bind HSV-1 gD peptide was determined by ELISA.
Identification of HSV-1 gD epitopes that are recognized by MAb CH42 or CH43.
Alanine scanning mutagenesis was used to identify amino acid epitopes as described previously (62). ELISA was performed as described previously (21), and purified antibody was tested by ELISA against the wild-type peptide and peptides with substituted alanine.
Cells and viruses.
B78H1-A10 and B78H1-C10 mouse melanoma cell lines that stably express human HVEM or nectin-1, respectively (63), were provided by Gary Cohen and Roselyn Eisenberg (University of Pennsylvania). B78H1-A10 and B78H1-C10 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) containing 5% fetal bovine serum (FBS) and 250 or 500 μg/ml G418, respectively. Vero cells and HFF were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM containing 10% FBS. SK-N-SH human neuroblastoma cells (from the ATCC) were cultured in minimal essential medium (MEM) (Life Technologies) with 10% FBS and 1% nonessential amino acids. NK-92-CD16 cells are NK-92 cells stably expressing human CD16-176V and green fluorescent protein (GFP) (64, 65) and were kindly provided by Kerry Campbell at Fox Chase Cancer Center and NantKwest, Inc. NK-92-CD16 cells were grown in MEM alpha (Gibco) supplemented with 0.2 mM Myo-insitol Prep (Sigma-Aldrich), nonessential amino acids, 1 mM sodium pyruvate, penicillin-streptomycin, 0.0025 mM folic acid, 0.1 mM beta-mercaptoethanol, 10% heat-inactivated FBS (HyClone), 10% heat-inactivated horse serum (Life Technologies), and 200 IU/ml recombinant human IL-2 (Peprotech). The cells were cultured for a maximum of 12 passages and split 1:3 every 3 days. HSV-1 McKrae and HSV-2 333 (plaque purified) (66) were propagated and titrated in Vero cells, aliquoted, and stored at −80°C.
HSV neutralization assays.
Stock concentrations of MAbs and human IVIG were 1 mg/ml and 10 mg/ml, respectively. Neutralizing assays were performed by incubating 2-fold serial dilutions of MAbs in PBS containing 2% FBS with HSV-1 McKrae or HSV-2 333 at room temperature for 1 h. The mixtures were then added to cell monolayers in 6-well plates, and when plaques were of sufficient size to visualize, they were counted, and 50% neutralizing titers (reciprocal of dilution) were determined by nonlinear regression with Prism software as described previously (66).
HSV-1-specific ADCC assay.
Human SK-N-SH cells were infected with HSV-1 McKrae at a multiplicity of infection (MOI) of 1 and incubated at 37°C for 16 h. The infected cells were dissociated with TrypLE, (Gibco) and resuspended at 107 cells/ml in complete MEM, and 5 × 105 cells (in 50 μl) were added to each well of 96-well U bottom plates. Uninfected SK-N-SH cells were included as a negative control. MAbs were serially diluted 10-fold in MEM from 100 μg/ml to 0.1 ng/ml, and 100 μl of diluted antibody was added to the wells containing SK-N-SH cells. Each MAb dilution with HSV-1-infected or uninfected SK-N-SH cells was performed in triplicate. The plates were incubated at 37°C for 15 min, and 1 × 105 NK-92-CD16 cells in 50 μl were added to each well and further incubated for 5 h at 37°C. To determine the percentage of NK-92-CD16 cells that were activated in the presence of the MAb and HSV-1-infected cells, cultures were stained with allophycocyanin (APC)-Cy7-conjugated anti-CD107a (clone H4A3; BioLegend) for 30 min, washed with PBS, and fixed with 10% paraformaldehyde. The cells were analyzed by flow cytometry on an LSRII (Becton Dickinson), and the percentage of GFP-positive cells (which represents the NK-92-CD16 population) with surface staining for CD107a was determined using FlowJo analysis software. To determine the percentage of SK-N-SH cells infected with HSV-1, cells were stained with mouse anti-gD MAb MC23 (a gift from Gary Cohen) and then incubated with goat anti-mouse IgG Alexa Fluor 488 (Life Technologies), fixed in paraformaldehyde, and analyzed by flow cytometry.
HSV gD-specific ADCC assay.
Ninety-six-well ELISA plates (Maxisorp; Nunc) were coated with recombinant HSV-1 gD protein, gD-1(Δ290-299t) (67), provided by Gary Cohen and Roselyn Eisenberg at the University of Pennsylvania, at 400 ng/well. Serial 10-fold dilutions of antibody were added to the wells and incubated for 15 min, and 5 × 105 NK-92-CD16 cells/well were added and incubated for 5 h. After washing with PBS, the cells were stained with 4 μg/ml APC-Cy7-conjugated anti-CD107a antibody in 5 mM EDTA-PBS buffer for 30 min at room temperature and fixed with 10% paraformaldehyde-PBS. The frequency of CD16-GFP+ NK cells expressing CD107a was analyzed by flow cytometry.
Mouse experiments.
All mouse studies were conducted under a protocol approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases. Female 8-week-old BALB/c mice (5 per group) were injected i.p. with MAb CH42 or CH43 at 450, 150, 30, or 6 μg/mouse. Human MAb A32, an IgG1 antibody that recognizes HIV gp120 (68), was used at 150 μg/mouse as an isotype control. Human IVIG (Gamunex C; Grifols Therapeutics Inc.) was used at 5 mg/mouse as a positive control. Two days later, mouse eyes were infected with HSV-1 McKrae at 2 ×105 PFU/eye bilaterally after corneal scarification. Eye swabs were taken to monitor virus shedding on days 1, 2, and 3 postinfection, and the swabs were stored in 0.5 ml of medium at −80°C. The virus titer of the swabs was determined in Vero cells by plaque assay. The severity of eye disease was scored from 0 to 4 based on prior criteria (69, 70) with minor modifications: 0, no sign of infection; 1, noticeably puffy eyelids and modest eye discharge; 2, eye swollen with blepharospasms, about 50% shut with moderate discharge; 3, eye nearly closed with discharge and crust; and 4, eye totally swollen and crusted shut. Four weeks postinfection, the mice were euthanized, their trigeminal ganglia were harvested, and DNA was isolated with a QIAgen blood and tissue DNA kit. HSV-1 genomic DNA was quantified by real-time PCR (TaqMan; Applied Biosystems) using primers and a probe specific for the HSV-1 gG gene, as previously described (71).
Statistics.
Virus titers of eye swabs were log10 transformed, and the t test was performed to compare eye swab titers and ocular disease scores between groups. Correlations of MAb dose with eye disease scores or eye swab virus titers were analyzed by linear regression.
ACKNOWLEDGMENTS
This work was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases (NIAID) and by the Center for HIV/AIDS Vaccine Immunology (CHAVI) (AI0678501, funded by the National Institutes of Health [NIH] NIAID Division of AIDS), and by the Duke University Center for AIDS Research (CFAR) (5P30 AI064518, funded by NIH). S.J. was funded by a National Health and Medical Research Council (NHMRC) Australia Early Career Fellowship (APP1072127).
We thank Gary Cohen and Roselyn Eisenberg for B78H1 cell lines and gD-1(Δ290-299t) protein; Carter Lee (Executive Director) and Faruk Sinangil (Director), Research Collaborations, Global Solutions for Infectious Diseases (GSID), South San Francisco, CA, for assistance with obtaining vaccine; and Jing Qin for help with statistics.
REFERENCES
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Berman PW. 1998. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res Hum Retroviruses 14(Suppl 3):S277–S289. [PubMed] [Google Scholar]
- 3.Lasky LA, Groopman JE, Fennie CW, Benz PM, Capon DJ, Dowbenko DJ, Nakamura GR, Nunes WM, Renz ME, Berman PW. 1986. Neutralization of the AIDS retrovirus by antibodies to a recombinant envelope glycoprotein. Science 233:209–212. doi: 10.1126/science.3014647. [DOI] [PubMed] [Google Scholar]
- 4.Berman PW, Huang W, Riddle L, Gray AM, Wrin T, Vennari J, Johnson A, Klaussen M, Prashad H, Kohne C, deWit C, Gregory TJ. 1999. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology 265:1–9. doi: 10.1006/viro.1999.0031. [DOI] [PubMed] [Google Scholar]
- 5.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL Jr, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. 2013. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol 87:1554–1568. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert PB, Excler JL, Tomaras GD, Carpp LN, Haynes BF, Liao HX, Montefiori DC, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Kijak GH, Tovanabutra S, Francis DP, Lee C, Sinangil F, Berman PW, Premsri N, Kunasol P, O'Connell RJ, Michael NL, Robb ML, Morrow R, Corey L, Kim JH. 2017. Antibody to HSV gD peptide induced by vaccination does not protect against HSV-2 infection in HSV-2 seronegative women. PLoS One 12:e0176428. doi: 10.1371/journal.pone.0176428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume G, Cocchi F, Menotti L, Lopez M. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev Med Virol 10:305–319. [DOI] [PubMed] [Google Scholar]
- 9.Spear PG. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol 6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 10.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. 1998. LIGHT, a new member of the TNF superfamily, and lymphotoxin alpha are ligands for herpesvirus entry mediator. Immunity 8:21–30. doi: 10.1016/S1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 11.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. 2005. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol 6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 12.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. 2008. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 13.Sakisaka T, Taniguchi T, Nakanishi H, Takahashi K, Miyahara M, Ikeda W, Yokoyama S, Peng YF, Yamanishi K, Takai Y. 2001. Requirement of interaction of nectin-1alpha/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J Virol 75:4734–4743. doi: 10.1128/JVI.75.10.4734-4743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8:169–179. doi: 10.1016/S1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J Virol 77:8127–8140. doi: 10.1128/JVI.77.14.8127-8140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karaba AH, Kopp SJ, Longnecker R. 2012. Herpesvirus entry mediator is a serotype specific determinant of pathogenesis in ocular herpes. Proc Natl Acad Sci U S A 109:20649–20654. doi: 10.1073/pnas.1216967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaba AH, Kopp SJ, Longnecker R. 2011. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J Virol 85:10041–10047. doi: 10.1128/JVI.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopp SJ, Banisadr G, Glajch K, Maurer UE, Grunewald K, Miller RJ, Osten P, Spear PG. 2009. Infection of neurons and encephalitis after intracranial inoculation of herpes simplex virus requires the entry receptor nectin-1. Proc Natl Acad Sci U S A 106:17916–17920. doi: 10.1073/pnas.0908892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. 2007. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe 2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, Parks R, Whitesides J, Marshall DJ, Hwang KK, Yang Y, Chen X, Gao F, Munshaw S, Kepler TB, Denny T, Moody MA, Haynes BF. 2009. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods 158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Kappel JD, Canders C, Davila WF, Sayre D, Chavez M, Pesnicak L, Cohen JI. 2012. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM. J Virol 86:12891–12902. doi: 10.1128/JVI.01055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balachandran N, Bacchetti S, Rawls WE. 1982. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun 37:1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, Sengupta M, Herold BC, Jacobs WR. 10 March 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 4. doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alter G, Malenfant JM, Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar J, Tiwari V, Oh MJ, Kovacs M, Jani A, Kovacs SK, Valyi-Nagy T, Shukla D. 2008. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest Ophthalmol Vis Sci 49:4026–4035. doi: 10.1167/iovs.08-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farooq AV, Valyi-Nagy T, Shukla D. 2010. Mediators and mechanisms of herpes simplex virus entry into ocular cells. Curr Eye Res 35:445–450. doi: 10.3109/02713681003734841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. 2010. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol Vis 16:2476–2486. [PMC free article] [PubMed] [Google Scholar]
- 30.Casadevall A, Pirofski LA. 2011. A new synthesis for antibody-mediated immunity. Nat Immunol 13:21–28. doi: 10.1038/ni.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krummenacher C, Baribaud F, Ponce de Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ. 2004. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 322:286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Campbell KS, Hasegawa J. 2013. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol 132:536–544. doi: 10.1016/j.jaci.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shore SL, Cromeans TL, Romano TJ. 1976. Immune destruction of virus-infected cells early in the infectious cycle. Nature 262:695–696. doi: 10.1038/262695a0. [DOI] [PubMed] [Google Scholar]
- 34.Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM. 1989. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis 160:770–776. doi: 10.1093/infdis/160.5.770. [DOI] [PubMed] [Google Scholar]
- 35.Howell KA, Qiu X, Brannan JM, Bryan C, Davidson E, Holtsberg FW, Wec AZ, Shulenin S, Biggins JE, Douglas R, Enterlein SG, Turner HL, Pallesen J, Murin CD, He S, Kroeker A, Vu H, Herbert AS, Fusco ML, Nyakatura EK, Lai JR, Keck ZY, Foung SK, Saphire EO, Zeitlin L, Ward AB, Chandran K, Doranz BJ, Kobinger GP, Dye JM, Aman MJ. 2016. Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 15:1514–1526. doi: 10.1016/j.celrep.2016.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Servat E, Ro BW, Cayatte C, Gemmell L, Barton C, Rao E, Lin R, Zuo F, Woo JC, Hayes GM. 2015. Identification of the critical attribute(s) of EBV gp350 antigen required for elicitation of a neutralizing antibody response in vivo. Vaccine 33:6771–6777. doi: 10.1016/j.vaccine.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Du L, Zhao G, Yang Y, Qiu H, Wang L, Kou Z, Tao X, Yu H, Sun S, Tseng CT, Jiang S, Li F, Zhou Y. 2014. A conformation-dependent neutralizing monoclonal antibody specifically targeting receptor-binding domain in Middle East respiratory syndrome coronavirus spike protein. J Virol 88:7045–7053. doi: 10.1128/JVI.00433-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bian C, Zhang X, Cai X, Zhang L, Chen Z, Zha Y, Xu Y, Xu K, Lu W, Yan L, Yuan J, Feng J, Hao P, Wang Q, Zhao G, Liu G, Zhu X, Shen H, Zheng B, Shen B, Sun B. 2009. Conserved amino acids W423 and N424 in receptor-binding domain of SARS-CoV are potential targets for therapeutic monoclonal antibody. Virology 383:39–46. doi: 10.1016/j.virol.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, Dormitzer PR, Haynes BF, Walter EB, Moody MA, Kepler TB, Liao HX, Harrison SC. 2011. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci U S A 108:14216–14221. doi: 10.1073/pnas.1111497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Chumakov K, Dragunsky E, Kouiavskaia D, Makiya M, Neverov A, Rezapkin G, Sebrell A, Purcell R. 2011. Chimpanzee-human monoclonal antibodies for treatment of chronic poliovirus excretors and emergency postexposure prophylaxis. J Virol 85:4354–4362. doi: 10.1128/JVI.02553-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol 72:7064–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CC, Lin LL, Chan WE, Ko TP, Lai JS, Wang AH. 2013. Structural basis for the antibody neutralization of herpes simplex virus. Acta Crystallogr D Biol Crystallogr 69:1935–1945. doi: 10.1107/S0907444913016776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol 72:3595–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kovacs SK, Tiwari V, Prandovszky E, Dosa S, Bacsa S, Valyi-Nagy K, Shukla D, Valyi-Nagy T. 2009. Expression of herpes virus entry mediator (HVEM) in the cornea and trigeminal ganglia of normal and HSV-1 infected mice. Curr Eye Res 34:896–904. doi: 10.3109/02713680903184250. [DOI] [PubMed] [Google Scholar]
- 46.Valyi-Nagy T, Sheth V, Clement C, Tiwari V, Scanlan P, Kavouras JH, Leach L, Guzman-Hartman G, Dermody TS, Shukla D. 2004. Herpes simplex virus entry receptor nectin-1 is widely expressed in the murine eye. Curr Eye Res 29:303–309. doi: 10.1080/02713680490516756. [DOI] [PubMed] [Google Scholar]
- 47.Edwards RG, Longnecker R. 2017. Herpes virus entry mediator (HVEM) and ocular herpes infection: more than meets the eye. J Virol 91:e00115-17. doi: 10.1128/JVI.00115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards RG, Kopp SJ, Karaba AH, Wilcox DR, Longnecker R. 2015. Herpesvirus entry mediator on radiation-resistant cell lineages promotes ocular herpes simplex virus 1 pathogenesis in an entry-independent manner. mBio 6:e01532–e01515. doi: 10.1128/mBio.01532-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wickham S, Ash J, Lane TE, Carr DJ. 2004. Consequences of CXCL10 and IL-6 induction by the murine IFN-alpha1 transgene in ocular herpes simplex virus type 1 infection. Immunol Res 30:191–200. doi: 10.1385/IR:30:2:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards RG, Kopp SJ, Ifergan I, Shui JW, Kronenberg M, Miller SD, Longnecker R. 2017. Murine corneal inflammation and nerve damage after infection with HSV-1 are promoted by HVEM and ameliorated by immune-modifying nanoparticle therapy. Invest Ophthalmol Vis Sci 58:282–291. doi: 10.1167/iovs.16-20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue Y, Ohashi Y, Watanabe H, Manabe R. 1992. Protective effects of anti-glycoprotein D monoclonal antibodies in murine herpetic keratitis. Curr Eye Res 11:53–60. doi: 10.3109/02713689209069167. [DOI] [PubMed] [Google Scholar]
- 52.Krawczyk A, Dirks M, Kasper M, Buch A, Dittmer U, Giebel B, Wildschutz L, Busch M, Goergens A, Schneweis KE, Eis-Hubinger AM, Sodeik B, Heiligenhaus A, Roggendorf M, Bauer D. 2015. Prevention of herpes simplex virus induced stromal keratitis by a glycoprotein B-specific monoclonal antibody. PLoS One 10:e0116800. doi: 10.1371/journal.pone.0116800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lausch RN, Oakes JE, Metcalf JF, Scimeca JM, Smith LA, Robertson SM. 1989. Quantitation of purified monoclonal antibody needed to prevent HSV-1 induced stromal keratitis in mice. Curr Eye Res 8:499–506. doi: 10.3109/02713688909000030. [DOI] [PubMed] [Google Scholar]
- 54.Lausch RN, Staats H, Metcalf JF, Oakes JE. 1990. Effective antibody therapy in herpes simplex virus ocular infection. Characterization of recipient immune response. Intervirology 31:159–165. [DOI] [PubMed] [Google Scholar]
- 55.Lousch RN, Staats H, Oakes JE, Cohen GH, Eisenberg RJ. 1991. Prevention of herpes keratitis by monoclonal antibodies specific for discontinuous and continuous epitopes on glycoprotein D. Invest Ophthalmol Vis Sci 32:2735–2740. [PubMed] [Google Scholar]
- 56.Metcalf JF, Koga J, Chatterjee S, Whitley RJ. 1987. Passive immunization with monoclonal antibodies against herpes simplex virus glycoproteins protects mice against herpetic ocular disease. Curr Eye Res 6:173–177. doi: 10.3109/02713688709020086. [DOI] [PubMed] [Google Scholar]
- 57.Metcalf JF, Chatterjee S, Koga J, Whitley RJ. 1988. Protection against herpetic ocular disease by immunotherapy with monoclonal antibodies to herpes simplex virus glycoproteins. Intervirology 29:39–49. doi: 10.1159/000150027. [DOI] [PubMed] [Google Scholar]
- 58.Ritchie MH, Oakes JE, Lausch RN. 1993. Passive transfer of anti-herpes simplex virus type 2 monoclonal and polyclonal antibodies protect against herpes simplex virus type 1-induced but not herpes simplex virus type 2-induced stromal keratitis. Invest Ophthalmol Vis Sci 34:2460–2468. [PubMed] [Google Scholar]
- 59.Su YH, Yan XT, Oakes JE, Lausch RN. 1996. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J Virol 70:1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonsignori M, Hwang KK, Chen X, Tsao CY, Morris L, Gray E, Marshall DJ, Crump JA, Kapiga SH, Sam NE, Sinangil F, Pancera M, Yongping Y, Zhang B, Zhu J, Kwong PD, O'Dell S, Mascola JR, Wu L, Nabel GJ, Phogat S, Seaman MS, Whitesides JF, Moody MA, Kelsoe G, Yang X, Sodroski J, Shaw GM, Montefiori DC, Kepler TB, Tomaras GD, Alam SM, Liao HX, Haynes BF. 2011. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol 85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z, Qin HR, Chen W, Zhao Q, Shen X, Schutte R, Wang Y, Ofek G, Streaker E, Prabakaran P, Fouda GG, Liao HX, Owens J, Louder M, Yang Y, Klaric KA, Moody MA, Mascola JR, Scott JK, Kwong PD, Montefiori D, Haynes BF, Tomaras GD, Dimitrov DS. 2011. Cross-reactive HIV-1-neutralizing human monoclonal antibodies identified from a patient with 2F5-like antibodies. J Virol 85:11401–11408. doi: 10.1128/JVI.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther 3:160–168. doi: 10.1006/mthe.2000.0240. [DOI] [PubMed] [Google Scholar]
- 64.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. 2008. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol 180:6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sobhanie M, Matsuoka Y, Jegaskanda S, Fitzgerald T, Mallory R, Chen Z, Luke C, Treanor J, Subbarao K. 2016. Evaluation of the safety and immunogenicity of a candidate pandemic live attenuated influenza vaccine (pLAIV) against influenza A(H7N9). J Infect Dis 213:922–929. doi: 10.1093/infdis/jiv526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang K, Goodman KN, Li DY, Raffeld M, Chavez M, Cohen JI. 2015. A herpes simplex virus 2 (HSV-2) gD mutant impaired for neural tropism is superior to an HSV-2 gD subunit vaccine to protect animals from challenge with HSV-2. J Virol 90:562–574. doi: 10.1128/JVI.01845-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nicola AV, Willis SH, Naidoo NN, Eisenberg RJ, Cohen GH. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol 70:3815–3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liao HX, Alam SM, Mascola JR, Robinson J, Ma B, Montefiori DC, Rhein M, Sutherland LL, Scearce R, Haynes BF. 2004. Immunogenicity of constrained monoclonal antibody A32-human immunodeficiency virus (HIV) Env gp120 complexes compared to that of recombinant HIV type 1 gp120 envelope glycoproteins. J Virol 78:5270–5278. doi: 10.1128/JVI.78.10.5270-5278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandt CR, Spencer B, Imesch P, Garneau M, Deziel R. 1996. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob Agents Chemother 40:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saied AA, Chouljenko VN, Subramanian R, Kousoulas KG. 2014. A replication competent HSV-1 (McKrae) with a mutation in the amino-terminus of glycoprotein K (gK) is unable to infect mouse trigeminal ganglia after cornea infection. Curr Eye Res 39:596–603. doi: 10.3109/02713683.2013.855238. [DOI] [PubMed] [Google Scholar]
- 71.Wang K, Lau TY, Morales M, Mont EK, Straus SE. 2005. Laser-capture microdissection: refining estimates of the quantity and distribution of latent herpes simplex virus 1 and varicella-zoster virus DNA in human trigeminal ganglia at the single-cell level. J Virol 79:14079–14087. doi: 10.1128/JVI.79.22.14079-14087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]