Abstract
Duplication and segregation of chromosomes involves dynamic reorganization of their internal structure by conserved architectural proteins, including the structural maintenance of chromosomes (SMC) complexes cohesin and condensin. Despite active investigation of the roles of these factors, a genome‐wide view of dynamic chromosome architecture at both small and large scale during cell division is still missing. Here, we report the first comprehensive 4D analysis of the higher‐order organization of the Saccharomyces cerevisiae genome throughout the cell cycle and investigate the roles of SMC complexes in controlling structural transitions. During replication, cohesion establishment promotes numerous long‐range intra‐chromosomal contacts and correlates with the individualization of chromosomes, which culminates at metaphase. In anaphase, mitotic chromosomes are abruptly reorganized depending on mechanical forces exerted by the mitotic spindle. Formation of a condensin‐dependent loop bridging the centromere cluster with the rDNA loci suggests that condensin‐mediated forces may also directly facilitate segregation. This work therefore comprehensively recapitulates cell cycle‐dependent chromosome dynamics in a unicellular eukaryote, but also unveils new features of chromosome structural reorganization during highly conserved stages of cell division.
Keywords: chromosome segregation, Hi‐C, loop extrusion, replication profile, SMC
Subject Categories: Cell Cycle; Chromatin, Epigenetics, Genomics & Functional Genomics; DNA Replication, Repair & Recombination
Introduction
The chromosomes of prokaryotes and eukaryotes display multiple levels of hierarchical organization, whose dynamic changes influence or regulate metabolic processes including gene expression and DNA replication and repair (Taddei & Gasser, 2012; Wang et al, 2013; Dekker & Mirny, 2016). The improper coordination of chromosome condensation and segregation during the cell cycle can lead to important structural abnormalities and result in cell death or diseases such as cancer (Valton & Dekker, 2016). In recent years, major advances in imaging and chromosome conformation capture approaches (Dekker et al, 2002; Lieberman‐Aiden et al, 2009; 3C, Hi‐C) have complemented earlier work by describing at an unprecedented resolution the multiple hierarchical layers of genome organization. A variety of remarkable 3D chromosomal structures have been described in a number of species, including in unicellular organisms such as bacteria and yeasts.
The genome of budding yeast Saccharomyces cerevisiae presents a Rabl organization driven by (i) centromeres clustering at the spindle pole body (SPB, S. cerevisiae microtubule organizing center), (ii) telomeres tethering to the nuclear envelope, (iii) the nucleolus where the rDNA is sequestered opposite to the SPB, and (iv) chromosome arm length (Burgess & Kleckner, 1999; Taddei & Gasser, 2012). Hi‐C experiments have confirmed this Rabl organization, but the existence of sub‐megabase structures within yeast chromosomes similar to mammalian topological associated domains or their bacterial equivalent is still controversial (Duan et al, 2010; Hsieh et al, 2015; Eser et al, 2017). Importantly, genomic analysis of chromosome 3D architectures has usually been done using asynchronous populations, in which cells are found in various stages of the cell cycle. However, the initiation and progression of replication, followed by the segregation of the sister chromatids (SCs) into daughter cells, is expected to modify the genome higher‐order organization. Recent studies have unveiled cell‐cycle stage‐specific genome‐wide topological variations in bacteria, yeast, fly, and mammals (Naumova et al, 2013; Guidi et al, 2015; Marbouty et al, 2015; Hug et al, 2017). As expected, in all species the largest reorganization transition is associated with SC condensation, a fundamental process occurring concomitantly to their individualization, and facilitating their proper segregation.
Pioneer studies on yeasts proved essential to study these processes. Mutations in cell‐division cycle (cdc; Hartwell et al, 1973) genes can block the cell cycle progression, enabling the study of global and/or local chromosome reorganization at specific cycle phases (Hartwell et al, 1973; Guacci et al, 1994; Sullivan et al, 2004; Renshaw et al, 2010; Rock & Amon, 2011). The evolutionary conserved structural maintenance of chromosomes (SMC) proteins bind to chromosomes and modify their structure in spatially and temporarily regulated manner during the cell cycle (Aragon et al, 2013; Uhlmann, 2016). Cohesins, such as Scc1, promote SC cohesion during DNA replication (Blat & Kleckner, 1999; Glynn et al, 2004) and get cleaved at the metaphase‐to‐anaphase transition (Uhlmann et al, 1999). At the same time, condensins such as Smc2 are loaded onto SCs to facilitate their segregation (Renshaw et al, 2010; Stephens et al, 2011; Hirano, 2012). In fission yeast, the binding of SMCs modifies the level of chromosome compaction defined as the ratio between long (> 10 kb)‐ and short‐range (< 10 kb) contacts at specific loci (Mizuguchi et al, 2014; Kim et al, 2016).
While Hi‐C studies on mammalian and drosophila cells have confirmed this compaction change and provided important insights on the organization of mitotic chromosomes’ internal structure (Naumova et al, 2013; Hug et al, 2017), no comprehensive analysis of the 4D dynamics of the chromosomes during an entire eukaryotic cell cycle has been achieved. To explore new chromosomal structural features over the cell cycle progression, we analyzed the internal folding and overall organization of S. cerevisiae genome over 15 synchronized time points and the role of cohesin and condensin using Hi‐C (Dekker et al, 2002; Lieberman‐Aiden et al, 2009). This analysis provides a broad overview and in‐depth insight on SMC‐dependent structural transitions resulting in chromosome individualization and segregation, including a potential role for a condensin‐dependent loop in contributing to the segregation of the rDNA cluster.
Results
Comparison of chromosome contact maps of synchronized cells
Hi‐C libraries were generated from cell cultures synchronized in G1 with elutriation (Marbouty et al, 2014) and/or arrested at different stages of the cell cycle through thermosensitive (ts) cdc mutations (Fig 1A; Hartwell et al, 1973). After sequencing, the corresponding normalized genome‐wide contact maps were computed (bin: 5 kb; Fig 1C and D, left panels; Fig EV1; Materials and Methods; Cournac et al, 2012).
Figure 1. Comparison of genome structures recovered from five synchronized stages over the cell cycle.

-
AOverview of the different synchronization time points with corresponding FACS profiles and representative images of DAPI‐stained cells.
-
B3D average representation of the Hi‐C contact map of a yeast G1 population. The color code reflects chromosomal arm lengths, and centromeres, telomeres, and rDNA are highlighted.
-
C, DComparison of contact maps. The 16 yeast chromosomes are displayed atop the maps. Black arrowheads: inter‐telomere contacts. Yellow arrowheads: inter‐centromeric contacts. Left panels: Hi‐C maps obtained from two G1 cell populations synchronized independently (C) and from G1 and G0 populations (D). Brown to yellow color scales reflect high to low contact frequencies, respectively (log10). Right panels: log‐ratio between each pair of maps. Insets display magnifications of chr4. Blue to red color scales reflect the enrichment in contacts in one population with respect to the other (log2).
-
EPairwise Euclidian distances between contact maps of populations of G0, G1 either synchronized with elutriation or blocked using a cdc6 mutant, metaphase (cdc20 mutant), and anaphase (cdc15 mutant) cells. Color code: contact map similarity.
-
FPrincipal component analysis (PCA) of the distance matrix in (E).
Figure EV1. Contact maps and 3D genome representations of the five cell cycle synchronization states.

-
A, BContact maps generated from synchronized cell populations described in this study, with each vector (or bin) corresponding to 5 kb. x‐ and y‐axis represent the 16 chromosomes of the yeast genome, displayed atop the maps. Brown to yellow color scales reflect high to low contact frequencies, respectively (log10). Magnification panels in (B) show variations of the contact frequencies between synchronized populations. Yellow and pink arrowheads point at centromeres and rDNA positions, respectively.
-
C3D average representations of the Hi‐C contact maps of synchronized cell populations of panel (A). The color code represents the chromosomal arm length, and centromeres, telomeres, and rDNA flanking regions are highlighted.
These 2D maps were translated into 3D representations to visualize the main folding features (Lesne et al, 2014; e.g., centromeres and telomeres clustering in G1; Figs 1B and EV1). These 3D structures are average representations of the contact frequencies quantified over a population of cells and therefore do not represent the exact structure found in individual cells. For instance, on these 3D representations all the telomeres loosely cluster together. In a single nucleus, telomeres rather form small groups scattered all around the nuclear membrane (Taddei & Gasser, 2012). Since in different cells the composition of these clusters differs, all telomeres end up being regrouped together in the average 3D structure that reflects the population average of contacts. In addition, they are not polymer models and cannot be interpreted as such. Nevertheless, these representations conveniently highlight important structural features not readily apparent in the 2D maps (Mercy et al, 2017).
The differences between two conditions were determined by computing the log‐ratio between the maps (bin: 5 kb † ; Fig 1C; Materials and Methods). The color scale reflects the variations in contact frequency for each bin between two different contact maps. The ratio of contact maps generated from two independent G1 cell populations (experimental replicates) displays a relatively homogenous white (i.e., null) signal, corresponding to little differences between them (Fig 1C, right panel). These minor variations between the maps result in occasional faint colored areas and reflect experimental noise (Appendix and Materials and Methods). On the other hand, the ratio between exponentially growing G1 and quiescent G0 cells contact maps (Fig 1D, right panel) shows a strong difference in inter‐telomere contact frequencies, reflecting the formation of the telomeres hyper‐cluster characteristic of the G0 metabolic state (Guidi et al, 2015; Fig 1D, black arrowheads).
Multiple maps can also be compared altogether by computing their pairwise distance matrix, showing that the genome organization of cells in anaphase (cdc15) differs the most compared to other time points (Fig 1E; Materials and Methods). The overall similarities/differences between datasets can then be summarized using principal component analysis (PCA; Fig 1F). This 2D representation shows that the experimental duplicates (such as G1, or anaphase cdc15) clustered together, while the distance increases progressively between G1 (obtained with either elutriation or cdc6 ts mutant), metaphase (cdc20), and the distant anaphase (cdc15) datasets.
Altogether, these comparisons highlight major changes in chromosome higher‐order architecture taking place in cells progressing throughout the cell cycle into metaphase and anaphase.
Cohesin‐mediated compaction during S phase
To decipher the chromosome structural changes that take place during replication, synchronized G1 cells were released into S phase and Hi‐C maps generated for six time points sampled from two independent kinetics (Figs 2A and EV2; Materials and Methods). The PCA reveals a progressive structural evolution from G1 to late S/G2 phase (Fig 2B). The dependency of the contact probability P on genomic distance reflects the chromosome compaction state (Lieberman‐Aiden et al, 2009; Naumova et al, 2013; Mizuguchi et al, 2014). The P(s) shows a gradual and consistent enrichment in long‐range intra‐chromosomal contacts (> 20 kb) with respect to short‐range (< 10 kb) during replication (Fig 2C). This compaction change is absent when replication is impaired, for instance, in the absence of the replication‐checkpoint regulator cdc6 (Piatti et al, 1995), even though cells enter mitosis and engage into segregation of non‐replicated chromosomes (Fig 2D, left panel). The progressive increase in long‐range contacts stops with the completion of S phase, when it reaches the level observed in cells arrested at the G2/metaphase transition (G/M) with the microtubule‐depolymerizing drug nocodazole (Jacobs et al, 1988; Fig 2D, middle panel). The crossing of the P(s) slopes from the early to late replication time points occurs around 10–20 kb (Fig 2C, highlighted in gray), a window within the range of the spacing reported between cohesin binding sites (~11 kb on average; Glynn et al, 2004), suggesting that this change in compaction could be due to cohesin activity. In agreement with the key role of cohesin in sister‐chromatid folding during replication, Scc1 depletion using an auxin‐inducible degron scc1‐aid strain prevents the enrichment in long‐range contacts in S/G2 (Fig 2D, right panel). This result supports the hypothesis that distant regions enriched in cohesin are tethered together, resulting in chromatin loops (Guillou et al, 2010).
Figure 2. Dynamic reorganization of chromosomes during replication.
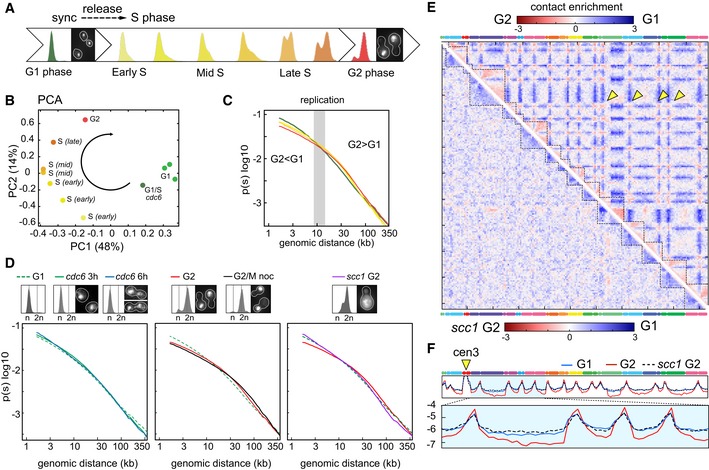
- FACS profiles and representative DAPI‐stained cells of G1 synchronized cells released in S phase.
- PCA of the distance matrix between the contact maps of the population displayed in (A).
- P(s), that is, average intra‐chromosomal contact frequency P between two loci with respect to their genomic distance s along the chromosome (log–log scale) during replication (color code identical to FACS profiles and PCA).
- Left panel: P(s) of replication‐defective cells (cdc6 thermosensitive mutant). G1 elutriated cells were released for 3 h and 6 h in non‐permissive conditions. The corresponding FACS profiles show no S‐phase progression. Middle panel: P(s) of cells that completed replication. G1 elutriated cells were released in S phase in the absence or presence of nocodazole (G2/M noc). Right panel: P(s) of cohesin‐depleted (scc1 G2) and nocodazole‐arrested cells.
- Log‐ratio of contact maps between G2 and G1 cells (top right) and scc1 G2 and G1 cells (bottom left). Blue to red color scales reflect the enrichment in contacts in one population with respect to the other (log2). Yellow arrowheads: inter‐centromere contacts.
- Normalized contact frequencies between chr3 centromere (cen3; yellow arrowhead) and the rest of the genome for G1, G2, and scc1 G2.
Figure EV2. Contact maps and 3D genome representations during replication.

-
A, BContact maps recovered from cell populations undergoing replication after G1 release. For each contact map, the FACS profile is displayed. x‐ and y‐axis represent the 16 chromosomes of the yeast genome. The same color code as in Fig EV1. Magnification panels in (B) highlight changes of the contact frequencies during S‐phase progression. Yellow and pink arrowheads point at centromeres and rDNA positions, respectively.
-
C3D average representations of the Hi‐C contact maps of synchronized cell populations of panel (A). The color code represents the chromosomal arm length, and centromeres, telomeres, and rDNA flanking regions are highlighted.
Chromosome compaction is concomitant with chromosome individualization
The Scc1‐dependent compaction occurs concomitantly with a gradual individualization of the SC pairs throughout replication, as shown by the overall increase in the ratio between intra‐ and inter‐chromosomal contacts from 63 ± 10% in G1 (six time points) to 73 ± 4% in S/G2 (four time points) and illustrated by the ratio between G1 and G2 maps (Fig 2E, top right ratio). In sharp contrast to this overall decrease in inter‐chromosomal contacts, the centromeres of different chromosome tend to strongly cluster in G2. In the absence of the cohesin Scc1, intra‐chromosomal contacts in G2 cells decrease to levels similar to or even below G1 (Fig 2E, bottom left ratio), while the major binding sites for cohesin (i.e., centromeres; Glynn et al, 2004) also exhibit a reduced level of contacts (Fig 2F; Appendix Fig S1). These results suggest that cohesins affect the genome organization through the gradual compaction of SC, the clustering of centromeres, and chromosome individualization. Although yeast chromosomes are shorter than mammalian chromosomes, they similarly change their internal conformation and individualize themselves prior to entering metaphase.
Spatial resolution of the replication timing program
In budding yeast, replication initiates at discrete autonomously replicating sequences (ARSs; Brewer & Fangman, 1987). ARSs display partially stochastic activation, with only a subset of origins activated early during S phase. The distribution of early origins is uneven, with an enrichment in pericentromeric regions, and a depletion in subtelomeric regions. The genome‐wide pattern of ARS activation timing defines a population‐average replication timing program (Raghuraman et al, 2001). To investigate the link between genome organization and replication timing, the read coverage of the Hi‐C libraries was used to compute the replication timing profile of the cell population for each of the time point, and follow their progression through S phase. The average profile correlates well with previously published pattern (Raghuraman et al, 2001; McCune et al, 2008; Fig 3A; Materials and Methods). To visualize the progression of replication on the higher‐order architecture of the genome, we colored the 3D structures recovered from three early replication time points according to their replication progression status. The superimposition of the three structures recapitulates intuitive properties of yeast replication program, with a “replication wave” propagating from the centromeric regions enriched in early origins, through chromosomal arms, and toward the late replicating subtelomeric regions (Fig 3B and C; red and blue signal, respectively).
Figure 3. 3D replication profile.

- Comparison of replication profiles of the synchronized populations used for the analysis displayed in Fig 2G. The read coverage of raw Hi‐C libraries reflects the replication progression throughout S phase, plotted along the 16 chromosomes of the yeast genome (top axis; blue curve). The replication timing obtained in this study is highly similar to the one from McCune et al (2008) (yellow curve).
- Superposition of three 3D representations of chromosomes in early replication (I, II, III). The color scale indicates the replication timing. Centromeres and telomeres are highlighted. Different views of the structure are presented.
- Pattern of the replication profile for each of the chromosomal arms. The color code reflects the timing of replication.
We also asked whether our data support the proposed co‐localization of adjacent early replication origins (Kitamura et al, 2006; Knott et al, 2012; Saner et al, 2013). We found a statistically significant enrichment in contacts between these positions and their surrounding regions, but whether it results from an active co‐localization or from their positioning in the pericentromeric regions co‐localized due to the Rabl organization remains unclear (not shown). More analyses are required to solve this question and integrate the different observations.
Global structural changes during mitotic transitions
After replication, cells progress into mitosis (M phase). During metaphase, microtubules originating from opposite SPBs attach to the kinetochores of the two SCs (London & Biggins, 2014). The anaphase‐promoting complex (APC) co‐activator Cdc20 is essential for the proper activation of separase, resulting in the cleavage of cohesin and SC segregation in anaphase (Uhlmann et al, 1999; Visintin et al, 1997; 20). In the absence of Cdc20, cohesins are not cleaved and cells remain blocked in metaphase. Another key player in mitosis progression is the Cdc15 kinase which promotes mitotic exit at the end of anaphase by activating cytokinesis (Rock & Amon, 2011). In the absence of Cdc15, cells are therefore blocked into late anaphase. The higher‐order changes in the organization of chromosomes that take place during metaphase and anaphase were investigated using populations of cells synchronized with conditional mutants of cdc20 and cdc15, respectively. Contact maps of cdc20‐, cdc15‐, and cdc15‐arrested cells released into permissive conditions were generated to characterize chromosome reorganization throughout M phase (Figs 4A and EV3; Materials and Methods). PCA shows that the major structural change occurs during mitotic exit and that cells released from the cdc15 arrest display after 60 min a G1‐like genome structure, reflecting the fact that the entire cell cycle is now covered by our analysis (Fig 4B). The P(s) reveals a strong increase in short‐range contacts (< 10–20 kb) from G2 to anaphase, exceeding G1 levels which are only restored after anaphase completion (Fig 4C, left panel). This increase in short‐range contacts and the accompanying drop in long‐range contacts suggest the formation of an elongated, stretched structure. Upon spindle destabilization using the microtubule‐depolymerizing drug nocodazole in cdc15‐arrested cells (cdc15 noc), the two segregated chromosomal masses get closer as shown by imaging of DAPI‐stained cells (Fig 4C, inset; Fig EV4; Materials and Methods), in agreement with former reports (Jacobs et al, 1988). In these cells, the stretched chromosomal structure disappears as shown by a P(s) that now overlaps the G2 curve (Fig 4C, right panel). Besides the change in P(s), the global contact pattern of cdc15‐arrested cells remains unaltered following nocodazole treatment (Fig 4D, upper right ratio). Altogether, these results show that microtubule‐dependent segregation forces contribute to the stretching the chromosomes in anaphase, possibly in combination with additional constraints resisting this force such as the cohesion of SC arm extremities (see Discussion).
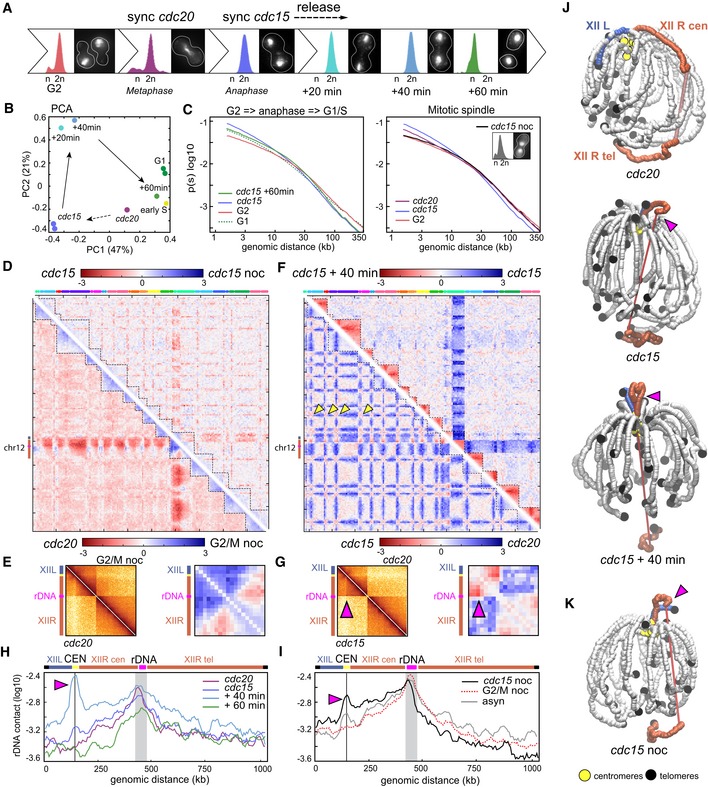
Figure 4. Dynamic reorganization of chromosomes during mitosis.
-
AFACS profiles and representative DAPI‐stained cells of synchronized and/or released populations, from G2 until re‐entry in G1/S.
-
BPCA of the distance matrix between the contact maps of the populations described in (A).
-
CLeft panel: P(s) of cells in G1, G2, and anaphase (cdc15) and released from a cdc15 arrest (cdc15+60 min). Right panel: P(s) of G2, cdc20‐, and cdc15‐arrested cells in the absence or presence of nocodazole (cdc15 noc).
-
DLog‐ratio of contact maps. Bottom left: ratio between cells arrested in metaphase (cdc20) or at the G2/M transition with nocodazole (G2/M noc). Top right: ratio of cells blocked in anaphase and treated or not with nocodazole (cdc15 noc and cdc15, respectively). Blue to red color scales reflect the enrichment in contacts in one population with respect to the other (log2).
-
ELeft: chr12‐normalized contact maps of cells arrested at the G2/M transition and cdc20‐arrested cells. Right: magnification of the log‐ratio map from (D, bottom left).
-
FLog‐ratio of contact maps. Bottom left: log‐ratio between cdc20‐ and cdc15‐arrested cells. Top right: log‐ratio of cdc15‐arrested and cdc15‐released (40 min) cells. Blue to red color scales reflect the enrichment in contacts in one population with respect to the other (log2). Yellow arrowheads: inter‐centromere contacts.
-
GLeft: chr12‐normalized contact maps in cdc20‐ and cdc15‐arrested cells. Right: magnification of the log‐ratio map from (F, bottom left). Pink arrowheads point at the right arm anaphase loop.
-
H, IDistributions of intra‐chromosomal contacts made by a 20‐kb cen‐proximal rDNA flanking region (highlighted in gray) with the rest of chr12 in cdc20‐, cdc15‐, cdc15‐released (+40 min, +60 min), nocodazole‐treated (G2/M noc, cdc15 noc), and asynchronous (asyn) cells. Schematic representations of chr12 are displayed atop the graphs. Gray lines indicate centromere position. Pink arrowheads point at the right arm anaphase loop.
-
J3D representations of the contact maps from cdc20‐ and cdc15‐arrested and cdc20‐ and cdc15‐released (+40 min) cells. The right (XIIR) and left (XIIL) arms of chr12 are highlighted in red and blue, respectively. Pink arrowheads point at the right arm anaphase loop. Centromeres and telomeres are highlighted.
-
K3D representation of the contact map from cdc15 noc cells. Pink arrowhead points at the right arm anaphase loop.
Figure EV3. Contact maps and 3D genome representations during M phase.

-
A, BContact maps of cell populations synchronized in metaphase (cdc20) and anaphase (cdc15) and released into mitosis from cdc15 block (+20 min, +40 min, and +60 min). The corresponding FACS profiles and representative DAPI‐stained cells are displayed on the left on the maps. x‐ and y‐axis represent the 16 chromosomes of the yeast genome. The same color code as in Fig EV1. Magnification panels in (B) display variations of the contact frequencies during mitotic progression. Yellow and pink arrowheads point at centromeres and rDNA positions, respectively.
-
C3D average representations of the Hi‐C contact maps of panel (A). The color code represents the chromosomal arm length, and centromeres, telomeres, and rDNA flanking regions are highlighted.
Figure EV4. Nocodazole affects chromosome 12 conformation.

-
A, BContact maps of G1 synchronized cell populations released either in the presence of nocodazole (G2/M noc) or at cdc15 non‐permissive temperature followed by a nocodazole treatment (cdc15 noc). The corresponding FACS profiles and representative DAPI‐stained cells are displayed on the left on the maps. x‐ and y‐axis represent the 16 chromosomes of the yeast genome. The same color code as in Fig EV1. Magnification panels in (B) display variations of the contact frequencies. Yellow and pink arrowheads point at centromeres and rDNA positions, respectively.
-
C3D average representations of the Hi‐C contact maps of panel (A). The color code represents the chromosomal arm length, and centromeres, telomeres, and rDNA flanking regions are highlighted.
Nocodazole affects chromosome 12 conformation
Nocodazole is commonly used to synchronize cells at the G2/M transition. We took advantage of having contact maps of cdc20‐arrested cells in metaphase to compare them with those obtained from nocodazole‐arrested cells (Fig EV4; Materials and Methods). The ratio map appeared globally similar, although we noticed in the presence of nocodazole a small drop in inter‐chromosomal contacts (Fig 4D, bottom left ratio). Chromosome 12 (chr12) also presents a peculiar signal at the level of the rDNA cluster (Fig 4E, left panel), with an enrichment in contacts between the two flanking regions of the rDNA cluster in G2/M nocodazole‐treated cells compared to cdc20‐arrested cells (Fig 4E, right panel). These results indicate that the G2/M nocodazole arrest is associated with a destabilization of the chr12 structure at the level of the rDNA locus. The intra‐chromosomal contact increase within chr12 is also accompanied by a global decrease in inter‐chromosomal contacts in the presence of nocodazole (Fig 4D, bottom left ratio). Remarkably, chr12 organization was not affected when cdc15‐arrested cells were treated with nocodazole (Fig 4D, top right ratio). Altogether, these observations point to a role for the microtubule array in maintaining the organization of the nucleolus inside the nucleus, before its segregation in anaphase. In summary, while chromosome structures are overall similar in cell synchronized in G2 by nocodazole or in a cdc20 ts mutant, nocodazole‐arrested cells present a slightly different nucleolus structure (and, by extension, chr12). One interpretation could be that the condensation of the rDNA is not yet completed in G2/M nocodazole arrest and that as a result, rDNA flanking regions are freer to contact each other's.
Chromosome 12 looping during anaphase
The comparison of cdc15 and cdc20 maps shows an increase in centromere clustering in anaphase, leading to the formation of a prominent polymer brush structure (Daoud & Cotton, 1982; Fig 4F, bottom left ratio, yellow arrowheads). Such increase is in agreement with the role of condensin in forming a “spring” of chromatin at pericentromeric regions at the metaphase‐to‐anaphase transition (Stephens et al, 2011). Surprisingly, a peculiar loop pattern appears on chr12 in cdc15‐arrested cells, bridging the centromere and the centromere–proximal left flanking region of the rDNA cluster (see pink arrowheads in Fig 4F, G and H). Upon release from the cdc15 arrest, the telomere–proximal right flanking region of the rDNA cluster becomes strongly isolated from the rest of the genome (Fig 4F, upper right ratio; cdc15+40 min), while the contacts of the centromere–rDNA loop intensify (Fig 4H; cdc15+40 min). After completion of mitosis and re‐entry in interphase (cdc15+60 min), the loop disappears (Fig 4H). Interestingly, this loop can be seen in asynchronous populations while it is only present in anaphase (Fig 4I). 3D representations illustrate the dramatic reorganization of chr12 and the formation of the loop bridging centromeric region and the rDNA (Fig 4J, pink arrowheads). Microtubules are not required to maintain this loop in anaphase, since it remains present in cdc15‐arrested cells treated with nocodazole (Fig 4D, upper right ratio; Fig 4I and K), suggesting that the left flanking region of the rDNA is physically bound through an unknown mechanism to the centromeric regions. These results complement imaging studies showing that the rDNA exhibits a dense, line‐like shape that extends throughout the nucleus at anaphase (2.1 SD, 0.2 μm; Sullivan et al, 2004).
Condensin promotes dramatic reorganization of chromosomes during anaphase
The proper condensation and segregation of the rDNA cluster requires the nucleolar release of the Cdc14 phosphatase. Cdc14 mediates a shutdown of rDNA transcription, facilitating the loading of the Smc2 condensin and hence the condensation of the cluster (Yoshida et al, 2002; D'Amours et al, 2004; Sullivan et al, 2004, 14; Machín et al, 2006; Clemente‐Blanco et al, 2009). In addition, topoisomerase II (Top2), which decatenates the intertwining structures that appear between SCs during replication, is also required for rDNA segregation to proceed (Sullivan et al, 2004; D'Ambrosio et al, 2008; Baxter et al, 2011; Leonard et al, 2015). We investigated the influence of those factors on the 3D structure of the rDNA locus during anaphase (Figs 5A and EV5; Materials and Methods).
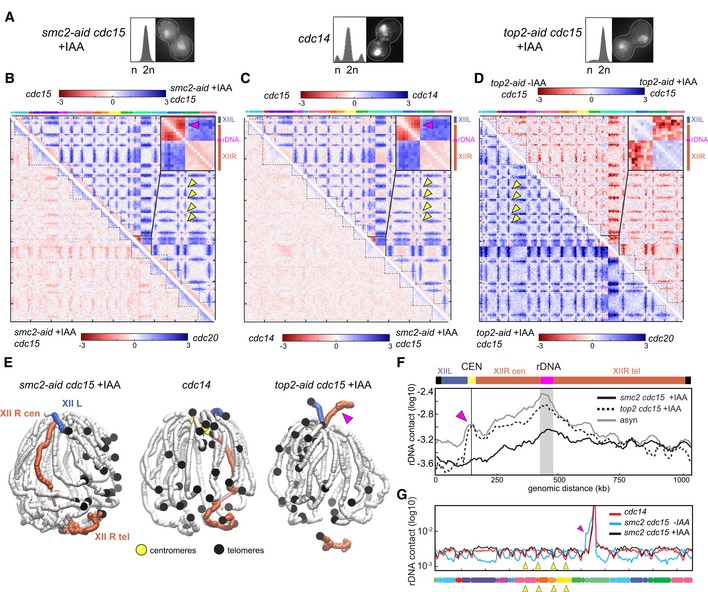
Figure 5. The anaphase rDNA loop is condensin‐dependent.
-
AFACS profiles and representative DAPI‐stained cells of cells blocked in anaphase, in the absence of condensin (smc2‐aid cdc15 +IAA and cdc14) or topoisomerase 2 (top2‐aid cdc15 +IAA).
-
B–DLog‐ratio of contact maps. Yellow arrowheads: inter‐centromere contacts. The pink arrowheads point at the right arm anaphase loop on chr12. Insets display magnification of the chr12 ratio map. (B) Ratio map between (bottom left) cdc20 and smc2‐aid cdc15 cells and between (top right) cells blocked in anaphase with our without condensin depletion (cdc15 and smc2‐aid cdc15 +IAA). (C) Ratio map between (bottom left) cdc14 and smc2‐aid cdc15 +IAA cells and between (top right) cdc14 and cdc15 cells. (D) Ratio map between (bottom left) top2‐aid cdc15 +IAA and cdc20 cells and (top right) top2‐aid cdc15 ‐IAA and top2‐aid cdc15 +IAA cells.
-
E3D representations of the contact maps from smc2‐aid cdc15 +IAA‐, cdc14‐, and top2‐aid cdc15 +IAA‐arrested cells. The right (XIIR) and left (XIIL) arms of chr12 are highlighted in red and blue, respectively. Pink arrowhead points at the right arm anaphase loop.
-
FDistribution of intra‐chromosomal contacts of a cen‐proximal rDNA flanking region (highlighted in gray) with the rest of chr12 in smc2 cdc15, top2 cdc15, and asynchronous (asyn) cells. Pink arrowhead points at the right arm anaphase loop.
-
GNormalized contact frequencies between the left rDNA flanking region (50 kb) and the rest of the genome in cdc15 smc2‐aid (‐IAA) and cdc15 smc2‐aid (+IAA) cells. Yellow arrowheads point at a subset of centromeric positions. Pink arrowhead points at the right arm anaphase loop.
Figure EV5. Condensin and decatenation influence on chromatin structure during mitosis.

-
A, BContact maps of cell populations arrested in anaphase, either defective with condensation (cdc14 or smc2‐aid cdc15 +IAA) or depleted of topoisomerase 2 (top2‐aid cdc15 +IAA). The corresponding FACS profiles and representative DAPI‐stained cells are displayed on the left on the maps. x‐ and y‐axis represent the 16 chromosomes of the yeast genome. The same color code as in Fig EV1. Magnification panels in (B) display variations of the contact frequencies. Yellow and pink arrowheads point at centromeres and rDNA positions, respectively.
-
C3D average representations of the Hi‐C contact maps of panel (A). The color code represents the chromosomal arm length, and centromeres, telomeres, and rDNA flanking regions are highlighted.
First, Smc2 depletion in smc2‐aid cdc15‐arrested strain affects anaphase genome organization by (i) reducing centromere clustering and (ii) suppressing the formation of the rDNA loop, with a resulting contact map highly similar to the cdc20 map (Fig 5B, bottom left ratio). Therefore, condensins are responsible for the observed increase in inter‐centromere contacts at anaphase compared to metaphase (Fig 4F, bottom left ratio), while they are also required for the formation of the loop bridging the centromere of chromosome 12 with the rDNA cluster (two loci enriched in condensin deposition). The smc2 cdc15 and cdc14 maps are strikingly similar (Fig 5C, bottom left ratio). The 3D representations of smc2 cdc15 and cdc14 cells (Fig 5E) and the rDNA contact plots with the rest of chr12 (Fig 5F) illustrate the loss of the rDNA loop in the absence of Smc2 and/or Cdc14. In addition to this effect, both mutants also display the same decrease in centromere clustering compared to cdc15 cells (Fig 5C, upper right ratio; Fig 5G), pointing at their functional relationship on the same pathway.
The organization of the genome was also compared in cdc15‐arrested cells in the presence or absence of Top2, top2‐aid cdc15‐arrested (Fig 5D, upper right ratio; Fig 5E and F; Materials and Methods). Top2‐depleted cells display a strong decrease in contacts between the telomere–proximal region of chr12R and the rest of the genome (including chr12L). The signal is consistent with the essential role played by Top2 in rDNA segregation, showing that the non‐segregated regions are isolated from the segregated chromosomal sets. The comparison between top2 cdc15 and cdc20 cells reveals an enrichment in contacts at centromeres and the persistence of the centromere–rDNA loop in the Top2 mutant (Fig 5D, bottom left ratio). These results indicate that the formation of these condensin‐dependent structures in anaphase is independent from the decatenation and/or the segregation of the rDNA cluster.
Discussion
This study consists of an experimental and analysis framework to systematically investigate and compare chromosome folding and organization at different stages of the cell cycle. We applied Hi‐C to populations of cells synchronized at different points of the cycle, generating genome‐wide, 5‐kb‐resolution contact maps which unveil their average 3D genome organization. The global influence of cohesin, condensin, and topoisomerase 2 has been investigated in the corresponding mutants, as well as the effects of the microtubule‐depolymerizing drug nocodazole. Comparative approaches between contact maps provided a global view of the structural transitions between the different stages of the cycle, some expected, such as chromosome compaction during replication, and others that had not been described before, such as topological structures involving the rDNA cluster.
An overview of chromosome structural changes during the cell cycle can be summarized from centromere contacts, intra‐/inter‐chromosomal contact ratio, and short‐/long‐range contact ratio computed for each of the time points (Fig 6A).
Figure 6. 4D reorganization of the yeast genome.

- Dynamics of centromere contacts (top panel), Short‐/long‐range contact ratio (middle panel) and intra‐/inter‐chromosomal contact ratio (bottom panel) for each of the 20 time points (blue dots; see bottom x‐axis) during the cell cycle. The three folding states (I, II, and III; Fig EV4) identified in the analysis are indicated under the panels, as well as interpretation with respect to individualization status.
- Illustration of the three chromatin folding states characteristic of each of the cell cycle phases. Establishment of sister‐chromatid (SC) cohesion during S phase increases intra‐SC long‐range contacts and leads to the individualization of the replicated chromosomes. Then during M phase, the two sisters are segregated and each chromatid (C) individualized thanks to the action combination of cohesin cleavage, condensin loading and spindle elongation. The chromosomes display a stretched internal structure, which relaxed upon destabilization of the spindle with nocodazole.
- Model of loop extrusion generating the condensin‐dependent loop formation between the centromere and the rDNA cluster, two regions enriched in condensin deposition. A loop formed in between the centromere and the rDNA cluster may extend until it reaches these two discrete positions, and stall because of mechanic impediment blocking further extrusion.
Centromere clustering gradually increases during the cell cycle, through the establishment of sister‐chromatid cohesion during replication, and through condensin‐dependent clustering during anaphase (Fig 6A, upper panel). A potential consequence of this increased clustering in anaphase could be the generation of a stronger polymer brush, that is, the mechanical phenomenon that leads to the self‐organization of a polymer tethered to a surface into stretched, non‐intermingling structure (de Gennes, 1987). Interestingly, the strengthening of the polymer brush organization could consequently contribute to chromosome individualization during anaphase. The intra‐/inter‐chromosomal contact variations reflect the successive phases of chromosome individualization and intermingling, with individualization taking place during replication (cohesin‐dependent) and during anaphase (spindle‐dependent; Fig 6A, bottom panel). The intra‐/inter‐chromosomal contact ratio correlates strongly with centromere clustering (c = 0.72, p = 10−4), with both ratios peaking during anaphase exit.
Short‐/long‐range contact ratio recapitulates the three different internal folding (I, II, and III) states of chromosomes (G1, G2, and anaphase; Fig 6B, middle panel). These three states can be determined based on a quantitative analysis of the significance of changes between P(s) curves obtained using several replicates in different phases of the cycle (Fig 7). During replication, cohesins mediate the compaction of chromosomes from state I to II. The chromosomes are then stretched by the mitotic apparatus during anaphase (state III) before returning to state I in G1. The mechanical constraint imposed by the anaphase spindle appears responsible for the state III stretching, as a nocodazole treatment results in relaxation of chromosomes, which switch back to state II. Imaging of the two sets of segregated chromosome during nocodazole treatment supports this spring relaxation effect, with the two masses being brought back together upon the depolymerization of microtubules. The nature of the mechanical constraints remains unknown, but it is tempting, in light of our observation of chr12 behavior (below), to propose a role for condensins in actively promoting this movement. In this scenario, condensins could favor the segregation of sister chromatids by pulling the chromosomes toward the centromere cluster. As a result, the loss of microtubule and tethering to the SPB may lead cohesin to actively pull back the segregated region together. We anticipate that whether condensins play an active role in the segregation of chromosomes in addition to the pulling force imposed by the microtubule spindle will be thoroughly investigated in the years to come.
Figure 7. Variation in P(s) for different phase of the cell cycle.

- P(s) for four different time points along the cycle. Each curve represents the average between three replicates with error bars corresponding to the standard deviation.
- To assess the statistical significance of the differences between short (resp. long)‐range contacts between these three time points, we computed a P‐value for each pairwise comparison between two time points using Wilcoxon signed‐rank test. The two distributions to compared were built by aggregating all the data points below (resp. above) 10 kb for the three replicates for each time point.
In addition, we also show that the two main regions of condensin deposition, that is, the centromeres and the rDNA locus, are bridged during anaphase through a condensin‐dependent mechanism resulting in a loop‐like structure on the right arm of chromosome 12. Whether this structure is systematically found in all cells, or only in a subset of the population, remains to be determined through single‐cell imaging approaches such as FISH analysis. Although the precise mechanisms of formation remain unknown as well as its functional importance, we show that the setting up of the loop depends on condensin. Several mechanisms can be envisioned for the generation of this loop. One possibility is that starting from regions with a high condensin density, an active mechanism such as DNA extrusion through the action of condensins would pull the centromere and the rDNA cluster together (Fig 6C). Condensin depletion (leading to disruption of the loop) is associated with segregation defects. Overall, this structure therefore appears to de facto play a role in the segregation of the rDNA cluster, potentially through the application of a force that would drag the rDNA region to the centromere cluster before the completion of anaphase. A consequence of this model, would be that a similar loop extrusion mechanism could facilitate the segregation of other chromosomes as well. In this case, one or more loops could actively facilitate the segregation of large regions of chromosomes toward the tethered centromeres, down the telomeric regions. Chromosome 12, in this scenario, would appear as an exception with the large rDNA cluster generating a physical barrier in the middle of the right arm that is not present in other chromosomes. More experiments are nevertheless needed to investigate this proposed role. Yeast chromosome 12 could therefore prove a convenient model to study the action of loop extrusion mechanism (Alipour & Marko, 2012).
The importance of the rDNA loop remains to be further characterized as well as its similarity with loops found in other eukaryotic species. Overall, our exhaustive dataset opens new avenues for the comprehensive analysis of the 3D chromosome choreography during replication and segregation and brings to light new perspectives regarding these fundamental processes.
Materials and Methods
Media and culture conditions
All strains were grown in rich medium (YPD: 1% bacto peptone (Difco), 1% bacto yeast extract (Difco), and 2% glucose), except for YKL051 (MET3‐HA‐CDC20) that was grown in synthetic complete medium deprived of methionine (SC: 0.67% yeast nitrogen base without amino acids (Difco), supplemented with a mix of amino acids, uracil and adenine, and 2% glucose). Cells were grown at either 30°C or 23–25°C (the later temperature corresponding to the permissive temperature of the conditional thermosensitive mutations cdc6‐1, cdc14‐3, and cdc15‐2; see below for details). Dataset corresponding to the quiescent state (G0) comes from already published data by Guidi et al (2015) and was obtained by carbon source exhaustion. All strains are described in Table EV1.
Elutriation (recovery of G1 cells)
To recover G1 daughter cells, the exponentially growing cultures were elutriated—a physical method of synchronization, used to separate cells according to their density and sedimentation velocity (see Appendix Supplementary Methods; Marbouty et al, 2014). The G1 daughter cells recovered through elutriation were suspended in fresh YPD at 30°C for 30 min, so they could recover from the elutriation procedure (i.e., stay in PBS). To minimize the potential variability introduced by the age heterogeneity of the bulk population, G1 daughter cells were used as starting point for all cell cycle synchrony and in combination with genetic and chemical synchronization methods (see below).
Release into S phase
G1 elutriated cells were released into S phase to analyze genome conformation during this stage. 2 × 109 G1 cells—originating from the same elutriated fraction to minimize heterogeneity in replication initiation—were inoculated into 150 ml YPD at 25°C (to slow down replication fork progression). Upon release, the synchronized cultures were sampled every 5 min and the cells analyzed through FACS, revealing an approximate lag of 130 min before replication restart. Therefore, aliquots were cross‐linked and processed into Hi‐C libraries at 135, 140, 145, 150, 155, 160, and 165 min. The progression of each fraction throughout the S phase (from G1 to G2) was monitored with flow cytometry.
Synchronization through thermosensitive mutations
Synchronizations using thermosensitive (ts) cdc strains (Hartwell et al, 1973) were all performed starting from elutriated G1 daughter cells growing in non‐permissive temperature conditions designed to arrest the progression of the cycle at specific phases. See Appendix Supplementary Methods for details of synchronization procedures of strains YKL052 (cdc14‐3), YKL053 (cdc15‐2), and YKL054 (cdc6‐1).
Synchronization through chemical compounds
Chemical synchronization was also performed on elutriated G1 daughter cells.
Synchronization at the G2/M transition was achieved by restarting G1 cells (strain YKL050) in YPD at 30°C for 1 h, followed by the addition of nocodazole (Calbiochem; 15 μg/ml) and incubation for another 2 h at 30°C. Cells arrested in G2/M with nocodazole were either processed into Hi‐C libraries, or washed and inoculated in fresh YPD medium at 30°C. The washing of nocodazole allowed G2/M synchronized cells to proceed into M phase (cells sampled after 20, 45, 60 and 90 min were processed into Hi‐C libraries).
To investigate the constraints imposed by the spindle during anaphase, elutriated YKL053 cells were elutriated and the recovered G1 daughter cells processed and blocked into anaphase using the cdc15‐2 thermosensitive mutation. A sample of the population was then incubated with nocodazole (15 μg/ml) for 20 min. A sample was released at permissive temperature in the presence of nocodazole for 20 min. Finally, a sample was released at the permissive temperature for 20 min before being incubated with nocodazole for 20 min.
For synchronization in metaphase, a system allowing induced depletion of cdc20 was used (MET3‐HA‐CDC20; strain YKL051). Elutriated G1 daughter cells were restarted in YPD complemented with 50 μg/ml methionine for 5 h at 30°C. Cells arrested in metaphase were split into different aliquots. One sample was immediately processed into a Hi‐C library, while two others were washed, suspended in SC medium without methionine, and processed into Hi‐C after 20 and 40 min.
To investigate the influence of SMC on chromosome organization, strains carrying auxin‐inducible degron (aid) versions of Scc1 (strain YKL055) and Smc2 (YKL056) proteins were processed into Hi‐C libraries. The degradation of these proteins is induced when auxin (IAA) is added to the medium at a final concentration of 2 mM. Both asynchronous populations of strains YKL055 and YKL056 were elutriated in the absence of IAA. G1 daughter cells were incubated in YPD supplemented with IAA at 30°C. A sample of the YKL055 population (scc1‐aid) was processed into a Hi‐C library in late S/G2 (see Release into S phase). For the YKL056 population (smc2‐aid), the cells were arrested in late anaphase using the cdc15‐2 mutation also present in the genome of this strain, before being processed into a Hi‐C library.
To study the influence of topoisomerase II‐mediated decatenation on chromosome organization, we used a strain (YKL057) in which TOP2 gene is tagged by aid (top2‐aid) and that also carries the cdc15‐2 mutation. An asynchronous exponentially growing culture of YKL057 cells was split into two fractions incubated for 3 h at the non‐permissive temperature of 37°C in either the presence or absence of IAA (20 mM). The synchrony of each time point was monitored with flow cytometry and microscopy, and the cells were processed by Hi‐C.
Flow cytometry
About 5 × 106 cells were fixed in ethanol 70% and stored at 4°C overnight. Cells were then pelleted, washed, and incubated in sodium citrate 50 mM (pH 7.4) complemented with RNase A (10 mg/ml; Roche) for 2 h at 37°C. Next, Sytox green (2 μM in sodium citrate 50 mM; ThermoFisher) was added and cells incubated for 1 h at 4°C. Flow cytometry was performed on a MACSQuant Analyzer (Miltenyi Biotec), and data were analyzed using FlowJo X 10.0.7 software (Tree Star).
Microscopy
Fractions of cells fixed in ethanol 70% and stored at 4°C overnight were pelleted and washed three times for 5 min in 1× PBS. Cells were permeabilized by immersion in 0.2% Triton X‐100 (Biosolve) for 5 min. To remove the Triton, cells were pelleted and washed three times in 1× PBS. The liquid was aspirated and cells were suspended in DAPI labeling solution (2 μg/ml in 1× PBS) for 10 min at room temperature. Before imaging acquisition, the labeling solution was aspirated and the cells were washed three times for 5 min in 1× PBS. Cells were imaged at 350 nm excitation wavelength with Nikon fluorescence microscope (Camera Andor Neo sCMOS, software Andor IQ2 2.7.1, LED Lumencor Spectra X).
Hi‐C libraries
Hi‐C libraries were generated using the four‐cutter enzyme DpnII through a protocol adapted from Belton et al (2012). The protocol is detailed in Appendix Supplementary Methods. The resulting libraries were used as template for the Illumina amplification by PE‐PCR primers and paired‐end‐sequenced on the NextSeq 500 or HiSeq 2000 Illumina platform (2 × 75 or 2 × 150 bp kits; see Table EV2 for details).
Generation and normalization of contact maps
Raw Hi‐C data were processed as follows. PCR duplicates were removed using the 6 Ns present on each of the custom‐made adapter and the 2 trimmed Ns. Paired‐end reads were mapped independently using Bowtie 2.1.0 (mode: –very‐sensitive –rdg 500,3 –rfg 500,3) against the S. cerevisiae reference genome (S288C). An iterative alignment, with an increasing truncation length of 20 bp, was used to maximize the yield of valid Hi‐C reads (mapping quality > 30). Only uniquely mapped reads were retained. On the basis of their DpnII restriction fragment assignment and orientation, reads were classified as either valid Hi‐C products or unwanted events to be filtered out (i.e., loops and non‐digested fragments; for details, see Cournac et al, 2012, 2016). To generate contact matrices for all time points along the cycle, filtered Hi‐C reads were binned into units of single restriction fragments, and then, successive fragments were assigned to fixed size bins of either 5 or 50 kb. Bins that exhibited a high contact frequency variance (< 1.5 Standard Deviation or 1.5–2 SD. from the mean) were filtered out for all maps to allow pairwise comparison of the data. On average, around 15 million of valid reads were used to build each contact map. To remove potential biases resulting from the uneven distribution of restriction sites and variation in GC content and mappability, the contact maps were normalized using the sequential component normalization (SCN) procedure (Cournac et al, 2012).
Similarity between contact maps
To assess the similarity between normalized matrices, these were binned at 50 kb and quantile‐normalized (Hicks & Irizarry, 2015). We then measured their similarity by computing the Euclidean distance between them. In order to visualize similarities between sets of matrices, we did a principal component analysis (PCA) of the pairwise distance matrix between samples.
Contact probability within increasing genomic distance
Polymers display a decrease in contact probability, P(s), as a function of the genomic distance, s. The degree of decay of P(s) was often interpreted as informative of the polymer state. To compute the intra‐chromosomal P(s) plots, pair of reads aligned in intra‐chromosomal positions were partitioned by chromosome arms. Reads oriented toward different directions or separated by < 1.5 kb were discarded to filter for self‐circularizing events. For each chromosome, read pairs were log‐binned in function of their genomic distance s (in kb), according to the following formula:
The P(s) plot is the histogram computed on the sum of read pairs for each bin. This sum is weighted by the bin size 1.1(1+bin) (because of the log‐binning), as well as the difference between the length of the chromosome and the genomic distance s. The difference acts as a proxy for the number of possible events.
4C‐like interaction plots
To obtain the 4C‐like intra‐ and inter‐chromosomal contact profiles for rDNA and centromeres, adjacent bins were indexed on the respective chromosomes. The resulting indexed and filtered matrices at either 5‐ or 50‐kb bin were normalized using SCN (see Generation and normalization of contact maps). The profiles for the selected bins were plotted and compared using Matlab (no smoothing was applied).
Computation of the replication profile from Hi‐C data
The replication profile was computed from the raw 5‐kb‐binned contact maps. Firstly, G1 replicates were averaged and the sum of contact over each 5‐kb bin was computed. The same computation was repeated for datasets obtained from cells released into S phase. To obtain the replication timing, we computed the ratio of these two signals and smoothed this ratio using a running‐average window of six bins.
3D representation of contact maps
The 3D representations of the contact maps were generated using ShRec3D (Lesne et al, 2014) on the normalized contact maps, filtered for low‐signal bins. First, the algorithm computes the distance matrix from the contact map, by assuming that distances are inversely proportional to the normalized contact counts. A shortest path algorithm is then used to insure that the distance matrix satisfies the triangular inequality. Finally, we used Sammon mapping to recover the optimal 3D coordinates from the distance matrix (Morlot et al, 2016). All the 3D structures presented here were rendered using VMD (Humphrey et al, 1996). Besides the cautiousness regarding the interpretation of 3D structure we mention in the main text, we also underline that the 3D structures are not used to compare datasets: All computational analyses are performed using the contact map data.
Comparison of centromeres, intra‐/inter‐, and short‐/long‐range contacts between datasets
To compare contacts between centromeric regions, the sum of normalized inter‐chromosomal contacts between 100‐kb regions centered on centromeres was computed and divided by the total number of normalized inter‐chromosomal contacts between all chromosomes. To compare short‐ versus long‐range contacts, a ratio of intra contacts was computed as follows. The number of intra contacts involving fragments positioned < 30 kb apart was divided by the number of intra contacts involving fragments positioned more than 30 kb apart, for all chromosomes. For intra‐ versus inter‐chromosomal contacts, the total number of normalized intra‐chromosomal contacts was divided by the sum of normalized inter‐chromosomal contacts.
Quantification of variability between replicates
To assess for the contribution of experimental variability to the variations in contacts between different conditions, we proceeded as follows. Density histograms displaying the distribution of the log2 contact ratio of all elements of Hi‐C matrices (50‐kb bins) between pairs of biological and experimental replicates (3×G1, 2×G2, 3×M) were computed and compared to similar histograms computed from pairs of Hi‐C matrix obtained in different experimental conditions (see Appendix Fig S2).
An estimation of the replicate variability at the centromeres was obtained by plotting the boxplots representing the distribution of the log2 contact ratios between pairs of biological and experimental replicates only of the bins encompassing the centromeres (50‐kb bins; see mask; Appendix Figs S3 and S4). The same computation was performed on pairs of matrices obtained in different conditions to estimate the statistical significance of the variations. All replicates were taken into account. P‐values were obtained by the Wilcoxon signed‐rank test.
Author contributions
LL‐S and RK designed research. LL‐S performed the experiments, with contributions from GM, AT, and HM. TMG generated the smc2‐aid mutant. VFS and JM analyzed the data, with contributions from LL‐S. LL‐S, JM, and RK interpreted the data and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Review Process File
Acknowledgements
We thank Martial Marbouty and Axel Cournac for contributions to the early stage of this project. Sample description and raw contact maps are accessible on the GEO database through the following accession number: GSE90902. Raw sequences are accessible on SRA database through the following accession number: SRP094582. We are also grateful to Armelle Lengronne, Stephane Marcand, Philippe Pasero, Etienne Schwob, Emmanuelle Fabre, Nancy Kleckner, and Angela Taddei for sharing strains and for discussions. Vittore Scolari and Heloise Muller were partly supported by Pasteur‐Roux‐Cantarini postdoctoral fellowships. This research was supported by funding to R.K. from the European Research Council under the 7th Framework Program (FP7/2007‐2013, ERC grant agreement 260822), from Agence Nationale pour la Recherche (MeioRec ANR‐13‐BSV6‐0012‐02), and from ERASynBio and Agence Nationale pour la Recherche (IESY ANR‐14‐SYNB‐0001‐03).
The EMBO Journal (2017) 36: 2684–2697
See also: C Barrington et al (September 2017)
Note
Correction added on 15 September 2017 after first online publication: Bin size was corrected from 5 to 50 kb.
Contributor Information
Julien Mozziconacci, Email: mozziconacci@lptmc.jussieu.fr.
Romain Koszul, Email: romain.koszul@pasteur.fr.
References
- Alipour E, Marko JF (2012) Self‐organization of domain structures by DNA‐loop‐extruding enzymes. Nucleic Acids Res 40: 11202–11212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon L, Martinez‐Perez E, Merkenschlager M (2013) Condensin, cohesin and the control of chromatin states. Curr Opin Genet Dev 23: 204–211 [DOI] [PubMed] [Google Scholar]
- Baxter J, Sen N, Martínez VL, Carandini MEMD, Schvartzman JB, Diffley JFX, Aragón L (2011) Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 331: 1328–1332 [DOI] [PubMed] [Google Scholar]
- Belton J‐M, McCord RP, Gibcus JH, Naumova N, Zhan Y, Dekker J (2012) Hi–C: a comprehensive technique to capture the conformation of genomes. Methods 58: 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blat Y, Kleckner N (1999) Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98: 249–259 [DOI] [PubMed] [Google Scholar]
- Brewer BJ, Fangman WL (1987) The localization of replication origins on ARS plasmids in Saccharomyces cerevisiae . Cell 51: 463–471 [DOI] [PubMed] [Google Scholar]
- Burgess SM, Kleckner N (1999) Collisions between yeast chromosomal loci in vivo are governed by three layers of organization. Genes Dev 13: 1871–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente‐Blanco A, Mayán‐Santos M, Schneider DA, Machín F, Jarmuz A, Tschochner H, Aragón L (2009) Cdc14 inhibits transcription by RNA polymerase I during anaphase. Nature 458: 219–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac A, Marie‐Nelly H, Marbouty M, Koszul R, Mozziconacci J (2012) Normalization of a chromosomal contact map. BMC Genom 13: 436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac A, Marbouty M, Mozziconacci J, Koszul R (2016) Generation and analysis of chromosomal contact maps of yeast species In Yeast functional genomics, Devaux F. (ed) Methods in Molecular Biology, Vol 1361, pp 227–245. New York: Humana Press; Available at: https://doi.org/10.1007/978-1-4939-3079-1_13 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio C, Kelly G, Shirahige K, Uhlmann F (2008) Condensin‐dependent rDNA decatenation introduces a temporal pattern to chromosome segregation. Curr Biol 18: 1084–1089 [DOI] [PubMed] [Google Scholar]
- D'Amours D, Stegmeier F, Amon A (2004) Cdc14 and condensin control the dissolution of cohesin‐independent chromosome linkages at repeated DNA. Cell 117: 455–469 [DOI] [PubMed] [Google Scholar]
- Daoud M, Cotton JP (1982) Star shaped polymers: a model for the conformation and its concentration dependence. J Phys 43: 531–538 [Google Scholar]
- Dekker J, Rippe K, Dekker M, Kleckner N (2002) Capturing chromosome conformation. Science 295: 1306–1311 [DOI] [PubMed] [Google Scholar]
- Dekker J, Mirny L (2016) The 3D genome as moderator of chromosomal communication. Cell 164: 1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Andronescu M, Schutz K, Mcllwain S, Kim YJ, Lee C, Shendure J, Fields S, Blau CA, Noble WS (2010) A three‐dimensional model of the yeast genome. Nature 465: 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser U, Chandler‐Brown D, Ay F, Straight AF, Duan Z, Noble WS, Skotheim JM (2017) Form and function of topologically associating genomic domains in budding yeast. Proc Natl Acad Sci USA 114: E3061–E3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gennes PG (1987) Polymers at an interface; a simplified view. Adv Colloid Interface Sci 27: 189–209 [Google Scholar]
- Glynn EF, Megee PC, Yu H‐G, Mistrot C, Unal E, Koshland DE, DeRisi JL, Gerton JL (2004) Genome‐wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae . PLoS Biol 2: e259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guacci V, Hogan E, Koshland D (1994) Chromosome condensation and sister chromatid pairing in budding yeast. J Cell Biol 125: 517–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi M, Ruault M, Marbouty M, Loïodice I, Cournac A, Billaudeau C, Hocher A, Mozziconacci J, Koszul R, Taddei A (2015) Spatial reorganization of telomeres in long‐lived quiescent cells. Genome Biol 16: 206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou E, Ibarra A, Coulon V, Casado‐Vela J, Rico D, Casal I, Schwob E, Losada A, Méndez J (2010) Cohesin organizes chromatin loops at DNA replication factories. Genes Dev 24: 2812–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell LH, Mortimer RK, Culotti J, Culotti M (1973) Genetic control of the cell division cycle in yeast: V. Genetic analysis of cdc mutants. Genetics 74: 267–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SC, Irizarry RA (2015) Quantro: a data‐driven approach to guide the choice of an appropriate normalization method. Genome Biol 16: 117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T (2012) Condensins: universal organizers of chromosomes with diverse functions. Genes Dev 26: 1659–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T‐HS, Weiner A, Lajoie B, Dekker J, Friedman N, Rando OJ (2015) Mapping nucleosome resolution chromosome folding in yeast by micro‐C. Cell 162: 108–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug CB, Grimaldi AG, Kruse K, Vaquerizas JM (2017) Chromatin architecture emerges during zygotic genome activation independent of transcription. Cell 169: 216–228.e19 [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14: 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- Jacobs CW, Adams AE, Szaniszlo PJ, Pringle JR (1988) Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol 107: 1409–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K‐D, Tanizawa H, Iwasaki O, Noma K (2016) Transcription factors mediate condensin recruitment and global chromosomal organization in fission yeast. Nat Genet 48: 1242–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura E, Blow JJ, Tanaka TU (2006) Live‐cell imaging reveals replication of individual replicons in eukaryotic replication factories. Cell 125: 1297–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott SRV, Peace JM, Ostrow AZ, Gan Y, Rex AE, Viggiani CJ, Tavaré S, Aparicio OM (2012) Forkhead transcription factors establish origin timing and long‐range clustering in Saccharomyces cerevisiae . Cell 148: 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J, Sen N, Torres R, Sutani T, Jarmuz A, Shirahige K, Aragón L (2015) Condensin relocalization from centromeres to chromosome arms promotes Top2 recruitment during anaphase. Cell Rep 13: 2336–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne A, Riposo J, Roger P, Cournac A, Mozziconacci J (2014) 3D genome reconstruction from chromosomal contacts. Nat Methods 11: 1141–1143 [DOI] [PubMed] [Google Scholar]
- Lieberman‐Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J (2009) Comprehensive mapping of long‐range interactions reveals folding principles of the human genome. Science 326: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Biggins S (2014) Signalling dynamics in the spindle checkpoint response. Nat Rev Mol Cell Biol 15: 736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machín F, Torres‐Rosell J, Piccoli GD, Carballo JA, Cha RS, Jarmuz A, Aragón L (2006) Transcription of ribosomal genes can cause nondisjunction. J Cell Biol 173: 893–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbouty M, Ermont C, Dujon B, Richard G‐F, Koszul R (2014) Purification of G1 daughter cells from different Saccharomycetes species through an optimized centrifugal elutriation procedure. Yeast Chichester Engl 31: 159–166 [DOI] [PubMed] [Google Scholar]
- Marbouty M, Le Gall A, Cattoni DI, Cournac A, Koh A, Fiche J‐B, Mozziconacci J, Murray H, Koszul R, Nollmann M (2015) Condensin‐ and replication‐mediated bacterial chromosome folding and origin condensation revealed by Hi‐C and super‐resolution imaging. Mol Cell 59: 588–602 [DOI] [PubMed] [Google Scholar]
- McCune HJ, Danielson LS, Alvino GM, Collingwood D, Delrow JJ, Fangman WL, Brewer BJ, Raghuraman MK (2008) The temporal program of chromosome replication: genomewide replication in clb5{Delta} Saccharomyces cerevisiae . Genetics 180: 1833–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercy G, Mozziconacci J, Scolari VF, Yang K, Zhao G, Thierry A, Luo Y, Mitchell LA, Shen M, Shen Y, Walker R, Zhang W, Wu Y, Xie ZX, Luo Z, Cai Y, Dai J, Yang H, Yuan YJ, Boeke JD et al (2017) 3D organization of synthetic and scrambled chromosomes. Science 355: eaaf4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi T, Fudenberg G, Mehta S, Belton J‐M, Taneja N, Folco HD, FitzGerald P, Dekker J, Mirny L, Barrowman J, Grewal SIS (2014) Cohesin‐dependent globules and heterochromatin shape 3D genome architecture in S. pombe . Nature 516: 432–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlot J‐B, Mozziconacci J, Lesne A (2016) Network concepts for analyzing 3D genome structure from chromosomal contact maps. EPJ Nonlinear Biomed Phys 4: 2 [Google Scholar]
- Naumova N, Imakaev M, Fudenberg G, Zhan Y, Lajoie BR, Mirny LA, Dekker J (2013) Organization of the mitotic chromosome. Science 342: 948–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti S, Lengauer C, Nasmyth K (1995) Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a “reductional” anaphase in the budding yeast Saccharomyces cerevisiae . EMBO J 14: 3788–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman MK, Winzeler EA, Collingwood D, Hunt S, Wodicka L, Conway A, Lockhart DJ, Davis RW, Brewer BJ, Fangman WL (2001) Replication dynamics of the yeast genome. Science 294: 115–121 [DOI] [PubMed] [Google Scholar]
- Renshaw MJ, Ward JJ, Kanemaki M, Natsume K, Nédélec FJ, Tanaka TU (2010) Condensins promote chromosome recoiling during early anaphase to complete sister chromatid separation. Dev Cell 19: 232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JM, Amon A (2011) Cdc15 integrates Tem1 GTPase‐mediated spatial signals with Polo kinase‐mediated temporal cues to activate mitotic exit. Genes Dev 25: 1943–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saner N, Karschau J, Natsume T, Gierliński M, Retkute R, Hawkins M, Nieduszynski CA, Blow JJ, de Moura APS, Tanaka TU (2013) Stochastic association of neighboring replicons creates replication factories in budding yeast. J Cell Biol 202: 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens AD, Haase J, Vicci L, Taylor RM, Bloom K (2011) Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring. J Cell Biol 193: 1167–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M, Higuchi T, Katis VL, Uhlmann F (2004) Cdc14 phosphatase induces rDNA condensation and resolves cohesin‐independent cohesion during budding yeast anaphase. Cell 117: 471–482 [DOI] [PubMed] [Google Scholar]
- Taddei A, Gasser SM (2012) Structure and function in the budding yeast nucleus. Genetics 192: 107–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F, Lottspeich F, Nasmyth K (1999) Sister‐chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400: 37–42 [DOI] [PubMed] [Google Scholar]
- Uhlmann F (2016) SMC complexes: from DNA to chromosomes. Nat Rev Mol Cell Biol 17: 399–412 [DOI] [PubMed] [Google Scholar]
- Valton A‐L, Dekker J (2016) TAD disruption as oncogenic driver. Curr Opin Genet Dev 36: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A (1997) CDC20 and CDH1: a family of substrate‐specific activators of APC‐dependent proteolysis. Science 278: 460–463 [DOI] [PubMed] [Google Scholar]
- Wang X, Montero Llopis P, Rudner DZ (2013) Organization and segregation of bacterial chromosomes. Nat Rev Genet 14: 191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Asakawa K, Toh‐e A (2002) Mitotic exit network controls the localization of Cdc14 to the spindle pole body in Saccharomyces cerevisiae . Curr Biol 12: 944–950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Table EV1
Table EV2
Review Process File


