Abstract
[Purpose] Counteracting the systemic cytokine release and its inflammatory effects by improving respiratory muscle strength and controlling lung inflammation may be important for improving immune system in patients with chronic obstructive pulmonary disease, So the aim of the present study was to evaluate the effect of low level laser therapy and inspiratory muscle training on interleukin-6 (IL-6) as a marker of inflammation and CD4+/CD8+ ratio as a marker for T Lymphocytes in these patients. [Subjects and Methods] Forty male patients with stable COPD participated in the study, their ages ranged between 55−65 years. They were randomly divided into group (A) who received inspiratory muscle training and group (B) who received low level laser (LLL) acupuncture stimulation for about 8 week. [Results] There was a reduction in the concentration of plasma IL-6 associated with an increase in CD4+/CD8+ ratio in both groups, but laser was superior to inspiratory muscle training. IL-6 and CD4+/CD8+ were negatively correlated. [Conclusion] Both inspiratory muscle training and low level laser therapy are effective physical therapy modalities in promoting immune disturbances. The results also supported the superior role of LLLT over IMT in managing immune disturbances.
Key words: Low level laser therapy, Inspiratory muscle training, Immunity
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major public health problem with a higher level of incidence. Despite this fact, it had received little attention in the Middle East to be still under recognized and underdiagnosed. So the patient was represented in an advanced stage1).
The most important risk factors for COPD in this region are tobacco smoke, passive smoking, shisha (water Pipe), low socioeconomic status associated with indoor pollutant, and out air pollution2, 3).
COPD is defined as progressive and partially reversible airflow obstruction. It is a preventable and treatable disease, characterized by small airways disease as a result of inflammation, excess mucus production and peribronchial fibrosis associated with parenchymal destruction4).
The inspiratory muscles in patients with COPD face a mechanical disadvantage of hyperinflation with a consequence of disturbed length-tension relationship5). The end result is a change in the intrinsic properties of the inspiratory muscles and loss of strength, with the release of lung inflammatory mediators (cytokines) such as IL-6, tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP)6, 7).
Patients with COPD hard pulmonary and extra pulmonary systemic inflammation that can be explained based on multiple theories8).
These mechanisms or theories include cytokines spill over from the lung into systemic circulation, tobacco smoke with its role in oxidative stress and endothelial dysfunction9). Another supposed theories include hypoxia and dynamic hyperinflation, both of them are associated with an increase in the systemic biomarkers of inflammation10, 11). Aging may also be a contributing factor, as aging is associated with low-grade systemic inflammation and as the disease progress slowly so the majority of the patients are elderly12).
Cluster of differentiation 8 (CD8+) T-lymphocytes are suppressor cells of the immune system that induce apoptosis of epithelial and endothelial cells and reflects lung parenchyma destruction13). On the other hand, cluster of differentiation 4 (CD4+) is expressed on the surface of T-helper cells (T-lymphocytes) Normally there are about 1–2 CD4+ cells for every CD8+ cell, so the CD4+/CD8+ ratio ranges between 0.9 and 1.914).
In COPD, repeated stimulation of T-Lymphocyte as a result of chronic infection and exacerbation can decrease the number of CD4+ cells, and so the ratio of CD4+/CD8+ is decreased15).
IL-6 is a glycoprotein and one of the cytokines that was markedly affected by the exercise duration and intensity. Cytokines such as IL-6 can be upregulated within the lungs of animals exposed to inspiratory resistive loading, which can then enter the systemic circulation16). The reason for this release may include depletion of glycogen in the diaphragm, and so energy crisis that needs signals for the liver to increase glucose output17). It is still unknown whether the respiratory muscles contribute to this systemic inflammation.
On the other hand, low level laser therapy is a non-invasive, has no side effects, available, and cost-saving physical therapy modality with antioxidant and anti-inflammatory effects18,19,20). Its anti-inflammatory effects had been studied by many authors, who examined the cellular signaling responsible for these effects and concluded that reduction in calcium sensitivity may be responsible for its anti-inflammatory effect21,22,23). So the purpose of the present study was to investigate the effect of low level laser therapy on acupuncture points and inspiratory muscle training on modulating the immune disturbances, such as chronic inflammation in patients with chronic obstructive pulmonary disease, aiming to manage this problem and reduce its systemic manifestations, hence reducing exacerbation attacks, hospital admission, and mortality rates for these patients.
SUBJECTS AND METHODS
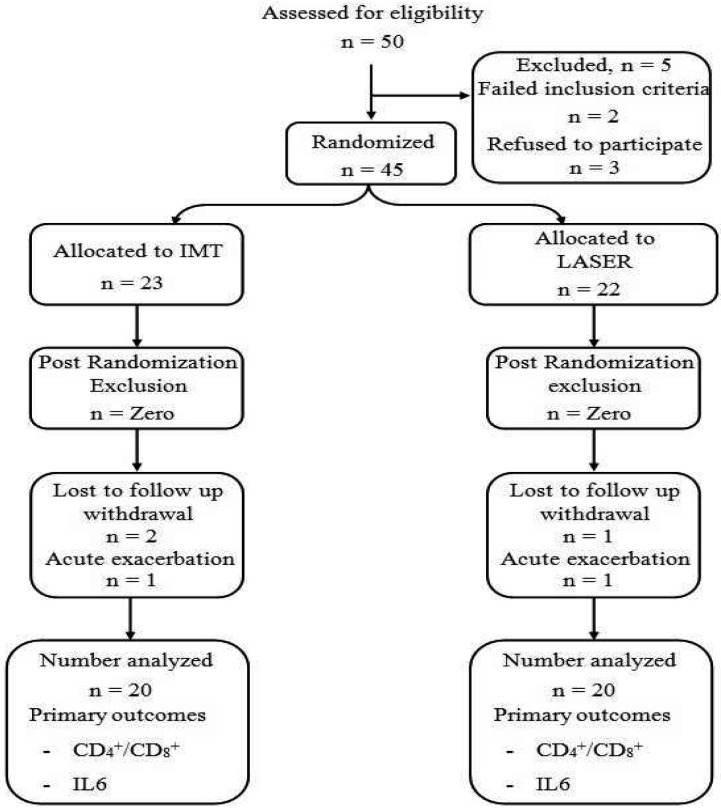
Forty male patients with mild to moderate COPD were recruited for this study from Imbaba National Institute for Allergy and Chest Diseases, Giza, out-patient clinic (Fig. 1). Patients were diagnosed according to American Thoracic Society24) standards and all of them had forced expiratory volume in the first second to be equal to 70−50% of the predicted value Their ages ranged between 55−65 years old and they were medically stable. All of them had a previous history of smoking; with smoking index <400. Patients were excluded from the study if they were heavy smoker, FEV was less than 50% of the of the practiced value, a requirement for supplemental oxygen, chest infection or pulmonary hypertension, cardiac diseases, obesity or diabetes mellitus as these diseases could affect measurement of immune system and inflammatory markers, and mental disorders that could affect the understanding and cooperation of the patient. This study was approved by the National Institute for Allergy and Chest Diseases Ethics Committee. The study procedure was explained for all patients and informed consent was obtained from them. The study was complying with the ethical standards of Declaration of Helsinki. Patients were randomly assigned into two groups; group (A) who received inspiratory muscle training in addition to their medical treatment and group (B) who received low level laser therapy on the acupuncture points in addition to the same medical treatment. Randomization process was performed using closed envelopes. The investigator prepared 50 closed envelopes with each envelope containing a card labeled with either the group A or the group B. Finally, each patient was asked to draw a closed envelope that contains whether he was allocated to the group A or the group B. The patients were randomly allocated to either a study (n=25) or control (n=25) group. The randomization was done by a colleague independent and blind to the study using concealed envelopes within which the group description was randomly placed within them.
Fig. 1.
Flow chart for study procedure
Spirometry was used to select COPD patients with mild to moderate airways obstruction (FEV1, was in the range of 70−50% of the predicted value). It was also used to measure the maximum inspiratory pressure as an indicator for inspiratory muscle strength, to be used for adjusting the intensity of inspiratory muscle training program for group (A). Kits and standard anticoagulant tube (EDTA) of blood samples was used for measuring interleukin 6 (IL-6), flow cytometer to measure CD4+/CD8+ ratio.
Inspiratory muscle trainer (REF-HS730, Respironic, NJ, USA) was used in group (A) for applying resisted inspiratory muscle training. Pointer laser (LLL3A, GALAS, He-Ne Laser Acupuncture) Point laser was applied on acupuncture points for group (B); with the following parameters, peak power 5 watts wave length 904 nanometer, pulse length 200 nanoseconds.
Medical history and demographic data of the patients was collected. The severity of smoking index was calculated according to the number of cigarette/day × number of years of smoking). Pulmonary function test was conducted to select the eligible patients before entry the study. In all patients, several practice tests were performed before obtaining the baseline value to avoid the possible training and learning effects. All the data were collected by the same investigator who was blinded to the patient assignment and also to the mode of treatment they received. For spirometry measurement, flow-volume loop measurement (FEV1) was obtained from sitting position, with a straight posture. Patient was asked to breathe normally at first until his breathing was rhythmic, then takes complete expiration, followed by deep, complete inspiration, and then forced rapid expiration. The test was repeated three times, and the largest FEV1 obtained was recorded.
Maximal inspiratory pressure was measured using spirometry after calibrating the mouth pressure of the device, and the patient was instructed to inhale maximally from a residual volume, through a specialized mouthpiece that had a small air leak to prevent pressure generation by glottis closure and was connected to pressure transducer. The patient was asked to maintain the pressure for about one second. The best value of three repeated trials was recorded as the maximal inspiratory pressure25).
Plasma or serum samples were obtained by venipuncture and stored on ice. The collections were taken between 9 and 12 am, at least 24 hours and not more than 5 days after the last treatment session.
IL-6 was measured using sample which centrifuged at 4°C for 10 minutes and then stored at between −75°C and −80°C. Concentration of IL-6 was measured by commercially available enzyme linked immunosorbent assays (ELLIS As).
The percentage of positive lymphocytes in the blood CD4+ and CD8+ cells were collected from venous blood. Ten milliliters of venous blood was drawn in an anticoagulant tube (EDTA) for each patient.
Treatment procedure was explained for patients in both groups. Group (A) received inspiratory muscle training program for about 2 months aiming to improve the strength of the inspiratory muscle. The intensity was adjusted to be about 30% of the maximal inspiratory pressure measured before entry the treatment program and the resistance was increased by about 5% each week, till reaching 60% and was maintained at this intensity till the end of the two months. The inspiratory training program was applied through a pressure threshold device which had a spring loaded valve, requiring the patient to inspire hard enough to open the valve and permit inspiration against that force.
The program was applied for about 30 minutes in the form of sets about 6 sets, and each set consisted of 5 deep breaths and in between rest for about 1 minute26). Also this group received placebo laser therapy while the device was off. As there is no heating effect and the patient could not detect if the device was on or off.
During the training sessions, rating for the perceived dyspnea according to modified Borg scale was used to assign the dyspnea during training. The intensity of training should be adjusted according to score 3 for patients in group (A)27).
For group (B) low level laser therapy was applied from comfortable sitting position, on the following acupuncture points; large interesting 11, kidney meridian 27, large intestine 4, lung meridian 1, and lung meridian 7. Each point was exposed to LLLT for about 90 seconds for twice per day, 3 times per week for 2 months. Before application, the target areas were cleaned with alcohol to minimize any back scatter or reflection. For protection from the laser’s beam, patient and physiotherapist wore protective glasses. This group also received placebo inspiratory muscle training with a fixed low load about 7 cm H2O that was not changed through-out the study period.
Subject characteristics compared between both groups using t test. Mixed MANOVA was conducted to compare the mean values of IL6 and CD4+/CD8+ ratio between the laser and IMT groups and between pre and post treatment in each group. Pearson Correlation Coefficient was conducted to determine the correlation between IL6 and CD4+/CD8+ ratio. The level of significance for all statistical tests was set at p<0.05. All statistical analysis was conducted through SPSS (statistical package for social sciences, version 19).
RESULTS
Table 1 showed the mean ± SD age, weight, height and BMI of laser and IMT groups. There was no significant difference between both groups in the mean age, weight, height and BMI (p<0.05).
Table 1. Comparison of mean age, weight, height and BMI between laser and IMT groups.
| Laser group | IMT group | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | MD | p-value | |
| Age (years) | 60.3 ± 3.4 | 60.1 ± 3.5 | –60.05 | 0.85 |
| Weight (kg) | 74.1 ± 10.1 | 78.2 ± 10.7 | –78.2 | 0.22 |
| Height (cm) | 169.5 ± 7.7 | 171.5 ± 5.7 | –171.5 | 0.35 |
| BMI (kg/m²) | 25.7 ± 2.6 | 26.5 ± 2.4 | –26.5 | 0.34 |
| FEV1 | 64.2 ± 9.0 | 65.55± 8.1 | –1.35 | 0.62 |
SD: standard deviation; MD: mean difference; p-value
Mixed MANOVA revealed that there was a significant interaction between treatment and time (Wilks’ Lambda=0.38; F (2.37)=29.33, p=0.0001). There was a significant main effect of time (Wilks’ Lambda=0.04; F (2.37)=393.71, p=0.0001) and a significant main effect of treatment (Wilks’ Lambda=0.84; F (2.37)=3.48, p=0.04). Table 2 showed descriptive statistics of dependent variables as well as the significant level of comparison between groups at pre and post treatment as well as significant level of comparison between pre and post treatment in each group.
Table 2. IL6 and CD4+/CD8+ ratio pre and post treatment in laser and IMT groups.
| Pre treatment | Post treatment | Repeated measures (Group A) |
Repeated measures (Group B) |
|||||
|---|---|---|---|---|---|---|---|---|
| Laser group (A) | IMT group (B) | Laser group | IMT group | |||||
| Mean ± SD | Mean ± SD | p value | Mean ± SD | Mean ± SD | p | p | p | |
| IL6 (pg/ml) | 90.2 ± 15.1 | 92.2 ± 13.0 | 0.66 | 49.4 ± 10.1 | 62.8 ± 11.4 | * | * | * |
| CD4/CD8 ratio | 0.47 ± 0.2 | 0.49 ± 0.2 | 0.71 | 0.95 ± 0.2 | 0.75 ± 0.2 | * | * | * |
SD: standard deviation; p-value, level of significance; *Significant
There was no significant difference in IL6 and CD4+/CD8+ ratio between the laser and the IMT groups pre-treatment (p>0.05). At post treatment measurements, Laser group showed significant decrease in IL6 compared with the control group (p>0.0001), also laser group showed significant increase in CD4+/CD8+ ratio compared with the control group (p>0.01),
Comparison between pre and post treatment in the laser group revealed that, there was a significant decrease in IL6 and significant increase in CD4+/CD8+ ratio post treatment compared with pre-treatment (p>0.0001). Comparison between pre and post treatment in the IMT group revealed that, there was a significant decrease in IL6 and significant increase in CD4+/CD8+ ratio post treatment compared with pre-treatment (p>0.0001).
There was a negative moderate significant correlation between IL6 and CD4+/CD8+ ratio (r=−0.63, p=0.0001) (Table 3).
Table 3. Correlation between IL6 and CD4+/CD8+ ratio.
| r value | p value | ||
|---|---|---|---|
| IL6 (pg/ml) | CD4/CD8 ratio | –0.63 | 0.0001* |
r value: correlation coefficient value; p value: probability value, *Significant
DISCUSSION
Patients with COPD had reduced inspiratory muscle strength and endurance associated with increased systemic cytokines. This is may be the result of inspiratory resistive loading and so the higher contractile demand imposed on the inspiratory muscles)5, 28, 29).
Stated that there is an inverse relationship between respiratory muscles strength and cytokines transcription and concluded that there was a synergistic action between the different cytokines such as tumor necrosis factor and interleukins, which magnifies the inflammation within the respiratory muscles. Their study relied the dysfunction of the respiratory muscles to pulmonary hyperinflation which induces changes in length-tension relationship. As seen in the results of the present study, patients had immunity disturbances at the baseline represented as an increase in interleukin 6 (IL-6) associated with a reduction in CD4+/CD8+ (about 0.47 in LASER group and 0.49 in the IMT group) which coincided with the study done by Casadevall et al5).
Many studies such as were conducted by Oh-Ishi et al.30) and Fanti et al.31), in an attempt to examine the effect of whole body endurance training (treadmill training) on lowering interleukin in the respiratory muscles after training, but unfortunately no data was available to illustrate the effect of inspiratory muscle training (IMT) on reducing the systemic inflammatory response.
The results of the present study showed a reduction in interleukin-6 associated with an increase in CD8+/CD4+ ratio which reflects the regression of the inflammatory response after inspiratory muscle training. In patients with COPD, the respiratory muscles did not complain from reduction in the oxidative capacity, as hypoxia acted as stimulus for endurance training due to the increased ventilation. So the diaphragm suffers from lack of strength, rather than endurance32). The positive immunomodulator effects of IMT seen in the present study may be the result of (1) Elevation of the antioxidant enzymes of the diaphragm as reported by, Sigala et al.33,34); Barreiro et al.35), (2) increased glycogen storage in the diaphragm as IL-6 secretion is increased after glycogen depletion to act as a stimulus for the liver to increase glucose out-put17); (3) stimulation for slowly adapting stretch receptors within the lung which can affect autonomic nervous system, decreasing sympathetic output36).
Attenuation of chemoreceptors activities within the diaphragm after IMT which aims to improve strength as a result of repeated exposure to high metabolite during training, may be another mechanism responsible for positive immunity effect seen in the present study. Witt et al.37) and Romer38) had concluded that improved respiratory muscle strength may eliminate the inhibitory feedback signals that cause recruitment of another accessory muscles of respiration with a resultant decrease in the work of breathing.
The results of the present study were supported by Mills et al.26) Who examined the effect of cycling exercise at lactate steady state on IL-6 in healthy males after 6 weeks of IMT with intensity about 50% of maximum inspiratory pressure (MIP). Their results showed a reduction in IL6 and they attributed that to reduction in metaboreflex of the respiratory muscles that could increase blood flow to the liver, facilitating IL-6 uptake and also could increase leg blood flow with less glycogen utilization. It had been assumed that the strenuous contraction of respiratory muscles in patients with COPD could activate afferent nerve fibers within the phrenic nerve. These impulses can travel through hypothalamic-pituitary-adrenal axis with a resultant secretion of β-endorphin in an attempt to decrease the activation and so tidal volume is reduced29).
The improvement of inspiratory muscle strength after IMT applied in the present study may eliminate the activity of β-endorphin with a more deep breathing and oxygenation to the liver and peripheral muscles.
The reduction in CD4+/CD8+ ratio observed in COPD patients in the present study may be another aspect of immune system disturbances in these patients as a result of increased cytotoxic-T cells (CD8+) and reduction of helper-T cells (CD4+)39). Patients with COPD had defect in macrophages phagocytic action (airway clearance) associated with an increase in its lifespan with a defect in their elimination or apoptosis. CD8+ is responsible for this apoptosis and should be also eliminated upon their activation. As a result of autoimmune deficiency in these patients, the old macrophages and CD8+ accumulate within the lung along with an increase in the inflammatory cytokines (IL-6) in a trial to eliminate them. The previous findings shed the light on apoptosis dysregulation40, 41)
The reduction in CD4+/CD8+ in IMT group may be a promising feature of the effective intensity used in this type of training to be efficient to stimulate regulating T-cells capable of down regulating autoimmunity disturbances42). The results of the present study coincided with that reported by Davidson et al.43), who concluded that acute incremental exercise (one shoot) had anti-inflammatory effect in patients with COPD. Their study revealed an immediate increase in CD8+ after exercise which returned to the baseline after 2 hours. In the previous study, the patients had a normal baseline value for CD4+/CD8+ (2.4 + 1.6).
Another physical therapy modality that is known by approved anti-inflammatory effect; LLLT, as seen in the results of the present study, it was superior to IMT in achieving better adaptive (Cellular) immunity response, CD4+/CD8+ ratio was nearly normalized (about 0.95) after LLT application. The results of the present study were supported by previous animal studies such as that done by De-Lima et al. 44), who examined the effect of LLLT on acute lung inflammation induced by intestinal ischemic −reperfusion in rat model. Infrared laser was applied (660 nm), power out-put of 30 mw, on the skin over the upper trachea. Their results revealed an increase in the anti-inflammatory mediators (interleukin-10) to counteract the proinflammatory mediators (tumor necrosis factor, TNF) which was also reduced after laser irradiation.
De-Silva et al.45), also examined the effect of LLLT on acute lung inflammation induced by formaldehyde inhalation in rats. Infrared laser (660 nm), with power out of 30 mw was applied at the trachea (3 Points) and at each lung lobe (3 points for each lung). The previous study concluded the anti-inflammatory effect of LLLT (increase in the level of IL-10) associated with a reduction in IL-6 as observed in the present study. The study of De-Silva et al.45) shed the light on the possible complex role of IL-6 in lung diseases associated with chronic inflammation.
Low level laser therapy might induce a reduction in mucus over production, cytokine release and collagen deposition44, 46, 47).
REFERENCES
- 1.Khan JH, Lababidi HM, Al-Moamary MS, et al. : The Saudi guidelines for the diagnosis and management of COPD. Ann Thorac Med, 2014, 9: 55–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben Abdallah FC, Taktak S, Chtourou A, et al. : Burden of chronic respiratory diseases (CRD) in Middle East and North Africa (MENA). World Allergy Organ J, 2011, 4: S6–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daldoul H, Denguezli M, Jithoo A, et al. : Prevalence of COPD and tobacco smoking in Tunisia—results from the BOLD study. Int J Environ Res Public Health, 2013, 10: 7257–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatipoğlu U, Aboussouan LS: Chronic obstructive pulmonary disease: an update for the primary physician. Cleve Clin J Med, 2014, 81: 373–383. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall C, Coronell C, Ramírez-Sarmiento AL, et al. : Upregulation of pro-inflammatory cytokines in the intercostal muscles of COPD patients. Eur Respir J, 2007, 30: 701–707. [DOI] [PubMed] [Google Scholar]
- 6.Pinto-Plata VM, Livnat G, Girish M, et al. : Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest, 2007, 131: 37–43. [DOI] [PubMed] [Google Scholar]
- 7.Karadag F, Kirdar S, Karul AB, et al. : The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur J Intern Med, 2008, 19: 104–108. [DOI] [PubMed] [Google Scholar]
- 8.Bailey KL, Goraya J, Rennard SL: The role of systemic inflammation in COPD. In: Nici L, Zu-wallack R, Chronic obstructive pulmonary disease co morbidities and systemic consequences. New York: Human Press, 2012, pp 15–30. [Google Scholar]
- 9.Hansson GK: Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med, 2005, 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 10.Sabit R, Thomas P, Shale DJ, et al. : The effects of hypoxia on markers of coagulation and systemic inflammation in patients with COPD. Chest, 2010, 138: 47–51. [DOI] [PubMed] [Google Scholar]
- 11.Pini L, Valsecchi A, Boni E,, et al. : Acute dynamic hyperinflation and systemic inflammation in stable COPD patients. Am J Respir Crit Care Med, 2010, 181: A 2907. [Google Scholar]
- 12.Sharma G, Hanania NA, Shim YM: The aging immune system and its relationship to the development of chronic obstructive pulmonary disease. Proc Am Thorac Soc, 2009, 6: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese F, Giacometti C, Beghe B, et al. : Marked alveolar apoptosis/proliferation imbalance in end-stage emphysema. Respir Res, 2005, 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jelley-Gibbs DM, Dibble JP, Filipson S, et al. : Repeated stimulation of CD4 effector T cells can limit their protective function. J Exp Med, 2005, 201: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekberg-Jansson A, Andersson B, Avrå E, et al. : The expression of lymphocyte surface antigens in bronchial biopsies, bronchoalveolar lavage cells and blood cells in healthy smoking and never-smoking men, 60 years old. Respir Med, 2000, 94: 264–272. [DOI] [PubMed] [Google Scholar]
- 16.Toumpanakis D, Kastis GA, Zacharatos P, et al. : Inspiratory resistive breathing induces acute lung injury. Am J Respir Crit Care Med, 2010, 182: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen BK, Febbraio MA: Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev, 2008, 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- 18.Kashanskaia EP, Fedorov AA: [Low-intensity laser radiation in the combined treatment of patients with chronic obstructive bronchitis]. Vopr Kurortol Fizioter Lech Fiz Kult, 2009, (2): 19–22 (in Russian). [PubMed] [Google Scholar]
- 19.de Lima FM, Villaverde AB, Albertini R, et al. : Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg Med, 2011, 43: 410–420. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira MC, Jr, Greiffo FR, Rigonato-Oliveira NC, et al. : Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J Photochem Photobiol B, 2014, 134: 57–63. [DOI] [PubMed] [Google Scholar]
- 21.Bjordal JM, Johnson MI, Iversen V, et al. : Photoradiation in acute pain: a systematic review of possible mechanisms of actions and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg, 2006, 24: 158–168. [DOI] [PubMed] [Google Scholar]
- 22.Chow RT, Johnson MI, Lopes-Martins RA, et al. : Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet, 2009, 374: 1897–1908. [DOI] [PubMed] [Google Scholar]
- 23.Aimbire F, Lopes-Martins RA, Castro-Faria-Neto HC, et al. : Low-level laser therapy can reduce lipopolysaccharide-induced contractile force dysfunction and TNF-alpha levels in rat diaphragm muscle. Lasers Med Sci, 2006, 21: 238–244. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society / European Respiratory Society Task Force: Standards for the diagnosis and management of patients with COPD, version 1.2. New York: American Thoracic Society, 2009, pp 100 −132. [Google Scholar]
- 25.Mesquita R, Donária L, Genz IC, et al. : Respiratory muscle strength during and after hospitalization for COPD exacerbation. Respir Care, 2013, 58: 2142–2149. [DOI] [PubMed] [Google Scholar]
- 26.Mills DE, Johnson MA, McPhilimey MJ, et al. : The effects of inspiratory muscle training on plasma interleukin-6 concentration during cycling exercise and a volitional mimic of the exercise hyperpnea. J Appl Physiol 1985, 2013, 115: 1163–1172. [DOI] [PubMed] [Google Scholar]
- 27.Borg E, Borg G, Larsson K, et al. : An index for breathlessness and leg fatigue. Scand J Med Sci Sports, 2010, 20: 644–650. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro JP, Chiappa GR, Neder JA, et al. : Respiratory muscle function and exercise intolerance in heart failure. Curr Heart Fail Rep, 2009, 6: 95–101. [DOI] [PubMed] [Google Scholar]
- 29.Vassilakopoulos T, Divangahi M, Rallis G, et al. : Differential cytokine gene expression in the diaphragm in response to strenuous resistive breathing. Am J Respir Crit Care Med, 2004, 170: 154–161. [DOI] [PubMed] [Google Scholar]
- 30.Oh-ishi S, Kizaki T, Ookawara T, et al. : Endurance training improves the resistance of rat diaphragm to exercise-induced oxidative stress. Am J Respir Crit Care Med, 1997, 156: 1579–1585. [DOI] [PubMed] [Google Scholar]
- 31.Yfanti C, Fischer CP, Nielsen S, et al. : Role of vitamin C and E supplementation on IL-6 in response to training. J Appl Physiol 1985, 2012, 112: 990–1000. [DOI] [PubMed] [Google Scholar]
- 32.Dumitru L, Iliescu A, Dinu H, et al. : Disability in COPD and chronic heart failure is the skeletal muscle the final common pathway? Maedica (Buchar), 2013, 8: 206–213. [PMC free article] [PubMed] [Google Scholar]
- 33.Sigala L, Zacharatos P, Toumpanakis D, et al. : Differentially regulate cytokine expression in the diaphragm in response to resistive breathing: the role off oxidative stress. Am J Physiol Integr Comp Physiol, 2011, 300: R 1152–R 1162. [DOI] [PubMed] [Google Scholar]
- 34.Sigala I, Zacharatos P, Boulia S, et al. : Nitric oxide regulates cytokine induction in the diaphragm in response to inspiratory resistive breathing. J Appl Physiol 1985, 2012, 113: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 35.Barreiro E, de la Puente B, Minguella J, et al. : Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med, 2005, 171: 1116–1124. [DOI] [PubMed] [Google Scholar]
- 36.Brilla L R: Perspectives on breathing in sports and health. J Sport Med Doping Stud, 2012, 2: 1000 e 121. [Google Scholar]
- 37.Witt JD, Guenette JA, Rupert JL, et al. : Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J Physiol, 2007, 584: 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romer LM, McConnell AK: Specificity and reversibility of inspiratory muscle training. Med Sci Sports Exerc, 2003, 35: 237–244. [DOI] [PubMed] [Google Scholar]
- 39.Katsaounou P, Karatza MH, Kollintza A, et al.: The immune response to strenuous resistive breathing. Am J Respir Crit Care Med, 2001, 163: A 621. [Google Scholar]
- 40.Domagała-Kulawik J, Hoser G, Dabrowska M, et al. : Increased proportion of Fas positive CD8+ cells in peripheral blood of patients with COPD. Respir Med, 2007, 101: 1338–1343. [DOI] [PubMed] [Google Scholar]
- 41.Bolton CE, Evans M, Ionescu AA, et al. : Insulin resistance and inflammation—a further systemic complication of COPD. COPD, 2007, 4: 121–126. [DOI] [PubMed] [Google Scholar]
- 42.Rafiq R, Aleva FE, Schrumpf JA, et al. : Prevention of exacerbations in patients with COPD and vitamin D deficiency through vitamin D supplementation (PRECOVID): a study protocol. BMC Pulm Med, 2015, 15: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson WJ, Verity WS, Traves SL, et al. : Effect of incremental exercise on airway and systemic inflammation in patients with COPD. J Appl Physiol 1985, 2012, 112: 2049–2056. [DOI] [PubMed] [Google Scholar]
- 44.de Lima FM, Aimbire F, Miranda H, et al. : Low-level laser therapy attenuates the myeloperoxidase activity and inflammatory mediator generation in lung inflammation induced by gut ischemia and reperfusion: a dose-response study. J Lasers Med Sci, 2014, 5: 63–70. [PMC free article] [PubMed] [Google Scholar]
- 45.Miranda da Silva C, Peres Leal M, Brochetti RA, et al. : Low level laser therapy reduces the development of lung inflammation induced by formaldehyde exposure. PLoS One, 2015, 10: e0142816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva VR, Marcondes P, Silva M, et al. : Low-level laser therapy inhibits bronchoconstriction, Th2 inflammation and airway remodeling in allergic asthma. Respir Physiol Neurobiol, 2014, 194: 37–48. [DOI] [PubMed] [Google Scholar]
- 47.Peron JP, de Brito AA, Pelatti M, et al. : Human tubal derived mesenchymal stromal cells associated with low level laser therapy significantly reduces cigarette smoke-induced COPD in C57 BL/ 6 mice. PLoS One, 2015, 10: e0136942. [DOI] [PMC free article] [PubMed] [Google Scholar]