Abstract
[Purpose] Histopathological investigation of the effects of low-intensity pulsed ultrasound (LIPUS) on joint components using a rat knee joint contracture model. [Subjects and Methods] Nineteen, 9-week-old Wistar male rats were divided into a control group (n=6) and an experimental group. Rats in the experimental group underwent cast immobilization of the right rear limb for 8 weeks. They were then randomly divided into a non-treatment group (n=6), which was raised under normal conditions for 4 weeks, and a treatment group (n=7), which underwent LIPUS for 4 weeks. LIPUS irradiation was performed at a frequency of 3 MHz, an intensity of 30 mW/cm2, and a pulse rate of 20% duty cycle. Irradiation was performed once daily for 10 min, 5 days per week. At the end of this period, tissue specimens in which the knee sagittal plane could be observed were prepared and observed using an optical microscope. [Results] The extension-limiting angle of the knee joint was significantly less in the treatment group compared with the non-treatment group. The posterior joint capsule was significantly thicker only in the non-treatment group, and the density was 53.5 ± 7.5% for the control group, 77.2 ± 5.7% for the non-treatment group, and 69.2 ± 2.9% for the treatment group, with significant differences existing across all groups. [Conclusion] LIPUS may widen the space between collagen fiber bundles of the joint capsule, thereby improving the range of motion.
Keywords: Contracture, Joint capsule, LIPUS
INTRODUCTION
Limitations in joint range of motion can interfere with activities of daily living and quality of life. In orthopedic medicine, limitations in range of motion are classified according to the responsible lesion. However, it has become clear that limitations in range of motion that result from prolonged periods of immobilization of a joint are due to alterations in both soft tissues, such as muscles and skin that are not joint components, and joint components, such as the joint capsule and ligaments1, 2). In this way, it is difficult to identify the responsible lesion. Therefore, rehabilitation medicine has developed the term “joint contracture” to describe this phenomenon, thereby better reflecting the pathological condition of a limited range of motion in comparison to the classification system that describes the responsible part. The present study also uses this approach.
For treatment of joint contracture, in addition to exercise therapy and manual therapy, electrophysical agents are also used, either alone or in combination with other such methods. Reports related to the treatment of joint contracture using ultrasound first appeared in the 1960s and are now widely presented3,4,5,6). There have also been some studies using experimental animals7, 8), but this has mostly verified the effects of muscle and tendon heating due to ultrasound irradiation, with little verification focused on non-heat treatment. Furthermore, to the best of our knowledge, there have been no reports in which the object of intervention is the joint capsule. Sugama et al.9) used ultrasound irradiation with an intensity of 0.5 W/cm2 on the soleus muscle after immobilization of rat leg joints. The speculated action of collagen cross-linking observed on the ultrasound was related to tissue flexibility due to the observed decrease in the amount of insoluble collagen. In their report, it was unclear whether the tissue temperature was elevated and so it is uncertain if this result was due to non-heat ultrasound treatment. However, Kondo et al.10) used the same rat leg joint immobilization model in a hot water bath (42°C) and found that there was no significant difference in the amount of insoluble collagen in the soleus muscle. Thus, ultrasound properties beyond heating may have an effect on collagen fibers after immobilization.
One type of ultrasound that does not involve thermal activity is low-intensity pulsed ultrasound (LIPUS). LIPUS is defined as ultrasound in the form of low-intensity pulses. Following the release of a report on synostosis in pseudoarthrosis11), there have been repeated verifications of bone healing. Furthermore, its use in treating some types of bone fractures is covered by insurance. Recently, there have been various reports involving its use beyond bone repair, including that of soft tissues, such as muscle, tendons, and ligaments, in which the effectiveness of LIPUS has been reported12). Hence, it may have an effect on the joint capsule after joint immobilization; however, to date, there have been no reports testing this hypothesis. Therefore, the purpose of the present study was to use a rat knee joint contracture model to investigate the effect of LIPUS irradiation by analyzing changes in the range of motion and histopathological changes in the joint capsule.
SUBJECTS AND METHODS
Nineteen, 9-week-old Wistar male rats were divided into control (n=6) and experimental (n=13) groups. The right hind legs of the experimental group were subsequently fixed with casts for 8 weeks, after which they were randomly divided into a non-treatment group (n=6) that was raised normally for 4 weeks, and a treatment group (n=7) that underwent LIPUS treatment for 4 weeks. The control group was raised normally for 12 weeks. The present study was performed with the approval of the Animal Care Committee of Nagoya Gakuin University (Approval number: 2007-003). All procedures for animal care and treatment were performed in accordance with the regulations on Animal Experiments of Nagoya Gakuin University.
Cast immobilization was performed as described previously13). Under inhalation anesthesia with isoflurane, the rats were fitted with custom-made jackets produced from Velfoam (Velcro USA Inc., Manchester, NH, USA) and secured at the back with Velcro. An area from the pelvic girdle to the distal foot joint was immobilized with a cast with full extension of the hip joint, full flexion of the knee joint, and full plantar flexion of the foot joint. The cast was then covered with gauze to prevent excoriation of the animal from the cast. The area between the distal foot joint and the toe on the immobilized limb was exposed to monitor the development of edema and to confirm the absence of congestion. The patella and its surrounding area on the immobilized limb were also exposed to permit normal bone growth during the immobilization period. The contralateral posterior limb was not modified. Rats were able to move freely in their cages and had sufficient supply of water and food. The casts were replaced every 2 weeks. If pedal edema developed or the casts loosened, they were replaced immediately to maintain adequate immobilization.
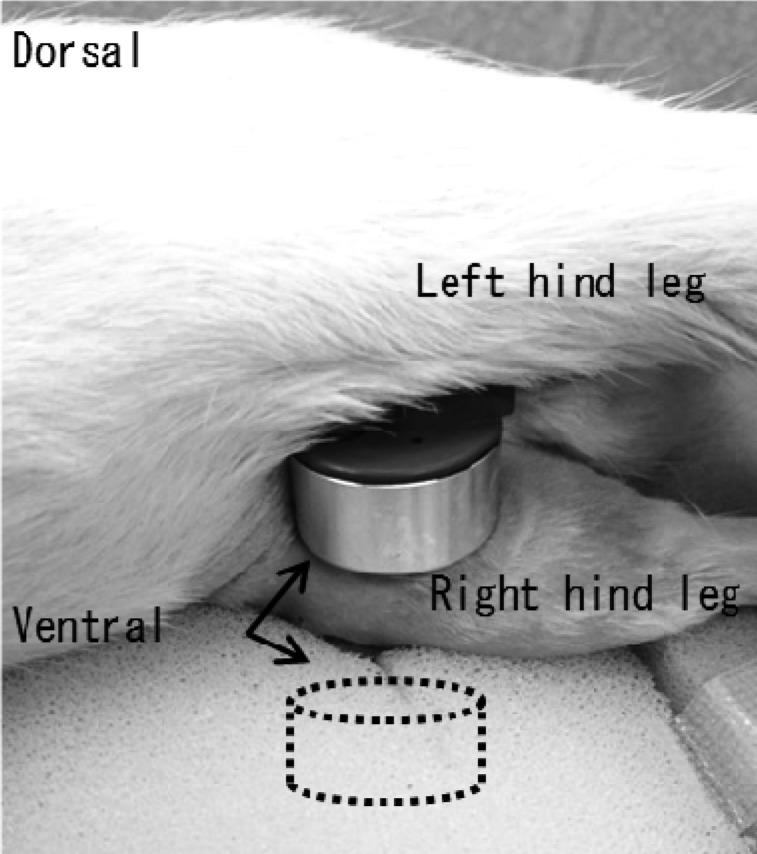
An ultrasound treatment machine (Ito Co., Ltd., ST-SONIC) was used to perform LIPUS. The rats were irradiated while lying on their side under inhalation anesthesia using two electrodes fixed on both the inside and outside of the knee joint (Fig. 1). The irradiation conditions were: frequency of 3 MHz, spatial-average temporal average intensity (ISATA) of 30 mW/cm2, and pulsed 1:4 irradiation ratio of 20% (2 ms on and 8 ms off). Irradiation was performed daily for 10 min, five times per week. The non-treatment group was irradiated with a placebo using the same method.
Fig. 1.
Low-intensity pulsed ultrasound (LIPUS) for knee joint. Two electrodes fixed on the inside and outside (dotted line, in the sponge) of the knee (arrows).
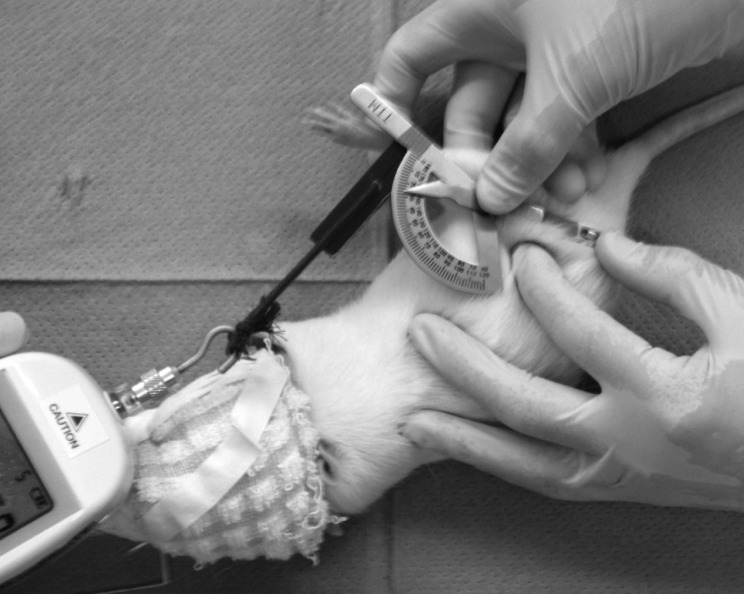
To measure the range of motion (ROM) of the knee joint, a goniometer designed for human fingers was fitted with a custom-made attachment, which was used in conjunction with a digital push-pull gauge (Shiro Co., Ltd., RX-1) to apply a force while the rats were under inhalation anesthesia. The angle was read when it was pulled with a force of approximately 0.04 Nm (Fig. 2). The measurements were read before and after cast immobilization, and at the end of the intervention period. The difference between the angle before and after cast immobilization served as a measure of the extension-limiting angle of the knee joint.
Fig. 2.
Measurement of range of motion
The knee joint ROM was measured by goniometer for use in finger with original attachment. The knee joint was pulled in 0.04Nm by tension meter.
After the breeding period, all groups underwent perfusion fixation using 4% paraformaldehyde under intra-abdominal Nembutal anesthesia. The right posterior limb was disarticulated at the hip joint, and specimens were permeated and fixed for 72 h, followed by decalcification with Plank-Rychlo solution for 72 h at 4°C. The knee joints were then excised, neutralized with 5% sodium sulfate solution, delipidated with 100% ethanol, and paraffin-embedded to study the sagittal plane. Using a sliding microtome, approximately 3–5 μm slices were obtained from the prepared paraffin block, which were then stained with hematoxylin-eosin (HE) and visualized using light microscopy (Olympus Corporation, BX-51).
A posterior joint capsule tissue image was then obtained using a microscope digital camera (Olympus Corporation, DP73), which was then analyzed used image processing software to measure the thickness and density of the joint capsule. The thickness of the joint capsule was then measured using an image of the entire joint taken with a magnification of 10×. A line was drawn along the path of the patellar ligament, through which a perpendicular line was drawn such that it passed as close as possible to the center of the space between the femur and the tibia. The length of the posterior joint capsule was the measured along that line14). To calculate the density of the joint capsule, a 400× image was analyzed using Image J (ver. 1.45s for Windows, NIH). The area between the collagen fibers was measured and then divided by the total area excluding the clear blood vessels and joint cavity. This value was expressed as a percentage14). The photographed portion of the joint was located just below the meniscus near the center of the inner and outer sides. Images from 6 to 8 non-overlapping regions were used.
After checking the normality of the data obtained for each group, the difference in the extension-limiting angles was compared using Student’s t-test, while the thickness and density of the posterior joint capsule among groups were compared using Tukey’s multiple comparison test. The level of significance was set at α=0.05. All analyses were performed using R (2.8.1).
RESULTS
A significant difference was not found between the treatment group and the non-treatment group in the extension-limiting angle that occurred due to 8 weeks of immobility. A significant difference was observed in the final extension-limiting angle 4 weeks at the end of cast immobilization: 31.1 ± 9.2° in the treatment group, and 42.3 ± 5.6° in the non-treatment group (Table 1).
Table 1. Limitation of the knee joint range of motion (°).
| After immobilization for 8 weeks | After intervention for next 4 weeks | |
|---|---|---|
| Non-treatment group | 86.2 ± 2.7 | 42.3 ± 5.6 |
| Treatment group | 84.1 ± 10.4 | 31.1 ± 9.2* |
Mean ± SD. *p<0.05, significant difference according to the Student’s t-test.
In the histological findings for the posterior joint capsule, a tendency for thickening was found in the non-treatment group (Fig. 3A–C), with the thickness being significantly larger only in the non-treatment group (Table 2). Narrowing of the space between collagen fiber bundles was observed in both the treatment group and the non-treatment group compared to the control group. Meanwhile, such spaces tended to be wider in the treatment group compared to the non-treatment group (Fig. 3a–c). The densities were 53.3 ± 7.5% for the control group, 77.2 ± 5.7% for the non-treatment group, and 69.2 ± 2.9% for the treatment group. Furthermore, a significant difference was observed across all groups (Table 2).
Fig. 3.
Histopathological finding of posterior joint capsule (HE Staining). Right figure shows a high power field image of the posterior joint capsule. Each figure shows A/a: control group, B/b: non-treatment group, C/c: treatment group, respectively. The thickness of the joint capsule tended to increase in non-treatment group (A–C). The deeply colored area indicates collagen fiber bundles and the white area indicates the spaces between them (a–c). The narrowing of the space was observed in both non-treatment group and treatment group. But in the treatment group, the extent of the spaces seemed to come close to that in the control group. Scale; 500/50 µm (Left side/Right side), T: tibia; F: femur; M: meniscus; arrows: the thickness of the posterior joint capsule
Table 2. Measured values of the posterior joint capsule.
| Control groupa | Experimental group | ||
|---|---|---|---|
| Non-treatmentb | Treatmentc | ||
| Thickness (mm) | 0.74 ± 0.15 | 1.17 ± 0.25>a,c | 0.78 ± 0.09 |
| Density (%)* | 53.3 ± 7.5>b,c | 77.2 ± 5.7<a, c | 69.2 ± 2.9<a, >b |
Mean ± SD. a, b, c; p<0.05, significant difference according to the Tukey’s multiple comparison test. *A cross-sectional area of the collagen fiber bundles measured as an index of the density of the posterior joint capsule. The values measured were then divided by the total area, omitting any blood vessels apparent, and these values are shown as percentages.
DISCUSSION
In the present study, a model of contracture caused by 8 weeks of immobility was used to histologically study the effects of LIPUS irradiation on the range of motion and posterior joint capsule.
It is believed that the cause of the limitation observed in contracture is different depending on the joint immobilization period. Namely, a large portion of immobility is due to myogenic limitation at the beginning of the period of immobility, but is gradually caused more by articular limitations1, 2). Additionally, experimental cases in which contracture was induced by long-term immobilization, thickening of the posterior joint capsule and narrowing of the space between collagen fiber bundles were found during longer immobilization periods13). Additionally, in the present study, which involved an 8-week immobilization period using a similar immobilization model, articular limitations developed and were accompanied by changes in the joint capsule. Indeed, the limitations in range of motion were reduced in rats that were raised normally, as indicated by measurements of the range of motion at the end of immobilization and after the subsequent 4-week re-mobilization period, regardless of whether there was LIPUS intervention. Therefore, even if reduced range of motion accompanies an articular limitation, reversibility is observed due to natural healing. Furthermore, it is possible that LIPUS irradiation promotes reversibility for the joint components, as indicated by the therapy-dependent difference in the range of motion-limiting angle. Regarding the reversibility of contracture, Trudel et al.15) reported that, after internal fixation of rat knee joints for 8 weeks that is then followed by normal raising during the subsequent 4 weeks, the range of motion at the knee was an average of 51.9°. Meanwhile, the average range of motion at the knee in the control (sham fixing) group was 18.9°. Therefore, approximately 30° of the limitation was found to be irreversible. In the present study, an average limitation of 42.3° remained in the non-treatment group and, although there is a difference in the angle, it appears that similar results were obtained. Meanwhile, it is possible that the differences in creating the contracture model (i.e., internal fixing vs. external fixing) or in the torque when measuring the range of motion (0.065 Nm vs. 0.04 Nm), had an effect on the differences in the measured angle.
Regarding the histological findings for the posterior joint capsule, the spaces between the collagen fiber bundles exhibited increased width due to LIPUS irradiation. Although the density did not return to normal levels, it was significantly improved compared with the cases in which no treatment was given. Akeson et al.16), in an experiment involving a fixed rabbit knee joint in the flexed position, exhibited a reduction in the presence of water and glycosaminoglycan in the connective tissue around the knee joint. Furthermore, based on a reduction in the extracellular matrix, they reported that the collagen fibers were closer together, that their sliding ability was decreased, and that the flexibility of the connective tissue was decreased. Therefore, the observed widening of the spaces between the collagen fiber bundles may indicate a sliding motion of the collagen fiber bundles. Therefore, it is easier to extend the joint capsule, which results in a difference in the limiting angles depending on whether there was therapeutic intervention.
Some of the effects of LIPUS on soft tissue have been verified. For example, Yamamoto et al.17,18,19) reported the expression of fibroblast growth factor, platelet-derived growth factor, transforming growth factor-beta 1, and collagen I and III following LIPUS irradiation in a rat tendon injury model. Additionally, Takakura et al.20) reported a widening of collagen fiber diameter using electron microscopy after LIPUS irradiation in a rat medial collateral ligament injury model. Sparrow et al.21) reported an increase in the ratio of type I collagen in a rat medial collateral ligament extraction model that involved LIPUS irradiation. These results suggest the proliferation of fibroblasts and the promotion of collagen fibers synthesis following LIPUS. In vitro studies with human fibroblasts22) have demonstrated that mechanical stress due to LIPUS irradiation activates the extracellular signal-related kinase (ERK) pathway, thereby promoting cell proliferation, which supports the above reports. However, it has also been reported that LIPUS irradiation not only promotes, but also suppresses, cell proliferation. Nakamura et al.23) used LIPUS irradiation in a mouse arthritis model (MRL/lpr mice) and found that inflammation, pannus, and hyperplasia of the synovial membrane were significantly reduced based upon a histological analysis of the knee joint after 3 weeks of treatment. Sato et al.24) of the same group used LIPUS irradiation on synovial membrane cell stocks to study the amount of expression of integrin, FAK, and MAPK (ERK1/2, JNK, p38), and concluded that LIPUS irradiation regulates the apoptosis and survival of synovial cells through the integrin/FAK/MAPK pathway. These results suggest that the mechanical stress due to LIPUS promotes the adaptation of metabolism to the cell environment by controlling cell proliferation and apoptosis. Additionally, in the present study, it is hypothesized that the space between collagen fiber bundles is expanded to adapt to an environment that is required for greater joint mobility. Indeed, when Skutek et al.25) applied a periodic tensile stimulus of 15 min or 60 min to fibroblasts of the human patellar tendon, the rate of apoptosis was found to vary across the two conditions.
In treating joint contracture, manual methods, such as stretching and mobilization, are often used. While there are few reports that verify the effects of these techniques on the joint capsule, they do not indicate whether sufficient improvement is obtained. Similarly, in the present study, it is unclear if there is sufficient improvement in the joint capsule; however, LIPUS may be advantageous in that it can provide quantitative, deep (joint capsule) stimulation that does not depend on the skill of the technician. The present study demonstrates the use of a possible novel treatment method for joint contracture and, therefore, may have great significance.
In the present study, measurement of the range of motion was performed without extraction of skin or muscle. However, it is possible that LIPUS, given irradiation method and electrode size, incidentally applies stimulation to the surrounding skin, fascia, and several muscles. For this reason, the results of the present study included changes that result from immobilization of soft tissue beyond the joint components. Therefore, the effects of LIPUS on these tissues cannot directly correlate the change in the range of motion to the histological changes in the joint capsule. In addition, the effects on the joint capsule are limited to histological observations, while quantitative assessments require further refinement. Therefore, additional, multi-faceted verification is required.
The results of the present study indicate that LIPUS irradiation to treat contracture after joint immobilization can widen the space between collagen fiber bundles of the posterior joint capsule, and thereby improve the limitation on range of motion.
Conflict of interest
We have no COI with regard to this study.
Acknowledgments
We would like to deeply thank the staff of Department of Human Pathology, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University. This work was supported by JSPS KAKENHI Grant Number 15K16375.
REFERENCES
- 1.Trudel G, Uhthoff HK: Contractures secondary to immobility: is the restriction articular or muscular? An experimental longitudinal study in the rat knee. Arch Phys Med Rehabil, 2000, 81: 6–13. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto M, Okita M, Kasuya A, et al. : Effects of immobilization period on restriction of soft tissue and articulation in rat ankle joint. Phy Ther Jpn. 2004, 31: 36–42(in Japanese). [Google Scholar]
- 3.Lehmann JF, Fordyce WE, Rathbun LA, et al. : Clinical evaluation of a new approach in the treatment of contracture associated with hip fracture after internal fixation. Arch Phys Med Rehabil, 1961, 42: 95–100. [PubMed] [Google Scholar]
- 4.Rutjes AW, Nüesch E, Sterchi R, et al. : Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst Rev, 2010, (1): CD003132. [DOI] [PubMed] [Google Scholar]
- 5.Casimiro L, Brosseau L, Robinson V, et al. : Therapeutic ultrasound for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev, 2002, (3): CD003787. [DOI] [PubMed] [Google Scholar]
- 6.Dogru H, Basaran S, Sarpel T: Effectiveness of therapeutic ultrasound in adhesive capsulitis. Joint Bone Spine, 2008, 75: 445–450. [DOI] [PubMed] [Google Scholar]
- 7.Usuba M, Miyanaga Y, Miyakawa S, et al. : Effect of heat in increasing the range of knee motion after the development of a joint contracture: an experiment with an animal model. Arch Phys Med Rehabil, 2006, 87: 247–253. [DOI] [PubMed] [Google Scholar]
- 8.Okita M, Nakano J, Kataoka H, et al. : Effects of therapeutic ultrasound on joint mobility and collagen fibril arrangement in the endomysium of immobilized rat soleus muscle. Ultrasound Med Biol, 2009, 35: 237–244. [DOI] [PubMed] [Google Scholar]
- 9.Sugama S, Tachino K, Haida N, et al. : The effect of ultrasound irradiation and static stretching on immobilized muscle—Biochemical studies on collagen from rat soleus muscle—. Phy Ther Jpn, 1998, 25: 368–375(in Japanese). [Google Scholar]
- 10.Kondo Y, Nakano J, Sakamoto J, et al. : Effects of prolonged stretching and thermotherapy on muscle contracture of immobilized rat soleus muscle. J Phys Ther Sci, 2012, 24: 541–547. [Google Scholar]
- 11.Duarte LR: The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg, 1983, 101: 153–159. [DOI] [PubMed] [Google Scholar]
- 12.Khanna A, Nelmes RT, Gougoulias N, et al. : The effects of LIPUS on soft-tissue healing: a review of literature. Br Med Bull, 2009, 89: 169–182. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, Hoso M, Hibino I, et al. : Histopathological changes of joint capsule after joint immobility, compared with aging in rats. J Phys Ther Sci, 2010, 22: 369–374. [Google Scholar]
- 14.Watanabe M, Hoso M, Hibino I, et al. : Histopathological changes in joint components in a rat knee joint contracture model following mobilization. J Phys Ther Sci, 2012, 24: 1199–1203. [Google Scholar]
- 15.Trudel G, Zhou J, Uhthoff HK, et al. : Four weeks of mobility after 8 weeks of immobility fails to restore normal motion: a preliminary study. Clin Orthop Relat Res, 2008, 466: 1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akeson WH, Woo SL, Amiel D, et al. : The connective tissue response to immobility: biochemical changes in periarticular connective tissue of the immobilized rabbit knee. Clin Orthop Relat Res, 1973, (93): 356–362. [DOI] [PubMed] [Google Scholar]
- 17.Asahi S, Yamamoto K, Masaoka T, et al. : Effect of low-intensity ultrasound stimulation on the repair process after Achilles tendon injury. J Tokyo Med Univ, 2005, 63: 144–153(in Japanese). [Google Scholar]
- 18.Iwasaki T, Masaoka T, Asahi S, et al. : Treatment effect of low-intensity ultrasound stimulation on Achilles tendon injury. J Tokyo Med Univ, 2007, 65: 178–186(in Japanese). [Google Scholar]
- 19.Kosaka T, Masaoka T, Yamamoto K: Possible molecular mechanism of promotion of repair of acute Achilles tendon rupture by low intensity-pulsed ultrasound treatment in a rat model. West Indian Med J, 2011, 60: 263–268. [PubMed] [Google Scholar]
- 20.Takakura Y, Matsui N, Yoshiya S, et al. : Low-intensity pulsed ultrasound enhances early healing of medial collateral ligament injuries in rats. J Ultrasound Med, 2002, 21: 283–288. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow KJ, Finucane SD, Owen JR, et al. : The effects of low-intensity ultrasound on medial collateral ligament healing in the rabbit model. Am J Sports Med, 2005, 33: 1048–1056. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Schmelz A, Seufferlein T, et al. : Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem, 2004, 279: 54463–54469. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Fujihara S, Yamamoto-Nagata K, et al. : Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng, 2011, 39: 2964–2971. [DOI] [PubMed] [Google Scholar]
- 24.Sato M, Nagata K, Kuroda S, et al. : Low-intensity pulsed ultrasound activates integrin-mediated mechanotransduction pathway in synovial cells. Ann Biomed Eng, 2014, 42: 2156–2163. [DOI] [PubMed] [Google Scholar]
- 25.Skutek M, van Griensven M, Zeichen J, et al. : Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc, 2003, 11: 122–129. [DOI] [PubMed] [Google Scholar]