Abstract
[Purpose] Handgrip strength is a surrogate indicator for assessing disease-related and age-related skeletal muscle loss. Clinical utility as such a surrogate can be at least partially explained by the close relationship between handgrip strength and whole-body skeletal muscle mass. The handgrip strength is related to hand muscle size. Thus, the present study examined whether hand muscle thickness is associated with whole-body skeletal muscle mass. [Subjects and Methods] Thirty healthy male adults participated in this study. All subjects were right-hand dominant. Two muscle thicknesses (lumbrical and interosseous muscles) in the right hand were measured using ultrasonography. Whole-body and appendicular skeletal muscle masses were assessed using dual-energy X-ray absorptiometry. [Results] Although lumbrical muscle thickness was not correlated with whole-body skeletal muscle mass, there was a significant correlation with appendicular skeletal muscle mass. Furthermore, interosseous muscle thickness was significantly correlated with both whole-body and appendicular skeletal muscle masses. [Conclusion] The present findings suggest that two muscle thicknesses in the hand are related to whole-body and/or appendicular skeletal muscle mass in healthy adults. Therefore, we propose that despite being smaller than other limb muscles, hand muscle thickness may be useful as surrogate indicator for assessing disease-related and age-related skeletal muscle loss.
Keywords: Handgrip strength, Forearm muscle thickness, Ultrasonography
INTRODUCTION
Handgrip strength is known to be a surrogate indicator for assessing the risk of various chronic diseases1, 2). Moreover, the handgrip strength can be valuable in predicting the prognosis of several chronic diseases1,2,3), particularly cardiovascular diseases2), because it can potentially reflect cachexia, which is disease-related skeletal muscle loss4). In addition, handgrip strength is among the criteria for assessing sarcopenia5), which is age-related skeletal muscle loss. Thus, handgrip strength can be clinically useful for evaluating whole-body skeletal muscle mass (SMM) in several populations6, 7), including older individuals and patients with chronic diseases.
The magnitude of handgrip strength is largely determined by the sizes of the intrinsic (i.e., hand muscles) and extrinsic (i.e., forearm muscles) muscles8,9,10,11). A series by Abe et al.8,9,10,11) demonstrated that handgrip strength is related to forearm muscle size, which was assessed by muscle thickness (MT) measured using ultrasonography. Based on this relationship, they further determined that the forearm MTs, which measured from two parts (i.e., forearm radius and ulna MTs), correlated with whole-body SMM in older individuals9, 11), indicating that forearm MT measurement may be useful in evaluating whole-body SMM. However, Abe et al.11) reported that although forearm MTs correlated with whole-body SMM in older men, this relationship did not apply to older women. Thus, the forearm MT measurement alone may be insufficient for evaluating whole-body SMM. In a recent study, Abe et al.8) determined that hand MTs, which measured from lumbrical and interosseous muscles, correlated with handgrip strength in young individuals. Thus, it is hypothesized that hand MTs may be also useful in evaluating whole-body SMM; however, this relationship remains unknown. By clarifying this relationship in the present study, it may be possible to establish a new surrogate indicator for diagnosing cachexia and sarcopenia.
Appendicular SMM, which is defined as the sum of lean soft tissue mass of the arms and the legs, is widely used for identifying sarcopenia12). To compensable for the difference in body size among subjects, a height-adjusted whole-body or appendicular SMM index (SMMI) is also frequently used to assess sarcopenia13). With the potential for establish a new surrogate indicator for diagnosing cachexia and sarcopenia, the present pilot study examined whether hand MT is related to whole-body and appendicular SMM and SMMI in healthy adults.
SUBJECTS AND METHODS
Thirty healthy male adults (age, 22.1 ± 1.4 years; height, 172.1 ± 3.7 cm; weight, 67.1 ± 9.6 kg) participated in this study. Subjects were recreationally active, but did not include athletes of any specific sports, and had not habitually performed specific physical training. The subjects had no history of orthopedic injuries or previous surgery of the forearm and hand, and were free of any known neurologic, cardiovascular, or pulmonary disorders. All subjects were informed of the experimental procedures and potential risks and provided written consent to participate in the study. All procedures were approved by the Ethics Committee of Ritsumeikan University (BKC-IRB-2015-018).
All subjects were right-hand dominant. Handgrip strength of the right hand was measured using a handheld dynamometer (GRIP-D, Takei Scientific Instruments Co., Niigata, Japan). Subjects were instructed to maintain an upright standing position with arms straight. The size of the dynamometer’s handle was set to that subjects felt comfortable while squeezing the grip. Subjects were allowed to perform a test trial prior to 2 maximal effort trials. Two maximal effort trials were performed for each 3 s with a 1-min rest period. If the difference between the two values was more than 5% of the highest value, additional trials were performed until this was collected. The highest value of the two, or more than two, was used for analysis.
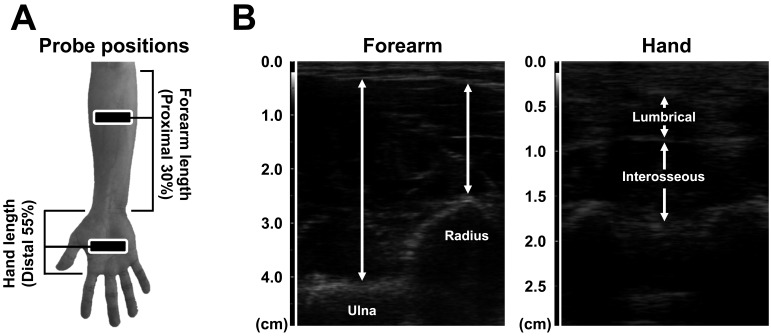
Representative ultrasonographic images of the forearm and hand MTs are showed in Fig. 1. The MTs of the right forearm and hand were measured using a B-mode ultrasonographic apparatus (SSD-3500SV; Aloka, Japan) with a linear scanner (scanning frequency; 7.5 MHz). The four MTs in the forearm (i.e., forearm radius and ulna MTs) and hand (i.e., lumbrical and interosseous MTs) were measured according to the method described in the previous studies8,9,10,11). In brief, for measuring two forearm MTs, subjects were instructed to stand quietly with the elbow extended and relaxed. Two forearm MTs were measured as the perpendicular distances between the subcutaneous adipose tissue–muscle interface and muscle–bone interface of the radius (i.e., forearm radius MT) and ulna (i.e., forearm ulna MT) at the proximal 30% of the forearm length. For measuring two hand MTs, subjects were instructed to be seated with the forearm and hand supinated. Two hand MTs were measured as the distances between the superficial and deep muscle fascia interfaces of the lumbrical and interosseous muscles on the middle and ring fingers at proximal 55% of the anterior hand length. All four MTs in the forearm and hand were each measured twice, and the mean value of two measurements was used for analysis. To assess the variability and repeatability of measurements of the forearm and hand MTs obtained in the present study, the coefficient of variation and intraclass correlation coefficient were calculated. The coefficient of variation of the two measurements of all four MTs in the forearm and hand were 0.9 ± 0.6% for forearm radius MT, 0.5 ± 0.3% for forearm ulna MT, 1.6 ± 1.5% for lumbrical MT, and 1.1 ± 0.8% for interosseous MT, respectively. The intraclass correlation coefficient of the two measurements was 0.996 for forearm radius MT, 0.998 for forearm ulna MT, 0.991 for lumbrical MT, and 0.990 for interosseous MT, respectively. Additionally, we measured the intraclass correlation coefficient of all four MTs in the forearm and hand on two separate days in 11 healthy men (age, 23.2 ± 1.3 years; height, 171.4 ± 4.1 cm, weight: 68.4 ± 8.9 kg). The intraclass correlation coefficients of the measurements performed on two separate days was 0.978 for forearm radius MT, 0.981 for forearm ulna MT, 0.985 for lumbrical MT, and 0.977 for interosseous MT, respectively. The intraclass correlation coefficient for >0.9 is classified as excellent14). Thus, the measurements of the forearm and hand MTs obtained in the present study can be considered as reliable.
Fig. 1.
Probe positions and representative ultrasonographic images of the forearm and hand muscle thicknesses
Panel A shows probe position for measurements of the forearm and hand muscle thicknesses. Forearm length was defined as the distance between the styloid process and the head of the radius. Hand length was defined as the distance between the palmar digital crease and flexion crease of the wrist.
Panel B shows representative ultrasonographic images of the forearm and hand muscle thicknesses. Forearm muscle thicknesses were measured as the perpendicular distances between the subcutaneous adipose tissue–muscle interface and muscle–bone interface of the radius (i.e., forearm radius muscle thickness) and ulna (i.e., forearm ulna muscle thickness) at the proximal 30% of the forearm length. Hand muscle thicknesses were measured as the distances between the superficial and deep muscle fascia interfaces of the lumbrical and interosseous muscles on the middle and ring fingers at proximal 55% of the anterior hand length.
Whole-body and appendicular SMM and SMMI were assessed using dual-energy X-ray absorptiometry (Lunar Prodigy; GE Healthcare, Tokyo, Japan). Subjects lay in a supine position on examination table with their limbs close to the body. Whole-body scans were performed in accordance with the manufacturer’s protocol. Whole-body SMM was divided as the sum of lean soft tissue mass of the whole-body, including the arms, legs, trunk, and head. Appendicular SMM was divided as the sum of lean soft tissue mass of the arms and legs. Whole-body and appendicular SMMI were calculated by using a body height to two square power13).
RESULTS
Mean values of body composition, handgrip strength, and forearm and hand MTs in subjects are listed in Table 1. Handgrip strength was significantly correlated with forearm radius and ulna MTs (r=0.705 and 0.661, respectively). Moreover, handgrip strength was significantly correlated with lumbrical and interosseous MTs (r=0.485 and 0.567, respectively).
Table 1. Mean values of body composition, handgrip strength, and forearm and hand muscle thicknesses in subjects.
| Mean ± SD | Range | ||
|---|---|---|---|
| Body composition | |||
| Whole body skeletal muscle mass (kg) | 53.9 ± 5.3 | 44.5−66.3 | |
| Whole body skeletal muscle mass index (kg/m2) | 18.2 ± 1.5 | 15.6−22.4 | |
| Appendicular skeletal muscle mass (kg) | 25.1 ± 3.2 | 20.5−33.6 | |
| Appendicular skeletal muscle mass index (kg/m2) | 8.5 ± 3.2 | 20.5−33.6 | |
| Handgrip strength (kg) | 41.4 ± 7.9 | 29.4−62.9 | |
| Muscle thickness | |||
| Forearm radius muscle thickness (cm) | 2.23 ± 0.29 | 1.67−2.83 | |
| Forearm ulna muscle thickness (cm) | 4.16 ± 0.40 | 3.31−5.04 | |
| Lumbrical muscle thickness (cm) | 0.43 ± 0.08 | 0.30−0.66 | |
| Interosseous muscle thickness (cm) | 0.96 ± 0.09 | 0.78−1.14 | |
Correlation coefficients between body composition and hand grip strength and forearm and hand MTs are shown in Table 2. Handgrip strength was significantly correlated with whole-body and appendicular SMM or SMMI (r=0.479–0.786). Similarly, forearm radius and ulna MTs were significantly correlated with whole-body and appendicular SMM or SMMI (r=0.448–0.565). Moreover, while a trend against such significant correlation was observed between lumbrical MT and whole-body SMM (r=0.362), it did not correlate with SMMI (r=0.305). However, lumbrical MT was significantly correlated with appendicular SMM and SMMI (r=0.434 and 0.403, respectively). Furthermore, interosseous MT was significantly correlated with whole-body and appendicular SMM or SMMI (r=0.410–0.483).
Table 2. Correlation coefficients among body composition and handgrip strength and forearm and hand muscle thicknesses.
| Whole body SMM | Whole body SMMI | Appendicular SMM | Appendicular SMMI | |
|---|---|---|---|---|
| Handgrip strength | 0.479** | 0.479** | 0.702*** | 0.786*** |
| Forearm radius muscle thickness | 0.466** | 0.448** | 0.553*** | 0.565*** |
| Forearm ulna muscle thickness | 0.542** | 0.503** | 0.662*** | 0.657*** |
| Lumbrical muscle thickness | 0.362† | 0.305 | 0.434* | 0.403* |
| Interosseous thickness | 0.410* | 0.417* | 0.457* | 0.483** |
***p<0.001, **p<0.010, *p<0.050, †p=0.050
DISCUSSION
The present findings demonstrated that handgrip strength and forearm and hand MTs were correlated with whole-body and appendicular SMM or SMMI. In large clinical cross-sectional studies, it has been shown that handgrip strength is related to whole-body and appendicular SMM or SMMI in middle-aged and older individuals6, 7). Moreover, a series by Abe et al.9, 11) determined that forearm MTs are related to whole-body and appendicular SMM or SMMI in middle-aged and older individuals. In the present study, we observed that handgrip strength and forearm MTs correlated with whole-body and appendicular SMM or SMMI in young individuals. However, to the best of our knowledge, the relationships between hand MTs and whole-body and appendicular SMM or SMMI had not determined prior to the present study. Thus, the primary findings in the present study were that hand MTs, particularly of the interosseous MT, are related to whole-body and appendicular SMM or SMMI.
The present findings showed that the correlations between handgrip strength and whole-body and appendicular SMM or SMMI in young men ranged from moderate to strong (r=0.48–0.79). In a large clinical cross-sectional study, Arden and Spector6) reported that the correlation between handgrip strength and whole-body SMM in middle-aged and older women was lower (r=0.33) than that in the present study (r=0.48). Similarly, Rolland et al.7) reported that the correlation between handgrip strength and appendicular SMM in older women was lower (r=0.24) than in the present study (r=0.70). Thus, compared with young individuals, the magnitude of handgrip strength in middle-aged and older individuals may be more affected by physiological and neurological factors (e.g., muscle recruitment and neural activation) than by morphological factors.
Abe et al.11) reported that the correlations between forearm MTs and whole-body SMM and SMMI in older men ranged from moderate to strong (r=0.49−0.56), which are similar to those of the present findings (r=0.45–0.54). Based on these findings, it indicates that forearm MTs may be useful in evaluating whole-body and appendicular SMM or SMMI. However, Abe et al.11) reported that relationships between forearm MTs and whole-body SMM could be not obtained in older women, which had low correlations (r=0.28–0.38). Thus, forearm MT measurement alone may be insufficient for evaluating whole-body and appendicular SMM or SMMI. In the present study, we found moderate correlations between hand MTs and whole-body and appendicular SMM and SMMI (r=0.31−0.48); however, these correlations were less than those of handgrip strength (r=0.48–0.79) and forearm MTs (r=0.45−0.66). Therefore, to understand the potential clinical utility of hand MT measurement, further studies are needed to examine the relationship between hand MT and whole-body and appendicular SMM or SMMI in older people and patients with chronic diseases.
The present study measured MTs of the forearm and hand using ultrasonography. Previous studies have determined that MTs of the trunk and limbs measured using ultrasonography correlated with the muscle cross-sectional area or volume measured using magnetic resonance imaging15, 16). Ultrasonography can be utilized more easily than magnetic resonance imaging in the clinical setting because it is non-invasive, higher portability, lower cost, and provides faster feedback. Thus, ultrasonography-measured MT is known to be a useful parameter for evaluating muscle size. In addition, previous studies have reported that despite handgrip exercise was performed by a low-intensity (e.g., 30% of maximum voluntary contraction), blood pressure was significantly raised at 30 sec after the onset of this type of exercise17, 18). Although handgrip strength measurement is performed in a relatively shorter time (i.e., 3–5 sec), the blood pressure during this measurement may be remarkably raised because maximal voluntary force output is required. Moreover, because older people and patients with chronic disease have mostly hypertension19), the increased blood pressure during handgrip in these individuals may be naturally higher than that in younger individuals20). Therefore, handgrip strength measurement may be associated with a slightly increased risk of cardiovascular events in older people and patients with chronic disease. Furthermore, because maximal voluntary force output is often affected by physiological and neuromuscular factors, the magnitude of handgrip strength is determined based on several factors in addition to morphological factors10).
In conclusion, the present study demonstrated that hand MT, in addition to handgrips strength and forearm MT, is related to whole-body and appendicular SMM and SMMI in healthy adults. Therefore, we suggest that despite being smaller than other limb muscles, hand MT may be useful as a possible surrogate indicator for assessing sarcopenia and cachexia.
REFERENCES
- 1.Al Snih S, Markides KS, Ray L, et al. : Handgrip strength and mortality in older Mexican Americans. J Am Geriatr Soc, 2002, 50: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 2.Leong DP, Teo KK, Rangarajan S, et al. Prospective Urban Rural Epidemiology (PURE) Study investigators: Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet, 2015, 386: 266–273. [DOI] [PubMed] [Google Scholar]
- 3.Rantanen T, Volpato S, Ferrucci L, et al. : Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc, 2003, 51: 636–641. [DOI] [PubMed] [Google Scholar]
- 4.Evans WJ, Morley JE, Argilés J, et al. : Cachexia: a new definition. Clin Nutr, 2008, 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. European Working Group on Sarcopenia in Older People: Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing, 2010, 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arden NK, Spector TD: Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res, 1997, 12: 2076–2081. [DOI] [PubMed] [Google Scholar]
- 7.Rolland Y, Lauwers-Cances V, Cournot M, et al. : Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc, 2003, 51: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 8.Abe T, Counts BR, Barnett BE, et al. : Associations between handgrip strength and ultrasound-measured muscle thickness of the hand and forearm in young men and women. Ultrasound Med Biol, 2015, 41: 2125–2130. [DOI] [PubMed] [Google Scholar]
- 9.Abe T, Fujita E, Thiebaud RS, et al. : Ultrasound-derived forearm muscle thickness is a powerful predictor for estimating DXA-derived appendicular lean mass in Japanese older adults. Ultrasound Med Biol, 2016, 42: 2341–2344. [DOI] [PubMed] [Google Scholar]
- 10.Abe T, Thiebaud RS, Loenneke JP: Age-related change in handgrip strength in men and women: is muscle quality a contributing factor? Age (Dordr), 2016, 38: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe T, Thiebaud RS, Loenneke JP, et al. : Association between forearm muscle thickness and age-related loss of skeletal muscle mass, handgrip and knee extension strength and walking performance in old men and women: a pilot study. Ultrasound Med Biol, 2014, 40: 2069–2075. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Wang Z, Heymsfield SB, et al. : Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr, 2002, 76: 378–383. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner RN, Koehler KM, Gallagher D, et al. : Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol, 1998, 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 14.Vincent WJ: Statistics in kinesiology. Northbridge: Human Kinetics, 1999. [Google Scholar]
- 15.Miyatani M, Kanehisa H, Ito M, et al. : The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur J Appl Physiol, 2004, 91: 264–272. [DOI] [PubMed] [Google Scholar]
- 16.Wachi M, Suga T, Higuchi T, et al. : Applicability of ultrasonography for evaluating trunk muscle size: a pilot study. J Phys Ther Sci, 2017, 29: 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito M, Mano T, Iwase S: Changes in muscle sympathetic nerve activity and calf blood flow during static handgrip exercise. Eur J Appl Physiol Occup Physiol, 1990, 60: 277–281. [DOI] [PubMed] [Google Scholar]
- 18.Vissing SF, Scherrer U, Victor RG: Stimulation of skin sympathetic nerve discharge by central command. Differential control of sympathetic outflow to skin and skeletal muscle during static exercise. Circ Res, 1991, 69: 228–238. [DOI] [PubMed] [Google Scholar]
- 19.Burt VL, Whelton P, Roccella EJ, et al. : Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension, 1995, 25: 305–313. [DOI] [PubMed] [Google Scholar]
- 20.Bakke EF, Hisdal J, Kroese AJ, et al. : Blood pressure response to isometric exercise in patients with peripheral atherosclerotic disease. Clin Physiol Funct Imaging, 2007, 27: 109–115. [DOI] [PubMed] [Google Scholar]