Abstract
Supervised high‐intensity interval training (HIIT) can rapidly improve cardiorespiratory fitness (CRF). However, the effectiveness of time‐efficient unsupervised home‐based interventions is unknown. Eighteen volunteers completed either: laboratory‐HIIT (L‐HIIT); home‐HIIT (H‐HIIT) or home‐isometric hand‐grip training (H‐IHGT). CRF improved significantly in L‐HIIT and H‐HIIT groups, with blood pressure improvements in the H‐IHGT group only. H‐HIIT offers a practical, time‐efficient exercise mode to improve CRF, away from the laboratory environment. H‐IHGT potentially provides a viable alternative to modify blood pressure in those unable to participate in whole‐body exercise.
Keywords: Blood Pressure, Cardiorespiratory, Exercise, HIIT, HIT
Introduction
The risk of developing cardiovascular (D'Agostino. et al. 2008) and metabolic (Veronica and Esther 2014) disease(s) increases with advancing age. However, aging is not the only risk factor for cardiovascular disease (CVD); sedentary middle‐aged adults have been identified as a specific high‐risk group, with inactive lifestyles associated with all‐cause mortality (Biddle et al. 2010). It therefore follows that exercise is the most well‐established nonpharmacological countermeasure to CVD risk (Myers 2003). Current guidelines state that adults should complete at least 150 min of moderate‐intensity aerobic physical activity throughout the week, or do at least 75 min of vigorous‐intensity aerobic physical per week (WHO 2015). However, less than 40% of men and 30% of women meet these guidelines (UK Department of Health 2011). Poor uptake and adherence to exercise is driven by a multitiude of factors, such as “lack of time,” aversion to exertion, and access to specialist equipment (Trost et al. 2002; Gillen and Gibala 2013). Moreover, these current physical activity guidelines do not consider the potential benefits of novel exercise modes, that is, short intense bouts of exercise, or static isometric training.
High intensity interval training (HIIT) (Trost et al. 2002; Gillen and Gibala 2013) is one such novel exercise mode (Kravitz 2011). Indeed, laboratory‐based (supervised) HIIT (L‐HIIT) has been shown to elicit improvements in cardiorespiratory fitness (CRF), over very short time‐periods (2–6 weeks) in athletes (Iaia et al. 2009), moderately trained (Helgerud et al. 2007; Little et al. 2010), sedentary (Trilk et al. 2011; Klonizakis et al. 2014) and patient groups (Gibala et al. 2012; Weston et al. 2014; Lanzi et al. 2015). These improvements were seen despite low exercise volume and minimal time commitments (Gillen et al. 2014).
Nonetheless, despite these findings supporting the benefits of L‐HIIT, the efficacy of home‐based unsupervised HIIT‐based strategies (H‐HIIT), which overcome the need for specialist equipment and personnel, is unknown. Previously most HIIT protocols have been studied in the laboratory setting, however, newer protocols requiring no specialist equipment have been investigated showing positive effects on CRF. Whole body aerobic resistance training (Mcrae et al. 2012) and more recently low volume intense stair climbing (Allison et al. 2017) has improved CRF in untrained females over a 4 week period while supervised but with no specialist equipment. Similarly, while home‐isometric handgrip training (H‐IHGT) is a promising, simple and rapid task that has been shown to lower resting blood pressure (RBP) within ~10 weeks (Millar et al. 2008; Garg et al. 2014), how it compares to HIIT‐based strategies in relation to modulating RBP is unknown. Herein, we aim to resolve this by comparing the effects of H‐HIIT to an already established efficacious supervised L‐HIIT protocol, on VO2 max and anaerobic threshold (AT). We also aim to compare the effects of H‐IHGT on RBP versus L‐HIIT and H‐HIIT.
Materials and Methods
Subjects
Eighteen middle‐aged (52 ± 5 year; 13:5 female:male) individuals (BMI 27.4 ± 3.9 kg/m2) not engaged in any formal exercise regime (<2 times per week) were recruited to the study and provided written informed consent (Table 1). Exclusion criteria were as per ATS/ACCP Guideline for CPET (American Thoracic & American College of Chest 2003). Inclusion criteria included no musculoskeletal limitations and availability for the whole study duration. Six subjects were randomly assigned to each intervention group (L‐HIIT, H‐HIIT or H‐IHGT) prior to baseline testing. The study was approved by the University of Nottingham Medical School ethics committee and complied with the Declaration of Helsinki.
Table 1.
Subject baseline characteristics
| L‐HIIT (n = 6) | H‐HIIT (n = 6) | H‐IHGT (n = 6) | |
|---|---|---|---|
| Age (years) | 51.5 (2.7) | 52.2 (2.0) | 51.5 (2.3) |
| BMI (kg/m2) | 27.5 (1.1) | 26.3 (2.0) | 28.3 (1.8) |
| VO2max (mL/kg/min) | 26.5 (2.6) | 27.8 (1.9) | 23.7 (2.4) |
| VO2AT (mL/kg/min) | 15.2 (1.1) | 13.93 (0.7) | 13.6 (1.5) |
| SBP (mmHg) | 127 (6.5) | 129 (5.4) | 138 (4.2) |
| DBP (mmHg) | 84 (0.9) | 81 (5.1) | 93 (2.7) |
Data depict mean (SD). There were no significant differences between the groups. Analysis via one‐way ANOVA.
Baseline and post‐training measures
All measurement equipment was calibrated and fully maintained throughout the study period. Subjects' height and weight was measured on arrival. Resting heart rate and noninvasive blood pressure was taken following 5 min seated rest with an automatic blood pressure monitor (A&D Medical, Saitama, Japan) prior to any exercise testing. All subjects then underwent cardiopulmonary exercise testing (CPET; Lode Corival, Lode, Groningen), with inline breath by breath data collected via a metabolic cart (nSpire Zan 600, Germany), using a modified Bruce ramp protocol as previously described (Boereboom et al. 2016). Tests were considered maximal if 3 or more of the following criteria were met: (1) plateau in the oxygen uptake curve (sustained flattening of VO2 curve despite rising VCO2); (2) a respiratory exchange ratio (RER) of >1.1; (3) HR over 85% age‐predicted maximum, and (4) a rating of perceived exertion (RPE); modified Borg scale (Borg 1982) ≥9 immediately following the test. CPET interpretation was performed by two independent experienced assessors blinded to time‐point (i.e., pre or post‐training) and group information. VO2 max values were taken as the highest reading in the last 30 sec of the test. AT was determined using a modified V‐slope and ventilatory equivalents method (Boereboom et al. 2016). All baseline measures were repeated >3 but <7 d after the last training session.
Training regimes
Volunteers performed their respective regime 3 times each week for 4 weeks. Compliance was monitored via a self‐report training diary (H‐HIIT, H‐IHGT) or attendance (L‐HIIT), and was 100% for each intervention.
L‐HIIT comprised a 2 min unloaded warm‐up, followed by 5 × 1 min exertions at 95–110% of the maximal load (watts (W)) achieved during subjects' baseline CPET (determined by an initial assessment session (Boereboom et al. 2016)), interspersed with 90 seconds unloaded cycling. A 2 min unloaded recovery completed each session. All participants underwent a 10% intensity increase at the mid‐way point of training (after session 6). Participants were given verbal encouragement throughout each session to ensure a rate of cadence sufficient to evoke a HR response greater than 85% predicted maximum (i.e., 220 – age (y)).
H‐HIIT comprised a 2 min jogging warm‐up, followed by 5 × 1 min exertions of three different equipment‐free exercises (star‐jumps, squat thrusts, and static sprints). To try and ensure that exercise intensity remained constant throughout each session, subjects were instructed to complete the maximum number of repetitions possible with good form during each exertion, and to match the number of repetitions achieved during exertions 1 (star‐jumps) and 2 (squat thrusts) during exertions 4 and 5 when these exercises were repeated. Each exertion was interspersed with 90 sec walking, with 2 min light static jogging completing each session.
H‐IHGT comprised 4 × 2 min isometric hand‐grip holds with their dominant hand at 30% of maximal voluntary contraction (MVC), interspersed with 2 min rest periods (Camry EH101 Electronic Hand dynamometer, USA). MVC was recorded as best of three maximal contractions on the dominant arm while stood in the anatomical position (Takei 5401 Grip strength dynamometer, Japan).
Statistical analysis
Descriptive data are presented as means ± standard deviation. ANCOVA was used to compare postintervention efficacy between groups with preintervention scores as a covariate. Results are presented with Bonferroni adjusted p values. We also tested for the assumption of homogeneity of regression slopes by testing the interaction of the independent variable with the covariate. Paired t‐tests were used for within group analyses. Pearson's correlation was used to test the association between change in blood pressure and baseline values. Statistical significance was set at P < 0.05. All analyses were conducted on STATA Version 14.2, SPSS Version 22, and Graphpad Prism Version 6.
Results
There were no adverse events during the study and all subjects completed all testing and training sessions. All subjects fulfilled our VO2 max criteria as outlined above. There were no significant differences in body weight (kg) in any group after the training period.
There was a significant mean improvement in CRF in both L‐HIIT (AT: 15.28 ± 2.73 to 18.23 ± 2.54 mL/kg/min, P < 0.01; VO2max: 26.50 ± 6.31 to 31.00 ± 6.69 mL/kg/min, P < 0.001) and H‐HIIT (AT: 13.93 ± 1.82 to 15.35 ± 2.27 mL/kg/min, P < 0.05; VO2 max: 27.77 ± 4.75 vs. 29.98 ± 6.094 mL/kg/min, P = <0.05), with no significant effect of H‐IHGT (AT: 13.55 ± 3.61 to 13.63 ± 3.25 mL/kg/min, P = 0.88; VO2 max: 23.65 ± 5.98 to 24.60 ± 4.80 mL/kg/min, P = 0.39 (Figs. 1 & 2)). L‐HIIT elicited significantly greater improvements in AT and VO2 max (both P < 0.05) when compared with H‐IHGT. There were no other significant differences between the groups. The assumption of homogeneity of regression slopes was not violated (P > 0.05 for interaction).
Figure 1.
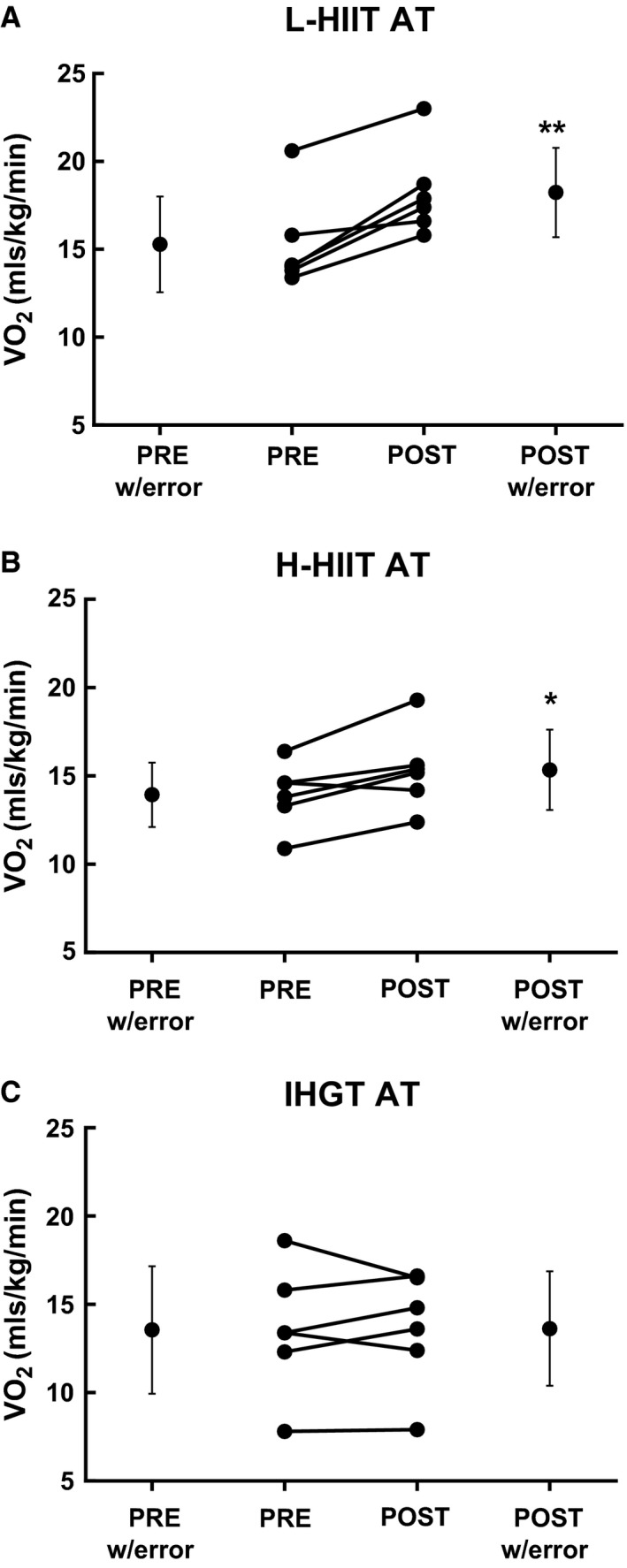
Anaerobic threshold (AT) before (PRE) and after (POST) 4 weeks laboratory‐based high intensity interval training (L‐HIIT; A), home‐based HIIT (H‐HIIT; B) or isometric hand‐grip training (H‐IHGT; C). Graphs depict mean±SD and individual changes. Analysis via paired Students t‐test. *=P < 0.05, **= P < 0.01 versus PRE training.
Figure 2.
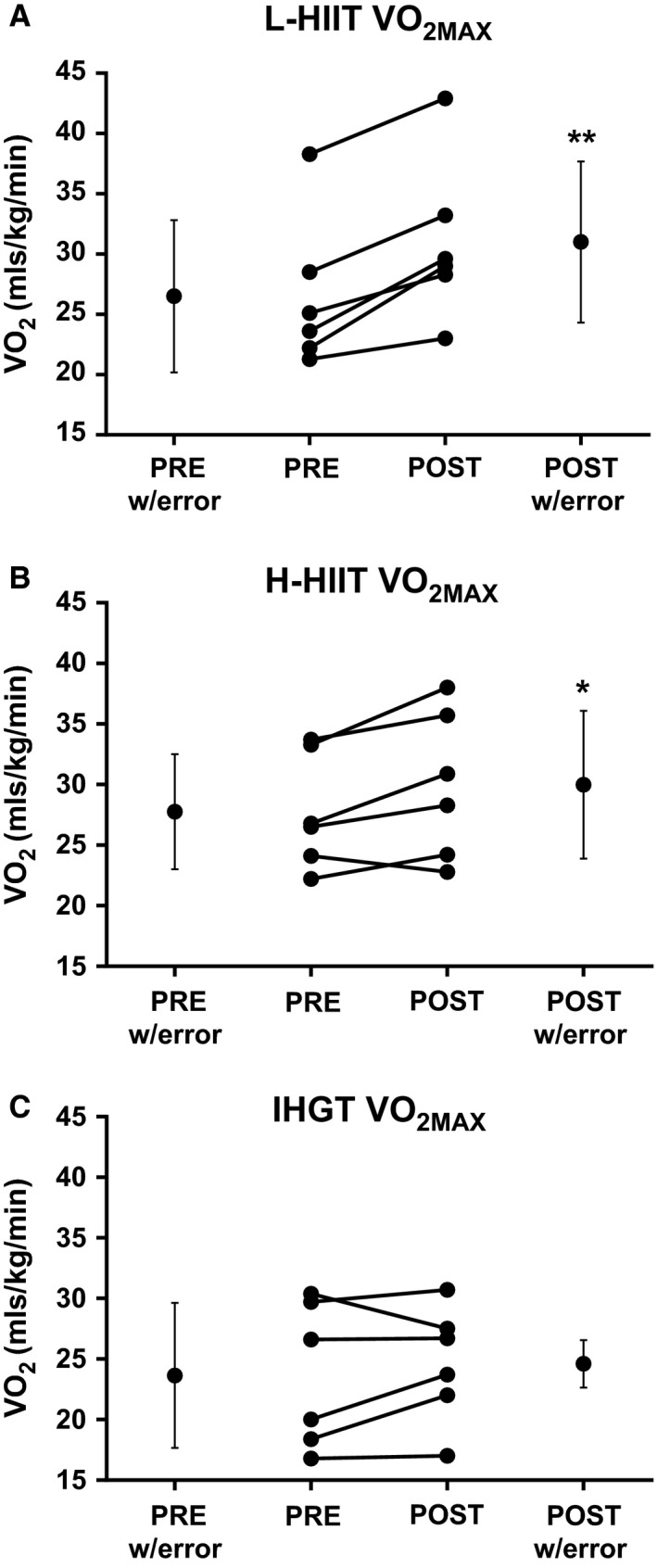
VO 2max before (PRE) and after (POST) 4 weeks laboratory‐based high intensity interval training (L‐HIIT; A), home‐based HIIT (H‐HIIT; B) or isometric hand‐grip training (H‐IHGT; C). Graphs depict mean±SD and individual changes. Analysis via paired Students t‐test. *= P < 0.05, **= P < 0.01 versus PRE training.
There were no significant differences between the groups' baseline systolic (SBP) or diastolic (DBP) blood pressures. When grouping all subjects together, there was a significant negative correlation between baseline systolic and diastolic blood pressures and change in these values after training (r = −0.72; P < 0.05 and r = −0.64; P < 0.05, respectively). SBP (139 ± 4 to 123 ± 3 mmHg, P < 0.01) and DBP (93 ± 3 to 82 ± 3 mmHg, P < 0.05) decreased significantly in the H‐IHGT group only, with no significant changes in the L‐HIIT or H‐HIIT groups (Figs. 3 & 4).
Figure 3.
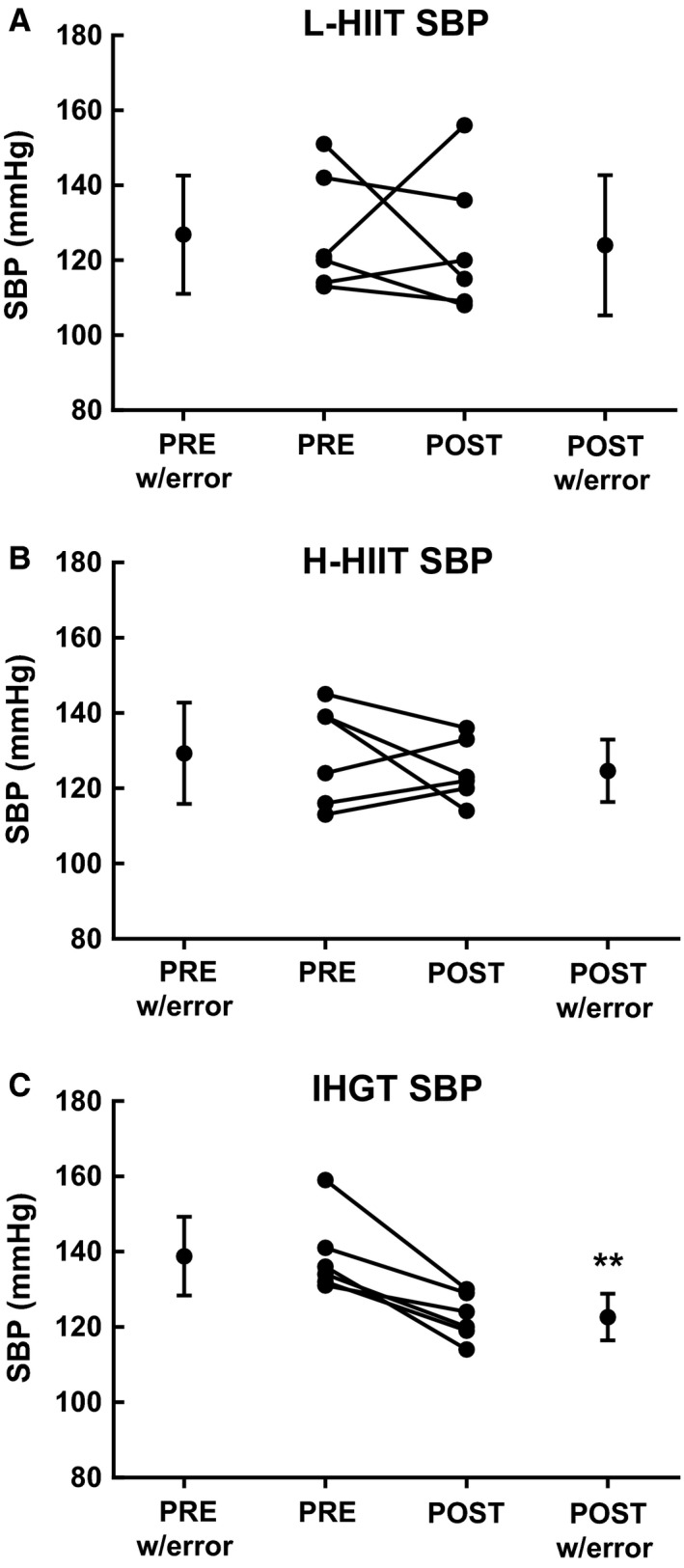
Systolic blood pressure (SBP) before (PRE) and after (POST) 4 weeks laboratory‐based high intensity interval training (L‐HIIT; A), home‐based HIIT (H‐HIIT; B) or isometric hand‐grip training (H‐IHGT; C). Graphs depict mean±SD and individual changes. Analysis via paired Students t‐test. **= P < 0.01 versus PRE training.
Figure 4.
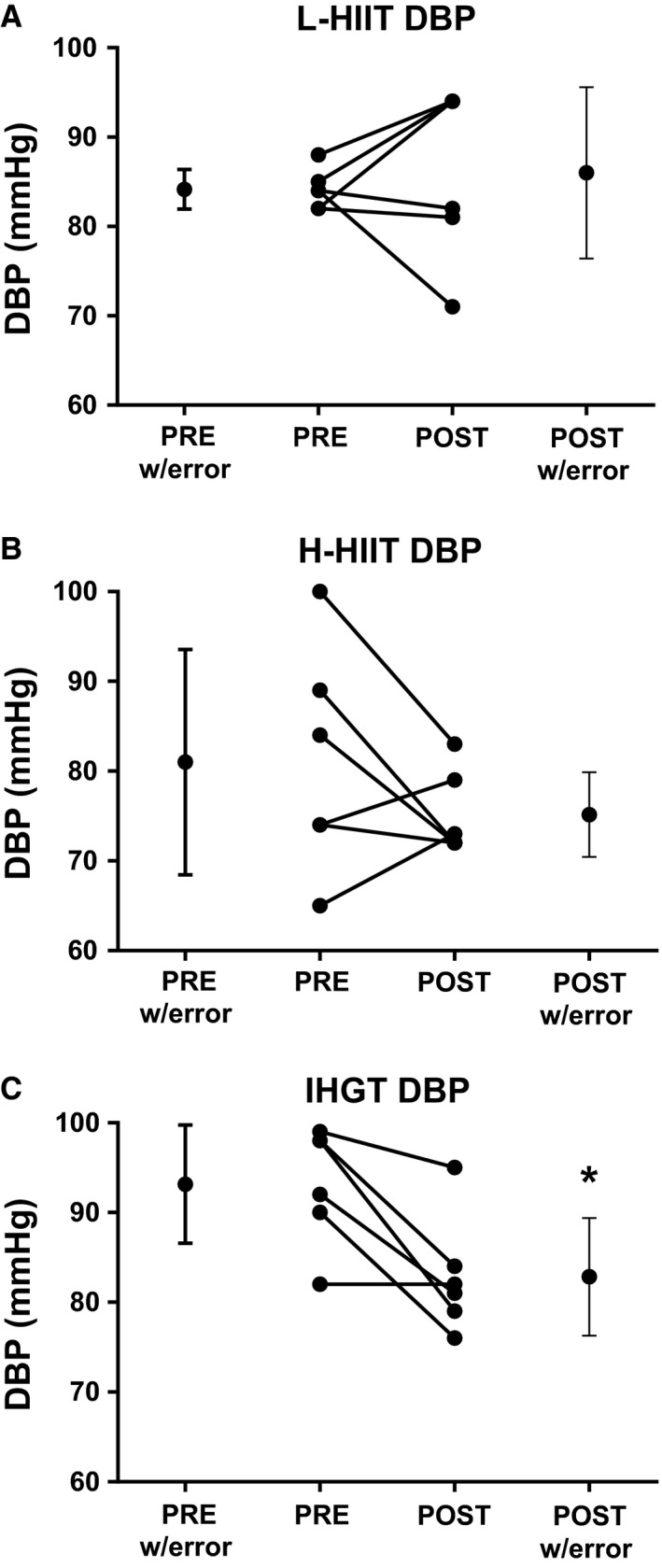
Diastolic blood pressure (DBP) before (PRE) and after (POST) 4 weeks laboratory‐based high intensity interval training (L‐HIIT; A), home‐based HIIT (H‐HIIT; B) or isometric hand‐grip training (H‐IHGT; C). Graphs depict mean±SD and individual changes. Analysis via paired Students t‐test. *= P < 0.05 versus PRE training.
Discussion
For the first time, both supervised L‐HIIT and unsupervised H‐HIIT have been shown to improve CRF in just 4 weeks using an identical work‐to‐rest ratio. H‐IHGT did not confer benefit in CRF, but did elicit a beneficial effect on SBP in this short 4‐week time frame.
As previously, and consistently shown (Little et al. 2010; Lanzi et al. 2015; Boereboom et al. 2016), L‐HIIT elicited improvements in indices of CRF in just 12 sessions. However, despite this solid and expanding evidence base the mechanistic basis of HIIT‐induced improvements in CRF are not fully elucidated. Increased skeletal muscle mitochondrial capacity (Little et al. 2010) and (central and peripheral) vascular adaptation (Wisløff et al. 2009) have both been postulated to account for improvements in VO2 max in previous studies, while improvements in muscle buffering capacity (Gibala et al. 2006) and reduced submaximal exercise energy expenditure (Iaia et al. 2009) may account for improvements in AT. Thus, L‐HIIT may represent a time‐efficient method to engage sedentary middle‐aged individuals, identified as at high risk for CVD (Biddle et al. 2010), in a regular physical activity regime with the aim of enhancing aerobic fitness and reducing BP. However, time‐efficacy only combats one of the cited reasons for poor exercise adherence. Indeed, the need for specialist equipment (cycle ergometers) and supervision are notable limitations for this method of training, demanding significant time, and financial commitments.
Interestingly this study demonstrates that unsupervised H‐HIIT, without the need for specialist equipment, can also improve CRF in middle‐aged sedentary individuals in just 4 weeks. With an identical time commitment to L‐HIIT, H‐HIIT induced significant gains in both VO2 max and AT, with no significant difference between the improvements made by these groups. Additionally, H‐HIIT can be easily adapted to account for injury and/or pathologies commonly occurring in middle‐age (e.g., osteoarthritis, urinary stress incontinence), potentially further improving adherence.
To the best of our knowledge the impact of H‐IHGT upon VO2 max and other indices of CRF was unknown, here we show no effects in middle‐aged sedentary adults. Perhaps, as would be predicted, in recruiting a significantly smaller muscle mass than both forms of HIIT and offering no significant cardiorespiratory challenge, H‐IHGT did not provide sufficient stimulus to promote improvements in CRF. Nonetheless, H‐IHGT was able to confer significant improvements in resting BP within this cohort. H‐IHGT may provide a viable alternative for those individuals who are unable to participate in dynamic exercise regimes who also have rising blood pressure not yet requiring medical management (accepted hypertension treatment threshold <140/90, (NICE 2016)); especially those with a tendency toward hypertension given the significant negative correlation between baseline BP and training‐induced change in BP observed in this study. Potential mechanisms for this improvement include reduced endothelial dysfunction due to increased nitric oxide bioavailability as well as decreased sympathetic nerve activity, both of which lead to reduced resting arterial pressure (Garg et al. 2014). With no recorded side effects, particularly versus pharmacological intervention, H‐IHGT is a very attractive option to reduce BP given the striking risk reduction in both coronary heart disease events (22%) and stroke (41%) with just 10 mmHg reduction in SBP or 5 mmHg reduction in DBP (Law et al. 2009).
In summary, advancing age, lack of time, climate, and perceived effort are all negatively associated with physical activity participation (Trost et al. 2002). All three of the interventions employed in this study potentially address these issues in that they are time‐efficient, suitable for all ages and can be performed indoors. Indeed, previous studies have also reported HIIT to be more enjoyable and less effortful than traditional endurance exercise for both healthy individuals (Bartlett et al. 2011) and patient groups (Kong et al. 2016). Ongoing debate exists as to the wider public health application of HIIT (Biddle and Batterham 2015), suggesting that, as in this study, low volume or reduced exertion HIIT (RE‐HIIT) may be a more practical and tolerable solution to promote extensive uptake of HIIT, versus the earlier Wingate style HIIT (Gillen and Gibala 2013).
Importantly, all three exercise interventions in this study required a total weekly time commitment of <45 mins. This is 30% less time than the current adult guidelines for vigorous activity and only one‐third of the time commitment recommended for moderate activity (WHO 2015). As a previously identified barrier to exercise, reduction in total time commitment, would likely lead to enhanced exercise adoption and adherence (Trost et al. 2002). Additionally, our findings suggest that the adaptations induced by H‐HIIT and H‐IHGT have potential, particularly as adjuvant home‐based strategies, to improve key aspects of CRF and BP.
We recognize limitations to this study design. The small sample size may increase type II errors, which may mask the potential of L‐HIIT to improve BP given that reductions in BP have previously been shown with L‐HIIT (Boereboom et al. 2016). Equally the improvements in BP noted in the H‐IHGT group may be reflective of regression to the mean and as such larger studies are required to remove this potential error. The intensity and compliance for the home‐based exercise interventions was monitored by self‐report, however, given the improvements in CRF in just 4‐weeks, volunteers in the H‐HIIT group were likely exercising at high‐intensity given the improvements seen despite low total workload, as seen previously (Iaia et al. 2009; Gibala et al. 2012; Gillen and Gibala 2013).
In conclusion, both L‐HIIT and H‐HIIT can safely elicit significant gains in CRF in sedentary middle‐aged individuals in just 4 weeks. Additionally, H‐IHGT can improve BP within the same timeframe with a similar low time commitment. Larger scale studies are required to fully assess the feasibility and effectiveness of these interventions, in healthy and clinical populations, while also exploring the mechanistic basis of adaptation.
Conflict of Interest
None declared.
Blackwell J., Atherton P. J., Smith K., Doleman B., Williams J. P., Lund J. N., Phillips B. E.. The efficacy of unsupervised home‐based exercise regimens in comparison to supervised lab‐based exercise training upon cardio‐respiratory health facets. Physiol Rep, 5 (17), 2017, e13390, https://doi.org/10.14814/phy2.13390
Funding Information
This work was supported by the Medical Research Council [grant number MR/K00414X] and Arthritis Research UK [grant number 19891] awarded to the MRC‐ARUK Centre for Musculoskeletal Ageing Research; and The Dunhill Medical Trust [grant number R468/0216]
References
- Allison, M. K ., Baglole J. H., Martin B. J., Macinnis M. J., Gurd B. J., and Gibala M. J.. 2017. Brief intense stair climbing improves cardiorespiratory fitness. Med. Sci. Sports Exerc. 49:298–307. [DOI] [PubMed] [Google Scholar]
- American Thoracic, S. and P. American College of Chest . 2003. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 167:211–277. [DOI] [PubMed] [Google Scholar]
- Bartlett, J. D. , G. L. Close,0 MacLaren D. P. M., Gregson W., Drust B., and Morton J. P.. 2011. High‐intensity interval running is perceived to be more enjoyable than moderate‐intensity continuous exercise: implications for exercise adherence. J. Sports Sci. 29:547–553. [DOI] [PubMed] [Google Scholar]
- Biddle, S. J. H. , and Batterham A. M.. 2015. High‐intensity interval exercise training for public health: a big HIT or shall we HIT it on the head?. Int. J. Behav. Nutr. Phys. Act. 12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle, S. , Cavill N., Ekelund U., Gorely T., Griffiths M. D., and Jago R.. 2010. Sedentary behaviour and obesity: review of the current scientific evidence.
- Boereboom, C. L ., Phillips B. E., Williams J. P., and J. N. Lund. 2016. A 31‐day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech. Coloproctol. 20:375–382. Available at: http://www.ncbi.nlm.nih.gov/pubmed/27015678. [DOI] [PubMed] [Google Scholar]
- Borg, G. A . 1982. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14:377–381. [PubMed] [Google Scholar]
- D'Agostino, Sr. R. B. , Vasan R. S., Pencina M. J., Wolf P. A., Cobain M., Massaro J. M., et al. 2008. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117:743–753. [DOI] [PubMed] [Google Scholar]
- Department of Health Physical Activity Health Improvement and Protection . 2011. Start Active, Stay Active. Report, p. 62.
- Garg, R ., Malhotra V., Kumar A., Dhar U., Y. Tripathi. 2014. Effect of isometric handgrip exercise training on resting blood pressure in normal healthy adults. J. Clin. Diagn. Res. 8:BC08–BC10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala, M. J ., Little J. P., van Essen M., Wilkin G. P., Burgomaster K. A., Safdar A., et al. 2006. Short‐term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 575(Pt 3):901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala, M. J ., Little J. P., Macdonald M. J., and J. A. Hawley. 2012. Physiological adaptations to low‐volume, high‐intensity interval training in health and disease. J. Physiol. 590(Pt 5):1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen, J. B. , and Gibala M. J.. 2013. Is high‐intensity interval training a time‐efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 39:409–412. [DOI] [PubMed] [Google Scholar]
- Gillen, J. B ., Percival M. E., Skelly L. E., Martin B. J., Tan R. B., Tarnopolsky M. A., et al. 2014. Three minutes of all‐out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS One 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgerud, J. , Hoydal K., Wang E., Karlsen T., Berg P., Bjerkaas M., et al. 2007. Aerobic high‐intensity intervals improve VO~ 2~ m~ a~ x more than moderate training. Med. Sci. Sports Exerc. 39:665. [DOI] [PubMed] [Google Scholar]
- Iaia, F. M. , Hellsten Y., Nielsen J. J., Fernstrom M., Sahlin K., and J. Bangsbo. 2009. Four weeks of speed endurance training reduces energy expenditure during exercise and maintains muscle oxidative capacity despite a reduction in training volume. J. Appl. Physiol. 1985:73–80. [DOI] [PubMed] [Google Scholar]
- Klonizakis, M. , Moss J., Gilbert S., Broom D., Foster J., and Tew G. A.. 2014. Low‐volume high‐intensity interval training rapidly improves cardiopulmonary function in postmenopausal women. Menopause 21:1099–1105. [DOI] [PubMed] [Google Scholar]
- Kong, Z. , Fan X., Sun S., Song L., Shi Q., and Nie J.. 2016. Comparison of High‐Intensity Interval Training and Moderate‐to‐Vigorous Continuous Training for Cardiometabolic Health and Exercise Enjoyment in Obese Young Women: a Randomized Controlled Trial. PLoS ONE 11:e0158589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz, L. , 2011. HigH‐intensity interval training. American College of Sports Medicine, pp.1–2. Available at: https://www.acsm.org/docs/brochures/high-intensity-interval-training.pdf.
- Lanzi, S. , Codecasa F., Cornacchia M., Maestrini S., Capodaglio P., Brunani A., et al. 2015. Short‐term HIIT and Fat max training increase aerobic and metabolic fitness in men with class II and III obesity. Obesity (Silver Spring) 23:1987–1994. [DOI] [PubMed] [Google Scholar]
- Law, M. , Morris J., and Wald N.. 2009. Use of blood pressure lowering drugs in the prevention of cardiovascular disease : meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, J. P. , Safdar A., Wilkin G. P., Tarnopolsky M. A., and Gibala M. J.. 2010. A practical model of low‐volume high‐intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J. Physiol. 588(Pt 6):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae, G. , Payne A., Zelt J. G., Scribbans T. D., Jung M. E., Little J. P., et al. 2012. Extremely low volume, whole‐body aerobic – resistance training improves aerobic fitness and muscular endurance in females. Appl. Physiol. Nutr. Metab 1131:1124–1131. [DOI] [PubMed] [Google Scholar]
- Millar, P. J. , Bray S. R., MacDonald M. J., and McCartney N.. 2008. The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. J. Cardiopulm. Rehabil. Prev. 28:203–207. [DOI] [PubMed] [Google Scholar]
- Myers, J. , 2003. Exercise and cardiovascular health. Circulation 107:e2–e5. [DOI] [PubMed] [Google Scholar]
- NICE , 2016. Hypertension in adults: diagnosis and management | Guidance and guidelines | NICE. Available at: https://www.nice.org.uk/guidance/CG127/chapter/1-Guidance#diagnosing-hypertension-2 [Accessed June 20, 2017].
- Trilk, J. L. , Singhal A., Bigelman K. A., and Cureton K. J.. 2011. Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur. J. Appl. Physiol. 111:1591–1597. [DOI] [PubMed] [Google Scholar]
- Trost, S. G. , Owen N., Bauman A. E., Sallis J. F., and Brown W.. 2002. Correlates of adults' participation in physical activity: review and update. Med. Sci. Sports Exerc.. 34:1996–2001. [DOI] [PubMed] [Google Scholar]
- Veronica, G. , and Esther R.‐R. M.. 2014. Aging, metabolic syndrome and the heart. Aging Dis. 3:269–279. [PMC free article] [PubMed] [Google Scholar]
- Weston, K. S. , Wisloff U., and Coombes J. S.. 2014. High‐intensity interval training in patients with lifestyle‐induced cardiometabolic disease: a systematic review and meta‐analysis. Br. J. Sports Med. 48:1227–1234. [DOI] [PubMed] [Google Scholar]
- WHO , 2015. World Health Organisation ‐ Physical Activity and Adults. Available at: http://www.who.int/dietphysicalactivity/factsheet_adults/en/ [Accessed May 3, 2017].
- Wisløff, U. , Ellingsen Ø., and Kemi O. J.. 2009. High‐intensity interval training to maximize cardiac benefits of exercise training? Exerc. Sport Sci. Rev. 37:139–146. [DOI] [PubMed] [Google Scholar]


