Summary
Background
Multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis are emerging worldwide. The Green Light Committee initiative supported programmatic management of drug-resistant tuberculosis in 90 countries. We used estimates from the Preserving Effective TB Treatment Study to predict MDR and XDR tuberculosis trends in four countries with a high burden of MDR tuberculosis: India, the Philippines, Russia, and South Africa.
Methods
We calibrated a compartmental model to data from drug resistance surveys and WHO tuberculosis reports to forecast estimates of incident MDR and XDR tuberculosis and the percentage of incident MDR and XDR tuberculosis caused by acquired drug resistance, assuming no fitness cost of resistance from 2000 to 2040 in India, the Philippines, Russia, and South Africa.
Findings
The model forecasted the percentage of MDR tuberculosis among incident cases of tuberculosis to increase, reaching 12·4% (95% prediction interval 9·4–16·2) in India, 8·9% (4·5–11·7) in the Philippines, 32·5% (27·0–35·8) in Russia, and 5·7% (3·0–7·6) in South Africa in 2040. It also predicted the percentage of XDR tuberculosis among incident MDR tuberculosis to increase, reaching 8·9% (95% prediction interval 5·1–12·9) in India, 9·0% (4·0–14·7) in the Philippines, 9·0% (4·8–14·2) in Russia, and 8·5% (2·5–14·7) in South Africa in 2040. Acquired drug resistance would cause less than 30% of incident MDR tuberculosis during 2000–40. Acquired drug resistance caused 80% of incident XDR tuberculosis in 2000, but this estimate would decrease to less than 50% by 2040.
Interpretation
MDR and XDR tuberculosis were forecast to increase in all four countries despite improvements in acquired drug resistance shown by the Green Light Committee-supported programmatic management of drug resistant tuberculosis. Additional control efforts beyond improving acquired drug-resistance rates are needed to stop the spread of MDR and XDR tuberculosis in countries with a high burden of MDR tuberculosis.
Funding
US Agency for International Development and US Centers for Disease Control and Prevention, Division of Tuberculosis Elimination.
Introduction
Tuberculosis is a leading cause of morbidity and mortality worldwide. In 2015, an estimated 10·4 million new cases of tuberculosis and 1·8 million deaths related to tuberculosis disease occurred globally.1 Intensive implementation of the Stop TB Strategy and its predecessor, directly observed treatment short-course (DOTS), led to the successful treatment of 56 million individuals with tuberculosis from 1995 to 2012, and prevented their premature deaths.2 Despite this progress however, tuberculosis that is resistant to first-line drugs is a growing problem worldwide. Multidrug-resistant (MDR) tuberculosis, resistant to at least isoniazid and rifampicin, accounted for 480 000 cases in 2015. Of additional concern, extensively drug-resistant (XDR) tuberculosis, defined as MDR tuberculosis with additional resistance to fluoroquinolones and second-line injectable drugs, has emerged globally.3 In 2015, 9·5% of MDR tuberculosis cases were estimated to be XDR tuberculosis.1 Patients with MDR or XDR tuberculosis, particularly those also living with HIV infection (PLHIVs), have worse outcomes than individuals with tuberculosis that is not MDR (ie, non-MDR tuberculosis).
Drug-resistant tuberculosis is primarily driven by acquired drug resistance (ADR) during treatment and transmission of drug-resistant tuberculosis from source cases to contacts. Drug resistance might develop spontaneously in a previously drug-susceptible strain before treatment and MDR tuberculosis might develop despite completion of first-line treatment.4 Findings from mathematical models5 have predicted that detection and treatment of tuberculosis could generate unexpectedly high proportions of MDR tuberculosis over time, and that the proportion of XDR tuberculosis cases among the MDR cases would rise rapidly if increases in detection and treatment of MDR tuberculosis were not accompanied with a simultaneous increase in cure rates.6
In 2000, the Stop TB Partnership and WHO formed the Green Light Committee (GLC) to increase access to high-quality medicines at greatly reduced prices and prevent further ADR by supporting countries implementing programmatic management of drug-resistant tuberculosis (PMDT).7 The Preserving Effective TB Treatment Study (PETTS) was launched in nine countries in 2005 to quantify the frequency of ADR to second-line drugs and treatment outcomes among patients with MDR tuberculosis, comparing GLC-approved and non-GLC programmes.8 Findings from PETTS showed that treatment of MDR tuberculosis was associated with a substantial risk of ADR to second-line drugs, but GLC-approved programmes had lower ADR risk and better treatment outcomes.9 However, whether this lower risk of ADR will assist in control of MDR or XDR tuberculosis in countries with GLC-approved programmes is unknown.
We therefore used a mathematical model to project the future burden of drug-resistant tuberculosis in four countries with a high MDR tuberculosis burden1 and different tuberculosis epidemiology, HIV prevalence, and GLC support: India, the Philippines, Russia, and South Africa. The Philippines and Russia had GLC-approved programmes, whereas the GLC programme in India did not expand beyond a pilot phase. South Africa did not participate in the GLC initiative. Data from PETTS on outcomes of treatment of MDR tuberculosis stratified by GLC support were used to estimate ADR to second-line tuberculosis medicines with the objectives of estimation of the proportion of MDR tuberculosis among incident cases of tuberculosis, estimation of the proportion of individuals with XDR tuberculosis among incident cases of MDR tuberculosis, estimation of the proportion of MDR or XDR tuberculosis attributable to ADR, and identification of which variables most affected the incidence of MDR or XDR tuberculosis.
Methods
Study design
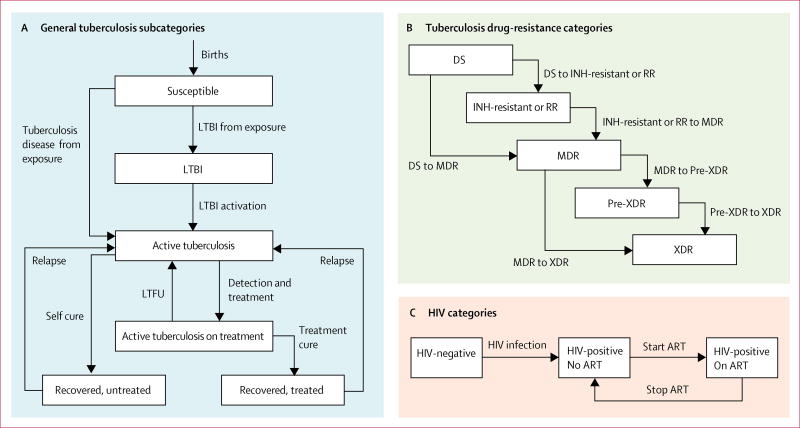
We developed a deterministic, population-level, compartmental model comprised of a system of ordinary differential equations based on characteristics of earlier models, and calibrated using a Bayesian approach to published reports of recent estimates of tuberculosis and MDR tuberculosis.10,11 The model incorporated six main tuberculosis states, subdivided by drug resistance and HIV status (figure 1). It accounted for transmission of both tuberculosis and HIV, progression of latent tuberculosis infection to active tuberculosis disease in HIV-infected and uninfected individuals, outcomes of treatment (or non-treatment) of both tuberculosis and HIV, and ADR during treatment. Population growth was incorporated to approximate UN country projections. Additional details of the model are in the appendix.
Figure 1. Model categories, subcategories, and transitions.
Re-infection and mortality events are not shown. DS=susceptible to isoniazid and rifampicin; INH=isoniazid. RR=rifampicin-resistant. MDR=multidrug-resistant. XDR=extensively drug-resistant. LTBI=latent tuberculosis infection. ART=antiretroviral therapy. LTFU=lost to follow-up.
The model simulated transfer between six main states of tuberculosis: (1) uninfected or susceptible; (2) latent tuberculosis infection; (3) active tuberculosis disease; (4) active tuberculosis disease that had been detected and patient was receiving the correct treatment on the basis of the underlying drug resistance (ie, non-MDR tuberculosis versus MDR tuberculosis); (5) tuberculosis disease that had been successfully treated; and (6) tuberculosis disease that spontaneously resolved without treatment (figure 1A). Individuals were entered into the model in a susceptible state, where they could be exposed to people with active tuberculosis disease. On exposure, susceptible individuals could acquire latent tuberculosis infection or proceed directly to tuberculosis disease. Individuals with tuberculosis disease might spontaneously resolve, die from untreated tuberculosis, or initiate treatment for active tuberculosis. Those who initiated treatment for tuberculosis disease might be cured, die during treatment, acquire drug resistance, or become lost to follow-up and stop treatment. Individuals for whom treatment failed and who did not acquire additional drug resistance were assumed to undergo retreatment. Individuals who recovered from tuberculosis might go on to relapse. Individuals with latent tuberculosis infection and those who recovered from tuberculosis disease might be infected during contact with persons with active tuberculosis and develop tuberculosis disease again. Individuals in states other than active tuberculosis were subject to a background mortality rate.
We also modelled five subdivisions of drug resistance: no resistance to isoniazid or rifampicin; resistance to either isoniazid or rifampicin; resistance to isoniazid and rifampicin (ie, MDR tuberculosis); resistance to isoniazid, rifampicin, and either fluoroquinolones or second-line injectable drugs (ie, pre-XDR tuberculosis); and resistance to isoniazid, rifampicin, fluoroquinolones, and second-line injectable drugs (ie, XDR tuberculosis; figure 1B). ADR could only occur during treatment for active tuberculosis disease. All tuberculosis transmission events were modelled to confer the drug resistance profile of the source case, and ADR was modelled to confer no cost to competitiveness.
A simplified HIV submodel accounted for HIV mortality, mortality due to tuberculosis disease among PLHIV, and treatment outcomes of tuberculosis–HIV co-infection (figure 1C). HIV transmission was modelled to occur among susceptible individuals, individuals with latent tuberculosis infection, and persons recovered from tuberculosis disease. PLHIV might initiate ART, and those on ART might stop treatment or die. PLHIV on ART were assumed to not contribute to HIV incidence. Tuberculosis states for PLHIV were similar to those for individuals without HIV infection, with the exception that active tuberculosis in PLHIV could not spontaneously resolve. PLHIV with tuberculosis were modelled to be able to transmit tuberculosis to individuals without HIV infection, and vice versa. HIV was modelled to emerge in different years for each country (appendix).
Roll-out of tuberculosis treatment and ART
Treatment for non-MDR tuberculosis and MDR tuberculosis was assumed to have become available in all modelled countries beginning in 1970 and 1990, respectively. Roll-out of non-MDR tuberculosis, MDR tuberculosis, and ART programmes was assumed to follow a sigmoidal growth curve with an inflection point of 10 years. Roll-out of DOTS and GLC-supported PMDT was assumed to follow a sigmoidal growth curve with an infection point of 5 years (appendix). ART expansion was modelled to achieve the UNAIDS target that 90% of PLHIV will receive ART by 2020.12 The proportion of individuals with incident tuberculosis who initiated treatment appropriate to the underlying drug resistance was calibrated to recently reported case-detection rates (appendix). The proportion of tuberculosis treated under DOTS-supported programmes was calibrated to fit incident MDR tuberculosis estimates from drug-resistance surveys (appendix).
Data from PETTS were used to construct proportions of outcomes based on drug resistance profiles (eg, for MDR tuberculosis, pre-XDR tuberculosis, and XDR tuberculosis) stratified by HIV status and GLC support. Values for additional variables and their 95% CIs were drawn from published scientific literature (table). For variables without CIs, we used Wilson’s method to calculate 95% CIs.28 Proportions were transformed into rates using the exponential assumption.
Table.
Description of main variables and corresponding ranges used for the mathematical model
| Range | Source | |
|---|---|---|
| Probability of acquiring active tuberculosis from index case, PLHIV contact | 0·088–0·441 | Fox et al, 201313 |
| Probability of acquiring LTBI from index case, PLHIV contact | 0·406–0·660 | Fox et al, 201313 |
| Probability of acquiring active tuberculosis from index case, HIV-negative contact | 0·022–0·044 | Fox et al, 201313 |
| Probability of acquiring LTBI from index case, HIV-negative contact | 0·471–0·558 | Fox et al, 201313 |
| Probability of reactivation of LTBI in 5 years, HIV-negative individuals | 0·012–0·047 | Sloot et al, 201414 |
| Probability of reactivation of LTBI in 1 year, PLHIV not on ART | 0·039–0·147 | Selwyn et al, 198915 |
| Proportion of HIV-infected individuals with tuberculosis who spontaneously recover | Imputed | Tiemersma et al, 201116 |
| Proportion of PLHIV with tuberculosis who spontaneously recover | 0 | Assumed |
| Probability of death without tuberculosis treatment in 10 years, HIV-negative individuals | 0·630–0·770 | Tiemersma et al, 201116 |
| Probability of death without tuberculosis treatment, PLHIV not on ART | 0·450–0·550 | Tiemersma et al, 201116 |
| Probability of relapse among individuals with spontaneously recovered tuberculosis in 1·5 years | 0·090–0·110 | National Tuberculosis Institute, 197417 |
| Probability of treatment success for DS* tuberculosis, HIV-negative individuals, DOTS† | 0·789–0·830 | Seung et al, 200418 |
| Probability of treatment success for INH-resistant or RR tuberculosis, HIV-negative individuals, DOTS | 0·625–0·748 | Seung et al, 200418 |
| Probability of treatment failure for DS tuberculosis, HIV-negative individuals, DOTS | 0·024–0·042 | Seung et al, 200418 |
| Probability of treatment failure for INH-resistant or RR tuberculosis, HIV-negative individuals, DOTS | 0·077–0·162 | Seung et al, 200418 |
| Probability of ADR from DS tuberculosis to MDR tuberculosis‡ among treatment failures | 0·221–0·491 | Seung et al, 200418 |
| Probability of ADR from DS tuberculosis to INH-resistant or RR tuberculosis among treatment failures | 0·146–0·394 | Seung et al, 200418 |
| Probability of ADR from INH-resistant or RR tuberculosis to MDR tuberculosis among treatment failures | 0·540–0·873 | Seung et al, 200418 |
| Probability of being lost to follow-up during treatment for DS tuberculosis and INH-resistant or RR tuberculosis | 0·035–0·099 | Seung et al, 200418 |
| Probability of death during treatment for DS tuberculosis and INH-resistant or RR tuberculosis | 0·044–0·113 | Seung et al, 200418 |
| Probability of treatment success for DS tuberculosis and INH-resistant or RR tuberculosis, PLHIV on ART, DOTS | 0·661–0·718 | El-Sadr et al, 200119 |
| Probability of treatment failure for DS tuberculosis and INH-resistant or RR tuberculosis, PLHIV on ART, DOTS | 0·018–0·046 | Khan et al, 201020 |
| Probability of being lost to follow-up during treatment for DS tuberculosis and INH-resistant or RR tuberculosis, PLHIV | 0·108–0·149 | El-Sadr et al, 200119 |
| Probability of death during treatment for DS tuberculosis and INH-resistant or RR tuberculosis, PLHIV on ART | 0·085–0·159 | Khan et al, 201020 |
| Probability of treatment success for MDR tuberculosis, non-GLC§ | 0·447–0·568 | PETTS |
| Probability of death during treatment for MDR tuberculosis, non-GLC | 0·079–0·156 | PETTS |
| Probability of ADR to pre-XDR tuberculosis¶ during treatment for MDR tuberculosis, non-GLC | 0·069–0·142 | PETTS |
| Probability of ADR to XDR tuberculosis‖ during treatment for MDR tuberculosis, non-GLC | 0·021–0·069 | PETTS |
| Probability of treatment success for pre-XDR tuberculosis, non-GLC | 0·129–0·270 | PETTS |
| Probability of death during treatment for pre-XDR tuberculosis, non-GLC | 0·114–0·251 | PETTS |
| Probability of ADR to XDR tuberculosis during treatment for pre-XDR tuberculosis, non-GLC | 0·280–0·453 | PETTS |
| Probability of treatment success for XDR tuberculosis, non-GLC | 0·044–0·161 | PETTS |
| Probability of death during treatment for XDR tuberculosis, non-GLC | 0·416–0·615 | PETTS |
| Probability of being lost to follow up during treatment for MDR, pre-XDR, or XDR tuberculosis, non-GLC | 0·118–0·182 | PETTS |
| Probability of treatment success for MDR tuberculosis, GLC | 0·502–0·612 | PETTS |
| Probability of death during treatment for MDR tuberculosis, GLC | 0·066–0·131 | PETTS |
| Probability of ADR to pre-XDR tuberculosis during treatment for MDR tuberculosis, GLC | 0·042–0·098 | PETTS |
| Probability of ADR to XDR tuberculosis during treatment for MDR tuberculosis, GLC | 0·005–0·033 | PETTS |
| Probability of treatment success for pre-XDR tuberculosis, GLC | 0·422–0·594 | PETTS |
| Probability of death during treatment for pre-XDR tuberculosis, GLC | 0·055–0·159 | PETTS |
| Probability of ADR to XDR tuberculosis during treatment for pre-XDR tuberculosis, GLC | 0·061–0·169 | PETTS |
| Probability of treatment success for XDR tuberculosis, GLC | 0·191–0·460 | PETTS |
| Probability of death during treatment for XDR tuberculosis, GLC | 0·117–0·359 | PETTS |
| Probability of being lost to follow up during treatment for MDR, pre-XDR, or XDR tuberculosis, GLC | 0·165–0·237 | PETTS |
| Probability of relapse for successfully treated tuberculosis, HIV-negative individuals | 0·011–0·021 | Houben et al, 201221 |
| Probability of relapse for successfully treated tuberculosis, PLHIV not on ART | 0·030–0·052 | Houben et al, 201221 |
| Probability of relapse for successfully treated tuberculosis, PLHIV on ART | 0·017–0·054 | Houben et al, 201221 |
| Probability of death, HIV-negative individuals | Imputed from life expectancy | Imputed from life expectancy |
| Probability of death, PLHIV not on ART | 0·084–0·105 | Anglaret et al, 201222 |
| Probability of death, PLHIV on ART | 0·030–0·037 | Anglaret et al, 2012,22 Gabillard et al, 201323 |
| Risk of stopping ART, PLHIV on ART | 0·026–0·033 | Anglaret et al, 2012,22 Gabillard et al, 201323 |
| Contact rate for individuals with tuberculosis | 2·30–5·00 | Fox et al, 201313 |
| Effective contact rate for PLHIV | 0·50–1·50 | Assumed |
| Relative risk of failure during treatment, INH-resistant or RR tuberculosis to DS tuberculosis | 6·83–12·17 | Lew et al, 200824 |
| Hazard ratio of tuberculosis incidence, PLHIV not on ART to PLHIV on ART | 2·50–3·10 | Alvarez-Uria et al, 201425 |
| Hazard ratio of tuberculosis treatment success, PLHIV on ART to PLHIV not on ART | 1·60–7·40 | Arentz et al, 201226 |
| Hazard ratio of death during tuberculosis treatment, PLHIV on ART to PLHIV not on ART | 0·30–0·60 | Arentz et al, 201226 |
| Hazard ratio of ADR during treatment, non-DOTS to DOTS | 3·69–12·47 | Weis et al, 199427 |
| Probability of initiating proper treatment for MDR, pre-XDR, or XDR tuberculosis | Calibrated | Calibrated |
| Probability of initiating proper treatment for DS, INH-resistant, or RR tuberculosis | Calibrated | Calibrated |
| Proportion of DS, INH-resistant, and RR tuberculosis treated in DOTS programmes | Calibrated | Calibrated |
| Proportion of MDR, pre-XDR, and XDR tuberculosis treated in GLC programmes | Calibrated | Calibrated |
| Proportion of PLHIV not on ART who initiate ART | Calibrated | Calibrated |
Outcomes for HIV-negative individuals and PLHIV on ART were assumed to be equivalent. Probabilities for all events were assumed to occur in 1 year unless otherwise specified. PLHIV=people living with HIV. LTBI=latent tuberculosis infection. ART=antiretroviral therapy. DS=drug-susceptible. DOTS=directly observed treatment short-course. INH=isoniazid. MDR=multidrug-resistant. ADR=acquired drug resistance. GLC=Green Light Committee. XDR=extensively drug-resistant.
Susceptible to isoniazid and rifampicin.
Treatment duration was assumed to be 6 months for both DOTS and non-DOTS.
Resistant to isoniazid and rifampicin.
Treatment duration was assumed to be 18 months for both GLC and non-GLC.
Tuberculosis resistant to isoniazid, rifampicin, and either fluoroquinolones or second-line injectable drugs.
Tuberculosis resistant to isoniazid, rifampicin, fluoroquinolones, and second-line injectable drugs.
Statistical analysis
We generated likelihood functions from several country-specific estimates: WHO estimates for overall tuberculosis incidence and tuberculosis incidence among PLHIV in 2000 and 2015, and percentages of incident tuberculosis cases with MDR tuberculosis and incident MDR tuberculosis cases with XDR from drug resistance surveys (appendix). Likelihood functions for each of these estimates were assumed to be independent and were multiplied to generate country-specific joint likelihood functions. Latin hypercube sampling was used to construct 100 000 unique variable sets using uniform prior distributions for all variables. Outcomes for each variable set were generated for all countries. A likelihood value was calculated for each parameter set by applying the joint likelihood function to modelled outputs corresponding to the country-specific estimates (prior and posterior distributions are in the appendix).
Sampling with replacement was done 100 000 times with likelihood values as sampling weights. The 2·5 and 97·5 centiles of the distribution of the 100 000 resampled outputs were used to construct prediction intervals for each country. Point estimates were derived from the median of distributions. To identify variables with the greatest effect on the modelled outputs, multivariate sensitivity analysis was done using partial-rank correlation coefficients (PRCC). PRCC measures monotonicity between a given parameter input and the outcome of interest after controlling for all other variables by calculating partial correlations of the rank-transformed inputs and outputs. Variables with significant PRCCs (p<0·05 with multiple testing correction) related to the percentage of individuals with MDR tuberculosis among those with incident tuberculosis, and the percentage of individuals with XDR tuberculosis among those with incident MDR tuberculosis in 2040 were identified. All analyses were programmed in R version 3.2.1.
Role of the funding source
The US Agency for International Development (USAID) had no role in study design, implementation, data collection, data analysis, data interpretation, or writing of the report. The US Centers for Disease Control and Prevention (CDC) led the model design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
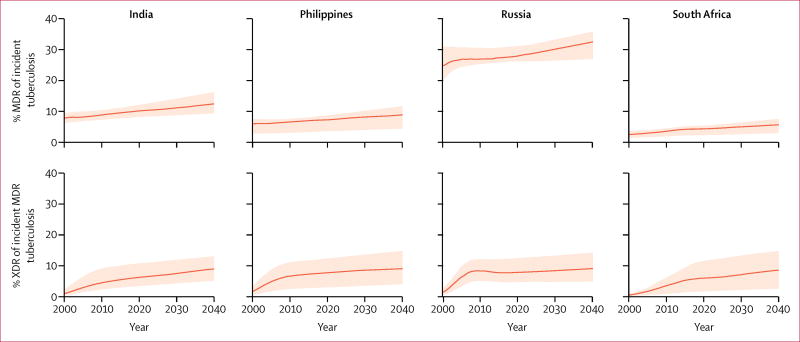
The percentage of incident tuberculosis cases with MDR increased in all modelled countries from 2000 to 2040 (figure 2). By the end of the time horizon, MDR tuberculosis among incident cases of tuberculosis was estimated to be 12·4% (95% prediction interval 9·4–16·2) in India, 8·9% (4·5–11·7) in the Philippines, 32·5% (27·0–35·8) in Russia, and 5·7% (3·0–7·6) in South Africa. Similarly, the percentage of incident MDR tuberculosis with XDR was estimated to increase for all countries, reaching 8·9% (5·1–12·9) in India, 9·0% (4·0–14·7) in the Philippines, 9·0% (4·8–14·2) in Russia, and 8·5% (2·5–14·7) in South Africa in 2040.
Figure 2. Projected trends of the proportion of individuals with MDR tuberculosis of those with incident tuberculosis, and the proportion with XDR tuberculosis of those with incident MDR tuberculosis.
Data are for India, the Philippines, Russia, and South Africa from 2000 to 2040. MDR=multidrug-resistant. XDR=extensively drug-resistant. Solid lines represent medians of projections. Shaded areas represent 95% prediction intervals.
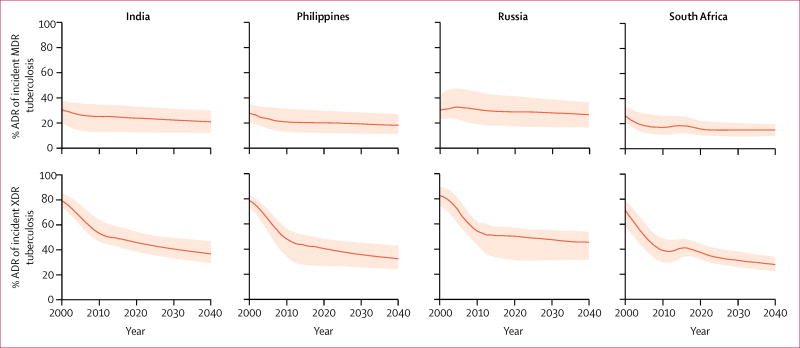
Trends for percentages of incident MDR tuberculosis caused by ADR were similar to trends for percentages of incident XDR tuberculosis caused by ADR across countries (figure 3). In the model, the percentage of MDR tuberculosis caused by ADR marginally decreased during 2000 to 2040, with median estimates indicating that ADR accounted for less than 30% of all incident MDR tuberculosis during this period. The magnitude of reduction of the percentage of incident XDR tuberculosis caused by ADR was greater than that for incident MDR tuberculosis. From median estimates of approximately 80% in 2000, the percentage of incident XDR tuberculosis caused by ADR decreased to approximately 35% in India and the Philippines, 46% in Russia, and 28% in South Africa.
Figure 3. Projected trends of proportions of incident MDR and XDR tuberculosis caused by acquired drug resistance.
Data are for India, the Philippines, Russia, and South Africa from 2000 to 2040. MDR=multidrug-resistant. XDR=extensively drug-resistant. ADR=acquired drug resistance. Solid lines represent medians of projections. Shaded areas represent 95% prediction intervals.
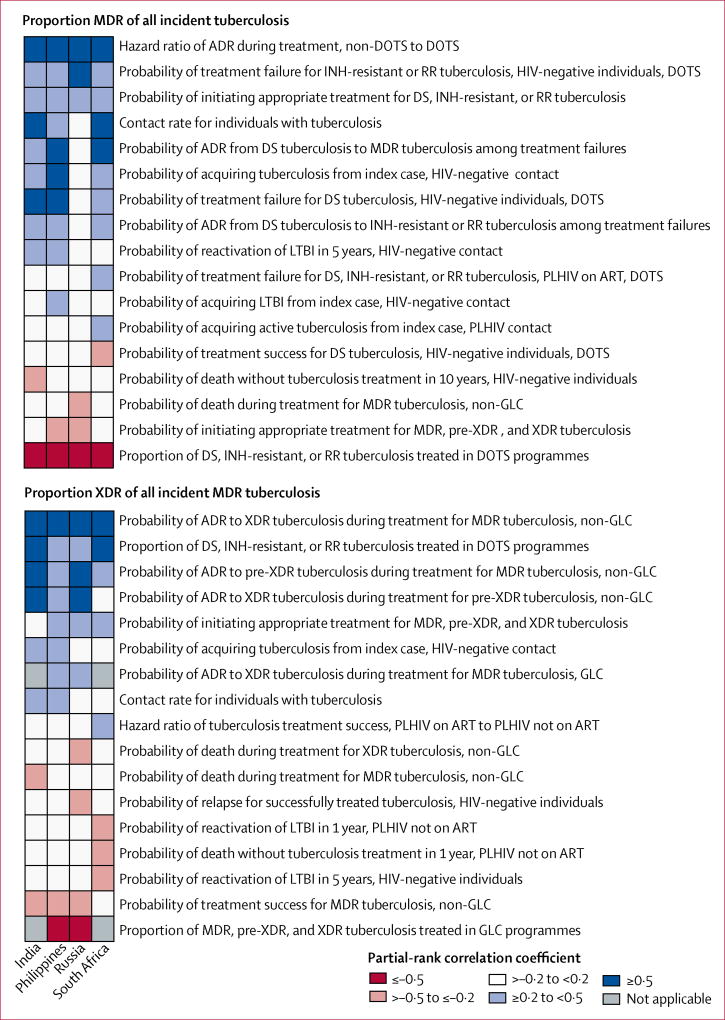
The results of the sensitivity analysis suggested that MDR tuberculosis is mostly driven by common factors across countries (figure 4). The proportion of individuals initiating appropriate treatment for non-MDR tuberculosis, the hazard ratio of ADR during treatment of non-MDR tuberculosis for non-DOTS relative to DOTS, the probability of treatment failure during DOTS, and the probability of ADR during treatment of non-MDR tuberculosis were positively associated with the proportion of MDR tuberculosis among incident cases of tuberculosis. Transmission of tuberculosis to HIV-negative contacts was positively associated with incident MDR tuberculosis in most countries; tuberculosis transmission to PLHIV contacts was associated with incident MDR tuberculosis in South Africa. The proportion of individuals initiating treatment for MDR tuberculosis and the proportion of DOTS coverage were negatively associated with incident MDR tuberculosis.
Figure 4. Main variables associated with the proportion of individuals with MDR tuberculosis of those with incident tuberculosis, and the proportion with XDR tuberculosis of those with incident MDR tuberculosis.
Data are partial-rank correlation coefficient values for variables, divided by country. MDR=multidrug-resistant. XDR=extensively drug-resistant. ADR=acquired drug resistance. DOTS=directly observed treatment short-course. GLC=Green Light Committee. INH=isoniazid. RR=rifampicin-resistant. DS=susceptible to isoniazid and rifampicin. LTBI=latent tuberculosis infection. PLHIV=people living with HIV. ART=antiretroviral therapy.
Several transmission and treatment variables were associated with the percentage of XDR among incident MDR tuberculosis (figure 4). ADR during treatment for MDR tuberculosis in HIV-negative people was associated with incident XDR tuberculosis in countries with and without GLC-approved programmes. The proportion of individuals initiating appropriate treatment for MDR tuberculosis was a significant driver of cases of incident XDR tuberculosis in most countries. In South Africa, transmission of tuberculosis among PLHIV contacts and treatment outcomes among PLHIV based on ART status were positively associated with incident MDR and XDR tuberculosis, whereas LTBI reactivation was negatively associated with these tuberculosis subgroups. Tuberculosis transmission was the major driver of incident XDR tuberculosis in India and the Philippines, and exposure to MDR tuberculosis treatment was positively associated with incident XDR tuberculosis in most countries. Among countries with GLC-approved PMDT, the proportion of MDR tuberculosis treated under GLC-approved conditions was inversely related with XDR tuberculosis burden. DOTS coverage was inversely related to the percentage of incident XDR tuberculosis across countries.
Discussion
In this study, we adapted recent mathematical models of tuberculosis to forecast trends of MDR and XDR tuberculosis in India, the Philippines, Russia, and South Africa—four countries with a high burden of MDR tuberculosis that represents a wide range of epidemiological conditions. We estimated that the proportion of MDR tuberculosis among incident cases of tuberculosis, and the proportion of XDR tuberculosis among incident MDR tuberculosis will increase in all modelled countries. We identified that most incident MDR tuberculosis is not caused by ADR, and that ADR will become a decreasing cause of incident MDR and XDR tuberculosis over time, regardless of whether a country’s MDR tuberculosis treatment programmes operate under GLC-approved conditions. Our observations suggest that incident MDR and XDR tuberculosis will increase despite improvements in ADR achieved by GLC-supported PMDT.
Our analysis builds on previous studies that modelled drug-resistant tuberculosis by using data about treatment outcomes from a rigorous multisite, prospective cohort study to generate country-specific forecasts for MDR and XDR tuberculosis. Country-specific projections for the percentages of incident MDR and XDR tuberculosis are consistent with aggregate findings described by Blower and colleagues.5 Our finding that ADR is a decreasing cause of incident MDR tuberculosis is similar to results described by Kendall and colleagues.10 To our knowledge, our study is the first to express and project trends of incident XDR tuberculosis caused by ADR using empirical data about treatment outcomes of individuals with MDR tuberculosis.
The results of this study define the expected trajectories for MDR and XDR tuberculosis in the context of the availability of new treatment regimens for non-MDR and MDR tuberculosis since 1970 and the subsequent genesis of tuberculosis strains with varying degree of drug resistance. The emergence of MDR tuberculosis occurred because of broadening availability of first-line drugs. As prevalence of MDR tuberculosis increased, the primary driver of incident MDR tuberculosis shifted from ADR during treatment to transmission to contacts of individuals with MDR tuberculosis. The benefits of DOTS expansion, which is efficient at reducing non-MDR tuberculosis, might have the unintended consequence of facilitating the spread of MDR and XDR tuberculosis due to decreased transmission of less-resistant tuberculosis strains. Similarly, XDR tuberculosis is a recent form of MDR tuberculosis that emerged due to expanded availability of second-line drugs for MDR tuberculosis. Our model suggests that as prevalence of XDR tuberculosis increases, increasing proportions of incident XDR tuberculosis will be caused by person-to-person transmission. Expansion of tuberculosis treatment programmes might reduce the overall incidence, but new cases of tuberculosis will become increasingly resistant as strains with lower resistance contribute less to transmission on a population scale.
Our findings have several implications for MDR tuberculosis. First, national tuberculosis programmes in countries with a high MDR tuberculosis burden should recognise that improved treatment outcomes conferred by GLC-approved PMDT might reduce, but will not eliminate, MDR tuberculosis resistant to second-line medicines. Implementation of GLC-approved PMDT might slow the rise of XDR tuberculosis in countries operating in non-GLC approved conditions. Second, the proportion of incident MDR tuberculosis will increase despite improved access to MDR tuberculosis treatment if there are no improvements in access to and enrolment in DOTS treatment programmes for non-MDR tuberculosis. LTBI treatment with isoniazid or rifampicin will reduce the incidence of non-MDR tuberculosis but at the consequence of increasing MDR and XDR tuberculosis as fewer non-MDR tuberculosis LTBI reactivate and subsequently contribute to person-to-person transmission. Next, to reduce burden of MDR and XDR tuberculosis, treatment of MDR tuberculosis must be coupled with methods to prevent general transmission of tuberculosis, including early detection, reducing the risk of pretreatment loss to follow-up among individuals diagnosed with tuberculosis, screening contacts for tuberculosis, and initiating proper treatment based on universal drug susceptibility testing.
The evidence for the fitness cost of ADR is mixed. Findings from in-vitro studies have shown that ADR confers a cost to competitive fitness but that MDR strains with low or no cost to fitness are the most commonly identified in clinical isolates.29 Contact investigations examining LTBI or active disease among household contacts of individuals with tuberculosis have shown lower, equal, and higher fitness of resistant strains.13,30 Considering this conflicting evidence, we chose to model a scenario in which ADR does not confer a penalty to fitness at a population level. Our model was calibrated to published estimates from several sources including reports from health ministries, World Bank, and WHO. The accuracy of our estimates is contingent on the accuracy of sources used for calibration. Our model did not account for possible systematic bias in published estimates to which modelled outputs were calibrated. In India and Russia, percentages of incident MDR and XDR tuberculosis used for calibration are based on subnational drug resistance surveys that might not represent nationwide MDR and XDR tuberculosis burden. Our study highlights the need for serial, nationally representative surveys to better track the spread of drug-resistant tuberculosis. Although we used a Bayesian approach to quantify uncertainty in our estimates, our findings are based on a single model structure. Alternative model structures that simulate tuberculosis transmission and ADR during treatment should be considered and compared with our results to better identify which factors drive MDR and XDR tuberculosis. We collapsed compartments for new and retreatment tuberculosis cases because of few data for clinical outcomes for MDR and XDR tuberculosis by previous treatment status. Differences in treatment outcomes for new compared to retreatment cases might have affected our estimates. Last, we did not consider changes in ecological factors, such as the human development index, population density, and migration that might affect tuberculosis transmission and estimates of MDR and XDR tuberculosis burden.
In summary, our findings suggest that the tuberculosis subgroups of MDR and XDR tuberculosis will become more common, given current treatment regimens and control methods. Expanding coverage for treatment of MDR tuberculosis will decrease the proportion of MDR tuberculosis among incident cases of tuberculosis, but at the expense of rising XDR tuberculosis. Our results also showed that improved treatment outcomes conferred by GLC-approved programmes might reduce acquired resistance to second-line drugs, but are unlikely to drive down the incidence of MDR tuberculosis, and do not appear to halt the rise of XDR tuberculosis at the population level. The results also suggest that current tools are insufficient to reverse the epidemics of MDR or XDR tuberculosis. Enhanced interventions to reduce tuberculosis transmission and expansion of improved treatment to minimise the risk of ADR are necessary to stop the spread of MDR and XDR tuberculosis.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for articles published up to Nov 1, 2016, on studies of drug-resistant tuberculosis with the search term “(tuberculosis OR TB) AND mathematical AND model AND resistan*”, and also reviewed citations of search results for additional articles of relevance. We found articles about eight mathematical modelling studies describing the emergence and spread of multidrug-resistant (MDR) or extensively drug-resistant (XDR) tuberculosis. Previous studies have described global predictions of replacement of tuberculosis with MDR tuberculosis, estimates of drug-resistant tuberculosis in specific cohorts such as children, and outcomes of scenario-based interventions in regions or countries with a high burden of MDR tuberculosis. We identified no study that used empirical data for acquired drug resistance to second-line antituberculosis drugs to forecast estimates of MDR or XDR tuberculosis across a range of countries with a high burden of MDR tuberculosis representing different epidemiological conditions and histories of tuberculosis control.
Added value of this study
Our deterministic, compartmental model of MDR and XDR tuberculosis used data from a rigorous longitudinal study of treatment outcomes in individuals with MDR tuberculosis including rates of acquired drug resistance to forecast estimates of incident MDR and XDR tuberculosis in four countries with a high burden of MDR tuberculosis: India, the Philippines, Russia, and South Africa. Our results estimated that MDR and XDR tuberculosis will rise and that acquired drug resistance will be a decreasing cause of incident MDR and XDR tuberculosis in all countries in our model. To our knowledge, ours is the first analysis to incorporate empirical data about acquired resistance to second-line antituberculosis drugs to generate national estimates of MDR and XDR tuberculosis in countries with a high burden of MDR disease.
Implications of all the available evidence
Improved outcomes during treatment of drug-resistant tuberculosis are unlikely to halt the spread of MDR or XDR tuberculosis at the population level. Additional strategies to prevent transmission will be necessary to stop MDR or XDR tuberculosis in countries with a high burden of MDR tuberculosis.
Acknowledgments
This work was supported by the US Agency for International Development (USAID; IAA: OGH09-011:CGH10-1010196) and the US Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination (CDC-OGH11-12021). Some authors from this Article are employed by the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of USAID or the US CDC.
Footnotes
Contributors
AS and AH undertook the mathematical modelling. AS, AH, EK, and PC contributed intellectually to the study concept, design, analysis, and responded to reviewers’ comments. PC was the principal investigator, and EK was the global coordinator for the parent study. All authors approved the study concept, contributed data, and critically reviewed draft and final manuscripts.
Declaration of interests
We declare no competing interests.
Contributor Information
Aditya Sharma, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Andrew Hill, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Ekaterina Kurbatova, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Martie van der Walt, South Africa Medical Research Council, Pretoria, South Africa.
Charlotte Kvasnovsky, University of Maryland Medical Center, Baltimore, MD, USA.
Thelma E Tupasi, Tropical Disease Foundation, Manila, Philippines.
Janice C Caoili, Tropical Disease Foundation, Manila, Philippines.
Maria Tarcela Gler, Tropical Disease Foundation, Manila, Philippines.
Grigory V Volchenkov, Vladimir Oblast Tuberculosis Dispensary, Vladimir, Russia.
Boris Y Kazennyy, Orel Oblast Tuberculosis Dispensary, Orel, Russia.
Olga V Demikhova, Central Tuberculosis Research Institute, Russian Academy of Medical Sciences, Moscow, Russia.
Jaime Bayona, Socios en Salud Sucursal, Lima, Peru.
Carmen Contreras, Socios en Salud Sucursal, Lima, Peru.
Martin Yagui, National Institute of Health, Lima, Peru.
Vaira Leimane, Riga East University Hospital Centre of Tuberculosis and Lung Diseases, Latvia.
Prof Sang Nae Cho, International Tuberculosis Research Center, Changwon and Yonsei University College of Medicine, Seoul, South Korea.
Hee Jin Kim, Korean Institute of Tuberculosis, Seoul, South Korea.
Kai Kliiman, Tartu University Hospital, Tartu, Estonia.
Somsak Akksilp, Ministry of Public Health, Bangkok, Thailand.
Ruwen Jou, Taiwan Centers for Disease Control, Taipei, Taiwan.
Julia Ershova, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Tracy Dalton, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Peter Cegielski, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.WHO. WHO/HTM/TB/2016.13. Geneva: World Health Organization; 2016. Global tuberculosis report. [Google Scholar]
- 2.UN. The Millenium Development Goals report. New York: United Nations; 2014. [Google Scholar]
- 3.Shah NS, Abigail W, Gill-Han B, et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–87. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colijn C, Cohen T, Ganesh A, Murray M. Spontaneous emergence of multiple drug resistance in tuberculosis before and during therapy. PLoS One. 2011;6:e18327. doi: 10.1371/journal.pone.0018327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blower SM, Chou T. Modeling the emergence of the hot zones: tuberculosis and the amplification dynamics of drug resistance. Nat Med. 2004;10:1111–16. doi: 10.1038/nm1102. [DOI] [PubMed] [Google Scholar]
- 6.Blower S, Supervie V. Predicting the future of XDR tuberculosis. Lancet Infect Dis. 2007;7:443. doi: 10.1016/S1473-3099(07)70143-3. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Geneva: World Health Organization; 2000. DOTS-Plus and the Green Light Committee: improving access to second-line anti-TB drugs. [Google Scholar]
- 8.Dalton T, Cegielski P, Akksilp S, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–17. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cegielski JP, Dalton T, Yagui M, et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2014;59:1049–63. doi: 10.1093/cid/ciu572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendall EA, Fofana MO, Dowdy DW. Burden of transmitted multidrug resistance in epidemics of tuberculosis: a transmission modelling analysis. Lancet Respir Med. 2015;3:963–72. doi: 10.1016/S2213-2600(15)00458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies NA, Cohen T, Lin HH, Murray M, Salomon JA. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med. 2012;9:e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNAIDS. Joint United Nations programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic (UNAIDS/JC2684) Geneva: United Nations; 2014. [Google Scholar]
- 13.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–56. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sloot R, Schim van der Loeff MF, Kouw PM, Borgdorff MW. Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med. 2014;190:1044–52. doi: 10.1164/rccm.201406-1159OC. [DOI] [PubMed] [Google Scholar]
- 15.Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–50. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 16.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV-negative patients: a systematic review. PLoS One. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Tuberculosis Institute. Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull World Health Organ. 1974;51:473–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Seung KJ, Gelmanova IE, Peremitin GG, et al. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clin Infect Dis. 2004;39:1321–28. doi: 10.1086/425005. [DOI] [PubMed] [Google Scholar]
- 19.El-Sadr WM, Perlman DC, Denning E, Matts JP, Cohn DL. A review of efficacy studies of 6-month short-course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clin Infect Dis. 2001;32:623–32. doi: 10.1086/318706. [DOI] [PubMed] [Google Scholar]
- 20.Khan FA, Minion J, Pai M, et al. Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clin Infect Dis. 2010;50:1288–99. doi: 10.1086/651686. [DOI] [PubMed] [Google Scholar]
- 21.Houben RM, Glynn JR, Mboma S, et al. The impact of HIV and ART on recurrent tuberculosis in a sub-Saharan setting. AIDS. 2012;26:2233–39. doi: 10.1097/QAD.0b013e32835958ed. [DOI] [PubMed] [Google Scholar]
- 22.Anglaret X, Minga A, Gabillard D, et al. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis. 2012;54:714–23. doi: 10.1093/cid/cir898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabillard D, Lewden C, Ndoye I, et al. Mortality, AIDS morbidity, and loss to follow-up by current CD4 cell count among HIV-1-infected adults receiving antiretroviral therapy in Africa and Asia: data from the ANRS 12222 collaboration. JAIDS. 2013;62:555–61. doi: 10.1097/QAI.0b013e3182821821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lew W, Pai M, Oxlade O, Martin D, Menzies D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med. 2008;149:123–34. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Uria G, Pakam R, Midde M, Naik PK. Incidence and mortality of tuberculosis before and after initiation of antiretroviral therapy: an HIV cohort study in India. J Int AIDS Soc. 2014;17:19251. doi: 10.7448/IAS.17.1.19251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arentz M, Pavlinac P, Kimerling ME, et al. Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: a systematic review. PLoS One. 2012;7:e47370. doi: 10.1371/journal.pone.0047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–84. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 28.Wilson E. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209. [Google Scholar]
- 29.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–46. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 30.Grandjean L, Gilman RH, Martin L, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med. 2015;12:e1001843. doi: 10.1371/journal.pmed.1001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.