Abstract
Objectives
Guided implant surgery (GIS) is performed with drilling guides that are produced on the virtual tooth model using CAD/CAM technology. The prerequisite for this workflow is the alignment of patients cone beam computed tomography CBCT and surface scan (registration). Dental restorations may cause deteriorating imaging artifacts in CBCT data, which in turn can have an impact on the registration process. The influence of the user and the preprocessing of data and of image artifacts on the registration accuracy were examined.
Material and Methods
CBCT data and intraoral surface scans of 36 patients were used for virtual implant planning in coDiagnostiX (Dentalwings, Montreal, Canada). CBCT data were reconstructed to a three‐dimensional anatomical model with the default settings provided by the software and also manually by four different examiners. Subsequently, the CBCT and intraoral surface models were registered by each examiner with the help of anatomical landmarks. Patients' data were subdivided into four groups (A–D) according to the number of metallic restorations: A = 0–2 restorations, B = 3–5 restorations, C = 6–8 restorations and D > 8 restorations. After registration, the distances between CBCT and dental surface models were measured. Linear regression models were used to assess the influence of the segmentation, the examiner and to the number of restorations (P < 0.05).
Results
The deviations between surface scan and CBCT models accounted to 0.54 mm (mean). The mean deviations were 0.69 mm (max. 24.8 mm) and 0.4 mm (max. 9.1 mm) for default and manual segmentation, respectively. Mean deviations of 0.36 mm (Group A), 0.43 mm (Group B), 0.67 mm (Group C) and 1.01 mm (Group D) were recorded.
The segmentation (P = 0.000), the user (P = 0.0052) and the number of restorations (P = 0.0337) had a significant influence on the registration accuracy.
Conclusions
The deviation between CBCT and surface scan model resulting from inaccurate registration is transferred to the surgical field and results in a deviation between the planned and actual implant position. The registration accuracy in commercial virtual implant planning software is significantly influenced by the preprocessing of imported data, by the user and by the number of restorations resulting in clinically non‐acceptable deviations encoded in drilling guides.
Keywords: CAD/CAM, cone beam computed tomography, guided implant surgery
Guided implant surgery involves the virtual placement of implants and the production of drilling guides using CAD/CAM procedures. With virtual implant planning systems, the surgical guide may be virtually designed on the surface model of the teeth and produced in‐house using a 3D printing device (Flugge et al. 2013a,b).
For the virtual planning of dental implants, anatomical data of the patient are required. Cone beam computed tomography (CBCT) or computed tomography (CT) are used to display a three‐dimensional image of the jaw for the identification of anatomical structures such as the inferior alveolar nerve, the maxillary sinus and the roots of neighboring teeth (Birkfellner et al. 2001; Bornstein et al. 2014).
For further use, DICOM data acquired with CBCT are processed: With segmentation, specific structures (e.g., teeth) are separated from the imaged volume with the help of an automatically or manually defined range of gray values (Dawant & Zijdenbos 2000). However, the virtual model reconstructed from CBCT data does not display the teeth accurately enough for the manufacturing of a drilling guide (Swennen et al. 2007a,b; Plooij et al. 2011). Therefore, the integration of a virtual model of the teeth derived from an intraoral surface scan or extraoral scan of a stone cast (Flugge et al. 2013a,b) is required. For the alignment of two data sets (virtual model and CBCT model), the process of transformation of the three‐dimensional images, defined as registration, is implemented (Fitzpatrick et al. 2000). The necessity for an accurate registration of data becomes evident when considering that implants are virtually placed based on the radiographic data, and the implant drilling guide is produced on the basis of the virtual model of the teeth (Vercruyssen et al. 2014a,b).
The accurate transfer of the virtually planned implant position to the surgical site is achieved, when all images and reproductions of one patient are aligned with each other in one virtual coordinate system. These are the three‐dimensional radiographic data and a virtual model of the teeth.
For the purpose of registration, areas on the tooth surface represented in the virtual stone cast and in the model produced from radiographic data are selected (Flugge et al. 2013a,b Widmann & Bale 2006). To reliably serve for registration, the areas must lie in clearly discernible features represented in the respective images to be registered (Fitzpatrick et al. 2000).
As an alternative to anatomical structures, markers placed in a radiographic template may serve as fiducial marks for registration. With a double‐scan technique, the actual position of the markers in the patient is fused with the marker position in the radiographic template (Birkfellner et al. 2001; Fortin et al. 2003; Gateno et al. 2003; Swennen et al. 2007a,b; Katsoulis et al. 2009; Behneke et al. 2012; Vercruyssen et al. 2014a,b). A radiographic template bears the disadvantages of additional time, effort and laboratory costs. Furthermore, an ill‐fitting template may cause inaccuracies during the registration process (van Steenberghe et al. 2002; Fortin et al. 2003; Di Giacomo et al. 2005).
The use of anatomical structures for registration is advantageous as the radiographic examination may be performed during the first consultation without the previous production of a radiographic template. If three‐dimensional data of the patient were already acquired in a different context, it may be used for implant planning.
The use of guided implant surgery was proven to be more accurate than freehand implant placement and freehand drilling, respectively, in vitro (Park et al. 2009) and in vivo (Behneke et al. 2012; Vercruyssen et al. 2014a,b). However, deviations between the planned and the actual implant position account for 1.12 mm on average with maximum deviations of 4.5 mm in the literature (Tahmaseb et al. 2014). The influence of data registration on this deviation has not been discussed to date.
The presence of artifacts caused by metallic restorations in radiographic data may mask anatomical structures or reference markers (Swennen et al. 2007a,b) and may therefore hamper an accurate registration of data contributing to these imprecisions (Nkenke et al. 2004).
Hitherto, the influence of imaging artifacts in CBCT data on the registration process has not been studied. This study investigates the hypothesis that the presence of imaging artifacts and their amount influences the registration accuracy, and therefore, the accuracy of image guided implant surgery. Furthermore, the accuracy of registration is compared between four different examiners with different levels of expertise with virtual implant planning and between automatically and manually segmented data.
Material and methods
Patient selection and group assignment
The database of the Academisch Centrum Tandheelkunde Amsterdam (ACTA) was scanned for patient data eligible to be included in this study. Included patients were partially edentulous with various numbers of dental restorations and teeth and required implant therapy. CBCT scans, acquired with 3D Accuitomo (Morita, Japan) (90 kV, 5 mA, 18 s, 360° rotation, 250 μm isotropic voxel size, interval distance 250 μm, FOV 8 × 8 cm) in non‐occlusion and intraoral scans, acquired by a trained dentist with an intraoral scanner (True Definition, 3M) were available. All patients had at least one tooth in the frontal region (incisors and canines) and the left and right posterior region (premolar and molar regions), respectively.
A total number of 36 patients were selected for evaluation and divided into four groups according to the following criteria. Patients with 0–2 dental restorations were assigned to Group A (seven patients); patients with 3–5 dental restorations were assigned to Group B (17 patients); patients with 6–8 dental restorations were assigned to Group C (seven patients); and patients with nine or more dental restorations were assigned to Group D (five patients).
Import and segmentation of imaging data
Three‐dimensional radiographic data available in a DICOM format and intraoral scans available in a STL format were imported into the software coDiagnostiX (Dentalwings, Montreal, Canada). Neither radiographic data nor intraoral scanning data were processed before import.
Four dentists blinded to the clinical data of the patients were asked to perform segmentation of CBCT data. Two dentists were experienced in the use of the implant planning software, and two dentists had little experience in the use of the implant planning software. An instruction to the software was provided prior to the experimental phase.
Two different segmentations of each CBCT data set were performed by each examiner: the first 3D model was created with the default gray values for bone segmentation provided by the software. This presetting includes gray values between 2250 HU and 350 HU for the imported CBCT data (Fig. 1a). The second segmentation of a 3D model was created according to parameters determined by each examiner. The examiner selected the range of gray values for segmentation and was allowed to manually adapt the model according to the individually favored representation of anatomical structures (Fig. 1b).
Figure 1.
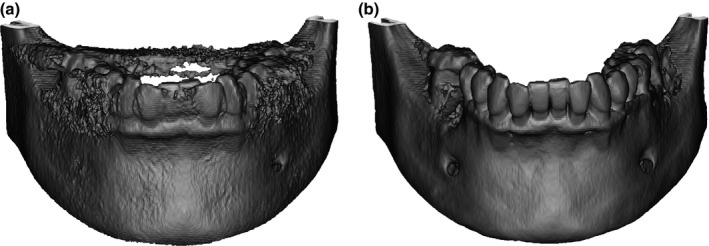
Two three‐dimensional reconstructions of CBCT data of the lower jaw of one patient. In a, the default segmentation provided by the software was used to create the model. In b, a manual segmentation with individualized gray value settings was used.
Registration of imaging data
Data registration was performed by each of the four examiners. Firstly, the intraoral scan was registered to the 3D model created with default segmentation values; secondly, the intraoral scan was registered to the 3D model created with manual segmentation.
The following specifications were used for image registration. Each examiner defined three to six corresponding surface areas on 3D models of CBCT and intraoral scan, of which a minimum of one landmark was placed in the anterior region and the left and right molar regions, respectively (Fig. 2). The surface areas were located on the coronal part of the teeth. In case of a lack of identifiable surface areas, the respective region was omitted and surface areas were only selected in distinct anatomical structures.
Figure 2.
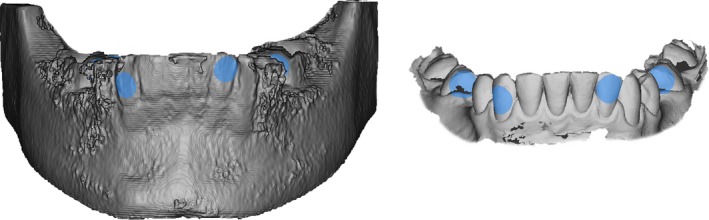
For the registration procedure, the examiner selects corresponding surfaces (blue) on the model reconstructed from CBCT (left) and the surface scan model (right). These areas are located on the tooth surfaces, as this is the only anatomical structure, that is displayed in both models.
Evaluation of data registration
The registration accuracy was evaluated by measuring the distance between intraoral scan and CBCT (Fig. 3). The distance between the intraoral scan and the 3D model created with default segmentation and the 3D model created with manual segmentation, respectively, was measured at defined landmarks. In the intraoral scan, one examiner selected landmarks on the disto‐buccal cusps of the left and right second molars (M_ri, M_le) and the cusps of the left and right canines (C_le, C_ri) (Fig. 4). If the landmark was not visible or not present because of a missing tooth, the most distal molar and the most distal incisal edge were selected. The corresponding marks were plotted to the CBCT model with a perpendicular line to the intraoral scan and the distances between each landmark pair was calculated. Therefore, four distances were recorded for each pair of registered models.
Figure 3.
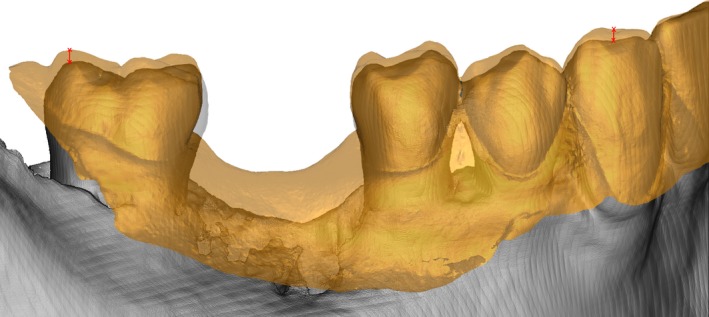
The registration of surface scan model (yellow outline) and CBCT model segmented with default gray values is displayed in A and in C. The individualized CBCT segmentation and the registered surface scan model (green outline) are displayed in B and in C.
Figure 4.
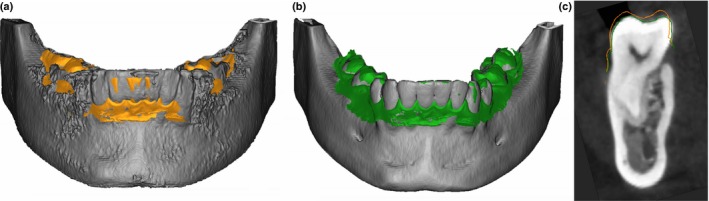
After registration of the model reconstructed from CBCT data (gray) and the surface scan model (yellow), the distances between the two models were measured at four points: left and right canine cusp and right and left disto‐buccal cusp of the second molar. The figure displays measured distances at the right canine (C_ri) and molar (M_ri).
Statistical evaluation
Linear mixed regression models with random intercepts for each patient and examiner were used to evaluate the influence of the segmentation, the examiner and the number of restorations on the accuracy of registration. This was performed separately for each variable of interest (examiner, image segmentation, number of restorations). The method of “Scheffe” was applied to correct for the multiple testing problem (adjustment of P ‐values).
To evaluate the segmentation, default segmentation and manual segmentation were considered. All examiners were evaluated separately and according to their experience with the software. Furthermore, the influence of the presence and number of restorations on the accuracy of registration was evaluated. The level of significance was defined as P = 0.05.
The calculations were performed with the statistical software STATA 14 (College Station, Texas, USA).
Results
Overall results show 0.54 mm deviation between models with a minimum deviation of 0 mm and maximum deviation of 24.8 mm. With default segmentation, a mean deviation of 0.69 mm (min 0 mm, max 24.8 mm) between models was recorded. With manual segmentation, a mean deviation of 0.4 mm (min 0 mm, max 9.1 mm) was recorded. The segmentation had a highly significant influence (P = 0.00) on the deviation between the models regardless of the examiner and the number of restorations. The distances between the corresponding models in Group A, B, C and D are displayed in Table 1.
Table 1.
The distances between all corresponding models in Group A (0–2 restorations), B (3–5 restorations), C (6–8 restorations) and D (>8 restorations) are displayed with mean values, standard deviation (SD); maximum and minimum values in mm, separately displayed for default and manual segmentations
| Group A default / manual | Group B default / manual | Group C default / manual | Group D default / manual | |
|---|---|---|---|---|
| Mean Dev | 0.5 / 0.3 | 0.6 / 0.3 | 0.7 / 0.7 | 1.5 / 0.5 |
| SD | 0.4 / 0.3 | 0.5 / 0.3 | 0.7 / 1.4 | 3.9 / 0.6 |
| Max Dev | 1.8 / 1.3 | 4.5 / 4.9 | 3.1 / 9.1 | 24.8 / 3.5 |
| Min Dev | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
Linear mixed regression analysis showed a significant influence of the total number of restorations and the segmentation mode as well as the number of metallic restorations on the deviation between the models (P < 0.001). The higher the number of restorations, the higher the deviations between the models. However, with pairwise comparison, registration accuracy was only significantly different between Group A and Group D (P = 0.03).
The distances between the corresponding models for each examiner are displayed in Table 2.
Table 2.
The distances between all corresponding models registered by examiner 1‐4 are displayed with mean values, standard deviation (SD); maximum and minimum values in mm, separately displayed for default and manual segmentations
| Examiner 1 default / manual | Examiner 2 default / manual | Examiner 3 default / manual | Examiner 4 default / manual | |
|---|---|---|---|---|
| Experienced | Inexperienced | |||
| Mean Dev | 0.7 / 0.3 | 0.5 / 0.2 | 0.5 / 0.3 | 1.1 / 6.9 |
| SD | 0.6 / 0.3 | 0.5 / 0.3 | 0.4 / 0.4 | 3 / 1.3 |
| Max Dev | 4.4 / 2.6 | 4.3 / 2.2 | 2.8 / 2.4 | 24.8 / 9.1 |
| Min Dev | 0 / 0 | 0 / 0 | 0 / 0 | 0 / 0 |
The registration accuracy among each of the four examiners was significantly different (P = 0.005). However, the expertise (unexperienced vs. experienced) did not have a significant influence (P = 0.07).
Discussion
This study showed a highly significant influence of the mode of segmentation on the accuracy of registration of surface imaging data with CBCT data. Inaccuracies of the registration occurred for all examiners and were significantly different among each other. The higher the number of dental restorations in each patient, the lower the registration accuracy.
The use of anatomical surfaces for the registration of data within the workflow of virtual implant planning is advantageous as it saves time, reduces patient appointments and prevents the preparation of stone casts, a prosthetic setup and a radiographic template in the dental laboratory. Nevertheless, the workflow of semiautomatic registration commonly implemented in virtual implant planning software requires the user to take a number of steps before the actual planning of the implant is performed. These steps include the segmentation of data and the selection of common areas for the registration of surface scans with CBCT data.
The user displays a three‐dimensional reconstruction of CBCT data with either default or manual gray value settings. The acceptance of default gray values saves time and does not require an individual procedure; however, imaging artifacts might mask anatomical features needed for registration in the subsequent step. Individual (manual) segmentation might improve the recognition of anatomical features; however, it takes time and either expertise with the software or a good visual awareness of the three‐dimensional model to be processed and displayed. The default gray value range provided by the software proved inaccurate for tooth segmentation from CBCT data, as the registration accuracy was significantly lower compared to individual segmentation. This is in accordance with Swennen et al. who stated that the use of default gray value thresholds defined for by Hounsfield units (HU) in CT may result in a compromised display of anatomical structures in CBCT due to the inherent inhomogeneity of gray values (Swennen et al. 2009). Image segmentation algorithms in implant planning software should be compatible with CBCT data presenting with variable gray value information, metal artifacts and further image artifacts, such as noise, to provide the user with accurate anatomical three‐dimensional models.
Following segmentation, image registration might be conducted along two pathways with different advantages and limitations either with the use of additional reference objects or using anatomical surface characteristics as shown in this study.
The presented results show an overall deviation of 0.54 mm between the virtual teeth model and the CBCT model and are comparable with a case report that used the tooth surfaces for registration of CT data with surface models (0.56 mm maxilla / 0.66 mm mandible) (Nkenke et al. 2004). The authors are not aware of further studies investigating registration accuracy using the tooth surface in clinical patient data, while investigating the segmentation, the number of restoration or the experience of the examiner, respectively, as an influencing factor. An in vitro study regarding the registration of surface scans and CT data derived from regular stone casts and stone casts with metal restorations (0.27 mm) suggested higher deviations between surface scans and CT data of the stone casts with restorations (Nkenke et al. 2004). This study confirmed the significant influence of artifacts caused by dental restorations in CT data of real patients. The accuracy of in vivo CBCT is not only limited by metallic artifacts, but by voxel size, contrast resolution, patient motion and further image artifacts (Al‐Rawi et al. 2010; Spin‐Neto et al. 2013). With a voxel size/nominal resolution of 0.25 mm as applied in this study, a base error of 0.25 mm might be assumed.
The use of a radiographic splint with reference objects for registration is common; however, the in vivo registration accuracy of that method was never evaluated. Reported overall deviations between the planned and the actual implant position included not only the possible registration error during implant planning, but also the surgical procedure and the second registration of pre‐ and postoperative data for evaluation (Birkfellner et al. 2001; Fortin et al. 2003; Gateno et al. 2003; Di Giacomo et al. 2005; Swennen et al. 2007a,b; Katsoulis et al. 2009; Behneke et al. 2012 Vercruyssen et al. 2014a,b). Using the double‐scan method under ideal conditions (no restorations, no missing teeth) using ten cadaver skulls yielded mean deviations of 0.14 mm (Swennen et al. 2007a,b). Among the significant influence of dental restorations, an imprecise positioning of the splint during try‐in or radiographic examination is more likely to occur in actual patients and may also account for a higher error in clinical situations.
As an alternative to a radiographic template, reference objects for registration might be glued to the patients' gingival surface during impression taking and radiographic examination. This procedure was evaluated in a case study for orthognathic planning in one patient (Rangel et al. 2012, 2013). The presented patient was fully dentate and did not have any restorations. The registration error of 0.1 mm has to be confirmed with multiple patients with various numbers of restorations to prove its practicability in clinical routine. The proximity of the markers to the teeth is prone to an inaccurate display or fading in the presence of restorations and artifacts, respectively.
Image registration with the help of anatomical landmarks does not depend on reference objects and is therefore most advantageous with regard to efficiency. This study showed that the registration accuracy is dependent on a number of other factors including the number of restorations and the experience of the examiner. According to the clinical procedure, the examiners performed segmentation and registration only once. This explains the maximum deviation of 24.8 mm in one patient. Repeated segmentation and registration might improve the registration accuracy, but does not represent the normal clinical routine and time factor relevant in clinical practice. One of the examiners (examiner 4) reached significantly higher deviations compared to all other three examiners. This fact might be attributed to the factor that excluding examiner 4, all other examiners were digital natives. The interindividual differences between the examiners might be furthermore attributed to the time and effort each examiner invested in the segmentation and registration process. In this context, the examiners represent the users confronted with the software in clinical practice.
To the knowledge of the authors, this is the first study to examine and confirm the significant influence of clinical parameters on the registration accuracy of three‐dimensional models derived from CBCT and surface scanning. The reported deviations of the models are directly transferred to the surgical site and might result in clinically relevant deviations between the planned and actual implant position.
The current data suggest that registration with the help of tooth surfaces should be limited to patients with a limited number of restorations and a manual correction of the three‐dimensional models. The preprocessing of CBCT data and segmentation options in software for virtual implant planning should be regarded in more detail, as they are a key factor for accuracy of the workflow of image guided implant surgery.
Conclusion
The manual segmentation of three‐dimensional models by the user proved significantly better than the automatic segmentation especially in patients with multiple restorations. The accuracy of guided implant surgery using commercial virtual implant planning software is therefore significantly influenced by the segmentation of the imported data and by the number of restorations resulting in clinically relevant deviations encoded in drilling guides. Specialized import algorithms for CBCT data and automated presettings might help to minimize the influence of the identified factors and improve reliability of the workflow of virtual implant planning.
Acknowledgements
The presented research was made possible with an ITI scholarship. We gratefully acknowledge Kirstin Vach, Institute for Medical Biometry and Statistics, Faculty of Medicine and Medical Center ‐ University of Freiburg, Germany for statistical analysis.
Flügge T, Derksen W, te Poel J, Hassan B, Nelson K, Wismeijer D. Registration of cone beam computed tomography data and intraoral surface scans – A prerequisite for guided implant surgery with CAD/CAM drilling guides. Clin. Oral Impl. Res. 28, 2017, 1113–1118. doi: 10.1111/clr.12925
References
- Al‐Rawi, B. , Hassan, B. , Vandenberge, B. & Jacobs, R. (2010) Accuracy assessment of three‐dimensional surface reconstructions of teeth from cone beam computed tomography scans. Journal of Oral Rehabilitation 37: 352–358. [DOI] [PubMed] [Google Scholar]
- Behneke, A. , Burwinkel, M. & Behneke, N. (2012) Factors influencing transfer accuracy of cone beam CT‐derived template‐based implant placement. Clinical Oral Implants Research 23: 416–423. [DOI] [PubMed] [Google Scholar]
- Birkfellner, W. , Solar, P. , Gahleitner, A. , Huber, K. , Kainberger, F. , Kettenbach, J. , Homolka, P. , Diemling, M. , Watzek, G. & Bergmann, H. (2001) In‐vitro assessment of a registration protocol for image guided implant dentistry. Clinical Oral Implants Research 12: 69–78. [DOI] [PubMed] [Google Scholar]
- Bornstein, M.M. , Scarfe, W.C. , Vaughn, V.M. & Jacobs, R. (2014) Cone beam computed tomography in implant dentistry: a systematic review focusing on guidelines, indications, and radiation dose risks. International Journal of Oral and Maxillofacial Implants 29(Suppl): 55–77. [DOI] [PubMed] [Google Scholar]
- Dawant, B.M. & Zijdenbos, A.P. (2000) Image segmentation In: Fitzpatrick J.M. & Sonka M., eds. Hand Book of Medical Imaging: Medical image processing and analysis, 2nd edition, 73–120. Bellingham: The International Society of Optics and Photonics. [Google Scholar]
- Di Giacomo, G.A. , Cury, P.R. , de Araujo, N.S. , Sendyk, W.R. & Sendyk, C.L. (2005) Clinical application of stereolithographic surgical guides for implant placement: preliminary results. Journal of Periodontology 76: 503–507. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, J. M. , Hill, D. L. G. & Maurer, C. RJr. (2000) Handbook of Medical Imaging: Medical Image Processing and Analysis, 2nd edition, 447–506. Bellingham: The International Society of Optics and Photonics. [Google Scholar]
- Flugge, T.V. , Nelson, K. , Schmelzeisen, R. & Metzger, M.C. (2013a) Three‐dimensional plotting and printing of an implant drilling guide: simplifying guided implant surgery. Journal of Oral and Maxillofacial Surgery 71: 1340–1346. [DOI] [PubMed] [Google Scholar]
- Flugge, T.V. , Schlager, S. , Nelson, K. , Nahles, S. & Metzger, M.C. (2013b) Precision of intraoral digital dental impressions with itero and extraoral digitization with the itero and a model scanner. American Journal of Orthodontics and Dentofacial Orthopedics: Official Publication of the American Association of Orthodontists, its Constituent Societies, and the American Board of Orthodontics 144: 471–478. [DOI] [PubMed] [Google Scholar]
- Fortin, T. , Bosson, J.L. , Coudert, J.L. & Isidori, M. (2003) Reliability of preoperative planning of an image‐guided system for oral implant placement based on 3‐dimensional images: an in vivo study. International Journal of Oral and Maxillofacial Implants 18: 886–893. [PubMed] [Google Scholar]
- Gateno, J. , Xia, J. , Teichgraeber, J.F. & Rosen, A. (2003) A new technique for the creation of a computerized composite skull model. Journal of Oral and Maxillofacial Surgery 61: 222–227. [DOI] [PubMed] [Google Scholar]
- Katsoulis, J. , Pazera, P. & Mericske‐Stern, R. (2009) Prosthetically driven, computer‐guided implant planning for the edentulous maxilla: a model study. Clinical Implant Dentistry and Related Research 11: 238–245. [DOI] [PubMed] [Google Scholar]
- Nkenke, E. , Zachow, S. , Benz, M. , Maier, T. , Veit, K. , Kramer, M. , Benz, S. , Hausler, G. , Neukam, F.W. & Lell, M. (2004) Fusion of computed tomography data and optical 3d images of the dentition for streak artefact correction in the simulation of orthognathic surgery. Dentomaxillofacial Radiology 33: 226–232. [DOI] [PubMed] [Google Scholar]
- Park, C. , Raigrodski, A.J. , Rosen, J. , Spiekerman, C. & London, R.M. (2009) Accuracy of implant placement using precision surgical guides with varying occluso gingival heights: an in vitro study. The Journal of Prosthetic Dentistry 101: 372–381. [DOI] [PubMed] [Google Scholar]
- Plooij, J.M. , Maal, T.J. , Haers, P. , Borstlap, W.A. , Kuijpers‐Jagtman, A.M. & Berge, S.J. (2011) Digital three‐dimensional image fusion processes for planning and evaluating orthodontics and orthognathic surgery. A systematic review. International Journal of Oral and Maxillofacial Surgery 40: 341–352. [DOI] [PubMed] [Google Scholar]
- Rangel, F.A. , Maal, T.J. , Berge, S.J. & Kuijpers‐Jagtman, A.M. (2012) Integration of digital dental casts in cone‐beam computed tomography scans. International Scholarly Research Network Dentistry 2012: 949086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel, F.A. , Maal, T.J. , Bronkhorst, E.M. , Breuning, K.H. , Schols, J.G. , Berge, S.J. & Kuijpers‐Jagtman, A.M. (2013) Accuracy and reliability of a novel method for fusion of digital dental casts and cone beam computed tomography scans. PLoS One 8: e59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spin‐Neto, R. , Gotfredsen, E. & Wenzel, A. (2013) Impact of voxel size variation on cbct‐based diagnostic outcome in dentistry: a systematic review. Journal of Digital Imaging: The Official Journal of the Society for Computer Applications in Radiology 26: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steenberghe, D. , Naert, I. , Andersson, M. , Brajnovic, I. , Van Cleynenbreugel, J. & Suetens, P. (2002) A custom template and definitive prosthesis allowing immediate implant loading in the maxilla: a clinical report. International Journal of Oral and Maxillofacial Implants 17: 663–670. [PubMed] [Google Scholar]
- Swennen, G.R. , Barth, E.L. , Eulzer, C. & Schutyser, F. (2007a) The use of a new 3d splint and double ct scan procedure to obtain an accurate anatomic virtual augmented model of the skull. International Journal of Oral and Maxillofacial Surgery 36: 146–152. [DOI] [PubMed] [Google Scholar]
- Swennen, G.R. , Mollemans, W. & Schutyser, F. (2009) Three‐dimensional treatment planning of orthognathic surgery in the era of virtual imaging. Journal of Oral and Maxillofacial Surgery 67: 2080–2092. [DOI] [PubMed] [Google Scholar]
- Swennen, G.R. , Mommaerts, M.Y. , Abeloos, J. , De Clercq, C. , Lamoral, P. , Neyt, N. , Casselman, J. & Schutyser, F. (2007b) The use of a wax bite wafer and a double computed tomography scan procedure to obtain a three‐dimensional augmented virtual skull model. Journal of Craniofacial Surgery 18: 533–539. [DOI] [PubMed] [Google Scholar]
- Tahmaseb, A. , Wismeijer, D. , Coucke, W. & Derksen, W. (2014) Computer technology applications in surgical implant dentistry: a systematic review. International Journal of Oral and Maxillofacial Implants 29(Suppl.): 25–42. [DOI] [PubMed] [Google Scholar]
- Vercruyssen, M. , Cox, C. , Coucke, W. , Naert, I. , Jacobs, R. & Quirynen, M. (2014a) A randomized clinical trial comparing guided implant surgery (bone‐ or mucosa‐supported) with mental navigation or the use of a pilot‐drill template. Journal of Clinical Periodontology 41: 717–723. [DOI] [PubMed] [Google Scholar]
- Vercruyssen, M. , Fortin, T. , Widmann, G. , Jacobs, R. & Quirynen, M. (2014b) Different techniques of static/dynamic guided implant surgery: modalities and indications. Periodontology 2000(66): 214–227. [DOI] [PubMed] [Google Scholar]
- Widmann, G. & Bale, R.J. (2006) Accuracy in computer‐aided implant surgery–a review. International Journal of Oral and Maxillofacial Implants 21: 305–313. [PubMed] [Google Scholar]


