ABSTRACT
Phaeodactylum tricornutum is a widely studied diatom and has been proposed as a source of oil and polyunsaturated fatty acids (PUFA), particularly eicosapentaenoic acid (EPA). Recent studies indicate that lipid accumulation occurs under nutritional stress. Aim of this research was to determine how changes in nitrogen availability affect productivity, oil yield, and fatty acid (FA) composition of P. tricornutum UTEX 640. After preliminary laboratory trials, outdoor experiments were carried out in 40‐L GWP® reactors under different nitrogen regimes in batch. Nitrogen replete cultures achieved the highest productivity of biomass (about 18 g m−2 d−1) and EPA (about 0.35 g m−2 d−1), whereas nitrogen‐starved cultures achieved the highest FA productivity (about 2.6 g m−2 d−1). The annual potential yield of P. tricornutum grown outdoors in GWP® reactors is 730 kg of EPA per hectare under nutrient‐replete conditions and 5,800 kg of FA per hectare under nitrogen starvation. Biotechnol. Bioeng. 2017;114: 2204–2210. © 2017 The Authors. Biotechnology and Bioengineering Published by Wiley Periodicals, Inc.
Keywords: Phaeodactylum tricornutum, nitrogen starvation, nitrogen limitation, eicosapentaenoic acid, fatty acids
Introduction
Microalgae are a promising alternative source of oil for biodiesel production because of their capacity to accumulate neutral lipids and achieve high productivities (Breuer et al., 2012; Caporgno et al., 2016; De Bhowmick et al., 2015; Guccione et al., 2014; Rawat et al., 2013; Rodolfi et al., 2009; Velmurugan et al., 2014). Moreover, marine microalgae cultivation does not require freshwater and fertile soils as it can be performed in saline waters and on marginal lands (Chini Zittelli et al., 2013; Chisti, 2007; Tredici, 2010). However, at present, the process is not yet economically and energetically sustainable (Ruiz et al., 2016; Tredici et al., 2015, 2016), unless other high‐value compounds present within the algal cell are exploited together with the storage components used for bioenergy (Biondi et al., 2016; Chew et al., 2017; Hariskos and Posten, 2014; Trivedi et al., 2015).
It is well known that under stress conditions, the so‐called oleaginous microalgae can increase cellular lipid content, mainly the neutral lipid fraction, although the response is species‐specific (Benvenuti et al., 2015; Piorreck et al., 1984; Pruvost et al., 2009; Rodolfi et al., 2009). Nitrogen limitation and starvation are the most frequently applied stresses for increasing lipid (oil) content, as this technique is cheap, easy to apply, and has a reliable and strong effect in oleaginous species (Benvenuti et al., 2015; Biondi et al., 2013; Bondioli et al., 2012; Converti et al., 2009; Guccione et al., 2014; Ho et al., 2012; Klok et al., 2013). However, nutrient deprivation decreases growth and may not necessarily improve lipid productivity (Griffith and Harrison, 2009). While nutrient starvation typically results in a first period of unbalanced growth, in which a reserve substance (e.g., starch or oil) is accumulated over the levels observed during nutrient sufficient growth, inevitably followed by growth termination, nutrient limitation can lead to an acclimated growth in which the growth rate is reduced and cell composition is stably modified (MacIntyre and Cullen, 2005; Parkhill et al., 2001).
Some marine microalgae are also able to synthesize ω‐3 polyunsaturated fatty acids (PUFA), and especially eicosapentaenoic acid (EPA), which are of great interest for nutraceuticals and feed applications (Borowitzka and Moheimani, 2013; Spolaore et al., 2006; Tredici et al., 2009). Among the main genera studied for this purpose are Nannochloropsis, Phorphyridium, Isochrysis, Tetraselmis, and Phaeodactylum; however few cases of commercial applications do exist (Borowitzka, 2013; Mata et al., 2010). While for oil accumulation nutrient stress is necessary, high EPA content and productivity are generally attained under nutrient replete growth as this fatty acid is a component of cell membranes (Cohen, 1999).
Because of its high EPA content, ability to accumulate lipids upon nutrient stress, and capacity to be mass cultivated, the diatom Phaeodactylum tricornutum has been widely studied both in the laboratory (Chrismadha and Borowitzka, 1994; Meiser et al., 2004; Yongmanitchai and Ward, 1991) and outdoors (Acién Fernández et al., 2000; Mata et al., 2010; Torzillo et al., 2012). Outdoor studies focused mainly on EPA production under optimal (nutrient replete) growth conditions.
While lipid metabolism under nitrogen starvation and limitation in P. tricornutum has been diffusely studied in the laboratory (Guihéneuf et al., 2011; Kaixian and Borowitzka, 1993; López Alonso et al., 2000; Parrish and Wangersky, 1987; Qiao et al., 2016), and transformants with enhanced fatty acid content have been recently developed (Shemesh et al., 2016), to our knowledge, the effects of nutrient starvation/limitation on oil and fatty acids synthesis and productivity have not been researched in outdoor cultures of this microalga.
In this work, after preliminary laboratory trials in which batch and semi‐continuous cultures of two P. tricornutum strains (UTEX 640 and UTEX 646) were compared under nitrogen starvation, the selected strain UTEX 640 was cultivated outdoors under different nitrogen regimes in Green Wall Panel (GWP®‐III) photobioreactors, and growth and fatty acid content and productivity were evaluated.
Materials and Methods
Organisms and Culture Conditions
P. tricornutum UTEX 640 and UTEX 646 were cultivated in the laboratory in F medium containing sodium nitrate as nitrogen source (Guillard and Ryther, 1962), which was prepared with artificial seawater (Adriatic Sea Aquarium & Equipment, Rimini, Italy) at 30 g L−1 salinity. The seawater was autoclaved, allowed to cool, and then added with sterile nutrient solutions. In a first laboratory trial, the strains were grown in nitrogen‐depleted medium in batch or in batch/semi‐continuous regimes. In batch, the cultures were not diluted for the whole length of the experiment (6 days), whereas in batch/semi‐continuous, the cultures were not diluted for the first 3 days, then a semi‐continuous regime followed during which a 50% daily dilution rate was applied. In the second laboratory trial, a 4‐day growth experiment with UTEX 640 was performed in which a semi‐continuous regime (50% daily dilution) was applied since the first day. All the trials were carried out in 300‐mL glass tubes illuminated from one side with continuous light (250 μmol photons m−2 s−1) supplied by daylight fluorescent tubes (OSRAM, 39W/865). Temperature was kept at 20°C by immersing the tubes in a thermoregulated water bath. Cultures were bubbled with an air/CO2 mixture (98/2, v/v) to provide mixing and maintain pH in the range 7.5–8 as well as to supply carbon for growth. Both trials were carried out in duplicate. In all the cultures, the small amount of nitrogen introduced with the inoculum was depleted during the first day. Nitrogen was not replenished. Other nutrients such as phosphorus and iron were reintegrated according to growth.
For outdoor trials, UTEX 640 was grown in 40‐L GWP®‐III reactors (see following section), using F medium prepared from tap water filtered through 10 and 1 μm polypropylene filters (Domnick Hunter, St Neots, UK) and then sterile nutrient solutions were added with sterile nutrient solutions. Two batch trials were performed, one in summer and one in spring. The cultures were cooled when temperature exceeded 28.5°C; pH was maintained at 8.2 ± 0.1. In the first trial, performed in July, growth and fatty acid (FA) content of UTEX 640 was evaluated under nitrogen sufficiency, nitrogen starvation, and nitrogen limitation in duplicate cultures. As the results of the replicates were highly comparable, only the data from the culture used for FA analyses are shown. In the nitrogen‐replete and nitrogen‐limited cultures, nitrogen concentration (expressed as mg N per liter) was measured three times per day (at 8:30, 12:30, and 18:00) and each time restored to 50 mg L−1 in the nutrient‐replete cultures and to 7.5 mg L−1 in the nitrogen‐limited (L3) culture. The second trial was performed in April under nitrogen sufficiency, nitrogen starvation, and nitrogen limitation. Three different nitrogen supply regimes were adopted in the nitrogen limited cultures: (i) nitrogen was restored to 7.5 mg L−1 once per day (L1), at 8:30; (ii) twice per day (L2), at 8:30 and 12:30; and (iii) three times per day (L3), at 8:30, 12:30, and 18:00. Besides that contained in the inoculum, the total nitrogen supplied during the first 6 days of the growth period was 193.7 and 130.6 mg L−1 in the nitrogen‐replete summer and spring cultures, 94.5 and 70.8 mg L−1 in the L3 summer and spring cultures, and 47.7 mg L−1 in the L2, and 28.7 mg L−1 in the L1 spring cultures. In both trials, nitrogen was never restored in the starved cultures and that brought with the inoculum was depleted during the first day. Other nutrients (P and Fe) were supplied according to their relative content in the biomass and to productivity.
The Outdoor Culture System
The outdoor trials were carried out at the experimental area of Fotosintetica & Microbiologica S.r.l. located in Sesto Fiorentino (Florence, Italy) using GWP®‐III (Green Wall Panel) photobioreactors. GWP® is a registered trademark for a series of flat photobioreactors composed of a disposable culture chamber enclosed in a rigid metal frame. A detailed description and an energy analysis of the GWP®‐II are reported in Tredici et al. (2015) and an economic analysis of the system at 1‐ha scale in Tredici et al. (2016). The GWP®‐III used in this work is entirely made of stainless steel, which provides increased stability and longer duration. As in the previous designs, the external metal structure encloses a plastic culture chamber and allows maintaining an average light‐path of 4.8–5.2 cm. The reactor can be easily opened to replace the plastic chamber when weathered or damaged. The culture chamber is closed to limit airborne contaminations and allows gas collection and recycling to maximize CO2 utilization. In the present study, the size of each panel was 1.24 × 0.05 × 0.70 m (length, width, height), the culture surface exposed to direct radiation was about 0.78 m2, and the culture volume varied between 37 L and 43 L. Compressed air was injected for mixing through a perforated plastic pipe, running at the bottom of the culture, at a flow‐rate of 0.35 L L−1 min−1. CO2 was injected by a small bubble diffuser via a valve regulated by a pH control system. The reactors faced south; had an inclination of 50° with respect to the vertical; and were arranged in a single line with dummies in the front, back, and lateral sides in order to simulate a full‐scale plant and thus allow to calculate productivity per unit occupied land area (Tredici, 2004). The distance between the photobioreactors and the dummy rows was 1 m (Fig. 1).
Figure 1.

GWP®‐III reactors installed at the experimental area of Fotosintetica & Microbiologica S.r.l. (Sesto Fiorentino, Florence, Italy). Dummies are placed in front, back, and sides of the reactors to simulate a full‐scale plant. The panels face south with an inclination of 50°. The distance between rows is 1 m.
Analytical Procedures
Microalgal growth was estimated by biomass dry weight determination as reported by Guccione et al. (2014), but using ammonium formate (0.5 M), instead of distilled water, for washing the cells collected on the filter membrane. For outdoor cultures, the sampling was performed in the early morning. For lipid (laboratory trials) and fatty acid (outdoor trials) analyses, the cultures were harvested by centrifugation and the resulting biomass was washed twice with NaCl solution (9 g L−1), then frozen and lyophilized. The lyophilized biomasses were extracted and analyzed according to Rodolfi et al. (2009) for lipids and according to Abiusi et al. (2014) for fatty acids. Nitrate nitrogen concentration in the medium was measured spectrophotometrically (Cary 60 UV‐Vis, Agilent Technologies, Santa Clara, CA) by means of an analytical kit (H135, Hanna Instruments, Woonsocket, RI).
The productivity of outdoor cultures was expressed as areal productivity (in g m−2 d−1), that is, productivity per unit of culture surface area exposed to solar radiation (considering only the South facing surface of the reactor) and was calculated as the difference between biomass (or product) amount in the culture at the end and at the beginning of the trial divided by the culture surface area (m2) and then divided by the number of days of the trial. The biomass (or product) amount in the culture was calculated by multiplying its volumetric concentration (g L−1) by the culture volume (L). The land areal productivity was calculated by multiplying the culture areal productivity by the coefficient 0.65, since in the full‐scale arrangement adopted in our trials, 1 m2 of land allows to deploy 0.7 m2 of panel with 0.65 m2 of culture exposed to solar radiation. Global radiation values were obtained from LaMMA Consortium–Environmental Modelling and Monitoring Laboratory for Sustainable Development (Sesto Fiorentino, Florence, Italy).
Results and Discussion
Preliminary Laboratory Trials
A laboratory trial was performed with both UTEX 640 and UTEX 646 in 300‐mL bubbled tubes under continuous light (250 μmol photons m−2 s−1) to compare biomass productivity and lipid accumulation under nitrogen starvation in two different regimes: a 6‐day batch (6B) and a 3‐day batch followed by a 3‐day semi‐continuous cultivation (3B+3S). In the first period (days 1–3), when under both regimes the cultures were in batch, biomass productivity of UTEX 640 was more than double than that of UTEX 646 (Table I). In the second period (days 4–6), productivity was strongly reduced in both strains: UTEX 640 produced about 80% less both in batch and in semi‐continuous; UTEX 646 produced 60% less in batch and 83% less in semi‐continuous (Table I). In the second period, there was no significant difference in productivity between batch and semi‐continuous cultivation with UTEX 640, while UTEX 646 was by far less productive in semi‐continuous than in batch.
Table I.
Biomass productivity (mg L−1 d−1) and lipid content (% dry biomass) of UTEX 640 and UTEX 646 cultivated in 300‐mL bubbled tubes under nitrogen starvation
| Days 1–3 | Days 4–6 | |
|---|---|---|
| Biomass productivity (mg L−1 d−1) | ||
| UTEX 640 | ||
| 6B | 368.3 | 73.3 |
| 3B+3S | 348.3 | 69.3 |
| UTEX 646 | ||
| 6B | 158.3 | 63.3 |
| 3B+3S | 150.0 | 25.3 |
| Lipid content (% dry biomass) a | ||
| UTEX 640 | ||
| 6B | 32.1 | 37.1 |
| 3B+3S | 30.0 | 31.5 |
| UTEX 646 | ||
| 6B | 30.4 | 30.8 |
| 3B+3S | 29.1 | 31.0 |
Two culture regimes were tested: a 6‐day batch (6B) and a 3‐day batch followed by a 3‐day semi‐continuous phase (3B+3S).
The values refer to the last day of the period.
Concerning lipid content, except a higher value of 37% measured in UTEX 640 cultures at the end of the 6‐day batch (Table I) and at day 5 in the 3B+3S regime (data not shown), no differences were observed in both conditions and with both strains (Table I), all cultures showing an average lipid content of about 31% on dry biomass. It is to be noted that the initial lipid content was about 25% in all the cultures.
A second trial under nitrogen starvation was carried out in the laboratory only with UTEX 640 adopting a semi‐continuous regime (daily dilution of 50%) since the first day. The average biomass productivity was about half than that obtained with the same strain in batch (whole 6‐day period), while lipid content did not change in comparison with the previous experiment. In conclusion, the laboratory trials indicated that UTEX 640 is by far better than UTEX 646 for biomass and lipid production both in batch and semi‐continuous cultivation, and that a daily culture dilution (i.e., a semi‐continuous regime), adopted either from the starting day or after a short batch of three days, does not increase biomass productivity nor lipid content compared to a batch regime. According to these results, UTEX 640 and batch conditions were selected for outdoor trials.
There are several laboratory studies with P. tricornutum under nitrogen starvation, but different strains and conditions have been used. Breuer et al. (2012) cultivated in batch UTEX 640 under nitrogen starvation in shaken flasks for 14 days. Growth ceased after about 1 week and a maximum productivity of about 400 mg L−1 d−1, similar to what we obtained in the first half of the batch with the same strain, was achieved. After 14 days of cultivation fatty acid reached about 30% of dry biomass. A much higher lipid content (41% on dry biomass) was reported for P. tricornutum‐starved batch cultures after 9 days of cultivation under low light intensity and light/dark cycles by Kaixian and Borowitzka (1993). Shemesh et al. (2016) cultivated UTEX 646 under nitrogen starvation in shaken flasks for 7 days achieving a productivity of about 160 mg L−1 d−1 and a final fatty acid content of 25% on dry biomass. Results from laboratory experiments are difficult to compare because of the very different culture conditions (use of axenic and non axenic cultures, light intensity and cycle, temperature, culture vessels, etc.) adopted. However, a general behavior can be observed: nitrogen‐starved P. tricornutum cultures can grow for 3–7 days, productivity decreases with time, and lipids (fatty acids) are accumulated reaching maximum values varying from 25% to about 40% on dry biomass.
Outdoor Trials
For the outdoor trials, UTEX 640 was grown in 40‐L, N‐S oriented, 50°‐inclined GWP®‐III reactors (Fig. 1) in summer and spring under different nitrogen regimes (N‐replete, N‐limited, and N‐starved cultures). In summer, the nitrogen‐replete (control) and the nitrogen‐limited cultures had the same growth until the third day, then the replete culture prevailed (Fig. 2, left). Growth of the starved culture was much lower. In the three conditions, productivity did not vary much when calculated after 6 or 8 days of growth (Table II). The nitrogen‐replete culture achieved the highest areal productivity (17.2–17.8 g m−2 d−1) followed by the limited (12.3–12.8 g m−2 d−1) and the starved (8.7–7.9 g m−2 d−1) culture (Table II). These productivities, expressed on culture surfacearea, correspond to land areal productivities of 11.2, 8.0 and 5.6 g m−2 d−1 after 6 days and of 11.6, 8.3 and 5.1 g m−2 d−1 after 8 days, for the replete, limited and starved culture, respectively.
Figure 2.
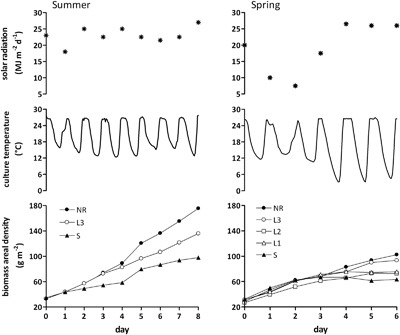
Growth of Phaeodactylum tricornutum UTEX 640 cultivated in 40‐L GWP®‐III reactors under different nitrogen supply regimes in summer (left) and spring (right). Global solar radiation and culture temperature are also shown. NR = nitrogen‐replete culture; L1, L2, L3 = limited cultures with nitrogen supplied once, twice, and thrice per day; S = starved culture. The data shown refer to one of two replicate cultures per each treatment. Differences between the replicates were not significant (P > 0.05).
Table II.
Biomass, total fatty acid (TFA), and EPA areal productivities (g m−2 d−1) of Phaeodactylum tricornutum UTEX 640 grown in 40‐L GWP®‐III reactors under different nitrogen supply regimes in summer and spring
| Biomass productivity (g m−2 d−1) | TFA productivity (g m−2 d−1) | EPA productivity (g m−2 d−1) | |
|---|---|---|---|
| Summer 6 days | |||
| NR | 17.20 | 1.24 | 0.38 |
| L3 | 12.25 | 1.51 | 0.37 |
| S | 8.68 | 2.62 | 0.13 |
| Summer 8 days | |||
| NR | 17.80 | 1.27 | 0.35 |
| L3 | 12.84 | 1.69 | 0.24 |
| S | 7.91 | 2.71 | 0.11 |
| Spring 6 days | |||
| NR | 12.30 | 0.87 | 0.29 |
| L3 | 10.30 | 1.60 | 0.28 |
| L2 | 7.62 | 1.53 | 0.22 |
| L1 | 7.39 | 1.96 | 0.18 |
| S | 5.20 | 2.34 | 0.16 |
NR = nitrogen‐replete culture; L1, L2, L3 = limited cultures with nitrogen supplied once, twice, and thrice per day; S = starved culture. In the summer trial productivities were measured both after 6 and 8 days.
In comparison with the 6‐day laboratory batch cultivation, where only starved cultures were tested and productivity was measured on a per volume basis, the outdoor starved batch culture of UTEX 640 obtained a 23% lower volumetric productivity (0.17 g L−1 d−1 in the 6‐day period). However, given the very different volume to surface ratio of the cultivation systems used in the laboratory (small bubbled tubes) and outdoors (the GWP®), any comparison in terms of volumetric productivity is of marginal value. For this reason productivity of laboratory cultures was expressed in terms of culture volume, while that of outdoor cultures in terms of culture (or land) area.
In spring, besides nutrient repletion and nitrogen starvation, three different nitrogen limitation regimes were tested: nitrogen was replenished once (L1), twice (L2), and thrice (L3) per day each time up to 7.5 mg L−1. This trial lasted only 6 days (Fig. 2, right). Culture growth was lower in all the conditions compared to the summer trial. All the cultures, including the starved one, had a similar growth until day 3, then started to differentiate and growth correlated with nitrogen availability. Biomass productivity during spring was always lower than in summer under the same conditions of nitrogen supply and, as expected, decreased with decreasing nitrogen availability (Table II).
To our knowledge, whereas P. tricornutum has been extensively cultivated in nutrient‐replete conditions, no studies are available with outdoor cultures under nitrogen deprivation. Chini Zittelli and Tredici (unpublished) cultivated UTEX 640 in a 93‐L tubular reactor (45° inclination) obtaining a culture areal productivity of 14 g m−2 d−1 in summer. In the same location, same season, and with the same strain, Benavides et al. (2013) attained land areal productivities of 11.7 and 13.1 g m−2 d−1 in circular ponds and a horizontal tubular photobioreactor adopting a daily dilution regime. In our study with UTEX 640, higher culture areal productivities (about 18 g m−2 d−1) compared to Chini Zittelli and Tredici (unpublished) and similar land areal productivities (about 12 g m−2 d−1) to those of Benavides et al. (2013) were measured. It is worth noting that in our work culture temperature was allowed to rise up to 28.5°C (Fig. 2), instead of 20°C (as in Benavides et al., 2013), the optimal growth temperature reported for Phaeodactylum. Much higher land areal productivities (32 g m−2 d−1) were obtained with the same strain in a two‐layer horizontal tubular photobioreactor operated under a continuous dilution regime by Molina et al. (2001). The reasons of this high productivities may reside in the high solar radiation of Southern Spain, where the study was conducted, the adoption of an optimal temperature of 20°C and light dilution by the two‐layer serpentine reactor (Tredici, 2010).
In the outdoor trials, instead of lipids, we determined total fatty acid (TFA) content because it better represents the potential for oil of production the microalga. Besides, the fatty acid profile better shows the suitability of the biomass for biodiesel (high content in saturated and monounsaturated fatty acids) or nutritional use (high content in PUFA). In both seasons, TFA content in the biomass at the end of the growth (Table III) was inversely proportional to the amount of nitrogen supplied, with the maximum value observed in the two starved cultures (24.6% in summer after 8 days and 25.6% in spring after 6 days). It is to be noted that in both seasons, nitrogen starvation more than tripled TFA content in comparison to the control (from 7% to 25%).
Table III.
Total fatty acid (TFA), EPA content (% dry biomass; mean values ± SD), and fatty acid composition (% of TFA) of Phaeodactylum tricornutum UTEX 640 cultivated in 40‐L GWP®‐III reactors under different nitrogen supply regimes in summer and spring
| TFA a (% dry biomass) | EPA (% dry biomass) | Sat (% TFA) | Mon (% TFA) | PUFA b (% TFA) | EPA (% TFA) | |
|---|---|---|---|---|---|---|
| Summer 6 days | ||||||
| NR | 7.17 ± 0.25 | 2.26 ± 0.16 | 20.92 | 17.29 | 61.92 | 31.52 |
| L3 | 10.68 ± 0.49 | 2.81 ± 0.31 | 24.91 | 25.75 | 49.34 | 26.31 |
| S | 20.91 ± 2.62 | 1.83 ± 0.38 | 39.93 | 43.95 | 16.21 | 8.75 |
| Summer 8 days | ||||||
| NR | 7.11 ± 0.66 | 2.06 ± 0.28 | 23.21 | 20.82 | 55.84 | 36.57 |
| L3 | 11.65 ± 1.33 | 1.98 ± 0.09 | 29.87 | 31.76 | 38.37 | 17.00 |
| S | 24.58 ± 1.07 | 1.78 ± 0.00 | 41.58 | 45.28 | 13.18 | 7.24 |
| Spring 6 days | ||||||
| NR | 7.05 ± 0.67 | 2.38 ± 0.24 | 22.13 | 18.72 | 59.00 | 33.76 |
| L3 | 12.65 ± 0.21 | 2.60 ± 0.17 | 32.25 | 31.78 | 35.97 | 20.55 |
| L2 | 15.24 ± 1.11 | 2.66 ± 0.30 | 34.91 | 34.19 | 30.38 | 17.45 |
| L1 | 18.48 ± 2.02 | 2.42 ± 0.40 | 39.39 | 36.04 | 24.03 | 13.09 |
| S | 25.63 ± 1.54 | 2.67 ± 0.23 | 41.47 | 40.11 | 18.03 | 10.42 |
NR = nitrogen‐replete culture; L1, L2, L3 = limited cultures with nitrogen supplied once, twice, and thrice per day; S = starved culture; Sat = saturated FA; Mon = monounsaturated FA; PUFA = polyunsaturated FA; EPA = eicosapentaenoic acid. Data refer to the last day of the period. In the summer trial, analyses were done both after 6 and 8 days.
TFA content at the beginning of the trial was about 7% on dry biomass both in summer and spring trials.
Including EPA.
Similarly to TFA content, also TFA productivity increased as nitrogen availability in the culture decreased and, in both seasons, reached the maximum value (2.3–2.7 g m−2 d−1) under starvation, despite the lower biomass productivities of starved cultures (Table II). In spring, the EPA content of biomass was rather stable (around 2.4–2.7%), while in summer, it decreased significantly in the starved culture (to about 1.8%) (Table III). EPA productivity was directly related with biomass productivity and thus with the amount of nitrogen added (Table II). The highest EPA productivity (0.35–0.38 g m−2 d−1) was obtained in the summer nitrogen‐replete culture, and the lowest in the summer‐starved cultures (0.11–0.13 g m−2 d−1) (Table II). In both summer and spring, saturated and monounsaturated fatty acids on biomass and on TFA increased as nitrogen availability in the culture decreased (Table III). The most suitable biomasses for biodiesel production were those attained under nitrogen starvation, where saturated and monounsaturated fatty acids surpassed 80% of TFA (Table III). The most valuable biomasses for their content of nutritionally important fatty acids were those produced under replete conditions, where PUFA reached about 60% of TFA and EPA more than 30% of TFA and 2.2% of dry biomass (Table III). A similar content of EPA on fatty acid was found by Benavides et al. (2013), and a similar EPA content in the biomass was reported by Acién Fernández et al. (2000).
In our work, the most suitable biomasses for biodiesel production were those attained under nitrogen starvation. Nitrogen limitation did not improve fatty acid productivity nor fatty acid content compared with starvation. Oil production under nitrogen limitation has been reported for indoor cultures of other microalgae, among these Neochloris oleoabundans (Klok et al., 2013) and Isochrysis sp. (Lacour et al., 2012), although in this latter study, neutral lipid productivity was enhanced more by nitrogen starvation, as it was in our case.
Taking into account the averages of summer and spring productivities per unit land area of EPA under nutrient replete conditions (0.2 g m−2 d−1) and of FA under nitrogen starvation (1.6 g m−2 d−1), we can estimate, for more suitable countries where cultures can be grown outdoors all year round, a potential annual yield of about 730 kg of EPA per hectare in nutrient sufficient cultures and of about 5,800 kg of fatty acids per hectare in nitrogen‐deprived cultures.
Conclusions
Laboratory experiments under suitable light intensity were good predictors of performance of outdoor P. tricornutum cultures, allowed to select the better strain (UTEX 640) and showed that nitrogen‐starved cultures can increase biomass and store lipid for about 1 week. The outdoor experiments in GWP® reactors showed that the most suitable biomasses for biodiesel are attained under nitrogen starvation (reaching 25% FA content and more than 80% saturated and monounsaturated fatty acids on TFA), while the most nutritionally valuable are those produced under nutrient replete conditions (which reach about 60% PUFA and 30% EPA on TFA).
This work was supported by the European Commission FP7 projects Fuel4Me (Grant No. 308983) and GIAVAP (Grant No. 266401). The authors wish to thank Lucilla Taddei and Giulia Padovani for their technical assistance in part of the laboratory experiments, and Filippo Bacci and Folco Tredici for their assistance in assembling and maintenance of the outdoor equipment.
Conflict of interest: Liliana Rodolfi, Niccolò Bassi, and Mario R. Tredici have a financial interest in F&M S.r.l.; Natascia Biondi, Alessia Guccione, Massimo D'Ottavio, and Gimena Arganaraz have no conflict of interest.
References
- Abiusi F, Sampietro G, Marturano G, Biondi N, Rodolfi L, D'Ottavio M, Tredici MR. 2014. Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M‐M33 grown with LEDs of different colors. Biotechnol Bioeng 111:956–964. [DOI] [PubMed] [Google Scholar]
- Acién Fernández FG, Sánchez Pérez JA, Fernández Sevilla JM, García Camacho F, Molina Grima E. 2000. Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. Biotechnol Bioeng 68:173–183. [DOI] [PubMed] [Google Scholar]
- Benavides AM, Torzillo G, Kopecký J, Masojídek J. 2013. Productivity and biochemical composition of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in tubular photobioreactors and open ponds. Biomass Bioenergy 54:115–122. [Google Scholar]
- Benvenuti G, Bosma R, Klok AJ, Ji F, Lamers PP, Barbosa MJ, Wijffels RH. 2015. Microalgal triacylglycerides production in outdoor batch operated tubular PBRs. Biotechnol Biofuels 8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi N, Bassi N, Chini Zittelli G, De Faveri D, Giovannini A, Rodolfi L, Allevi C, Macrì C, Tredici MR. 2013. Nannochloropsis sp. F&M‐M24: Oil production, effect of mixing on productivity and growth in an industrial wastewater. Environ Prog Sustain Energy 32:846–853. [Google Scholar]
- Biondi N, Fonseca D, Sampietro G, Santos E, Costa L, Verdelho V, Piana A, Carlini D, Mangini S, Bassi N, Rodolfi L, Prussi M, D'Ottavio M, Khozin‐Goldberg I, Boussiba S, Tredici MR. 2016. Cultivation of Nannochloropsis oceanica F&M‐M24 and Tetraselmis suecica F&M‐M33 in the two 0.5‐ha BIOFAT pilot plants for biofuel production. Madrid, Spain: Algae Europe, 13–15 December, 2016. Book of Abstracts. p 71–72.
- Bondioli P, Della Bella L, Rivolta G, Chini Zittelli G, Bassi N, Rodolfi L, Casini D, Prussi M, Chiaramonti D, Tredici MR. 2012. Oil production by the marine microalgae Nannochloropsis sp. F&M‐M24 and Tetraselmis suecica F&M‐M33. Bioresour Technol 114:567–572. [DOI] [PubMed] [Google Scholar]
- Borowitzka MA. 2013. High‐value products from microalgae—Their development and commercialization. J Appl Phycol 25:743–756. [Google Scholar]
- Borowitzka MA, Moheimani NR. 2013. Sustainable biofuels from algae. Mitig Adapt Strateg Glob Change 18:13–25. [Google Scholar]
- Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH. 2012. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226. [DOI] [PubMed] [Google Scholar]
- Caporgno MP, Olkiewicz M, Fortuny A, Stüber F, Fabregat A, Font J, Pruvost J, Lepine O, Legrand J, Bengoa C. 2016. Evaluation of different strategies to produce biofuels from Nannochloropsis oculata and Chlorella vulgaris . Fuel Process Technol 144:132–138. [Google Scholar]
- Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee D‐J, Chang J‐S. 2017. Microalgae biorefinery: High value products perspective. Bioresour Technol 229:53–62. [DOI] [PubMed] [Google Scholar]
- Chini Zittelli G, Rodolfi L, Bassi N, Biondi N, Tredici MR. 2013. Photobioreactors for microalgae biofuel production In: Borowitzka MA, Moheimani NR, editors. Algae for biofuels and energy. Developments in applied phycology. Vol. 5. Dodrecht: Springer; p 115–131. [Google Scholar]
- Chisti Y. 2007. Biodiesel from microalgae. Biotechnol Adv 25:294–306. [DOI] [PubMed] [Google Scholar]
- Chrismadha T, Borowitzka MA. 1994. Effect of cell density and irradiance on growth, proximate composition and eicosapentaenoic acid production of Phaeodactylum tricornutum grown in a tubular photobioreactor. J Appl Phycol 6:67–74. [Google Scholar]
- Cohen Z. 1999. Production of polyunsaturated fatty acids by the microalga Porphyridium cruentum In: Cohen Z, editor. Production of chemicals by microalgae. London: Taylor and Francis; p 1–24. [Google Scholar]
- Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M. 2009. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48:1146–1151. [Google Scholar]
- De Bhowmick G, Koduru L, Sen R. 2015. Metabolic pathway engineering towards enhancing microalgal lipid biosynthesis for biofuel application—A review. Renew Sustain Energy Rev 50:1239–1253. [Google Scholar]
- Griffith MJ, Harrison STL. 2009. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507. [Google Scholar]
- Guccione A, Biondi N, Sampietro G, Rodolfi L, Bassi N, Tredici MR. 2014. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol Biofuels 7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guihéneuf F, Leu S, Zarka A, Khozin‐Goldberg I, Khalilov I, Boussiba S. 2011. Cloning and molecular characterization of a novel acyl‐CoA:diacylglycerol acyltransferase 1‐like gene (PtDGAT1) from the diatom Phaeodactylum tricornutum . FEBS J 278:3651–3666. [DOI] [PubMed] [Google Scholar]
- Guillard RRL, Ryther JH. 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve). Can J Microbiol 8:229–239. [DOI] [PubMed] [Google Scholar]
- Hariskos I, Posten C. 2014. Biorefinery of microalgae—Opportunities and constraints for different production scenarios. Biotechnol J 9:739–752. [DOI] [PubMed] [Google Scholar]
- Ho S‐H, Chen C‐Y, Chang J‐S. 2012. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW‐N. Bioresour Technol 113:244–252. [DOI] [PubMed] [Google Scholar]
- Kaixian Q, Borowitzka M. 1993. Light and nitrogen deficiency effects on the growth and the composition of Phaeodactylum tricornutum . Appl Biochem Biotechnol 38:93–103. [Google Scholar]
- Klok AJ, Martens DE, Wijffels RH, Lamers PP. 2013. Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour Technol 134:233–243. [DOI] [PubMed] [Google Scholar]
- Lacour T, Sciandra A, Talec A, Mayzaud P. 2012. Neutral lipid and carbohydrate productivities as a response to nitrogen status in Isochrysis sp. (T‐iso; Haptophyceae): Starvation versus limitation. J Phycol 48:647–656. [DOI] [PubMed] [Google Scholar]
- López Alonso D, Belarbi E‐H, Fernández Sevilla JM, Rodríguez‐Ruiz J, Molina Grima E. 2000. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum . Phytochemistry 54:461–471. [DOI] [PubMed] [Google Scholar]
- MacIntyre HL, Cullen JJ. 2005. Using cultures to investigate the physiological ecology of microalgae In: Andersen RA, editor. Algal culturing techniques. Burlington: Academic Press; p 287–326. [Google Scholar]
- Mata TM, Martins AA, Caetano NS. 2010. Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev 14:217–232. [Google Scholar]
- Meiser A, Schmid‐Staiger U, Trosh W. 2004. Optimization of eicosapentaenoic acid production by Phaeodactylum tricornutum in the flat panel airlift (FPA) reactor. J Appl Phycol 16:215–225. [Google Scholar]
- Molina E, Fernández J, Acién FG, Chisti Y. 2001. Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Maillet G, Cullen JJ. 2001. Fluorescence‐based maximal quantum yield for PSII as a diagnostic of nutrient stress. J Phycol 37:517–529. [Google Scholar]
- Parrish C, Wangersky PJ. 1987. Particulate and dissolved lipid classes in cultures of Phaeodactylum tricornutum grown in cage culture turbidostats with a range of nitrogen supply rates. Mar Ecol Progr Ser 35:119–128. [Google Scholar]
- Piorreck M, Baasch K, Pohl P. 1984. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue‐green algae under different nitrogen regimes. Phytochemistry 23:207–216. [Google Scholar]
- Pruvost J, Van Vooren G, Cogne G, Legrand J. 2009. Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100:5988–5995. [DOI] [PubMed] [Google Scholar]
- Qiao H, Cong C, Sun C, Li B, Wang J, Zhang L. 2016. Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum . Aquaculture 452:311–317. [Google Scholar]
- Rawat I, Kumar RR, Mutanda T, Bux F. 2013. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl Energy 103:444–467. [Google Scholar]
- Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR. 2009. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low‐cost photobioreactor. Biotechnol Bioeng 102:100–112. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith H, Eppink MHM, Kleinegris DMM, Wijffels RH, Barbosa MJ. 2016. Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043. [Google Scholar]
- Shemesh Z, Leu S, Khozin‐Goldberg I, Didi‐Cohen S, Buossiba S. 2016. Inducible expression of Haematococcus oil globule protein in the diatom Phaeodactylum tricornutum: Association with lipid droplets and enhancement of TAG accumulation under nitrogen starvation. Algal Res 18:321–331. [Google Scholar]
- Spolaore P, Joannis‐Cassan C, Duran E, Isambert A. 2006. Commercial applications of microalgae. J Biosci Bioeng 2:87–96. [DOI] [PubMed] [Google Scholar]
- Torzillo G, Faraloni C, Silva AM, Kopecký J, Pilný J, Masojídeck J. 2012. Photoacclimation of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in photobioreactors and open ponds. Eur J Phycol 47:167–181. [Google Scholar]
- Tredici MR. 2004. Mass production of microalgae: Photobioreactors In: Richmond A, editor. Handbook of microalgal culture: Biotechnology and applied phycology. Oxford: Blackwell Science Ltd; p 178–214. [Google Scholar]
- Tredici MR. 2010. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 1:143–162. [Google Scholar]
- Tredici MR, Bassi N, Prussi M, Biondi N, Rodolfi L, Chini Zittelli G, Sampietro G. 2015. Energy balance of algae biomass production in a 1‐ha “Green Wall Panel” plant: How to produce algae biomass in a closed reactor achieving a high net energy ratio. Appl Energy 154:1103–1111. [Google Scholar]
- Tredici MR, Biondi N, Ponis E, Rodolfi L, Chini Zittelli G. 2009. Advances in microalgal culture for aquaculture feed and other uses In: Burnell G, Allan G, editors. New technologies in aquaculture: Improving production efficiency, quality and environmental management. Cambridge: Woodhead Publishing; p 610–676. [Google Scholar]
- Tredici MR, Rodolfi L, Biondi N, Bassi N, Sampietro G. 2016. Techno‐economic analysis of microalgal biomass production in a 1‐ha Green Wall Panel (GWP®) plant. Algal Res 19:253–263. [Google Scholar]
- Trivedi J, Aila M, Bangwal DP, Kaul S, Garg MO. 2015. Algae based biorefinery—How to make sense? Renew Sustain Energy Rev 47:295–307. [Google Scholar]
- Velmurugan N, Sung M, Yim SS, Park MS, Yang JW, Jeong KJ. 2014. Systematically programmed adaptive evolution reveals potential role of carbon and nitrogen pathways during lipid accumulation in Chlamydomonas reinhardtii . Biotechnol Biofuel 7:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongmanitchai W, Ward OP. 1991. Growth of and omega‐3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl Environ Microbiol 57:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]


