Abstract
Objective
To study the effect and mechanism of geraniin on the osteogenesis of tumour necrosis factor-alpha (TNF-α)-induced bone marrow-derived mesenchymal stem cells (BMSCs).
Methods
BALB/c mice aged 4–6 weeks were used to collect BMSC. Methyl thiazol tetrazolium assay and lactate dehydrogenase assay were used to analyse the effect of geraniin on the TNF-α-induced impairments of osteogenesis in BMSCs. BMSCs were stained using Oil red to measure the osteogenesis. Real-time reverse transcription PCR and western blot analysis were used to analyse the expression of RunX2 and Osx miRNA, and expression of NF-κB, IкB-α and p38 MAPK protein in BMSCs.
Results
2.5 µM geraniin significantly inhibited TNF-α-induced BMSCs cytotoxicity. 2.5 µM geraniin significantly reduced the expression of RunX2 and Osx miRNA in TNF-α-induced BMSCs. 2.5 µM geraniin suppressed the expression of NF-κB and p38 MAPK protein and promoted the expression of IкB-α protein in the TNF-α-induced BMSCs.
Conclusion
Geraniin inhibits TNF-α-induced impairments of osteogenesis through NF-κB/IкB-α and p38 MAPK signalling pathways in BMSCs.
Keywords: geraniin, TNF-α, osteogenesis, bone marrow stem cell
Introduction
The normal bone metabolism is extremely essential for maintaining the bone mineral density and bone strength, which is mainly determined by a delicate balance between bone formation by anabolic activities of osteoblasts and bone resorption by catabolic effects of osteoclasts.1 Since bone marrow is a good source of mesenchymal stem cells which have clonogenicity and multipotential characteristics, it is the most commonly used cell therapy for bone regeneration in the orthopaedics.2 Bone marrow-derived mesenchymal stem cells (BMSCs), a kind of adult stem cells, have the same hereditary basis with other tissues.3 BMSCs can be differentiated into osteoblasts, chondrocytes, adipocytes and myoblasts.4 Mankani et al reported that transplantation of BMSC-conjugated hydroxyapatite/tricalcium phosphate particles was able to heal craniofacial bone defects for a long term.5 Quarto et al first presented the preliminary clinical data of three patients with the treatment of autologous BMSCs and hydroxyapatite porous blocks in long bone defects. Complete fusion was observed in all three patients.6 Therefore, the proliferation and differentiation of BMSCs is critical for the osteogenesis.7
Increasing evidences proved that the inflammation could disturb the balance between bone formation and resorption resulting in osteoporosis or osteonecrosis.8 The proliferation and osteogenic differentiation of BMSCs could be also affected by inflammatory cytokines including tumour necrosis factor-alpha (TNF-α), interleukin 1 (IL-1), IL-6 and interferon γ (IFN-γ).9 TNF-α is an important inflammatory cytokine which can regulate cellular proliferation, differentiation and apoptosis by activating various signal pathways and molecular events.10 TNF-α can cause osteoclast-induced bone destruction, inhibit the osteoblastogenesis and regulate the migration of BMSCs in vivo.11 12 Lu et al demonstrated that TNF-α inhibited osteoblasts differentiation from precursor cells, reduced expression of RUNX2 and osteoblast-associated transcription factors (Osterix) and suppressed vitamin D-stimulated transcription owing to the activation of transcription factor Nuclear factor kappa-light-chain-enhancer of activated B cellI (NF-κB).13 Hess et al showed that TNF-α increased the expression of bone morphogenetic protein-2 (BMP-2) in BMSCs through NF-κB signalling pathway in early osteogenic differentiation and NF-κB stimulated key regulators of osteogenesis, such as BMP-2, RUNX2 and Osterix, resulting in enhanced mineralisation of the extracellular matrix.14 Huang et al reported that levels of Runx2, Osx and ALP were upregulated at lower concentration of TNF-α, but downregulated at higher concentration of TNF-α causing dose-dependent effects of TNF-α on BMSCs’ osteogenic differentiation.15 These findings suggested that the binding of TNF-α to its receptors resulted in activating multiple signalling pathways.
Geraniin is a kind of polyphenols extracted from under leaf pearl, which is widely used to treat hepatitis, enteritis, dysentery, nephritis and oedema.16 Recently, Okabe et al reported that geraniin could relieve pain and reduce blood pressure through inhibiting releasing TNF-α.17 Xiao et al demonstrated that geraniin inhibited RANKL-induced osteoclastogenesis in a dose-dependent manner through NF-κB and Extracellular-signal Regulated protein Kinase (ERK) signalling pathways, which might be one of the mechanisms of anti-osteoporosis effects.18
In this study, the effects of geraniin on TNF-α-induced impairments of osteogenesis in BMSCs were studied by using methyl thiazol tetrazolium (MTT) assay and lactate dehydrogenase (LDH) assay. Oil red was used to measure osteogenesis of BMSCs. Real-time PCR and western blot analysis were used to analyse the miRNA expression of RunX2 and Osx, and detect the protein expression of NF-κB/Inhibitor of nuclear factor kappa-B, alpha (IкB-α) and p38 Mitogen Activated Proteinkinase (MAPK). In summary, the aim of this study is to unveil the mechanisms of the inhibitory effects of geraniin on TNF-α-induced impairments of osteogenesis in BMSCs.
Materials and methods
Isolation and culture of BMSCs
All experiments were performed following the guidelines of Animal Use and Care Committee. BALB/c mice aged 4–6 weeks were aseptically raised in the clean animal room. The mice BMSCs were harvested from long bones in the aseptic condition after carefully trimming excess tissues and washing marrow cavity using Phosphate Buffered Solution at 4°C. Subsequently, BMSCs were centrifugated at 2000 rpm for 5 min prior to resuspending with 5 mL Dulbecco’s modified eagle medium (Gibco, USA) plus 10% fetal bovine serum (Gibco, USA) in 25 cm2 flasks. After 24 hours, the floating cells were removed and the adherent cells were cultured at 37°C with 5% humidified CO2.
The adherent cells were passaged by trypsinisation when reaching 80%–90% confluency and subcultured at a density of 4000–5000 cells/cm2. Meanwhile, adherent cells were incubated with 0.25% trypsin and 1 mM Ethylene Diamine Tetraacetic Acid at 37°C for 5 min. BMSC were subcultured four times and incubated for 3 days after labelling with 3 µg/mL of bromodeoxyuridine.19
MTT assay
BMSCs were seeded into a 96-well plate at a density of 2×104 cells/well, and the fresh medium was supplemented with 20 ng/mL TNF-α or 0, 1.25, 2.5, 5, 10, 20 µM geraniin for 5 days. Subsequently, 20 µL 5 mg/mL MTT (Sigma) was added into each well. After 4-hour incubation, the absorbancy of each well was measured by using an ELX800 absorbance microplate reader (Bio-Tek, USA) at 560 nm.
LDH assay
BMSCs were seeded into a 96-well plate at a density of 2×104 cells/well, and the fresh medium was supplemented with 20 ng/mL TNF-α or 2.5 µM geraniin for 1 day. Then, 50 µL LDH was added into each well at 37°C for 30 min protecting from light. The absorbancy was measured at 450 nm using an ELX800 absorbance microplate reader.
Oil red O stain
BMSCs were seeded into a 96-well plate at a density of 2×106 cells/well, and the fresh medium was supplemented with 20 ng/mL TNF-α or 2.5 µM geraniin for 1 day. BMSCs was fixed by using 5% precool paraformaldehyde for 30 min at 4°C. 0.6% (w/v) oil red was added into each well and stained for 15 min at 37°C. BMSCs were carefully washed to remove unbound dyes and then immersed with 1 mL isopropyl alcohol for 10 min at 37°C. BMSCs were observed through fluorescent microscope (D5300, Nikon, Japan) at 510 nm. Three different visual fields of control group and geraniin-treated group containing approximately 10 cells were analysed, and triplicate experiments were done at three independent time points. The mean fluorescence intensity (MFI) of the whole cell was separately analysed by Image J software.
Real-time reverse transcription PCR
BMSCs were seeded into a 96-well plate at a density of 2×106 cells/well and the fresh medium was supplemented with 20 ng/mL TNF-α or 2.5 µM geraniin for 1 day. RNeasy Mini kit (Qiagen, USA) was used to collect total RNA from each well according to the manufacturer’ s instructions. One microgram of total RNA was used to synthesise cDNA using reverse transcriptase (TaKaRa Biotechnology, Japan). Then, the expression of miRNA was detected by using Real-time PCR ABI 7500 Sequencing Detection System (Applied Biosystems, USA) and a SYBR Premix Ex Tag kit (TaKaRa Biotechnology, Japan). The primers of Runx2 were as follows: 5′-TCTTAGAACAAATTCTGCCCTTT-3′ and 5′-TGCTTTGGTCTTGAAATCACA-3′; the primers of Osx were as follows: 5′-CCTCCTCAGCTCACCTTCTC-3′ and 5′-GTTGGGAGCCCAAATAGAAA-3′; the primers of GAPDH were as follows: 5′-GAAGGTGAAGGTCGGAGTC-3′ and 5′-GAGATGGTGATGGGATTTC-3′. Real-time PCR was performed under the following conditions: 40 cycles of 95°C for 35 s and 60°C for 35 s.20
Western blot analysis
BMSCs were seeded into a 96-well plate at a density of 2×106 cells/well, and the fresh medium was supplemented with 20 ng/mL TNF-α or 2.5 µM geraniin for 1 day. BMSCs were lysed using radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, USA) for 20–30 min on ice. The protein concentration was measured using a bicinchoninic acid protein assay (Thermo Fisher Scientific, USA). Thirty microgram proteins were separated using 10%–12% SDS-PAGE (Beyotime Institute of Biotechnology, China), transferred onto polyvinylidene difluoride membranes, blocked with 5% skimmed milk in TBS–Tween (0.2% Tween-20) for 2 hour at 37°C and then incubated with primary antibodies overnight at 4°C including anti-NF-κB/p65 (1:2000, Cell Signaling Technology, USA), anti-IкB-α (1:2000, Cell Signaling Technology, USA), anti-p38 MAPK(1:2000, Cell Signaling Technology, USA) and β-actin (Beyotime Institute of Biotechnology, China). Membranes were incubated with the appropriate secondary antibodies (Beyotime Institute of Biotechnology, China) conjugated with an IRDye 800CW. Immunoblots were analysed with Image-Pro Plus 5.0 software (Media Cybernetics, USA).
Statistical analysis
All data in graphs are generated from at least three independent experiments and expressed as the mean±SD. Student’s t-test was used to determine the statistical significance, and values of p<0.05 were considered to be statistically significant.
Results
Identification of BMSCs
Cell morphology was observed by a light microscope with staining of bromodeoxyuridine. After 24 hours of incubation, most BMSCs had already adhered to the surface of culture flasks (figure 1A). BMSCs exhibited a fibrocyte-like form with the blue nucleus and a long spindle shape after being cultured for 4 days (figure 1B).
Figure 1.

The morphology of BMSCs was observed by light microscope. (A) Primary BMSCs cultured for 24 hours; (B) primary BMSCs cultured for 4 days. The scale bar is 10 µm. BMSCs, bone marrow-derived mesenchymal stem cells.
Effect of geraniin on TNF-α-induced BMSCs cytotoxicity
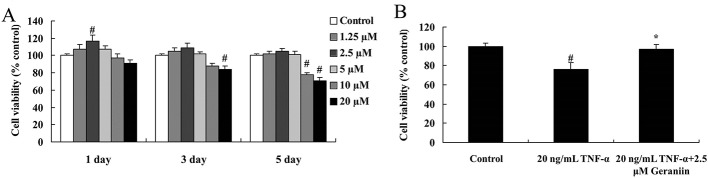
MTT assay was used to detect the effect of geraniin on TNF-α-induced BMSCs’ viability. After being treated with 2.5 µM geraniin for 1 day, the viability of BMSCs was significantly increased, compared with control group (p<0.01). However, the viability of BMSCs was significantly suppressed with treatment of 20 µM geraniin at 3 days (p<0.01). After being treated with 10 or 20 µM geraniin for 5 days, the BMSCs’ viability significantly decreased (p<0.01) (figure 2A). Therefore, treating with 2.5 µM geraniin for 1 day was selected as optimal condition in this study. The viability of BMSCs was significantly reduced after treating with 20 ng/mL TNF-α (p<0.01); however, 2.5 µM geraniin could significantly improve TNF-α-induced BMSCs cytotoxicity (p<0.05) (figure 2B). Moreover, LDH assay was used to investigate the effect of geraniin on TNF-α-induced BMSCs cytotoxicity. As shown in figure 3, TNF-α showed significant BMSCs cytotoxicity at the dose of 20 ng/mL. In contrast, 2.5 µM geraniin could significantly diminished TNF-α-induced BMSCs cytotoxicity (figure 3).
Figure 2.
(A) The effects of geraniin with different concentrations on BMSCs’ viability; (B) effect of geraniin on TNF-α-induced BMSC viability. # p<0.01 compared with control group, *p<0.05 compared with 20 ng/mL TNF-α group. BMSCs, bone marrow-derived mesenchymal stem cells; TNF-α, tumour necrosis factor-alpha.
Figure 3.
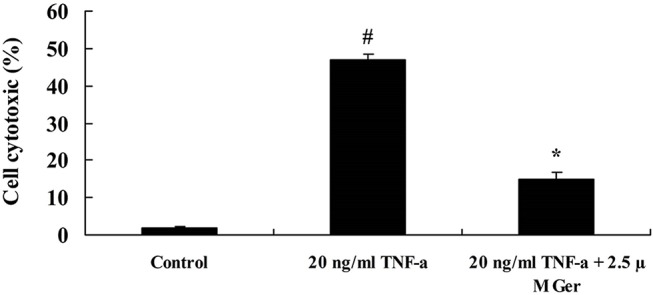
Effect of geraniin on TNF-α-induced BMSCs cytotoxicity. # p<0.01 compared with control group, *p<0.05 compared with 20 ng/mL TNF-α group. BMSCs, bone marrow-derived mesenchymal stem cells; Ger, geraniin; TNF-α, tumour necrosis factor-alpha.
Effect of geraniin on osteogenesis in BMSCs
To investigate the effect of geraniin on osteogenesis in BMSCs, Oil red O stain was used to detected osteogenesis of BMSCs. As shown in figure 4, the red area of control group (figure 4A) was lower than that of geraniin group (figure 4B). The MFI of BMSCs nucleus in the control group was significantly lower than that of geraniin group (p<0.05) (figure 4C).
Figure 4.
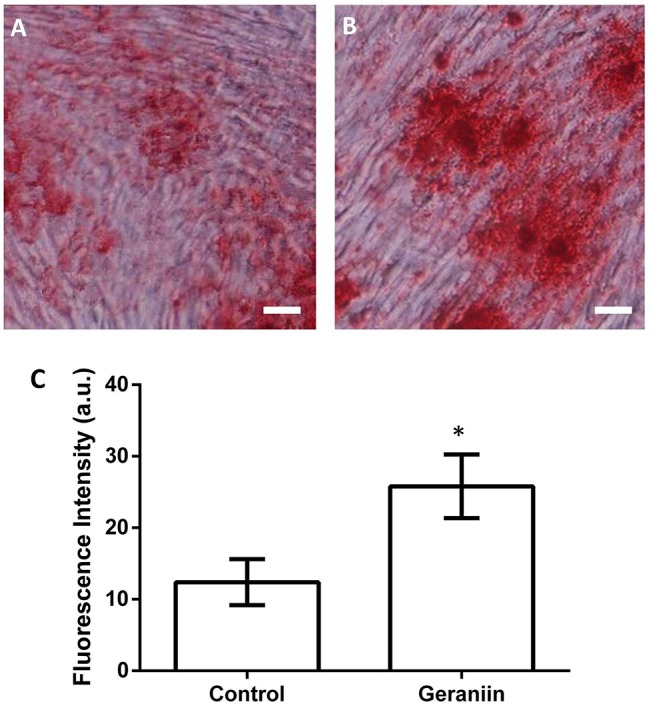
Effect of geraniin on osteogenesis in BMSCs. *p<0.01 compared with control group; the scale bar is 10 µm. BMSCs, bone marrow-derived mesenchymal stem cells.
The expression of miRNA Runx2 and Osx
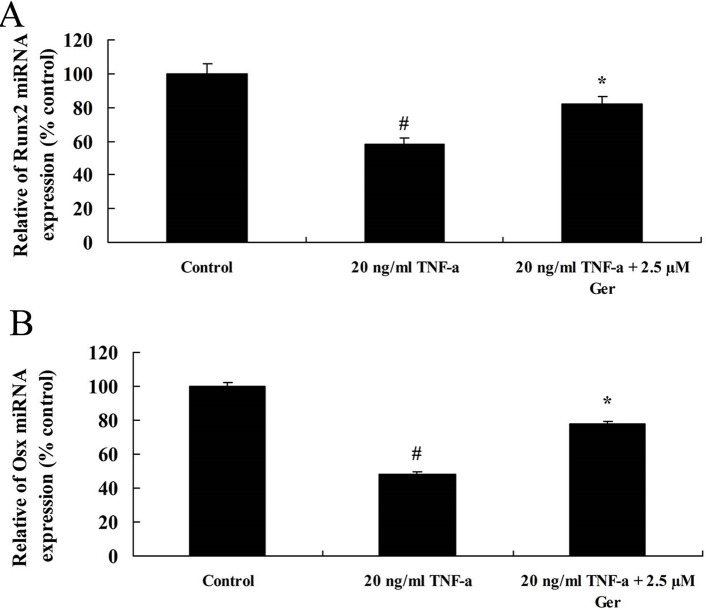
To unveil the mechanisms on the effect of geraniin on TNF-α-induced impairment of BMSCs, the expression of miRNA Runx2 and Osx were measured using real-time PCR in this study. The expression of miRNA Runx2 and Osx was significantly decreased in BMSCs after treating with 20 ng/mL TNF-α compared with control group. In contrast, the expression of miRNA Runx2 and Osx was significantly upregulated by treating with 2.5 µM geraniin (figure 5).
Figure 5.
The expression of Runx2 (A) and Osx (B) in BMSCs treating with TNF-α or TNF-α+Ger. # p<0.05 compared with control group, *p<0.05 compared with TNF-α group. BMSCs, bone marrow-derived mesenchymal stem cells; Ger, geraniin; TNF-α, tumour necrosis factor-alpha.
Effect of geraniin on TNF-α-activated signal pathways in BMSCs
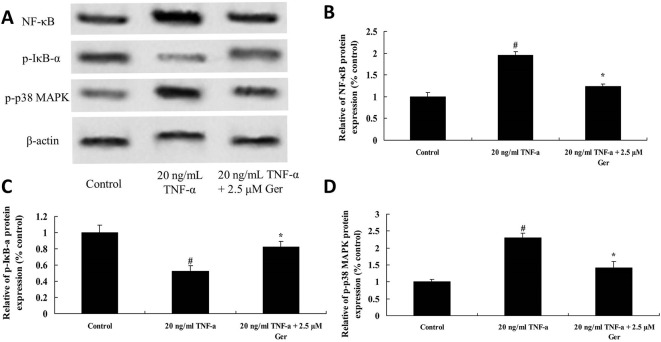
To investigate mechanisms on the effect of geraniin on TNF-α-induced impairment of BMSCs, NF-κB/p65, p-IкB-α and p38 MAPK protein expression was detected using western blot analysis. As shown in figure 6A, the expression of NF-κB/p65 and p38 MAPK protein in TNF-α-treated BMSCs was higher than that in control group; however, TNF-α significantly inhibited the expression of p-IкB-α protein in BMSCs. After treating with 2.5 µM geraniin, the expression of NF-κB/p65 and p38 MAPK significantly reduced in BMSCs. In contrast, geraniin significantly increased the expression of p-IкB-α protein in TNF-α-treated BMSCs (figure 6B-D).
Figure 6.
Effect of geraniin on TNF-α-activated NF-κB, p-IкB-α and p38 MAPK protein expression in BMSCs, including western blot analysis (A) and statistics of NF-κB (B), p-IкB-α (C) and p38 MAPK (D) protein expression. # p<0.01 compared with control group, *p<0.05 compared with TNF-α group. BMSCs, bone marrow-derived mesenchymal stem cells; Ger, geraniin; TNF-α, tumour necrosis factor-alpha.
Discussion
BMSCs are pluripotent stem cells derived from various organs or tissues. In the normal conditions, most of BMSCs stay in the dormant state; however, under pathological conditions, the renewability of BMSCs will be activated.21 BMSCs can be differentiated into osteoblasts, chondrocytes, adipocytes and myoblasts.4 Various evidences demonstrated that BMSCs played an essential role in osteogenesis and inflammatory cytokines could disrupt the balance between bone formation and bone resorption.1 2 TNF-α is an important inflammatory cytokine regulating proliferation and differentiation of BMSCs, resulting in osteoclast-induced bone destruction and inhibiting osteogenesis.10–12 In this study, we demonstrated that 2.5 µM geraniin could decrease TNF-α-induced BMSCs cytotoxicity. Lu et al indicated that geraniin was able to prevent ovariectomy-induced osteoporosis in rats.16 Park et al suggested that soybean-treated osteoblasts suppressed TNF-α-stimulated osteoclast formation and RANKL-induced osteoclast differentiation through increasing OPG/RANKL ratio.22 Previous study showed that geraniin played a role of TNF-α inhibitor on impairments of osteogenesis.18 In this study, we found that geraniin could significantly improve expression of Runx2 and Osx miRNA in BMSCs. Velázquez-González et al also showed that geraniin possessed antinociceptive and anti-inflammatory activities.23 As we all know, Runx2 is the most important osteogenic transcriptional factor playing a determinant role in the early stage of osteogenesis and is a significant marker of early osteogenic differentiation.24 It has been proved that TNF-α can stimulate osteoclast formation via NF-κB pathway.25 Recently, Fujii et al reported that TNF-α promoted the differentiation of osteogenesis through activation of NF-κB pathways.26 In this study, geraniin significantly suppressed TNF-α-induced expression of NF-κB/p65 protein and promoted p-IкB-α protein expression in BMSCs. Similarly, Xiao et al suggested that geraniin suppressed RANKL-induced NF-κB expression.18 Moreover, we suggested that geraniin could significantly reduce the TNF-α-induced p38 MAPK protein expression in BMSCs. Adhesion molecules are produced through stimulation of BMSCs by p38 MAPK signalling pathways.27 TNF-α can promote the secretion of Hepatocyte growth factor by BMSCs through p38 MAPK and PI3K/Akt pathways.28 Thus, NF-κB, ERK and c-Jun-NH2-terminal Kinase (JNK) pathways play an essential role for migration homing and adhesion of BMSCs.29
In summary, geraniin can inhibit TNF-α-induced impairments in BMSCs. Moreover, the mechanisms about protective effect of geraniin are mediated through NF-κB, IкB-α and p38 MAPK signalling pathways in BMSCs. It suggests that geraniin may be a new candidate drug for treating TNF-α-induced impairment of osteogenesis.
Footnotes
Contributors: CL: guarantor of integrity of the entire study, study concepts and manuscript review; CL and SG: study design, statistical analysis and manuscript editing; GX: definition of intellectual content; SG and GX: experimental studies, data acquisition, data analysis and manuscript preparation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kotake S, Nanke Y. Effect of TNFA on osteoblastogenesis from mesenchymal stem cells. Biochim Biophys Acta 2014;1840:1209–13. doi:10.1016/j.bbagen.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 2. Stanovici J, Le Nail LR, Brennan MA, et al. . Bone regeneration strategies with bone marrow stromal cells in orthopaedic surgery. Curr Res Transl Med 2016;64:83–90. doi:10.1016/j.retram.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 3. Wang X, Xi WC, Wang F. The beneficial effects of intracoronary autologous bone marrow stem cell transfer as an adjunct to percutaneous coronary intervention in patients with acute myocardial infarction. Biotechnol Lett 2014;36:2163–8. doi:10.1007/s10529-014-1589-z [DOI] [PubMed] [Google Scholar]
- 4. Bianco P, Riminucci M, Gronthos S, et al. . Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001;19:180–92. doi:10.1634/stemcells.19-3-180 [DOI] [PubMed] [Google Scholar]
- 5. Mankani MH, Kuznetsov SA, Wolfe RM, et al. . In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells 2006;24:2140–9. doi:10.1634/stemcells.2005-0567 [DOI] [PubMed] [Google Scholar]
- 6. Quarto R, Mastrogiacomo M, Cancedda R, et al. . Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001;344:385–6. doi:10.1056/NEJM200102013440516 [DOI] [PubMed] [Google Scholar]
- 7. Tan J, Xu X, Tong Z, et al. . Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res 2015;3:15003 doi:10.1038/boneres.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther 2016;7:44 doi:10.1186/s13287-016-0300-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev 2005;208:207–27. doi:10.1111/j.0105-2896.2005.00334.x [DOI] [PubMed] [Google Scholar]
- 10. Bradley JR. TNF-mediated inflammatory disease. J Pathol 2008;214:149–60. doi:10.1002/path.2287 [DOI] [PubMed] [Google Scholar]
- 11. Delmas PD. The use of bisphosphonates in the treatment of osteoporosis. Curr Opin Rheumatol 2005;17:512–6. doi:10.1097/01.bor.0000163448.51661.87 [DOI] [PubMed] [Google Scholar]
- 12. Kim H, Choi HK, Shin JH, et al. . Selective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in mice. J Clin Invest 2009;119:813–25. doi:10.1172/JCI36809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu X, Farmer P, Rubin J, et al. . Integration of the NfκB p65 subunit into the vitamin D receptor transcriptional complex: identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem 2004;92:833–48. doi:10.1002/jcb.20143 [DOI] [PubMed] [Google Scholar]
- 14. Hess K, Ushmorov A, Fiedler J, et al. . TNFD promotes osteogenic differentiation of human mesenchymal stem cells by triggering the Nf-κB signaling pathway. Bone 2009;45:367–76. doi:10.1016/j.bone.2009.04.252 [DOI] [PubMed] [Google Scholar]
- 15. Huang H, Zhao N, Xu X, et al. . Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif 2011;44:420–7. doi:10.1111/j.1365-2184.2011.00769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Y, He B, Zhang X, et al. . Osteoprotective effect of geraniin against ovariectomy-induced bone loss in rats. Bioorg Med Chem Lett 2015;25:673–9. doi:10.1016/j.bmcl.2014.11.081 [DOI] [PubMed] [Google Scholar]
- 17. Okabe S, Suganuma M, Imayoshi Y, et al. . New TNF-a releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense, Megusurino-ki. Biol Pharm Bull 2001;24:1145–8. doi:10.1248/bpb.24.1145 [DOI] [PubMed] [Google Scholar]
- 18. Xiao F, Zhai Z, Jiang C, et al. . Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp Cell Res 2015;330:91–101. doi:10.1016/j.yexcr.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 19. Yang N, Wang G, Hu C, et al. . Tumor necrosis factor a suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 2013;28:559–73. doi:10.1002/jbmr.1798 [DOI] [PubMed] [Google Scholar]
- 20. Wang N, Zhou Z, Wu T, et al. . TNF-α-induced NF-κB activation upregulates microRNA-150-3p and inhibits osteogenesis of mesenchymal stem cells by targeting ß-catenin. Open Biol 2016;6:150258 doi:10.1098/rsob.150258 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Cho DI, Kim MR, Jeong HY, et al. . Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 2014;46:e70 doi:10.1038/emm.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park K, Ju WC, Yeo JH, et al. . Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int J Mol Med 2014;33:178–84. doi:10.3892/ijmm.2013.1557 [DOI] [PubMed] [Google Scholar]
- 23. Velázquez-González C, Cariño-Cortés R, Gayosso de Lucio JA, et al. . Antinociceptive and anti-inflammatory activities of geranium bellum and its isolated compounds. BMC Complement Altern Med 2014;14:506 doi:10.1186/1472-6882-14-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu J, Li Z, Hou Y, et al. . Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res 2015;7:2527–35. [PMC free article] [PubMed] [Google Scholar]
- 25. Rementer CW, Wu M, Buranaphatthana W, et al. . An inducible, ligand-independent receptor activator of NF-κB gene to control osteoclast differentiation from monocytic precursors. PLoS One 2013;8:2 2 00 00. doi:10.1371/journal.pone.0084465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujii T, Kitaura H, Kimura K, et al. . IL-4 inhibits TNF-α-mediated osteoclast formation by inhibition of RANKL expression in TNF-α-activated stromal cells and direct inhibition of TNF-α-activated osteoclast precursors via a T-cell-independent mechanism in vivo. Bone 2012;51:771–80. doi:10.1016/j.bone.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 27. Xiao WL, Zhang DZ, Fan CH, et al. . Intermittent stretching and osteogenic differentiation of bone marrow derived mesenchymal stem cells via the p38MAPK-Osterix signaling pathway. Cell Physiol Biochem 2015;36:1015–25. doi:10.1159/000430275 [DOI] [PubMed] [Google Scholar]
- 28. Bocelli-Tyndall C, Trella E, Frachet A, et al. . FGF2 induces RANKL gene expression as well as IL1ß regulated MHC class II in human bone marrow-derived mesenchymal progenitor stromal cells. Ann Rheum Dis 2015;74:260–6. doi:10.1136/annrheumdis-2013-204235 [DOI] [PubMed] [Google Scholar]
- 29. Yang F, Chen H, Liu Y, et al. . Doxorubicin caused apoptosis of mesenchymal stem cells via p38, JNK and p53 pathway. Cell Physiol Biochem 2013;32:1072–82. doi:10.1159/000354507 [DOI] [PubMed] [Google Scholar]