Abstract
Purpose:
The characteristics of fetal thyroid on magnetic resonance (MR) imaging, including normal thyroid and disorders other than goiter have not been fully evaluated. Our aim was to assess fetal thyroid using three dimensional (3D) gradient echo (GRE) T1-weighted MR imaging and to examine the usefulness of this modality.
Materials and Methods:
The study included 27 3D GRE T1-weighted images from 26 fetuses. The largest possible region of interest (ROI) within the thyroid at the slice level depicting the thyroid was manually defined and three circular ROIs on neck muscle were manually defined on the image slices showing the highest signal intensity (SI) of the thyroid. Maximum and mean thyroid-to-muscle SI ratios (SIRs) were then calculated as SIR = maximum or mean thyroid SI/muscle SI.
Results:
The thyroid could not be identified in two cases. Fetal thyroid function was normal in 17 cases, and there were 7 cases of hypothyroidism (6 transient and 1 thyroid dysgenesis). There was no linear relationship between mean and maximum SIR and gestational age. The mean and maximum SIR in the cases of normal fetal thyroid were 1.85 ± 0.20 and 2.61 ± 0.39, and the mean and maximum SIR in fetal hypothyroidism were 1.58 ± 0.20 and 2.13 ± 0.37. Mean (P = 0.0088) and maximum (P = 0.0221) SIR values were significantly different between euthyroid and hypothyroid fetuses.
Conclusion:
Thyroid SIR measurement provided useful information regarding fetal thyroid function.
Keywords: magnetic resonance imaging, fetus, thyroid, T1-weighted imaging
Introduction
Fetal magnetic resonance (MR) imaging has become more widely available for the evaluation of the fetus, although fetal ultrasonography remains the primary imaging modality during pregnancy. Fetal MR imaging has mainly been applied for imaging of the central nervous system, but has also become accepted for the evaluation of other organs including neck, lungs, gastrointestinal and genitourinary systems.1
Congenital hypothyroidism is the most common congenital endocrine disorder, affecting 1 in 3000 to 4000 newborns. Congenital hypothyroidism is classified into permanent and transient hypothyroidism. Permanent hypothyroidism is a persistent deficiency of thyroid hormone. Transient congenital hypothyroidism is a temporary deficiency of thyroid hormone at birth, but then recovering to normal thyroid hormone production. Congenital hypothyroidism is caused by thyroid dysgenesis or dyshormonogenesis.2 Dysgenesis encompasses the defects of thyroid development including hemiagenesis, ectopy, and athyreosis. It is often sporadic and has a genetic origin in only 3% of cases. Dyshormonogenesis comprises defects in any step of hormonal synthesis, including hyporesponsiveness to thyroid-stimulating hormone (TSH), defects in thyroidal iodide transport and organification, and thyroglobulin synthesis, which are all characterized by a normally located thyroid gland with normal or increased volume. Thyroid development is essential for normal fetal growth and maturation of the central nervous system,3 and many reports have shown that congenital hypothyroidism is associated with an increased prevalence of congenital malformations.4–7
Fetal thyroid status can be examined by invasive techniques such as amniotic fluid or fetal blood sampling.8,9 In terms of imaging methods, previous studies have proposed the intrauterine evaluation of fetal thyroid using various ultrasound parameters such as thyroid diameter, circumference, and volume.10–12 However, even careful assessment of routine sonograms may fail to detect some cases of congenital hypothyroidism because thyroid dyshormonogenesis does not always result in a goiter, even at birth.13 To the best of our knowledge, there are only a few reports of fetal thyroid evaluation by MR imaging.14,15 Fetal thyroid can be identified as a hyperintense structure in the neck on T1-weighted imaging, and this finding can differentiate goiter from other neck masses more readily than ultrasonography.14,15 However, the characteristics of fetal thyroid, including normal thyroid and disorders other than goiter, have not been fully described. Herein, we assessed fetal thyroid using three dimensional (3D) gradient echo (GRE) T1-weighted imaging and evaluated the usefulness this imaging modality.
Materials and Methods
Patients
Fetal MR imaging was underwent between October 2006 and November 2009 in 55 cases in which fetal anomaly was suspected at second- or third-trimester fetal sonography screenings or in those in which sonography results were equivocal or undetermined. All patients were informed about the procedure and the safety of the technique by their obstetricians, and all provided their informed consent. Of 58 MR images from 55 patients, 27 MR images of 26 fetuses (gestational age 18 weeks 2 days to 41 weeks, mean 31 weeks 4 days) were performed using 3D GRE T1-weighted imaging through the fetal thyroid. Final diagnosis was determined by imaging, operation, and/or clinical course. In addition, a pediatric endocrinologist with 19 years of experience retrospectively evaluated the thyroid function by neonatal screening measurements of TSH and/or by history and physical examination, measurements of serum TSH and free-T3 and free-T4, bone maturation by knee X-ray, or autopsy findings.
Institutional Review Board approval was obtained for the study, and the requirement for informed consent for inclusion in the study was waived.
Imaging technique
Fetal MR imaging was performed with a 1.5-T Siemens Symphony MR unit (Siemens, Erlangen, Germany) with a phased-array body coil. For routine pelvic MR imaging, half-Fourier single shot turbo spin echo (HASTE) T2-weighted images (TR/TEeff, 1000–1400/96–104; flip angle, 160°; field of view, 320 mm; slice thickness, 3.0 to 5.0 mm; section gap, 0.9–1.5 mm; number of slices, 15–20; image matrix, 256 × 164; bandwidth, 476 Hz/pixel; 1 signal acquisition; scanning time, 20 seconds) were obtained through the fetus in the transverse, sagittal, and coronal planes. Next, T1-weighted images were obtained through the fetus in the coronal plane using 3D GRE (volumetric interpolated breath-hold examination [VIBE] TR/TE, 3.4–4.5/1.36–1.73; flip angle, 15°; field of view, 320 mm; slice thickness, 2–3 mm; number of slices, 32–72; image matrix, 256 × 208 [interpolation, 512 × 512]; bandwidth, 490 Hz/pixel; 1 signal acquisition; scanning time, 24–28 seconds), and parallel imaging (sense factor = 2) was used in some patients. All images were to be obtained during a single breath-hold. Neither sedatives nor IV gadolinium-based contrast material was used.
Image analysis
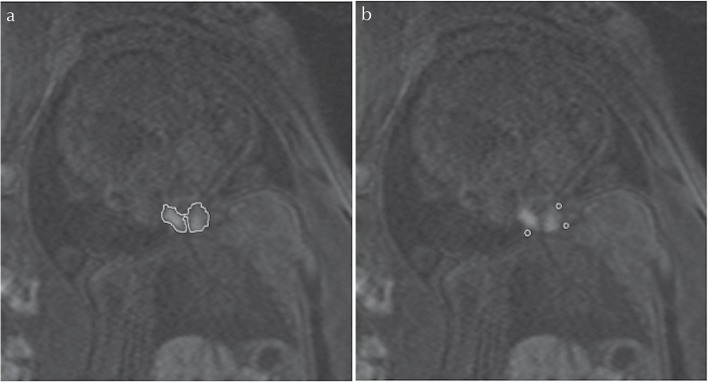
HASTE T2-weighted and 3D GRE T1-weighted MR images were analyzed on the monitor (EV Insite, PSP Corporation, Tokyo, Japan). All of the images were retrospectively reviewed by two radiologists (8 years and 1 year of experience in fetal MR imaging) who were without knowledge of the thyroid function. The largest possible region of interest (ROI) was manually defined within the thyroid at the slice level depicting the thyroid as the largest area (Fig. 1). The ROI provided quantitative values of mean and highest signal intensities (SIs). In addition, to obtain SIs of the neck muscle, three circular ROIs were manually defined on the slice image showing the greatest SI for the thyroid (Fig. 1). Muscle SI was calculated as mean SI of the three circular ROIs. Maximum and mean thyroid-to-muscle SI ratios (SIRs) were calculated for each case as follows: SIR = mean or maximum thyroid SI/muscle SI.
Fig 1.
Coronal 3D-gradient echo (GRE) images of a fetus at 41 weeks gestation with cervical lymphangioma show the regions of interest in thyroid (manual region of interest) and cervical muscle (circular region of interest) from which the signal intensity ratio (SIR) was calculated.
Statistical Analysis
Interobserver variability for ROI measurements was analyzed by an interclass correlation coefficient (ICC) as follows: ICC 0.00–0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.00, excellent.
Relationships between gestational age and thyroid volume in fetuses with normal thyroid were plotted and compared by standard regression techniques. We performed a statistical comparison of both mean and maximum SIR values and gestational ages of fetuses with normal thyroid and hypothyroidism using the Mann-Whitney U-test. A P-value of < 0.05 was considered to be statistically significant. Statistical analyses were performed using MedCalc for Windows, version 12.2.1 (MedCalc Software, Mariakerke, Belgium). Additionally, the sensitivity, specificity and accuracy for detection of congenital hypothyroidism were calculated.
Results
The thyroid was not identified in two cases, one (21 weeks 2 days) with cloacal exstrophy and the other (18 weeks 2 days) with the renal cystic disease. Both were intrauterine fetal deaths without autopsy. Thus, 25 MR images from 24 fetuses were finally included in this retrospective study. The gestational ages ranged from 25 weeks 4 days to 41 weeks (mean, 32 weeks). Five of the 24 fetuses were normal. Fetal abnormalities were as follows: central nervous system (CNS) abnormalities (n = 5), cervical lymphangioma (n = 1), chylothorax (n = 1), right aortic arch (n = 1), congenital diaphragmatic hernia (n = 1), congenital pulmonary airway malformation (n = 1), duodenal and jejunal atresia (n = 3), gastroschisis (n = 1), multicystic renal disease (n = 2, including one case of Down syndrome), fetal hydrops (n = 2, including one case of Down syndrome), and trisomy 18 (n = 1).
In terms of thyroid function, 17 of 24 fetuses had normal thyroids, and seven were diagnosed with congenital hypothyroidism, six of them with transient hypothyroidism and the seventh, by autopsy, with Down syndrome and thyroid dysgenesis. The gestational ages of the fetuses with normal thyroid function ranged from 28 weeks to 41 weeks (mean, 33 weeks 2 days), while those in cases of hypothyroidism ranged from 25 weeks 4 days to 34 weeks 4 days (mean, 30 weeks 6 days). A summary of the cases of fetal hypothyroidism is presented in Table 1.
Table 1.
Cases of fetal hypothyroidism
| GA at MRI | Diagnosis | Hypothyroidism | Mean SIR | Highest SIR | |
|---|---|---|---|---|---|
| 1 | 31w 0d | jejunal atresia | transient | 1.456 | 1.899 |
| 2 | 27w 6d | fetal | transient | 1.525 | 2.004 |
| 33w 0d | hydrops, CNS abnormality | 1.625 | 2.378 | ||
| 3 | 34w 4d | gastroschisis | transient | 2.000 | 2.865 |
| 4 | 25w 4d | IUGR, MCDK, Down synd. | dysgenesis | 1.522 | 1.778 |
| 5 | 34w 1d | jejunal atresia | transient | 1.618 | 2.194 |
| 6 | 31w 0d | 18 trisomy | transient | 1.595 | 2.217 |
| 7 | 29w 4d | fetal hydrops, Down synd. | transient | 1.324 | 1.718 |
GA, gestational age; MRI, MR imaging; SIR, signal intensity ratios; CNS, Central nervous system; IUGR, Intrauterine growth restriction; MCDK, multicystic dysplastic kidney.
The fetal thyroid was identified as a hyperintense structure in the neck on 3D GRE T1-weighted MR imaging, while the thyroid gland was not identified on HASTE T2-weighted imaging because it was isointense to surrounding structures.
The ICC was 0.651 for mean SIR and for 0.829 maximum SIR, indicating good and excellent correlation, respectively.
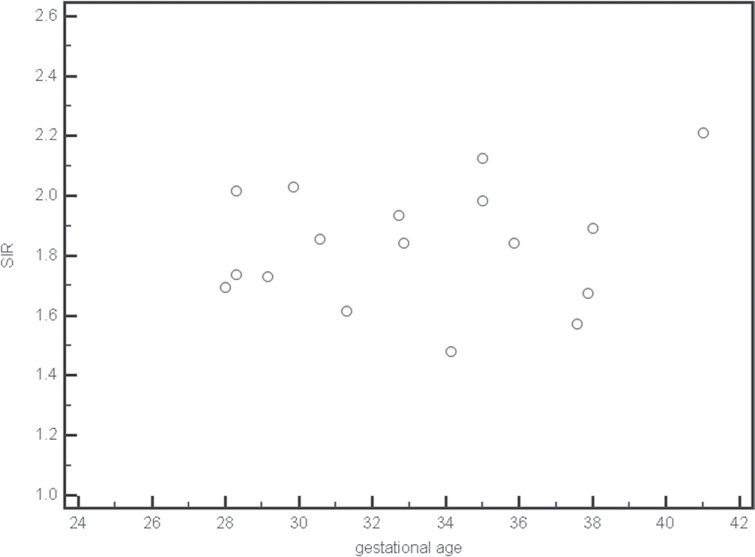
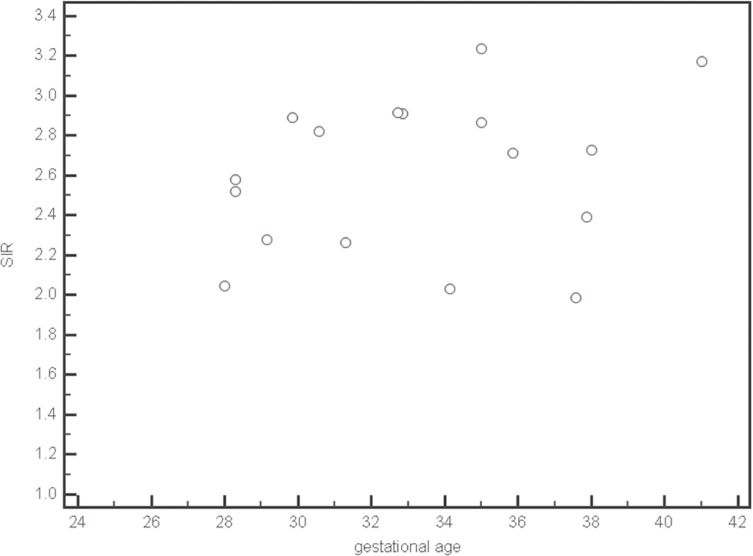
Figures 2 and 3 show gestational age plotted against the corresponding SIR for normal thyroid. There was no linear relationship between the mean and maximum SIR and gestational age.
Fig 2.
Scatter plots of mean signal intensity ratios (SIR) for normal thyroid at each gestational age.
Fig 3.
Scatter plots of maximum signal intensity ratios (SIR) of normal thyroid for each gestational age.
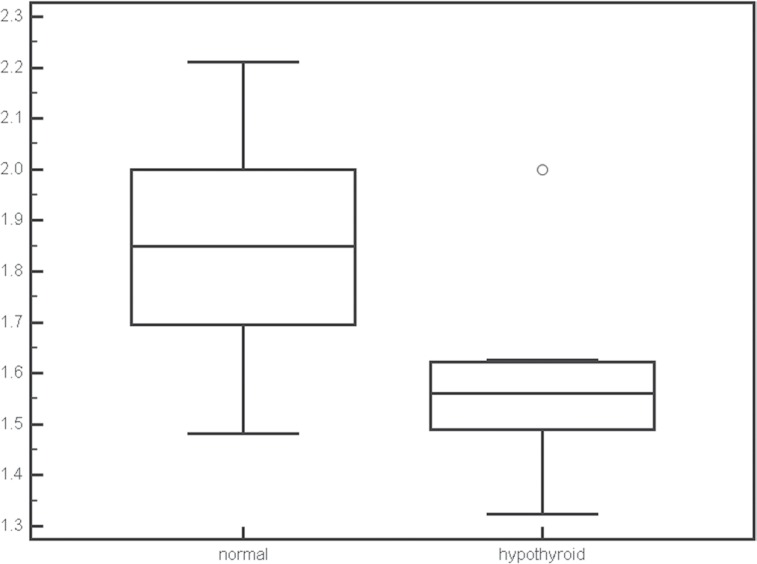
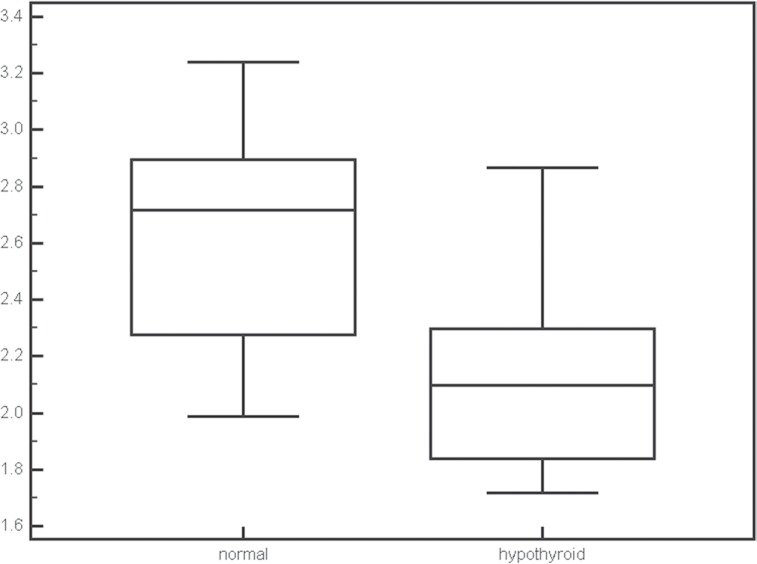
The mean and maximum SIRs in fetuses with normal thyroid were 1.85 ± 0.20 and 2.61 ± 0.39 and the mean and maximum SIRs in fetuses with hypothyroidism were 1.58 ± 0.20 and 2.13 ± 0.37. Both mean (P = 0.0088) and maximum (P = 0.0221) SIR values were significantly different between normal and hypothyroid fetuses (Figs. 4 and 5). The gestational ages were not significantly different between fetuses with normal thyroid and those with hypothyroid.
Fig 4.
Box plots of the mean signal intensity ratios (SIR) for normal thyroid and congenital hypothyroidism. The SIR was significantly different between normal thyroid and congenital hypothyroidism (P = 0.0088).
Fig 5.
Box plots of the maximum signal intensity ratios (SIR) for normal thyroid and congenital hypothyroidism. The signal intensity ratio (SIR) was significantly different between normal thyroid and congenital hypothyroidism (P = 0.0221).
We calculated the sensitivity, specificity, and accuracy using the data by one radiologist because the ICC was good or excellent. When we defined hypothyroidism as cases with a mean SIR value less than 1.70, the sensitivity, specificity, and accuracy were 87.5% (7/8), 70.6% (12/17), and 76% (19/25), respectively. When we defined hypothyroidism as cases with a higher SIR value less than 2.60, the sensitivity, specificity, and accuracy were 87.5% (7/8), 64.7% (11/17), and 72% (18/25), respectively.
Discussion
Although the fetal thyroid gland is indistinct and isointense to surrounding structures on HASTE T2-weighted imaging, it is identifiable on 3D GRE T1-weighted imaging as a hyperintense structure in the neck. This finding is consistent with previous reports.14 Single-shot fast spin-echo T2-weighted-imaging is a standard fetal MR imaging technique that provides excellent depictions of fetal anatomy at all gestational ages. This applies particularly to brain, fluid filled cavities (nasal and oral cavity, pharynx, trachea, stomach and small intestines, urinary system, gall bladder), lungs, placenta, and surface features including the profile.18 T1-weighted imaging may provide additional information, particularly in diagnosis of fetal gastrointestinal abnormalities, because meconium is more apparent on T1-weighted than T2-weighted images.19 In addition, recent reports have shown that 3D GRE T1-weighted imaging is useful for 3D understanding in the diagnosis and monitoring of fetal gastrointestinal tract malformations because the mean diameter of the fetal intestine is small.16,17 We consider that 3D GRE T1-weighted imaging is also suitable for the evaluation of fetal thyroid because the fetal thyroid is a small structure, but further study is needed to confirm the characteristics of the thyroid at early gestational ages.
Our results indicate that a calculation of thyroid SIRs may provide useful information for differentiating congenital hypothyroidism and normal thyroid, although there were some overlaps. This may be an advantage of fetal MR imaging particularly in cases of normal sized thyroid without goiter. Previous studies have also indicated that thyroid signal intensity on T1-weighted imaging may be a useful measure of thyroid function.20,21 T1 values were significantly higher in Hashimoto thyroiditis compared to those in healthy controls because the decrease in soluble thyroid protein and thyroglobulin content per unit weight of the gland due to the depletion of colloids causes prolongation of T1.21 Therefore, the hyperintensity of fetal thyroid might be attributed to viscosity of thyroid colloid, which consists primarily of thyroglobulin. Additionally, in normal fetuses with normal thyroid function, values of mean and maximum SIR were almost constant regardless of gestational age. The concentration of thyroglobulin does not change within the thyroid during fetal life.23,24 Consequently, the constant SIRs may be attributed to constant concentrations of thyroglobulin. However, the thyroids of two fetuses at early gestational ages were not identified. This may be due to the small size of the thyroid, thyroid dysgenesis, or insufficient concentration of thyroglobulin.
Mean and maximum SIR values were lower in cases of fetal hypothyroidism. We suggest that this finding reflected the prolongation of T1 values due to decreased thyroglobulin content.21 However, the clinical significance is not clear because 6 of the 7 cases of fetal hypothyroidism in our study were transient. Nevertheless, there were two cases of Down syndrome, and it is well known that infants with Down syndrome have an increased risk (up to 35-fold) of primary congenital hypothyroidism compared with infants in the general population.25,26 Although the cause of hypothyroidism in Down syndrome is not clear, van Trotsenburg et al. have suggested that it is thyroidal in origin because of a direct relation to the trisomic state of chromosome 21, hypothetically through the genomic dosage imbalance of dosage-sensitive genes interfering with thyroid hormone production, with IFNAR1 and IFNAR2 being possible candidate genes.27 In the present study, the SIRs in Down syndrome were particularly low. Consequently, we recommend that Down syndrome should be considered in the differential diagnosis for cases of suspected fetal hypothyroidism.
Among the cases of fetal hypothyroidism in our study, there were two cases of fetal hydrops. Fetal hydrops is characterized by an abnormal accumulation of fluid in two or more fetal compartments, including ascites, pleural effusion, pericardial effusion, and skin edema. In some cases, fetal hydrops may also be associated with polyhydramnios and placental edema. Fetal hydrops may be attributable to immune or nonimmune causes. Kessel et al. discussed the possible pathophysiologic association between congenital hypothyroidism and nonimmune fetal hydrops.28 Nonimmune fetal hydrops may be caused by lymphatic congestion attributable to an impairment of lymphatic flow and a delayed return of lymph to the vascular compartment. As the effects of thyroid hormone are mediated by adrenergic receptors, it is possible that thyroid hormone deficiency may be associated with reduced adrenergic stimulation of the lymphatic system, resulting in a sluggish flow of lymphatic fluid and engorgement of the lymphatic system, leakage of lymph into the pleura and the interstitial spaces, and, subsequently fetal hydrops.28 Although the cause of the two cases of fetal hydrops was not clear in the present study, we recommend evaluation of the thyroid by fetal MR imaging in cases of fetal hydrops.
The incidence of hypothyroidism in the present study was high (7/24). The incidence may be affected by the population in which fetal anomaly was suspected. Congenital hypothyroidism is associated with an increased prevalence of congenital malformations.4–7 As described above in discussion, congenital hypothyroidism has also some relationships with Down syndrome and fetal hydrops. Besides, the small population in our study may affect the incidence.
When necessary, fetal therapy can be instituted in cases of fetal hypothyroid. Fetal treatment options include intra-amniotic injection of L-thyroxine.29 Although preliminary, our data suggest that MR imaging of the fetal thyroid can be a useful tool for the evaluation of fetal thyroid function. Consequently, MR imaging evaluation may serve as a complementary modality to ultrasonography for deciding the timing of fetal blood sampling and for follow-up after treatment of fetal hypothyroid. Further study will be needed to support this.
Our study has some limitations. First, as described above, the study population was small. Therefore, additional studies will be required to validate the present results in a larger population. Second, our study did not evaluate the fetal thyroid function using cord blood or amniotic fluid in fetus, but only by examinations after birth. Therefore, we did not actually evaluate fetal thyroid function. Third, our study did not include any cases of fetal goiter. An additional study should be performed to evaluate the usefulness of this method for the clinical evaluation of fetal goiter.
Conclusion
Fetal thyroid was identifiable as a hyperintense structure in the neck on 3D GRE T1-weighted imaging. Our preliminary data suggest that thyroid SIR measurements based on 3D GRE T1-weighted images may provide useful information about fetal thyroid function.
Acknowledgments
We thank Eijirou Yamashita for technical assistance.
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Estroff JA. The growing role of MR imaging in the fetus. Pediatr Radiol 2009; 39 Suppl 2:S209–S210. [DOI] [PubMed] [Google Scholar]
- 2.Nagasaki K, Asami T, Ogawa Y, Kikuchi T, Uchiyama M. A study of the etiology of congenital hypothyroidism in the Niigata prefecture of Japan in patients born between 1989 and 2005 and evaluated at ages 5–19. Thyroid 2011; 21:361–365. [DOI] [PubMed] [Google Scholar]
- 3.Kratzsch J, Pulzer F. Thyroid gland development and defects. Best Pract Res Clin Endocrinol Metab 2008; 22:57–75. [DOI] [PubMed] [Google Scholar]
- 4.Oakley GA, Muir T, Ray M, Girdwood RW, Kennedy R, Donaldson MD. Increased incidence of congenital malformations in children with transient thyroid-stimulating hormone elevation on neonatal screening. J Pediatr 1998; 132:726–730. [DOI] [PubMed] [Google Scholar]
- 5.Kreisner E, Neto EC, Gross JL. High prevalence of extrathyroid malformations in a cohort of Brazilian patients with permanent primary congenital hypothyroidism. Thyroid 2005; 15:165–169. [DOI] [PubMed] [Google Scholar]
- 6.Gu YH, Harada S, Kato T, et al. Increased incidence of extrathyroidal congenital malformations in Japanese patients with congenital hypothyroidism and their relationship with Down syndrome and other factors. Thyroid 2009; 19:869–879. [DOI] [PubMed] [Google Scholar]
- 7.Kumar J, Gordillo R, Kaskel FJ, Druschel CM, Woroniecki RP. Increased prevalence of renal and urinary tract anomalies in children with congenital hypothyroidism. J Pediatr 2009; 154:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballabio M, Nicolini U, Jowett T, Ruiz de Elvira MC, Ekins RP, Rodeck CH. Maturation of thyroid function in normal human foetuses. Clin Endocrinol (Oxf) 1989; 31: 565–571. [DOI] [PubMed] [Google Scholar]
- 9.Thorpe-Beeston JG, Nicolaides KH, Snijders RJ, Felton CV, Vyas S, Campbell S. Relations between the fetal circulation and pituitary-thyroid function. Br J Obstet Gynaecol 1991; 98:1163–1167. [DOI] [PubMed] [Google Scholar]
- 10.Bromley B, Frigoletto FD, Cramer D, Osathanondh R, Benacerraf BR. The fetal thyroid: normal and abnormal sonographic measurements. J Ultrasound Med 1992; 11:25–28. [DOI] [PubMed] [Google Scholar]
- 11.Ho SS, Metreweli C. Normal fetal thyroid volume. Ultrasound Obstet Gynecol 1998; 11:118–122. [DOI] [PubMed] [Google Scholar]
- 12.Radaelli T, Cetin I, Zamperini P, Ferrazzi E, Pardi G. Intrauterine growth of normal thyroid. Gynecol Endocrinol 2002; 16:427–430. [PubMed] [Google Scholar]
- 13.Cavarzere P, Castanet M, Polak M, et al. Clinical description of infants with congenital hypothyroidism and iodide organification defects. Horm Res 2008; 70:240–248. [DOI] [PubMed] [Google Scholar]
- 14.Shinmoto H, Kashima K, Yuasa Y, et al. MR imaging of non-CNS fetal abnormalities: a pictorial essay. Radiographics 2000; 20:1227–1243. [DOI] [PubMed] [Google Scholar]
- 15.Kondoh M, Miyazaki O, Imanishi Y, Hayakawa M, Aikyou M, Doi H. Neonatal goiter with congenital thyroid dysfunction in two infants diagnosed by MRI. Pediatr Radiol 2004; 34:570–573. [DOI] [PubMed] [Google Scholar]
- 16.Brugger PC, Prayer D. Fetal abdominal magnetic resonance imaging. Eur J Radiol 2006; 57:278–293. [DOI] [PubMed] [Google Scholar]
- 17.Inaoka T, Sugimori H, Sasaki Y, et al. VIBE MRI for evaluating the normal and abnormal gastrointestinal tract in fetuses. AJR Am J Roentgenol 2007; 189:W303–W308. [DOI] [PubMed] [Google Scholar]
- 18.Brugger PC, Stuhr F, Lindner C, Prayer D. Methods of fetal MR: beyond T2-weighted imaging. Eur J Radiol 2006; 57:172–181. [DOI] [PubMed] [Google Scholar]
- 19.Farhataziz N, Engels JE, Ramus RM, Zaretsky M, Twickler DM. Fetal MRI of urine and meconium by gestational age for the diagnosis of genitourinary and gastrointestinal abnormalities. AJR Am J Roentgenol 2005; 184:1891–1897. [DOI] [PubMed] [Google Scholar]
- 20.Charkes ND, Maurer AH, Siegel JA, Radecki PD, Malmud LS. MR imaging in thyroid disorders: correlation of signal intensity with Graves disease activity. Radiology 1987; 164:491–494. [DOI] [PubMed] [Google Scholar]
- 21.Takashima S, Fukuda H, Tomiyama N, Fujita N, Iwatani Y, Nakamura H. Hashimoto thyroiditis: correlation of MR imaging signal intensity with histopathologic findings and thyroid function test results. Radiology 1995; 197: 213–219. [DOI] [PubMed] [Google Scholar]
- 22.Andreisek G, Froehlich JM, Hodler J, et al. Direct MR arthrography at 1.5 and 3.0 T: signal dependence on gadolinium and iodine concentrations—phantom study. Radiology 2008; 247:706–716. [DOI] [PubMed] [Google Scholar]
- 23.Costa A, De Filippis V, Panizzo M, et al. Development of thyroid function between VI–IX month of fetal life in humans. J Endocrinol Invest 1986; 9:273–280. [DOI] [PubMed] [Google Scholar]
- 24.van den Hove MF, Beckers C, Devlieger H, de Zegher F, De Nayer P. Hormone synthesis and storage in the thyroid of human preterm and term newborns: effect of thyroxine treatment. Biochimie 1999; 81:563–570. [DOI] [PubMed] [Google Scholar]
- 25.Fort P, Lifshitz F, Bellisario R, et al. Abnormalities of thyroid function in infants with Down syndrome. J Pediatr 1984; 104:545–549. [DOI] [PubMed] [Google Scholar]
- 26.Roberts HE, Moore CA, Fernhoff PM, Brown AL, Khoury MJ. Population study of congenital hypothyroidism and associated birth defects, Atlanta, 1979–1992. Am J Med Genet 1997; 71:29–32. [DOI] [PubMed] [Google Scholar]
- 27.van Trotsenburg AS, Kempers MJ, Endert E, Tijssen JG, de Vijlder JJ, Vulsma T. Trisomy 21 causes persistent congenital hypothyroidism presumably of thyroidal origin. Thyroid 2006; 16:671–680. [DOI] [PubMed] [Google Scholar]
- 28.Kessel I, Makhoul IR, Sujov P. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics 1999; 103:E9. [DOI] [PubMed] [Google Scholar]
- 29.Ribault V, Castanet M, Bertrand AM, et al. French Fetal Goiter Study Group Experience with intraamniotic thyroxine treatment in nonimmune fetal goitrous hypothyroidism in 12 cases. J Clin Endocrinol Metab 2009; 94: 3731–3739. [DOI] [PubMed] [Google Scholar]