Abstract
Purpose:
To evaluate the cranial pachymeningeal involvement of polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes (POEMS) syndrome using pre- and post-contrast fluid-attenuated inversion recovery (FLAIR) and T1-weighted imaging (T1WI).
Methods:
The appearance of pachymeningeal involvement in nine cases of POEMS syndrome was evaluated using pre- and post-contrast FLAIR and T1WI. The degree of pachymeningeal thickening was graded as normal or abnormal using pre-contrast FLAIR. The degrees of contrast enhancement effect were evaluated based on pre- and post-contrast images, and recorded in each of three separate anatomical areas, i.e., the falx cerebri, cerebral convexity, and tentorium cerebelli. The degrees of contrast enhancement of pachymeninges were graded as not detected (ND), positive, or prominent on post-contrast FLAIR, and normal range (NR), positive, and prominent on post-contrast T1WI.
Results:
Pre-contrast FLAIR demonstrated 41% of pachymeningeal anatomical regions as areas of thickening. Post-contrast FLAIR did not detect any contrast enhancement on 26% of the regions but showed positive enhancement on 30% and prominent enhancement on 44%. Post-contrast T1WI showed normal range enhancement on 48%, positive enhancement on 11%, and prominent enhancement on 41% of the regions. Post-contrast FLAIR showed the highest percentage for detection of pachymeningeal abnormalities (74%).
Conclusion:
Post-contrast FLAIR may contribute to objective judgment in the evaluation of pachymeningeal involvement in POEMS syndrome.
Keywords: POEMS syndrome, magnetic resonance imaging, pachymeninges, contrast enhancement, FLAIR
Introduction
Polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes (POEMS) syndrome is a rare systemic disease associated with underlying plasma cell dyscrasia. Although its pathogenesis is not yet fully understood, its clinical manifestations have been attributed to increased concentrations of serum or plasma vascular endothelial growth factor (VEGF).1 Although a diagnosis of POEMS syndrome can be suspected based on the clinical course, the infrequency of this disease has often resulted in diagnostic and therapeutic delay.2 No single test is sufficient to establish a diagnosis of POEMS syndrome. In addition, even with the previously established diagnostic criteria,1 there have been reports of patients not satisfying the criteria but for whom a therapeutic strategy also taking into consideration the possibility of POEMS syndrome is needed.2 In contrast, other cases have been described that despite satisfying these criteria continue to show a clinically benign course inconsistent with POEMS syndrome.3 The existence of such cases suggests that the recent criteria may be inappropriate in some situations but too strict in others to identify patients that require specific treatment for POEMS syndrome.
Therefore, there is a need for an examination that is easy to perform and that can reveal abnormalities specific to POEMS syndrome. The clinical manifestations of POEMS syndrome are thought to be associated with microangiopathy, neovascularization, and accelerated vasopermeability. Tissue vascular permeability is considered to increase in parallel with serum VEGF level. Therefore, VEGF released from plasma cells and/or platelets may induce edema and microangiopathy/macroangiopathy, potentially resulting in the various clinical features of POEMS syndrome. POEMS syndrome can be associated with cerebral infarction as a result of cerebral vasculopathy.4,5 Although contrast-enhanced cranial magnetic resonance imaging (MRI) of POEMS syndrome has revealed a high frequency of pachymeningeal enhancement,6,7 to our knowledge, there have been no previous studies using pre- and post-contrast fluid-attenuated inversion recovery (FLAIR) in the evaluation of pachymeningeal involvement of POEMS syndrome. This suggested that contrast-enhanced cranial MRI using both FLAIR and T1-weighted images (T1WI) may contribute to accurate and more specific evaluation of pachymeningeal involvement in POEMS syndrome.
In this study, we evaluated the distribution and imaging manifestations of cranial pachymeningeal involvement in POEMS syndrome using both pre- and post-contrast FLAIR and T1WI.
Materials and Methods
This study was approved by the local institutional review board, and informed consent was obtained from each patient for MRI examination using contrast medium. We encountered nine cases of POEMS syndrome diagnosed according to the clinical criteria of Dispenzieri.8 We performed cranial MRI to evaluate ischemic brain damage in POEMS syndrome. In addition, we also performed contrast-enhanced MRI using both pre- and post-contrast FLAIR and T1WI to evaluate pachymeningeal involvement.
Clinical and laboratory data of the nine cases of POEMS syndrome are listed in Table 1. Most clinical and laboratory examinations were performed before the MRI examination, but cerebrospinal fluid (CSF) examination was performed after cranial MRI. In our institution, the normal serum VEGF level is < 38 pg/ml, the normal CSF protein concentration range is 10–40 mg/dl, and the normal CSF pressure range is 50–150 mmH2O.
Table 1.
Patients’ characteristics and laboratory studies of 9 cases of POEMS syndrome
| Case | Age/Sex | Polyneuropathy | Organomegaly | Endocrinopathy | M protein | Systemic edema | Bone lesions | VEGF (pg/ml) | CSF protein (mg/dl) | CSF pressure (mmH2O) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64/M | + | + | + | IgAλ | + | Sclerotic | 609 | 105 | 150 |
| 2 | 69/F | + | + | + | IgGλ | + | Sclerotic | 2000 | 56 | 205 |
| 3 | 71/M | + | + | + | IgGλ | + | Sclerotic | 319 | 115 | 150 |
| 4 | 49/M | + | + | + | IgAλ | + | Lytic | 1690 | 65 | 200 |
| 5 | 65/F | + | + | + | IgAλ | + | Sclerotic | 1400 | 80 | 120 |
| 6 | 73/F | + | + | + | IgAλ | + | Sclerotic | 5020 | 210 | 195 |
| 7 | 56/M | + | + | + | IgAλ | + | Sclerotic | 6900 | 150 | 170 |
| 8 | 55/F | + | + | + | IgAλ | + | Sclerotic | 322 | 50 | 230 |
| 9 | 57/F | + | + | + | IgAλ | + | Sclerotic | 1160 | 101 | 140 |
CSF, cerebrospinal fluid; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes; VEGF, vascular endothelial growth factor.
MRI was performed using a 1.5T imager in seven cases and 3.0T imager in two cases (both Signa; GE Medical Systems, Milwaukee, WI). FLAIR imaging was performed using a fast FLAIR sequence with a repetition time/effective echo time/inversion time of 10000/175/2200 ms on 1.5T MRI and 9000/150/2400 ms on 3.0T MRI. T1WI was performed using the 2-dimensional spin echo (SE) method (TR, TE: 500, 8) in both 1.5T and 3.0T MRI examinations. We did not use a fat suppression technique. All of the imaging sequences were acquired with a slice thickness/interslice gap = 5/1 mm. In our institution, the routine MRI protocol for meningeal diseases includes both axial and coronal pre- and post-contrast FLAIR and T1WI, including diffusion-weighted, sagittal T1WI, and axial T2-weighted images. We adopted this protocol to evaluate the pachymeningeal involvement of POEMS syndrome. Post-contrast images were obtained after slow intravenous administration (manual injection) of 0.1 mmol/kg body weight gadopentetate dimeglumine (Gd-DTPA; Magnevist, Bayer Healthcare Pharmaceutical, Berlin, Germany, or Gd-DTPA- bismethylamide (Gd [DTPA-BMA]); Omniscan Daiichi-Seiyaku, Tokyo, Japan). Post-contrast FLAIR imaging was performed before post-contrast T1WI in all MRI examinations.
Three neuroradiologists (O.M., H.A., and Y.Y.) each with > 10 years of experience who were unaware of the diagnosis of POEMS syndrome reviewed all of the pre- and post-contrast MR images. The pachymeningeal thickness was evaluated using pre-contrast FLAIR and classified as normal or abnormal. Assessments of the degree and extent of the contrast enhancement effect using a pair of pre- and post-contrast FLAIR and T1WI were performed. Pre- and post-contrast images were compared side by side to determine the degree of enhancement effect. The degree of contrast enhancement effect in all cases was evaluated by all of the reviewers and recorded independently in each of three separate anatomical regions, i.e., falx cerebri, cerebral convexity, and tentorium cerebelli. After independent review, a consensus was reached regarding the results.
On post-contrast FLAIR, the normal dural sinus is occasionally enhanced,9 but the normal pachymeninges and cortical vessels are usually not enhanced; therefore, we determined the degree of enhancement as “not detected,” “positive” (i.e., detectable), or “prominent.” In addition, the degrees of contrast enhancement of the pachymeninges were graded as “normal,” “positive,” or “prominent” on post-contrast T1WI. As normal pachymeninges show segmental enhancement on post-contrast T1WI,10,11 we determined the degree of contrast enhancement based on accumulated past experience of normal range enhancement of post-contrast T1WI in our institution. Positive enhancement was determined as “more conspicuous” or “continuous enhancement” on T1WI evaluated by viewing several adjacent slices images and determined based on the consensus of all three reviewers. Prominent enhancement was determined as definitely continuous thick pachymeningeal enhancement on each post-contrast-FLAIR or T1WI.
Results
The results of the consensus regarding the range of pachymeningeal thickness of pre-contrast FLAIR and degree of post-contrast enhancement of FLAIR and T1WI of the pachymeninges by the three reviewers are shown in Tables 2–4. The detectability of abnormalities in each examination of different anatomical parts and summary data are shown in Table 5. Pre-contrast FLAIR showed 41% (11/27) abnormal regions. Although post-contrast T1WI showed enhancement in all examinations in all regions, 48% (13/27) of MRI examinations were judged to show a normal range in each of the regions (Fig. 1). Post-contrast FLAIR did not detect any contrast enhancement on 26% (7/27) of the regions, but showed positive enhancement on 30% (8/27) and prominent enhancement on 44% (12/27) (Figs. 2 and 3). Post-contrast T1WI showed prominent enhancement on 41% (11/27) (Fig. 3), and positive enhancement on 11% (3/27) of the regions. Post-contrast T1WI did not show prominent enhancement on the regions where post-contrast FLAIR did not reveal any enhancement. Detectability of pachymeningeal abnormality was highest in post-contrast FLAIR74% (20/27).
Table 2.
Pachymeningeal thickeness of 9 cases of POEMS syndrome: evaluation by pre-contrast FLAIR
| Case | Age/Sex | Magnet | Location and degree of contrast enhancement | ||
|---|---|---|---|---|---|
| Falx cerebri | Cerebral convexity | Tentorium cerebelli | |||
| 1 | 64/M | 1.5T | + | + | NR |
| 2 | 69/F | 1.5T | + | + | NR |
| 3 | 71/M | 1.5T | + | + | NR |
| 4 | 49/M | 3T | NR | NR | NR |
| 5 | 65/F | 1.5T | + | NR | NR |
| 6 | 73/F | 3T | + | + | + |
| 7 | 56/M | 1.5T | NR | NR | NR |
| 8 | 55/F | 1.5T | NR | NR | + |
| 9 | 57/F | 1.5T | NR | NR | NR |
NR, Normal range; +, Thickening; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes; FLAIR, fluid-attenuated inversion recovery.
Table 4.
Degree of pachymeningeal enhancement in 9 cases of POEMS syndrome: evaluation by post-contrast T1WI
| Case | Age/Sex | Magnet | Location and degree of contrast enhancement | ||
|---|---|---|---|---|---|
| Falx cerebri | Cerebral convexity | Tentorium cerebelli | |||
| 1 | 64/M | 1.5T | + + | + | NR |
| 2 | 69/F | 1.5T | + | + + | NR |
| 3 | 71/M | 1.5T | + + | + + | NR |
| 4 | 49/M | 3T | NR | NR | NR |
| 5 | 65/F | 1.5T | + + | NR | NR |
| 6 | 73/F | 3T | + + | + + | + + |
| 7 | 56/M | 1.5T | + + | NR | NR |
| 8 | 55/F | 1.5T | + + | NR | + + |
| 9 | 57/F | 1.5T | NR | + | NR |
NR, Normal range enhancement; +, Positive enhancement; + +, Prominent enhancement; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes.
Table 5.
Detectablility of pachymeningeal abnormality of POEMS syndrome
| Image modalitiy location | Falx cereberi | Crebral convexity | Tentorium cerebelli | Total |
|---|---|---|---|---|
| Pre-contrast FLAIR | 5/9(56%) | 4/9(44%) | 2/9(22%) | 11/27(41%) |
| Gd-FLAIR | 9/9(100%) | 7/9(78%) | 4/9(33%) | 20/27(74%) |
| Gd-T1WI | 7/9(78%) | 5/9(56%) | 13/9(22%) | 11/27(48%) |
POEMS, polyneuropathy, organomegaly, endocr monoclonal gammopathy, skin changes; FLAIR, fluid-attenuated inversion recovery.
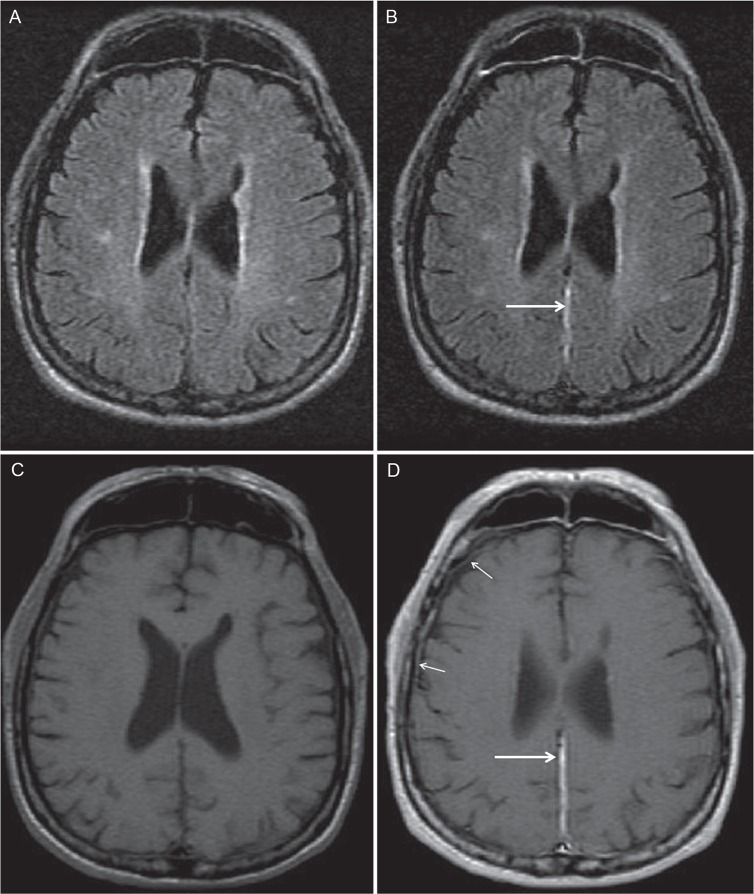
Fig 1.
Case 4. Coronal pre- and post-contrast FLAIR (A, B) and T1WI (C, D). Pre-contrast FLAIR showed normal range thickness of pachymeninges in both falx and convexity. Post-contrast FLAIR imaging shows positive enhancement of the falx cerebri, where post-contrast T1WI showed normal range enhancement (arrows). FLAIR, fluid-attenuated inversion recovery.
Fig 2.
Case 7. Axial pre- and post-contrast FLAIR (A, B) and T1WI (C, D). Pre-contrast FLAIR showed normal range thickness of pachymeninges in both falx and convexity. Both post-contrast FLAIR and T1WI showed prominent enhancement of the falx cerebri (arrows). Post-contrast T1WI showed normal range enhancement of the convexity, whereas post-contrast FLAIR showed negative enhancement (small arrows).FLAIR, fluid-attenuated inversion recovery.
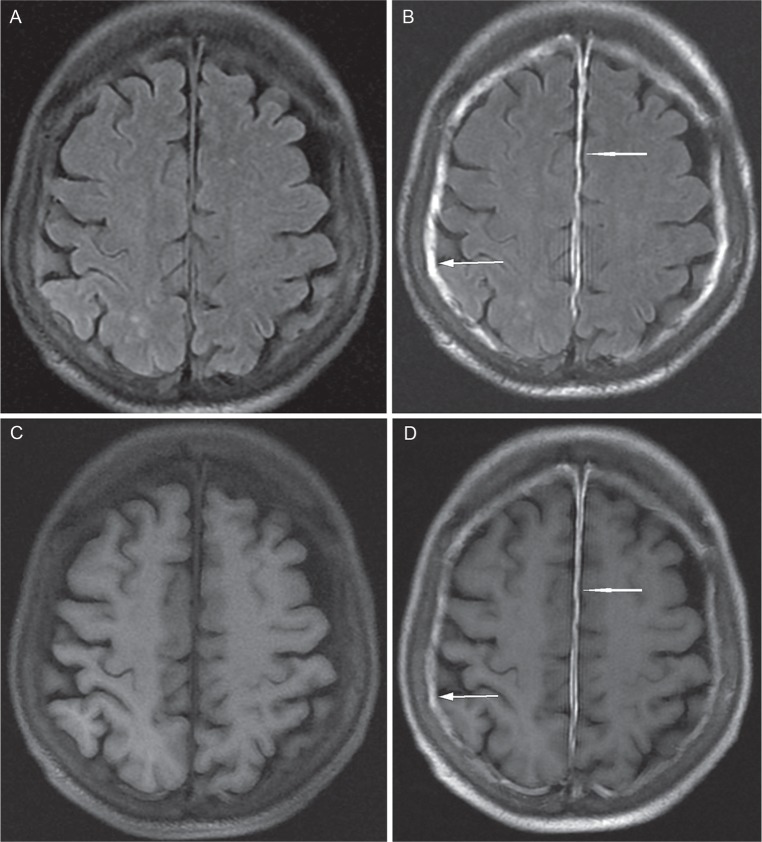
Fig 3.
Case 6. Axial pre- and post-contrast FLAIR (A, B) and T1WI (C, D). Pre-contrast FLAIR revealed thickened pachymeninges (arrows). Both pre-contrast FLAIR and T1WI showed prominent enhancement of the pachymeninges of both the falx cerebri and cerebral convexity (arrows). FLAIR, fluid-attenuated inversion recovery.
Discussion
Pachymeningeal involvement is an abnormality associated with POEMS syndrome that has already been discussed from the viewpoints of both imaging and histopathological features, with the former showing prominent enhancement and the latter showing hyperplasia of meningothelial cells, neovascularization, and obstructive vessel remodeling, without inflammation.6 POEMS syndrome has also been noted to manifest arterial and venous thrombosis that may result in cerebral infarction, and so some reports have attributed the MRI findings of POEMS syndrome to the vascular insults of intracranial vessel involvement.4,12 The performance of contrast-enhanced cranial MRI after routine brain examination can provide additional information in cases of probable POEMS syndrome. We consider there to be two aspects to the clinical impact of pachymeningeal involvement of POEMS syndrome revealed as prominent enhancement, the first being to help improve the diagnostic accuracy and the second to assess the therapeutic results in follow-up, because successful chemotherapy may be reflected by a reduction in the degree of pachymeningeal enhancement corresponding with that in the serum VEGF level, which is an index representing disease severity.7
With regard to pachymeningeal contrast enhancement on MRI, normal pachymeninges are highly vascularized but do not usually show enhancement on post-contrast FLAIR. However, in practice post-contrast FLAIR images showed more conspicuous linear pachymeningeal enhancement around the operation site than on post-contrast T1WI.9
Proliferation of meningothelial cells, narrowing or occlusion of arterioles, thickening of the media and endothelial hyperplasia, and neovascularization with prominent capillary endothelial cells within loose extracellular matrix were reported as characteristics of cranial pachymeningeal involvement in POEMS syndrome.6 Another ultrastructural study indicated opening of endothelial cell tight junctions in POEMS syndrome patients and suggested that alteration of microvascular hyperpermeability may be induced by VEGF.13 Gd distribution remodeling occurring with opening of endothelial cell tight junctions in the involved pachymeninges may reveal stronger pachymeningeal enhancement on both post-contrast FLAIR and T1WI. However, it is difficult to discuss the signal intensity in dural tissue correlated with Gd concentration.
Delayed enhancement of the dura has been reported in even spin-density- and T2-weighted images,14 meaning that when we evaluate the contrast enhancement superiority or inferiority between post-contrast FLAIR and post-contrast T1WI, it is important to consider image acquisition timing from intravenous injection. We performed post-contrast FLAIR first followed by post-contrast T1WI, and so the delayed enhancement effect was not more conspicuous in post-contrast FLAIR than post-contrast T1WI.
Although post-contrast T1WI usually shows enhancement of the pachymeninges because of the rich vascularity of the dura mater, discrimination between normal and abnormal enhancement is not easy. One reason is that the extent of normal pachymeningeal enhancement has a vague range even on standard post-contrast spin echo (SE) T1WI.10,11,14 Another reason is that the imaging appearance of post-contrast T1WI depends on the pulse sequence and parameters selected and the magnetic field strength of the machine.15,16 With regard to the contrast enhancement effect when using the same amount of contrast medium, MRI at 3.0T showed greater contrast effects than that using a 1.5T machine.16 Moreover, on comparison of contrast enhancement patterns on 3D gradient-echo and SE images, long segment enhancement of the normal pachymeninges is routinely observed on contrast-enhanced 3D gradient-echo images.11
The elevated VEGF level is thought to be one major causative factor of POEMS syndrome. Neovascular formation may induce prominent thick enhancement of the pachymeninges. Although we do not feel that prominent pachymeningeal enhancement is specific to POEMS syndrome, the thick pachymeningeal enhancement in POEMS syndrome may be related to the opening of tight junction microvasculature induced by VEGF. Therefore, in patients with POEMS syndrome-like symptoms not satisfying the current criteria, determination of a pachymeningeal abnormal enhancement by post-contrast FLAIR may contribute to decision-making on starting appropriate therapy for POEMS syndrome.
Our study had several limitations. First, evaluation of pachymeningeal involvement in POEMS syndrome was performed without pathological confirmation, and therefore the actual correlation between pathology and contrast enhancement effect was not determined. This study was retrospective in nature, and was therefore subject to bias. The sample size allowed descriptive analysis, but was too small for statistical analysis. We did not perform fat-suppressed MRI in T1WI, which is considered to be superior to FLAIR for depicting intracranial meningeal diseases,17 and so the pachymeninges on post-contrast T1WI may have been more conspicuous if fat suppression had been used. In addition, 3D imaging methods, such as 3D T2-FLAIR and 3D gradient-echo T1WI, are commonly used in the clinical MRI acquisition. 3D T2-FLAIR has high sensitivity to flow,17 and 3D gradient-echo T1WI shows normal dural enhancement more prominently than that revealed by SE T1WI.11 A correlation study of these two pulse sequences may further develop the discussion.
Conclusions
Pachymeningeal enhancement can be seen on both post-contrast FLAIR and T1WI in POEMS syndrome. Although the detectability of contrast enhancement is superior in post-contrast T1WI, post-contrast FLAIR may contribute to objective judgment in the evaluation of pachymeningeal involvement in POEMS syndrome.
Table 3.
Degree of pachymeningeal enhancement in 9 cases of POEMS syndrome: evaluation by post-contrast FLAIR
| Case | Age/Sex | Magnet | Location and degree of contrast enhancement | ||
|---|---|---|---|---|---|
| Falx cerebri | Cerebral convexity | Tentorium cerebelli | |||
| 1 | 64/M | 1.5T | + + | + + | ND |
| 2 | 69/F | 1.5T | + | + + | ND |
| 3 | 71/M | 1.5T | + + | + + | + |
| 4 | 49/M | 3T | + | + | ND |
| 5 | 65/F | 1.5T | + + | + | + |
| 6 | 73/F | 3T | + + | + + | + + |
| 7 | 56/M | 1.5T | + + | ND | ND |
| 8 | 55/F | 1.5T | + + | ND | + + |
| 9 | 57/F | 1.5T | + | + | ND |
ND, Not detected; +, Positive enhancement; + +, Prominent enhancement; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes; FLAIR, fluid-attenuated inversion recovery.
References
- 1.Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 2011; 86:591–601. [DOI] [PubMed] [Google Scholar]
- 2.Morizane R, Sasamura H, Minakuchi H, et al. A case of atypical POEMS syndrome without polyneuropathy. Eur J Haematol 2008; 80:452–455. [DOI] [PubMed] [Google Scholar]
- 3.Yishay O, Eran E. POEMS syndrome: failure of newly suggested diagnostic criteria to anticipate the development of the syndrome. Am J Hematol 2005; 79:316–318. [DOI] [PubMed] [Google Scholar]
- 4.Kang K, Chu K, Kim DE, Jeong SW, Lee JW, Roh JK. POEMS syndrome associated with ischemic stroke. Arch Neurol 2003; 60:745–749. [DOI] [PubMed] [Google Scholar]
- 5.Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology 2009; 73:1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briani C, Fedridgo M, Manara R, et al. Pachymeningeal involvement in POEMS syndrome: MRI and histopathological study. J Neurol Neurosurg Psychiatry 2012; 83:33–37. [DOI] [PubMed] [Google Scholar]
- 7.Briani C, Manara R, Lessi F, Citton V, Zambello R, Adami F. Pachymeningeal involvement in POEMS syndrome: dramatic cerebral MR improvement after lenalidomide therapy. Am J Hematol 2012; 87:539–541. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A. POEMS syndrome. Blood Rev 2007;21: 285–299. [DOI] [PubMed] [Google Scholar]
- 9.Goo HW, Choi CG. Post-contrast FLAIR MR imaging of the brain in children: normal and abnormal intracranial enhancement. Pediatr Radiol 2003; 33:843–849. [DOI] [PubMed] [Google Scholar]
- 10.Sze G, Soletsky S, Bronen R, Krol G. MR imaging of the cranial meninges with emphasis on contrast enhancement and meningeal carcinomatosis. AJNR Am J Neuroradiol 1989; 10:965–975. [PMC free article] [PubMed] [Google Scholar]
- 11.Farn JW, Mirowitz SA. MR imaging of the normal meninges: comparison of contrast-enhancement patterns on 3D gradient-echo and spin-echo images. AJR Am J Roentgenol 1994; 162:131–135. [DOI] [PubMed] [Google Scholar]
- 12.Garcia T, Dafer R, Hocker S, Schneck M, Barton K, Biller J. Recurrent strokes in two patients with POEMS syndrome and Castleman’s disease. J Stroke Cerebrovasc Dis 2007; 16:278–284. [DOI] [PubMed] [Google Scholar]
- 13.Scarlato M, Previtali SC, Carpo M, et al. Polyneuropathy in POEMS syndrome: role of angiogenic factors in the pathogenesis. Brain 2005; 128:1911–1920. [DOI] [PubMed] [Google Scholar]
- 14.Wessbecher FW, Maravilla KR, Dalley RW. Optimizing brain MR imaging protocols with gadopentetate dimeglumine: enhancement of intracranial lesions on spin-density- and T2-weighted images. AJNR Am J Neuroradiol 1991; 12:675–679. [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer CC, Fukui MB, Kanal E, Smirniotopoulos JG. MR imaging of the meninges part 1. normal anatomic features and nonneoplastic disease. Radiology 1996; 201: 297–308. [DOI] [PubMed] [Google Scholar]
- 16.Krautmacher C, Willinek WA, Tschampa HJ, et al. Brain tumors: full- and half-dose contrast-enhanced MR imaging at 3.0T compared with 1.5T—Initial experience. Radiology 2005; 237:1014–1019. [DOI] [PubMed] [Google Scholar]
- 17.Galassi W, Phuttharak W, Hesselink JR, Healy JF, Dietrich RB, Imbesi SG. Intracranial meningeal disease: comparison of contrast-enhanced MR imaging with fluid-attenuated inversion recovery and fat-suppressed T1-weighted sequences. AJNR Am J Neuroradiol 2005; 26:553–559. [PMC free article] [PubMed] [Google Scholar]