Abstract
The present case is the first report in Japan in which a breast cancer was discovered as a result of prospective magnetic resonance imaging (MRI) screening study for BRCA1/2 mutation carriers who were free of breast or ovarian cancer. This case is significant and it verifies the importance of MRI screening in breast or ovarian cancer-free BRCA1/2 mutation carriers who do not exhibit positive mammographic or ultrasonographic findings.
Keywords: hereditary breast and ovarian cancer syndrome, BRCA2, magnetic resonance imaging, ductal carcinoma in situ
Introduction
Hereditary breast cancer generally accounts for approximately 5% to 10% of all cases of breast cancer, and BRCA1/2 are widely known as representative genes that can cause hereditary cancer.1,2 Since the occurrence of BRCA1/2 pathogenic mutations in the germline is strongly related to the development of breast cancer or ovarian cancer, the condition is referred to as hereditary breast and ovarian cancer (HBOC) syndrome. Currently, attempts aimed at optimizing the clinical management of HBOC are being made by registering data on HBOC-related Japanese women.
We recently treated a patient with breast cancer whose tumor was discovered during a prospective magnetic resonance imaging (MRI) screening study for BRCA1/2 mutation carriers who were free of breast or ovarian cancer. The presently reported case is significant in that it verifies the importance of MRI screening in breast or ovarian cancer-free BRCA1/2 mutation carriers who do not exhibit positive mammographic or ultrasonographic findings.
Case Report
A 48-year-old woman presented with no particular chief complaints and no right nipple discharge.
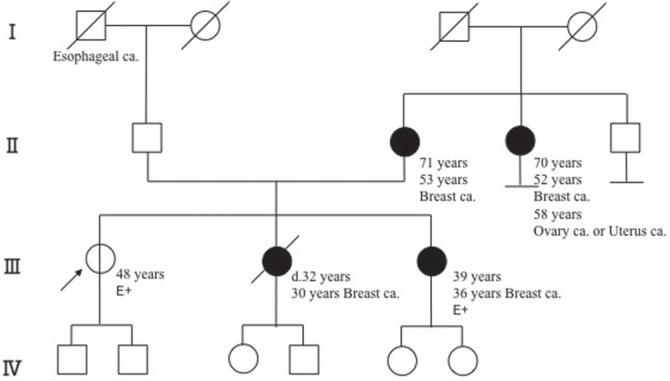
Family History: Her sister (second daughter) had developed breast cancer at the age of 30 years, another sister (third daughter) also had breast cancer at the age of 36 years and had tested positive for a BRCA2 mutation, her mother had suffered breast cancer at the age of 53 years, and her mother’s sister (second daughter) also had breast cancer at the age of 52 years and ovarian or uterine cancer at the age of 58 years (Fig. 1).
Fig 1.
Pedigree of a proband individual with a BRCA2 mutation. The arrow indicates outpatient (proband). E+ represents BRCA2 mutation positive. The numbers represent age and organs represent the site of cancer origin. Roman numerals on the left edge represent generations. Squares, males; circles, females; oblique line, deceased. Open circle is a mutation carrier with no onset of cancers.
Past History: No cancer and no particular disease.
Present Illness: After her younger sister was diagnosed as having breast cancer, the patient sought medical advice at the Department of Clinical Genetic Oncology, Cancer Institute Hospital, where she underwent a genetic checkup; the results revealed a BRCA2 pathologic mutation (in exon10, 1506delA, c. 1278delA, p. Lys426KfsX4). She provided informed consent and participated in a prospective study regarding the usefulness of MRI screening, among other studies aided by a Health Labour Sciences Research grant, and underwent imaging examinations at an entrusted clinic. She also underwent mammography (MicroDose mammography SI; Philips Digital Mammography Sweden AB), breast ultrasonography (US) (ACUSON S2000 US system; Siemens Medical Solutions, Mountain View, CA, USA), and contrast-enhanced dynamic MRI on the same day. All MRI images were acquired using a 3-T system (MAGNETOM Skyra 3T, Siemens Healthcare GmbH, Erlangen, Germany). A body coil was used for transmission and a double breast coil (16-channel breast array coil) for receiving. Dynamic MRI using a three dimensional (3D) fat-suppressed volumetric interpolated breath-hold examination (VIBE) sequence with parallel acquisition was performed before and three times after injection of a bolus of gadoterate meglumine (0.1 mmol/kg; Magnescope, Terumo) at a rate of 2 mL/s, followed by a 20 mL saline flush administered using an automatic injector. Both breasts were examined in the coronal plane on the first-, second-, and third-phase dynamic images acquired at 30 seconds, 1.5 minutes, and 4.5 minutes after contrast injection, respectively. The parameters for dynamic MRI were as follows: 4.1/1.7; flip angle, 10°; field of view, 33 cm; matrix, 480 × 384; receiver bandwidth, 390 Hz per pixel; interpolated slice thickness, 1.5 mm; partitions, 128; and time of acquisition, 61 seconds. The right and left breasts were examined in the sagittal plane using the VIBE sequence at 2.5 and 3.5 minutes after contrast injection—that is, between the second- and third- phase images, respectively (4.3/1.8; flip angle, 10°; field of view, 16 cm; matrix, 256 × 256; receiver bandwidth, 430 Hz per pixel; interpolated slice thickness, 1.2 mm; partitions, 88; time of acquisition, 60 seconds).
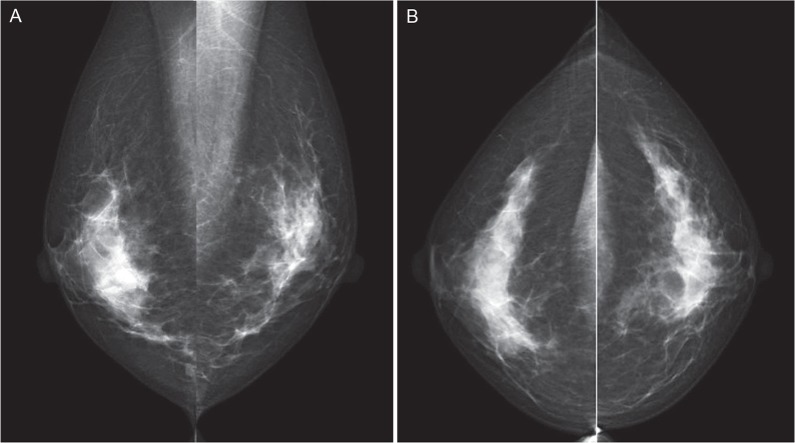
The mammograms (Fig. 2) and ultrasonograms (Fig. 3) were negative, but contrast-enhanced dynamic MRI scans disclosed an extensive segmental non-mass lesion primarily involving the 12 o’clock position of the right breast that was visualized even during the first-phase scan (Fig. 4a). The internal enhancement of the lesion showed branching ductal patterns (Fig. 4b) suggestive of ductal carcinoma in situ (DCIS). The lesion continuously extended directly to the nipple. The final diagnosis is Category 4 according to the breast imaging reporting and data system (BI-RADS).3
Fig 2.
No abnormal findings were detected on these bilateral mediolateral oblique (MLO) (A) and craniocaudal (CC) (B) mammograms.
Fig 3.
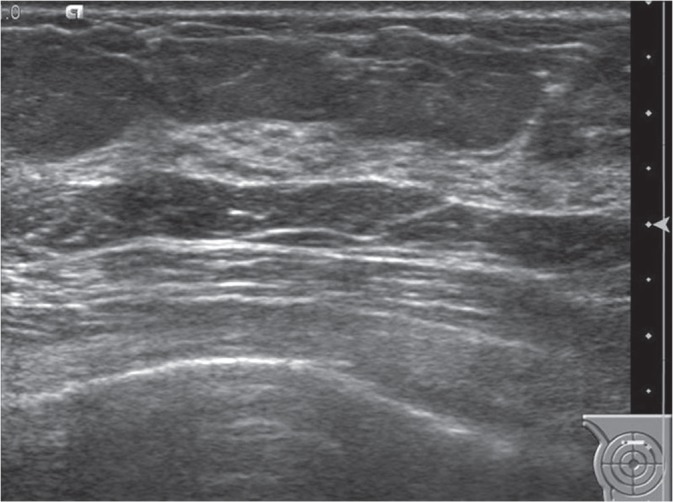
Ultrasonographic image of the right breast. There were no suspicious findings on the upper portion of the right breast.
Fig 4.
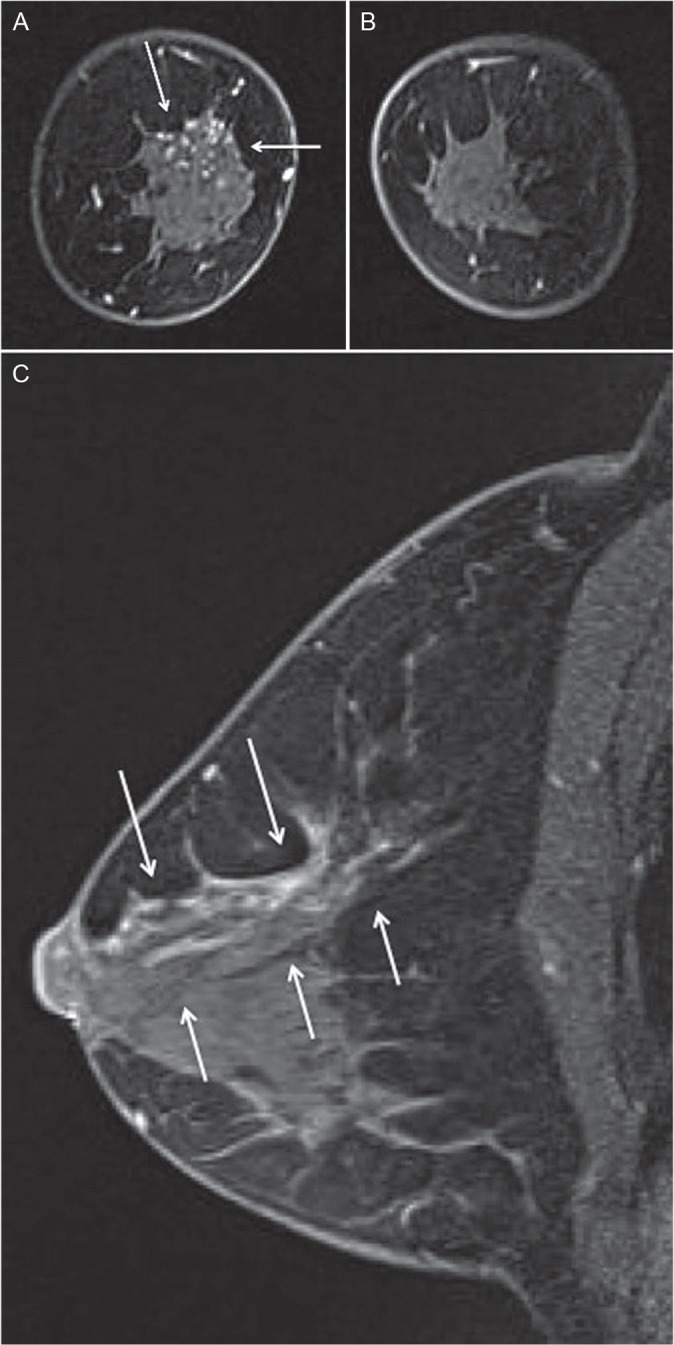
(A, B) Magnetic Resonance (MR) image of both breasts. Background parenchymal enhancement (BPE) is minimal. Coronal first contrast-enhanced fat-suppressed T1-weighted MR image showed segmental non-mass lesions (arrows) located at the upper portion of the right breast. (C) MR image of the right breast. Sagittal contrast-enhanced fat-suppressed T1-weighted MR image showed branching ductal patterns (arrows) located at the upper portion of the right breast.
At 1 month after the MRI examination, she underwent a second look US examination at the original facility, where an irregular-shaped hypoechoic area with ductal dilation was noted on the upper portion of the right breast (Fig. 5). Therefore, a checkup was performed using fine needle aspiration to identify changes in cellular atypia, but the results were uncertain; hence malignancy could not be ruled out. Within the next month, a US-guided vacuum-assisted biopsy was performed, leading to a diagnosis of DCIS (Fig. 6).
Fig 5.
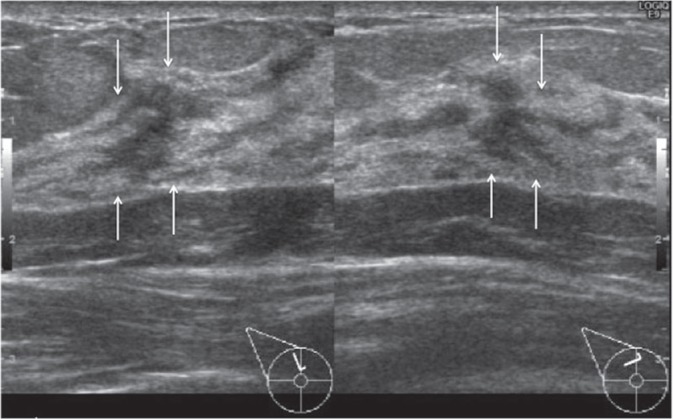
Second look ultrasonographic image of the right breast. There was an irregular-shaped hypoechoic area with ductal dilation (arrows) on the upper portion of the right breast.
Fig 6.
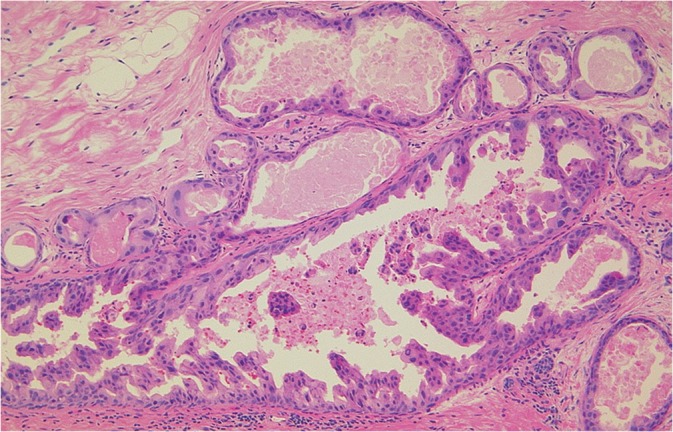
Hematoxylin and eosin staining sections showed ductal carcinoma in situ (DCIS), micropapillary type, intermediate grade nuclei (NG2).
At 3 months after the MRI examination, she underwent a right-sided mastectomy plus a sentinel lymph node biopsy. The lesion was pathologically diagnosed as DCIS, micropapillary type, intermediate grade nuclei (NG2), Estrogen receptor (ER) negative, progesterone receptor (PgR) negative, human epidermal growth factor receptor–2 (HER2) score 3+, with no evidence of lymph node metastasis. The extent of the lesion coincided with that depicted by the MRI study. At present, 3 months after the operation, the patient is healthy and has been progressing satisfactorily without the development of any other malignancy.
Discussion
Until relatively recently, the incidence of hereditary breast cancer in Japan was thought to be lower than that in Europe and the United States. The prevalence of BRCA1/2 germline mutations in Japanese patients was initially reported by Sugano et al. in 2008.4 They examined 135 cases using a full sequence analysis of the BRCA1/2 gene and found 28 types of deleterious mutations among 36 cases (26.7%), including 13 types of BRCA1 mutations in 17 cases (12.6%) and 15 types of BRCA2 mutations in 19 cases (14.1%). They reported that the prevalence of such mutations in Japanese subjects was significantly higher than that among non-Ashkenazi individuals (P = 0.005; odds ratio, 1.87; 95% confidence interval, 1.22–2.88). Accordingly, a study group entitled “Research on countermeasures for patients with hereditary breast cancer and disease-free carriers in Japan” was established by the Japanese Breast Cancer Society in 2010, leading to the subsequent inauguration of the Japanese HBOC Consortium. Through these activities, the results of an analysis of data on 320 subjects at 8 medical institutions were reported in 2015.5 They found that HBOC may have nearly the same prevalence in Japan as in the US or Europe. Thus, a nationwide database of HBOC is thought to be very important for developing risk models for BRCA1/2 carriers in Japan.
It had been presumed that mammographic screening might not be sufficient for the early detection of breast cancer, since the age at the onset of breast cancer is relatively low among BRCA mutation-positive individuals and since dense breasts are more frequently observed among young subjects. As well, because breast tissue harboring a BRCA mutation might be more vulnerable to ionizing radiation than genetically intact parenchyma, non-ionising radiation imaging techniques, such as MRI, are thought to be the main tool for surveillance in BRCA1/2 mutation carriers. In Europe and the United States, a vast amount of research on breast MRI screening in high-risk groups has been performed, with a paper published by Kuhl et al.6 in 2000 providing momentum.7–10 An analysis of data obtained in 3818 subjects (52 centers in the United States, Canada, UK, Holland, Germany, and Italy) revealed that the breast cancer detection rate for MRI was overwhelmingly higher (77–100%) than that for mammography (16–40%) or US (16–40%).8 In a prospective multicenter cohort study conducted to compare breast cancer detection rates for mammography, US and MRI in high-risk individuals whose blood relatives had breast cancer (the EVA trial),9 687 high-risk women underwent screenings using mammography, US, and MRI once yearly (1679 occasions). The significance of semi-annually ultrasonographic screening was assessed in 371 women. The combination of MRI and mammography yielded the highest diagnosis rate with a 100% sensitivity, whereas the sensitivity was 92.6% (25/27) for MRI alone and 92.6% (25/27) for a combination of MRI and US. Furthermore, for mammography alone, US alone, and a mammography-US combination, the diagnostic sensitivities were 33.3% (9/27), 37% (10/27), and 48.1% (13/27), respectively; these results were not considered satisfactory. Riedl et al. reported similar results demonstrating that the combination of MRI with mammography yielded the highest diagnosis rate of 95% (38/40); the authors concluded that additional US examinations did not contribute significant results.10
Furthermore, the EVA trial report9 furnished several lessons concerning MRI screening. The most important matter was the term “quality-assured breast MRI.” This term refers to three key points: performing Magnetic Resonance (MR) imaging using an appropriate method, the feasibility of an MRI-guided biopsy, and a sufficient skill in interpreting images using BI-RADS.3 In the EVA trial report, Kuhl et al. cited past reports concerning the high rates of false-negative cases obtained with MRI and the low sensitivity for DCIS detection, and they commented that the standardization of BI-RADS-MRI terms and methods for MRI image interpretation would improve these drawbacks.
Meanwhile, under the general recognition that we have already entered an era where breast MRI is needed as a mandatory tool for screening high-risk women in Japan, a guideline was released by the Japan Association of Breast Cancer Screening in 201311 The guideline provides descriptions with primary references to appropriate imaging methods for breast MRI and includes a description stressing the importance of image interpretation using BI-RADS. The usefulness of breast MRI screening for women bearing BRCA1 or BRCA2 gene mutations has been newly adopted as a clinical question (CQ) in the Japanese Breast Cancer Society Clinical Practice Guideline.12
The present case is the first report in Japan in which a breast cancer was discovered as a result of prospective MRI screening in a BRCA1/2 mutation gene carrier who was considered to be free of breast or ovarian cancer. It is clearly stated in the European and US guidelines that an environment amenable to the practice of MRI-guided biopsy is essential for MRI screening.8,13 MRI-guided biopsy is generally thought to be essential because MRI is utilized to detect early breast cancer that is undetectable using mammography or US. In Japan, however, MRI-guided biopsy is not covered by national health insurance. Therefore, such biopsies must be performed at the patients’ own expense or as part of clinical trials at a limited number of facilities.14 In the present case, an MRI-guided biopsy should have been performed. Instead, a US-guided biopsy was performed while referring to the MRI findings, leading to an accurate diagnosis.
The experience we gained in this case suggests that an accurate histologic diagnosis may be achieved using a US-guided biopsy even if an MRI-guided biopsy is not performed insofar as a detailed second look US can be performed. Using such an approach, a vacuum-assisted biopsy, which yields a greater volume of harvested tissue, might be preferable to fine needle aspiration.
In conclusion, we reported a case of breast cancer that was discovered as a result of prospective MRI screening study for BRCA1/2 mutation carriers who were free of breast or ovarian cancer. Further examination of accumulated cases is needed to verify the significance of MRI screening for BRCA1/2 mutation gene carriers who are considered to be free of breast cancer or ovarian cancer. Verifying the significance of MRI screening according to the type of breast cancer susceptibility gene (BRCA1 vs. BRCA2), patient age, cost performance, and frequency/interval of MRI scanning will also be important.
Footnotes
Funding
This work was supported by Health Labour Sciences Research grant (H26-policy for cancer-general-012).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994; 266:66–71. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda N, Miyoshi Y, Yoneda K, et al. Frequency of BRCA1 and BRCA2 germline mutations in Japanese breast cancer families. Int J Cancer 2001; 91:83–88. [DOI] [PubMed] [Google Scholar]
- 3.American College of Radiology Breast imaging reporting and data system (BI-RADS). 5th edn Reston, VA; 2013. [Google Scholar]
- 4.Sugano K, Nakamura S, Ando J, et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci 2008; 99:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura S, Takahashi M, Tozaki M, et al. Prevalence and differentiation of hereditary breast and ovarian cancers in Japan. Breast Cancer 2015; 22:462–468. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl CK, Schmutzler RK, Leutner CC, et al. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology 2000; 215:267–279. [DOI] [PubMed] [Google Scholar]
- 7.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004; 292:1317–1325. [DOI] [PubMed] [Google Scholar]
- 8.Saslow D, Boetes C, Burke W, et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin 2007; 57:75–89. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010; 28:1450–1457. [DOI] [PubMed] [Google Scholar]
- 10.Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015; 33:1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidelines for Breast MRI Screening for Breast Cancer High-Risk Groups. Japan Association of Breast Cancer Screening. 2013. (http://www.jabcs.jp/images/mri_guideline_fix.pdf). (Accessed Dec.19.2016).
- 12.Taira N, Arai M, Ikeda M, et al. The Japanese Breast Cancer Society clinical practice guideline for epidemiology and prevention of breast cancer. Breast Cancer 2015; 22:16–27. [DOI] [PubMed] [Google Scholar]
- 13.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 2008; 18:1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tozaki M, Yamashiro N, Sakamoto M, Sakamoto N, Mizuuchi N, Fukuma E. Magnetic resonance-guided vacuum-assisted breast biopsy: results in 100 Japanese women. Jpn J Radiol 2010; 28:527–533. [DOI] [PubMed] [Google Scholar]