Abstract
Purpose:
To investigate temporal changes in brain metabolites during the first year of life in preterm infants using multivoxel proton magnetic resonance spectroscopy (1H-MRS).
Methods:
Seventeen infants born at 29 (25–33) gestational week (median, range) weighing 1104 (628–1836) g underwent 1.5-T multivoxel 1H-MRS at 42 postconceptional week (PCW) and at 3, 6, 9, and 12 months after. We measured N-acetyl aspartate (NAA)/creatine (Cr), choline (Cho)/Cr, myo-inositol (Ins)/Cr, NAA/Cho, and Ins/Cho ratios in the frontal lobe (FL) and basal ganglia and thalamus (BG + Th). Linear regression analyses were performed to identify longitudinal changes in infants showing normal imaging findings and normal development. We also evaluated ratios of subjects with abnormal imaging findings and/or development using the 95% confidence intervals (CIs) of regression equations in normal subjects.
Results:
In the 13 infants with normal development, NAA/Cr and NAA/Cho ratios showed significant positive correlations with PCWs in the FL (r = 0.64 and 0.83, respectively, both P < 0.01) and BG + Th (r = 0.79 and 0.87, respectively, both P < 0.01), while Cho/Cr and Ins/Cr ratios revealed significant negative correlations with PCWs in the FL (r =−0.69 and −0.58, respectively, both P < 0.01) and BG + Th (r = −0.74 and −0.72, respectively, both P < 0.01). Ins/Cho ratios in the FL did not significantly correlate with PCWs (r = −0.19, P = 0.18), while those in the BG + Th showed significant negative correlation with PCWs (r = −0.44, P < 0.01). The metrics in the abnormal group were within the normal group 95% CIs in all periods except a few exceptions.
Conclusions:
Longitudinal multivoxel MRS is able to detect temporal changes in major brain metabolites during the first year of life in preterm infants.
Keywords: multivoxel magnetic resonance spectroscopy, preterm infants, brain metabolites
Introduction
Predicting psychomotor development delays is a major issue in the clinical management of preterm infants. Cranial ultrasonography (US) is the most effective imaging modality for identifying intraventricular hemorrhage and/or periventricular leukomalacia (PVL) to predict severe motor consequences such as spastic diplegia or quadriparesis in preterm infants.1 However, US is not sensitive enough to detect changes associated with less severe psychomotor retardation in preterm infants,1 and the utilities of several imaging techniques including structural magnetic resonance imaging (MRI), diffusion tensor imaging, and magnetic resonance spectroscopy (MRS) have been explored.1 Among these, proton MRS (1H-MRS) is hypothesized to provide additional diagnostic value because it can noninvasively measure various brain metabolites that are dramatically altered during rapid brain development that occurs within the first year of life.1,2 In term infants, MRS can reportedly identify changes in brain metabolites associated with normal development and perinatal asphyxia and to predict neurological outcomes.3–6 Although there are several reports with short observation periods,7–11 no study has investigated longitudinal changes in brain metabolites of preterm infants during the first year of life.
Here, we used multivoxel 1H-MRS to investigate temporal changes in the ratios of brain metabolites in preterm and low-birth-weight infants with normal/abnormal psychomotor development every 3 months during the first year of life.
Materials and Methods
Patients
This study was approved by the institutional ethics committee (number 23–37). From May to October 2011, we prospectively recruited 29 preterm infants (gestational age ≤34 weeks and birth weight ≤2000 g) who were admitted to the neonatal intensive care unit. Written informed consent to participate in the study was obtained from the parents of 21 (72%) infants, but 4 were excluded due to major anomaly (n = 1), renal failure (n = 1), voicelessness (n = 1), and severe maternal renal failure (n = 1). Ultimately, 17 infants were enrolled in the study. They were born at 29 (25–33) weeks of gestation (median, range) and weighed 1104 (628–1836) g. Their Apgar scores were 5 (1–8) at 1 min and 8 (3–9) at 5 min. The infants underwent cranial US and conventional MRI with multivoxel 1H-MRS at around 42 postconceptional weeks (PCW). Follow-up MRS examinations were performed 3, 6, 9, and 12 months after the baseline assessment (57, 70, 83, and 96 median PCWs, respectively). We also administered two batteries to evaluate infant psychomotor development. The developmental quotient (DQ) using the Enjoji Scale of Infant Analytical Development12 was assessed at 12 months. A DQ score ≥70 was defined as normal development.12,13 In addition, the Denver Developmental Screening Test II (DDST II) adopted for Japanese children was administered at 12 months.14 Subjects who failed at least 2 out of 46 items were defined as having abnormal development as previously described.14
Imaging protocol
MRI examinations were performed using a 1.5-Tesla scanner (Echelon Vega, Hitachi Medical Corporation, Tokyo) equipped with an eight-channel head coil. All subjects were sedated with oral triclofos sodium (50 mg/kg) 30 min before the examination. Multivoxel 1H-MRS data of the axial section through the frontal lobe (FL) and the basal ganglia and thalamus (BG + Th) were acquired using spin-echo with a point-resolved spectroscopy sequence with the following parameters: repetition time, 1500 ms; echo time, 35 ms; field of view, 180 × 180 mm; matrix size, 12 × 12; volume of interest, 90 × 90 × 15 mm; voxel size, 15 × 15 × 15 mm; number of excitations, one; acquisition time, 4 min 3 s. High-order global shimming, low-order local shimming, and optimization of radio frequency power for water suppression were automatically performed.
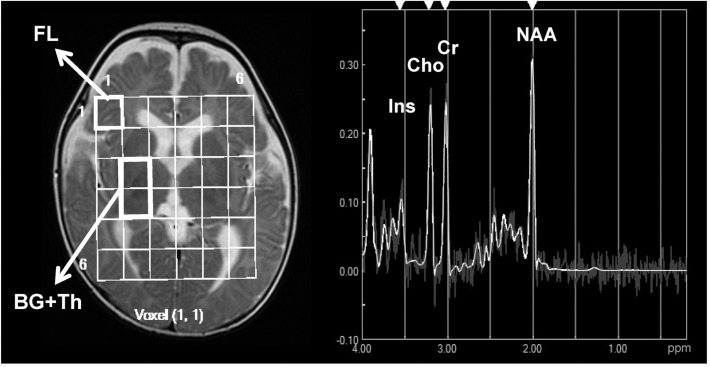
N-acetyl aspartate (NAA)/creatine (Cr), choline (Cho)/Cr, myo-inositol (Ins)/Cr, NAA/Cho, and Ins/Cho ratios were automatically measured in the FL and BG +Th voxels using built-in software (LCModel Ver. 6.1, S-Provencher Inc., Oakville, Ontario, Canada) (Fig. 1), and the mean values of the bilateral voxels were used for further analyses.
Fig. 1.
Representative multivoxel magnetic resonance spectroscopy output for a preterm infant with normal development (male, birth weight of 1142 g at 57 weeks postconception). The images show regions of interest (15 × 15 mm) in the frontal lobe (FL) and basal ganglia and thalamus (BG + Th) and the spectrum obtained from the FL using a spin-echo with a point-resolved spectroscopy sequence with a 1500-ms repetition time and 35-ms echo time. Ins, myoinositol; Cho, choline; Cr, creatine; NAA, N-acetyl aspartate.
Statistical analyses
Mann-Whitney U or Fisher’s exact tests were used to compare clinical characteristics between the two subject groups. Linear regression analyses were used to estimate temporal changes in NAA/Cr, Cho/Cr, Ins/Cr, NAA/Cho, and Ins/Cho ratios in subjects with normal imaging findings measured every 3 months. We obtained correlation coefficient, regression equation, and 95% confidence interval (CI) of the regression equation in each ratios. The values obtained in subjects with abnormal imaging findings and/or development were compared with the 95% CIs of the regression equations calculated from the normal subject data. Statistical analyses were performed using SPSS Ver. 20 (SPSS IBM Inc., Armonk, New York, USA). A P-value less than 0.05 was considered statistically significant. Data were presented as median (range) unless otherwise indicated.
Results
Thirteen of the 17 subjects (76%) showed no sonographic or MRI findings suggesting PVL, subependymal hemorrhage (SEH), or other central nervous system abnormalities at baseline. All 13 had a DQ score ≥ 70 according to the Enjoji Scale of Infant Analytical Development and a normal DDST II score and were classified as the normal group. The remaining four subjects showed abnormal imaging findings of PVL and/or SEH, and two had a DQ <70 and abnormal DDST II results. These subjects were classified as the abnormal group. Except for sex, clinical characteristics at birth such as birth weight, and Apgar scores were not significantly different between the two groups (Table 1).
Table 1.
Clinical characteristics of preterm infants group by development status
| Subjects with normal development (n = 13) | Subjects with abnormal imaging findings and/or abnormal development (n = 4) | P-value* | |
|---|---|---|---|
| Sex (male) | 9 (69%) | 0 (0%) | 0.03 |
| Gestational age (weeks) | 29 (25–33) | 28 (27–29) | 0.16 |
| Birth weight (g) | 1142 (638–1836) | 713 (628–1174) | 0.06 |
| Apgar score at 1 min | 5 (1–8) | 5 (3–6) | 0.56 |
| Apgar score at 5 min | 8 (3–9) | 8 (7–9) | 1.00 |
| DQ at 1 year | 97 (80–105) | 69 (60–85) [<70, 2] | <0.01 |
| DDST II at 1 year | 46 (45–46) | 42 (39–45) [<45, 2] | <0.01 |
| Abnormal MRI findings | 0 (0%) | 4 (100%) [PVL 2, SEH 1, PVL + SEH 1] | <0.01 |
Data are shown as n (%) or median (range).
Mann-Whitney U test or Fisher exact test. DQ, developmental quotient; DDST II, Denver Developmental Screening Test II; MRI, magnetic resonance imaging; PVL, periventricular leukomalacia; SEH, subependymal hemorrhage.
Because of parent/patient unavailability, the time points of the baseline and follow-up MRI examinations were distributed as follows: baseline, 42 (39–49) PCW, 15 cases (88%); 3 months, 57 (53–64) PCW, 15 cases (88%); 6 months, 70 (66–77) PCW, 16 cases (94%); 9 months, 83 (80–90) PCW, 7 cases (41%); and 12 months, 96 (92–104) PCW, 14 cases (82%).
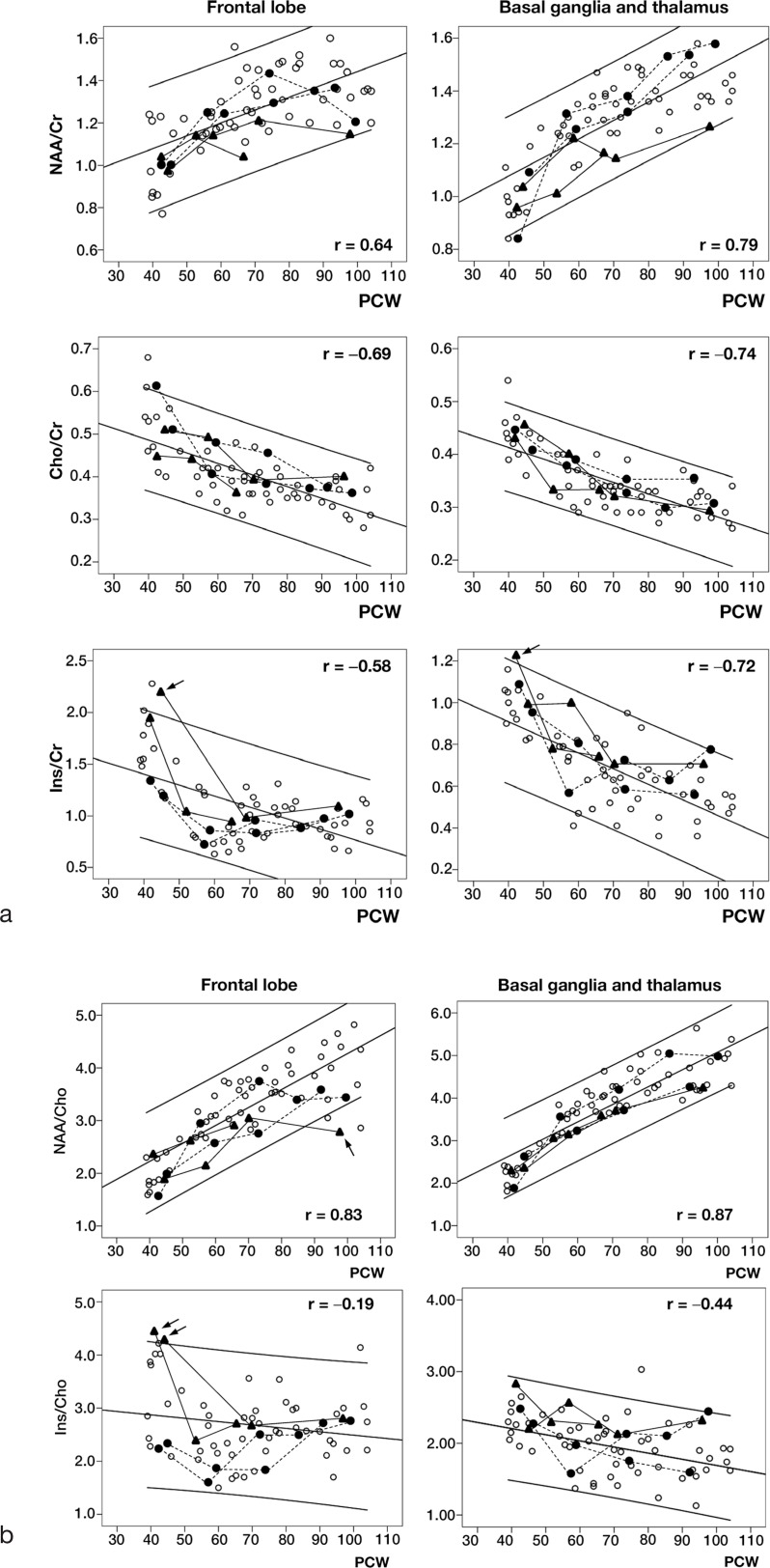
In the normal group, NAA/Cr ratios in the FL and BG + Th showed significant positive correlation with PCWs during the first year (r = 0.64 and 0.79, both P < 0.01; y = 0.0061x + 0.84 and y = 0.0070x + 0.80, respectively) (Fig. 2a). NAA/Cho ratios in the FL and BG + Th also revealed significant positive correlation with PCWs (r = 0.83 and 0.87, both P < 0.01; y = 0.0034x + 0.86 and y = 0.0041x + 0.98, respectively) (Fig. 2b). In contrast, significant negative correlations were observed in the FL and BG + Th for Cho/Cr ratios (r = −0.69 and −0.74, both P < 0.01; y = −0.0027x + 0.60 and y = −0.0022x + 0.50, respectively) and Ins/Cr ratios (r = −0.58 and −0.72, both P < 0.01; y = −0.011x + 1.83 and y = −0.0076x + 1.21, respectively) (Fig. 2a). The Ins/Cho ratios in the FL did not significantly correlate with PCWs (r = −0.19, P = 0.18), while those in the BG + Th demonstrated significant negative correlation with PCWs (r = −0.44, P < 0.01, y = −0.0086x + 2.55) (Fig. 2b).
Fig. 2.
Temporal changes in (a) NAA/Cr, Cho/Cr, Ins/Cr ratios, and (b) NAA/Cho, Ins/Cho ratios in the frontal lobe (FL) and basal ganglia/thalamus (BG + Th) in preterm infants during the first year of life. Open circles, infants with normal imaging findings and normal psychomotor development; closed circles with dotted lines, infants with abnormal imaging findings and normal development; triangles with solid lines, infants with abnormal imaging findings and abnormal development; parallel lines, regression equations and 95% confidence intervals of the regression equations; PCW, postconceptional week; r, correlation coefficient. Arrow indicates an infant with abnormal development whose metabolites ratio showed above or below the normal 95% confidence intervals.
The metrics in the abnormal group were within the normal group 95% CIs in all periods with a few exceptions (Fig. 2a, b). The baseline Ins/Cr ratio in one infant and Ins/Cho ratios in two infants with abnormal development were above the normal 95% CIs, and the NAA/Cho ratio in one infant with abnormal development at 100 PCWs was below the normal 95% CI range.
Discussion
Our results show that there are temporal changes in brain metabolites in preterm infants with normal or abnormal psychomotor development during the first year of life. In normally developing infants, NAA/Cr and NAA/Cho ratios showed significant positive correlation with PCWs, while Cho/Cr and Ins/Cr ratios revealed significant negative correlation with PCWs in both FL and BG + Th until 12 months of age. These findings are comparable to those reported for term infants.3
Among the metabolites that can be measured by 1.5-T 1H-MRS, NAA is considered to be related to neurons and myelinated axons.2 NAA can be detected in gray and white matter even in the early fetal period (16 weeks gestation); its level increases gradually during the late fetal period and rapidly during infancy.15 Hence, the temporal positive correlations with PCWs in NAA/Cr and NAA/Cho ratios in the FL and BG + Th in this study could reflect neuronal development and axon myelination.15 In contrast, the Cho/Cr ratio negatively correlated with PCWs in the FL and BG + Th. Cho is involved in membrane synthesis and is considered to be a marker of cell membrane turnover.2 The decrease in Cho observed during early brain development is commonly observed,6 although no clear explanation for this phenomenon has been provided. Furthermore, Ins, which is considered a marker of glial cells,2 negatively correlated with PCWs during the first year of life, suggesting a relative decrease in glial process volume during brain maturation.
We calculated ratios against Cr and Cho to standardize metabolite concentrations. Although Cr is considered a relatively stable metabolite in adult brain and is usually used as a reference compound for semi-quantitative analyses,2,6 its concentration within the brain is reported to increase during infancy.11,16 Therefore, we also calculated ratios with Cho, which is purported to decrease during infancy.6 The quantitative approaches are considered suboptimal in infants given the age-dependent alterations in these metabolites; however, there are no alternative methods because the levels of free water and other metabolites are dramatically altered during infancy.6
Several short-term 1H-MRS studies of preterm infants8,10,11,16 have reported increased NAA and/or Cr, decreased or stable Ins, and stable Cho levels. These results were somewhat different from our findings, but this could be due to the short time windows of MRS examinations (i.e., approximately 30–54 PCW). To our knowledge, the present study is the first to investigate temporal changes in major metabolites of the preterm infant brain during the first year of life, and our results can serve as reference data when assessing MRS abnormalities in preterm infants.
The metabolite ratios in infants with abnormal imaging findings and/or abnormal development were largely within the 95% CIs calculated for the normal group. However, the Ins/Cr ratio in one infant and Ins/Cho ratio in two infants with abnormal development were above the normal 95% CIs at baseline. The elevated Ins level around 40 PCW may reflect gliosis due to substantial neuronal damage17–21 and might be a useful marker for predicting psychomotor development in preterm infants. The observed decrease in NAA/Cho (and probably NAA/Cr) at 100 PCWs in one infant with abnormal development may support this hypothesis. Previous studies of neonatal encephalopathy reported that lactate/NAA is the most accurate marker for predicting developmental outcome.22 However, subjects in this study did not have substantial brain damage and thus showed no substantial lactate peaks on multivoxel MRS. Further research is needed to identify accurate imaging markers to predict psychomotor development in preterm infants.
The findings of this study should be interpreted in the context of several limitations. First, the parents of several subjects did not consent to enroll their children in the study, and <60% of consecutive candidates were included, which could have resulted in subject selection bias. In addition, the MRI examination time points were variable due to subject condition and/or scheduling conflicts. This made it difficult to compare metabolites at precise time points. We performed regression analyses to overcome this problem. Second, reliable tests for evaluating infant psychomotor development have not been fully established. Intelligence quotients (IQs) measured with the Wechsler Intelligence Scale for Children are widely used to assess psychomotor development. However, this battery cannot be administered in subjects younger than 4 years. Although we administered both the DQ and DDST II to improve reliability, the assessment of psychomotor development in this study has not been fully validated. Finally, there were some limitations with regard to our imaging technique. We used a multivoxel MRS technique instead of single- or dual-voxel MRS because the former can simultaneously obtain spectra in bilateral white matter and deep nuclei during a relatively short examination time. However, it was difficult to optimize voxel location compared with single/dual-voxel MRS. This issue may have resulted in contamination by the surrounding brain tissue. In addition, we adopted a relatively large voxel size because the 1.5-Tesla scanner we used has a relatively low signal-to-noise ratio and small chemical shift compared with higher field scanners. Further studies with larger sample sizes and smaller voxels with 3-Tesla scanners may improve metabolite concentration-measuring accuracy and allow clear discrimination between preterm infants with and without psychomotor abnormalities.
Conclusion
Longitudinal multivoxel MRS is able to detect temporal changes in major brain metabolites during the first year of life in preterm infants with normal and abnormal psychomotor development.
Acknowledgments
This work was supported by JSPS KAKENHI (Grant Number 23591513) and a Grant-in-Aid for Strategic Medical Science Research (S1491001) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Panigrahy A, Wisnowski JL, Furtado A, Lepore N, Paquette L, Bluml S. Neuroimaging biomarkers of preterm brain injury: toward developing the preterm connectome. Pediatr Radiol 2012; 42(Suppl 1):S33–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker PB, Bizzi A, De Stefano N, Gullapalli R, Lin DDM. eds. Introduction to MR spectroscopy in vivo. In: Clinical MR Spectroscopy: Techniques and Applications. Cambridge, UK: Cambridge University Press, 2010; 1–18. [Google Scholar]
- 3.Pi SY. Application of in vivo MR spectroscopy in neonatology. J Jpn Soc Premature Newborn Med 2003; 15:172–182. [Google Scholar]
- 4.van Doormaal PJ, Meiners LC, ter Horst HJ, van der Veere CN, Sijens PE. The prognostic value of multivoxel magnetic resonance spectroscopy determined metabolite levels in white and grey matter brain tissue for adverse outcome in term newborns following perinatal asphyxia. Eur Radiol 2012; 22:772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigrahy A, Nelson MD, Jr, Blüml S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol 2010; 40:3–30. [DOI] [PubMed] [Google Scholar]
- 6.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 1993; 30:424–437. [DOI] [PubMed] [Google Scholar]
- 7.Hüppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N. Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res 1991; 30:574–578. [DOI] [PubMed] [Google Scholar]
- 8.Hüppi PS, Schuknecht B, Boesch C, et al. Structural and neurobehavioral delay in postnatal brain development of preterm infants. Pediatr Res 1996; 39:895–901. [DOI] [PubMed] [Google Scholar]
- 9.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Hüppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2002; 48:949–958. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Bonifacio SL, Charlton NN, et al. MR spectroscopy of normative premature newborns. J Mag Reson Imaging 2011; 33:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blüml S, Wisnowski JL, Nelson MD, Jr, Paquette L, Panigrahy A. Metabolic maturation of matter is altered in preterm infants. PLoS One 2014; 9:e85829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Numata Y, Onuma A, Kobayashi Y, et al. Brain magnetic resonance imaging and motor and intellectual functioning in 86 patients born at term with spastic diplegia. Dev Med Child Neurol 2013; 55:167–172. [DOI] [PubMed] [Google Scholar]
- 13.Sherr EH, Shevell MI. Global developmental delay and mental retardation/intellectual disability. In: Swaiman KF, Ashwal S, Ferriero DM, Schor NF. eds. Pediatric Neurology, 5th ed Philadelphia, PA: Elsevier Saunders, 2012; 554–574. [Google Scholar]
- 14.Hallioglu O, Topalpglu AK, Zenciroglu A, Duzovali O, Yilgor E, Saribas S. Denver developmental screening test II for early identification of the infants who will develop major neurological deficit as sequelae of hypoxic-ischemic encephalopathy. Pediatr Int 2001; 43:400–404. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Nishina M, Matsushita K, Hori E, Mito T, Takashima S. Neuronal maturation and N-acetyl-L-aspartic acid development in human fetal and child brains. Brain Dev 1997; 19:131–133. [DOI] [PubMed] [Google Scholar]
- 16.Hüppi PS, Fusch C, Boesch C, et al. Regional metabolic assessment of human brain during development by proton magnetic resonance spectroscopy in vivo and by high performance liquid chromatography/gas chromatography in autopsy tissue. Pediatr Res 1995; 37:145–150. [DOI] [PubMed] [Google Scholar]
- 17.Robertson NJ, Kuint J, Counsell SJ, et al. Characterization of cerebral white matter damage in preterm infants using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab 2000; 20:1446–1456. [DOI] [PubMed] [Google Scholar]
- 18.Ashwal S, Holshouser B, Tong K, et al. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res 2004; 56:630–638. [DOI] [PubMed] [Google Scholar]
- 19.Robertson NJ, Lewis RH, Cowan FM, et al. Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr Res 2001; 50:692–700. [DOI] [PubMed] [Google Scholar]
- 20.Takado Y. Assessment of brainstem myo-inositol in multiple system atrophy using proton magnetic resonance spectroscopy on a 3.0 T system [in Japanese]. J Niigata Med Assoc 2010; 124:377–385. [Google Scholar]
- 21.Rutherford MA, Supramaniam V, Ederies A, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 2010; 52:505–521. [DOI] [PubMed] [Google Scholar]
- 22.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 2010; 125:e382–e395. [DOI] [PubMed] [Google Scholar]